Submitted:
20 November 2025
Posted:
21 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
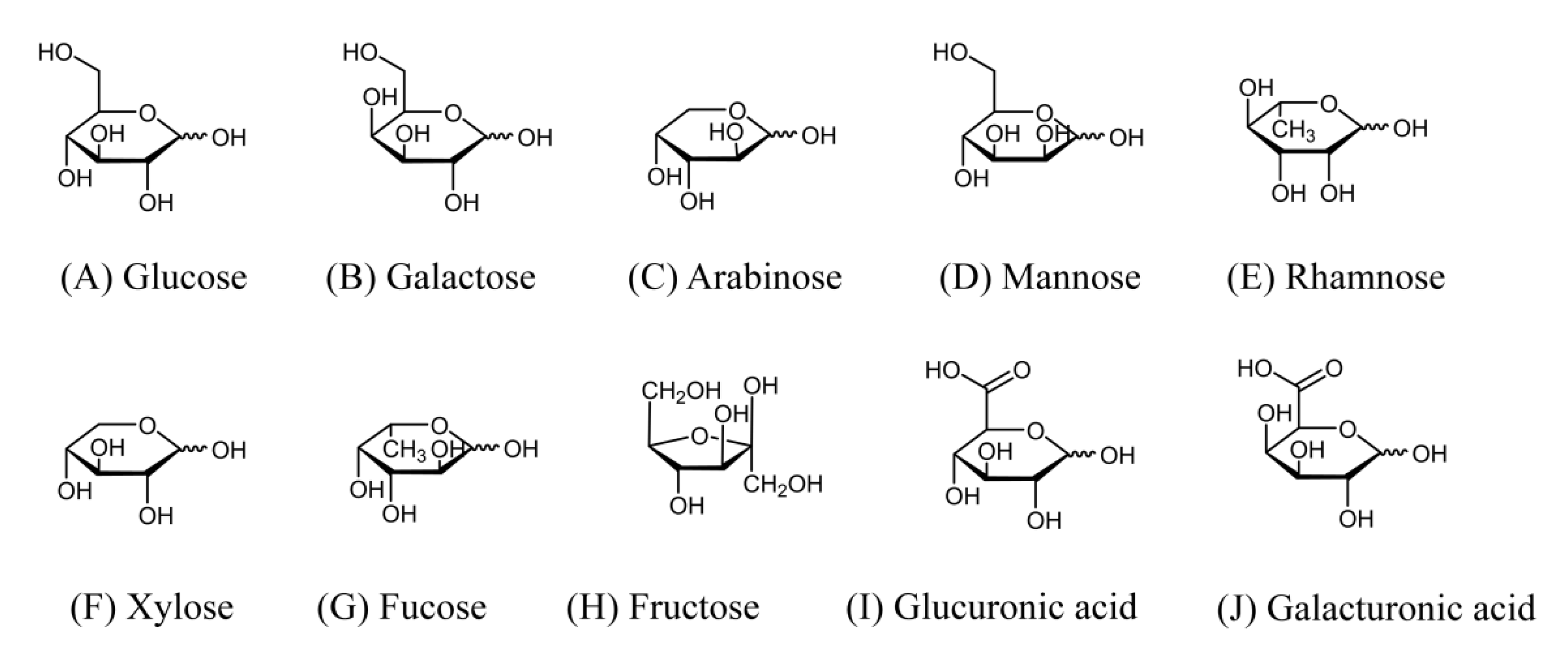
2. Main Monosaccharides Description
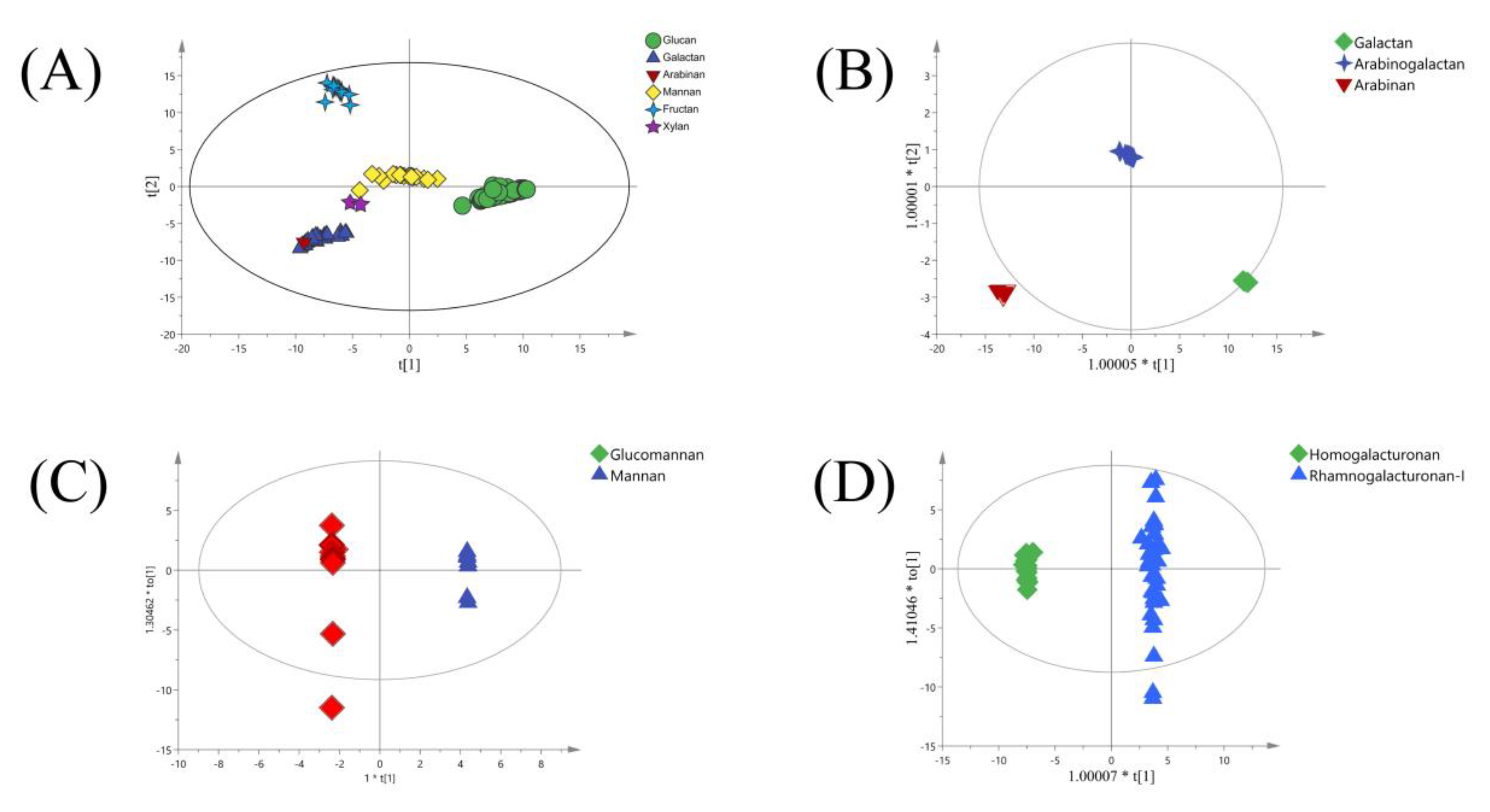
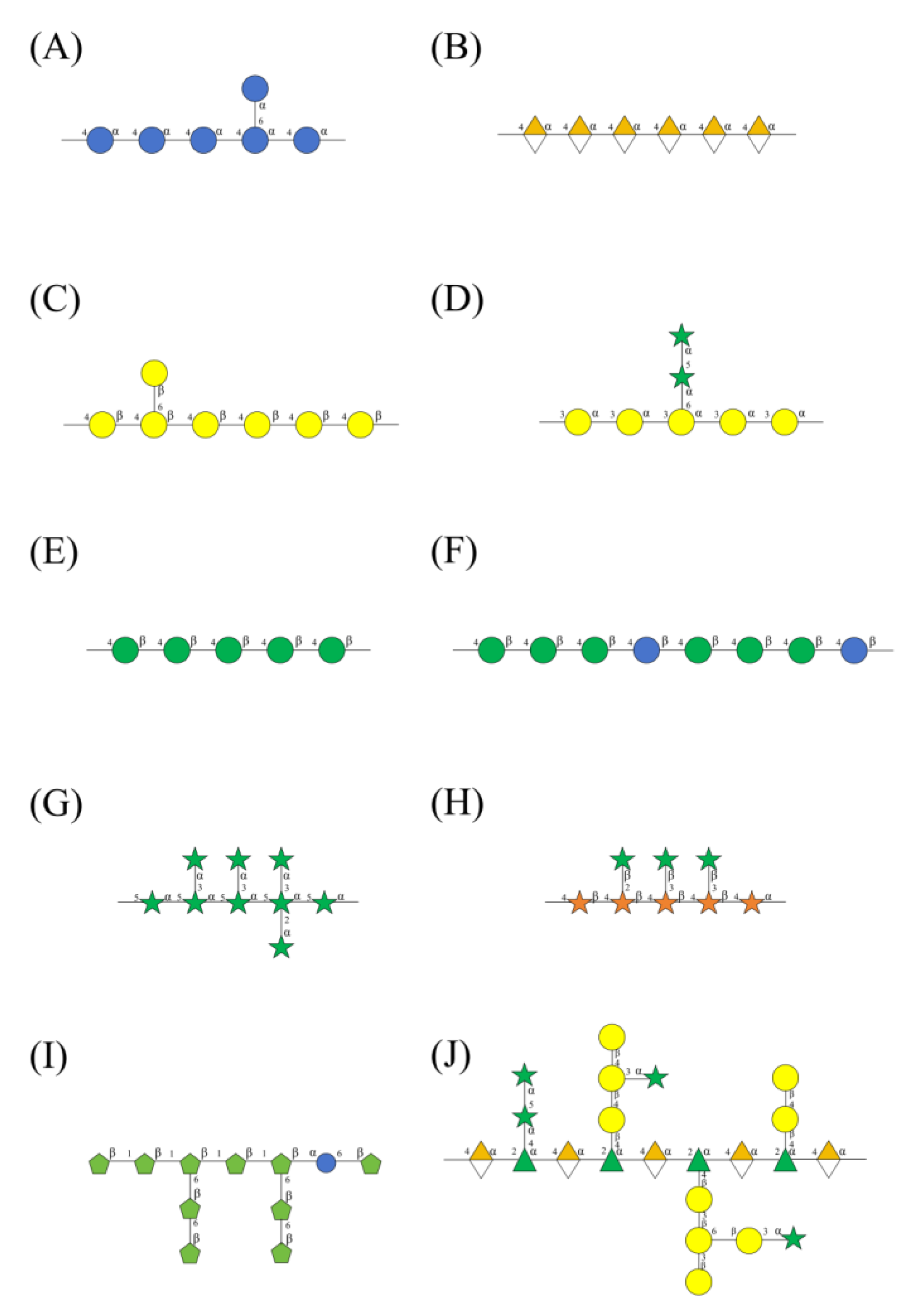
3. Classification of Medicinal Plant Polysaccharides
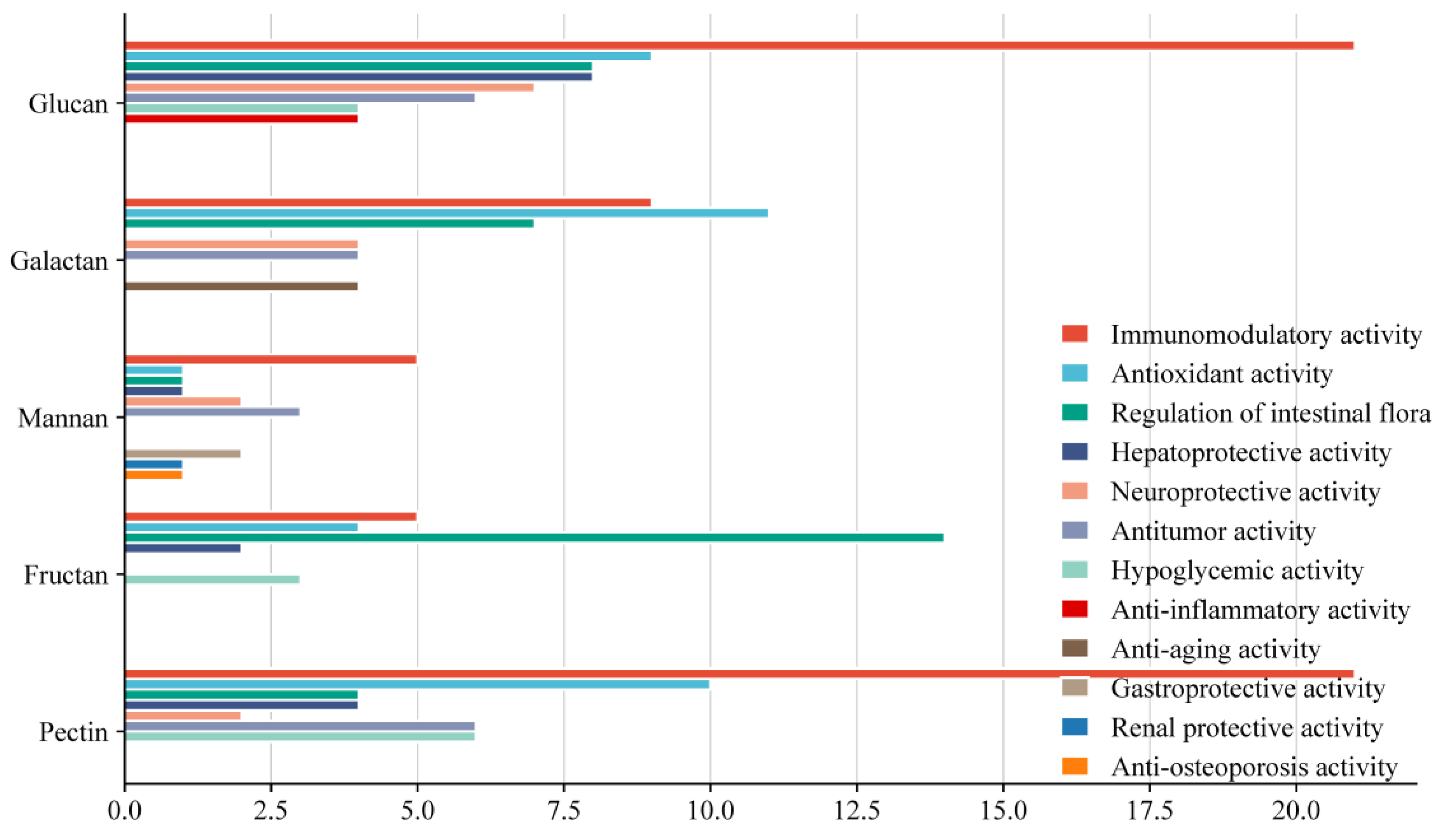
5.1. Immunomodulatory Activity
5.2. Antioxidant Activity
5.3. Antitumor Activity
5.4. Regulation of Intestinal Flora
5.5. Hypoglycemic Activity
6. Conclusions and Prospects
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- S.I. Choi, I.J. La, X. Han, X. Men, S.J. Lee, G. Oh, H.Y. Kwon, Y.D. Kim, G.S. Seong, S.H. Kim, O.H. Lee, Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice, Foods 11(13) (2022).
- S. Wu, H. Liu, S. Li, H. Sun, X. He, Y. Huang, H. Long, Transcriptome Analysis Reveals Possible Immunomodulatory Activity Mechanism of Chlorella sp. Exopolysaccharides on RAW264.7 Macrophages, Mar Drugs 19(4) (2021).
- X. Fan, K. Li, X. Qin, Z. Li, Y. Du, Advances in the Preparation and Bioactivity of Polysaccharides From Medicinal Plants With Different Molecular Weights: A Review, Chem Biodivers (2025) e03031.
- Q. Liu, J. Wu, P. Wang, Y. Lu, X. Ban, Neutral Polysaccharides From Hohenbuehelia serotina With Hypoglycemic Effects in a Type 2 Diabetic Mouse Model, Front Pharmacol 13 (2022) 883653.
- Z. Wang, Y. Zheng, Z. Lai, X. Hu, L. Wang, X. Wang, Z. Li, M. Gao, Y. Yang, Q. Wang, N. Li, Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review, Int J Biol Macromol 254(Pt 2) (2024) 127955.
- Z. Wang, X. Zhou, Z. Shu, Y. Zheng, X. Hu, P. Zhang, H. Huang, L. Sheng, P. Zhang, Q. Wang, X. Wang, N. Li, Regulation strategy, bioactivity, and physical property of plant and microbial polysaccharides based on molecular weight, Int J Biol Macromol 244 (2023) 125360.
- X. Bao, H. Yuan, C. Wang, J. Liu, M. Lan, Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi, Carbohydr Polym 98(1) (2013) 1236-43.
- Y. Ruan, C. Niu, P. Zhang, Y. Qian, X. Li, L. Wang, B. Ma, Acid-Catalyzed Water Extraction of Two Polysaccharides from Artemisia argyi and Their Physicochemical Properties and Antioxidant Activities, Gels 8(1) (2021).
- Y. Zhang, N. Li, H.X. Gong, C.J. Zhao, X.R. Bao, W. Liu, J. Gao, J.L. Zhang, H.S. Yin, Z.Q. Dong, Structural characterization and anti-tumor immunomodulatory effects of polysaccharides from Astragalus mongholicus with different cultivation modes, Int J Biol Macromol 318(Pt 4) (2025) 145233.
- Y. Li, J. Zheng, Y. Wang, H. Yang, L. Cao, S. Gan, J. Ma, H. Liu, Immuno-stimulatory activity of Astragalus polysaccharides in cyclophosphamide-induced immunosuppressed mice by regulating gut microbiota, Int J Biol Macromol 242(Pt 2) (2023) 124789.
- G. Chen, N. Jiang, J. Zheng, H. Hu, H. Yang, A. Lin, B. Hu, H. Liu, Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus, Int J Biol Macromol 241 (2023) 124386.
- M. Ye, M. Fan, Y. Zhao, F. Wang, X. Yang, W. Yao, X. Gao, J. Yu, W. Liu, Low molecular weight Astragalus membranaceus polysaccharides alleviates dextran sulfate sodium-induced colitis in mice, Carbohydr Polym 367 (2025) 124050.
- J. Fang, Y.X. Li, H.Y. Luo, W.H. Zhang, K.C. Chan, Y.M. Chan, H.B. Chen, Z.Z. Zhao, S.L. Li, C.X. Dong, J. Xu, Impacts of sulfur fumigation on the chemistry and immunomodulatory activity of polysaccharides in ginseng, Int J Biol Macromol 247 (2023) 125843.
- J. Zhang, M. Chen, C. Wen, J. Zhou, J. Gu, Y. Duan, H. Zhang, X. Ren, H. Ma, Structural characterization and immunostimulatory activity of a novel polysaccharide isolated with subcritical water from Sagittaria sagittifolia L, Int J Biol Macromol 133 (2019) 11-20.
- X. Zhang, L. Yu, H.T. Bi, X.H. Li, W.H. Ni, H. Han, N. Li, B.Q. Wang, Y.F. Zhou, G.H. Tai, Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer, Carbohydrate Polymers 77(3) (2009) 544-552.
- K.K. Hao, Q.J. Wang, S.X. Wei, H.Y. Si, J.W. Hao, N.F. Chen, N.D. Chen, X.Y. Gao, S.J. Liao, S.J. Zheng, M.M. Zhang, Structural characterization and anti-inflammatory activity of a neutral polysaccharide from Dendrobium huoshanense C. Z. Tang et S. J. Cheng, Int J Biol Macromol 302 (2025) 140339.
- M. Li, H. Yue, Y. Wang, C. Guo, Z. Du, C. Jin, K. Ding, Intestinal microbes derived butyrate is related to the immunomodulatory activities of Dendrobium officinale polysaccharide, Int J Biol Macromol 149 (2020) 717-723.
- J. Wang, H. Wang, H. Zhang, Z. Liu, C. Ma, W. Kang, Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-κB/MAPK signaling pathway, Int J Biol Macromol 132 (2019) 1024-1030.
- J. Wang, B. Ge, Z. Li, F. Guan, F. Li, Structural analysis and immunoregulation activity comparison of five polysaccharides from Angelica sinensis, Carbohydr Polym 140 (2016) 6-12.
- Q. Zhang, Y. Xu, J. Lv, M. Cheng, Y. Wu, K. Cao, X. Zhang, X. Mou, Q. Fan, Structure characterization of two functional polysaccharides from Polygonum multiflorum and its immunomodulatory, Int J Biol Macromol 113 (2018) 195-204.
- X. Yang, Y. Wu, C. Zhang, S. Fu, J. Zhang, C. Fu, Extraction, structural characterization, and immunoregulatory effect of a polysaccharide fraction from Radix Aconiti Lateralis Preparata (Fuzi), Int J Biol Macromol 143 (2020) 314-324.
- J. Liang, Y.X. Huang, X.H. Zhu, F.Y. Zhou, T.Y. Wu, J.F. Jia, X. Liu, H.X. Kuang, Y.G. Xia, Structural identification, rheological properties and immunological receptor of a complex galacturonoglucan from fruits of Schisandra chinensis (Turcz.) Baill, Carbohydr Polym 346 (2024) 122644.
- T. Zhao, F. Wang, Y. Guo, H. Ji, W. Zhang, G. Mao, W. Feng, Y. Chen, L. Yang, X. Wu, Structural characterization of a novel Schisandra polysaccharides and nutritional intervention in immunotoxicity to PCBs, Carbohydr Polym 258 (2021) 117380.
- J.Q. Chen, W.Y. Yuan, W. Miao, S.L. Gong, J. Zhou, Y. Liu, J.L. Wu, N. Li, In vitro and in vivo immune-enhancing effects of polysaccharides with different molecular weights and structural characteristics from Gastrodia elata Blume, Int J Biol Macromol 295 (2025) 139526.
- G. Cai, C. Wu, T. Zhu, S. Peng, S. Xu, Y. Hu, Z. Liu, Y. Yang, D. Wang, Structure of a Pueraria root polysaccharide and its immunoregulatory activity on T and B lymphocytes, macrophages, and immunosuppressive mice, Int J Biol Macromol 230 (2023) 123386.
- Z. Dong, M. Zhang, H. Li, Q. Zhan, F. Lai, H. Wu, Structural characterization and immunomodulatory activity of a novel polysaccharide from Pueraria lobata (Willd.) Ohwi root, Int J Biol Macromol 154 (2020) 1556-1564.
- B. Du, Y. Fu, X. Wang, H. Jiang, Q. Lv, R. Du, Y. Yang, R. Rong, Isolation, purification, structural analysis and biological activities of water-soluble polysaccharide from Glehniae radix, Int J Biol Macromol 128 (2019) 724-731.
- K. Li, X. Ran, J. Han, H. Ding, X. Wang, Y. Li, W. Guo, X. Li, W. Guo, S. Fu, J. Bi, Astragalus polysaccharide alleviates mastitis disrupted by Staphylococcus aureus infection by regulating gut microbiota and SCFAs metabolism, Int J Biol Macromol 286 (2025) 138422.
- Z. Huo, J. Li, X. Li, H. Xiao, Y. Lin, Y. Ma, J. Li, H. Yang, C. Zhang, Functional fractions of Astragalus polysaccharides as a potential prebiotic to alleviate ulcerative colitis, Int J Biol Macromol 271(Pt 1) (2024) 132580.
- B. Yang, Z. Xiong, M. Lin, Y. Yang, Y. Chen, J. Zeng, X. Jia, L. Feng, Astragalus polysaccharides alleviate type 1 diabetes via modulating gut microbiota in mice, Int J Biol Macromol 234 (2023) 123767.
- Y. Zhang, W. Ji, H. Qin, Z. Chen, Y. Zhou, Z. Zhou, J. Wang, K. Wang, Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner, Carbohydr Polym 349(Pt A) (2025) 122829.
- Y. Gao, M. Guo, J. Chen, Y. Sun, M. Wang, A ginseng polysaccharide protects intestinal barrier integrity in high-fat diet-fed obese mice, Int J Biol Macromol 277(Pt 1) (2024) 133976.
- L. Luo, X. Meng, S. Wang, R. Zhang, K. Guo, W. Wang, Z. Zhao, Hawthorn (Crataegus pinnatifida Bunge) polysaccharide improves the adaptability of crucian carp (Carassius auratus) to high alkalinity by regulating immunity, intestinal microbiota, and intestinal metabolomics, Int J Biol Macromol 320(Pt 4) (2025) 146088.
- S. Zhang, R. Zhou, X. Xie, S. Xiong, L. Li, Y. Li, Polysaccharides from Lycium barbarum, yam, and sunflower ameliorate colitis in a structure and intrinsic flora-dependent manner, Carbohydr Polym 349(Pt A) (2025) 122905.
- S. Huang, H. He, H. Li, C. Li, F. Wang, Y. Hu, Y. Liu, L. Chen, H. Chen, Modulating the intestinal flora involves the effect of Atractylodis macrocephalae Rhizoma polysaccharide on spleen deficiency diarrhea, Int J Biol Macromol 321(Pt 4) (2025) 146562.
- Y. Zhou, Y. Duan, S. Huang, X. Zhou, L. Zhou, T. Hu, Y. Yang, J. Lu, K. Ding, D. Guo, X. Cao, G. Pei, Polysaccharides from Lycium barbarum ameliorate amyloid pathology and cognitive functions in APP/PS1 transgenic mice, Int J Biol Macromol 144 (2020) 1004-1012.
- M. Xu, T. Yan, G. Gong, B. Wu, B. He, Y. Du, F. Xiao, Y. Jia, Purification, structural characterization, and cognitive improvement activity of a polysaccharides from Schisandra chinensis, Int J Biol Macromol 163 (2020) 497-507.
- M. Wen, M. Liu, Y. Zhang, B. Qiao, T. Luo, G. Liu, D. Li, B. Zhou, Gastrodia elata polysaccharide alleviates depression via gut microbiota modulation and Keap1-Nrf2/BDNF-TrkB pathway activation, Int J Biol Macromol 317(Pt 1) (2025) 144630.
- H. Liu, X. Zhi, J. Sun, Y. Li, L. Tao, B. Xiong, W. Lan, L. Yu, S. Song, Y. Zhou, Structural characterization of two polysaccharides from Gastrodia elata Blume and their neuroprotective effect on copper exposure-induced HT-22 cell damage, Int J Biol Macromol 311(Pt 3) (2025) 144019.
- C. Zhu, S. Ma, S. Zhang, F. Ma, B. Li, S. Wang, Z. Sun, Structural characterisation of a homogeneous polysaccharide from Gastrodia elata Bl. and its effects on two models of Alzheimer’s disease, Int J Biol Macromol 311(Pt 3) (2025) 143987.
- P. Wang, W. Liao, J. Fang, Q. Liu, J. Yao, M. Hu, K. Ding, A glucan isolated from flowers of Lonicera japonica Thunb. inhibits aggregation and neurotoxicity of Aβ42, Carbohydr Polym 110 (2014) 142-7.
- Y. He, W. Xu, Y. Qin, Structural characterization and neuroprotective effect of a polysaccharide from Corydalis yanhusuo, Int J Biol Macromol 157 (2020) 759-768.
- L. Xu, X. Zeng, Y. Liu, Z. Wu, X. Zheng, X. Zhang, Inhibitory effect of Dendrobium officinale polysaccharide on oxidative damage of glial cells in aging mice by regulating gut microbiota, Int J Biol Macromol 247 (2023) 125787.
- W. Tuo, S. Wang, Y. Shi, W. Cao, Y. Liu, Y. Su, M. Xiu, J. He, Angelica sinensis polysaccharide extends lifespan and ameliorates aging-related diseases via insulin and TOR signaling pathways, and antioxidant ability in Drosophila, Int J Biol Macromol 241 (2023) 124639.
- L. Lv, Y. Cheng, T. Zheng, X. Li, R. Zhai, Purification, antioxidant activity and antiglycation of polysaccharides from Polygonum multiflorum Thunb, Carbohydr Polym 99 (2014) 765-73.
- Z. Cheng, Q. Zheng, Y. Duan, M. Cai, H. Zhang, Effect of subcritical water temperature on the structure, antioxidant activity and immune activity of polysaccharides from Glycyrrhiza inflata Batalin, Int J Biol Macromol 261(Pt 1) (2024) 129591.
- P. Mutaillifu, K. Bobakulov, A. Abuduwaili, H. Huojiaaihemaiti, R. Nuerxiati, H.A. Aisa, A. Yili, Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra, Int J Biol Macromol 144 (2020) 751-759.
- J.S. Ma, H. Liu, C.R. Han, S.J. Zeng, X.J. Xu, D.J. Lu, H.J. He, Extraction, characterization and antioxidant activity of polysaccharide from Pouteria campechiana seed, Carbohydr Polym 229 (2020) 115409.
- L. Cai, B. Chen, F. Yi, S. Zou, Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity, Int J Biol Macromol 140 (2019) 907-919.
- Q. Zhang, J. Yu, L. Zhang, M. Hu, Y. Xu, W. Su, Extraction, characterization, and biological activity of polysaccharides from Sophora flavescens Ait, Int J Biol Macromol 93(Pt A) (2016) 459-467.
- L. Chen, Y. Zhang, L. Jin, R. Gao, J. Bao, B. Cui, Preparation, characterization and antioxidant activity of polysaccharide from Fallopia multiflora (Thunb.) Harald, Int J Biol Macromol 108 (2018) 259-262.
- J. Long, M. Li, C. Yao, W. Ma, H. Liu, D. Yan, Structural characterization of Astragalus polysaccharide-D1 and its improvement of low-dose metformin effect by enriching Staphylococcus lentus, Int J Biol Macromol 272(Pt 1) (2024) 132860.
- W. Liu, Z. Li, C. Feng, S. Hu, X. Yang, K. Xiao, Q. Nong, Q. Xiao, K. Wu, X.Q. Li, W. Cao, The structures of two polysaccharides from Angelica sinensis and their effects on hepatic insulin resistance through blocking RAGE, Carbohydr Polym 280 (2022) 119001.
- W. Liu, X. Lv, W. Huang, W. Yao, X. Gao, Characterization and hypoglycemic effect of a neutral polysaccharide extracted from the residue of Codonopsis Pilosula, Carbohydr Polym 197 (2018) 215-226.
- D. Guo, X. Yin, D. Wu, J. Chen, X. Ye, Natural polysaccharides from Glycyrrhiza uralensis residues with typical glucan structure showing inhibition on α-glucosidase activities, Int J Biol Macromol 224 (2023) 776-785.
- L.L. Gao, J.M. Ma, Y.N. Fan, Y.N. Zhang, R. Ge, X.J. Tao, M.W. Zhang, Q.H. Gao, J.J. Yang, Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation, Int J Biol Macromol 183 (2021) 1379-1392.
- G. Yuan, Y. Wang, H. Niu, Y. Ma, J. Song, Isolation, purification, and physicochemical characterization of Polygonatum polysaccharide and its protective effect against CCl(4)-induced liver injury via Nrf2 and NF-κB signaling pathways, Int J Biol Macromol 261(Pt 2) (2024) 129863.
- Y.Y. Chi, J.Y. Xiang, H.M. Li, H.Y. Shi, K. Ning, C. Shi, H. Xiang, Q. Xie, Schisandra chinensis polysaccharide prevents alcohol-associated liver disease in mice by modulating the gut microbiota-tryptophan metabolism-AHR pathway axis, Int J Biol Macromol 282(Pt 2) (2024) 136843.
- W. Cao, J. Wu, X. Zhao, Z. Li, J. Yu, T. Shao, X. Hou, L. Zhou, C. Wang, G. Wang, J. Han, Structural elucidation of an active polysaccharide from Radix Puerariae lobatae and its protection against acute alcoholic liver disease, Carbohydr Polym 325 (2024) 121565.
- Q. Li, W. Liu, H. Zhang, C. Chen, R. Liu, H. Hou, Q. Luo, Q. Yu, H. Ouyang, Y. Feng, W. Zhu, α-D-1,3-glucan from Radix Puerariae thomsonii improves NAFLD by regulating the intestinal flora and metabolites, Carbohydr Polym 299 (2023) 120197.
- Q. Li, W. Liu, Y. Feng, H. Hou, Z. Zhang, Q. Yu, Y. Zhou, Q. Luo, Y. Luo, H. Ouyang, H. Zhang, W. Zhu, Radix Puerariae thomsonii polysaccharide (RPP) improves inflammation and lipid peroxidation in alcohol and high-fat diet mice by regulating gut microbiota, Int J Biol Macromol 209(Pt A) (2022) 858-870.
- X. Meng, Z. Wang, S. Liang, Z. Tang, J. Liu, Y. Xin, H. Kuang, Q. Wang, Hepatoprotective effect of a polysaccharide from Radix Cyathulae officinalis Kuan against CCl(4)-induced acute liver injury in rat, Int J Biol Macromol 132 (2019) 1057-1067.
- S. Liang, Z. Yao, J. Chen, J. Qian, Y. Dai, H. Li, Structural characterization of a α-d-glucan from Ginkgo biloba seeds and its protective effects on non-alcoholic fatty liver disease in mice, Carbohydr Polym 349(Pt B) (2025) 123022.
- H.Y. Wang, J.C. Ge, F.Y. Zhang, X.Q. Zha, J. Liu, Q.M. Li, J.P. Luo, Dendrobium officinale polysaccharide promotes M1 polarization of TAMs to inhibit tumor growth by targeting TLR2, Carbohydr Polym 292 (2022) 119683.
- W. Cao, X.Q. Li, L. Liu, T.H. Yang, C. Li, H.T. Fan, M. Jia, Z.G. Lv, Q.B. Mei, Structure of an anti-tumor polysaccharide from Angelica sinensis (Oliv.) Diels, Carbohydrate Polymers 66(2) (2006) 149-159.
- X. Wu, S.S.F. Ali, S. Jiang, Y. Zhong, Y. Xu, L. Wei, S. Zhang, Z. Feng, X. Huang, X. Shi, Y. Mu, X. Wang, C. Gan, C. Yang, Ultrasound-assisted combined with natural deep eutectic solvents for Platycodon grandiflorum polysaccharides extraction: Process optimization and evaluation of anti-lung cancer activity, Int J Biol Macromol 313 (2025) 144190.
- Y.Y. Feng, H.Y. Ji, X.D. Dong, A.J. Liu, An alcohol-soluble polysaccharide from Atractylodes macrocephala Koidz induces apoptosis of Eca-109 cells, Carbohydr Polym 226 (2019) 115136.
- J. Wu, W. Gao, Z. Song, Q. Xiong, Y. Xu, Y. Han, J. Yuan, R. Zhang, Y. Cheng, J. Fang, W. Li, Q. Wang, Anticancer activity of polysaccharide from Glehnia littoralis on human lung cancer cell line A549, Int J Biol Macromol 106 (2018) 464-472.
- Y. Pu, J. Zhu, J. Xu, S. Zhang, Y. Bao, Antitumor effect of a polysaccharide from Pseudostellaria heterophylla through reversing tumor-associated macrophages phenotype, Int J Biol Macromol 220 (2022) 816-826.
- W. Luo, B. Huang, M. Lei, Z. Wu, Q. Yu, D. Zhang, C. Yan, Structural characterization and anti-inflammatory effects of Angelica pubescens polysaccharide APRP50-2-1 in rheumatoid arthritis, Int J Biol Macromol 318(Pt 2) (2025) 144896.
- P. Li, N. Xiao, L. Zeng, J. Xiao, J. Huang, Y. Xu, Y. Chen, Y. Ren, B. Du, Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice, Carbohydr Polym 250 (2020) 116958.
- Z. Chen, L. Li, L. Guo, C. Kang, X. Cui, S. Pu, C. Wang, Y. Yang, A Gastrodia elata polysaccharide for restoring intestinal immunocompromise, Int J Biol Macromol 307(Pt 1) (2025) 141781.
- Y. Tan, W. Cao, L. Yang, X. Gong, H. Li, Structural characterization of the glucan from Gastrodia elata Blume and its ameliorative effect on DSS-induced colitis in mice, Int J Biol Macromol 275(Pt 2) (2024) 133718.
- Q. Peng, H. Liu, S. Shi, M. Li, Lycium ruthenicum polysaccharide attenuates inflammation through inhibiting TLR4/NF-κB signaling pathway, Int J Biol Macromol 67 (2014) 330-5.
- J. Gu, M. Zhao, L. You, L. Lin, Demonstration of the effective intestinal immunity activity of a high branched rhamnogalacturonan-I type pectin polysaccharide from wolfberry via exploration its interaction with mechanical barrier, Carbohydr Polym 362 (2025) 123698.
- S. Xiong, N. Li, S. Shi, Y. Zhao, J. Chen, M. Ruan, Y. Xu, R. Liu, S. Wang, H. Wang, Structural characterization of a polysaccharide from Scutellaria baicalensis Georgi and its immune-enhancing properties on RAW264.7 cells, Int J Biol Macromol 283(Pt 3) (2024) 137890.
- Y. Zhou, S. Wang, W. Feng, Z. Zhang, H. Li, Structural characterization and immunomodulatory activities of two polysaccharides from Rehmanniae Radix Praeparata, Int J Biol Macromol 186 (2021) 385-395.
- J. Qin, H.Y. Wang, D. Zhuang, F.C. Meng, X. Zhang, H. Huang, G.P. Lv, Structural characterization and immunoregulatory activity of two polysaccharides from the rhizomes of Atractylodes lancea (Thunb.) DC, Int J Biol Macromol 136 (2019) 341-351.
- K. Wang, Y. Zhou, M. Li, Z. Chen, Z. Wu, W. Ji, J. Wang, Y. Zhang, Structural elucidation and immunomodulatory activities in vitro of type I and II arabinogalactans from different origins of Astragalus membranaceus, Carbohydr Polym 333 (2024) 121974.
- J.Y. Yin, B.C. Chan, H. Yu, I.Y. Lau, X.Q. Han, S.W. Cheng, C.K. Wong, C.B. Lau, M.Y. Xie, K.P. Fung, P.C. Leung, Q.B. Han, Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali, Carbohydr Polym 87(1) (2012) 667-675.
- Y. Shen, Y.L. Guo, Y. Zhang, Y. Li, J. Liang, H.X. Kuang, Y.G. Xia, Structure and immunological activity of an arabinan-rich acidic polysaccharide from Atractylodes lancea (Thunb.) DC, Int J Biol Macromol 199 (2022) 24-35.
- C. Jin, M. Li, L. Duo, H. Chen, X. Guo, S. Li, C. Wen, C. Xie, K. Ding, Arabinogalactan-like polysaccharide isolated from the flowers of Dendrobium officinale regulates the gut microbes and promotes vitamin K1, vitamin A, and vitamin B6 products, Carbohydr Polym 366 (2025) 123660.
- Y. Yang, Y. Chang, Y. Wu, H. Liu, Q. Liu, Z. Kang, M. Wu, H. Yin, J. Duan, A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota, Int J Biol Macromol 183 (2021) 2074-2087.
- Y. Ding, Y. Yan, Y. Peng, D. Chen, J. Mi, L. Lu, Q. Luo, X. Li, X. Zeng, Y. Cao, In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum, Int J Biol Macromol 125 (2019) 751-760.
- J. Mou, J. Yang, Y. Sun, J. Liu, Y. Zhao, H. Lin, J. Yang, An arabinogalactan from Lycium barbarum mitigated DSS caused intestinal injury via inhibiting mucosal damage and regulating the gut microbiota disorder, Carbohydr Polym 352 (2025) 123155.
- W. Zhou, T. Yang, W. Xu, Y. Huang, L. Ran, Y. Yan, J. Mi, L. Lu, Y. Sun, X. Zeng, Y. Cao, The polysaccharides from the fruits of Lycium barbarum L. confer anti-diabetic effect by regulating gut microbiota and intestinal barrier, Carbohydr Polym 291 (2022) 119626.
- J. Luo, Q. Yang, W. Jiang, Y. Liu, Q. Hu, X. Peng, The interaction between Angelica sinensis polysaccharide ASP-2pb and specific gut bacteria alleviates rheumatoid arthritis in rats, Int J Biol Macromol 301 (2025) 140473.
- J. Sun, Y. Jiang, B. Wang, J. Yang, Y. Chen, H. Luo, T. Chen, C. Xiao, L. Weng, Structural characterization of the polysaccharides from Atractylodes chinensis (DC.) Koidz. and the protective effection against alcohol-induced intestinal injury in rats, Int J Biol Macromol 282(Pt 1) (2024) 136641.
- Z. He, T. Guo, Z. Cui, J. Xu, Z. Wu, X. Yang, H. Hu, H. Mei, J. Zhou, Y. Zhang, K. Wang, New understanding of Angelica sinensis polysaccharide improving fatty liver: The dual inhibition of lipid synthesis and CD36-mediated lipid uptake and the regulation of alcohol metabolism, Int J Biol Macromol 207 (2022) 813-825.
- K. Wang, J. Wang, M. Song, H. Wang, N. Xia, Y. Zhang, Angelica sinensis polysaccharide attenuates CCl(4)-induced liver fibrosis via the IL-22/STAT3 pathway, Int J Biol Macromol 162 (2020) 273-283.
- P. Cao, J. Sun, M.A. Sullivan, X. Huang, H. Wang, Y. Zhang, N. Wang, K. Wang, Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro, Int J Biol Macromol 111 (2018) 1133-1139.
- L.W. Sun, K. Feng, R. Jiang, J.Q. Chen, Y. Zhao, R. Ma, H.B. Tong, Water-soluble polysaccharide from Bupleurum chinense DC: Isolation, structural features and antioxidant activity, Carbohydrate Polymers 79(1) (2010) 180-183.
- L. Zhang, X. Liu, Y. Wang, G. Liu, Z. Zhang, Z. Zhao, H. Cheng, In vitro antioxidative and immunological activities of polysaccharides from Zizyphus Jujuba cv. Muzao, Int J Biol Macromol 95 (2017) 1119-1125.
- X. Li, W. Ji, S. Wu, C. Qian, J. Zhou, Z. Zhang, D. Li, The isolation, characterization and biological activities of the non-glucan polysaccharides from the high-starch-content plant Pueraria mirifica, Int J Biol Macromol 261(Pt 2) (2024) 129709.
- G. Du, Y. Liu, J. Zhang, S. Fang, C. Wang, Microwave-assisted extraction of dandelion root polysaccharides: Extraction process optimization, purification, structural characterization, and analysis of antioxidant activity, Int J Biol Macromol 299 (2025) 139732.
- P. Chen, E. Sang, H. Chen, Q. Meng, H. Liu, Effects of different extraction temperatures on the structural characteristics and antioxidant activity of polysaccharides from dandelion leaves, Int J Biol Macromol 283(Pt 3) (2024) 137726.
- Q. Ren, J. Chen, Y. Ding, J. Cheng, S. Yang, Z. Ding, Q. Dai, Z. Ding, In vitro antioxidant and immunostimulating activities of polysaccharides from Ginkgo biloba leaves, Int J Biol Macromol 124 (2019) 972-980.
- J. Wang, Y. Zhou, Y. Yu, Y. Wang, D. Xue, Y. Zhou, X. Li, A ginseng-derived rhamnogalacturonan I (RG-I) pectin promotes longevity via TOR signalling in Caenorhabditis elegans, Carbohydr Polym 312 (2023) 120818.
- X. Li, Q. Chen, G. Liu, H. Xu, X. Zhang, Chemical elucidation of an arabinogalactan from rhizome of Polygonatum sibiricum with antioxidant activities, Int J Biol Macromol 190 (2021) 730-738.
- X. Liang, M. Liu, Y. Wei, L. Tong, S. Guo, H. Kang, W. Zhang, Z. Yu, F. Zhang, J.A. Duan, Structural characteristics and structure-activity relationship of four polysaccharides from Lycii fructus, Int J Biol Macromol 253(Pt 5) (2023) 127256.
- W. Huang, M. Zhao, X. Wang, Y. Tian, C. Wang, J. Sun, Z. Wang, G. Gong, L. Huang, Revisiting the structure of arabinogalactan from Lycium barbarum and the impact of its side chain on anti-ageing activity, Carbohydr Polym 286 (2022) 119282.
- R. Shi, S. Yang, S. Zeng, J. Lin, X. Wang, J. Yu, Y. Liang, J. Li, T. Zhou, Y. Deng, X. Duan, C. Chen, M. Yu, G. Sun, J. Dong, Z. Shu, Effect of structural changes of Rehmannia glutinosa polysaccharide before and after processing on anti-aging activity, Int J Biol Macromol 309(Pt 4) (2025) 143168.
- L. Liang, Y. Yue, L. Zhong, Y. Liang, R. Shi, R. Luo, M. Zhao, X. Cao, M. Yang, J. Du, X. Shen, Y. Wang, Z. Shu, Anti-aging activities of Rehmannia glutinosa Libosch. crude polysaccharide in Caenorhabditis elegans based on gut microbiota and metabonomic analysis, Int J Biol Macromol 253(Pt 8) (2023) 127647.
- J. Wu, T. Chen, F. Wan, J. Wang, X. Li, W. Li, L. Ma, Structural characterization of a polysaccharide from Lycium barbarum and its neuroprotective effect against β-amyloid peptide neurotoxicity, Int J Biol Macromol 176 (2021) 352-363.
- F. Zhang, X. Zhang, S. Guo, F. Cao, X. Zhang, Y. Wang, J. Liu, B. Qian, Y. Yan, P. Chen, C. Xu, C. Liu, D. Qian, J.A. Duan, An acidic heteropolysaccharide from Lycii fructus: Purification, characterization, neurotrophic and neuroprotective activities in vitro, Carbohydr Polym 249 (2020) 116894.
- L. Zhou, W. Liao, X. Chen, H. Yue, S. Li, K. Ding, An arabinogalactan from fruits of Lycium barbarum L. inhibits production and aggregation of Aβ(42), Carbohydr Polym 195 (2018) 643-651.
- Y. Yang, P. Liu, L. Chen, Z. Liu, H. Zhang, J. Wang, X. Sun, W. Zhong, N. Wang, K. Tian, J. Zhao, Therapeutic effect of Ginkgo biloba polysaccharide in rats with focal cerebral ischemia/reperfusion (I/R) injury, Carbohydr Polym 98(2) (2013) 1383-8.
- G. Gong, Q. Liu, Y. Deng, T. Dang, W. Dai, T. Liu, Y. Liu, J. Sun, L. Wang, Y. Liu, T. Sun, S. Song, Z. Wang, L. Huang, Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis, Int J Biol Macromol 149 (2020) 639-650.
- F. He, S. Zhang, Y. Li, X. Chen, Z. Du, C. Shao, K. Ding, The structure elucidation of novel arabinogalactan LRP1-S2 against pancreatic cancer cells growth in vitro and in vivo, Carbohydr Polym 267 (2021) 118172.
- Y. Zhang, T. Zhou, H. Wang, Z. Cui, F. Cheng, K.P. Wang, Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels, Carbohydr Polym 147 (2016) 401-408.
- P. Wang, L. Zhang, J. Yao, Y. Shi, P. Li, K. Ding, An arabinogalactan from flowers of Panax notoginseng inhibits angiogenesis by BMP2/Smad/Id1 signaling, Carbohydr Polym 121 (2015) 328-35.
- D. Gu, L. Huang, X. Chen, Q. Wu, K. Ding, Structural characterization of a galactan from Ophiopogon japonicus and anti-pancreatic cancer activity of its acetylated derivative, Int J Biol Macromol 113 (2018) 907-915.
- H. Gong, X. Gan, B. Qin, J. Chen, Y. Zhao, B. Qiu, W. Chen, Y. Yu, S. Shi, T. Li, D. Liu, B. Li, S. Wang, H. Wang, Structural characteristics of steamed Polygonatum cyrtonema polysaccharide and its bioactivity on colitis via improving the intestinal barrier and modifying the gut microbiota, Carbohydr Polym 327 (2024) 121669.
- J. Zhang, H. Chen, L. Luo, Z. Zhou, Y. Wang, T. Gao, L. Yang, T. Peng, M. Wu, Structures of fructan and galactan from Polygonatum cyrtonema and their utilization by probiotic bacteria, Carbohydr Polym 267 (2021) 118219.
- Y. Wang, N. Liu, X. Xue, Q. Li, D. Sun, Z. Zhao, Purification, structural characterization and in vivo immunoregulatory activity of a novel polysaccharide from Polygonatum sibiricum, Int J Biol Macromol 160 (2020) 688-694.
- T. Sun, H. Zhang, Y. Li, Y. Liu, W. Dai, J. Fang, C. Cao, Y. Die, Q. Liu, C. Wang, L. Zhao, G. Gong, Z. Wang, L. Huang, Physicochemical properties and immunological activities of polysaccharides from both crude and wine-processed Polygonatum sibiricum, Int J Biol Macromol 143 (2020) 255-264.
- H. Zhang, Y. Yue, Q. Zhang, L. Liang, C. Li, Y. Chen, W. Li, M. Peng, M. Yang, M. Zhao, X. Cao, L. Zhong, J. Du, Y. Wang, X. Zhou, Z. Shu, Structural characterization and anti-inflammatory effects of an arabinan isolated from Rehmannia glutinosa Libosch, Carbohydr Polym 303 (2023) 120441.
- W. Liu, K. Li, H. Zhang, Y. Li, Z. Lin, J. Xu, Y. Guo, An antitumor arabinan from Glehnia littoralis activates immunity and inhibits angiogenesis, Int J Biol Macromol 263(Pt 2) (2024) 130242.
- H. Wang, X. Wang, Y. Li, S. Zhang, Z. Li, Y. Li, J. Cui, X. Lan, E. Zhang, L. Yuan, D.Q. Jin, M. Tuerhong, M. Abudukeremu, J. Xu, Y. Guo, Structural properties and in vitro and in vivo immunomodulatory activity of an arabinofuranan from the fruits of Akebia quinata, Carbohydr Polym 256 (2021) 117521.
- C. Yang, J. Li, M. Luo, W. Zhou, J. Xing, Y. Yang, L. Wang, W. Rao, W. Tao, Unveiling the molecular mechanisms of Dendrobium officinale polysaccharides on intestinal immunity: An integrated study of network pharmacology, molecular dynamics and in vivo experiments, Int J Biol Macromol 276(Pt 2) (2024) 133859.
- M. Cai, H. Zhu, L. Xu, J. Wang, J. Xu, Z. Li, K. Yang, J. Wu, P. Sun, Structure, anti-fatigue activity and regulation on gut microflora in vivo of ethanol-fractional polysaccharides from Dendrobium officinale, Int J Biol Macromol 234 (2023) 123572.
- Z.Z. Shang, D.Y. Qin, Q.M. Li, X.Q. Zha, L.H. Pan, D.Y. Peng, J.P. Luo, Dendrobium huoshanense stem polysaccharide ameliorates rheumatoid arthritis in mice via inhibition of inflammatory signaling pathways, Carbohydr Polym 258 (2021) 117657.
- T.B. He, Y.P. Huang, L. Yang, T.T. Liu, W.Y. Gong, X.J. Wang, J. Sheng, J.M. Hu, Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale, Int J Biol Macromol 83 (2016) 34-41.
- R.A. Cao, R. Ji, M. Tabarsa, J. Zhang, L. Meng, C. Zhang, J. Zhang, L. Wang, R. Wu, C. Wang, C. Jin, S. You, Purification, characterization and immunostimulatory effects of polysaccharides from Anemarrhena asphodeloides rhizomes, Int J Biol Macromol 172 (2021) 550-559.
- G. Ye, J. Li, J. Zhang, H. Liu, Q. Ye, Z. Wang, Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum, Carbohydr Polym 269 (2021) 118253.
- K. Zhang, X. Zhou, J. Wang, Y. Zhou, W. Qi, H. Chen, S. Nie, M. Xie, Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway, Carbohydr Polym 264 (2021) 118018.
- J. Zhang, Y. Li, Y. Li, Y. Li, X. Gong, L. Zhou, J. Xu, Y. Guo, Structure, selenization modification, and antitumor activity of a glucomannan from Platycodon grandiflorum, Int J Biol Macromol 220 (2022) 1345-1355.
- J. Fu, Z. Liang, Z. Chen, W. Chen, Y. Zhou, F. Xiong, L. Meng, Q. Liang, H. Gao, Mechanism of Dendrobium officinale polysaccharide in alleviating Alzheimer’s disease: Insights from metabolomics, lipidomics, and proteomics analysis, Int J Biol Macromol 319(Pt 2) (2025) 145531.
- X. Zhang, R. Ge, J. Wu, X. Cai, G. Deng, J. Lv, M. Ma, N. Yu, L. Yao, D. Peng, Structural characterization and improves cognitive disorder in ageing mice of a glucomannan from Dendrobium huoshanense, Int J Biol Macromol 269(Pt 1) (2024) 131995.
- H. Wang, H. Jin, Y. Dong, Z. Wang, Y. Wang, F. Wei, Structural characterization of Dendrobium huoshanense polysaccharides and its gastroprotective effect on acetic acid-induced gastric ulcer in mice, Int J Biol Macromol 311(Pt 2) (2025) 143361.
- H.Y. Ye, Z.Z. Shang, F.Y. Zhang, X.Q. Zha, Q.M. Li, J.P. Luo, Dendrobium huoshanense stem polysaccharide ameliorates alcohol-induced gastric ulcer in rats through Nrf2-mediated strengthening of gastric mucosal barrier, Int J Biol Macromol 236 (2023) 124001.
- M. Tao, Y. Xie, X. Fan, X. Yan, W. Fan, R. Cao, M. Li, R. Li, L. Wang, Neutral polysaccharide from Dendrobium officinale alleviates acute alcohol-induced liver injury via the gut microbiota-short chain fatty acids-liver axis, Int J Biol Macromol 317(Pt 1) (2025) 144719.
- Y. Shi, L. Zhou, G. Zheng, Y. Jing, X. Zhang, J. Yuan, Q. Zhang, H. Li, S. Huang, T. Xie, Q. Xiong, Therapeutic mechanism exploration of polysaccharides from Dendrobium officinale on unilateral ureteral obstruction operation-induced renal fibrosis based on improving oxidative stress injury mediated by AhR/NOX4 pathway, Int J Biol Macromol 253(Pt 3) (2023) 126920.
- Y. Sun, X. Zeng, Y. Liu, S. Zhan, Z. Wu, X. Zheng, X. Zhang, Dendrobium officinale polysaccharide attenuates cognitive impairment in circadian rhythm disruption mice model by modulating gut microbiota, Int J Biol Macromol 217 (2022) 677-688.
- X. Li, Q. Zhang, Y. Zhu, Y. Li, S. Mei, H. Luo, K. Wu, Structural characterization of a mannoglucan polysaccharide from Dendrobium huoshanense and evaluation of its osteogenesis promotion activities, Int J Biol Macromol 211 (2022) 441-449.
- L. Yue, W. Wang, Y. Wang, T. Du, W. Shen, H. Tang, Y. Wang, H. Yin, Bletilla striata polysaccharide inhibits angiotensin II-induced ROS and inflammation via NOX4 and TLR2 pathways, Int J Biol Macromol 89 (2016) 376-88.
- J. Li, W. Tao, W. Zhou, J. Xing, M. Luo, Y. Yang, The comprehensive analysis of gut microbiome and spleen transcriptome revealed the immunomodulatory mechanism of Dendrobium officinale leaf polysaccharide on immunosuppressed mice, Int J Biol Macromol 278(Pt 4) (2024) 134975.
- F. Yuan, R. Yu, Y. Yin, J. Shen, Q. Dong, L. Zhong, L. Song, Structure characterization and antioxidant activity of a novel polysaccharide isolated from Ginkgo biloba, Int J Biol Macromol 46(4) (2010) 436-9.
- J. Zhou, X. Zhang, C. Wang, X. Xu, J. Zhang, Y. Ge, J. Li, F. Yang, J. Gao, An inulin-type fructan CP-A from Codonopsis pilosula combined with 5-Fluorouracil alleviates colitis-associated tumorigenesis via inhibition of EGFR/AKT/ERK signaling pathway and regulation of intestinal flora, Int J Biol Macromol 308(Pt 3) (2025) 142655.
- X. Rong, Q. Shu, Modulating butyric acid-producing bacterial community abundance and structure in the intestine of immunocompromised mice with neutral polysaccharides extracted from Codonopsis pilosula, Int J Biol Macromol 278(Pt 3) (2024) 134959.
- L. Zhang, Y. Wang, F. Wu, X. Wang, Y. Feng, Y. Wang, MDG, an Ophiopogon japonicus polysaccharide, inhibits non-alcoholic fatty liver disease by regulating the abundance of Akkermansia muciniphila, Int J Biol Macromol 196 (2022) 23-34.
- X. Wang, L. Shi, X. Wang, Y. Feng, Y. Wang, MDG-1, an Ophiopogon polysaccharide, restrains process of non-alcoholic fatty liver disease via modulating the gut-liver axis, Int J Biol Macromol 141 (2019) 1013-1021.
- L.L. Shi, Y. Li, Y. Wang, Y. Feng, MDG-1, an Ophiopogon polysaccharide, regulate gut microbiota in high-fat diet-induced obese C57BL/6 mice, Int J Biol Macromol 81 (2015) 576-83.
- Y. Wang, Y. Zhu, K. Ruan, H. Wei, Y. Feng, MDG-1, a polysaccharide from Ophiopogon japonicus, prevents high fat diet-induced obesity and increases energy expenditure in mice, Carbohydr Polym 114 (2014) 183-189.
- H. Fan, H. Zhao, Y. Zheng, G. Chen, Y. Ji, W. Yu, J. Yan, H. Yang, X. Liang, Y. Chen, Polygonati kingianum polysaccharide alleviates dextran sulfate sodium-induced colitis by modulating gut microbiota and metabolic homeostasis, Int J Biol Macromol 316(Pt 2) (2025) 144836.
- C. Xue, M. Lu, Y. Qin, X. Zhao, J. Yang, S. Yuan, Z. Wang, N. Cho, C. Jiang, Polygonati Rhizoma polysaccharide suppresses microglial activation and promotes functional recovery of spinal cord via improving intestinal microbiota, Int J Biol Macromol 313 (2025) 143934.
- J.Y. Shi, Y.J. Wang, Q.W. Bao, Y.M. Qin, P.P. Li, Q.Q. Wu, C.K. Xia, D.L. Wu, S.Z. Xie, Polygonatum cyrtonema Hua polysaccharide alleviates ulcerative colitis via gut microbiota-independent modulation of inflammatory immune response, Carbohydr Polym 356 (2025) 123387.
- Q. Yuan, W. Liu, H. Wu, X. Yang, H. Li, Y. Chen, M. Shui, Y. Ding, S. Wang, Fructans with various molecular weights from Polygonatum cyrtonema Hua differentially ameliorate intestinal inflammation by regulating the gut microbiota and maintaining intestinal barrier, Int J Biol Macromol 285 (2025) 138359.
- X. Xu, M. Shan, C. Chu, S. Bie, H. Wang, S. Cai, Polysaccharides from Polygonatum kingianum Collett & Hemsl ameliorated fatigue by regulating NRF2/HO-1/NQO1 and AMPK/PGC-1α/TFAM signaling pathways, and gut microbiota, Int J Biol Macromol 266(Pt 2) (2024) 131440.
- Y. Cheng, S. Tian, Y. Chen, J. Xie, X. Hu, Y. Wang, J. Xie, H. Huang, C. Yang, J. Si, Q. Yu, Structural characterization and in vitro fermentation properties of polysaccharides from Polygonatum cyrtonema, Int J Biol Macromol 258(Pt 1) (2024) 128877.
- Y. Chen, S. Ma, J. Cui, A. Huang, Z. Chen, D. Pi, Z. Yi, H. Ye, M. Ma, M. Ouyang, Atractylodes lancea (Thunb.) DC polysaccharide structural properties and its protective impact on cisplatin-induced gastrointestinal harm by restoring the intestinal barrier and gut flora, Int J Biol Macromol 318(Pt 4) (2025) 145232.
- Y. Meng, Y. Xu, C. Chang, Z. Qiu, J. Hu, Y. Wu, B. Zhang, G. Zheng, Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula, Int J Biol Macromol 163 (2020) 1677-1686.
- N. Chen, Y. Ding, X. Li, J. Li, Y. Cheng, Y. Tian, Y. Tian, M. Wu, Chemical structures and immunomodulatory activities of polysaccharides from Polygonatum kingianum, Int J Biol Macromol 279(Pt 3) (2024) 135406.
- P. Zhao, H. Zhou, C. Zhao, X. Li, Y. Wang, Y. Wang, L. Huang, W. Gao, Purification, characterization and immunomodulatory activity of fructans from Polygonatum odoratum and P. cyrtonema, Carbohydr Polym 214 (2019) 44-52.
- X.J. Li, S.J. Xiao, J. Chen, H.R. Xu, Inulin-type fructans obtained from Atractylodis Macrocephalae by water/alkali extraction and immunoregulatory evaluation, Int J Biol Macromol 230 (2023) 123212.
- S. Zhang, Q. Zhang, L. An, J. Zhang, Z. Li, J. Zhang, Y. Li, M. Tuerhong, Y. Ohizumi, J. Jin, J. Xu, Y. Guo, A fructan from Anemarrhena asphodeloides Bunge showing neuroprotective and immunoregulatory effects, Carbohydr Polym 229 (2020) 115477.
- Q.M. Li, H. Xu, X.Q. Zha, F.Y. Zhang, J.P. Luo, Polygonatum cyrtonema polysaccharide alleviates dopaminergic neuron apoptosis in Parkinson’s disease mouse model via inhibiting oxidative stress and endoplasmic reticulum stress, Int J Biol Macromol 311(Pt 3) (2025) 143986.
- Y. Qin, G. Zhao, Z. Wang, M. Liu, H. Deng, L. Guo, L. Cao, Y. Zhang, Y. Qiao, X. Zhang, Y. Li, Polygonatum sibiricum polysaccharide attenuates cyclophosphamide-induced testicular damages and sperm defects in male mice via Nrf2 mediating antioxidant protective mechanisms, Int J Biol Macromol 307(Pt 3) (2025) 141968.
- W. Liu, Y.M. Qin, J.Y. Shi, D.L. Wu, C.Y. Liu, J. Liang, S.Z. Xie, Effect of ultrasonic degradation on the physicochemical characteristics, GLP-1 secretion, and antioxidant capacity of Polygonatum cyrtonema polysaccharide, Int J Biol Macromol 274(Pt 2) (2024) 133434.
- J. Huang, Y. Chen, Y. Su, W. Yuan, D. Peng, Z. Guan, J. Chen, P. Li, B. Du, Identification of carbohydrate in Polygonatum kingianum Coll. et Hemsl and inhibiting oxidative stress, Int J Biol Macromol 261(Pt 2) (2024) 129760.
- Y. Gong, J. Zhang, F. Gao, J. Zhou, Z. Xiang, C. Zhou, L. Wan, J. Chen, Structure features and in vitro hypoglycemic activities of polysaccharides from different species of Maidong, Carbohydr Polym 173 (2017) 215-222.
- J. Xu, Y. Wang, D.S. Xu, K.F. Ruan, Y. Feng, S. Wang, Hypoglycemic effects of MDG-1, a polysaccharide derived from Ophiopogon japonicas, in the ob/ob mouse model of type 2 diabetes mellitus, Int J Biol Macromol 49(4) (2011) 657-62.
- J. Yu, L. Zhao, Z. Wang, T. Yue, X. Wang, W. Liu, Correlations between the structure and anti-diabetic activity of novel polysaccharides from raw and “Nine Steaming Nine Sun-Drying” Polygonti rhizome, Int J Biol Macromol 260(Pt 2) (2024) 129171.
- K. Ma, X. Yi, S.T. Yang, H. Zhu, T.Y. Liu, S.S. Jia, J.H. Fan, D.J. Hu, G.P. Lv, H. Huang, Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and its therapeutic effects on non-alcoholic fatty liver disease in vitro and in vivo, Int J Biol Macromol 265(Pt 2) (2024) 130988.
- W. Liu, L. Zhang, X. Wei, Y. Xu, Q. Fang, S. Qi, J. Chen, C. Wang, S. Wang, L. Qin, P. Liu, J. Wu, Structural characterization of an inulin neoseries-type fructan from Ophiopogonis Radix and the therapeutic effect on liver fibrosis in vivo, Carbohydr Polym 327 (2024) 121659.
- F. Li, P. Du, W. Yang, D. Huang, S. Nie, M. Xie, Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line, Int J Biol Macromol 164 (2020) 2134-2140.
- J.L. Hu, S.P. Nie, C. Li, M.Y. Xie, In vitro effects of a novel polysaccharide from the seeds of Plantago asiatica L. on intestinal function, Int J Biol Macromol 54 (2013) 264-9.
- C. Li, Q. Huang, X. Fu, X.J. Yue, R.H. Liu, L.J. You, Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn, Int J Biol Macromol 75 (2015) 298-305.
- L. Feng, P. Shi, L. Zhao, M. Shang, Y. Han, N. Han, Z. Liu, S. Li, J. Zhai, J. Yin, Structural characterization of polysaccharides from Panax ginseng C. A. Meyer root and their triggered potential immunoregulatory and radioprotective activities, Int J Biol Macromol 280(Pt 3) (2024) 135993.
- C. Li, Z.N. Tian, J.P. Cai, K.X. Chen, B. Zhang, M.Y. Feng, Q.T. Shi, R. Li, Y. Qin, J.S. Geng, Panax ginseng polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1 pathway in human gastric cancer, Carbohydr Polym 102 (2014) 103-9.
- Y.Y. Fan, H.R. Cheng, S.S. Li, J. Wang, D. Liu, M.A. Hao, X.G. Gao, E.X. Fan, G.H. Tai, Y.F. Zhou, Relationship of the inhibition of cell migration with the structure of ginseng pectic polysaccharides, Carbohydrate Polymers 81(2) (2010) 340-347.
- Y. Fan, L. Sun, S. Yang, C. He, G. Tai, Y. Zhou, The roles and mechanisms of homogalacturonan and rhamnogalacturonan I pectins on the inhibition of cell migration, Int J Biol Macromol 106 (2018) 207-217.
- L. Feng, N. Han, Y.B. Han, M.W. Shang, T.W. Liang, Z.H. Liu, S.K. Li, J.X. Zhai, J. Yin, Structural analysis of a soluble polysaccharide GSPA-0.3 from the root of Panax ginseng C. A. Meyer and its adjuvant activity with mechanism investigation, Carbohydr Polym 326 (2024) 121591.
- H. Xue, Z. Zhao, Z. Lin, J. Geng, Y. Guan, C. Song, Y. Zhou, G. Tai, Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis, Carbohydr Polym 219 (2019) 121-129.
- Q.L. Sun, Y.X. Li, Y.S. Cui, S.L. Jiang, C.X. Dong, J. Du, Structural characterization of three polysaccharides from the roots of Codonopsis pilosula and their immunomodulatory effects on RAW264.7 macrophages, Int J Biol Macromol 130 (2019) 556-563.
- P. Zhang, L. Hu, R. Bai, X. Zheng, Y. Ma, X. Gao, B. Sun, F. Hu, Structural characterization of a pectic polysaccharide from Codonopsis pilosula and its immunomodulatory activities in vivo and in vitro, Int J Biol Macromol 104(Pt A) (2017) 1359-1369.
- J. Qiao, Z. Gao, C. Zhang, Hennigs, B. Wu, L. Jing, R. Gao, Y. Yang, Structural characterization and immune modulation activities of Chinese Angelica polysaccharide (CAP) and selenizing CAP (sCAP) on dendritic cells, Int J Biol Macromol 277(Pt 2) (2024) 132628.
- S. Liu, Y. Yang, Y. Qu, X. Guo, X. Yang, X. Cui, C. Wang, Structural characterization of a novel polysaccharide from Panax notoginseng residue and its immunomodulatory activity on bone marrow dendritic cells, Int J Biol Macromol 161 (2020) 797-809.
- J. Zhang, W. Pan, J. Cui, Z. Lin, J. Gao, J. Lin, Purified polysaccharides from Plantago asiatica L.: Preparation, characterization and immune-activating effects, Int J Biol Macromol 318(Pt 1) (2025) 144771.
- X.H. Yu, Y. Liu, X.L. Wu, L.Z. Liu, W. Fu, D.D. Song, Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng, Carbohydr Polym 156 (2017) 9-18.
- G. Cai, C. Wu, N. Mao, Z. Song, L. Yu, T. Zhu, S. Peng, Y. Yang, Z. Liu, D. Wang, Isolation, purification and characterization of Pueraria lobata polysaccharide and its effects on intestinal function in cyclophosphamide-treated mice, Int J Biol Macromol 218 (2022) 356-367.
- Y.S. Cui, Y.X. Li, S.L. Jiang, A.N. Song, Z. Fu, C.X. Dong, Z. Yao, W. Qiao, Isolation, purification, and structural characterization of polysaccharides from Atractylodis Macrocephalae Rhizoma and their immunostimulatory activity in RAW264.7 cells, Int J Biol Macromol 163 (2020) 270-278.
- J. Shao, T. Li, S. Zeng, J. Dong, X. Chen, C. Zang, X. Yao, H. Li, Y. Yu, The structures of two acidic polysaccharides from Gardenia jasminoides and their potential immunomodulatory activities, Int J Biol Macromol 248 (2023) 125895.
- Y. Li, Y. Sheng, J. Liu, G. Xu, W. Yu, Q. Cui, X. Lu, P. Du, L. An, Hair-growth promoting effect and anti-inflammatory mechanism of Ginkgo biloba polysaccharides, Carbohydr Polym 278 (2022) 118811.
- X. He, H. Fan, M. Sun, J. Li, Q. Xia, Y. Jiang, B. Liu, Chemical structure and immunomodulatory activity of a polysaccharide from Saposhnikoviae Radix, Int J Biol Macromol 276(Pt 1) (2024) 133459.
- H. Fan, M. Sun, J. Li, S. Zhang, G. Tu, K. Liu, Q. Xia, Y. Jiang, B. Liu, Structure characterization and immunomodulatory activity of a polysaccharide from Saposhnikoviae Radix, Int J Biol Macromol 233 (2023) 123502.
- R. Yao, C. Huang, X. Chen, Z. Yin, Y. Fu, L. Li, B. Feng, X. Song, C. He, G. Yue, B. Jing, C. Lv, G. Su, G. Ye, Y. Zou, Two complement fixing pectic polysaccharides from pedicel of Lycium barbarum L. promote cellular antioxidant defense, Int J Biol Macromol 112 (2018) 356-363.
- F. Zhang, X. Zhang, X. Liang, K. Wu, Y. Cao, T. Ma, S. Guo, P. Chen, S. Yu, Q. Ruan, C. Xu, C. Liu, D. Qian, J.A. Duan, Defensing against oxidative stress in Caenorhabditis elegans of a polysaccharide LFP-05S from Lycii fructus, Carbohydr Polym 289 (2022) 119433.
- Y.F. Zou, Y.Y. Zhang, B.S. Paulsen, F. Rise, Z.L. Chen, R.Y. Jia, L.X. Li, X. Song, B. Feng, H.Q. Tang, C. Huang, Z.Q. Yin, Structural features of pectic polysaccharides from stems of two species of Radix Codonopsis and their antioxidant activities, Int J Biol Macromol 159 (2020) 704-713.
- Zhao, C. Liu, C. Du, P. Gao, X. Liu, D. Li, Study on characterization of Bupleurum chinense polysaccharides with antioxidant mechanisms focus on ROS relative signaling pathways and anti-aging evaluation in vivo model, Int J Biol Macromol 266(Pt 2) (2024) 131171.
- Y.P. Fu, X. Peng, C.W. Zhang, Q.X. Jiang, C.Y. Li, B.S. Paulsen, F. Rise, C. Huang, B. Feng, L.X. Li, X.F. Chen, R.Y. Jia, Y.P. Li, X.H. Zhao, G. Ye, H.Q. Tang, X.X. Liang, C. Lv, M.L. Tian, Z.Q. Yin, Y.F. Zou, Salvia miltiorrhiza polysaccharide and its related metabolite 5-methoxyindole-3-carboxaldehyde ameliorate experimental colitis by regulating Nrf2/Keap1 signaling pathway, Carbohydr Polym 306 (2023) 120626.
- X. Ji, C. Hou, Y. Yan, M. Shi, Y. Liu, Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit, Int J Biol Macromol 149 (2020) 1008-1018.
- X. Zhao, Y. Meng, Y. Liu, Z. Sun, K. Cui, L. Zhu, X. Yang, K.H. Mayo, L. Sun, S. Cui, Pectic polysaccharides from Lilium brownii and Polygonatum odoratum exhibit significant antioxidant effects in vitro, Int J Biol Macromol 257(Pt 2) (2024) 128830.
- Q. Yuan, Y. Xie, W. Wang, Y. Yan, H. Ye, S. Jabbar, X. Zeng, Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves, Carbohydr Polym 128 (2015) 52-62.
- X. Ning, Y. Liu, M. Jia, Q. Wang, Z. Sun, L. Ji, K.H. Mayo, Y. Zhou, L. Sun, Pectic polysaccharides from Radix Sophorae Tonkinensis exhibit significant antioxidant effects, Carbohydr Polym 262 (2021) 117925.
- C. Yang, Y. Gou, J. Chen, J. An, W. Chen, F. Hu, Structural characterization and antitumor activity of a pectic polysaccharide from Codonopsis pilosula, Carbohydr Polym 98(1) (2013) 886-95.
- X. Wang, J. Gan, M. Han, Y. Wu, L. Liu, Y. Zhao, R. Zhao, Comparison of structure and the synergistic anti-hepatocellular carcinoma effect of three polysaccharides from vinegar-baked Radix Bupleuri, Int J Biol Macromol 282(Pt 1) (2024) 136755.
- M.Q. Liu, C.J. Bao, X.F. Liang, X.Y. Ji, L.Q. Zhao, A.N. Yao, S. Guo, J.L. Duan, M. Zhao, J.A. Duan, Specific molecular weight of Lycium barbarum polysaccharide for robust breast cancer regression by repolarizing tumor-associated macrophages, Int J Biol Macromol 261(Pt 1) (2024) 129674.
- S. Zhang, F. He, X. Chen, K. Ding, Isolation and structural characterization of a pectin from Lycium ruthenicum Murr and its anti-pancreatic ductal adenocarcinoma cell activity, Carbohydr Polym 223 (2019) 115104.
- W. Zhu, X. Xue, Z. Zhang, Structural, physicochemical, antioxidant and antitumor property of an acidic polysaccharide from Polygonum multiflorum, Int J Biol Macromol 96 (2017) 494-500.
- Y. Bian, H. Zeng, H. Tao, L. Huang, Z. Du, J. Wang, K. Ding, A pectin-like polysaccharide from Polygala tenuifolia inhibits pancreatic cancer cell growth in vitro and in vivo by inducing apoptosis and suppressing autophagy, Int J Biol Macromol 162 (2020) 107-115.
- L. Jiao, X. Zhang, M. Wang, B. Li, Z. Liu, S. Liu, Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing, Carbohydr Polym 114 (2014) 567-573.
- J. Zhu, W. Liu, J. Yu, S. Zou, J. Wang, W. Yao, X. Gao, Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L, Carbohydr Polym 98(1) (2013) 8-16.
- S. Zou, X. Zhang, W.B. Yao, Y.G. Niu, X.D. Gao, Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L, Carbohydrate Polymers 80(4) (2010) 1161-1167.
- K. Ko, C. Guo, E. Kim, G. Zhang, Y. Lee, Structural features of galacturonic acid-rich polysaccharides from Ziziphus jujuba and their protective effects against insulin resistance and muscle atrophy, Int J Biol Macromol 322(Pt 3) (2025) 146287.
- X. Guo, Y. Su, Y. Du, F. Zhang, W. Yu, W. Ren, S. Li, H. Kuang, L. Wu, Vinegar-processed Schisandra chinensis polysaccharide ameliorates type 2 diabetes via modulation serum metabolic profiles, gut microbiota, and fecal SCFAs, Int J Biol Macromol 294 (2025) 139514.
- J. Chen, W. Pang, Y. Kan, L. Zhao, Z. He, W. Shi, B. Yan, H. Chen, J. Hu, Structure of a pectic polysaccharide from Pseudostellaria heterophylla and stimulating insulin secretion of INS-1 cell and distributing in rats by oral, Int J Biol Macromol 106 (2018) 456-463.
- W. Zhou, X. Kan, G. Chen, Y. Sun, L. Ran, Y. Yan, J. Mi, L. Lu, X. Zeng, Y. Cao, The polysaccharides from the fruits of Lycium barbarum L. modify the gut community profile and alleviate dextran sulfate sodium-induced colitis in mice, Int J Biol Macromol 222(Pt B) (2022) 2244-2257.
- H. Yan, C.J. Fan, Y.J. Wang, Z.L. Liu, J.Q. Wang, S.P. Nie, Structural characterization and in vitro fermentation of the polysaccharide from fruits of Gardenia jasminoides, Int J Biol Macromol 309(Pt 1) (2025) 142678.
- T.Y. Liu, Z.W. Han, S.S. Jia, K. Ma, M. Li, X. Yi, H. Zhu, J.H. Fan, H.W. Qiu, G.P. Lv, H. Huang, An acidic polysaccharide from Lycium barbarum L: Isolation, purification, structural characterization, and therapeutic effects on ulcerative colitis, Int J Biol Macromol 319(Pt 4) (2025) 145602.
- Y. Wang, S. Shao, C. Guo, S. Zhang, M. Li, K. Ding, The homogenous polysaccharide SY01-23 purified from leaf of Morus alba L. has bioactivity on human gut Bacteroides ovatus and Bacteroides cellulosilyticus, Int J Biol Macromol 158 (2020) 698-707.
- Y. Li, X. Duan, Y. Wang, Y. Deng, J. Zhang, Structural characterization and in vitro hepatoprotective activity of an acidic polysaccharide from Dendrobium nobile Lindl. flower, Int J Biol Macromol 284(Pt 1) (2025) 138100.
- C. Wang, L. Zheng, S. Liu, X. Guo, Y. Qu, M. Gao, X. Cui, Y. Yang, A novel acidic polysaccharide from the residue of Panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice, Int J Biol Macromol 149 (2020) 1084-1097.
- Y. Zhang, J. Sun, X. Zhang, Q. Zhong, P. Hao, W. Yin, B. Chen, Q. Wei, H. Shen, Y. Zhang, X. Yang, X. Yu, X. Wu, W. Qu, Y. Wu, Structural characterisation of hawthorn polysaccharide and its mechanisms of action against non-alcoholic fatty liver disease, Int J Biol Macromol 319(Pt 4) (2025) 145713.
- X. Ma, W. Zhou, Y. Nie, X. Jing, S. Li, C. Jin, A. Zhu, J. Su, W. Liao, K. Ding, A novel branched galacturonan from Gardenia jasminoides alleviates liver fibrosis linked to TLR4/NF-κB signaling, Int J Biol Macromol 245 (2023) 125540.
- S. Ma, X. Liu, B. Cheng, Z. Jia, H. Hua, Y. Xin, Chemical characterization of polysaccharides isolated from scrophularia ningpoensis and its protective effect on the cerebral ischemia/reperfusin injury in rat model, Int J Biol Macromol 139 (2019) 955-966.
- H. Zeng, P. Li, L. Zhou, K. Ding, A novel pectin from Polygala tenuifolia blocks Aβ(42) aggregation and production by enhancing insulin-degradation enzyme and neprilysin, Int J Biol Macromol 161 (2020) 35-43.
- J. Song, Y. Zhang, Y. Zhu, X. Jin, L. Li, C. Wang, Y. Zhou, Y. Li, D. Wang, M. Hu, Structural characterization and anti-osteoporosis effects of polysaccharide purified from Eucommia ulmoides Oliver cortex based on its modulation on bone metabolism, Carbohydr Polym 306 (2023) 120601.
- M. Wang, X. Meng, R. Yang, T. Qin, Y. Li, L. Zhang, C. Fei, W. Zhen, K. Zhang, X. Wang, Y. Hu, F. Xue, Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken, Int J Biol Macromol 59 (2013) 178-83.
- M. Schieber, N.S. Chandel, ROS function in redox signaling and oxidative stress, Curr Biol 24(10) (2014) R453-62.
- J. Devi, B. Kumar, B. Taxak, Recent advancements of organotin(IV) complexes derived from hydrazone and thiosemicarbazone ligands as potential anticancer agents, Inorganic Chemistry Communications 139 (2022).
- J.L. Han, H.L. Lin, Intestinal microbiota and type 2 diabetes: from mechanism insights to therapeutic perspective, World J Gastroenterol 20(47) (2014) 17737-45.
- T. Mrudula, P. Suryanarayana, P.N. Srinivas, G.B. Reddy, Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina, Biochem Biophys Res Commun 361(2) (2007) 528-32.
| No. | Source | Types of polysaccharides | Glucose | Galactose | Arabinose | Mannose | Rhamnose | Xylose | Fucose | Glucuronic acid | Galacturonic acid | Fructose | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Astragalus membranaceus | Glucans | 89.00 | 3.00 | 3.50 | 1.00 | - | - | - | - | - | - | Immunomodulatory activity | [9] |
| 2 | Astragalus membranaceus | Glucans | 95.76 | 1.83 | 2.41 | - | - | - | - | - | - | - | Immunomodulatory activity | [10] |
| 3 | Astragalus membranaceus | Glucans | 95.76 | 1.83 | 2.41 | - | - | - | - | - | - | - | Immunomodulatory activity | [11] |
| 4 | Astragalus membranaceus | Glucans | 91.69 | 4.18 | 4.13 | - | - | - | - | - | - | - | Immunomodulatory activity | [12] |
| 5 | Panax ginseng | Glucans | 88.60 | 3.80 | 3.70 | 0.70 | 0.60 | - | - | 0.80 | 1.80 | - | Immunomodulatory activity | [13] |
| 6 | Sagittaria sagittifolia | Glucans | 91.76 | 7.72 | 0.27 | - | - | - | - | - | - | - | Immunomodulatory activity | [14] |
| 7 | Panax ginseng | Glucans | 95.30 | 3.30 | 1.30 | - | - | - | - | - | - | - | Immunomodulatory activity | [15] |
| 8 | Dendrobium huoshanense | Glucans | 95.46 | - | - | 4.54 | - | - | - | - | - | - | Immunomodulatory activity | [16] |
| 9 | Dendrobium officinale | Glucans | 94.17 | - | - | - | - | - | 5.82 | - | - | Immunomodulatory activity | [17] | |
| 10 | Angelica dahurica | Glucans | 97.50 | 0.41 | - | 0.82 | - | 1.15 | - | - | - | - | Immunomodulatory activity | [18] |
| 11 | Angelica sinensis | Glucans | 97.83 | - | - | 1.19 | - | - | - | - | - | - | Immunomodulatory activity | [19] |
| 12 | Polygonum multiflorum | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Immunomodulatory activity | [20] |
| 13 | Radix Aconiti Lateralis Preparata | Glucans | 92.50 | - | 7.50 | - | - | - | - | - | - | - | Immunomodulatory activity | [21] |
| 14 | Schisandra chinensis | Glucans | 89.10 | - | - | - | - | - | - | - | 10.90 | - | Immunomodulatory activity | [22] |
| 15 | Schisandra chinensis | Glucans | 89.80 | 10.20 | - | - | - | - | - | - | - | - | Immunomodulatory activity | [23] |
| 16 | Gastrodia elata | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Immunomodulatory activity | [24] |
| 17 | Pueraria lobata | Glucans | 95.74 | 2.19 | 1.25 | 0.30 | - | 0.43 | 0.09 | - | - | - | Immunomodulatory activity | [25] |
| 18 | Pueraria lobata | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Immunomodulatory activity | [26] |
| 19 | Glehnia littorali | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Immunomodulatory activity | [27] |
| 20 | Astragalus membranaceus | Glucans | 96.43 | 1.40 | 2.17 | - | - | - | - | - | - | - | Regulation of intestinal flora | [28] |
| 21 | Astragalus membranaceus | Glucans | 85.30 | 7.70 | 7.00 | - | - | - | - | - | - | - | Regulation of intestinal flora | [29] |
| 22 | Astragalus membranaceus | Glucans | 85.96 | - | 4.45 | 0.83 | - | 6.15 | - | 2.52 | - | - | Regulation of intestinal flora | [30] |
| 23 | Astragalus membranaceus | Glucans | 79.07 | 6.63 | 8.56 | 0.79 | 1.70 | - | - | - | 3.28 | - | Regulation of intestinal flora | [31] |
| 24 | Panax ginseng | Glucans | 96.64 | 3.20 | - | - | - | - | - | - | - | - | Regulation of intestinal flora | [32] |
| 25 | Crataegus pinnatifida | Glucans | 95.37 | 0.42 | 0.79 | 0.15 | 0.70 | - | - | - | 2.34 | - | Regulation of intestinal flora | [33] |
| 26 | Lycium barbarum | Glucans | 98.10 | - | - | - | - | - | - | - | - | - | Regulation of intestinal flora | [34] |
| 27 | Atractylodis macrocephalae | Glucans | 84.16 | 6.51 | 9.33 | - | - | - | - | - | - | - | Regulation of intestinal flora | [35] |
| 28 | Lycium barbarum | Glucans | 81.83 | 2.02 | 3.46 | 6.52 | 6.06 | - | - | - | - | - | Neuroprotective activity | [36] |
| 29 | Schisandra chinensis | Glucans | 87.80 | 12.30 | - | - | - | - | - | - | - | - | Neuroprotective activity | [37] |
| 30 | Gastrodia elata | Glucans | 99.10 | 0.90 | - | - | - | - | - | - | - | - | Neuroprotective activity | [38] |
| 31 | Gastrodia elata | Glucans | 97.90 | - | 2.10 | - | - | - | - | - | - | - | Neuroprotective activity | [39] |
| 32 | Gastrodia elata | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Neuroprotective activity | [40] |
| 33 | Lonicera japonica | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Neuroprotective activity | [41] |
| 34 | Corydalis yanhusuo | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Neuroprotective activity | [42] |
| 35 | Dendrobium officinale | Glucans | 77.87 | - | - | 22.12 | - | - | - | - | - | - | Antioxidant activity | [43] |
| 36 | Angelica sinensis | Glucans | 76.34 | 14.81 | 2.63 | 5.78 | - | - | - | - | - | - | Antioxidant activity | [44] |
| 37 | Polygonum multiflorum | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Antioxidant activity | [45] |
| 38 | Glycyrrhiza inflata | Glucans | 79.08 | 10.50 | 10.42 | - | - | - | - | - | - | - | Antioxidant activity | [46] |
| 39 | Glycyrrhiza glabra | Glucans | 98.03 | - | - | - | - | - | - | - | - | - | Antioxidant activity | [47] |
| 40 | Pouteria campechiana | Glucans | 86.65 | - | - | 4.62 | - | - | - | - | - | - | Antioxidant activity | [48] |
| 41 | Taraxacum officinale | Glucans | 79.30 | 10.00 | 8.80 | - | 1.50 | - | - | - | - | - | Antioxidant activity | [49] |
| 42 | Sophora flavescens | Glucans | 78.75 | 9.17 | 8.34 | 2.49 | 0.30 | 0.95 | - | - | - | - | Antioxidant activity | [50] |
| 43 | Fallopia multiflora | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Antioxidant activity | [51] |
| 44 | Astragalus membranaceus | Glucans | 97.51 | 1.56 | 0.93 | - | - | - | - | - | - | - | Hypoglycemic activity | [52] |
| 45 | Angelica sinensis | Glucans | 84.59 | 8.90 | - | - | 6.36 | - | - | - | - | - | Hypoglycemic activity | [53] |
| 46 | Codonopsis Pilosula | Glucans | 71.38 | 24.98 | 3.60 | - | - | - | - | - | - | - | Hypoglycemic activity | [54] |
| 47 | Glycyrrhiza uralensis | Glucans | 78.38 | 7.51 | 5.55 | 2.82 | 0.65 | 3.96 | 0.65 | - | 0.48 | - | Hypoglycemic activity | [55] |
| 48 | Lycium barbarum | Glucans | 81.83 | 2.02 | 3.46 | 6.52 | 6.06 | - | - | - | - | - | Hepatoprotective activity | [56] |
| 49 | Polygonatum sibiricum | Glucans | 98.10 | - | - | - | - | - | - | - | - | - | Hepatoprotective activity | [57] |
| 50 | Schisandra chinensis | Glucans | 77.80 | 4.10 | 7.74 | - | - | - | - | - | 8.98 | - | Hepatoprotective activity | [58] |
| 51 | Puerariae lobatae | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Hepatoprotective activity | [59] |
| 52 | Puerariae thomsonii | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Hepatoprotective activity | [60] |
| 53 | Puerariae thomsonii | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Hepatoprotective activity | [61] |
| 54 | Cyathulae officinalis | Glucans | 93.34 | 6.65 | - | - | - | - | - | - | - | - | Hepatoprotective activity | [62] |
| 55 | Ginkgo biloba | Glucans | 98.12 | 1.10 | 0.80 | - | - | - | - | - | - | - | Hepatoprotective activity | [63] |
| 56 | Dendrobium officinale | Glucans | 81.80 | - | - | 18.20 | - | - | - | - | - | - | Antitumor activity | [64] |
| 57 | Angelica sinensis | Glucans | 93.15 | - | 6.75 | - | - | - | - | - | - | - | Antitumor activity | [65] |
| 58 | Platycodon grandiflorus | Glucans | 92.80 | 2.85 | 1.11 | 0.26 | - | - | - | 1.14 | 1.83 | - | Antitumor activity | [66] |
| 59 | Atractylodes macrocephala | Glucans | 82.10 | - | 17.90 | - | - | - | - | - | - | - | Antitumor activity | [67] |
| 60 | Glehnia littoralis | Glucans | 92.10 | 5.30 | 2.60 | - | - | - | - | - | - | - | Antitumor activity | [68] |
| 61 | Pseudostellaria heterophylla | Glucans | 93.10 | 1.00 | 0.90 | - | - | - | - | 2.50 | 0.50 | - | Antitumor activity | [69] |
| 62 | Angelica pubescens | Glucans | 85.10 | 4.50 | 3.20 | 7.30 | - | - | - | - | - | - | Anti-inflammatory activity | [70] |
| 63 | Dioscorea opposita | Glucans | 79.72 | 3.03 | 1.45 | 14.90 | 0.22 | 0.42 | - | - | - | - | Anti-inflammatory activity | [71] |
| 64 | Gastrodia elata | Glucans | 99.30 | 0.20 | - | - | - | - | - | - | 0.40 | - | Anti-inflammatory activity | [72] |
| 65 | Gastrodia elata | Glucans | 100.00 | - | - | - | - | - | - | - | - | - | Anti-inflammatory activity | [73] |
| 66 | Lycium ruthenicum | Arabinogalactans | - | 39.52 | 56.62 | - | 3.80 | - | - | - | - | - | Immunomodulatory activity | [74] |
| 67 | Lycium barbarum | Arabinogalactans | - | 31.07 | 63.79 | - | 1.23 | - | - | - | 3.89 | - | Immunomodulatory activity | [75] |
| 68 | Scutellaria baicalensis | Arabinogalactans | - | 22.20 | 67.10 | - | 4.40 | - | - | 1.20 | 6.30 | - | Immunomodulatory activity | [76] |
| 69 | Rehmannia glutinosa | Arabinogalactans | 0.05 | 56.60 | 38.10 | - | - | - | - | - | - | - | Immunomodulatory activity | [77] |
| 70 | Atractylodes lancea | Arabinogalactans | - | 35.00 | 50.00 | - | 14.50 | 4.00 | - | - | - | - | Immunomodulatory activity | [78] |
| 71 | Astragalus membranaceus | Arabinogalactans | 6.34 | 27.39 | 48.39 | 1.61 | 6.05 | - | - | - | 10.21 | - | Immunomodulatory activity | [79] |
| 72 | Astragalus membranaceus | Arabinogalactans | 13.77 | 18.36 | 51.00 | - | 1.53 | - | - | - | 15.30 | - | Immunomodulatory activity | [80] |
| 73 | Atractylodes lancea | Arabinogalactans | 3.01 | 11.21 | 70.82 | - | 8.84 | 1.84 | - | - | 4.28 | - | Immunomodulatory activity | [81] |
| 74 | Dendrobium officinale | Arabinogalactans | - | 46.79 | 29.79 | - | 11.68 | - | - | - | 11.80 | - | Regulation of intestinal flora | [82] |
| 75 | Lycium barbarum | Arabinogalactans | - | 35.50 | 55.60 | - | 8.00 | - | - | - | - | - | Regulation of intestinal flora | [83] |
| 76 | Lycium barbarum | Arabinogalactans | 2.15 | 39.67 | 40.66 | - | - | - | - | 5.12 | 12.40 | - | Regulation of intestinal flora | [84] |
| 77 | Lycium barbarum | Arabinogalactans | 8.26 | 33.49 | 45.87 | - | - | - | - | 3.21 | 9.17 | - | Regulation of intestinal flora | [85] |
| 78 | Lycium barbarum | Arabinogalactans | 2.15 | 39.67 | 40.66 | - | - | - | - | 5.12 | 12.40 | - | Regulation of intestinal flora | [86] |
| 79 | Angelica sinensis | Arabinogalactans | - | 62.08 | 30.36 | - | - | - | - | - | 7.57 | - | Regulation of intestinal flora | [87] |
| 80 | Atractylodes chinensis | Arabinogalactans | - | 44.10 | 55.90 | - | - | - | - | - | - | - | Regulation of intestinal flora | [88] |
| 81 | Angelica sinensis | Arabinogalactans | 17.75 | 52.41 | 19.31 | - | - | - | - | 10.44 | - | - | Antioxidant activity | [89] |
| 82 | Angelica sinensis | Arabinogalactans | 17.75 | 52.40 | 19.31 | - | - | - | - | 10.44 | - | - | Antioxidant activity | [90] |
| 83 | Angelica sinensis | Arabinogalactans | 17.75 | 52.40 | 19.31 | - | - | - | - | 10.44 | - | - | Antioxidant activity | [91] |
| 84 | Bupleurum chinense | Arabinogalactans | 17.80 | 44.50 | 37.38 | - | - | - | - | - | - | - | Antioxidant activity | [92] |
| 85 | Zizyphus Jujuba | Arabinogalactans | 3.41 | 55.40 | 33.30 | 2.44 | 4.06 | - | - | - | 1.42 | - | Antioxidant activity | [93] |
| 86 | Pueraria mirifica | Arabinogalactans | 4.50 | 58.50 | 27.80 | 0.60 | 7.40 | - | - | 0.80 | 0.20 | - | Antioxidant activity | [94] |
| 87 | Taraxacum officinale | Arabinogalactans | 9.43 | 42.24 | 43.84 | 2.35 | 2.07 | - | - | - | - | - | Antioxidant activity | [95] |
| 88 | Taraxacum officinale | Arabinogalactans | 8.07 | 52.94 | 25.95 | 7.33 | 1.84 | - | - | 1.47 | 2.40 | - | Antioxidant activity | [96] |
| 89 | Ginkgo biloba | Arabinogalactans | 5.94 | 54.00 | 17.28 | 4.32 | 6.48 | - | - | 8.64 | 3.24 | - | Antioxidant activity | [97] |
| 90 | Panax ginseng | Arabinogalactans | - | 22.40 | 53.80 | - | 10.30 | - | - | - | 13.20 | - | Antioxidant activity | [98] |
| 91 | Polygonatum sibiricum | Arabinogalactans | - | 73.64 | 21.04 | - | 5.26 | - | - | - | - | - | Antioxidant activity | [99] |
| 92 | Lycium barbarum | Arabinogalactans | 6.48 | 40.85 | 44.99 | 1.06 | 2.97 | 3.65 | - | - | - | - | Anti-aging activity | [100] |
| 93 | Lycium barbarum | Arabinogalactans | - | 45.90 | 46.10 | - | - | - | - | - | - | - | Anti-aging activity | [101] |
| 94 | Rehmannia glutinosa | Arabinogalactans | 6.68 | 37.83 | 55.49 | - | - | - | - | - | - | - | Anti-aging activity | [102] |
| 95 | Rehmannia glutinosa | Arabinogalactans | 15.39 | 61.36 | 18.19 | 0.80 | 3.31 | - | - | - | 0.96 | - | Anti-aging activity | [103] |
| 96 | Lycium barbarum | Arabinogalactans | - | 60.93 | 39.06 | - | - | - | - | - | - | - | Neuroprotective activity | [104] |
| 97 | Lycium barbarum | Arabinogalactans | 6.89 | 37.64 | 34.88 | 1.03 | 3.68 | 2.46 | - | 0.73 | 12.67 | - | Neuroprotective activity | [105] |
| 98 | Lycium barbarum | Arabinogalactans | 1.40 | 49.80 | 47.80 | - | 1.20 | - | - | - | - | - | Neuroprotective activity | [106] |
| 99 | Ginkgo biloba | Arabinogalactans | 3.00 | 5.00 | 82.00 | 5.00 | - | - | - | - | - | - | Neuroprotective activity | [107] |
| 100 | Lycium barbarum | Arabinogalactans | - | 44.44 | 48.15 | - | - | - | - | - | - | - | Antitumor activity | [108] |
| 101 | Lycium ruthenicum | Arabinogalactans | 4.00 | 46.20 | 40.20 | 1.70 | 5.10 | - | - | 2.30 | 0.50 | - | Antitumor activity | [109] |
| 102 | Angelica sinensis | Arabinogalactans | 17.75 | 52.41 | 19.31 | - | - | - | - | 10.44 | - | - | Antitumor activity | [110] |
| 103 | Panax notoginseng | Arabinogalactans | - | 43.70 | 56.30 | - | - | - | - | - | - | - | Antitumor activity | [111] |
| 104 | Ophiopogon japonicus | Galactans | - | 100.00 | - | - | - | - | - | - | - | - | Antitumor activity | [112] |
| 105 | Polygonatum cyrtonema | Galactans | - | 100.00 | - | - | - | - | - | - | - | - | Regulation of intestinal flora | [113] |
| 106 | Polygonatum cyrtonema | Galactans | - | 100.00 | - | - | - | - | - | - | - | - | Regulation of intestinal flora | [114] |
| 107 | Polygonatum sibiricum | Galactans | 2.13 | 82.91 | - | 14.96 | - | - | - | - | - | - | Immunomodulatory activity | [115] |
| 108 | Polygonatum sibiricum | Galactans | - | 78.77 | - | 5.50 | - | - | - | - | 13.84 | - | Immunomodulatory activity | [116] |
| 109 | Rehmannia glutinosa | Arabinans | - | - | 100.00 | - | - | - | - | - | - | - | Immunomodulatory activity | [117] |
| 110 | Glehnia littoralis | Arabinans | - | - | 100.00 | - | - | - | - | - | - | - | Antitumor activity | [118] |
| 111 | Akebia quinata | Arabinans | - | - | 100.00 | - | - | - | - | - | - | - | Immunomodulatory activity | [119] |
| 112 | Dendrobium officinale | Glucomannans | 33.31 | 1.00 | - | 59.31 | 0.51 | - | - | - | - | - | Immunomodulatory activity | [120] |
| 113 | Dendrobium officinale | Glucomannans | 20.00 | - | - | 80.00 | - | - | - | - | - | - | Immunomodulatory activity | [121] |
| 114 | Dendrobium huoshanense | Glucomannans | 25.78 | - | - | 74.22 | - | - | - | - | - | - | Immunomodulatory activity | [122] |
| 115 | Dendrobium officinale | Glucomannans | 14.50 | - | - | 85.50 | - | - | - | - | - | - | Immunomodulatory activity | [123] |
| 116 | Anemarrhena asphodeloides | Glucomannans | 10.90 | 2.60 | 7.30 | 79.00 | - | 0.20 | - | - | - | - | Immunomodulatory activity | [124] |
| 117 | Dendrobium wardianum | Glucomannans | 22.85 | - | - | 76.66 | - | - | - | - | - | - | Antitumor activity | [125] |
| 118 | Dendrobium officinale | Glucomannans | 12.65 | - | - | 87.34 | - | - | - | - | - | - | Antitumor activity | [126] |
| 119 | Platycodon grandiflorum | Glucomannans | 42.00 | - | - | 57.96 | - | - | - | - | - | - | Antitumor activity | [127] |
| 120 | Dendrobium officinale | Glucomannans | 17.92 | - | - | 82.08 | - | - | - | - | - | - | Neuroprotective activity | [128] |
| 121 | Dendrobium huoshanense | Glucomannans | 24.19 | - | - | 75.81 | - | - | - | - | - | - | Neuroprotective activity | [129] |
| 122 | Dendrobium huoshanense | Glucomannans | 33.47 | 0.48 | 0.26 | 65.79 | - | - | - | - | - | - | Gastroprotective activity | [130] |
| 123 | Dendrobium huoshanense | Glucomannans | 24.75 | - | - | 75.25 | - | - | - | - | - | - | Gastroprotective activity | [131] |
| 124 | Dendrobium officinale | Glucomannans | 24.00 | - | - | 76.00 | - | - | - | - | - | - | Hepatoprotective activity | [132] |
| 125 | Dendrobium officinale | Glucomannans | 17.24 | - | - | 82.76 | - | - | - | - | - | - | Renal protective activity | [133] |
| 126 | Dendrobium officinale | Glucomannans | 28.17 | - | - | 71.83 | - | - | - | - | - | - | Regulation of intestinal flora | [134] |
| 127 | Dendrobium huoshanense | Glucomannans | 36.07 | 1.65 | - | 62.25 | - | - | - | - | - | - | Anti-osteoporosis activity | [135] |
| 128 | Bletilla striata | Glucomannans | 25.00 | - | - | 75.00 | - | - | - | - | - | - | Antioxidant activity | [136] |
| 129 | Dendrobium officinale | Mannans | 5.09 | 2.29 | 1.46 | 91.15 | - | - | - | - | - | - | Immunomodulatory activity | [137] |
| 130 | Ginkgo biloba | Mannans | - | 2.91 | - | 97.08 | - | - | - | - | - | - | Antioxidant activity | [138] |
| 131 | Codonopsis pilosula | Fructans | 3.40 | - | - | - | - | - | - | - | - | 96.60 | Regulation of intestinal flora | [139] |
| 132 | Codonopsis pilosula | Fructans | 2.72 | - | - | - | - | - | - | - | - | 97.28 | Regulation of intestinal flora | [140] |
| 133 | Ophiopogon japonicus | Fructans | - | - | - | - | - | - | - | - | - | 100.00 | Regulation of intestinal flora | [141] |
| 134 | Ophiopogon japonicus | Fructans | - | - | - | - | - | - | - | - | - | 100.00 | Regulation of intestinal flora | [142] |
| 135 | Ophiopogon japonicus | Fructans | - | - | - | - | - | - | - | - | - | 100.00 | Regulation of intestinal flora | [143] |
| 136 | Ophiopogon japonicus | Fructans | - | - | - | - | - | - | - | - | - | 100.00 | Regulation of intestinal flora | [144] |
| 137 | Polygonati kingianum | Fructans | 6.90 | - | - | 0.90 | - | - | - | - | - | 91.30 | Regulation of intestinal flora | [145] |
| 138 | Polygonatum kingianum | Fructans | 6.44 | - | - | - | - | - | - | - | - | 93.56 | Regulation of intestinal flora | [146] |
| 139 | Polygonatum cyrtonema | Fructans | 3.44 | - | - | - | - | - | - | - | - | 96.32 | Regulation of intestinal flora | [147] |
| 140 | Polygonatum cyrtonema | Fructans | 7.50 | - | - | 7.40 | - | - | - | - | - | 77.40 | Regulation of intestinal flora | [148] |
| 141 | Polygonatum kingianum | Fructans | 7.10 | - | - | - | - | - | - | - | - | 92.90 | Regulation of intestinal flora | [149] |
| 142 | Polygonatum cyrtonema | Fructans | 5.84 | - | - | 3.18 | - | - | - | - | - | 89.48 | Regulation of intestinal flora | [150] |
| 143 | Atractylodes lancea | Fructans | 5.52 | - | - | - | - | - | - | - | - | 94.48 | Regulation of intestinal flora | [151] |
| 144 | Codonopsis pilosula | Fructans | - | - | - | - | - | - | - | - | - | 100.00 | Immunomodulatory activity | [152] |
| 145 | Polygonatum kingianum | Fructans | 4.98 | - | - | - | - | - | - | - | - | 95.01 | Immunomodulatory activity | [153] |
| 146 | Polygonatum odoratum | Fructans | 3.30 | - | - | - | - | - | - | - | - | 96.70 | Immunomodulatory activity | [154] |
| 147 | Atractylodis Macrocephalae | Fructans | 11.00 | - | - | - | - | - | - | - | - | 89.00 | Immunomodulatory activity | [155] |
| 148 | Anemarrhena asphodeloides | Fructans | 5.50 | - | - | - | - | - | - | - | - | 94.50 | Immunomodulatory activity | [156] |
| 149 | Polygonatum cyrtonema | Fructans | 3.44 | - | - | - | - | - | - | - | - | 96.32 | Antioxidant activity | [157] |
| 150 | Polygonatum sibiricum | Fructans | 5.40 | - | - | 3.60 | - | - | - | - | - | 91.00 | Antioxidant activity | [158] |
| 151 | Polygonatum cyrtonema | Fructans | 3.85 | - | - | - | - | - | - | - | - | 95.89 | Antioxidant activity | [159] |
| 152 | Polygonatum kingianum | Fructans | 7.20 | 0.80 | - | - | - | - | - | - | - | 92.00 | Antioxidant activity | [160] |
| 153 | Liriope spicata | Fructans | 3.33 | - | - | - | - | - | - | - | - | 96.57 | Hypoglycemic activity | [161] |
| 154 | Ophiopogon japonicas | Fructans | - | - | - | - | - | - | - | - | - | 100.00 | Hypoglycemic activity | [162] |
| 155 | Polygonatum kingianum | Fructans | - | 11.20 | - | 1.10 | - | - | - | - | - | 87.70 | Hypoglycemic activity | [163] |
| 156 | Codonopsis pilosula | Fructans | 3.17 | - | 2.40 | - | - | - | - | - | - | 94.21 | Hepatoprotective activity | [164] |
| 157 | Ophiopogon japonicus | Fructans | 3.13 | - | - | - | - | - | - | - | - | 96.86 | Hepatoprotective activity | [165] |
| 158 | Plantago asiatica | Araboxylans | - | - | 32.20 | - | - | 61.10 | - | - | - | - | Regulation of intestinal flora | [166] |
| 159 | Plantago asiatica | Araboxylans | - | - | 32.20 | - | - | 61.10 | - | - | - | - | Hypoglycemic activity | [167] |
| 160 | Prunella vulgaris | Araboxylans | 8.30 | 9.70 | 24.20 | 1.90 | - | 55.90 | - | - | - | - | Immunomodulatory activity | [168] |
| 161 | Panax ginseng | Pectins | 18.20 | 19.40 | 7.90 | - | 5.20 | - | - | - | 49.30 | - | Immunomodulatory activity | [169] |
| 162 | Panax ginseng | Pectins | 4.46 | 33.03 | 14.28 | - | - | - | - | - | 48.21 | - | Immunomodulatory activity | [170] |
| 163 | Panax ginseng | Pectins | 2.00 | 5.90 | - | - | - | - | - | - | 92.10 | - | Immunomodulatory activity | [171] |
| 164 | Panax ginseng | Pectins | 3.00 | 19.50 | 9.20 | 0.40 | 21.80 | - | - | 2.20 | 33.80 | - | Immunomodulatory activity | [172] |
| 165 | Panax ginseng | Pectins | 12.28 | 14.58 | 15.53 | - | 9.86 | - | - | - | 47.74 | - | Immunomodulatory activity | [173] |
| 166 | Panax ginseng | Pectins | 3.00 | 19.50 | 9.20 | - | 21.80 | - | - | - | 33.80 | - | Immunomodulatory activity | [174] |
| 167 | Codonopsis pilosula | Pectins | - | - | 3.50 | - | 5.70 | - | - | - | 90.80 | - | Immunomodulatory activity | [175] |
| 168 | Codonopsis pilosula | Pectins | - | 4.92 | 2.92 | - | 7.59 | - | - | - | 84.55 | - | Immunomodulatory activity | [176] |
| 169 | Angelica sinensis | Pectins | 4.30 | 21.60 | 22.40 | 7.50 | 3.50 | - | - | - | 39.00 | - | Immunomodulatory activity | [177] |
| 170 | Panax notoginseng | Pectins | 4.50 | 33.30 | 25.20 | - | 15.50 | - | - | - | 17.10 | - | Immunomodulatory activity | [178] |
| 171 | Plantago asiatica | Pectins | 5.67 | 24.00 | 15.89 | 3.79 | 17.89 | 7.12 | 1.11 | 1.86 | 22.68 | - | Immunomodulatory activity | [179] |
| 172 | Panax quinquefolius | Pectins | 11.50 | 15.20 | 19.20 | 12.00 | 2.10 | 9.60 | - | 4.10 | 26.30 | - | Immunomodulatory activity | [180] |
| 173 | Pueraria lobata | Pectins | 4.05 | 16.60 | 16.52 | 0.48 | 6.14 | 4.75 | 2.54 | 1.47 | 47.44 | - | Immunomodulatory activity | [181] |
| 174 | Atractylodis Macrocephalae | Pectins | - | 4.20 | 6.80 | - | 11.00 | - | - | - | 77.90 | - | Immunomodulatory activity | [182] |
| 175 | Gardenia jasminoides | Pectins | 6.03 | 18.52 | 20.30 | - | 5.02 | - | - | - | 50.14 | - | Immunomodulatory activity | [183] |
| 176 | Ginkgo biloba | Pectins | 1.97 | 6.00 | 7.86 | 0.44 | 6.95 | 0.57 | 0.61 | 2.43 | 73.18 | - | Immunomodulatory activity | [184] |
| 177 | Saposhnikovia divaricata | Pectins | - | 5.80 | 7.60 | - | 1.60 | - | - | - | 85.60 | - | Immunomodulatory activity | [185] |
| 178 | Saposhnikovia divaricata | Pectins | - | 43.00 | 35.00 | - | 2.00 | - | - | - | 20.00 | - | Immunomodulatory activity | [186] |
| 179 | Lycium barbarum | Pectins | - | 7.60 | 26.60 | - | 20.80 | 1.90 | - | - | 43.10 | - | Antioxidant activity | [187] |
| 180 | Lycium barbarum | Pectins | 7.37 | 9.95 | 8.93 | 2.47 | 7.00 | 1.16 | - | - | 60.55 | - | Antioxidant activity | [188] |
| 181 | Codonopsis pilosula | Pectins | - | 11.00 | 8.90 | - | 9.30 | - | - | - | 70.10 | - | Antioxidant activity | [189] |
| 182 | Bupleurum chinense | Pectins | 2.50 | 16.70 | 12.90 | - | 14.20 | 1.60 | - | - | 49.20 | - | Antioxidant activity | [190] |
| 183 | Salvia miltiorrhiza | Pectins | 4.00 | 5.60 | 5.60 | - | 5.20 | - | - | - | 78.80 | - | Antioxidant activity | [191] |
| 184 | Ziziphus jujuba | Pectins | - | 4.26 | 8.52 | - | 7.41 | - | - | - | 79.61 | - | Antioxidant activity | [192] |
| 185 | Polygonatum odoratum | Pectins | - | 10.90 | 6.10 | - | 4.40 | 1.10 | - | 1.00 | 76.50 | - | Antioxidant activity | [193] |
| 186 | Morus alba | Pectins | - | 12.70 | 8.90 | - | 15.70 | - | - | 2.00 | 61.00 | - | Antioxidant activity | [194] |
| 187 | Sophorae Tonkinensis | Pectins | 1.20 | 9.70 | 7.30 | 2.00 | 18.40 | 0.90 | - | - | 60.40 | - | Antioxidant activity | [195] |
| 188 | Codonopsis pilosula | Pectins | - | 4.16 | 4.16 | - | 8.32 | - | - | - | 83.20 | - | Antitumor activity | [196] |
| 189 | Bupieurum chinense | Pectins | - | 4.42 | 11.51 | - | 7.18 | - | - | - | 76.89 | - | Antitumor activity | [197] |
| 190 | Lycium barbarum | Pectins | 15.47 | 14.67 | 27.95 | 4.10 | 3.19 | - | - | - | 34.62 | - | Antitumor activity | [198] |
| 191 | Lycium ruthenicum | Pectins | - | 26.60 | 24.90 | - | 14.40 | 16.40 | - | - | 17.70 | - | Antitumor activity | [199] |
| 192 | Polygonum multiflorum | Pectins | - | 29.60 | 24.60 | - | 26.40 | - | - | - | 20.00 | - | Antitumor activity | [200] |
| 193 | Polygala tenuifolia | Pectins | - | 18.90 | 65.60 | - | 7.30 | - | - | - | 8.20 | - | Antitumor activity | [201] |
| 194 | Panax ginseng | Pectins | 12.30 | 11.24 | 23.68 | - | 9.92 | - | 7.38 | - | 35.47 | - | Hypoglycemic activity | [202] |
| 195 | Lycium barbarum | Pectins | 5.29 | 19.53 | 23.10 | 3.49 | 2.77 | 3.46 | - | - | 42.33 | - | Hypoglycemic activity | [203] |
| 196 | Lycium barbarum | Pectins | - | 3.09 | 37.29 | 14.30 | 4.75 | 1.76 | - | - | 38.76 | - | Hypoglycemic activity | [204] |
| 197 | Ziziphus jujuba | Pectins | 4.05 | 9.48 | 3.29 | - | 9.13 | - | - | - | 68.71 | - | Hypoglycemic activity | [205] |
| 198 | Schisandra chinensis | Pectins | 1.10 | 1.29 | 0.89 | 0.71 | 0.88 | - | - | 2.56 | 90.06 | - | Hypoglycemic activity | [206] |
| 199 | Pseudostellaria heterophylla | Pectins | - | 7.00 | 20.50 | - | 5.10 | - | - | - | 63.20 | - | Hypoglycemic activity | [207] |
| 200 | Lycium barbarum | Pectins | 3.13 | 17.92 | 19.96 | - | 8.71 | - | - | - | 50.29 | - | Regulation of intestinal flora | [208] |
| 201 | Gardenia jasminoides | Pectins | 3.18 | 4.16 | 4.72 | - | 5.91 | - | - | - | 82.03 | - | Regulation of intestinal flora | [209] |
| 202 | Lycium barbarum | Pectins | 3.90 | 20.42 | 43.84 | 0.97 | 6.20 | - | - | - | 24.67 | - | Regulation of intestinal flora | [210] |
| 203 | Morus alba | Pectins | 5.74 | 17.28 | 24.13 | - | 23.00 | 1.12 | - | 4.12 | 24.60 | - | Regulation of intestinal flora | [211] |
| 204 | Dendrobium nobile | Pectins | 0.43 | 3.55 | 4.47 | - | 2.38 | 17.84 | 0.26 | 1.26 | 69.80 | - | Hepatoprotective activity | [212] |
| 205 | Panax notoginseng | Pectins | 1.60 | 3.00 | 4.50 | 0.20 | 3.80 | - | - | - | 86.80 | - | Hepatoprotective activity | [213] |
| 206 | Crataegus pinnatifida | Pectins | - | 3.98 | - | 4.50 | - | - | - | - | 85.10 | - | Hepatoprotective activity | [214] |
| 207 | Gardenia jasminoides | Pectins | - | 3.22 | 4.14 | - | 5.77 | - | - | - | 86.87 | - | Hepatoprotective activity | [215] |
| 208 | scrophularia ningpoensis | Pectins | - | 24.00 | 13.50 | 5.40 | 1.20 | 0.50 | - | 4.40 | 51.10 | - | Neuroprotective activity | [216] |
| 209 | Polygala tenuifolia | Pectins | - | 19.80 | 63.50 | - | 8.30 | - | - | - | 8.40 | - | Neuroprotective activity | [217] |
| 210 | Eucommia ulmoides | Pectins | 1.54 | 9.43 | 34.22 | 0.97 | 18.32 | - | 0.14 | 1.27 | 34.11 | - | Anti-osteoporosis activity | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





