1. Introduction
Chronic stress is known to dysregulate the HPA axis, leading to altered secretion of glucocorticoids such as corticosterone in rodents and cortisol in humans [
1].
Chronic social defeat stress (CSDS) is commonly employed to model stress-related disorders such as depression in animals. As a stress-related illness, major depressive disorder (MDD) is also associated with an abnormal HPA axis response to stress. Both people with depression and animals after CSDS typically show alterations in the sensitivity and amplitude of the HPA axis response, altered diurnal patterns of hormone secretion and resistance of immune system to glucocorticoids [
2,
3,
4,
5]. Elevated basal glucocorticoid levels are a key indicator of HPA axis hyperactivity, a condition frequently seen in both stressed animals and depressed individuals [
6,
7,
8]. Elevated corticosterone levels in the CSDS model are shown starting from 10 days of stress [
9], after 30 days [
7], and even extended protocol of CSDS for 7 weeks showed elevated morning corticosterone levels in male mice [
10].
To evaluate the feedback mechanism of the HPA axis the dexamethasone suppression test (DST) is often used. Low-dose treatment of dexamethasone, a synthetic glucocorticoid, typically suppresses intrinsic corticosterone/cortisol secretion. In chronically stressed animals, this suppression may be incomplete or delayed in time. Short-term social stress (7 days) on male rats had no effect on dexamethasone suppression sensitivity [
11]. However after 21 days of social stress weaker suppression by dexamethasone was observed in rats [
12] and CD1 mice [
13]. This impaired feedback mechanism is a significant marker of HPA axis dysregulation, which parallels findings in humans with depression, where cortisol suppression is often incomplete following dexamethasone administration [
14,
15].
Interestingly, there is substantial individual variability in how both humans and animals respond to the DST [
16], indicating that while HPA axis dysregulation is a consistent finding in chronic stress and depression, the exact nature of the dysregulation can differ. Some individuals may show a complete resistance to glucocorticoid feedback, while others exhibit only partial suppression, suggesting that the degree of HPA axis impairment may vary depending on factors such as stress duration, severity, and individual vulnerability.
Similarly, rodent studies show that different stress paradigms impair the HPA axis in distinct ways. As an example, after 14 days of foot shock rats exhibited impaired glucocorticoid feedback and higher corticosterone secretion in response to acute stress [
17], but not 7 days of foot shock. Conversely, 14 days of forced swim did not affect amplitude of corticosterone response to acute stress or feedback inhibition. Chronic dexamethasone treatment also showed variable effects: while 14 days of dexamethasone administration impaired negative feedback without exaggerating response to an acute stressor in rats [
17]. In one study 21 days of dexamethasone treatment lead to low basal corticosterone in C57BL/6J mice [
18]. These findings indicate that both the severity and duration of stress play a critical role in modulating the HPA axis.
Moreover, individual variability in how mice respond to chronic stress is reported in the studies, C57BL/6 mice are often categorized as either stress-sensitive or stress-resistant, with reported differences in behavior and even neurobiological adaptations [
19,
20,
21]. As an example, one study found that resilient C57BL/6J mice displayed increased glucocorticoid receptor (
Nr3c1) expression and nuclear translocation in the hippocampus [
22], suggesting this mechanism is crucial for stress resilience. Another study found that increased plasma corticosterone levels after stress predicted susceptibility to depression-like behaviors [
20]. Genomic studies have shown that epigenetic modifications play an important role in distinguishing resilience from susceptibility to CSDS. In a study by Kuehner et al. [
23], alterations in 5mC and 5hmC levels in the brain were found to differentiate resilient mice, which displayed neuroprotective molecular signatures, from susceptible mice, which exhibited markers of neurodegeneration. Similarly, a study by O’Toole et al. (2019) demonstrated differential DNA methylation patterns after CSDS, with CG hypermethylation being more prominent in susceptible mice. Genes such as
Esr1 and
Cacna1c, linked to psychiatric conditions, were among those with the most differentially methylated sites [
24].
Beyond stress vulnerability, intrinsic variability, even in isogenic populations, is well-documented [
25]. This variation underscores the importance of understanding the heterogeneity in stress responses, particularly in relation to the HPA axis, which may help elucidate why some individuals or animals are more resilient to stress, while others are more susceptible to its negative effects.
Research consistently demonstrates that chronic stress induces HPA axis dysregulation at multiple levels. For instance, stress can enhance Crh expression in the hypothalamus [
26,
27], which drives the HPA axis to secrete more glucocorticoids and reduce glucocorticoid receptor expression in key brain regions involved in feedback regulation, such as hippocampus and prefrontal cortex, contributing to disrupted feedback regulation. While the brain is primarily affected, chronic stress also impacts peripheral tissues, especially those involved in glucocorticoids synthesis. Changes in adrenal gland size and altered glucocorticoid receptor activity in peripheral tissues contribute to the systemic hormonal dysregulation observed under chronic stress. However, while stress response heterogeneity, such as the distinction between susceptible and resilient mice, has been observed, only few studies explore heterogeneity of HPA axis response. As a result, the impact of individual differences on HPA axis dysregulation remains unclear, yet understanding the variability in glucocorticoid sensitivity is important, as this variability may contribute to differences in antidepressant response and help guide personalized treatment choices.
Which is especially important since depression is a heterogeneous disorder with several phenotypes described in the literature [
28], yet all available treatments have similar mechanisms of action and in many cases fail to succeed.
To address these gaps, in this study, we aim to evaluate the variability in the sensitivity of the HPA axis following chronic social defeat stress. We used an extended 30-day social defeat protocol, incorporating dexamethasone suppression tests on both days 10 and 30 to assess transitional and sustained HPA axis states. We increased the sample size to enhance statistical power, allowing us to detect potential variability in corticosterone levels and individual differences in HPA axis response. Moreover, to determine whether differences in HPA axis sensitivity correlate with behavioral traits, we performed a series of behavioral tests (open field, elevated plus maze, social interaction test, partition, social defeat, forced swimming test, sucrose preference test). To uncover the molecular mechanisms linked to different HPA axis sensitivity, we analyzed expression of genes related to the HPA axis in the hypothalamus, pituitary and adrenal glands using qPCR.
2. Results
2.1. Effects of Behavioral Testing on Basal Corticosterone Levels in Control
We used a three-way ANOVA to analyze the effects of experiment, behavioral testing, and dexamethasone administration on corticosterone levels in control animals (
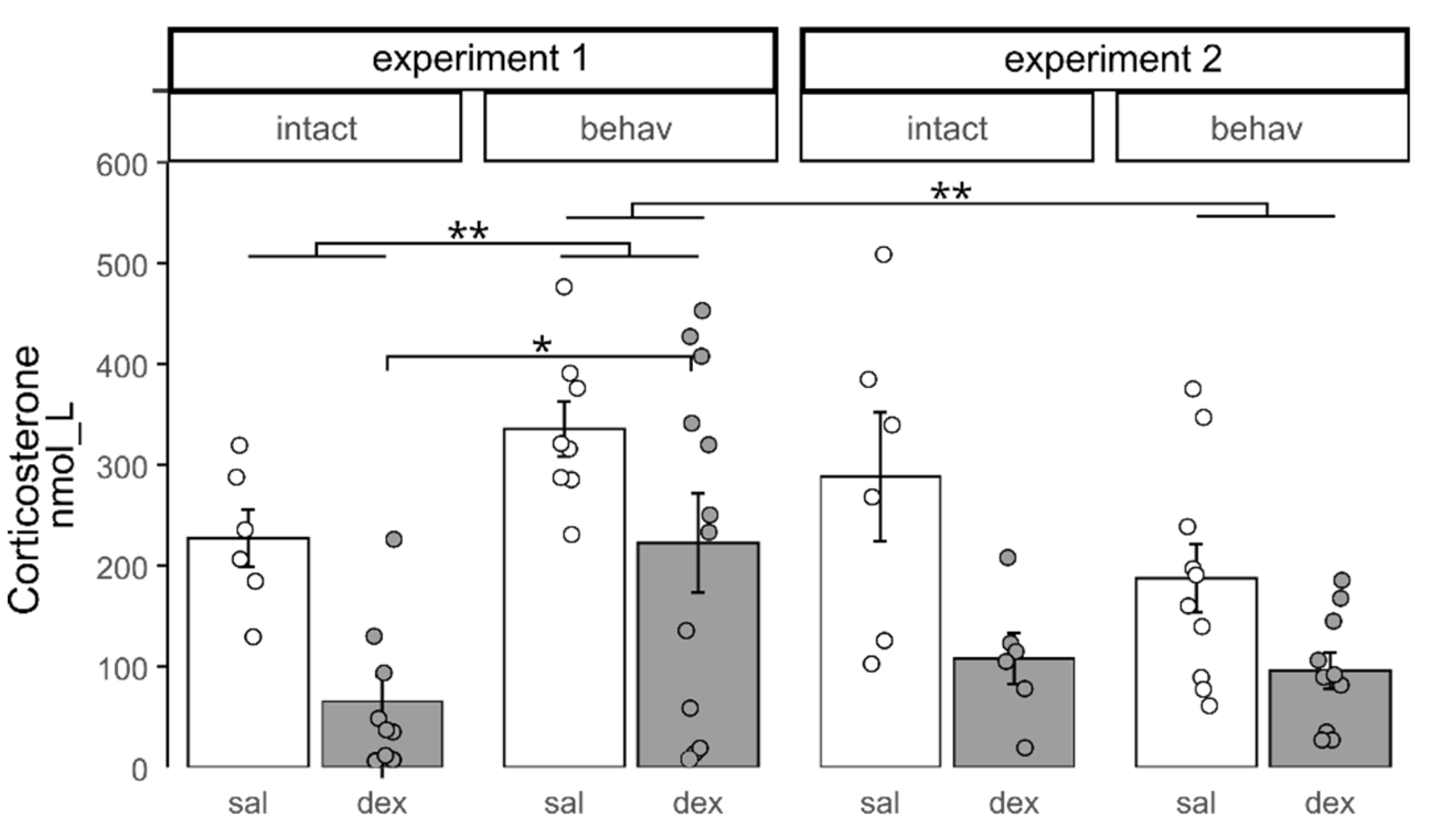
Figure 1). A significant reduction in hormone levels was observed following dexamethasone administration [F(1,59) = 23.13, p < 0.001] in both experiments.
Importantly, there was a significant interaction between factors of experiment and behavioral testing [F(1,59) = 12.60, p < 0.001], indicating that the impact of behavioral procedures on corticosterone levels depended on the nature and intensity of the tests. Specifically, in Experiment 1, animals exposed to a battery of behavioral tests (elevated plus maze, open field, social interaction, and partition test) had significantly higher corticosterone levels compared to intact animals ( p < 0.01). In contrast, in Experiment 2, where only the sucrose preference test was used, no significant differences were detected between control intact and behavioral testing controls.
Dexamethasone reduced corticosterone levels regardless of behavioral testing experience, with no significant interaction between these factors [F(1,59) = 1.49, p = 0.23].
Thus, we have demonstrated that behavioral testing affects the basal corticosterone level in control animals. To further characterize the results obtained in Experiment 1, we used an intact control group, whereas in Experiment 2, the control groups were combined.
When comparing the basal corticosterone levels of animals in the CSDS groups between Experiment 1 and Experiment 2, no significant differences were found (
Figure 2), despite the variation in the number of behavioral tests conducted between the groups. This suggests that the stress induced by behavioral testing is likely negligible compared to the daily social defeat stress in stressed animals. Therefore, we can conclude that, for chronically stressed groups, participation in behavioral tests does not influence resting corticosterone levels.
2.2. HPA Axis State Evaluation After 10 and 30 Days of CSDS with DST
Under prolonged stress, continuous HPA axis activation can lead to elevated basal (resting) glucocorticoids levels, while further HPA activation may result in compensatory suppression of the axis and impaired negative feedback. In this experiment, using the dexamethasone test, we assessed the ability of the glucocorticoid system in mice with CSDS experience to effectively suppress HPA activity in response to stress after administration of synthetic glucocorticoid dexamethasone.
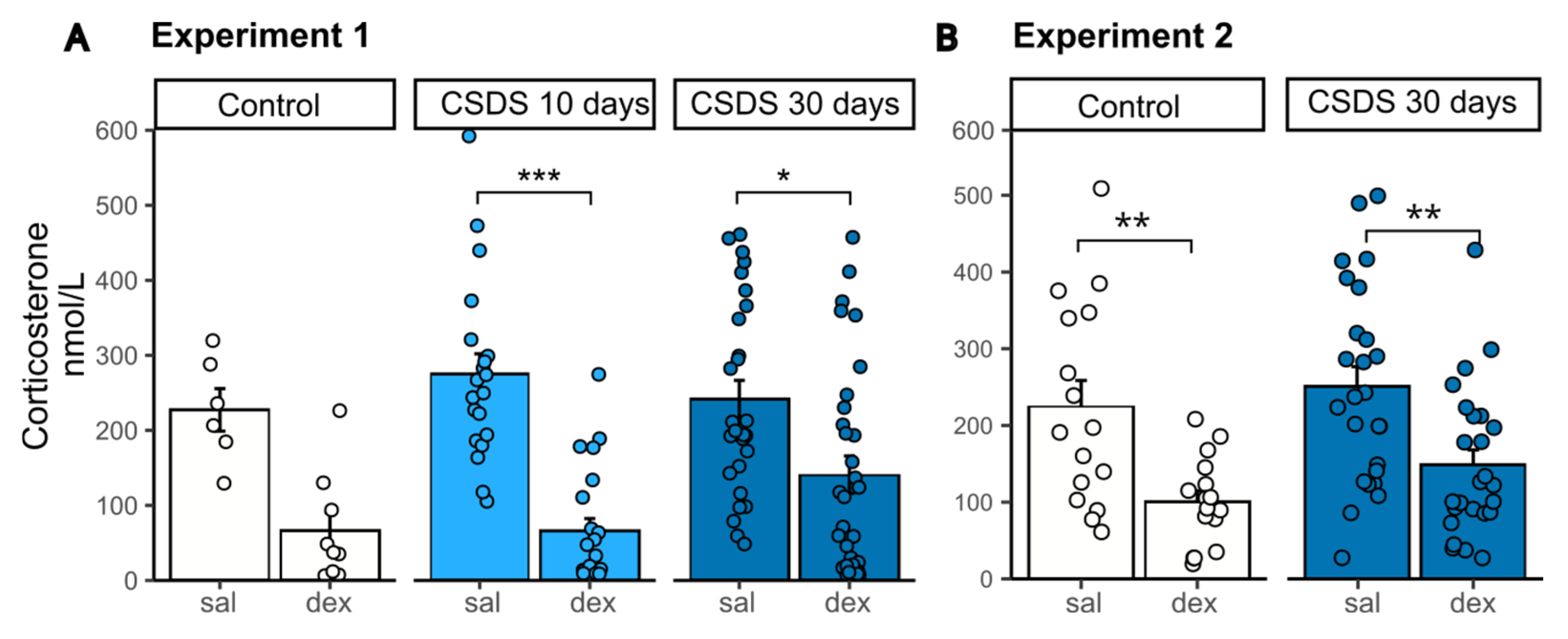
In Experiment 1, we measured corticosterone levels and their response to dexamethasone injection after 10 and 30 days of СSDS. Basal corticosterone in СSDS groups did not differ from controls and was unaffected by stress duration. We previously reported elevated morning corticosterone after 30 days of СSDS [
7], but the difference between groups likely diminishes during the daytime hormone peak. Dexamethasone administration reduced corticosterone in all groups, but suppression was more pronounced in the 10-day stress group (
Figure 2A). Two-way ANOVA revealed only a treatment effect [F(1,109) = 46.1, p < 0.001]. In Experiment 2, we observed the same pattern (control vs CSDS 30 days)—corticosterone reduction 6 hours post-dexamethasone regardless of group, with only a significant treatment effect [F(1,78) = 21.1, p < 0.001] (
Figure 2B).
2.3. Clustering by Corticosterone Response in DST
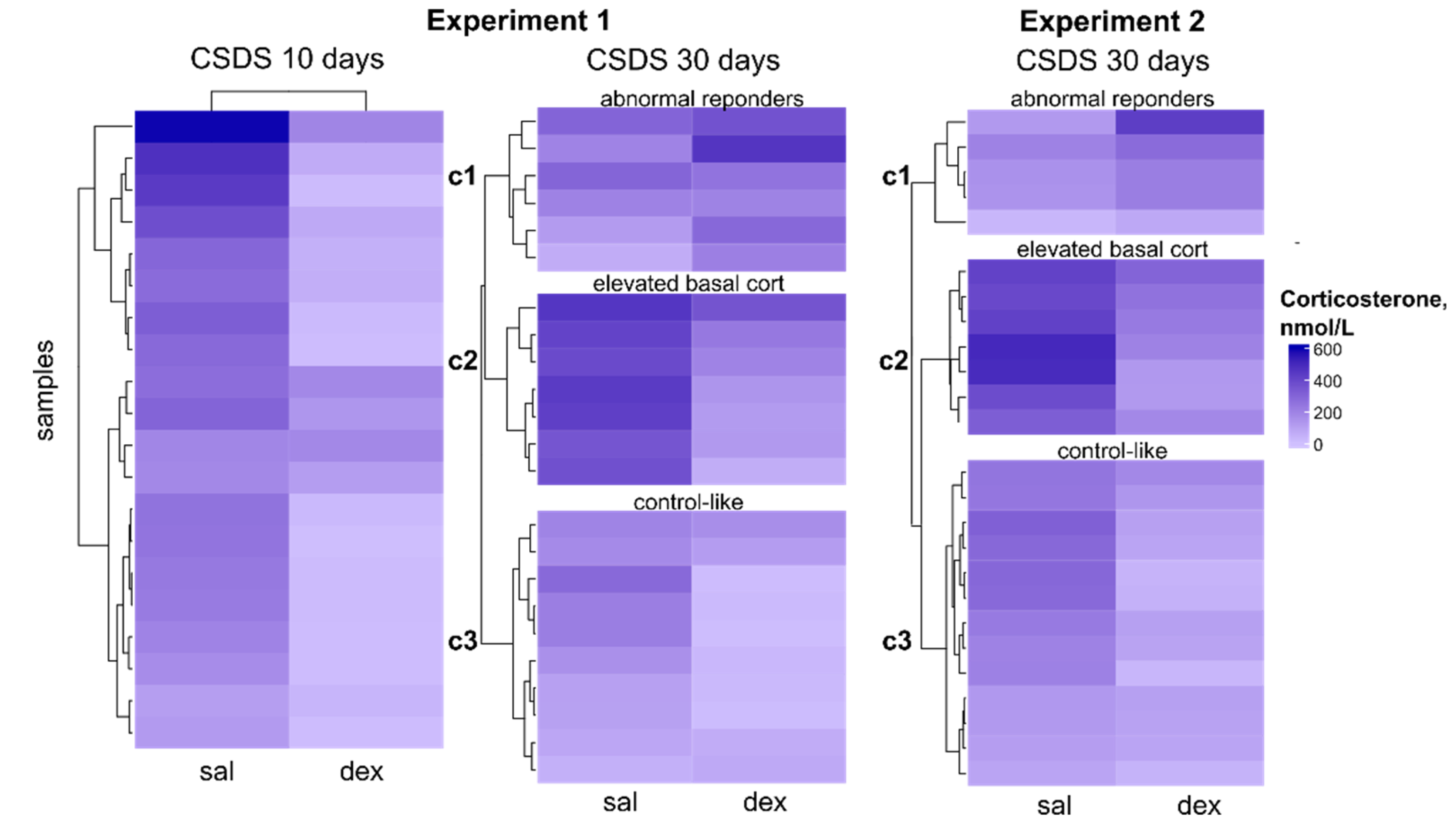
In both experiments the group of animals with 30-day CSDS experience exhibited high variability in corticosterone levels. Specifically, the CSDS group showed a standard deviation (SD) of 142 and 124 for Experiment 1 and 2 respectively, whereas the control group had an SD of 107 and 119. The heatmap reveals that by day 10 of stress, most animals showed reduced corticosterone levels in the dexamethasone suppression test. However, by day 30, both experiments included animals with either no change or even elevated corticosterone levels after dexamethasone treatment (
Figure 3).
The large sample size in both experiments (23 mice in Experiment 1 and 25 mice in Experiment 2, with sufficient serum collected for corticosterone measurement at both stages of the dexamethasone test) allowed us to perform k-means clustering to identify distinct response patterns to dexamethasone administration and define functional subgroups.
CSDS mice were clustered based on their dexamethasone test results after 30 days of stress (basal corticosterone levels and levels 6 hours post-dexamethasone injection). Clustering was performed on Z-scaled values.
In both Experiment 1 and 2, we identified three distinct subgroups, with nearly identical variation in corticosterone dynamics. 1) Abnormal responders (exp1: 26%, n=6; exp2: 20%, n=5), animals showing an atypical increase in corticosterone after dexamethasone, with basal levels consistently below controls. 2) High basal corticosterone (exp1: 30%, n=7; exp2: 29%, n=7), mice with elevated basal corticosterone (relative to controls) and only a mild (2-fold) reduction after dexamethasone, regardless of stress duration. 3) Strong suppressors (exp1: 44%, n=10; exp2: 50%, n=12), mice with low basal corticosterone and a pronounced (4-fold) dexamethasone-induced suppression, reaching levels comparable to intact controls.
Notably, the proportional distribution of animals across clusters was highly consistent between experiments, despite methodological differences (e.g., blood collection protocols, timing of measurements, and ELISA kits from different manufacturers). This reproducibility strongly suggests that the observed clusters reflect genuine, stress-induced alterations in dexamethasone sensitivity.
2.4. Behavior Analysis
To assess stress-induced behavioral changes, we performed a series of standardized tests across two experimental series. In Experiment 1, on days 25-28, animals underwent elevated plus maze, open field, social interaction and partition tests, with additional analysis of social defeat session on day 24. Experiment 2 included sucrose preference test (days 28-30) and forced swim test (day 34).
Chronic stress affected anxiety-related behavior parameters in the elevated plus maze test (
Figure 5A), as shown by one-way ANOVA (factor “group”) with post-hoc Tukey HSD. Animals from the CSDS group spent significantly less time in the center (p < 0.001) and in the open arms (p < 0.001), and significantly more time in the closed arms (p < 0.001) compared to controls, indicating increased anxiety-like behavior.
In the open field test (
Figure 5B) (one-way ANOVA, factor “group”), animals from the CSDS group explored a smaller area of the open field (p < 0.001) and spent less time on exploratory behavior (rearing, p < 0.001). Additionally, animals with chronic stress experience spent less time in the center of the field (p < 0.001) compared to the control group, confirming the increased anxiety level detected in the elevated plus maze test.
In the partition test (
Figure 5C), which assesses social behavior in the home cage under familiar conditions, a two-way ANOVA (factors “group” and “partner type”) revealed significant main effects of both “group” [F(1,95) = 20.39, p < 0.001] and “partner type” [F(1,95) = 46.72, p < 0.001] on time spent near the partition, but no interaction between these factors. Mice exposed to CSDS spent less time near the partition next to a social partner compared to the control, regardless of whether the partner was familiar or unfamiliar (familiar: p < 0.01, unfamiliar: p < 0.01). Both the control and CSDS groups showed greater interest in the unfamiliar social partner than in the familiar one, spending more time near the partition when the unfamiliar partner was presented (control: p < 0.01, CSDS: p < 0.001). Latent time to approach partition didn’t differ significantly between control and CSDS groups. Next, in the partition test, a two-way ANOVA (factors “group” and “partner type”) revealed an effect of the “group” factor on grooming [F(1,95) = 11.9, p < 0.001] and rearing durations [F(1,95) = 40.1, p < 0.001]. Animals from the CSDS group spent more time grooming (p < 0.05 when familiar partner was present, p < 0.001 unfamiliar) and significantly less time engaged in exploratory activity—rearings (p < 0.05 familiar partner, p < 0.001 unfamiliar) compared to control. The “partner type” factor was also significant [F(1,95) = 91.6, p < 0.001], with animals spending less time on rearing activity when an unfamiliar partner was introduced (p < 0.05 familiar partner, p < 0.001 unfamiliar).
In the social interaction test (
Figure 5D), where social behavior was assessed in an unfamiliar neutral cage, a two-way ANOVA (factors “group” and “trial”) revealed effects of the “group” factor [F(1,90) = 10.9, p < 0.01], the “trial” factor [F(1,90) = 24.4, p < 0.001], and their interaction [F(1,90) = 4.7, p < 0.05] on time spent in the target zone, as well as effects of the “group” factor [F(1,87) = 54.4, p < 0.001] and the “trial” factor [F(1,87) = 17.1, p < 0.001] on rearing duration. Animals with chronic stress experience approached the box with a social partner significantly less (second trial of the test) than control animals (p < 0.01), although their interest in the social partner was still significantly higher than in the empty box during the first trial (p < 0.05). The latency to approach the target zone did not differ between trials or groups. In this test, we also observed a decrease in exploratory activity (rearing) in the control group when a social partner was presented (trial 2, p < 0.01), which was not observed in the CSDS group, where exploratory activity was reduced compared to the control in both trials (trial 1: p < 0.001, trial 2: p < 0.01).
The sucrose preference test (
Figure 5E) did not reveal significant changes between control and CSDS groups.
The forced swim test (
Figure 5F) showed increased depressive-like behavior in the CSDS group, with significantly reduced active time (p < 0.05) and increased immobility time (p < 0.01) compared to the control.
Thus, chronic stress exposure led to anxiety- and depression-like behaviors, as well as reduced social activity in male C57BL/6 mice, which is consistent with previous studies [
7,
13,
33,
36] and confirms the validity of the chronic stress model used in the experiments.
2.5. Behavioral Characteristics of Animals with Different HPA Axis Response in DST
We aimed to identify behavioral markers that could reflect cluster segregation in CSDS mice (
Figure 5). Analysis of behavior in animals clustered based on their dexamethasone response revealed notable patterns.
Mice from the first cluster (~20% animals), exhibiting an abnormal increase in corticosterone levels in DST, displayed the lowest social activity in the partition test (toward both familiar and unfamiliar partners). However, in the forced swim test, their behavior was nearly indistinguishable from controls, whereas other subgroups (сluster 2 and 3) showed increased immobility. Notably, during confrontation with an aggressor, mice from this cluster exhibited more freezing behavior than others, with freezing persisting even after the aggressor retreated and contact ceased (
Figure 5G).
Mice from the second cluster (~30% animals), characterized by elevated basal corticosterone levels and a weak dexamethasone suppression, developed anhedonia (reduced sucrose preference, p < 0.001 vs. control), decreased immobility time (p < 0.01) in the forced swim test, and control-like social activity levels in the home cage (partition test).
The third cluster (~50% animals) did not exhibit any distinct behavioral features, displaying typical stress-induced phenotype. Their behavior largely mirrored that of the overall CSDS group, showing reduced time spent in the open arms of the elevated plus maze and in the center of the open field, along with a smaller explored area and elevated rearing activity. In the forced swim test, these mice exhibited increased immobility and a shorter latency to immobility. During the social defeat test, they demonstrated more frequent freezing near the aggressor and spent more time away from it, and in the partition test, they spent less time near the partition.
Thus, mice with an abnormal dexamethasone response (cluster 1) demonstrated a more pronounced passive defense strategy (freezing), accompanied by reduced social behavior even in the neutral home cage environment. At the same time, these animals did not exhibit pronounced depressive-like behavior in classical depression tests (sucrose preference and forced swim test). Mice from the second cluster, with high basal corticosterone levels, displayed characteristic depression-like behaviors while maintaining a control-like response in the dexamethasone suppression test. In contrast, mice with control-like dexamethasone response exhibited a typical CSDS-like phenotype, characterized by anxiety and depression-like behaviors such as reduced exploration, increased immobility and enhanced avoidance of aggressor.
2.6. qPCR Results
In addition in Experiment 2 we performed qPCR gene expression analysis on key HPA axis genes in hypothalamus (
Crh, Crhr1, Crhbp, Fkbp5, Nr3c1), pituitary gland (
Pomc, Crhr1, Nr3c1, Nr3c2), adrenal glands (
Cyp11a1, Cyp11b1, Hsd11b1, Mc2r, Star, Fkbp5, Nr3c1) and additionally
Gh (growth hormone) and
Prl (prolactine) in pituitary gland (
Figure 6).
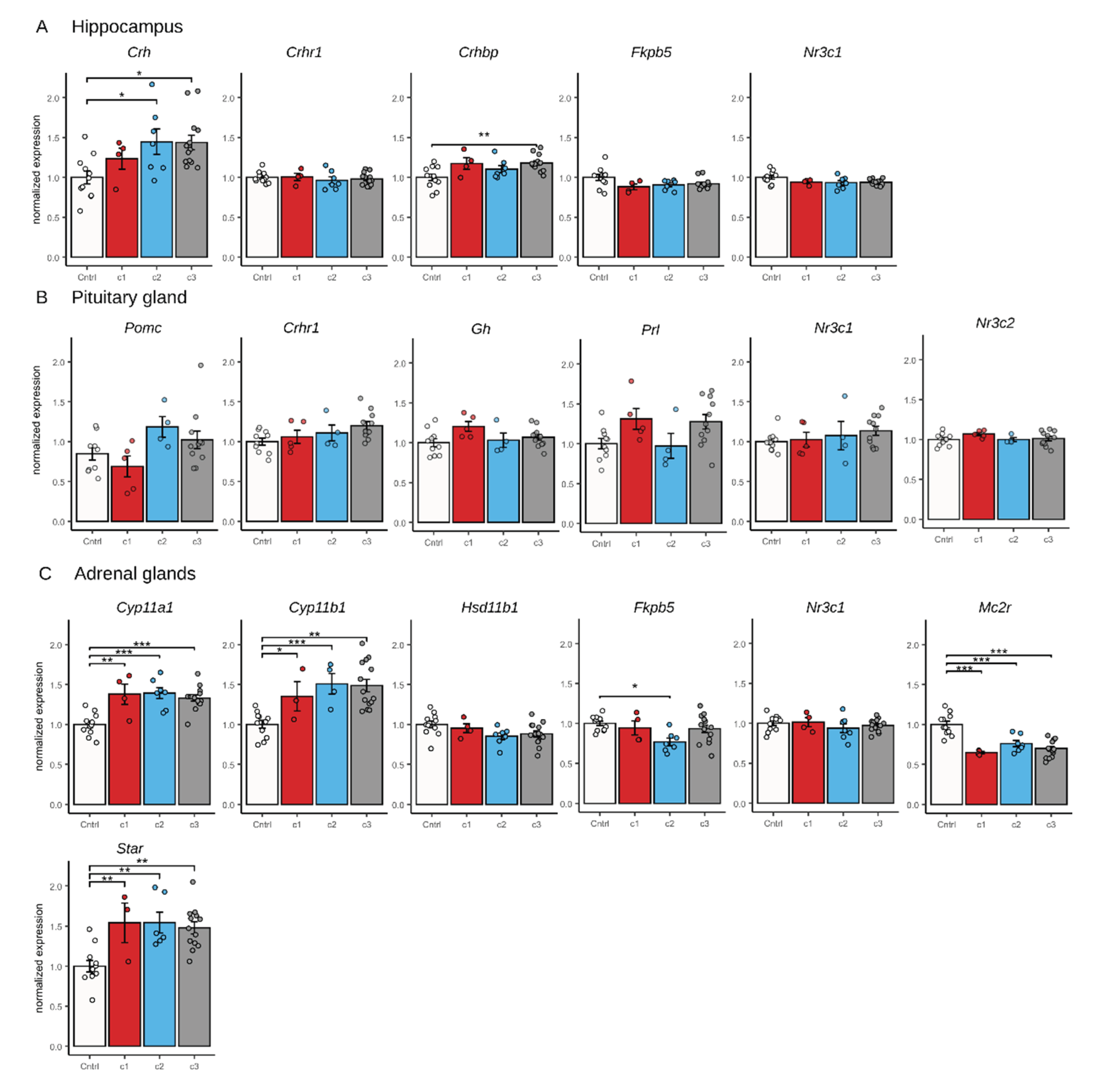
Chronic 30 day stress had a significant effect on expression of central HPA axis regulator Crh [F(3,31)=4.425, p < 0.05] in hypothalamus and its binding protein Crhbp [F(3,31)=4.754, p < 0.01], however this effect vary between clusters. In comparison with control Crh is upregulated in cluster 2 (p < 0.05) and 3 (p < 0.05), while Crhbp significantly upregulated only in cluster 3 (p < 0.01). Interestingly, cluster 1 didn’t significantly differ from control.
In the pituitary gland, we observed no significant effects of stress on gene expression. However, there was a non-significant trend toward increased Crhr1 expression in cluster 3 versus control [F(3,26)=2.58, p = 0.075].
We showed that changes in expression of genes coding for corticosterone synthesis enzymes in adrenal glands are present in all stressed animals regardless of cluster. Such changes are typical for animals after chronic stress exposure [
37] (upregulation of
Cyp11a1 [F(3,32)=12.14, p < 0.001],
Cyp11b1 [F(3,32)=10.46, p < 0.001] and
Star [F(3,32)=7.613, p < 0.001]; downregulation of
Mc2r [F(3,32)=18.2, p < 0.001]).
Downregulation of Fkbp5 [F(3,32)=3.887, p < 0.05] in adrenal glands is present only in cluster 2 (p < 0.05) compared to control. For remaining genes we didn’t show significant changes in expression.
3. Discussion
In this study, we examined how chronic social stress affects the response to dexamethasone administration, assessing the activity of the HPA axis in C57BL6 mice and its ability to respond to glucocorticoid stimulation. These findings provide insights into how chronic stress and glucocorticoids impact the molecular pathways that regulate stress adaptation and neurobiological functions.
Chronic social stress affects corticosterone levels depending on the duration of stress exposure. Initially, it increases due to repeated acute stress responses, but with continued exposure, corticosterone levels normalize or even decrease as a result of HPA axis adaptation. The results of the dexamethasone suppression test reflect these changes: in the early phase of chronic stress (10 days), the negative feedback system remains resistant to glucocorticoid elevation, but with prolonged stress, the regulation either becomes impaired or remains adaptive depending on individual variability in stress responses. This dynamic is critical for understanding the physiological and psychological consequences of chronic stress and has implications for the treatment of stress-related disorders [
38,
39].
Our results show that mice subjected to 10 days of chronic social stress, similar to control animals, significantly reduce corticosterone levels in response to dexamethasone administration (by 76% for 10 days and 73% for 30 days). However, 30 days of stress leads to a much weaker response — a reduction of only 20%. This indicates that despite prolonged stress, the glucocorticoid feedback mechanism required to limit the stress response remains functional in the early stages of stress exposure. Nevertheless, Buwalda et al. [
40] demonstrated that even brief episodes of social stress (2 days) can alter HPA axis regulation, as dexamethasone reduced corticosterone levels in stressed rats, yet ACTH remained elevated, indicating that reduction of pituitary sensitivity to glucocorticoids in stressed animals begins early and possibly intensifies with chronic exposure.
The variation we observed in corticosterone levels (both baseline and dexamethasone-suppressed) by day 30 in the CSDS group may reflect progressive adaptation or dysregulation of the HPA axis under prolonged stress exposure. This variability supports clinical findings in depressed patients, suggesting potential HPA axis dysregulation under chronic stress. Individuals with severe depression or chronic stress often show altered cortisol regulation, which may manifest as either an exaggerated or blunted cortisol response to stress [
41,
42].
Different subtypes of MDD have been proposed to account for variability in HPA axis activity, particularly the division into melancholic and atypical subtypes [
43]. Melancholic depression, characterized by low mood, anhedonia, loss of appetite, weight loss, and insomnia, is associated with elevated basal cortisol levels and a stronger cortisol response in the dexamethasone test compared to atypical depression. Some patients also exhibit impaired HPA axis inhibition (lack of cortisol suppression) via negative feedback [
43]. In contrast, atypical MDD is marked by heightened emotional reactivity to external stimuli and at least two of the following: overeating, hypersomnia, leaden paralysis, and dysphoria. In this subtype, basal cortisol levels are either normal or reduced, and the dexamethasone test typically shows either no significant differences from controls or a more pronounced suppression [
43].
The subgroups of stressed animals we identified based on their dexamethasone response may correspond to these depression subtypes. For example, a group of mice showing an abnormal (increased) corticosterone response to dexamethasone and reduced social activity may resemble atypical depression, where paradoxical responses to stress and medication are common [
44,
45],and social withdrawal is prominent [
46], despite milder classical depressive symptoms [
47].
A second cluster of stressed animals, characterized by high basal corticosterone and anhedonia, may resemble melancholic depression, which is marked by elevated physiological stress markers (high cortisol) and reduced capacity for pleasure. Elevated corticosterone, alongside increased immobility in the forced swim test, may reflect neurobiological adaptations to chronic stress, akin to the psychomotor retardation seen in melancholic individuals despite high stress hormone levels [
42].
A third cluster, which shows typical stress-induced behavior and a dexamethasone response indistinguishable from controls, may reflect features of mild or moderate depressive episodes, where stress reactivity is evident but without pronounced neuroendocrine dysfunction.
Studies using the same stress model have shown that mice exposed to chronic social stress and exhibiting passive behavior during confrontations displayed elevated corticosterone levels by day 21, which then dropped below baseline after cessation of daily confrontations [
48]. In contrast, animals with active coping behavior during confrontations had basal corticosterone levels comparable to controls. Active coping was also associated with more stable sympatho-adrenomedullary axis activity, as indicated by higher catecholamine levels compared to passively coping animals.
Our findings of variable behavioral and corticosterone responses point to the underlying heterogeneity that frequently manifests during prolonged stress exposure. Even organisms as simple as yeast, exposed to uniform conditions, display a wide range of phenotypic responses to environmental changes, as shown by single-cell RNA-seq data [
25]. This heterogeneity likely arises from the internal state of cells [
25], which can be reflected in transcript and protein levels as well as epigenetic modifications and is affected by random molecular events and environmental factors. Although this phenotypic variability is often temporary, it can still direct long-term outcomes.
Genes involved in vital processes, such as housekeeping genes, show less variation of expression levels, while stress-responsive genes display higher variability. This may be because fluctuations in stress-related proteins help cells adapt to changing environments [
49]. Stress has been shown to significantly impact heterogeneity, increasing noise levels in gene expression, protein production, and physiological responses, as seen in both yeast [
50] and mammalian studies [
51].
The diverse patterns in behavioral responses and corticosterone levels we observed underscore the individual variability that chronic stress tends to amplify. qPCR provides an additional layer of information, revealing the molecular underpinnings of variability in HPA axis regulation in animals exposed to chronic social stress.
In cluster 1, animals exhibit abnormal corticosterone elevation in the dexamethasone suppression test, indicating impaired negative feedback regulation of the HPA axis. Consistent adrenal upregulation of corticosterone synthesis genes (Cyp11a1, Cyp11b1, Star) reflects an increased capacity for corticosterone production that is only activated under stress or challenge conditions. The absence of significant Crh or Crhbp dysregulation in the hypothalamus suggests that central HPA axis regulators are not the primary drivers of this phenotype, but adrenal dysregulation plays a critical role.
Cluster 2 displayed elevated basal corticosterone, anhedonia, and reduced immobility in the forced swim test, suggesting a hyperactive HPA axis state with altered coping strategies. Anhedonia, as evidenced by reduced sucrose preference, may result from disruptions in reward system pathways sensitive to chronic corticosterone exposure and glucocorticoid receptor activity. Molecularly, elevated
Crh promotes HPA axis hyperactivation, while reduced
Fkbp5 may enhance glucocorticoid receptor sensitivity, leading to prolonged transcriptional effects of corticosterone and contributing to sustained hypercortisolemia. In humans and animal models there is evidence of
Fkbp5 involvement in depression phenotypes. Generally research shows that demethylation, especially observed in patients with history of childhood adversity [
52] and subsequent higher
Fkbp5 levels in blood samples contribute to an increased stress sensitivity and altered emotional processing [
53].
In cluster 3, with normal DST results, we observe the upregulation of Crh and Crhbp expression which could represent a homeostatic mechanism for maintaining HPA axis balance. Crhbp buffers excessive Crh activity, limiting overstimulation of Crh receptors, thereby preventing the development of stress-related phenotypes. This balanced regulation of the HPA axis corresponds with the absence of pronounced behavioral abnormalities, suggesting that this cluster represents an adaptive response to chronic stress.
Basal expression of HPA axis related genes showed only subtle changes, suggesting that differences in stress reactivity may be mediated by epigenetic or other regulatory mechanisms of gene expression rather than changes in basal gene expression. For example, DNA methylation has been implicated in chronic stress, although promoter-specific data is limited. Another possibility is that potential differences in gene expression may emerge under challenge rather than at rest, and since all the tissues were collected after chronic stress exposure without additional acute stress or dexamethasone administration, we only detect slight alterations.
Both animal and human studies highlight the critical role of the HPA axis in stress response and psychopathology. Our findings suggest that the degree of HPA axis dysregulation may correlate with the severity of behavioral and physiological symptoms. The complex interplay between stress responses and glucocorticoid sensitivity/resistance represents an important area in understanding the pathophysiology of stress-related disorders, including depression [
54].
It is important to note that depression remains a uniquely human disorder. Not all symptoms observed in patients with MDD can be replicated in animal models. However, stress-induced animal models of depression, such as CSDS, serve as valuable tools for research, enabling the investigation of molecular mechanisms in the brain and the analysis of response heterogeneity in large cohorts.
Another limitation is that synthetic glucocorticoids used in the DST differ from endogenous ones in terms of pharmacokinetics, bioavailability, blood-brain barrier penetration, and mineralocorticoid receptor cross-reactivity [
55,
56]. Consequently, when studying stress responses using synthetic glucocorticoids, tissue reactions may differ from those occurring under natural stress conditions.
This work highlights the importance of considering heterogeneity in stress responses. It underscores that depression cannot be accurately represented by a single physiological model, as multiple patterns of stress response exist. Future research may focus on deeper analysis of individual differences in chronic stress responses.
Figure 1.
Plasma corticosterone levels in control with behavior testing experience (“behav”) and intact (“intact”) animals in Experiments 1 and 2. Corticosterone levels were assessed using a dexamethasone test 6 hours after administration of saline (at 15:00, Zeitgeber time 8). Three-way ANOVA, TukeyHSD, * p adj <0.05, ** p adj <0.01, *** p adj <0.001.
Figure 1.
Plasma corticosterone levels in control with behavior testing experience (“behav”) and intact (“intact”) animals in Experiments 1 and 2. Corticosterone levels were assessed using a dexamethasone test 6 hours after administration of saline (at 15:00, Zeitgeber time 8). Three-way ANOVA, TukeyHSD, * p adj <0.05, ** p adj <0.01, *** p adj <0.001.
Figure 2.
Evaluation of HPA axis state in dexamethasone suppression test after 10 and 30 days of chronic social defeat stress. A. Experiment 1. B. Experiment 2. Two-way ANOVA, TukeyHSD, * p adj <0.05, ** p adj <0.01, *** p adj <0.001.
Figure 2.
Evaluation of HPA axis state in dexamethasone suppression test after 10 and 30 days of chronic social defeat stress. A. Experiment 1. B. Experiment 2. Two-way ANOVA, TukeyHSD, * p adj <0.05, ** p adj <0.01, *** p adj <0.001.
Figure 3.
Heatmaps of plasma corticosterone concentrations (nmol/L) in C57BL/6 mice after chronic social defeat stress. From left to right: after 10 days of CSDS in Experiment 1, after 30 days of CSDS in Experiment 1, and after 30 days of CSDS in Experiment 2. Lower concentrations are shown in lighter blue, higher concentrations in darker blue. CSDS30 heatmaps separated into clusters discussed below. sal—saline, dex—dexamethasone, CSDS—chronic social defeat stress. с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone + blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone.
Figure 3.
Heatmaps of plasma corticosterone concentrations (nmol/L) in C57BL/6 mice after chronic social defeat stress. From left to right: after 10 days of CSDS in Experiment 1, after 30 days of CSDS in Experiment 1, and after 30 days of CSDS in Experiment 2. Lower concentrations are shown in lighter blue, higher concentrations in darker blue. CSDS30 heatmaps separated into clusters discussed below. sal—saline, dex—dexamethasone, CSDS—chronic social defeat stress. с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone + blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone.
Figure 4.
Comparative evaluation of HPA axis state in dexamethasone suppression test after 10 and 30 days of chronic social defeat stress. On the left is Experiment 1, on the right is Experiment 2. A.Clustering of CSDS mice by response to DST using Z-scaled values of corticosterone serum levels after saline and dexamethasone injection. Numbers on edges of polygons are mice IDs. B. Corticosterone level by cluster, nmol/L. C. Change in corticosterone level by cluster, log2FC—log2 fold change in corticosterone levels (dexamethasone vs. saline injection). Clusters c1, c2 and c3 are CSDS subgroups identified based on k-means cluster analysis by response to dexamethasone administration. sal—saline, dex—dexamethasone, CSDS—chronic social defeat stress. с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone + blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone. Two-way ANOVA, TukeyHSD, ** p adj < 0.01.
Figure 4.
Comparative evaluation of HPA axis state in dexamethasone suppression test after 10 and 30 days of chronic social defeat stress. On the left is Experiment 1, on the right is Experiment 2. A.Clustering of CSDS mice by response to DST using Z-scaled values of corticosterone serum levels after saline and dexamethasone injection. Numbers on edges of polygons are mice IDs. B. Corticosterone level by cluster, nmol/L. C. Change in corticosterone level by cluster, log2FC—log2 fold change in corticosterone levels (dexamethasone vs. saline injection). Clusters c1, c2 and c3 are CSDS subgroups identified based on k-means cluster analysis by response to dexamethasone administration. sal—saline, dex—dexamethasone, CSDS—chronic social defeat stress. с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone + blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone. Two-way ANOVA, TukeyHSD, ** p adj < 0.01.
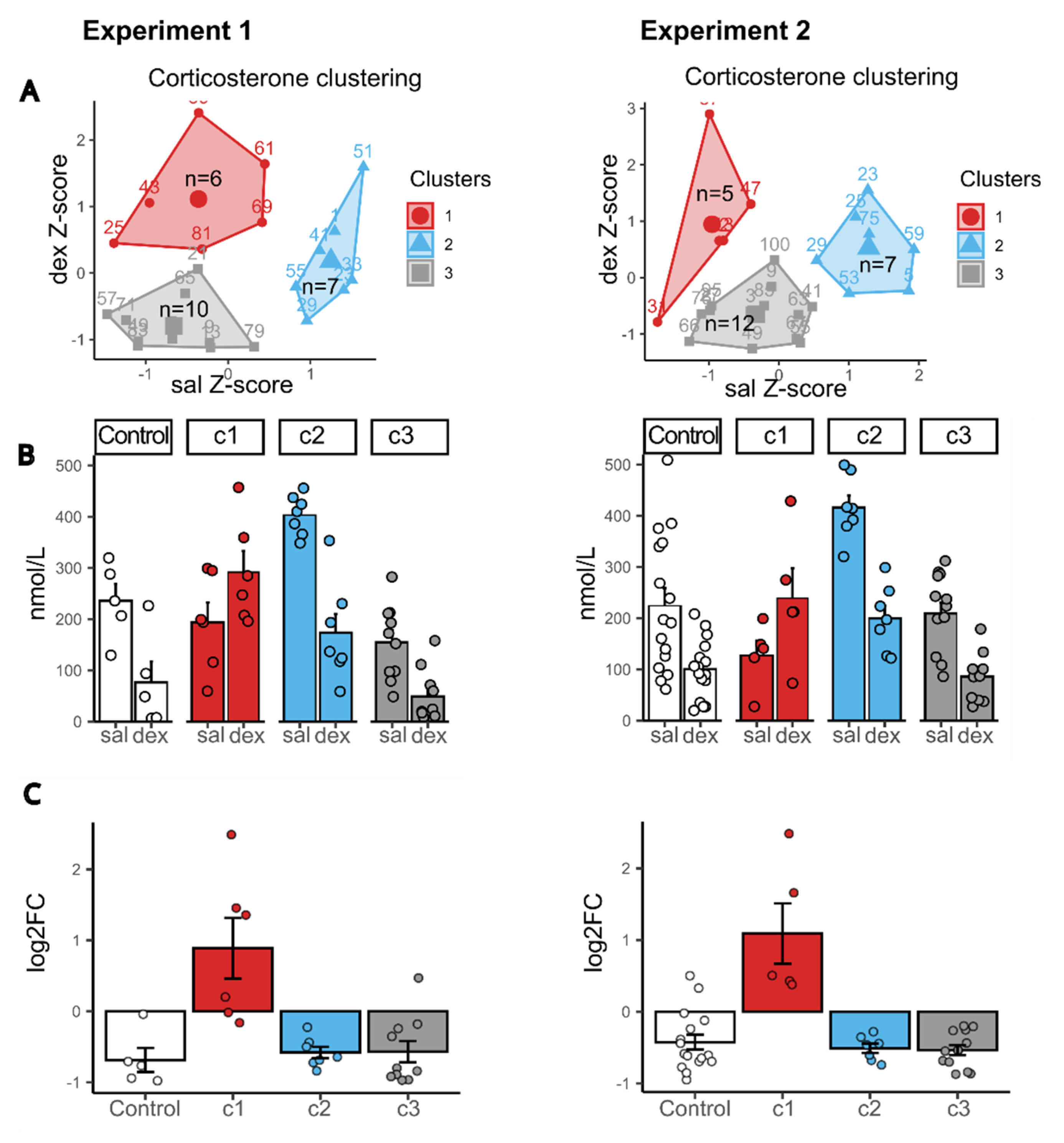
Figure 5.
Characteristics of mice behavior after chronic social defeat stress exposure. A. Elevated plus maze. B. Open field. C. Partition. Familiar partner—male CD1 with whom the experimental mice were kept in the home cage for the last day, unfamiliar—intact CD1 males. D. Social interaction test. In the first trial, testing was carried out without a social partner, in the second trial, CD1 aggressor was placed. t1—trial 1, t2—trial 2. E. Sucrose preference test. F. Forced swimming test. G. Social defeat session. The parameters include the percentage of total freezes occurring during the aggressor’s attack, during non-aggressive contact, time spent near or away from the aggressor. Clusters c1, c2 and c3 are CSDS subgroups identified based on k-means cluster analysis by response to dexamethasone administration . с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone and blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone. One- or two-way ANOVA, TukeyHSD, * p adj < 0.05, ** p adj < 0.01, *** p adj < 0.001.
Figure 5.
Characteristics of mice behavior after chronic social defeat stress exposure. A. Elevated plus maze. B. Open field. C. Partition. Familiar partner—male CD1 with whom the experimental mice were kept in the home cage for the last day, unfamiliar—intact CD1 males. D. Social interaction test. In the first trial, testing was carried out without a social partner, in the second trial, CD1 aggressor was placed. t1—trial 1, t2—trial 2. E. Sucrose preference test. F. Forced swimming test. G. Social defeat session. The parameters include the percentage of total freezes occurring during the aggressor’s attack, during non-aggressive contact, time spent near or away from the aggressor. Clusters c1, c2 and c3 are CSDS subgroups identified based on k-means cluster analysis by response to dexamethasone administration . с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone and blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone. One- or two-way ANOVA, TukeyHSD, * p adj < 0.05, ** p adj < 0.01, *** p adj < 0.001.
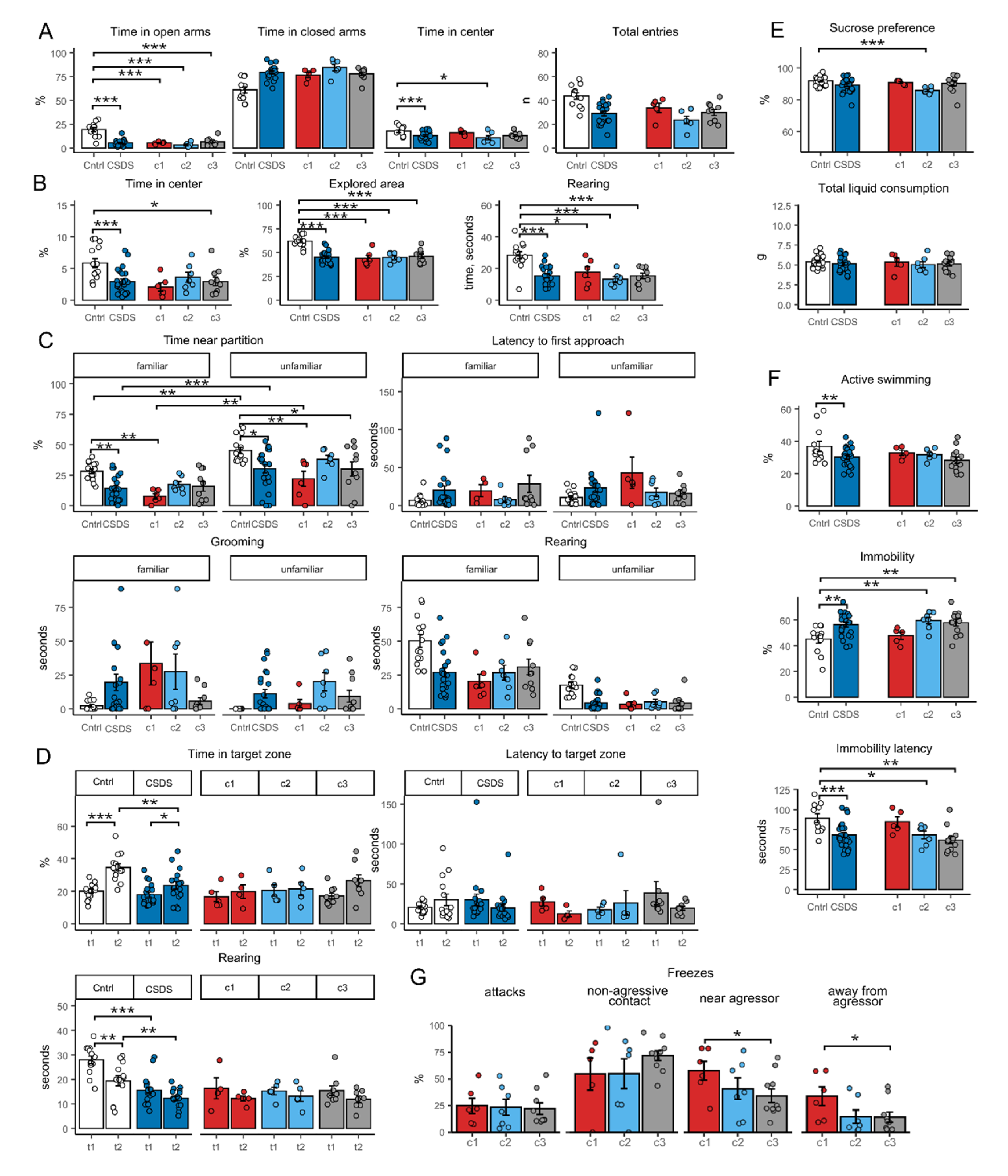
Figure 6.
Expression of key HPA axis genes in subgroups of animals identified after CSDS exposure as shown by qPCR analysis. A.Hypothalamus. B. Pituitary gland. C. Adrenal glands. Clusters c1, c2 and c3 are CSDS subgroups identified based on k-means cluster analysis by response to dexamethasone administration. с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone and blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone. One-way ANOVA, TukeyHSD, t—p adj < 0.1, * p adj < 0.05, ** p adj < 0.01, *** p adj < 0.001.
Figure 6.
Expression of key HPA axis genes in subgroups of animals identified after CSDS exposure as shown by qPCR analysis. A.Hypothalamus. B. Pituitary gland. C. Adrenal glands. Clusters c1, c2 and c3 are CSDS subgroups identified based on k-means cluster analysis by response to dexamethasone administration. с1 (сluster 1): low basal corticosterone + abnormal response to dexamethasone (paradoxical increase). с2 (сluster 2): high basal corticosterone and blunted suppression after dexamethasone (≤2-fold decrease). с3 (сluster 3): low basal corticosterone and control-like suppression after dexamethasone. One-way ANOVA, TukeyHSD, t—p adj < 0.1, * p adj < 0.05, ** p adj < 0.01, *** p adj < 0.001.
Figure 7.
Experimental design for Experiments 1 and 2. Arrows indicate the days on which saline or dexamethasone was administered (dexamethasone suppression test). Blood samples were collected six hours after administration on the same day. EPM—elevated plus maze, OF—open field, SI—social interaction, P—partition, FST—forced swim test; SPT—sucrose preference test; SD—social defeat test; CSDS—group of male mice was subjected to 30 days of chronic social defeat stress; Control_behav—animals without CSDS experience but with behavior testing; Intact—animals without CSDS or behavior testing experience.
Figure 7.
Experimental design for Experiments 1 and 2. Arrows indicate the days on which saline or dexamethasone was administered (dexamethasone suppression test). Blood samples were collected six hours after administration on the same day. EPM—elevated plus maze, OF—open field, SI—social interaction, P—partition, FST—forced swim test; SPT—sucrose preference test; SD—social defeat test; CSDS—group of male mice was subjected to 30 days of chronic social defeat stress; Control_behav—animals without CSDS experience but with behavior testing; Intact—animals without CSDS or behavior testing experience.