1. Introduction
Goose astrovirus (GoAstV) is an emerging avian pathogen in China, having disseminated across major goose-farming regions [
1,
2]. Goslings infected with GoAstV develop visceral and articular gout, accompanied by anorexia, lethargy, and excretion of chalky droppings [
3,
4,
5]. The infection induces rapid mortality in goslings, with case fatality rates of 20%–50%, causing substantial economic losses to the poultry industry [
6]. Although the gout-inducing mechanisms have been investigated, effective control strategies remain elusive [
5,
7,
8].
GoAstV is a single-stranded, positive-sense RNA virus within the family Astroviridae and genus Avastrovirus [
9]. Similar to other astroviruses, GoAstV possesses an approximately 7 kb genome comprising two untranslated regions (the 5′-UTR and 3′-UTR) and three open reading frames (ORF1a, ORF1b, and ORF2) [
1,
10,
11]. ORF1a and ORF1b encode non-structural proteins that mediate viral replication and transcription [
12,
13]. ORF2, encoding the structural protein, constitutes the viral capsid [
14]. During host cell invasion, the ORF2-encoded polyprotein undergoes trypsin-mediated proteolytic cleavage into subunits including VP25, VP27, and VP34; notably, VP27 governs viral infectivity and serves as the dominant antigenic protein [
15].
Vaccine development represents one of the most effective strategies for disease control, wherein neutralizing antibody titers serve as a critical metric for evaluating vaccine-induced immunoprotection. To date, several ELISA-based methods for detecting GoAstV-specific serum antibodies have been established, enabling diagnostic confirmation, seroepidemiological surveillance, and immune monitoring [
16,
17]. Furthermore, methods that could detect neutralizing antibodies should also be established to evaluate vaccine efficacy in future applications.
In this study, a murine monoclonal antibody (mAb) was generated against the recombinant GoAstV VP27 protein, and its immunoreactivity was characterized by western blot and immunofluorescence assay (IFA). An indirect competitive ELISA (ic-ELISA) was subsequently developed using this anti-VP27 monoclonal antibody, with subsequent evaluation of the sensitivity, specificity, and reproducibility. This established method was then applied to conduct an epidemiological investigation of GoAstV using serum samples. The present study provided a reliable serological tool for the detection of GoAstV-specific antibodies and facilitates future vaccine evaluation.
2. Materials and Methods
2.1. Cells, Virus, Sera and Animals
SP2/0 murine myeloma cells were obtained from Procell Life Science & Technology Co., Ltd. (Wuhan, China) and maintained in RPMI-1640 medium supplemented with 20% fetal bovine serum (FBS; Thermo Fisher Scientific, USA). LMH cells were preserved in our laboratory and cultured in DMEM containing 10% FBS and used for the propagation of GoAstV. One-day old geese were acquired from Guiliu Livestock Farming Co., Ltd. (Qihe, China). GoAstV-negative sera were obtained from goslings confirmed negative by PCR, while positive sera were collected at 7 days post-infection. BALB/c mice were procured from Shandong Pengyue Experimental Animal Breeding Co., Ltd. (Jinan, China).
2.2. Expression and Purification of VP27 Protein
Total RNA of GoAstV was extracted using the Simply P Total RNA Extraction Kit (BioFlux, Hangzhou, China). The VP27 gene segment was amplified by one-step RT-PCR using the AccurSTART One Step RT-PCR Kit (Vazyme, Nanjing, China) with the primer pair 5′-gaattcCAGGTTACTCCCTCGCT-3′ and 5′-gcggccgcAGAGGTCTTGAGCGAGAC-3′, which incorporate EcoRI and NotI restriction sites, respectively. The amplified VP27 sequence was verified by sequencing and subsequently codon-optimized for expression in E. coli by Sangon Biotech Co., Ltd. (Qingdao, China). The optimized VP27 gene was cloned into the pET-28a (+) vector, and the resulting recombinant plasmid, pET-28a-VP27, was confirmed by restriction digestion and DNA sequencing. The validated plasmid was then transformed into E. coli BL21(DE3) competent cells (TransGen, Beijing, China). Recombinant protein expression was induced by the addition of IPTG. VP27 expression was analyzed by SDS-PAGE, and the protein was purified under native conditions using His-Tagged Protein Purification Kit (CWBIO, Beijing, China). The purified VP27 was verified by SDS-PAGE and Western blot analysis using anti-His monoclonal antibody (Biodragon, Beijing, China).
2.3. Development of Anti-VP27 mAbs
The purified VP27 protein was emulsified with an equal volume of incomplete Freund's adjuvant (Sigma-Aldrich, Shanghai, China). Six-week-old female BALB/c mice were subcutaneously injected in the cervical region with the emulsion containing 50 μg of VP27 protein. Immunizations were administered three times at two-week intervals. Fourteen days following the final immunization, antibody titers in serum were detected by indirect ELISA using VP27 protein as the coating antigen following procedures reported before [
18]. Mice with high serum antibody titers were euthanized by cervical dislocation, and splenocytes were harvested for fusion with SP2/0 murine myeloma cells. The fused cells were cultured in HAT selection medium in 96-well plates. Cell culture supernatants were collected seven days post-fusion and screened for anti-VP27 antibodies by indirect ELISA using GoAstV as coating antigen. Hybridoma clones secreting VP27-specific antibodies were subsequently subjected to three rounds of limiting dilution to establish monoclonal lines. Monoclonal antibodies were produced as ascites by inoculating ten-week-old female BALB/c mice with the selected hybridoma cells.
2.4. Characterization of Anti-VP27 mAbs
The reactivity of monoclonal antibodies against the VP27 protein was assessed by Western blot analysis. Purified VP27 protein was separated by electrophoresis on a 12.5% SDS-polyacrylamide gel and subsequently transferred onto a nitrocellulose membrane. The membrane was then blocked by incubating with 5% skimmed milk for 2 h at room temperature. Following blocking, the membrane was incubated with anti-VP27 monoclonal antibodies (hybridoma supernatant) for 2 h at room temperature. After three washes with PBST, the membrane was incubated with HRP-conjugated goat anti-mouse IgG (CWBIO, Beijing, China) for 1 h at room temperature. Following a final washing step, protein bands were visualized using Amersha ImageQuant 800 (Cytiva, Shanghai, China).
An IFA was performed on GoAstV-infected LMH cells. LMH cells were seeded in 96-well plates and infected with GoAstV at a multiplicity of infection (MOI) of 0.1. At 48 hours post-infection, the cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature and stored at 4 °C until use. Fixed cells were incubated with anti-VP27 monoclonal antibodies, followed by incubation with AF488-labeled goat anti-mouse IgG (H+L) (Beyotime, Shanghai, China). Immunofluorescence images were acquired using a Nikon ECLIPSE Ts2R fluorescence microscope (Nikon, Tokyo, Japan).
2.5. Neutralization Test
Viral titer was measured with IFA, and calculated and expressed as TCID50. For the neutralization test, serum samples were inactivated at 56 °C for 30 min, after which 100 μl of each serum was mixed with an equal volume of GoAstV suspension containing 10⁵ TCID₅₀. Following incubation at 37 °C for 1 h, the residual viral titer of each mixture was determined. Samples demonstrating a reduction in virus titer to below 10⁴ TCID₅₀ were considered positive for neutralizing antibodies.
2.6. Development of mAb-Based ic-ELISA
GoAstV viral suspensions were diluted and coated onto 96-well plates (100 µL/well, 105 TCID₅₀, 104 TCID₅₀, 103 TCID₅₀) and incubated overnight at 4 °C. Following coating, the plates were washed three times with PBST and blocked with 5% non-fat milk (Beyotime, Shanghai, China) in PBS at 37 °C for 60, 90 and 120 min. After washing, 100 µL of GoAstV positive (P) and negative (N) serum (diluted 1:10 in dilution buffer) were added to the plates and incubated at 37 °C for 45, 60 and 75 min. After washing, 100 µL of anti-VP27 mAb (diluted from 1:1000 to 1:16000 in PBST) were added to the plates and incubated at 37 °C for 45, 60 and 75 min. Subsequently, 100 µL of HRP-conjugated goat anti-mouse IgG (CWBIO, Beijing, China) was added and incubated for 50 minutes at 37 °C. After three additional washes with PBST, the plates were developed with TMB substrate for 15 minutes, and the reaction was terminated using TMB stop solution (Beyotime, Shanghai, China). Absorbance was measured at 450 nm. Optimum reaction parameters and composition were optimized based on the maximum the P/N ratio.
2.7. Determination of the ic-ELISA Cut-Off Percent Inhibition Value
Thirty GoAstV-negative serum samples were analyzed to establish the cut-off percent inhibition (PI) value. The PI for each sample was calculated using the following formula: PI (%) = [1 - (OD₍₄₅₀₎ of sample - minimum OD₍₄₅₀₎) / (maximum OD₍₄₅₀₎ - minimum OD₍₄₅₀₎)] × 100% [
19]. The cut-off PI value was defined as the mean PI of these negative samples plus twice the standard deviation (mean + 2 × SD) [
20].
2.8. Sensitivity, Specificity and Reproducibility Assessments of ic-ELISA
The sensitivity of the developed ELISA was evaluated using known positive serum samples subjected to serial dilutions ranging from 1:10, 1:100, 1:1000, and 1:10,000. Specificity was assessed by testing the method against antisera positive for goose Parvovirus (GPV), Tembusu virus (TMUV), avian Reovirus (ARV), avian influenza virus (AIV, H5), and duck Avastrovirus (DAstV), which were generated using vaccine strain or natural infection. To determine reproducibility, five serum samples (comprising three positive and two negative) were analyzed in triplicate, and the intra-assay coefficient of variation (CV) was calculated.
2.9. Application of the ic-ELISA
Using the newly established serological method, we tested goose serum samples collected from major goose-farming regions in Shandong province between October 2024 to May 2025 to analyze epidemiology of this virus.
3. Results
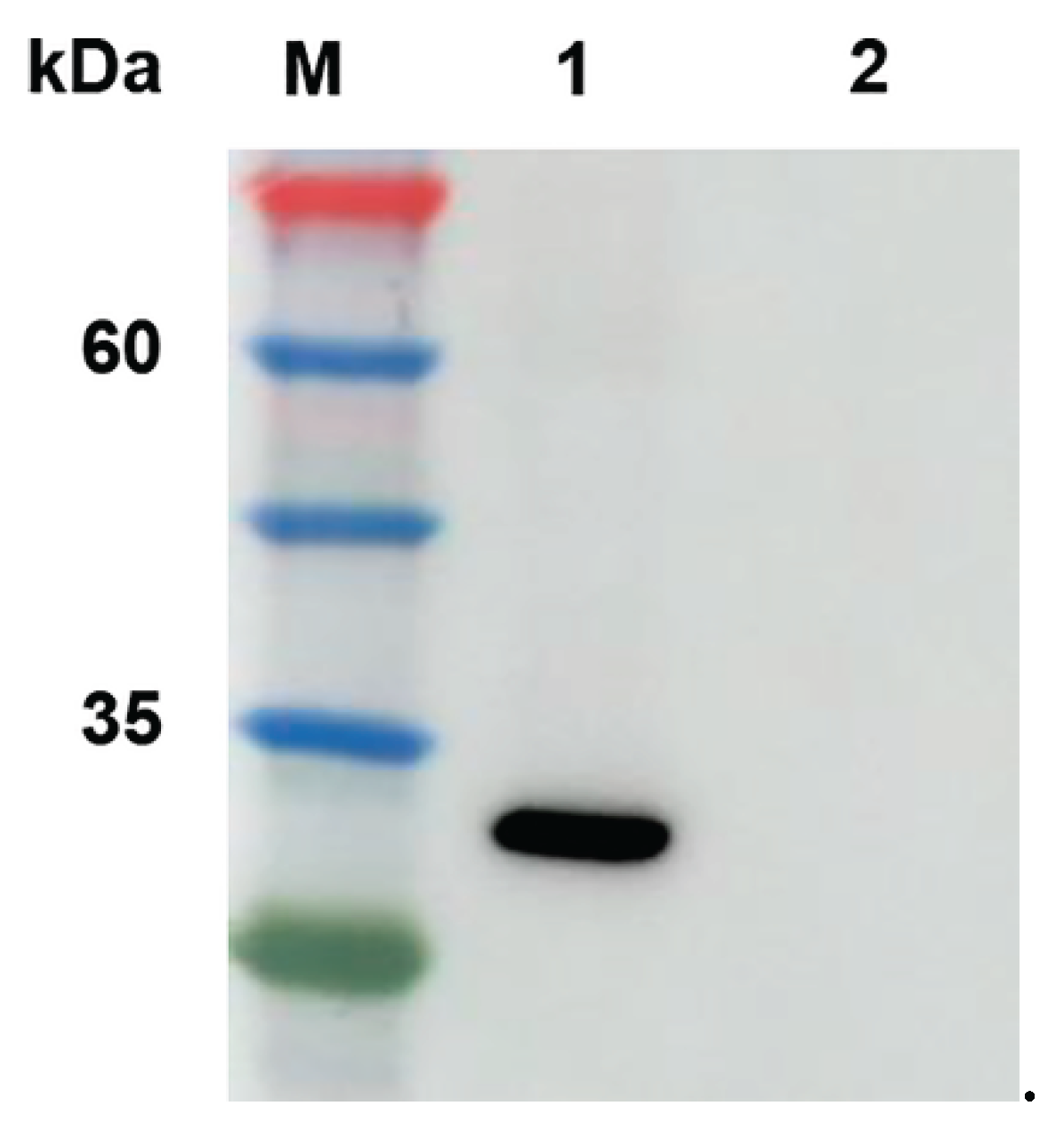
3.1. Preparation of VP27 Protein
The VP27 protein was expressed using recombinant plasmid of pET-28a-VP27 in
E. coli BL21(DE3) cells and purified using His-Tagged Protein Purification Kit. The proteins were identified with SDS-PAGE and western blot with an approximate 27 kDa molecular mass (
Figure 1).
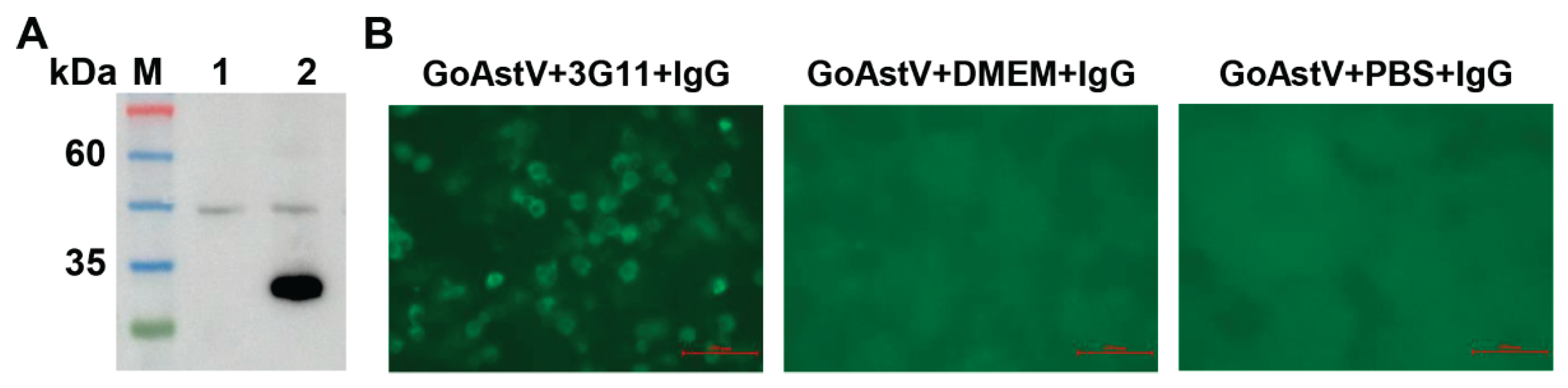
3.2. Identification of mAbs Specific for VP27
The purified VP27 protein served as an immunogen to generate mAbs in mice. Splenocytes were subsequently isolated and fused with SP2/0 murine myeloma cells. Following hybridoma formation and culture, the resulting clones were systematically screened for antigen-specific antibody production. After three rounds of screening, hybridoma clone 3G11 demonstrated superior immunoreactivity. When culture supernatants from 3G11 cells were employed as primary antibodies in western blot analysis, they specifically recognized the VP27 protein (
Figure 2A). Furthermore, these antibodies effectively detected GoAstV antigens in infected LMH cells by IFA (
Figure 2B). For large-scale antibody production, mAbs were subsequently prepared as ascites fluid by inoculating 3G11 cells into female BALB/c mice.
3.3. Optimization of the ic-ELISA
The optimal reaction conditions for the ic-ELISA were established using a checkerboard titration method. Based on the positive-to-negative (P/N) ratio, the optimal antigen coating concentration was determined to be 10⁴ TCID₅₀ per well, while the optimal dilution of the 3G11 mAbs was 1:8000 (
Table 1). The incubation times for blocking, serum samples, and monoclonal antibodies were standardized at 120 min, 60 min, and 60 min, respectively (
Table 2). The working dilution of HRP-conjugated goat anti-mouse IgG and the development times for TMB substrate and stop solution were implemented according to the manufacturer's recommended protocols.
3.4. Determination of Cut-Off Value
The cut-off value for the ic-ELISA was established using thirty negative serum samples. The mean PI value and SD of these samples were determined to be 29.30% and 7.61%, respectively. Accordingly, the cut-off value was calculated as 44.52% (
Table 3). Serum samples exhibiting PI values below this threshold were classified as negative, while those with values equal to or exceeding 44.52% were considered positive.
3.5. Sensitivity, Specificity, Reproducibility and Accuracy Assessments of ic-ELISA
The analytical sensitivity of the ic-ELISA was evaluated using five known positive serum samples subjected to serial dilution. All samples tested positive at dilutions of 1:10 and 1:100, while two remained positive at a 1:1000 dilution. All samples yielded negative results at the 1:10,000 dilution (
Table 4), demonstrating acceptable analytical sensitivity for this assay.
To assess specificity, the assay was challenged with positive antisera against GPV, TMUV, ARV, AIV H5 and DAstV. All antisera against these common avian viruses tested negative (
Table 5), indicating that the method exhibits high specificity for GoAstV antibody detection.
The reproducibility of the assay was determined by intra- and inter-assay testing of three positive and two negative serum samples, with results evaluated based on the coefficient of variation (CV). The intra-assay CV ranged from 3.53% to 9.82%, and the inter-assay CV varied from 3.19% to 8.42% (
Table 6). All CV values were below the 10% threshold established in previous studies [
18] (Gao et al., 2022), confirming satisfactory methodological reproducibility.
3.6. Epidemiological Investigation of GoAstV
A total of 196 serum samples were collected from major goose-farming regions in Shandong Province. The overall seropositivity rate for GoAstV was 11.7%, with the highest incidence (15.6%) observed in Liaocheng (
Table 7). These findings indicate an established circulation of GoAstV within the provincial poultry industry.
4. Discussion
VP27 represents a major antigenic capsid protein in many astroviruses, forming the outer spike structures of mature infectious virions. It primarily mediates host cell interaction and viral entry, while also eliciting potent immune responses [
15,
21,
22]. In contrast to the capsid precursor VP90, which is released from cells as part of immature viral particles, VP27 contains a higher density of immunodominant epitopes [
21,
23]. In this study, the recombinant VP27 protein was produced using an E. coli expression system and subsequently employed to generate mAbs in BALB/c mice. Western blot and IFA demonstrated that the resulting mAbs 3G11 exhibited specific immunoreactivity against both VP27 and native GoAstV particles (
Figure 2), indicating their utility for developing serological detection methods.
The ELISA represents one of the most widely utilized serological techniques in veterinary diagnostics due to its operational simplicity, high throughput, and favorable sensitivity and specificity [
16,
24,
25]. In the present study, a monoclonal antibody against VP27 (clone 3G11) was employed to develop an ic-ELISA. Optimal assay conditions were established via checkerboard titration, identifying an antigen coating concentration of 10⁴ TCID₅₀ per well and mAbs working dilution of 1:8000 (
Table 1). A cut-off value of 44.52% was determined based on PI analysis of 30 negative serum samples to differentiate positive and negative results. The assay demonstrated satisfactory analytical sensitivity, specificity, and reproducibility upon comprehensive evaluation. Taken together, an ic-ELISA was successfully established for the detection of GoAstV-specific antibodies in geese, providing a reliable tool for serological surveillance.
The use of intact viral particles during mAb screening increases the probability of obtaining neutralizing antibodies [
26,
27]. In this study, GoAstV particles served as the coating antigen throughout three rounds of subcloning, and the resulting mAbs 3G11 demonstrated strong immunoreactivity with viral particles (
Figure 2B). This finding suggested that 3G11 might recognize surface epitopes on the virion surface, a premise that warranted further validation. Similarly, since GoAstV particles were also employed as the coating antigen in the ic-ELISA, this method primarily detected neutralizing antibodies in serum, which were critical for evaluating vaccine-induced immune protection. Given that vaccine development represents the most effective strategy for controlling GoAstV infection, the established ic-ELISA provides a valuable tool for assessing vaccine efficacy in future applications.
5. Conclusions
In conclusion, a murine mAb (designated 3G11) was generated against the GoAstV VP27 protein. Western blot and IFA confirmed its specific immunoreactivity with both recombinant VP27 and native GoAstV particles. Subsequently, an ic-ELISA was developed using mAb 3G11, whose analytical performance in terms of sensitivity, specificity, and reproducibility was validated through comprehensive assessment. Collectively, this study establishes a reliable serological tool for detecting GoAstV-specific antibodies and provides a foundation for evaluating vaccine efficacy in future applications.
Author Contributions
Conceptualization, JL, YL, and YT; Formal analysis, ZL and WS; Methodology, JL, ZW and ZL; Data curation, YL, WS and CL; Writing-original draft preparation, JL and CL; Writing- review and editing, YL and YT; Funding acquisition, YT; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agricultural Science and Technology Innovation Project of Shandong Academy of Agricultural Sciences, grant number CXGC2025H10, Shandong Provincial Key Research and Development Project, grant number 2024LZGC021 and Shandong Agricultural Industrial Technology System, grant number SDAIT-11-01.
Institutional Review Board Statement
All animal husbandry and experimental procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Poultry Institute, Shandong Academy of Agricultural Sciences (SAAS-2025-S021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GoAstV |
Goose astrovirus |
| ic-ELISA |
indirect competitive ELISA |
| mAb |
monoclonal antibody |
| IFA |
immunofluorescence assay |
| VP |
viral protein |
| TCID₅₀ |
50% tissue culture infective dose |
| UTR |
untranslated region |
| ORF |
open reading frame |
| P |
positive |
| N |
negative |
| PI |
percent inhibition |
| GPV |
goose Parvovirus |
| TMUV |
Tembusu virus |
| ARV |
avian Reovirus |
| AIV |
avian influenza virus |
| DAstV |
duck Avastrovirus |
| CV |
coefficient of variation |
References
- Zhang, Y.; Wang, F.; Liu, N.; Yang, L.; Zhang, D. Complete genome sequence of a novel avastrovirus in goose. Arch. Virol. 2017, 162, 2135–2139. [CrossRef]
- Li, H.; Su, Q.; Fu, D.; Huang, H.; Lu, Z.; Huang, C.; Chen, Y.; Tan, M.; Huang, J.; Kang, Z.; Wei, Q.; Guo, X. Alteration of gut microbiome in goslings infected with goose astrovirus. Poult. Sci. 2024, 103, 103869. [CrossRef]
- An, D.; Zhang, J.; Yang, J.; Tang, Y.; Diao, Y. Novel goose-origin astrovirus infection in geese: the effect of age at infection. Poult. Sci. 2020, 99, 4323–4333. [CrossRef]
- Shen, Q.; Zhuang, Z.; Lu, J.; Qian, L.; Li, G.; Kanton, A.G.; Yang, S.; Wang, X.; Wang, H.; Yin, J.; Zhang, W. Genome analysis of goose-origin astroviruses causing fatal gout in Shanghai, China reveals one of them belonging to a novel type is a recombinant strain. Front. Vet. Sci. 2022, 9, 878441. [CrossRef]
- Lu, Z.; Li, H.; Gao, X.; Fu, D.; Huang, H.; Huang, C.; Wu, M.; Guo, X. Goose astrovirus induces apoptosis and endoplasmic reticulum stress in gosling hepatocytes. Poult. Sci. 2025, 104, 104600. [CrossRef]
- Wang, A.; Xie, J.; Wu, Z.; Liu, L.; Wu, S.; Feng, Q.; Dong, H.; Zhu, S. Pathogenicity of a goose astrovirus 2 strain causing fatal gout in goslings. Microb. Pathog. 2023, 184, 106341. [CrossRef]
- Wu, W.; Xu, R.; Lv, Y.; Bao, E. Goose astrovirus infection affects uric acid production and excretion in goslings. Poult. Sci. 2020, 99, 1967–1974. [CrossRef]
- Wang, Z.; Chen, H.; Gao, S.; Song, M.; Shi, Z.; Peng, Z.; Jin, Q.; Zhao, L.; Qiao, H.; Bian, C.; Yang, X.; Zhang, X.; Zhao, J. Core antigenic advantage domain-based ELISA to detect antibody against novel goose astrovirus in breeding geese. Appl. Microbiol. Biotechnol. 2022, 106, 2053–2062. [CrossRef]
- Zhang, X.; Ren, D.; Li, T.; Zhou, H.; Liu, X.; Wang, X.; Lu, H.; Gao, W.; Wang, Y.; Zou, X.; Sun, H.; Ye, J. An emerging novel goose astrovirus associated with gosling gout disease, China. Emerg. Microbes. Infect. 2018, 7, 152. [CrossRef]
- Niu, X.; Tian, J.; Yang, J.; Jiang, X.; Wang, H.; Chen, H.; Yi, T.; Diao, Y. Novel goose astrovirus associated gout in gosling, China. Vet. Microbiol. 2018, 220, 53–56. [CrossRef]
- Lan, J.; Zhang, R.; Li, P.; Chen, J.; Xie, Z.; Jiang, S. Identification of a type-specific epitope in the ORF2 protein of Duck Astrovirus type 1. Animals (Basel) 2019, 9, 1069. [CrossRef]
- Pantin-Jackwood, M.J.; Strother, K.O.; Mundt, E.; Zsak, L.; Day, J.M.; Spackman, E. Molecular characterization of avian astroviruses. Arch. Virol. 2011, 156, 235–244. [CrossRef]
- Wohlgemuth, N.; Honce, R.; Schultz-Cherry, S. Astrovirus evolution and emergence. Infect. Genet. Evol. 2019, 69, 30–37. [CrossRef]
- Cortez, V.; Meliopoulos, V.A.; Karlsson, E.A.; Hargest, V.; Johnson, C.; Schultz-Cherry, S. Astrovirus biology and pathogenesis. Annu. Rev. Virol. 2017, 4, 327–48. [CrossRef]
- Aguilar-Hernández, N.; López, S.; Arias, C.F. Minimal capsid composition of infectious human astrovirus. Virology 2018, 521, 58–61. [CrossRef]
- He, D.; Sun, M.; Jiang, X.; Zhang, S.; Wei, F.; Wu, B.; Diao, Y.; Tang, Y. Development of an indirect competitive ELISA method based on ORF2 detecting the antibodies of novel goose astrovirus. J. Virol. Methods 2023, 311, 114643. [CrossRef]
- Ren, D.; Zhang, X.; Zhang, W.; Lian, M.; Meng, X.; Li, T.; Xie, Q.; Shao, H.; Wan, Z.; Qin, A.; Gao, W.; Ye, J. A peptide-based ELISA for detection of antibodies against novel goose astrovirus type 1. J. Virol. Methods 2023, 312, 114646. [CrossRef]
- Gao, Y.; Xia, T.; Bai, J.; Zhang, L.; Zheng, H.; Jiang, P. Preparation of monoclonal antibodies against the viral p54 protein and a blocking ELISA for detection of the antibody against African Swine Fever virus. Viruses 2022, 14, 2335. [CrossRef]
- Zhang, Y.; Wang, R.; Bai, M.; Wang, X.; Dong, H.; Li, J.; Mu, S.; Miao, H.; Song, J.; Sun, S.; Guo, H. Development of a competitive ELISA method based on VLPs detecting the antibodies of serotype A FMDV. J. Virol. Methods 2022, 300, 114406. [CrossRef]
- Lee, H.; Kim, E.J.; Cho, I.S.; Song, J.Y.; Choi, J.S.; Lee, J.Y.; Shin, Y.K. A serological study of severe fever with thrombocytopenia syndrome using a virus neutralization test and competitive enzyme-linked immunosorbent assay. J. Vet. Sci. 2017, 18:33–38. [CrossRef]
- York, R.L.; Yousefi, P.A.; Bogdanoff, W.; Haile, S.; Tripathi, S.; DuBois, R.M. Structural, mechanistic, and antigenic characterization of the human astrovirus capsid. J. Virol. 2015, 90, 2254–2263. [CrossRef]
- Zhang, W.; Pan, L.; Huang, Y.; Dong, Q.; Liu, T.; Du, Y.; Lu, L.; Yang, D.; Liu, J.; Ouyang, K.; Chen, Y.; Wei, Z.; Liu, H.; Huang, W. Proteolytic processing of the capsid precursor by trypsin is essential for porcine astrovirus infectivity and isolation in vitro. Vet. Microbiol. 2025, 307, 110598. [CrossRef]
- De Benedictis, P.; Schultz-Cherry, S.; Burnham, A.; Cattoli, G. Astrovirus infections in humans and animals - molecular biology, genetic diversity, and interspecies transmissions. Infect. Genet. Evol. 2011, 11, 1529–1544. [CrossRef]
- Wang, W.; Xiao, S.; Zhang, M.; Liu, J.; Tian, J.; Chang, C.; Li, Y.; Zhang, Y.; Zhang, F.; Li, G.; Yuan, X.; Wang, W. A pan-genotypic indirect competitive ELISA for serological detection of pigeon circovirus antibodies. Front. Microbiol. 2025, 16, 1612715. [CrossRef]
- Niu, X.; Liu, Q.; Wang, P.; Zhang, G.; Jiang, L.; Zhang, S.; Zeng, J.; Yu, Y.; Wang, Y.; Li, Y. Establishment of an indirect ELISA method for the detection of the Bovine Rotavirus VP6 protein. Animals (Basel) 2024, 14, 271. [CrossRef]
- Qu, S.; Wang, X.; Yang, L.; Lv, J.; Meng, R.; Dai, W.; Li, Q.; Liu, H.; Zhang, B.; Zhang, D. Identification of a neutralizing monoclonal antibody that recognizes a unique epitope on domain III of the envelope protein of Tembusu virus. Viruses 2020, 12, 647. [CrossRef]
- Yang, B.; Meng, R.; Feng, C.; Huang, J.; Li, Q.; Wang, X.; Zhang, D. An antibody neutralization determinant on domain III and the first α-helical domain in the stem-anchor region of Tembusu virus envelope protein. J. Immunol. 2022, 209, 684–695. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).