Submitted:
11 February 2025
Posted:
11 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Newcastle Disease Virus (NDV) Antigen
2.3. Red Blood Cell (RBC) Suspension
2.4. Serum Samples
2.5. Test Controls
2.6. Standardization of Experimental Conditions from HI
2.7. Experimental Strategy for the Validation of the HI Assay
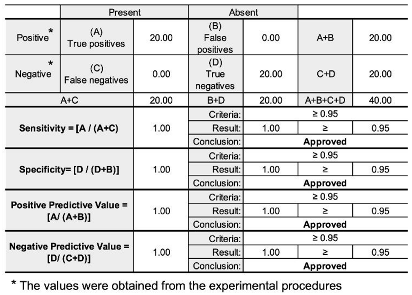
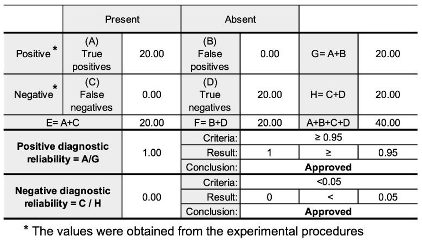
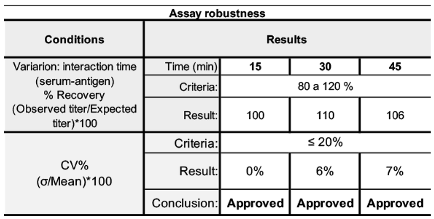
2.8. Parameters and Validation Criteria
2.9. Analysts
2.10. Statistical Treatment
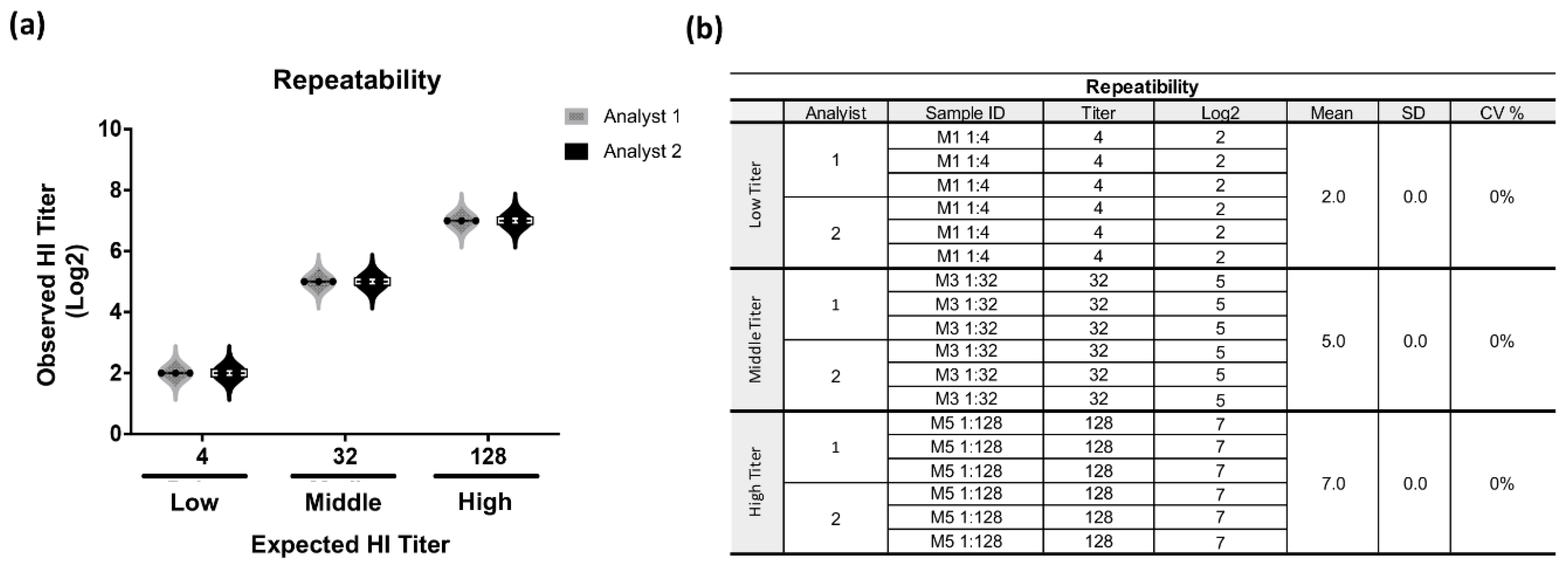
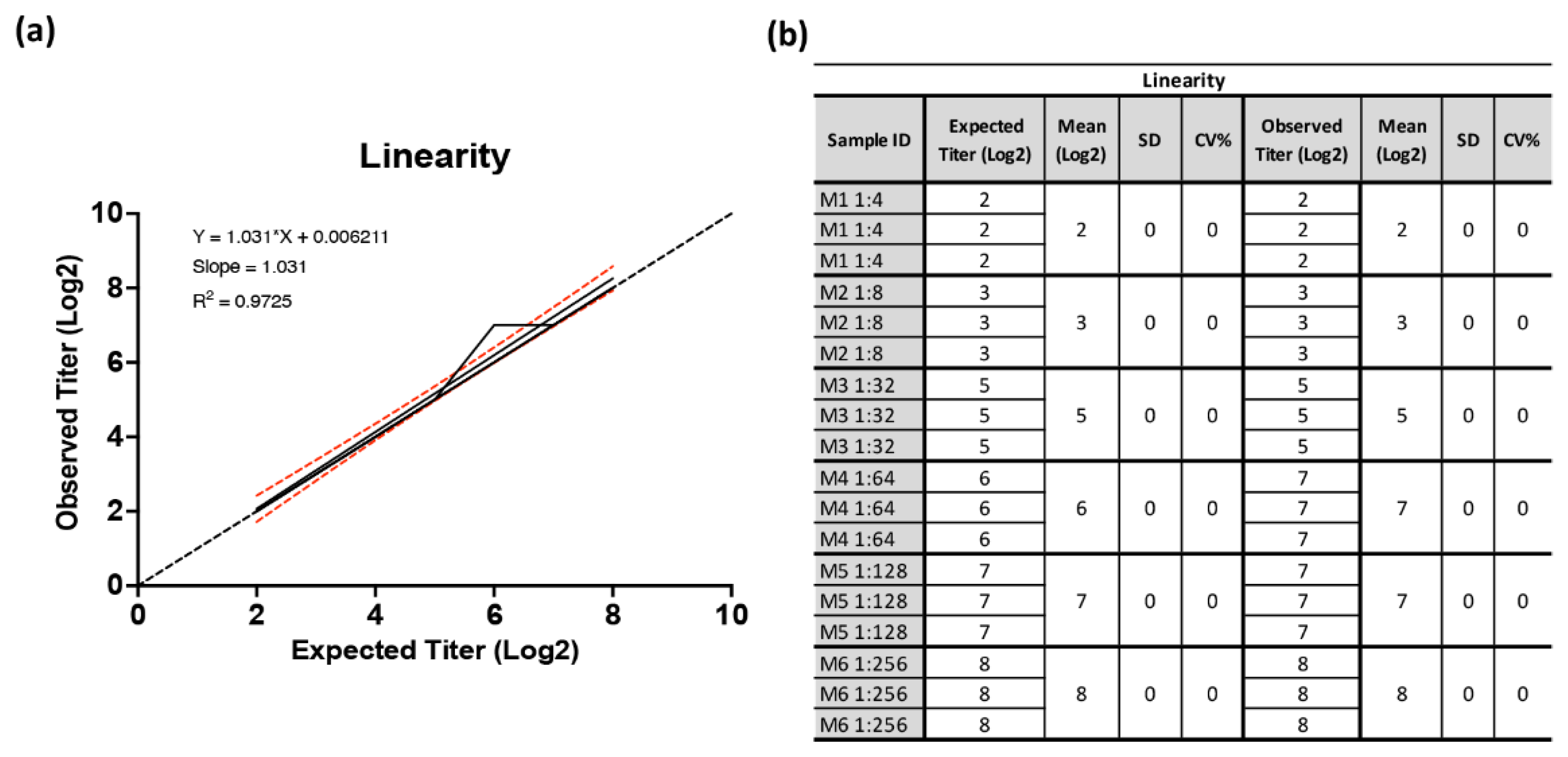
3. Results
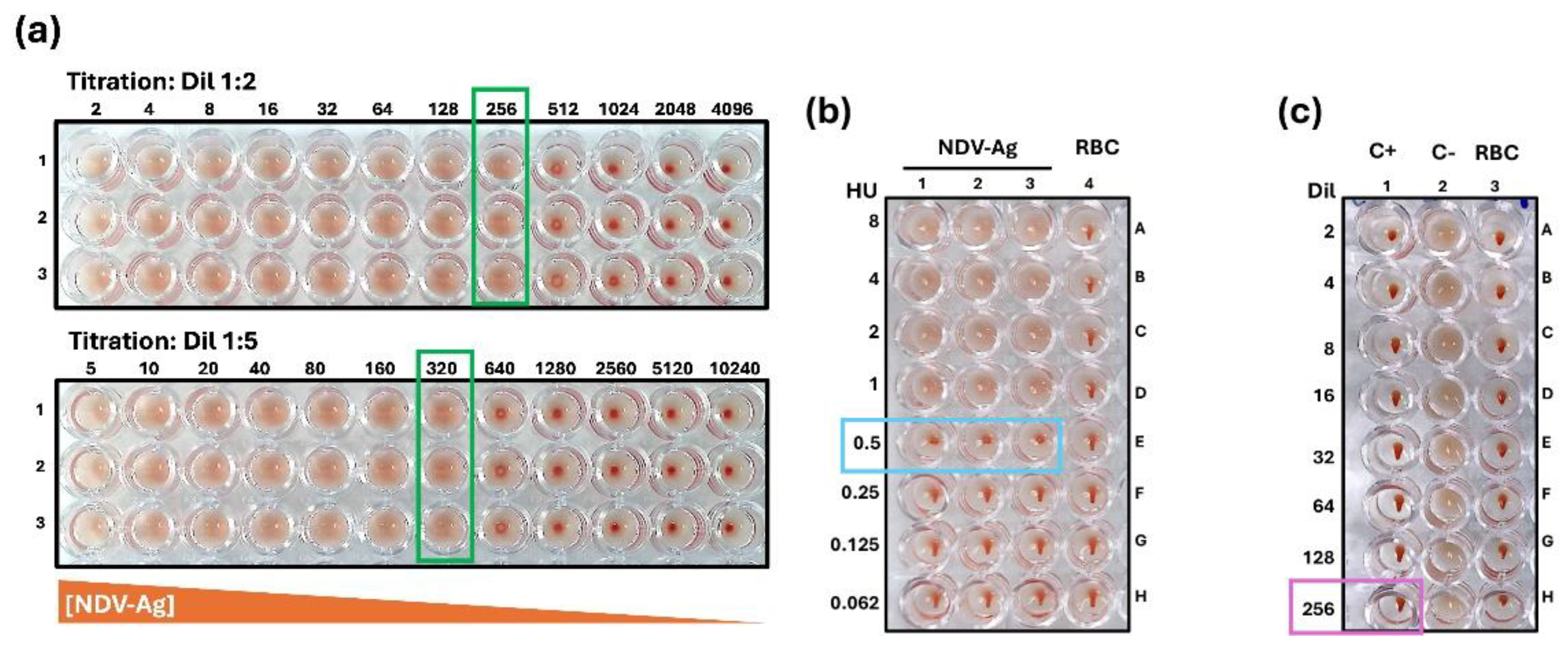
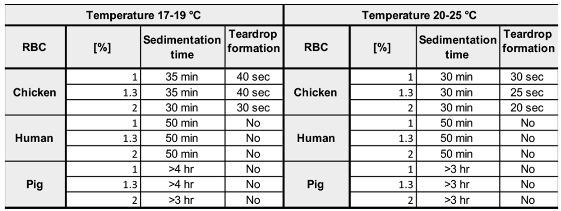
3.1. Standardization of Test Conditions
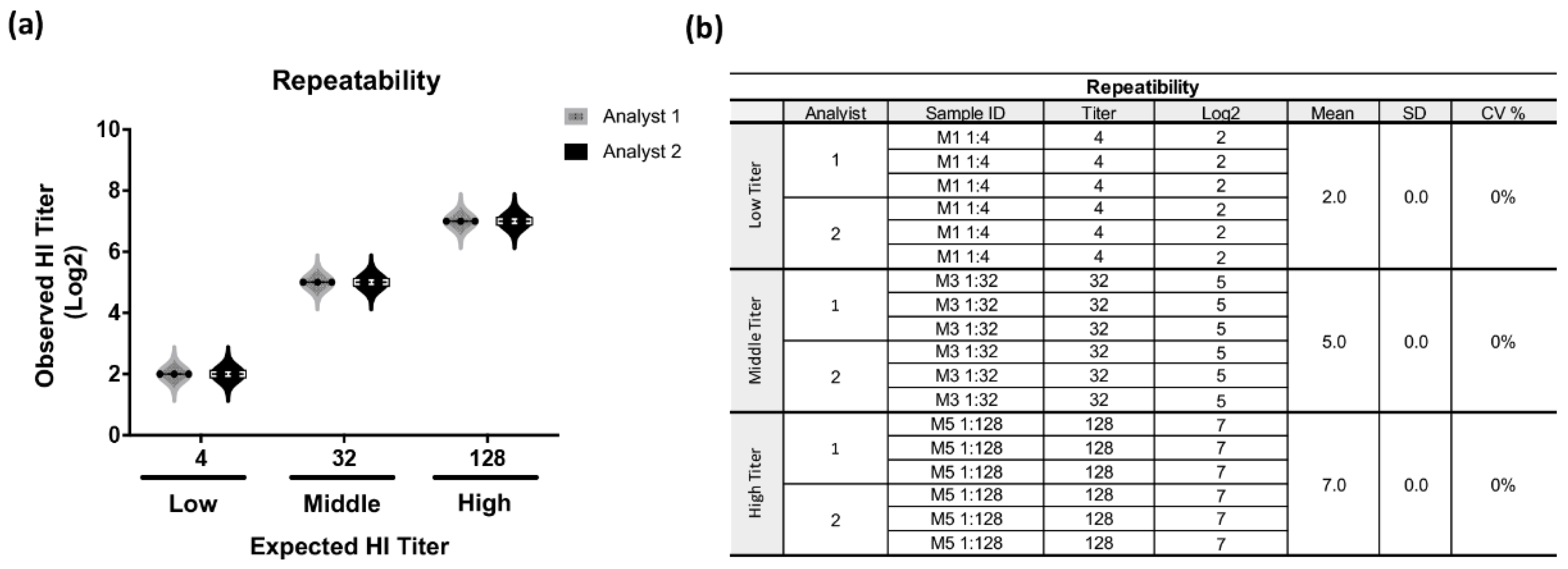
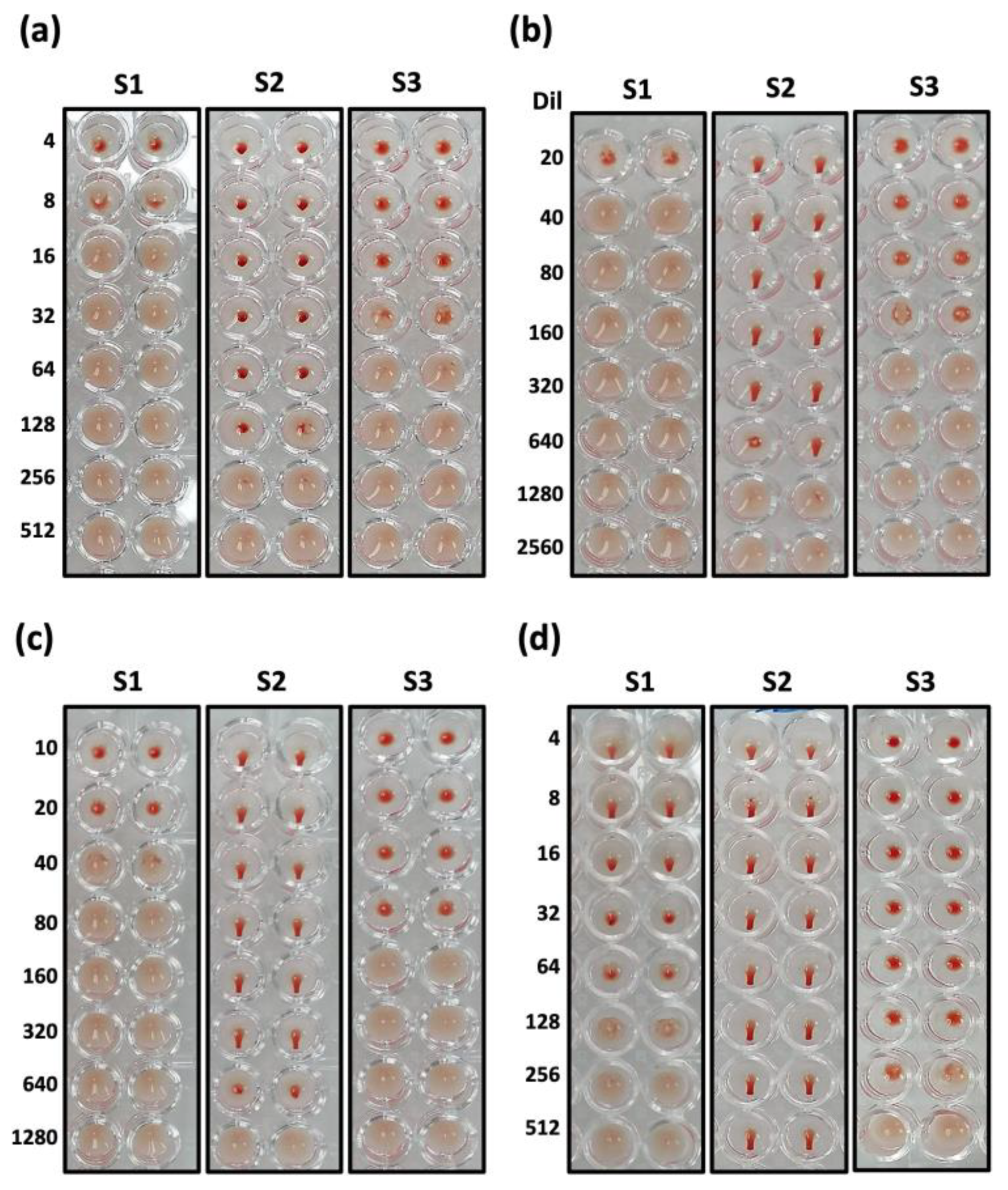
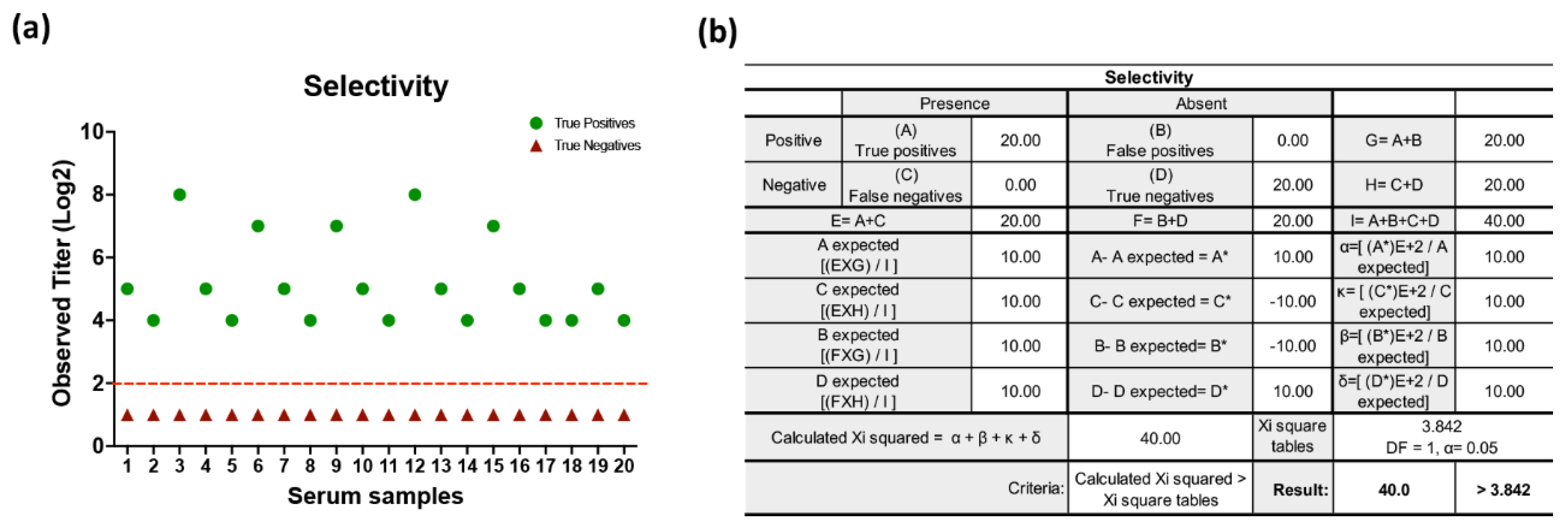
3.2. Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses (ICTV). Paramyxoviridae: Orthoavulavirus. [Online]. https://ictv.global/report/chapter/paramyxoviridae/paramyxoviridae/orthoavulavirus (accessed Jan 29, 2025).
- Rasekhi Kazeruni, A.; Babaei, N.; Esmaeili Gouvarchin Ghaleh, H.; Doosti, A.; Farzanehpour, M. Newcastle Disease Virus Enhances the Antitumor Efficacy of Doxorubicin in a Cervical Cancer Mouse Model. BMC Cancer 2024, 24 (1), 1253. [CrossRef]
- Suarez, D. L.; Miller, P. J.; Koch, G.; Mundt, E.; Rautenschlein, S. Newcastle Disease, Other Avian Paramyxoviruses, and Avian Metapneumovirus Infections. In Diseases of Poultry, 14th ed.; Swayne, D. E., Boulianne, M., Logue, C. M., McDougald, L. R., Nair, V., Suarez, D. L., Wit, S., Grimes, T., Johnson, D., Kromm, M., Prajitno, T. Y., Rubinoff, I., Zavala, G., Eds.; John Wiley & Sons: Hoboken, NJ, 2020; Chapter 3. [CrossRef]
- Yusoff, K.; Tan, W. S. Newcastle disease virus: macromolecules and opportunities. Avian Pathol. 2001, 30 (5), 439-455. ). [CrossRef]
- Morokutti, A., Redlberger-Fritz, M., Nakowitsch, S., Krenn, B. M., Wressnigg, N., Jungbauer, A., Romanova, J., Muster, T., Popow-Kraupp, T., & Ferko, B. Validation of the modified hemagglutination inhibition assay (mHAI), a robust and sensitive serological test for analysis of influenza virus-specific immune response. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology, 2013. 56(4), 323–330. [CrossRef]
- Makkoch, J., Prachayangprecha, S., Payungporn, S., Chieochansin, T., Songserm, T., Amonsin, A., & Poovorawan, Y. Erythrocyte binding preference of human pandemic influenza virus a and its effect on antibody response detection. Annals of laboratory medicine. 2012. 32(4), 276–282. [CrossRef]
- Kaufmann, L., Syedbasha, M., Vogt, D., Hollenstein, Y., Hartmann, J., Linnik, J. E., & Egli, A. An Optimized Hemagglutination Inhibition (HI) Assay to Quantify Influenza-specific Antibody Titers. Journal of visualized experiments : JoVE. 2017. (130), 55833. [CrossRef]
- Fulber JPC, Kamen AA. Development and Scalable Production of Newcastle Disease Virus-Vectored Vaccines for Human and Veterinary Use. Viruses. 2022. May 6;14(5):975. [CrossRef]
- Duan, Z., Xu, H., Ji, X., & Zhao, J. Recombinant Newcastle disease virus-vectored vaccines against human and animal infectious diseases. 2015. Future Microbiology, 10(8), 1307–1323. [CrossRef]
- Kim, S.-H.; Samal, S.K. Newcastle Disease Virus as a Vaccine Vector for Development of Human and Veterinary Vaccines. Viruses 2016, 8, 183. [CrossRef]
- Nelson, C. B., Pomeroy, B. S., Schrall, K., Park, W. E., & Linderman, R. J. An outbreak of conjunctivitis due to Newcastle disease virus (NDV) occurring in poultry workers. American journal of public health and the nation’s health. 1952. 42(6), 672–678. [CrossRef]
- López-Macías, C.; Torres, M.; Armenta-Copca, B.; Wacher, N. H.; Castro-Castrezana, L.; Colli-Domínguez, A. A.; Rivera-Hernández, T.; Torres-Flores, A.; Damián-Hernández, M.; Ramírez-Martínez, L.; la Rosa, G. P.; Rojas-Martínez, O.; Suárez-Martínez, A.; Peralta-Sánchez, G.; Carranza, C.; Juárez, E.; Zamudio-Meza, H.; Carreto-Binaghi, L. E.; Viettri, M.; Romero-Rodríguez, D.; ... Lozano-Dubernard, B. Phase II Study on the Safety and Immunogenicity of Single-Dose Intramuscular or Intranasal Administration of the AVX/COVID-12 "Patria" Recombinant Newcastle Disease Virus Vaccine as a Heterologous Booster Against COVID-19 in Mexico. Vaccine 2025, 43 (Pt 2), 126511. [CrossRef]
- Santra S, Seaman MS, Xu L, Barouch DH, Lord CI, Lifton MA, Gorgone DA, Beaudry KR, Svehla K, Welcher B, Chakrabarti BK, Huang Y, Yang ZY, Mascola JR, Nabel GJ, Letvin NL. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005. May;79(10):6516-22. [CrossRef]
- Bautista-Juárez J. Factores que intervienen en la reacción antígeno-anticuerpo y clasificación antigénica eritrocitaria. Revista Médica del IMSS. 2005. (43) 9-12. https://www.medigraphic.com/pdfs/imss/im-2005/ims051c.pdfBautista-Juárez J. Factores que intervienen en la reacción antígeno-anticuerpo y clasificación antigénica eritrocitaria. Revista Médica del IMSS. 2005. (43) 9-12. https://www.medigraphic.com/pdfs/imss/im-2005/ims051c.pdf.
- Food and Agriculture Organization of the United Nations. Manual of Procedures for the Implementation of the Mobile Desert Locust Information Surveillance System. [Online]; FAO: Rome, 2002; https://www.fao.org/4/AC802E/ac802e00.htm (accessed Apr 01, 2024).
- Boliar, S., Stanislawek, W., & Chambers, T. M. Inability of kaolin treatment to remove nonspecific inhibitors from equine serum for the hemagglutination inhibition test against equine H7N7 influenza virus. Journal of veterinary diagnostic investigation: official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. 2006. 18(3), 264–267. [CrossRef]
- Kim, H. R., Lee, K. K., Kwon, Y. K., Kang, M. S., Moon, O. K., & Park, C. K. Comparison of serum treatments to remove nonspecific inhibitors from chicken sera for the hemagglutination inhibition test with inactivated H5N1 and H9N2 avian Influenza A virus subtypes. Journal of veterinary diagnostic investigation : official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. 2012. 24(5), 954–958. [CrossRef]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza [Online]; WHO, 2011. https://www.who.int/publications/i/item/manual-for-the-laboratory-diagnosis-and-virological-surveillance-of-influenza (accessed Apr 01, 2024).
- Hidalgo-Lara, D. R., De la Luz-Armendáriz, J., Rivera-Benítez, J. F., Gomez-Nuñez, L., Salazar-Jiménez, E. N., Madrigal-Valencia, T. L., & Ramírez-Mendoza, H. Comparison of hemagglutination inhibition tests, immunoperoxidase monolayer assays, and serum neutralizing tests in detecting antibodies against blue eye disease in pigs. Journal of immunological methods. 2021. 496, 113088. [CrossRef]
- Rabenau, H. F., Kessler, H. H., Kortenbusch, M., Steinhorst, A., Raggam, R. B., & Berger, A. Verification and validation of diagnostic laboratory tests in clinical virology. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2007. 40(2), 93–98. [CrossRef]
- EURACHEM. Quantifying Uncertainty in Analytical Measurement, 3rd ed.; EURACHEM: London, 2012; https://www.eurachem.org/index.php/publications/guides/mv (accessed Apr 01, 2024).
- European Medicines Agency. ICH Q2(R2) Validation of Analytical Procedures - Scientific Guideline [Online]. https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline(accessed Apr 01, 2024).
- Sánchez, R. Validación de Métodos Analíticos no Cuantitativos. Rev. Mex. Cienc. Farm. 2010, 4 (12), 15-24.
- Trombetta, C. M., Remarque, E. J., Mortier, D., & Montomoli, E. Comparison of hemagglutination inhibition, single radial hemolysis, virus neutralization assays, and ELISA to detect antibody levels against seasonal influenza viruses. Influenza and other respiratory viruses, 2018. 12(6), 675–686. [CrossRef]
- Yang, H., Tian, J., Zhao, J., Zhao, Y., & Zhang, G. The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy. Viruses. 2024. 16(6), 886. [CrossRef]
- Cervantes-Torres, J., Cabello-Gutiérrez, C., Ayón-Núñez, D. A., Soldevila, G., Olguin-Alor, R., Diaz, G., Acero, G., Segura-Velázquez, R., Huerta, L., Gracia-Mora, I., Cobos, L., Pérez-Tapia, M., Almagro, J. C., Suárez-Güemes, F., Bobes, R. J., Fragoso, G., Sciutto, E., & Laclette, J. P. Caveats of chimpanzee ChAdOx1 adenovirus-vectored vaccines to boost anti-SARS-CoV-2 protective immunity in mice. Applied microbiology and biotechnology. 2024. 108(1), 179. [CrossRef]
- Byazrova, M. G., Astakhova, E. A., Minnegalieva, A. R., Sukhova, M. M., Mikhailov, A. A., Prilipov, A. G., Gorchakov, A. A., & Filatov, A. V. Anti-Ad26 humoral immunity does not compromise SARS-COV-2 neutralizing antibody responses following Gam-COVID-Vac booster vaccination. NPJ vaccines. 2022. 7(1), 145. [CrossRef]
- European Medicines Agency. ICH M4Q - Common technical document for the registration of pharmaceuticals for human use - Quality (scientific guideline). EMA [Online]. https://www.ema.europa.eu/en/ich-m4q-common-technical-document-registration-pharmaceuticals-human-use-quality-scientific-guideline. (accessed Apr 01, 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





