Submitted:
11 November 2025
Posted:
12 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Anti-Inflammatory
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
|---|---|---|---|---|---|
|
Annona cacans |
Leaves Pulp Seeds |
Hydromethanolic extract (HME) Ethyl acetate fraction (EAF) |
Myeloperoxidase (MPO) activity carrageenan-induced paw oedema | After 6 h, 28% (300 mg/kg HME-Leaves) 53% (100 mg/kg HME-Pulp) 58% (300 mg/kg HME-Pulp) 43% (30 mg/kg EAF), 51% (100 mg /kg EAF) |
(Volobuff et al. 2019) |
| Annona crassiflora | Fruit peel Leaves Leaves Leaves Leaves |
Polyphenol-enriched fraction (PEF) Methanolic extract Methanolic extract Methanolic extract Methanolic extract |
Wound closure in C57 mice Carrageenan-induced edema MPO activity Total leukocytes Carrageenan-induced leukocyte migration |
75% (2% PEF topical) 84%(6% PEF topical) 53% (100mg/kg) 47% (300mg/kg) 60% (100mg/kg) 78% (100mg/kg) 90% (300mg/kg) 43% (300mg/kg) |
(de Moura et al. 2019) (Rocha et al. 2016) (Rocha et al. 2016) (Rocha et al. 2016) (Rocha et al. 2016) (Rocha et al. 2016) |
| Annona glabra | Fruits | 7β,16α,17-trihydroxy-ent-kauran-19-oic acid | NO production in LPS-stimulated RAW264.7 cells | IC50= 0.39 ± 0.12 μM | (Nhiem et al. 2015) |
| Fruits | 16β,17-dihydroxy-ent-kauran-19-al | NO production in LPS-stimulated RAW264.7 cells | IC50 = 0.32 ± 0.04 μM | (Nhiem et al. 2015) | |
|
Annona muricata |
Fruits | Lyophilized extract | Xylene-induced ear edema | 34.04% (50μg/mL) 63.83(100μg/mL) 80.85(200μg/mL) |
(Ishola et al. 2014) |
| Annona muricata | Fruits | Lyophilized extract | Cyclooxygenase (COX)-1 activity | 39.44% (100μg/mL) | (Ishola et al. 2014) |
| Fruits | Lyophilized extract | Cyclooxygenase (COX)-2 activity | 55.71% (100μg/mL) | (Ishola et al. 2014) | |
|
Annona nutans |
Fruits | Lyophilized extract | Cyclooxygenase (COX)-2 activity | 55.71% (100μg/mL) | (Ishola et al. 2014) |
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
|
Annona Senegalensis |
Seeds | N-cerotoyltryptamine | ROS production in zymosan stimulated human whole blood phagocytes | IC50 = 2.7 ± 0.1 μg/mL | (Tamfu et al. 2021) |
| Seeds | Methanolic extract | ROS production in zymosan stimulated human whole blood phagocytes | IC50 =8.7 ± 10.2 μg/mL | (Tamfu et al. 2021) | |
| Seeds | Acetogenin | NO production in lipopolysaccharide (LPS) stimulated J774.2 mouse macrophages | IC50 = 3.9 ± 0.2 μg/mL | (Tamfu et al. 2021) | |
|
Annona squamosa |
Bark | Caryophyllene oxide | Carrageenan-induced paw edema | After 2 hours 45% (12.5mg/kg) 51% (25mg/kg) |
(Chavan et al. 2011) |
| Bark | Sesquiterpene fraction (copaene (35.40%), patchoulane (13.49%) and 1H-cycloprop(e)azulene (22.77%)) | Carrageenan-induced paw edema | After 2 hours 38% (12.5mg/kg) 34% (25mg/kg) |
(Chavan et al. 2012) | |
| Bark | 18-acetoxy-ent-kaur-16-ene | Carrageenan-induced paw edema | After 2 hours 51.6% (12.5mg/kg) 60.9% (25mg/kg) |
(Chavan et al. 2011) | |
| Annona vepretorum | Leaves | Ethanolic extract | leukocyte migration to the peritoneal cavity |
62%(25 mg/kg), 76% (50 mg/kg) 98% (100 mg/kg) |
(Silva et al. 2015) |
| Leaves | Ethanolic extract | Carrageenan-induced paw edema | After 2 hours 58%(25 mg/kg) 45% (50 mg/kg) 72% (100 mg/kg) |
(Silva et al. 2015) | |
| Annona vepretorum | Leaves | Ethanolic extract | Histamine-induced paw edema | After 1 hour >65% (100mg/kg) |
(Silva et al. 2015) |
| Cyathocalyx pruniferus | Leaves | Spathulenol Cyclopropa-azulene Polycarpol Koetjapic acid 2-Octaprenyl-benzoquinone 14-methylloctadec-1-ene 1-Docosene β-Sitosterol |
PGE2 | 71.4 (IC50 = 25.8) 8.6 (IC50 = -) 70.1 (IC50 = 24.7) 80.4 (IC50 = 13.1) 86.1 (IC50 = 11.2) 3.5 (IC50 = -) 5.8 (IC50 = -) 21.6 (IC50 = -) |
(Attiq et al. 2021) |
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
| Cyathocalyx pruniferus | Leaves | α-Tocopherol 11-prenol Quercetin Epicatechin Chrysin Indomethacin (Positive control) |
PGE2 | 15.9 (IC50 = -) 2.1 (IC50 = -) 79.8 (IC50 = 15.4) 77.3 (IC50 =17.3) 73.8 (IC50 = 21.8 88.1 (IC50 = 11.8) |
(Attiq et al. 2021) |
| Cyathocalyx pruniferus | Leaves | Spathulenol Cyclopropa-azulene Polycarpol Koetjapic acid 2-Octaprenyl-benzoquinone 14-methylloctadec-1-ene 1-Docosene β-Sitosterol α-Tocopherol 11-prenol |
COX-2 |
21.1 (IC50 = -) 4.6 (IC50 = -) 29.6 (IC50 = -) 85.6 (IC50 = 8.1) 88.1 (IC50 = 6.6) 2.1 (IC50 = -) 2.4 (IC50 = -) 11.1 (IC50 = -) 10.5 (IC50 = -) |
(Attiq et al. 2021) |
| Cyathocalyx pruniferus | Leaves | Quercetin Epicatechin Chrysin Dexamethasone (Positive control) |
COX-2 | 3.3 (IC50 = -) 80.1 (IC50 = 10.3) 74.7 (IC50 =12.5) 70.5 (IC50 =15.7) 92.8 (IC50 = 5.1) |
(Attiq et al. 2021) |
|
Duguetia furfuracea |
stem bark | Essential oil | LPS-induced paw edema | After two hours 41.67% (3mg/kg) 86.11% (10mg/kg) |
(Saldanha et al. 2019) |
| stem bark | Essential oil | LPS-induced paw edema | After four hours 45.45% (1mg/kg) 63.64% (3mg/kg) 92.42% (10mg/kg) |
(Saldanha et al. 2019) | |
| Leaves | Methanolic extract | Carrageenan-induced paw edema | After two hours 39% (300mg/kg) 22% (100mg/kg) 17.5% (30mg/kg) |
(do Santos et al. 2018) | |
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
| Duguetia furfuracea | Leaves | Methanolic extract | Carrageenan-induced paw edema | After four hours 40% (300mg/kg) 25% (100mg/kg) |
(do Santos et al. 2018) |
| Leaves | Dicentrinone | Carrageenan-induced paw edema | For 100 mg/kg 69.1% (2 hours) 50.4% (4 hours), |
(do Santos et al. 2018) | |
| Leaves | Methanolic extract | Zymosan-induced edema | 38.1% (300mg/kg) | (do Santos et al. 2018) | |
| Leaves | Dicentrinone | Zymosan-induced edema | 27.1% (300mg/kg) | (do Santos et al. 2018) | |
| Leaves | Enriched phenylpropanoid extract | LPS-induced paw edema | After two hours 90.91% (3mg/kg) 92.42% (10mg/kg) |
(Saldanha et al. 2021) | |
| Duguetia furfuracea | Leaves | Enriched phenylpropanoid extract | LPS-induced paw edema | After four hours 77.78% (1mg/kg) 77.78% (3mg/kg) 81.48% (10mg/kg) |
(Saldanha et al. 2021) |
| Leaves | α-asarone | LPS-induced paw edema | After two hours 62.12% (3mg/kg) 69.70% (10mg/kg) 69.70% (30mg/kg) |
(Saldanha et al. 2020) | |
| Leaves | α-asarone | LPS-induced paw edema | After four hours 72.22% (3mg/kg) 81.48% (10mg/kg) 81.48% (30mg/kg) |
(Saldanha et al. 2020) | |
|
Duguetia moricandiana |
Fruits | Discretamine | NO production in LPS-stimulated macrophages | Around 50%. (100 and 200 μg/mL) |
(Lemos et al. 2017) |
| Fruits | Discretamine | IL-6 production in LPS-stimulated macrophages | 74.1% (50μg/mL) 76.6% (100μg/mL) 75.1% (200 μg/mL) |
(Lemos et al. 2017) | |
| Fruits | Discretamine | IL1-b production in LPS-stimulated macrophages | 89.4% (50μg/mL) 87.4% (100μg/mL) 71.8% (200 μg/mL) |
(Lemos et al. 2017) | |
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
|
Duguetia moricandiana |
Fruits | Discretamine | TNF-α production in LPS-stimulated macrophages | 61.0% (50μg/mL) 45.2% (100μg/mL) 52.6% (200 μg/mL) |
(Lemos et al. 2017) |
| Fruits | Discretamine | Carrageenan-induced paw edema | After one hour 42% (10mg/kg) 62% (20mg/kg) |
(Lemos et al. 2017) | |
| Fruits | Discretamine | Carrageenan-induced paw edema | After two hours 44% (10mg/kg) 67% (20mg/kg) |
(Lemos et al. 2017) | |
|
Duguetia moricandiana |
Fruits | Discretamine | Carrageenan-induced paw edema | After four hours 59% (5mg/kg) 49% (10mg/kg) 48% (20mg/kg) |
(Lemos et al. 2017) |
|
Duguetia staudtii |
Stem bark | Pachypodol | Myeloperoxidase dependent (luminol/zymosan) oxidative burst | IC50 = 8.32 μg/mL | (Ngouonpe et al. 2019) |
| Stem bark | Kumatakenin | Myeloperoxidase dependent (luminol/zymosan) oxidative burst | IC50 = 10.64 μg/mL | (Ngouonpe et al. 2019) | |
| Stem bark | 5,4′-dihydroxy-3,7,3′,5′-tetramethoxyflavone | Myeloperoxidase dependent (luminol/zymosan) oxidative burst | IC50 = 6.44 μg/mL | (Ngouonpe et al. 2019) | |
| Stem bark | Pachypodol | Myeloperoxidase independent (lucigenin/PMA) oxidative burst | IC50 = 11.04 μg/mL | (Ngouonpe et al. 2019) | |
| Stem bark | Kumatakenin | Myeloperoxidase independent (lucigenin/PMA) oxidative burst | IC50 = 14.13 μg/mL | (Ngouonpe et al. 2019) | |
| Stem bark | 5,4′-dihydroxy-3,7,3′,5′-tetramethoxyflavone | Myeloperoxidase independent (lucigenin/PMA) oxidative burst | IC50 = 8.55 μg/mL | (Ngouonpe et al. 2019) | |
| Enicosanthum membranifolium |
2β-methoxyhardwickiic acid (-)-Hardwicckiic acid 2β-acetoxyhardwickiic acid 2β-hidroxyhardwickiic acid 15-methozypatagonic acid Indomethacin (Positive control) |
NO production | IC50 μM 65.4 38.9 16.1 82.4 28.9 32.2 |
(Polbuppha et al. 2022) | |
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
| Isolona dewevrei | Leaves | Essential oil Nordihydroguaiaretic (Positive control) |
Inhibit lipoxygenases (LOX) | 0.0020 (mg/mL) 0.013 (mg/ml) |
(Kambiré et al. 2021) |
| Melodorum fruticosum | Leaves | Melodamide A | Inhibition of superoxide anion generation | IC50 = 5.25 μM | (Chan et al. 2013) |
| Leaves | Melodamide A derivate (2-Cl) |
Inhibition of superoxide anion generation | IC50 = 7.49 μM | (Chan et al. 2013) | |
| Leaves | Melodamide A derivate (3-F) |
Inhibition of superoxide anion generation | IC50 = 5.59 μM | (Chan et al. 2013) | |
| Leaves | Melodamide A derivate (2-Br) |
Inhibition of superoxide anion generation | IC50 = 5.19 μM | (Chan et al. 2013) | |
|
Phaeanthus vietnamensis |
Leaves | spathulenol | NO production in LPS-stimulated BV2 cells | IC50 = 15.7 μM | (Nhiem et al. 2017) |
| Leaves | (8R,80R)-bishydrosyringenin | NO production in LPS-stimulated BV2 cells | IC50 = 25.3 μM | (Nhiem et al. 2017) | |
| Leaves | 1αH,5βH-aromandendrane-4α,10α-diol | NO production in LPS-stimulated BV2 cells | IC50 = 23.0 μM | (Nhiem et al. 2017) | |
| Leaves | 1βH,5βH-aromandendrane-4α,10β-diol | NO production in LPS-stimulated BV2 cells | IC50 = 22.6 μM | (Nhiem et al. 2017) | |
|
Polyalthia longifolia |
Seeds | 16-oxo-cleroda-3,13(14)E-dien-15-oic acid | COX-1, COX-2, and 5-LOX inhibitory activities | 62.85% (COX-2) 26.41% (LOX-5) |
(Nguyen et al. 2020) |
| Seeds | 16-hydroxy-cleroda-3,13-dien-15-oic acid | COX-1, COX-2, and 5-LOX inhibitory activities | 84.98% (COX-2) 30.51% (LOX-5) |
(Nguyen et al. 2020) | |
| Seeds | 16-hydroxy-cleroda-4(18),13-dien-16,15-olide | COX-1, COX-2, and 5-LOX inhibitory activities | 82.97% (COX-2) 12.73% (LOX-5) |
(Nguyen et al. 2020) | |
| Seeds | 3α,16α-dihydroxy-cleroda-4(18),13(14)Z-dien-15,16-olide | COX-1, COX-2, and 5-LOX inhibitory activities | 75.14% (COX-2) 14.38% (LOX-5) |
(Nguyen et al. 2020) | |
|
Polyalthia longifolia |
Seeds | 16α-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide | COX-1, COX-2, and 5-LOX inhibitory activities | 92.94% (COX-1) 79.41% (COX-2) 16,94% (LOX-5) |
(Nguyen et al. 2020) |
| Bark |
16-oxocleroda-4(18),13-dien-15,16-olide |
fMLP/CB induced superoxide generation by neutrophils |
IC50 = 0.60 mg/mL |
(Chang et al. 2006) | |
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
|
Diphenyleneiodonium (positive control) 16(R&S)-3,13-kolavadien-15,16-olide-2-one |
fMLP/CB induced superoxide generation by neutrophils Phorbol 12-myristate 13 acetate (PMA)-induced action |
IC50 = 0.11 mg/mL IC50 = 10μg/mL |
(Chang et al. 2006) | ||
| Stem bark | Ethyl acetate (EA) extract | Carrageenan-induced paw edema | After four hours 27.5%(50mg/kg) 39.1% (100mg/kg) |
(Kabir et al. 2019) | |
| Leaves | Diethyl ether extract | Xylene-induced ear edema | 42.70% (200mg/kg) 62.67% (400mg/kg) |
(Yasmen et al. 2018) | |
| Leaves | n-hexane extract | Xylene-induced ear edema | 48.54% (200mg/kg) 65.92% (400mg/kg) |
(Yasmen et al. 2018) | |
| Polyalthia viridis | Leaves Stem |
Leaf Essential oil Stem Essential oil Butein (Positive control) |
NO production in LPS stimulated BV2 cells | 80.8 (IC50 = 76.7 μg/mL) 87.2 (IC50 = 57.6 μg/mL) 91.8 (IC50 = 16.1 μg/mL) |
(Son et al. 2021) |
|
Uvaria flexuosa |
Leaves | Flexuvarol B | Superoxide anion generation assay | IC50 = 4.72 mM | (Hsu et al. 2016) |
| Leaves | Chrysin | Superoxide anion generation assay | IC50 = 2.25 mM | (Hsu et al. 2016) | |
| Leaves | Flexuvarol B | Elastase release assay | IC50 = 5.55 mM | (Hsu et al. 2016) | |
| Leaves | Chrysin | Elastase release assay | IC50 = 2.44 mM | (Hsu et al. 2016) | |
| Uvaria grandiflora | Stems | (-) zeylenol | EPP-induced rat ear edema | 90% (15min) 69% (30min) 52% (1 hour) 52% (2 hours) |
(Seangphakdee et al. 2013) |
|
Xylopia aethiopica |
Fruits | Methanolic extract | Prostaglandin synthetase activity | - | (Ezekwesili et al. 2010) |
| Fruits | Ethanolic extract | Mouse pinnal inflammation in carrageenan-induced paw oedema | 23% (30 μg/mL) 62% (300 μg/mL) |
(Obiri and Osafo 2013) | |
| Annonaceae Species | Used Material | Substances/Extracts | Methodology | Inhibition | Ref. |
| Xylopia aethiopica | Fruits | Extract 30 100 300 |
Superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), myeloperoxidase (MPO) and malondialdehyde (MDA) activity | 23.06 40.91 62.83 |
(Osafo et al. 2016) |
| Leaves | Hydroethanolic extract | TNF-α in (Lipopolysaccharide) LPS challenged THP-1-derived macrophages | >90% (500 mg/kg) | (Macedo et al. 2020) | |
| Leaves | Hydroethanolic extract | Inhibition of IL-6 production, in a LPS challenged THP-1-derived macrophages, | 84.6% (250 μg/mL) 96.3% (500 μg/mL) |
(Macedo et al. 2020) | |
| Leaves | Hydroethanolic extract | Interferences of 5-LOX in a LPS challenged THP-1-derived macrophages | IC50 = 85 μg/mL | (Macedo et al. 2020) | |
| Fruits | Essential oil | NO production in LPS-stimulated RAW264.7 cells | - | (Alolga et al. 2019) | |
|
Xylopia parviflora |
Fruits | Volatile oil | NO production in LPS-stimulated RAW264.7 cells | 37% (12μg/mL) | (Woguem et al. 2014) |
|
Xylopia sericea |
Leaves | Ethanolic extract | NO production in LPS-stimulated RAW264.7 cells | 76% | (Gomes et al. 2022) |
| Leaves | Ethanolic extract | IL-6 production in LPS-stimulated RAW264.7 cells | 85% | (Gomes et al. 2022) |
3.2. Insecticidal, Larvicidal and Pesticidal
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
|---|---|---|---|---|---|---|---|
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona atemoya | Seed | Seed extract | Trichoplusia ni (Lep.) | 197.67 | 301.30 | (de Cássia Seffrin et al. 2010) | |
| Annona squamosa | Seed | Seed extract | Trichoplusia ni (Lep.) | 382.37 | 167.48 | (de Cássia Seffrin et al. 2010) | |
|
Annona cherimola |
Seed |
Squamocin Molvizarin Almunequin Itrabin Deltamethrin (Positive control) |
Oncopeltus fasciatus |
0.16 0.34 11.23 14.91 7.4 |
(Colom et al. 2008) | ||
| Annona cherimola | Seed | Neoannonin Itrabin Almunequin Asimicin Squamocin Motrilin Cherimolin-1 Cherimolin-2 Tucumanin Control |
Spodoptera Frugiperda | 15.5 18.8 19.7 17.3 Instant death 18.0 14.0 17.7 14.7 12.1 |
10 30 30 30 100 20 0 10 20 10 |
0.90 0.59 1.10 0.77 0.16 1.19 0.91 0.97 0.81 1.02 |
(Alvarez Colom et al. 2007) |
| Annona coriaceae |
Seed |
Seed Extract |
Aedes aegypti (Dip.) |
0.01 |
- |
- |
(Costa et al. 2012) |
| Annona coriaceae | Seed | 100 ppm 50 ppm DMSO 0.1% Água (Control) |
Aedes aegypti (Dip.) | 0.50 0.40 0.30 0.20 |
3.00 6.00 - - |
3.5 5.5 - - |
(Dill et al. 2012) |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona coriaceae | Seed | Seed Extract 0 ppm 50 ppm 100 ppm 200 ppm 300 ppm 400 ppm 500 ppm |
Aedes aegypti (Dip.) |
0.0 0.7 0.7 1.0 2.2 4.5 6.2 |
0.0 7.5 7.5 10.0 22.5 45.0 62.5 |
10.0 9.25 9.25 9.00 7.75 5.50 3.75 |
(de Moraes et al. 2011) |
| Annona coriaceae | Seed | Seed Extract Methanol (des. Hexane) Hexane Dichloromethane Methanol (des. DCM) |
Aedes aegypti (Dip.) |
0.1 0.1 0.1 0.1 |
100.0 100.0 58.75 0.0 |
0.007 0.007 0.805 0.0 |
(Costa, Marilza da Silva, Mônica Josene Barbosa Pereira, Simone Santos de Oliveira, Paulo Teixeira de Souza, Evandro Luiz Dall’oglio 2013) |
| Annona coriaceae | Seed | Diet | Anagasta kuehniella (Lep.) | 0.0 2.0 |
26.3 16.8 |
71.8 81.9 |
(Coelho et al. 2007) |
| Annona coriaceae | Seed | Diet | Corcyra Cephalonica (Lep.) | 0.0 2.0 |
23.16 32.25 |
69.7 49.3 |
(Coelho et al. 2007) |
| Annona coriaceae | Seed | Seed Extract Hexanic 8.0% 4.0% 2.0% 1.0% 0.5% Methanolic 8.0% 4.0% |
Dichelops melacanthus (Hem.) |
78.00 86.00 68.00 58.00 42.00 96.00 |
(Souza, E. M.; Cordeiro, J. R.; Pereira 2007) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona coriaceae | Seed | Seed Extract Methanolic 2.0% 1.0% 0.5% Ethanolic 8.0% 4.0% 2.0% 1.0% 0.5% Distilled water (Positive Control) C 01 C 02 |
94.00 94.00 70.00 40.00 100.00 100.00 90.00 84.00 80.00 6.00 4.00 6.00 12.00 0.00 2.00 |
(Souza, E. M.; Cordeiro, J. R.; Pereira 2007) | |||
| Annona coriaceae | Seed | Seed Extract Preview 2 Days 5 Days 7 Days DMSO 20% (Positive Control 01) Water (Positive Control 02) |
Euschistus heros (Hem.) |
3.0 4.6 3.4 3.7 3.1, 5.0, 3.4 and 5.0 4.1, 4.4, 2.8 and 5.2 |
- 8.91 - 26.73 - - |
(Da Silva et al. 2013b) | |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona coriaceae | Seed | Seed Extract Methanolic 0.5 1.0 2.0 4.0 8.0 DMSO 20% Water |
Tuta absoluta (Lep.) |
- - - - - - - |
8.0 100 100 86.4 86.6 6.6 13.2 |
- - - - - - - |
(da Silva et al. 2007) |
| Annona cornifolia | Leaves | Leave extract 2.5 2.0 1.5 1.0 0.5 |
Anticarsia gemmantalis (Lep.) |
- - - - - |
0.41 0.38 -0.10 -0.25 0.01 |
- - - - - |
(Saito et al. 2004) |
| Annona cornifolia | Leaves | Leave extract 2.5 2.0 1.5 1.0 0.5 |
Spodoptera frugiperda (Lep.) |
- - - - - |
0.96 0.68 0.55 0.66 0.36 |
- - - - - |
(Saito et al. 2004) |
| Annona cornifolia | Leaves | Leave extract 2.5 2.0 1.5 1.0 0.5 |
Spodoptera frugiperda (Lep.) |
- - - - - |
0.96 0.68 0.55 0.66 0.36 |
- - - - - |
(Saito et al. 2004) |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona cornifolia | Leaves | Leave extract 2.5 2.0 1.5 1.0 0.5 |
Spodoptera frugiperda (Lep.) |
- - - - - |
0.96 0.68 0.55 0.66 0.36 |
- - - - - |
(Saito et al. 2004) |
| Annona Crassiflora | Fruits/ Twigs/ Roots | Extract Hexanic SB RW RB Ethanolic RW RB |
Aedes aegypti (Dip.) |
192.57 154.02 264.15 26.89 23.06 |
- - - - - |
- - - - - |
(Rodrigues et al. 2006) |
| Annona Crassiflora | Roots | Extract Root bark Root wood Stem |
Aedes aegypti (Dip.) |
0.71 8.94 16.1 |
- - - |
- - - |
(de Omena et al. 2007) |
| Annona Crassiflora | Seeds | Seeds Extract Methanol (Des. DCM) Hexanic Hydroalcoholic Fraction Ethyl Acetate |
Aedes aegypti (Dip.) |
1.0 1.0 1.0 1.0 |
0.0 91.25 0.0 0.0 |
- 0.433 - - |
(Costa, Marilza da Silva, Mônica Josene Barbosa Pereira, Simone Santos de Oliveira, Paulo Teixeira de Souza, Evandro Luiz Dall’oglio 2013) |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona Crassiflora | Seeds | Fraction Chloroform Crude Methanolic |
Aedes aegypti (Dip.) |
1.0 1.0 |
0.0 11.25 |
- 3.189 |
(Costa, Marilza da Silva, Mônica Josene Barbosa Pereira, Simone Santos de Oliveira, Paulo Teixeira de Souza, Evandro Luiz Dall’oglio 2013) |
| Annona Crassiflora | Seeds | Seeds Extract 1% 2% 4% DMSO 40% (Positive control) |
Euschistus heros (Hem.) |
481.50 542.00 372.00 683.00 |
1.25 1.50 2.00 1.25 |
353.00 396.00 306.00 309.20 |
(Oliveira and Pereira 2009) |
| Annona Crassiflora | Seeds | Seeds Extract Preview 2 Days 5 Days 7 Days DMSO 20% (Positive control 01) Water (Positive control 02) |
Euschistus heros (Hem.) |
2.4 4.1 3.0 4.2 3.1 5.0 3.4 5.0 4.1 4.4 2.8 5.2 |
17.82 13.04 16.83 - - - - - - - - |
(Da Silva et al. 2013b) |
|
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona Crassiflora | Seeds | Seed Extract 24 h 8.0% 4.0% 2.0% 1.0% 0.5% 72 h 8.0% 4.0% 2.0% 1.0% 0.5% 120h 8.0% 4.0% 2.0% 1.0% 0.5% Water + Tween 80 (Positive Control 01) Water (Positive Control 02) |
Tibraca limbativentris (Hem.) |
70.0 64.0 44.0 20.0 6.0 78.0 72.0 54.0 30.0 10.0 81.0 76.0 58.0 34.0 10.0 2.0 4.0 9.0 0.0 2.0 6.0 |
4.46 3.30 |
4.34 3.16 |
(Krinski and Massaroli 2014) |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona dioica | Seeds | Seeds Extract Fraction Dichloromethane Methanol |
Aedes Aegypti (Dip.) |
1.0 1.0 |
10.00 3.75 |
2.447 5.196 |
(Costa, Marilza da Silva, Mônica Josene Barbosa Pereira, Simone Santos de Oliveira, Paulo Teixeira de Souza, Evandro Luiz Dall’oglio 2013) |
| Annona dioica | Seeds | Seed Extract Topical application method 25 50 100 200 Cantate application method 25 50 100 200 |
Rhodnius neglectus (Hem.) |
6.2 85 90 100 88.2 91.6 95.6 96.0 |
(Carneiro 2010) | ||
| Annona diversifolia | Leaves/ Branches | Extract Stem Aqueous Ethanolic Leaf Aqueous Ethanolic |
Anastrepa ludens (Dip.) |
588.685 409.139 >1000 52.0284 |
(González-Esquinca et al. 2012) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona foetida | Seeds | Seed Extract Methanolic 24h 48h |
Aedes aegypti (Dip.) |
76.15 62.28 |
(Guarido 2009) |
||
| Annona foetida | Seeds | Hexanic 24h 48h Dichloromethanic 24h 48h |
Aedes aegypti (Dip.) |
15.17 6.72 0.73 0.33 |
(Guarido 2009) |
||
| Annona glabra | Seeds | Seed Extract | Aedes aegypti (Dip.) | 0.06 | (de Omena et al. 2007) | ||
| Annona montana | Leaves/ Branches | Annonacin Cis-annonacin-10-one Densicomacin-1 Gigantetronenin Murihexocin-B Tucupentol Control |
Spodoptera frugiperda (Hem.) |
49.20 44.75 60.00 55.20 55.12 59.00 27.12 |
50 60 40 70 30 30 0.0 |
50 40 60 30 70 70 10 |
(Toto Blessing et al. 2010) |
| Annona mucosa | Fruits and branches | Ethanolic extract Rollinicin Rolliniastacin-1 |
Aedes aegypti |
2.60 0.78 0.43 |
- |
(Rodrigues et al. 2021) |
|
| Annona mucosa | Fruits and branches | Ethanolic extract Rollinicin Rolliniastacin-1 |
Aedes albopictus |
0.55 1.13 0.20 |
- |
(Rodrigues et al. 2021) |
|
| Annona mucosa | Seeds | Seed Extract 0.5 1.0 2.0 |
Chrysodeixis includens (Lep.) |
< 55% < 55% < 55% |
(Massaroli et al. 2013) |
||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% CL - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona mucosa | Seeds | Seed Extract 4.0 8.0 |
Chrysodeixis includens (Lep.) |
86.6% 93.3% |
(Massaroli et al. 2013) | ||
| Annona mucosa | Seeds/ Branches/ Leaves | Extract Seeds 300 mg kg 1500 mg kg Leaves 300 mg kg 1500 mg kg Branches 300 mg kg 1500 mg kg Control |
Sitophilus zeamais (Col.) |
0.80 0.00 36.80 5.60 34.70 39.10 36.90 37.00 |
98.00 100.00 0.50 61.50 1.50 0.00 0.00 0.00 |
7.84 1.12 81.97 57.90 83.71 64.97 81.94 81.74 |
(Ribeiro et al. 2013) |
| Annona mucosa | Seeds | Seed Extract 24h 8.0% 4.0% 2.0% 1.0% 0.5% 72h 8.0% 4.0% 2.0% |
Timbraca limbativentris (Hem.) |
100.0 92.0 90.0 76.0 28.0 100.0 96.0 96.0 |
1.59 1.18 |
0.49 -0.24 |
(Krinski and Massaroli 2014) |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona mucosa | Seeds | 1.0% 0.5% 120h 8.0% 4.0% 2.0% 1.0% 0.5% Water + Tween80 (Positive Control 01) Water (Positive Control 02) |
Timbraca limbativentris (Hem.) |
86.0 42.0 100.0 98.0 96.0 88.0 56.0 2.0 4.0 9.0 0.0 2.0 6.0 |
0.91 |
-0.76 |
(Krinski and Massaroli 2014) |
| Annona muricata | Seeds | Seed Extract | Aedes aegypti (Dip.) | 236.23 | 74.68 | - | (Morales et al. 2004) |
| Annona muricata | Seeds | Seed Extract 12h 24h 36h 48h |
Aedes aegypti (Dip.) |
0.18 0.06 0.04 0.02 |
0.10 0.05 0.03 0.01 |
(Bobadilla et al. 2005) |
|
| Annona muricata | Seeds | Seed Extract | Aedes aegypti (Dip.) | 900.0 | 380.0 | (Henao et al. 2007) | |
| Annona muricata | Leaves/ Branches | Extract Leaves Ethanolic Aqueous |
Anastrepha ludens (Dip.) |
831.445 >1000 |
2058.3 3852.6 |
(González-Esquinca et al. 2012) | |
| Annona muricata | Leaves/ Branches | Stems Ethanolic Aqueous |
Anastrepha ludens (Dip.) |
865.0 >1000 |
4539 3984.2 |
(González-Esquinca et al. 2012) |
|
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona muricata | Seed | Seed extract | Anopheles albimanus (Dip.) | 16.20 | 0.82 | (Morales et al. 2004) | |
| Annona muricata | Seed | Food consumption (%) Low dose Medium dose Water (Positive Control 01) 10% Ethanol (Positive Control 02) |
Anticarsia gemmantallis (Lep.) |
10.0 30.0 0.0 0.0 |
25.0 29.3 19.9 19.3 |
(Fontana et al. 1998) |
|
| Annona muricata | Seed | Parviflorin Asimicin Sylvaticin Bullatalicin Annomontacin Gigantetrocin A Cypermethrin Chlorpyrifos Hydramethylnon Propoxur Bendiocarb |
Blatella germanica (Blat.) |
0.6 1.8 1.5 6.5 3.6 4.1 0.003 0.3 5.6 39.9 43.2 |
6 10 8 23 23 34 6 3 12 - - |
(Alali et al. 1998) |
|
| Annona muricata | Leaves | Leaves Extract |
Culex Quinquefascintus |
20.87 | 56.47 | (Magadula et al. 2009) | |
| Annona muricata | Seed | Compounds 1 2 Rotenone |
Leptinotarsa dercemlineata (Col.) |
92.18 29.68 100 |
(Guadaño et al. 2000) |
||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona muricata | Seeds | Seed Extract | Plutella xylostella (Lep.) | 43.0 | 60.0 | (Sinchaisri et al. 1991) | |
| Annona muricata | Seeds | Seed Extract Hexanic 24h 48h 72h Ethyl Acetathe 24h 48h 72h |
Sithophillus zeamais (Col.) |
11.447 - - - - - |
4.009 3.854 3.760 3.280 2.667 2.542 |
(Llanos et al. 2008) |
|
| Annona muricata | Seeds | Seeds Extract | Zabrotes subsfasciattus (Col.) | 46.0 | 39.1 | 36.4 | (Araújo 2010) |
| Annona reticulata | Seeds | Seed Extract (95% of Methanol) In two periods: 24h and 48h. g/l 2.5 g/l 5.0 g/l 7.5 g/l 10.0 g/l 15.0 g/l 20.0 g/l |
Epilachna vigintioctopunctata (Col.) |
(%) 24h and 48h 6.7 - 13.4 40.0 – 53.4 80.0 – 100 100 – 100 100 – 100 100 – 100 100 – 100 |
(Karunaratne and Arukwatta 2009) |
||
| Annona reticulata | Uninformed | Petroleum ether extract Ethanolic extract |
Rhodnius neglectus (Hem.) |
35.0 Not significant |
(Schmeda-Hirschmann and de Arias 1992) |
||
| Annona reticulata | Seeds | Methanolic extract | Spodoptera litura (Lep.) |
301.30 (259.15-326.33) |
50.0% |
167.48 (110.43-383.65) |
(de Cássia Seffrin et al. 2010) |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona salzmannii | Barks |
Hexanic Extract (8,68 g (0,48%) Methanolic Extract 143,29 g (7,96%) CHCl3 alkaloid fraction – FCA |
Aedes aegypti (Dip.) |
615.18 >700.00 163.53 |
(473.71 -981.44) (00.00-00.00) (107.90-238.82) (13.31-19.40) (130.00-218.00) (0.035-0.050) |
(Cruz 2011) |
|
| Annona salzmannii | Barks | Neutral CHCl3 fraction – FCN caryophyllene oxide Temephos |
Aedes aegypti (Dip.) |
15.92 167.00 0.042 |
(Cruz 2011) |
||
| Annona senegalensis | Root | Root Extract Control |
Callosobruchus maculatus (Col.) | 18.7 4.7 |
3.7 98.0 |
0.1 79.5 |
(Aku et al. 1998) |
| Annona senegalensis | Fruits | Ethanolic extract | Culex quinquefascintus (Dip.) | 0.67 | 23.42 | 29.78 |
(Magadula et al. 2009) |
| Annona senegalensis | Uninformed | Extract | Sitophilus zeamais (Col.) | 220.71 | 0.19 – 0.06 | (LS et al. 2004) | |
| Annona squamosa | Leaves | Aqueous extract (g/100 ml) 100.0 50.0 25.0 12.5 6.25 3.125 1.5625 Control |
Aedes aegypti (Dip.) |
100.0 100.0 83.3 90.0 70.0 76.6 73.3 5.7 |
(Monzon et al. 1994) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Seeds | Extract (µg/ml) 1 |
Aedes albopictus (Dip.) |
388.3 |
397.4 |
486.6 |
(Kempraj and Bhat 2011) |
| Annona squamosa | Seeds | Extract (µg/ml) 2 4 6 8 10 20 |
Aedes albopictus (Dip.) |
231.3 162.3 114.0 64.9 45.6 0.0 |
240.3 163.8 112.0 72.4 47.8 0.0 |
268.5 185.4 121.1 84.9 56.3 0.0 |
(Kempraj and Bhat 2011) |
| Annona squamosa | Leaves | Ethanol extract (mg/ml) at 24h, 48h and 72h. 5 10 20 30 |
Anopheles gambiae (Dip.) |
3.33 (24h) 6.67 (48h) 23.33 (72h) 16.67 (24h) 33.33 (48h) 63.33 (72h) 40.0 (24h) 66.67 (48h) 76.67 (72h) 53.33 (24h) 73.33 (48h) 90.0 (72h) |
(Allison et al. 2013) |
||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Leaves | Ethanol extract (mg/ml) at 24h, 48h and 72h. 40 |
Anopheles gambiae (Dip.) |
70.0 (24h) 90.0 (48h) 1000 (72h) |
(Allison et al. 2013) | ||
| Annona squamosa | Whole plant | Extract (ppm) 50 100 150 200 |
Anopheles stephensi (Dip.) | (%) 58 60 70 74 |
(%) 4 6 16 18 |
(%) 52 76 86 92 |
(Saxena et al. 1993) |
| Annona squamosa | Leaves | Extract (mgl) 500 250 125 62.5 31.25 15.63 7.82 |
Anopheles subpictus (Dip.) | (%) 100 – 0.0 82.6 – 2.46 63.0 – 1.84 48.2 – 4.62 16.4 – 2.04 92.0 – 3.28 4.6 – 4.60 |
(Kamaraj et al. 2011) | ||
| Annona squamosa | Leaves | Ethanolic extract Aqueous extract |
Bemisia tabaci (Hem.) |
100 – 0.0 99.3 – 1.05 |
(Cruz-Estrada et al. 2013) | ||
| Annona squamosa | Seeds | Extract (mg/ml) 0.01 0.03 0.05 |
Callasobruchus chinensis (Col.) | (%) 9.66 9.66 41.00 |
(Kotkar et al. 2002) | ||
| Annona squamosa | Seeds | Extract (mg/ml) 0.07 0.09 |
Callasobruchus chinensis (Col.) | % 81.33 99.00 |
(Kotkar et al. 2002) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Seeds | Extract (mg/ml) 0.07 0.09 |
Callasobruchus chinensis (Col.) | % 81.33 99.00 |
(Kotkar et al. 2002) | ||
| Annona squamosa | Seeds | Extract (µg/cm2) Petroleo ether Ethanol Acethone Methanol |
Ceratitis capitata (Dip.) | (%) 0.031 0.632 0.591 4.038 |
(%) 198.57 614.26 1000.40 135.25 |
(Epino and Chang 1993) | |
| Annona squamosa | Seeds | Extract (%) 0.05 0.1 Deltamethrin |
Crocidiolomia pavonana (Lep.) |
10.4 ± 1.2 6.3 ± 2.7 11.0 ± 3.6 |
32.0 ± 6.9 69.0 ± 4.8 62.4 ± 5.2 |
(Dadang and Prijono 2009) | |
| Annona squamosa | Seeds |
Aqueous extract (25%) |
Culex quinquefasciatus (Dip.) |
33.6% |
(Pérez-Pacheco et al. 2004) | ||
| Annona squamosa | Leaves | Ethanol extract (mg/ml) at 24h, 48h and 72h. 5 10 |
Culex quinquefasciatus (Dip.) | (%) 0.0 (24h) 13.33 (48h) 46.67 (72h) 33.33 (24h) 56.67 (48h) 73.33 (72h) |
(Allison et al. 2013) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Leaves | Ethanol extract (mg/ml) at 24h, 48h and 72h. 20 30 40 |
Culex quinquefasciatus (Dip.) |
56.67 (24h) 70.0 (48h) 90.0 (72h) 80.0 (24h) 96.67 (48h) 100 (78h) 93.33 (24h) 100 (48h) 1000 (72h) |
(Allison et al. 2013) | ||
| Annona squamosa | Leaves | Aqueous extract (g/100 ml) 100.0 50.0 25.0 12.5 6.25 3.125 1.5625 |
Culex quinquefasciatus (Dip.) | (%) 100.0 60.0 50.0 36.7 26.7 33.3 10.0 |
(Monzon et al. 1994) | ||
| Annona squamosa | Leaves | Extract | Culex quinquefasciatus (Dip.) | 0.64 | 14.69 | (Magadula et al. 2009) | |
| Annona squamosa | Leaves | Methanol Extract (mg/l) 500 |
Culex tritaeniorhynchus (Dip.) | (%) 100.0 ± 00.0 |
(Kamaraj et al. 2011) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Leaves | Methanol Extract (mg/l) 250 125 62.5 31.25 15.63 7.82 |
Culex tritaeniorhynchus (Dip.) | (%) 88.4 ± 1.64 52.6 ± 4.63 34.2 ± 2.84 20.6 ± 1.67 12.4 ± 2.45 6.8 ± 1.87 |
(Kamaraj et al. 2011) | ||
| Annona squamosa | Seeds | Diet (µg/2g) 48h |
Drosophila melanogaster (Dip.) |
62.5 |
(Kawazu et al. 1989) | ||
| Annona squamosa | Seeds | Extract (g/l) 1.0 2.5 5.0 10.0 15.0 20.0 |
Epilachna vigintioctopunctata (Col.) | 24h and 48h (%) 6.7 and13.4 40.0 and 53.4 80.0 and 53.4 100 and 100 100 100 |
(%) 64.8 83.4 92.3 95.9 95.9 100.0 |
(Karunaratne and Arukwatta 2009) | |
| Annona squamosa | Branches | Extract (%) | Musca domestica (Dip.) | 41.00 | (Sharma et al. 2011) | ||
| Annona squamosa | Leaves | Extract (mg/l) 0 200 400 600 800 1000 |
Musca domestica (Dip.) |
(%) 100 80 65 50 30 0 |
(%) 100 62.5 53.85 40.0 33.33 0.0 |
(Begum et al. 2010) | |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Leaves | Extract (% plant poder) 5 10 20 |
Oryctes rhinoceros (Col.) | (%) 10 30 50 |
(%) 10 20 20 |
(Sreeletha and Geetha 2012) | |
| Annona squamosa | Leaves | Extract (%) 100 75 50 25 10 5 0.1 |
Periplaneta americana (Blat.) | (%) 80 60 50 20 10 10 0 |
Average 4.00 ± 0.0 3.00 ± 0.0 2.5 ± 0.71 1.00 ± 0.0 0.5 ± 0.701 0.5 ± 0.701 0.0 ± 0.0 |
(Kesetyaningsih 2012) | |
| Annona squamosa | Seeds | Extract (mg/ml) 5 10 |
Plutella xylostella (Lep.) | (%) 46.7 70.0 |
(Sinchaisri et al. 1991) | ||
| Annona squamosa | Seeds | Aqueous extract Larval instar (Time h) 3rd: 24h 48h 72h 4th: 24 48 72 |
Plutella xylostella (Lep.) | (%) 5.2 (3.1-8.5); 1.7 (1.3-2.2); 0.9 (0.7-1.2). 8.7 (6.6-11.3); 4.2 (3.5-5.1); 2.0 (1.7-2.4). |
(%) 2.5 ± 1.4 10.0 ± 6.8 12.5 ± 6.0 0 1.3 ± 1.3 5.0 ± 2.0 |
(Leatemia and Isman 2004) | |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods |
Ref. |
||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Seeds | Aqueous extract 1% 5% Control 1 (Acetone) Control 2 (Methanol) Control 3 (Without solvente) |
Sitophilus oryzae (Col.) | (LD 50 Min) 23.1 (22.1-23.9) 11.4 (10.7-12.2) 0.0 0.0 0.0 |
(% min) 39.6±1.4 14.5±1.1 - - - |
(Kumar et al. 2010) | |
| Annona squamosa | Seeds | Extract (%) 0.5 |
Spodoptera litura (Lep.) |
21.66±1.66 |
0.0 |
28.33±1.66 |
(Deshmukhe et al. 2010) |
| Annona squamosa | Seeds | Extract (%) 1 5 10 15 20 25 |
Spodoptera litura (Lep.) |
23.33±1.66 38.33±1.66 48.33±1.66 56.66±1.66 51.66±1.66 61.66±1.66 |
0.0 0.0 0.0 0.0 1.66±1.66 1.66±1.66 |
33.33±1.66 51.66±1.66 58.33±1.66 78.33±3.33 75.00±0.0 80.00±0.0 |
(Deshmukhe et al. 2010) |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Seeds | Extract Petroleum ether EtOH Acetone Methanol |
Tribolium castaneum (Col.) | LD50 (µg/cm2) 0.031 0.632 0.591 4.038 |
95% (Lower and Upper) 0.006 and 0.150; 0.315 and 1.265; 0.285 and 1.224; 1.727 and 9.440. |
(Khalequzzaman and Sultana 2006) | |
| Annona squamosa | Seeds | Extracts using two methods of application: Topical (µg/larva) Oral (ppm fresh weight in diet. |
Trichoplusia ni (Lep.) |
301.30 (259.15-326.33) |
167.48 (110.43-383.65) |
(de Cássia Seffrin et al. 2010) | |
| Annona squamosa | Seeds | Extract (ppm) Hexane extract 250 500 750 1000 1250 1500 Ethyl Acetate extract 50 250 500 750 1000 1250 |
Trogoderma granarium (Dip.) | (%) 10th day and 15th day 0.0 and 11.13 4.47 and 11.13 11.13 and 17.8 17.8 and 28.87 48.9 and 53.33 75.33 and 82.2 4.47 and 20.00 24.5 and 33.33 26.7 and 55.53 51.13 and 57.8 64.47 and 80.0 84.5 and 91.13 |
(mg) 10th day and 15th day. 66.1 and 80.5 75.1 and 84.6 64.9 and 70.9 63.6 and 71.8 64.9 and 70.1 58.3 and 61.5 73.0 and 86.2 65.6 and 73 72.4 and 81.0 62.9 and 69.5 59.8 and 66.0 56.0 and 61.0 |
(Rao et al. 2005) | |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Annona squamosa | Seeds | Extract (ppm) Methanol extract 500 750 1000 1250 1500 2000 Acetone control Control |
Trogoderma granarium (Dip.) | (%) 10th day and 15th day 6.67 and 8.87 13.34 and 17.8 22.2 and 24.47 20.0 and 26.67 48.87 and 57.7 66.67 and 77.7 0.0 and 0.0; 0.0 and 0.0. |
(mg) 10th day and 15th day. 78.0 and 96.6 74.1 and 93.1 75.2 and 98.2 74.6 and 92.4 58.0 and 85.0 53.4 and 62.5 100.9 and 150.6. 94.9 and 161.6. |
(Rao et al. 2005) | |
| Artabotrys odoratissimus | Bark | Larval instar and exposure periods (h) Second 12h 24h |
Culex quinquefascintus (Dip.) | LC50 52.92 42.03 |
95% (Lower and Upper) 33.59 and 83.87 26.18 and 67.47 |
(Kabir 2010) | |
| Artabotrys odoratissimus | Bark | Larval instar and exposure periods (h) Third 12h 24h Fouth 12h 24h |
Culex quinquefascintus (Dip.) |
110.03 99.13 170.12 110.41 |
72.51 and 166.9 60.2 and 163.29 137.6 and 210.26 89.6 and 135.95 |
(Kabir 2010) | |
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Asimina triloba | Roots | Ethanolic extract (Fraction nº.) 017 018 019 020 021 Asimicin |
Acalymma vittatum (Col.) | LC50 (p.p.m.) 7.56 >1000 1.67 0.04 715 0.03 |
(Mikolajczak et al. 1988) |
||
| Cardiopetalum calophyllum | Seeds |
Methanolic extract (1.0 mg/mL) |
Aedes aegypti (Dip.) | (%) 5.00 |
(mg/mL) 1.789 |
(Costa, Marilza da Silva, Mônica Josene Barbosa Pereira, Simone Santos de Oliveira, Paulo Teixeira de Souza, Evandro Luiz Dall’oglio 2013) | |
| Dennettia tripetala | Leaves and roots | Ethanol extract (5mL/100g) in 1, 3 and 7 days. 0.0 |
Dermestes maculatus (Col.) | (%) In 1, 3 and 7 days. 0.0 ± 0.0 0.0 ± 0.0 0.0 ± 0.0 |
(Akinwumi et al. 2007) | ||
| Dennettia tripetala | Leaves and roots | Ethanol extract (5mL/100g) in 1, 3 and 7 days. 2.50 5.00 |
Dermestes maculatus (Col.) | (%) In 1, 3 and 7 days. 26.67 ± 0.88 71.67 ± 0.88 100.0 ± 0.0 26.67 ± 0.67 75.0 ± 1.16 100.0 ± 0.0 |
(Akinwumi et al. 2007) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Dennettia tripetala | Leaves and roots | Ethanol extract (5mL/100g) in 1, 3 and 7 days. 7.50 10.0 |
Dermestes maculatus (Col.) | (%) In 1, 3 and 7 days. 48.33 ± 0.88 91.67 ± 0.33 100.0 ± 0.0 51.67 ± 0.66 98.33 ± 0.33 100.0 ± 0.0 |
(Akinwumi et al. 2007) | ||
| Dennettia tripetala | Seeds | Concentration of plant powder/25g fish Extract Untreated control |
Dermestes maculatus (Col.) | (%) 87.0 100 |
(g) 0.25 0.50 |
(Okonkwo and Okoye 2001) | |
| Dennettia tripetala | Uninformed | Extracts (ppm) 0 10 100 1000 |
Ostrinia nubialis (Lep.) |
5.79 3.13 3.87 3.84 |
(Ewete et al. 1996) | ||
| Dennettia tripetala | Seeds | Extract (ml/25g) 0.1 0.2 0.3 |
Necrobia rufipes (Col.) | (%) 100 100 100 |
(Okonkwo and Okoye 2001) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Dennettia tripetala | Leaves and roots | Extract (%) at 24h, 48h and 72h. 0% |
Sitophilus zeamais (Col.) | (%) at 24h, 48h and 72h. 1.83 1.09 1.75 |
(Umeotok et al. 2013) | ||
| Dennettia tripetala | Leaves and roots | Extract (%) at 24h, 48h and 72h. 1% 5% 10% |
Sitophilus zeamais (Col.) | (%) at 24h, 48h and 72h. 1.50 2.09 2.58 1.50 2.09 3.08 2.83 3.58 4.67 |
(Umeotok et al. 2013) | ||
| Duguetia furfuraceae | Leaves/barks and roots | Extract | Aedes aegypti (Dip.) | (µg/ml) 56.6 |
(Rodrigues et al. 2006) | ||
| Duguetia furfuraceae | Leaves | Extract | Sitophilus zeamais (Col.) | (Proportion of insects in the treated area (SDM)) 0.500 (0.113) |
(Luciana et al. 2013) | ||
| Guatteria blephrophylla | Leaves | Extract (ppm) | Aedes aegypti (Dip.) | 85.74 | (74.05 – 112.78) | 4.48±0.89 | (Aciole et al. 2011) |
| Guatteria friesiana | Leaves | Extract (ppm) | Aedes aegypti (Dip.) | 52.60 | (50.11 – 55.17) | 6.48±0.55 | (Aciole et al. 2011) |
| Guatteria hispida | Leaves | Extract (ppm) | Aedes aegypti (Dip.) | 85.74 | (74.05 – 112.78) | 4.48±0.89 | (Aciole et al. 2011) |
| Mikilua fragans | Aerial parts | Essential oil | Anopheles gambiae (Dip.) |
RC75 (x10-5 mgcm-2) 481 (11, 145) |
(Odalo et al. 2005) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Oxandra cf xylopioides | Leaves | Extract 24h 48h 72h |
Spodoptera frugiperda (Lep.) | ppm 319.61 311.47 294.13 |
(Rojano et al. 2007) | ||
| Rollinia occidentalis | Seeds | Extract (ppm) 50 100 250 |
Spodoptera frugiperda (Lep.) | (%) 5 35 50 |
(%) 30 45 50 |
(%) - 20 - |
(Tolosa et al. 2012) |
| Uvaria scheflerri |
Roots |
Essential oil 5% 1% 0.5% 0.1 |
Anisakis L3 |
- 5 - - |
(Anza et al. 2021) |
||
| Xylopia aethiopica | Leaves and Roots | Extract Ethanolic Water |
Anopheles gambiae (Dip.) | LC50 3.57 4.50 |
(%) 125 (34.72%) 106 (29.44%) |
(Aina et al. 2009) | |
| Xylopia aethiopica | Uninformed | Extract (ppm) 0 10 100 1000 |
Ostrinia nubilalis (Lep.) | (mg) 3.78 3.69 3.87 3.84 |
(Ewete et al. 1996) | ||
| Annonaceae species | Used Material | Substances / Extracts | Insect species (Order) | Methods | Ref. | ||
| Topical (LC50 µg/ larva) | Topical (LD50 – 95% LC - µg/nymph) | Oral (LC50 ppm fresh weight in diet) | |||||
| Xylopia aromatica | Leaves/Barks and Roots | Extract | Aedes aegypti (Dip.) | (µg/ml) 384.37 |
(Rodrigues et al. 2006) | ||
| Xylopia caudata | Leaves | Oil | Aedes aegypti (Dip.) | 29.83 (21.87-37.45) | 60.33 (48.04-82.47) | 4.19±0.69 | (Zaridah et al. 2006) |
| Xylopia ferruginea | Leaves | Oil | Aedes aegypti (Dip.) | 74.51 (68.39-86.52) | 106.45 (90.23-159.78) | 8.27±1.88 | (Zaridah et al. 2006) |
3.3. Antimicrobial
| Annonaceae species | Used Material | Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Annona foetida |
Leaves |
Essential oil Chloramphenicol Nystatin (Positive control) |
ATCC 6538 200 20 - |
ATCC 10231 60 - 50 |
(Costa et al. 2009) |
|||||||||||||
|
Annona hypoglauca Mart. |
Bark |
Alkaloid phases FA5 (isoboldine) FA6 (actinodaphnine) |
ATCC 29213 70 70 |
ATCC 25922 - 90 |
ATCC 299212 70 80 |
(Rinaldi et al. 2017) |
||||||||||||
|
Annona muricata |
Leaves |
Dried leaf powder: AM0 (Size bands < 2000) AM1 (Size bands between 0.500 and 0.350) |
ATCC 25923 3120 1560 |
ATCC 25922 1560 1560 |
ATCC 27853 3120 3120 |
ATCC 4352 3120 3120 |
(de Andrade et al. 2019) |
|||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Annona senegalensis |
Root bark |
methanol-methylene chloride extract kaur-16-en-19-oic acid F1 (Subfraction) Ciprofloxacin Gentamicin (Positive controls) |
8750 150 - 1.18 0.23 |
1080 - 40 3.6 0.79 |
(Akah et al. 2012) |
|||||||||||||
|
Annona squamosa |
Leaves |
11-hydroxy-16-hentriacontanona + 10-hydroxy-hentricontanona Palmitone Ciprofloxacin (positive control) |
MTCC 96 25 12.5 0.78 |
MTCC 741 25 6.25 0.78 |
(Shanker et al. 2007) |
|||||||||||||
|
Annona vepretorum |
Leaves |
Crude ethanolic extract Hexanic extract |
ATCC 25923 3120 780 |
ATCC 25922 390 390 |
ATCC 19433 3120 3120 |
ATCC 13883 3120 3120 |
(Almeida et al. 2014) |
|||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Annona vepretorum |
Leaves |
Chloroform extract |
ATCC 25923 1560 |
ATCC 25922 780 |
ATCC 19433 12500 |
ATCC 13883 6250 |
(Almeida et al. 2014) |
|||||||||||
|
Annonidium mannii |
Root Bark |
Crude extract Ciproflocaxin (Positive control) |
ATCC 11296 64 2 |
(Ngangoue et al. 2020) |
||||||||||||||
|
Bocageopsis pleiosperma Maas |
Stem Bark |
Polycarpol Chloramphenicol (Positive control) Ketoconazole (Positive control) |
ATCC 6538 25 25 - |
ATCC 1228 50 50 - |
ATCC 10538 50 50 - |
ATCC 27853 - >500 - |
ATCC 10231 250 - 12.5 |
(da Silva et al. 2015) |
||||||||||
|
Bocageopsis multiflora (Mart.) R.E. Fr |
Leaves |
Essential oil |
ATCC 6538 190 |
ATCC 8739 1500 |
ATCC 9027 3000 |
ATCC 29212 90 |
(Alcântara et al. 2017) |
|||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Bocageopsis multiflora |
Aerial parts |
Essential oil TIENAM (Positive control) |
ATCC 33591 4.68 4.68 |
CDC-EDL 933-171-0157:H3 4.68 1.17 |
ATCC 29336 4.68 2.34 |
(Bay et al. 2019a) |
||||||||||||
|
Cananga odorata |
Essential oil Thymus vulgaris (reference oil) |
ATCC 48274 170 60 |
(Sacchetti et al. 2005) |
|||||||||||||||
|
Cleistopholis patens |
Leaves |
Cleistetroside-8 Cleistetroside-5 Cleistetroside-2 Vancomycin (positive control) |
ATCC 33591 8 8 0.5 1 |
(Hu et al. 2006) |
||||||||||||||
|
Cyathocalyx zeylanicus |
Leaves |
Essential oil Gentamycin Muconazol (Positive control) |
ATCC 25923 250 250 9.0 |
ATCC 25922 125 125 12.0 |
ATCC 37853 250 250 9.1 |
ATCC 27853 250 250 8.0 |
ATCC 90028 250 16 4.5 |
(Hisham et al. 2012) |
||||||||||
|
Annonaceae species |
Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) |
Ref. |
||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Cleistochlamys kirkii (Benth) Oliv. |
Root Bark |
Chamanetin Isochamanetin Dichamanetin Cleistenolide Acetylmelodorinol Amoxicillin Oxacillin Vancomycin (Positive control) |
S. aureus MSSA MRSA VISA ATCC ATCC ATCC FFHB ATCC CIP 6538 43866 9144 29593 700699 106760 7.5 15 30 - 125 15 62 125 250 30 62 62 2 2 1 4 2 2 30 30 7.5 15 30 30 >250 >250 125 >250 >250 >250 0.2 62 250 >250 250 250 0.2 125 125 250 250 250 0.2 0.4 0.4 0.8 2 4 |
VRE HB164 15 30 7.5 30 >250 - - 32 |
(Pereira et al. 2016) |
|||||||||||||
|
Dennetia tripetala Baker f. |
Seed |
Dried seeds essential oil Streptomycin Acriflavin (Positive control) |
NCIB 8586 3.13 - 0.13 |
NCIB 86 6.25 0.13 |
NCIB 950 25.0 1.0 |
NCCYC 6 6.25 - 2.0 |
(Oyemitan et al. 2019) |
|||||||||||
|
Desmopsis bibracteata Desmopsis macrocarpa |
Leaves Leaves |
Essential oil Essential oil |
ATCC 29213 625 1250 |
ATCC 259222 2500 1250 |
(Palazzo et al. 2009) |
|||||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Desmos chinensis |
Leaves |
Essential oil Gentamycin Muconazol (Positive control) |
ATCC 25923 250 250 9.0 |
ATCC 25922 125 125 12.0 |
ATCC 37853 250 250 9.1 |
ATCC 27853 250 250 8.0 |
ATCC 90028 250 16 4.5 |
(Hisham et al. 2012) |
||||||||||
|
Duguetia lanceolata |
Stem bark |
Essential oil (T2) Essential oil (T4) Chloramphenicol (positive control) |
ATCC 6538 60 125 2 |
ATCC 10231 60 100 15 |
(Sousa et al. 2012) |
|||||||||||||
|
Enantia chlorantha |
Stem bark |
Palmitin Chloramphenicol (positive control) |
ATCC 25923 32 8 |
E.C 136 32 32 |
K.L 128 16 32 |
(Etame et al. 2019) |
||||||||||||
|
Ephedranthus amazonicus R.E. Fr. |
Leaves |
Essential oil Chlorhexidine digluconate (Positive controls) |
ATCC 6538 90 6 |
ATCC 8739 1500 6 |
ATCC 9027 3000 10 |
ATCC 29212 190 90 |
(Alcântara et al. 2017) |
|||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Fissistigma kwangsiensis |
Leaves |
Essential oil Nystatine Streptomycin Cycloheximide (Positive controls) |
ATCC 25923 55.67 - 3.2 - |
ATCC 27853 3.45 8.0 - - |
ATCC 299212 33.62 2.07 - - |
ATCC 10231 16.45 - - 3.2 |
(Tsiang et al. 2022) |
|||||||||||
|
Fusaea longifolia |
Aerial parts |
Essential oil TIENAM (positive control) |
ATCC 33591 37.5 4.68 |
ATCC 29336 37.5 2.34 |
(Bay et al. 2019a) |
|||||||||||||
|
Greenwayodendron suaveolens subs. usambaricum |
Root Bark Roots |
Methanol extracts Pentacyclindole Polyalthenol Ciprofloxacin (Positive control) |
ATCC 25923 1000 4 4 2.5 |
(Christopher et al. 2018) (Williams et al. 2010) (Williams et al. 2010) (Christopher et al. 2018) |
||||||||||||||
|
Goniothalamus gracilipes |
Leaves |
Gracilipin C Streptomycin (Positive control) |
ATCC 25923 32 32 |
(Trieu et al. 2021) |
||||||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Goniothalamus longistipetes |
Bark |
(+)-altholactone ((2S,3R,3aS,7aS)-3-hydroxy-2-phenyl-2,3,3a,7a-tetrahydrobenzo-5(4H)-5-one) (2S,3R,3aS,7aS)-3-hydroxy-2-phenyl-2,3,3a,7a-tetrahydrobenzo-5(4H)-5-one) 2,6-dimethoxyisonicotinaldehyde alkenyl-5-hydroxyl-phenyl benzoic acid |
eMRSA – 15 64 128 128 8-16 |
NCTC 10418 256 512 256 512 |
NCTC 10662 500 512 256 128 |
NCTC 10662 256 512 256 128 |
(Teo et al. 2020) |
|||||||||||
|
Guatteria blepharophylla |
Leaves |
Essential oil Isomoschatoline Chlorhexidine digluconate Nystatin (Positive control) |
ATCC 6538 50 - 6 |
ATCC 8739 1500 - 6 |
ATCC 9027 1500 - 10 |
ATCC 29212 50 - 90 |
ATCC 10231 - 50.81 µM L-1 54 µM L-1 |
(Costa et al. 2011c; Alcântara et al. 2017) |
||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Guatteria costaricensis Guatteria diospyroides Guatteria oliviformis |
Leaves |
Essential oil Essential oil Essential oil |
ATCC 29213 1250 312 1250 |
ATC 25922 1250 1250 625 |
(Palazzo et al. 2009) |
|||||||||||||
|
Guatteria selowiana Guatteria latifolia Guatteria ferruginea Guatteria australis |
Aerial parts Aerial parts Aerial parts Aerial parts |
Essential oil Essential oil Essential oil Essential oil Chloramphenicol (Positive control) |
ATCC 11775 600 600 600 >1000 40 |
(Santos et al. 2017) |
||||||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Guatteria citriodora |
Leaves and Stem bark |
Crude hydroalcoholic extract of leaves Alkaloid fraction of leaves Alkalidic fraction of stem bark Imipenem (Positive control) |
250 - - 15.6 |
ATCC 4083 - 125 - 62.5 |
(Rabelo et al. 2014) |
|||||||||||||
|
Guatteria punctata |
Aerial parts |
Essential oil TIENAM (positive control) |
ATCC 33591 - 4.68 |
CDC-EDL 933-171-0157:H3 - 1.17 |
ATCC 29336 - 2.34 |
(Bay et al. 2019a) |
||||||||||||
|
Guatteriopsis hispida |
Essential oil Oxide caryophyllene |
ATCC 6538 >1000 - |
ATCC 12228 >1000 - |
ATCC 11775 >1000 - |
ATCC 133388 >1000 - |
ATCC 10231 >1000 600 |
(Costa et al. 2008) |
|||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Guatteriopsis blepharopylla Guatteriopsis friesiana |
seed |
Essential oil β-eudesmol Ƴ-eudesmol α-eudesmol Essential oil β - pinene α – pinene (E)- caryophyllene Chloramphenicol Nystatin (Positive control) |
ATCC 6538 1000 >1000 600 250 125 - - - 20 - |
ATCC 12228 >1000 600 700 700 100 - - - 40 - |
ATCC 11775 >1000 >1000 >1000 - 900 - - - 40 |
ATCC 133388 >1000 >1000 300 200 900 - - - 850 |
ATCC 10231 700 125 500 125 500 100 250 - 50 |
(Costa et al. 2008) |
||||||||||
|
Mitrephora celebica |
Leaves Stem bark Twigs |
Crude hydroalcoholic extract of leaves Crude hydroalcoholic extract Crude hydroalcoholic extract Ent-trachyloban-19-oic acid |
ATCC 43300 >100 12.5 >100 6.25 |
ATCC 27853 >100 >100 >100 >100 |
(Zgoda-Pols et al. 2002) |
|||||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Mitrephora celebica |
ent-kaur-16-en-19-oic acid Gentamycin Oxacillin Vancomycin (Positive control) |
ATCC 43300 >100 >50 6.2-12.5 0.8 |
ATCC 27853 >100 0.8 - - |
(Zgoda-Pols et al. 2002) |
||||||||||||||
|
Monodora myristica |
Fruits |
Cyclohexane extract Ethyl acetate extract |
25 25 |
25 50 |
- 50 |
12.5 12.5 |
6.3 12.5 |
(Mbosso et al. 2010) |
||||||||||
|
Polyalthia cinnamomea |
Leaves |
Leaf extract Vancomycine Streptomicyne Kanamycine (Positive control) |
ATCC 25923 4000 - 30 - |
4000 30 - - |
ATCC 10536 1000 - - 30 |
(Mahmud et al. 2018) |
||||||||||||
|
Polyalthia longifolia |
Stem bark |
Butanol fraction 3-o-methyl ellagic |
ATCC 29213/ 512 (MRSA) 320/320 80/160 |
ATCC 29212 320 80 |
ATCC 27853 160 80 |
(Jain et al. 2014) |
||||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Polyalthia longifolia |
Stem bark Roots Leaves |
Vancomycin Oxacillin Ciprofloxacin (Controle positive) Pendulamine A Pendulamine B Penduline Kanamycin sulfate (Control positive) Methanol extract 16(R and S)-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide 16-oxo-cleroda-3,13(14)E-dien-15-oic acid |
ATCC 29213/ 512 (MRSA) 0.25/- -/8.0 - 0.2 0.2 12.5 0.31 125 7.8 500 |
ATCC 29212 - - 0.015 |
ATCC 27853 - - 0.25 2 - - 5 125 250 500 |
2 2 - 5 |
(Jain et al. 2014) (Jain et al. 2014) (Jain et al. 2014) (Faizi et al. 2003) (Faizi et al. 2003) (Faizi et al. 2003) (Faizi et al. 2003) (Faizi et al. 2008) (Faizi et al. 2008) (Faizi et al. 2008) |
|||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Toussaintia orientalis Verdc. |
Seeds |
Toussintine A Toussintine B Toussintine C Toussintine D Toussintine E Ampicilin (Positive control) |
ATCC 25923 - 10 - 5 - 2.5 |
DSM 1103 10 20 10 - - 2.5 |
(Samwel et al. 2011) |
|||||||||||||
|
Unonopsis duckei R.E. Fr. Unonopsis floribunda Diels Unonopsis rufescens (Baill.) R.E. Fr. Unonopsis stipitala Diels Onychopetalum amazonicum R.E. Fr. |
Stem Bark |
Polycarpol Chloramphenicol (Positive control) Ketoconazole (Positive control) |
ATCC 6538 25 25 - |
ATCC 1228 50 50 - |
ATCC 10538 50 50 - |
ATCC 27853 - >500 - |
ATCC 10231 250 - 12.5 |
(da Silva et al. 2015) |
||||||||||
|
Unonopsis costaricensis |
Leaves |
Essential oil |
ATCC 29213 625 |
ATCC 25922 1250 |
(Palazzo et al. 2009) |
|||||||||||||
| Annonaceae species | Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) | Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Uvaria hamiltonii |
Leaves |
Essential oil Nystatine Streptomycin Cycloheximide (Positive controls) |
ATCC 25923 20.34 - 1.07 - |
ATCC 27853 12.34 8.0 - - |
ATCC 29212 7.99 0.48 - - |
ATCC 10231 32.57 - - 3.2 |
(Tsiang et al. 2022) |
|||||||||||
|
Uvaria schefflera |
Leaves |
5,7,8-trimetoxiflavona 2’,6’-dihydroxy-4’-methoxychalcone and 5,7-dihydroxyflavone (mixture) Ampicillin Ketoconazole (positive control) |
NCTC 6571 - 125 0.01 |
NCTC 10418 125 - 0.25 |
HG 392 - 31.2 0.125 |
(Moshi et al. 2004) |
||||||||||||
|
Uvaria tanzaniae |
Root Bark |
Methanol extracts Ciprofloxacin (Positive control) |
ATCC 25923 1.25 2.5 |
(Christopher et al. 2018) |
||||||||||||||
|
Annonaceae species |
Used Material |
Substances/ Extracts |
Microorganisms / MIC (µg/mL) |
Ref. | ||||||||||||||
| S. aureus | S. epidermidis | E. coli | P. aeruginosa | E. faecalis | K. pneumoniae | C. krusei | C. albicans | C. tropicalis | ||||||||||
|
Uvariodendron usambarense |
Leaves Stem Bark |
Methanol extracts Methanol extracts Ciprofloxacin (Positive control) |
ATCC 25923 8000 4000 2.5 |
ATCC 8740 500 - 0.63 |
(Christopher et al. 2018) |
|||||||||||||
|
Xylopia staudtii |
Bark |
Hydroethanolic extract Ciprofloxacin (Positive control) |
ATCC 25922 83.33 0.97 |
(Pouofo Nguiam et al. 2021) |
||||||||||||||
|
Xylopia aromatica (Lam.) Mart. |
Leaves |
Essential oil Chlorhexidine digluconate (Positive control) |
ATCC 6538 1200 6 |
ATCC 8739 3000 6 |
ATCC 9027 3000 10 |
ATCC 29212 50 90 |
(Alcântara et al. 2017) |
|||||||||||
|
Xylopia sericea |
Fruits |
Essential oil Chloramphenicol Ciprofloxacin (positive control) |
ATCC 6538 7.8 12.5 0.39 |
ATCC 10536 1000 6.25 1.56 |
ATCC 19433 1000 6.25 1.56 |
ATCC 4552 62.5 12.5 12.5 |
(Mendes et al. 2017) |
|||||||||||
3.4. Leishmanicidal
| Annonaceae species | Used Material | Substances/Extracts | Leishmania spp. | Parasite form | IC50 | Ref. | |
|---|---|---|---|---|---|---|---|
| µM | µg/ml | ||||||
| Anaxagorea dolichocarpa | root | Sampangine Imbiline 3 Imbiline 1 EupolaUramine |
L. donovani | Promastigote | 24.06 16.91 18.20 19.90 |
5.59 5.45 5.32 5.26 |
(Lorenzo et al. 2016) |
| Annickia kummeriae | leave | Methanolic extract Lysicamine Trivalvone Palmatine Jatrorrhizine Jatrorrhizinne/Columbamine Palmatine/Tetrahydro-palmetine |
L. donovani | Amastigote | 9.26 5.24 22.13 60.28 19.35 9.88 |
9.25 2.7 2.9 7.8 20.4 13.1 7.0 |
(Malebo et al. 2013b) |
| Annona crassiflora | Stem bark, stem wood, root bark and root wood | Ethanolic extract Stem bark Stem wood Root bark Root wood |
L. donovani | Promastigote |
12.4 8.3 3.7 8.7 |
(De Mesquita et al. 2005; Brígido et al. 2020) | |
| Annona coriaceae | leave | Essential oil | L. chagasi | Promastigote | 39.93 | (Siqueira et al. 2011) | |
| Annona cornifolia | seed | Annofolin Annotacin Extract |
L. amazonensis | Amastigote | 6.4 7.2 |
175.0 |
(Lima et al. 2014; Brígido et al. 2020) |
| Annona foetida | bark | Hexane extract Dichlorometahne extract Alkaloid fraction (Dichlorometane extract) Methanolic extract Alkaloid fraction (Methanolic extract) N-hydroxyanno-montine |
L. braziliensis and L. guyanensis | Promastigote |
911.3 and 1577.8 |
>160.0 and 42.7 23.0 and 2.7 23.0 and 2.7 40.4 and 23.6 24.3 and 9.1 252.7 and 437.5 |
(Costa et al. 2009; Brígido et al. 2020) |
| Annonaceae species | Used Material | Substances/Extracts | Leishmania spp. | Parasite form | IC50 | Ref. | |
| µM | µg/ml | ||||||
| Annona foetida | Bark Leave |
O-methylmoschatolin Liriodenine Annomontine Essential oil |
L. braziliensis and L. guyanensis L. amazonensis, L. braziliensis, L. chagasi and L. guyanensis |
Promastigote | 998.35 and 322.7 212.52 and 78.10 >2346.15 |
320.8 and 103.7 58.5 and 21.5 34.8 and >613.0 16.2, 9.9, 27.2 and 4.1 |
(Costa et al. 2009; Brígido et al. 2020) |
| Annona glabra | leave | Hydroalcoolic extrac | L. amazonensis | Promastigote | 37.8 | (Brígido et al. 2020) | |
| Annona glauca | seed | Dichloromethane extract Hexane extract Annonacin A Goniothalamicin Glaucanisin Rolliniastatin-2 Squamocin Glaucafilin Molvizarin Parviflorin Annonacin |
L. braziliensis, L. amazonensis and L. donovani |
Promastigote |
16.75 8.37 40.13 40.13 40.13 41.88 >168.09 >168.09 21.61 |
IC100 25.0 >100.0 10.0 5.0 25.0 25.0 25.0 25.0 >100.0 >100.0 12.5 |
(Waechter et al. 1998) |
| Annona haematantha | root | Argentilactone | L. donovani, L. major and L. amazonensis | Promastigote | 51.47 | 10.0 | (Waechter et al. 1997) |
| Annona mucosa | Leave Seed |
Hexane extract Dichloromethane extrac Methanol extract Hexane extract Methanol extract Liriodenine |
L. amazonensis and L. braziliensis |
Promastigote |
5.19 and 203.15 |
24.24 and 65.17 9.32 and 27.42 28.32 and 44.74 44.2 and 170.15 46.54 and 133.8 1.43 and 55.92 |
(De Lima et al. 2012; Brígido et al. 2020) |
| Annona muricata | leave | Ethil acetate extract | L. amazonensis, L. donovani | Promastigote | 25.0 | (Osorio et al. 2007; Vila-Nova et al. 2011; Brígido et al. 2020) | |
| Annonaceae species | Used Material | Substances/Extracts | Leishmania spp. | Parasite form | IC50 | Ref. | |
| µM | µg/ml | ||||||
| Annona muricata | Stem | Hexane extract Methanolic extrac Hexane extract Ethil acetate extract Methanolic extrac |
L. amazonensis, L. brasiliensis and L. donovani | Promastigote | >100.0 >100.0 98.6 63.2 98.6 |
(Osorio et al. 2007; Vila-Nova et al. 2011; Brígido et al. 2020) | |
| seeds | Annonacinone | L. chagasi | Promastigote and Amastigote | 63.20 and 22.69 | 37.6 and 13.5 | ||
| L. donovani, L. mexicana and L. major | Promastigote | 12.87, 13.44 and 11.29 | 7.66, 8.00 and 6.72 | ||||
| Corossolone | L. chagasi | Promastigote and Amastigote | 44.74 and 49.57 | 25.9 and 28.7 | |||
| L. donovani, L. mexicana and L. major | Promastigote | 32.35, 32.19 and 27.88 | 18.73, 18.64 and 16.14 | ||||
| Scoparone | L. donovani, L. mexicana and L. major | Promastigote | 133.42, 44.18 and 69.69 | 27.51, 9.11 and 14.37 | |||
| Annona purpurea | Bark Seed Leave |
Methanolic extract Aqueous extract Hydroalcoolic fraction |
L. donovani L. panamensis |
Promastigote | 113.24 28.57 289.0 0.961 |
(Brígido et al. 2020) | |
| Annona spinescens |
Bark Root |
Annonaine Liriodenine |
L. braziliensis L. amazonensis L. donovani L. braziliensis |
Promastigote |
188.45 94.22 376.91 363.29 |
IC100 50.0 25.0 100.0 100.0 |
(Emerson F. Queiroz et al. 1996) |
| Annona squamosa | leave | O-methylarmepavine C37 trihydroxy adjacent bistetrahydrofuran acetogenin |
L. chagasi | Promastigote and Amastigote | 71.16 and 77.58 42.44 and 40.67 |
23.3 and 25.4 26.4 and 25.3 |
(Vila-Nova et al. 2011) |
| Annona senegalensis | leave Stem |
Ethanolic extract | L. donovani | Promastigote | 10.8 27.8 |
(Ohashi et al. 2018; Brígido et al. 2020) | |
| Annonaceae species | Used Material | Substances/Extracts | Leishmania spp. | Parasite form | IC50 | Ref. | |
| µM | µg/ml | ||||||
| Bocageopsis multiflora | leave | Essential oil | L. amazonensis | Promastigote | 14.6 | (Oliveira et al. 2014) | |
| Duguetia furfuracea | bark | Alkaloid extract Duguetine Duguetine β-N-oxide Dicentrinone N-methyltetrahydropalmatine N-methylglaucine |
L. braziliensis | Promastigote | 16.32 4.32 0.11 0.01 17.03 4.88 |
(da Silva et al. 2009) | |
| Duguetia lanceolata | leave | Glaucine | L. infatum | Amastigote and Promastigote |
21.10 and >281.37 | 7.5 and >100.0 | (Dantas et al. 2020) |
| Enantia chlorantha | stem bark | Aqueous extract | L. infatum | Promastigote | 10.08 | (Olivier et al. 2015) | |
| Guatteria amplifolia | leave | Xylopine Nornuciferine |
L. mexicana and L. panamensis | Promastigote | 3.0 and 6.0 14.0 and 28.0 |
(Montenegro et al. 2003) | |
| Guatteria australis | leave | Essential oil | L. infatum | Promastigote | 30.71 | (Siqueira et al. 2015) | |
| Guatteria boliviana | bark | Ethanolic extract Puertogaline A Puertogaline B Sepeerine |
L. amazonensis, L. braziliensis and L. donovani |
Promastigote |
177.74 177.74 168.15 |
100.0 100.0 100.0 100.0 |
(Mahiou et al. 2000a) |
| Guatteria dumetorum | leave | Cryptodorine Normantenine |
L. mexicana and L. panamensis | Promastigote | 3.0 and 6.0 24.0 and 15.0 |
(Montenegro et al. 2003) | |
| Guatteria latifolia | branch | Crude extract Buthanolic fraction 1 Buthanolic fraction 2 |
L. amazonensis | Promastigote and Amastigote | 51.7 and 30.5 25.6 and 10.4 16.0 and 7.4 |
(Ferreira et al. 2017) | |
| Greenwayodendron suaveolens | Fruit, leave, root bark and stem bark | Dichlorometahne fraction rich in alkaloids Petroleum ether fraction rich in lipids and waxes Methanolic fraction rich in steroids and terpenes |
L. infatum | Promastigote | 24.05, 34.56, 0.63 and 20.32 24.05, 8.0, 27.27 and 5.04 40.32, 32.46, 7.51 and 6.82 |
(Muganza et al. 2016) | |
| Leave, root, stem bark | Crude ethanolic extract | 43.07, 8.11 and 24.05 | |||||
| Stem bark | Polycarpol Dihydropolycarpol Polyathenol |
3.2 8.0 8.1 |
|||||
| Annonaceae species | Used Material | Substances/Extracts | Leishmania spp. | Parasite form | IC50 | Ref. | |
| µM | µg/ml | ||||||
| Isolona hexaloba | Leave Root bark Stem bark |
Aqueous extract Methanolic extract Dichloromethane fraction Dichloromethane fraction |
L. infatum | Promastigote | 2.0 6.35 6.96 8.0 |
(Musuyu Muganza et al. 2015) | |
| Polyathia macropoda | Stem bark | (4S,9R,1OR) methyl 18- carboxy-labda-8, 1 3(E)-diene-15-oate |
L. donovani | Promastigote | 0.75 | (Richomme et al. 1991) | |
| Polyathia suaveolens | stem bark | Methanolic extract | L. infatum | Promastigote | 1.8 | (Lamidi et al. 2005) | |
| Porcelia macrocarpa | Seeds | Docos-13-yn-21-enoic acid 3-hydroxy-4-methylene-2-(eicos-11’-yn-19’-enyl)but-2-enolide 3-hydroxy-4-methylene-2-(octadec-9’-yn-17’-enyl)but-2-enolide 3-hydroxy-4-methylene-2-(hexadec-7’-yn-15’-enyl)but-2-enolide (2S,3R,4R)-3-hydroxy- 4-methyl-2-(eicos-11’-yn-19’-enyl)butanolide Miltefosine (positive control) |
L. infantum | Amastigotes | 48.5 9.2 10.4 11.0 29.9 17.8 |
(Brito et al. 2021) | |
| Porcelia macrocarpa | Seeds | (2S,3R,4R)-3-hydroxy- 4-methyl-2-(n-eicos-11’-yn-19’-enyl)butanolide (1) (2S,3R,4R)-3-hydroxy-4-methyl-2-(n-eicos-11’- ynyl)butanolide (2) Mixture of 1 and 2 2:1 1:1 1:2 |
L. infantum | Amastigotes |
29.9 Non active 8.4 13.6 19.4 |
(Brito et al. 2022) | |
| Annonaceae species | Used Material | Substances/Extracts | Leishmania spp. | Parasite form | IC50 | Ref. | |
| µM | µg/ml | ||||||
| Porcelia macrocarpa | Seeds | (2S,3R,4R)-3-hydroxy-4-methyl-2-(n-eicosyl)butanolide (3) | L. infantum | Amastigotes | Non active | (Brito et al. 2022) | |
| Raimondia monoica | leave | (–)-argentilactone (6S)-(50-oxohepten-10E,30Edienyl)- 5,6-dihydro-2H-pyran-2-one (6R)- (50-oxohepten-10Z,30E-dienyl)-5,6-dihydro-2H-pyran-2-one |
L. panamensis | Promastigote | 51.47 9.2 2.03 |
10.0 1.9 0.42 |
(Carmona et al. 2003) |
| Rollinia emarginata | stem bark |
Hexanic extract Dichloromethane extract Methanolic extract Rollidecin B Rolliniastatin-1 Lirioresinol B Squamocin Liriodenine Sylvaticin |
L. braziliensis, L. amazonensis and L. donovani |
Promastigote |
78.25 8.02 >239.0 8.02 18.16 15.65 |
IC100 >100.0 100.0 >100.0 50.0 5.0 >100.0 5.0 5.0 10.0 |
(Février et al. 1999) |
| Rollinia exsucca | stem | Hexane extract |
L. amazonensis, L. braziliensis and L. donovani |
Promastigote | 20.8 | (Osorio et al. 2007) | |
| Rollina pittieri | leave | Hexane extract Ethyl acetate extract Metanolic extract |
L. amazonensis, L. braziliensis and L. donovani |
Promastigote | 12.6, 10.7 and 10.7 20.8 19.7, 31.4 and 43.8 |
(Osorio et al. 2007) | |
| stem | Hexane extract Ethyl acetate extract |
13.5, 15.1 and 15.1 20.8, 25.0 and 19.7 |
|||||
| Unonopsis buchtienii | Stem bark |
Petroleum ether extract Dichloromethane extract O-methylmoschatoline Lysicamine Fraction containing Unonopsine |
L. major and L. donovani | Promastigote |
155.61 85.82 |
IC100 50.0 100.0 50.0 25.0 25.0 |
(Waechter et al. 1999) |
| Annonaceae species | Used Material | Substances/Extracts | Leishmania spp. | Parasite form | IC50 | Ref. | |
| µM | µg/ml | ||||||
| Unonopsis buchtienii | Stem bark | β-Sitosterol Stigmasterol Liriodenine |
L. major and L. donovani | Promastigote | >241.13 >242.30 11.33 |
>100.0 >100.0 3.12 |
(Waechter et al. 1999) |
| Unonopsis duckei | Twigs, barks and leaves | Alkaloidal fraction | L. amazonensis | Promastigote | 155.61, 32.16 and 4.0 | (da Silva et al. 2012) | |
| Unonopsis guatteriodes | Twigs, barks and leaves | Alkaloidal fraction | L. amazonensis | Promastigote | 1.07, 1.90 and 2.79 | (da Silva et al. 2012) | |
| Uvaria afzelii | root | Bigervone | L. donovani and L. major | Promastigote | 38.9 and 44.4 | (Okpekon et al. 2015) | |
| Uvaria klaineana | stem | Klaivanolide | L. donovani | Promastigote | 1.75 | (Akendengue et al. 2002) | |
| Xylopia aromatica | leave | Methanolic extract |
L. amazonensis, L. braziliensis and L. donovani |
Promastigote | 20.8 | (Osorio et al. 2007) | |
| Xylopia discreta | Leave | Ethanol extract Ether petroleum extract Acetate extract Methanol extract Essential oil |
L. panamensis Promastigote |
25.0 50.0 50.0 37.5 6.25 |
(López et al. 2009) | ||
| seed | Ethanol extract | 6.25 | |||||
| Xylopia excellens | leave | 7β-O-β-D-glucopyranoside-ent-kaur-16-ene | L. amazonensis | Promastigote | 33.74 | 15.23 | (Christopher et al. 2018) |
| Xylopia parviflora | roots | Dichloromethane extract | L. donovani | Amastigote | 5.01 | (Bapela et al. 2017) | |
3.5. Cytotoxic
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaxagorea dolichocarpa | Stem barks |
Extract Ad-EtOH (Ethanolic) Ad-Hex (Hexanic) Ad-CHCl3 (Chloroform) Ad-AcOEt (Ethyl acetate) |
Human tumor cell lines, including OVCAR-8 (ovarian), SF-295 (brain) and HCT-116 (colon). | OVCAR-8 | SF-295 | HCT-116 | (Pinheiro et al. 2016) | |||||||||||||||||||||||||||||||
| 53.94 44.31 52.51 −5.38 |
65.49 62.68 58.82 0.09 |
50.19 89.02 67.15 3.40 |
||||||||||||||||||||||||||||||||||||
|
Annona cacans Warm |
The fruits and leaves |
PHME-AC (pulp extract) PEAF-AC (ethyl acetate fraction) Acetogenin – PAC-1 Positive control Doxorubicin chloride (0.025–25 lg/mL) |
MCF-7 (breast) OVCAR-3 (ovarian) K-562 (leukemia) |
GI50 - lg/mL | (Volobuff et al. 2019) | |||||||||||||||||||||||||||||||||
| MCF-7 | OVCAR-3 | K-562 | ||||||||||||||||||||||||||||||||||||
| - 11.3 - |
8.8 5.68 6.4 |
6.10 7.84 - |
||||||||||||||||||||||||||||||||||||
| Annona crassiflora | Leaves |
Crassiflorine Xylopine Stephalagine Doxorubicin (Positive control) |
Cells line: HCT-116 – Colon carcinoma MCF-7 – Breast |
IC50 µM | (Peña-Hidalgo et al. 2021) | |||||||||||||||||||||||||||||||||
| HCT-116 | MCF-7 | |||||||||||||||||||||||||||||||||||||
|
143.4 30.2 48.5 0.07 |
Not determined 32.9 Not determined 0.15 |
|||||||||||||||||||||||||||||||||||||
| Annona Mucosa | Leaves and Fruits |
Fraction Hexane Extrat (L); Dichloromethane extract (L); Methanol extract (L); |
Cytotoxicity was studied after 96 h incubation of peritoneal macrophages with concentrations ranging from 6 to 100 µg.mL-1 of each extract and liriodenine. Results are expressed as 50% lethal concentrations (LC50) |
Cytotoxicity LC50 (µg.mL-1) + SEMc | LC50/ IC50 | (De Lima et al. 2012) | ||||||||||||||||||||||||||||||||
| PH8 | M2903 | |||||||||||||||||||||||||||||||||||||
| 62.63 ± 4.10 24.07 ± 4.02 29.41 ± 0.89 |
62.63 ± 4.10 24.07 ± 4.02 29.41 ± 0.89 |
2.58 2.58 1.03 |
||||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Annona Mucosa | Leaves and Fruits |
Fraction Hexane extrat (S); Methanol extrat (S); Compounds Liriodenine Pentamiddine |
Cytotoxicity was studied after 96 h incubation of peritoneal macrophages with concentrations ranging from 6 to 100 µg.mL-1 of each extract and liriodenine. Results are expressed as 50% lethal concentrations (LC50) |
Cytotoxicity LC50 (µg.mL-1) + SEMc | LC50/ IC50 | (De Lima et al. 2012) | ||||||||||||||||||||||||||||||||
| PH8 | M2903 | |||||||||||||||||||||||||||||||||||||
| 262.33 ± 5.81 139.00 ± 3.13 19.11 ± 1.6 51.99 ± 0.58 |
262.33 ± 5.81 139.00 ± 3.13 19.11 ± 1.6 51.99 ± 0.58 |
5.93 2.98 13.36 742.71 |
||||||||||||||||||||||||||||||||||||
| Annona senegalensis Pers. | The bark |
Fractions MeOH Extract Compounds Adriamicina |
BST (brine shrimp lethality test) Human solid tumor cell lines: A-549 (lung carcinoma) MCF-7 (breast carcinoma) HT-29 colon (adenocarcinoma) A-498 (kidney carcinoma) PC-3 (prostate adenocarcinoma) PACA-2 (pancreatic carcinoma) |
ED50 (µg/mL) | (Fatope et al. 1996) | |||||||||||||||||||||||||||||||||
| MeOH Extract | Adriamicina | |||||||||||||||||||||||||||||||||||||
| LC50 <1,0 <10-2 <10-2 1,0 <10-2 <10-2 <10-2 |
- - 1,85 × 10-1 4,37 × 10-2 1 × 10-2 2,23 × 10-2 2,05 ×10-2 |
|||||||||||||||||||||||||||||||||||||
| Anonna squamosa | Mature leaves and fruits |
Leaves aqueous extract (LAq) Seeds aqueous extract (SAq) |
Vero cell lines, (Vero, ATCC-CL 81) kindly provided by Instituto Butantan (São Paulo, Brazil), were used to perform cytotoxic. | Cytotoxicity Assays | (Silva et al. 2016) | |||||||||||||||||||||||||||||||||
| Tree Age | CC50 (mg.mL-1) | |||||||||||||||||||||||||||||||||||||
| 4 14 4 14 |
0.32 0.42 1.43 1.51 |
|||||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Anonna squamosa | Mature leaves and fruits |
Seeds hexane extract (SHex) |
Vero cell lines, (Vero, ATCC-CL 81) kindly provided by Instituto Butantan (São Paulo, Brazil), were used to perform cytotoxic. | Cytotoxicity Assays | (Silva et al. 2016) | |||||||||||||||||||||||||||||||||
| Tree Age | CC50 (mg.mL-1) | |||||||||||||||||||||||||||||||||||||
| 4 14 |
0.49 0.40 |
|||||||||||||||||||||||||||||||||||||
| Annona vepretorum | Stem bark |
Compounds ent-3β-hydroxy-kaur-16-en-19-al ent-3β,19-dihydroxy-kaur-16-eno ent-3β-hydroxy-kaur-16-eno ent-3β-acetoxy-kaur-16-eno ent-3β-hydroxy-kaurenoic acid Kaurenoic acid Positive Control Doxorubicin |
Cytotoxic activities towards tumor and non-tumor cells lines were investigated for compounds 1–6 with Tumor cells: B16-F10, Hep-G2, HL-60, K562 and non-tumor cells: PBMC | IC50 in µg/mL (µM) | (Dutra et al. 2014) | |||||||||||||||||||||||||||||||||
| B16-F10 | Hep-G2 | HL-60 | K562 | PBMC | ||||||||||||||||||||||||||||||||||
| 21.02 >25 19.12 >25 >25 16.56 2.30 |
15.50 >25 19.38 >25 >25 15.33 0.23 |
9.92 >25 9.86 >25 24.21 13.33 0.83 |
2.49 >25 2.94 >25 20.21 21.92 0.68 |
7.20 8.93 6.49 >25 >25 24.41 5.09 |
||||||||||||||||||||||||||||||||||
| Annona vepretorum | Leaves |
Extract Av-MeOH Av-HexC Av-Hex |
Human tumor cell lines were plated in 96-well plates: HCT-116, SF-295, HL-60, Sarcoma-180. The concentration that caused 50% cell growth inhibition (IC50) was determined from the concentration-response curves by non-linear regression with a confidence interval of 95%. |
Cell proliferation inhibition (%) | (Araújo et al. 2017) | |||||||||||||||||||||||||||||||||
| HCT-116 | SF-295 | HL-60 | Sarcoma-180 | IC50 (µM) | ||||||||||||||||||||||||||||||||||
| 98.16 ±0.92 29.96 ±1.60 56.04 ±21.0 |
63.98 ±4.84 86.54 ±3.31 65.43 ±6.52 |
82.23 ±4.84 17.11 ±7.34 55.85 ±3.56 |
82.34 ±1.36 79.43 ±4.39 86.24 ±1.09 |
2.81 ±0.41 4.87 ±0.83 45.82 ±9.07 |
||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Annona vepretorum | Leaves |
Av-CHCl3 AV-AcOEt Av-H2O |
Human tumor cell lines were plated in 96-well plates: HCT-116, SF-295, HL-60, Sarcoma-180. The concentration that caused 50% cell growth inhibition (IC50) was determined from the concentration-response curves by non-linear regression with a confidence interval of 95%. |
HCT-116 | SF-295 | HL-60 | Sarcoma-180 | IC50 (µM) | (Araújo et al. 2017) | |||||||||||||||||||||||||||||
| 74.28 ±0.25 17.8 7±6.45 9.52 ±11.68 |
82.05 ±24.67 27.98 ±5.16 6.72 ±1.25 |
29.79 ±1.82 68.72 ±38.28 -8.20 ±3.33 |
81.32 ±6.79 63.15 ±6.57 78.57 ±5.91 |
2.88 ±1.39 22.82 ±3.76 71.18 ±1.69 |
||||||||||||||||||||||||||||||||||
| Artabotrys zeylanicus | Not specified |
Compounds N-methoxynorcepharadione A (1) Atherospennidine (2) Positive control Camptothecin |
Cytotoxicity assessed by IC50 by assay with cells: RS 322YK (rad52Y) RS 321N RS 188N (rad+) P-388 (wild-type) P-388 (Camptothecin resistant) |
IC50 (µg/ml) | (Kithsiri Wijeratne et al. 1995) | |||||||||||||||||||||||||||||||||
| 1 | 2 | Camptothecin | ||||||||||||||||||||||||||||||||||||
| 2.16 1.20 >200 159 1.12 |
16 27 >50 Not tested Not tested |
0.6 - 100 0.012 >20 |
||||||||||||||||||||||||||||||||||||
| Asimina triloba | Seeds |
Acetogenins 1 2 Adramicyn |
Cytotoxicity tests against human tumor cell lines: A-549 (human lung carcinoma), MCF-7 (human breast carcinoma), HT-29 (human colon adenocarcinoma), A-498 (human kidney carcinoma), PC-3 (human prostate adenocarcinoma) and MIA PaCa-2 (human pancreatic carcinoma). | Human cancer cell line (ED50 µg/ml) | (Woo et al. 1999) | |||||||||||||||||||||||||||||||||
| BST LC50 | A-549 | MCF-7 | HT-29 | A-498 | PC-3 | MIA PaCa-2 | ||||||||||||||||||||||||||||||||
| 0.131 0.00429 Not tested |
0.00439 0.00165 0.0174 |
0.00211 0.00169 0.440 |
2.09 0.440 0.0116 |
2.78 2.19 0.0116 |
2.28 1.06 0.0461 |
0.000039 0.000028 0.00781 |
||||||||||||||||||||||||||||||||
|
Desmopsis bibracteata Desmopsis macrocarpa |
Leaves | Essential oil | Human MDA-MB-231 breast adenocarcinoma cells and Human Hs 578T breast ductal carcinoma cells. | % Kill at 100 µg/mL | (Palazzo et al. 2009) | |||||||||||||||||||||||||||||||||
| MDA-MB-231 | Hs 578T | |||||||||||||||||||||||||||||||||||||
| 99.3 (0.7) 53.0 (9.6) |
100 8.2 (14.0) |
|||||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Dasymaschalon blumei | The combined leaves and twigs Stems |
Extract: Acetate de ethyl extract Acetate de ethyl extract Compounds: 3,5-diydroxy-2,4-dimethoxyaristolactam Aristolactam BI Goniopedaline Griffithinam Oxodiscoguattine Dicentrinone Duguevalline Positive control Elipticine |
Cell culture: P-388 (mouse lymphoid neoplasm), KB (human epidermoid carcinoma in the mouth), Col-2 (human colon cancer), MCF-7 (human breast cancer), Lu-1 (human lung cancer), ASK (rat glioma), Hek 293 (noncancerous human embryonic kidney cell). The Hek 293 cell assay was used as a primary assay for assessing the specificity of an anticancer agent toward cancer cell lines in comparison with the normal mammalian cell. |
ED50 values (μg/ml) | (Chanakul et al. 2011) | |||||||||||||||||||||||||||||||||
| P-388 | KB | Col-2 | MCF-7 | Lu-1 | ASK | Hek 293 | ||||||||||||||||||||||||||||||||
| <4 <4 2.13 11.18 2.59 13.82 0.60 10.28 9.43 0.65 |
<4 4.12 2.97 - 1.98 - 2.30 4.56 - 0.62 |
17.38 - - - - - 0.91 7.34 - 0.65 |
- - - 3.60 9.45 - 2.74 9.05 19.11 0.69 |
16.46 14.04 - - - - 0.76 4.0 - 0.17 |
- - 3.04 - 12.76 - 2.11 10.20 - 0.61 |
- - 14.75 - 17.97 - 1.6 3.76 - 0.56 |
||||||||||||||||||||||||||||||||
|
Diclinanona calycina |
Barks |
Compounds Thalifoline (S)-(+)-Reticuline 1S,2R-Reticuline Nβ-oxide 1S,2S-Reticuline Nα-oxide Bisnorargemonine Isochamanetin Dichamanetin Uvarinol + Isouvarinol Doxorubicin (Positive control) |
Cancer cells HL-60 – Human promyelocytic leukemia MCF-7 – Breast adenocarcinoma HepG2 – hepatocellular carcinoma HCT116 – colon carcinoma Non-cancerous cell: MRC-5 – Human lung fibroblast |
µM | (Costa et al. 2021) | |||||||||||||||||||||||||||||||||
| HL-60 | MCF-7 | HCT116 | HepG2 | MRC-5 | ||||||||||||||||||||||||||||||||||
| Not determined Not determined Not determined Not determined Not determined Not determined 15.78 9.74 0.04 |
Not determined Not determined Not determined Not determined Not determined Not determined 23.59 >25 3.08 |
>25 >25 >25 >25 >25 >25 18.99 17.31 0.85 |
20.08 22.54 23.11 >25 >25 19.79 >25 >25 2.05 |
>25 >25 >25 >25 >25 24.69 >25 >25 3.19 |
||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Duguetia chrysocarpa | Stem barks |
Dc-EtOH (Ethanolic) Dc-Hex (Hexanic) Dc-CHCl3 (Chloroform) Dc-AcOEt (Ethyl acetate) |
Human tumor cell lines, including OVCAR-8 (ovarian), SF-295 (brain) and HCT-116 (colon). | OVCAR-8 | SF-295 | HCT-116 | (Pinheiro et al. 2016) | |||||||||||||||||||||||||||||||
| 49.65 18.39 42.24 13.01 |
43.95 34.84 63.17 30.99 |
60.16 15.61 59.35 9.76 |
||||||||||||||||||||||||||||||||||||
|
Guatteria costaricensis Guatteria diospyroides Guatteria oliviformis |
Leaves | Essential oil | Human MDA-MB-231 breast adenocarcinoma cells and Human Hs 578T breast ductal carcinoma cells. | % Kill at 100 µg/mL | (Palazzo et al. 2009) | |||||||||||||||||||||||||||||||||
| MDA-MB-231 | Hs 578T | |||||||||||||||||||||||||||||||||||||
| 54.6 (5.7) 98.8 (1.2) 100 |
0 21.1 (8.2) 35.6 (1.9) |
|||||||||||||||||||||||||||||||||||||
| Guatteria megalophylla Diels | Leaves |
Essential oil (EO) Positive Control Doxorubicin (DOX) 5-Fluorouracil (5-FU) |
Toxicity Assays with Human cancer cell lines: HL-60 Promyelocytic leukemia; MCF-7 Breast adenocarcinoma; Cal27 Oral squamous cell carcinoma; HSC-3 Oral squamous cell carcinoma; HepG2 Hepatocellular carcinoma; HCT116 Colon carcinoma and Human non-cancer cell line: MRC-5 Lung fibroblast. | IC50 (µg/mL) | (Costa et al. 2020) | |||||||||||||||||||||||||||||||||
| HL-60 | MCF-7 | CAL-27 | HSC-3 | Hep-g2 | HCT116 | MRC-5 | ||||||||||||||||||||||||||||||||
| 12.51 0.02 1.85 |
33.45 6.16 10.13 |
7.58 1.09 2.56 |
14.90 0.86 1.01 |
21.62 0.02 13.71 |
30.07 0.02 0.53 |
29.85 3.32 5.96 |
||||||||||||||||||||||||||||||||
| Miliusa balansae | Leaves and branches |
Flavonoids Ombuine Crysosplenol B Pachypodol Crysosplenol C Control Eliptin (Sigma) |
Cell culture: IC50 (µg/mL) - KB (Human Epidermoide Carcinom); - Hep-G2 (Hepatoma G2); - RD (Rhabdosarcoma). |
KB | Hep-G2 | RD | (Huong et al. 2005) | |||||||||||||||||||||||||||||||
|
˃ 5 4.6 0.7 4.3 0.002 |
1.5 0.93 0.55 0.57 0.001 |
˃ 5 ˃ 5 3.01 2.09 0.001 |
||||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Neouvaria acuminatissima | Stem bark |
Compounds Acuminolide (1) 17-O-Acetylacuminolide (2) Spiroacuminolide (3) Positive Control Doxorubicin |
Screened for cytotoxicity against a panel of human cancer cell lines and murine P388 cells, according to established protocols. 14 ED50 values of >4 ~tg/ml were regarded as negative. Among the cell lines represented, a human lung cancer cell line (Lul) was used to guide the fractionation against the HT-29 human colorectal and KB human epidermoid carcinoma models, Doxorubicin was run as a positive control. | ED50 Values: tg/ml | (Ik-Soo Lee et al. 1995) | |||||||||||||||||||||||||||||||||
| Compounds I and 2 were broadly cytotoxic, exhibiting ED50 values, ranging from 10 -~ to 10 ° ttg/ml in several cell lines. With the human cell lines, the most potent activity was observed with melanoma (Mel2) (ED50: 0.7 ttg/ml) and prostate (LNCaP) (ED50: 0.8 ~tg/ml) cells for compounds 1 and 2, respectively. Compound 3 was not significantly active for any of the cancer cell lines tested. Acuminolide (1) was inactive when tested in vivo against a HT-29 human colorectal xenograft model in nude mice at 40-60 mg/kg (maximum tolerated dose 70 mg/kg). 17-O-Acetylacuminolide (2) showed no significant activity when tested in vivo against a KB human epidermoid carcinoma murine model at 110 mg/kg. | ||||||||||||||||||||||||||||||||||||||
| Polyalthia crassa | Leaves and twigs |
Compounds (+)-Crassalactone A (+)-Crassalactone B (+)-Crassalactone C (+)-Crassalactone D Aristolactam AII (+)-tricinnamate Positive control Ellipticine |
Cytotoxicity assays of compounds 1-4, 10, and 11 were performed employing the colorimetric method. | Cell line (ED50 µg/mL) | (Tuchinda et al. 2006) | |||||||||||||||||||||||||||||||||
| P-388 | KB | Col-2 | BCA-1 | Lu-1 | ASK | |||||||||||||||||||||||||||||||||
| 0.18 3.8 >5 1.1 2.7 3.1 0.52 |
1.7 >5 >5 3.3 >5 >5 0.65 |
1.9 >5 >5 4.0 >5 >5 0.53 |
0.92 >5 >5 3.2 >5 >5 0.53 |
1.9 >5 >5 >5 >5 >5 0.56 |
1.6 >5 >5 3.1 >5 >5 0.60 |
|||||||||||||||||||||||||||||||||
| Polyalthia jucunda | Dried and powdered stem bark. |
Compound 4-Hydroxy-4,7-dimethyl-α-tetralone 4,5-Dihydroblu- menol A N-trans-feruloyltyramine 24–Methy- lenelanosta-7,9(11)-dien-3-β,15α-diol |
The effects of compounds on the growth of the human tumor and non–tumor cell lines were evaluated according to the procedure adopted by the National Cancer Institute (USA) for the in vitro anticancer drug discovery |
GI50 (µM) | (Suedee et al. 2007) | |||||||||||||||||||||||||||||||||
| MCF-7 | MDA-MB-231 | SF-268 | NCL-H460 | MRC-5 | ||||||||||||||||||||||||||||||||||
| >150 >150 >150 19.3±1.2 |
>150 >150 >150 18.8±2.0 |
>150 >150 >150 21.8±0.6 |
>150 >150 >150 23.0±1.7 |
>150 >150 >150 40.3±3.4 |
||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Polyalthia longifolia var. pendula | Leaves |
16α-hydroxycleroda3,13-dien-15,16-olide 5-hydroxy-6- Methoxyonychine (-)-anonnaine |
Tested against four human cancer cell lines: AGS (gastric cancer cells), DLD1 (colon cancer cells), HepG2 (hepatoma cells), and HA59T (hepatoma cells). | IC50 (µM) | (Chen et al. 2000) | |||||||||||||||||||||||||||||||||
| AGS | DLD | HA597 | HepG2 | |||||||||||||||||||||||||||||||||||
| 26.9 >30 8.6 |
>30 >30 28.9 |
23.6 21.7 16.4 |
>30 >30 20.8 |
|||||||||||||||||||||||||||||||||||
| Polyalthia longifólia var. pendula | The bark samples |
Compounds 16(R&S)- 3,13Z-kolavadien-15,16-olide-2-one 16-hydroxycleroda- 3,13-dien-15,16-olide 16-hydroxycleroda-4(18),13-dien- 15,16-olide 16-oxocleroda-3,13(14)E-dien-15-oic acid methyl ester Solidagonal acid (4→2)-abeo- 16(R&S)-2,13Z-clerodadien-15,16-olide-3-al labd-13E-en-8-ol-15-oic acid Polylongine Liriodenine Lysicamine (+)-Stepharine (−)-Stepholidine N-trans-feruloyltyramine N-trans-p-coumaroyltyramine Positive control Doxorubicin |
Tested against four human cancer cell lines: MCF-7, MDA-MB-231, Hep-G2, Hep 38. | IC50 (µM) | (Chang et al. 2006) | |||||||||||||||||||||||||||||||||
| MCF-7 | MDA-MB-231 | Hep-G2 | Hep38 | |||||||||||||||||||||||||||||||||||
| 18.28 14.42 10.43 14.34 18.12 11.89 - 10.41 4.46 8.94 9.40 16.56 25.53 17.35 0.04 |
4.50 8.29 3.22 13.22 14.67 11.65 - 9.94 10.28 16.75 9.90 - 25.54 - 0.32 |
2.88 4.42 3.35 - - 2.36 18.33 - - - - - 21.17 - 0.18 |
2.96 2.83 1.97 - - 8.94 15.40 - - - - - 24.98 - 0.23 |
|||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
| Pseuduvaria trimera (Craib) | Leaves And twigs |
8-hydroxyartabonatine C Ouregidione Positive control Doxorubicin |
The human hepatocellular carcinoma HepG2 and breast cancer MDA-MB231 cells. | IC50 | (Sesang et al. 2014) | |||||||||||||||||||||||||||||||||
| HepG2 | MDA-MB231 | |||||||||||||||||||||||||||||||||||||
| Mean | SD | Mean | SD | |||||||||||||||||||||||||||||||||||
| 26.36 12.88 2.21 |
±5.18 ±2.49 ±1.72 |
64.75 67.06 1.83 |
±4.45 ±3.5 ±0.09 |
|||||||||||||||||||||||||||||||||||
| Uvaria pandensis | Leaves |
Pandensenol D Pandensone A (8’α,9’β-dihydroxy)-3-farnesylindole (6’,7’-dihydro-8’α,9’β-dihydroxy)- 3-farnesylindole |
Cell culture: MCF-7 (breast cancer cells) |
EC50 (µM) | (Maeda et al. 2022) | |||||||||||||||||||||||||||||||||
|
>523.4 349.8 117.1 >563.4 | ||||||||||||||||||||||||||||||||||||||
| Unonopsis costariensis | Leaves |
Essential oil |
Human MDA-MB-231 breast adenocarcinoma cells and Human Hs 578T breast ductal carcinoma cells. |
% Kill at 100 µg/mL | (Palazzo et al. 2009) | |||||||||||||||||||||||||||||||||
| MDA-MB-231 | Hs 578T | |||||||||||||||||||||||||||||||||||||
| 100 | 17.3 (10.3) | |||||||||||||||||||||||||||||||||||||
| Xylopia aethiopica | Fruits |
Aqueous extract Aqueous extrat in gold nanoparticles Positive control Fulvestrant 5-Fluororacil |
Cell culture: IC50 (µg/mL) - MCF-7 (Bresat cancer); - MDA-MB-231; - Caco-2 cells. |
MCF-7 | MDA-MB-231 | Caco-2 | (Anadozie et al. 2021) | |||||||||||||||||||||||||||||||
| 171.3 >200 >120 nM - |
94.5 141.4 <120 nM - |
199.8 >200 - >100 |
||||||||||||||||||||||||||||||||||||
| Xylopia langsdorffiana | Stems |
Compound ent-7r-Acetoxytrachyloban-18-oic acid |
Cytotoxic activity of compound 1 was evaluated against V79 cells and rat hepatocytes using the MTT method. | IC50 (µM) | (Tavares et al. 2006) |
|||||||||||||||||||||||||||||||||
| V79 | K562 | |||||||||||||||||||||||||||||||||||||
| 224 and 231 µM | 200 µM | |||||||||||||||||||||||||||||||||||||
| Annonaceae species | Used material | Substances/ Extracts | Methodology | Cell Proliferation inhibition (%) | Ref. | |||||||||||||||||||||||||||||||||
|
Xylopia aethiopica (Dunal) A. Rich; Xylopia paviflora A. Rich (Benth.); |
Fruits |
Essential oil Composition: Monoterpenes hydrocarbons; Oxygenated monoterpenes; Sesquiterpene hydrocarbons; Oxygenated sesquiterpenes. |
The cytotoxic activity of all essential oils was evaluated on human breast cancer (MCF-7) and normal epithelial (ARPE-19) cell lines using the MTT assay based on cell viability. Cells were exposed to the oils at concentrations ranging from 0.1 to 2 μL/mL. | IC50 values μL/mL | (Bakarnga-Via et al. 2014) | |||||||||||||||||||||||||||||||||
| The six essential oils exerted cytotoxic activity against cancer (MCF-7) and normal cell lines (ARPE-19), with more pronounced effect on neoplastic cells in most cases. The highest selectivity was obtained with the essential oils of X. parviflora from Chad and Cameroon (5.87 and 5.54) which were more cytotoxic against MCF-7 than against normal cell line (ARPE-19) with IC50 values of 0.155 μL/mL and 0.166 μL/mL respectively | ||||||||||||||||||||||||||||||||||||||
| Xylopia laevigata | Leaves |
Compounds (-)-Roemerine (+)-Anonaine Lanuginosine (+)-Glaucine (+)-Xylopine Oxoglaucine (+)-Norglaucine (-)-Xylopinine (+)-Norpurpureine (+)-N-Methyllaurotetanine (+)-Norpredicentrine (+)-Discretine (+)-Calycinine (+)-Laurotetanine (+)-Reticuline (-)-Corytenchine (+)-Discretamine (+)-Flavinantine Positive Control Doxorubicin |
B16-F10, HepG2, K562 and HL-60 tumor cell lines were kindly donated by Hospital A.C. Camargo, São Paulo, Brazil. Cell viability was quantified using the Alamar Blue assay. | IC50 µg/mL (µM) | (Menezes et al. 2016) | |||||||||||||||||||||||||||||||||
| B16-F10 | HepG2 | HL60 | K562 | PBMC | ||||||||||||||||||||||||||||||||||
| NA 18.80 8.46 NA 3.77 19.14 8.48 NA 21.08 NA NA 16.15 22.17 NA NA NA 18.80 NA 0.08 |
NA 14.04 3.89 NA 1.87 NA 3.78 NA NA NA NA 7.89 NA NA 15.35 NA 14.04 NA 0.08 |
NA 10.09 7.81 NA 1.87 5.90 6.84 NA 10.11 NA NA 12.97 18.59 NA 23.81 NA 10.09 NA 0.09 |
NA 10.62 6.61 NA 3.12 12.48 7.84 NA 16.72 NA NA 14.85 NA NA NA NA 10.62 NA 0.15 |
NA NA 24.53 NA 4.08 10.25 6.70 NA 17.94 NA NA NA NA NA NA NA NA NA 2.47 |
||||||||||||||||||||||||||||||||||
3.6. NA - Not Active Instead (IC50 > 25µg/mL)Antitumor
| Annonaceae species | Used material | Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annona crassiflora | The leaves | A. crassiflora (AC) | To evaluate the antimutagenic/ chemopreventive activity through the Allium cepa test, we used 5, 10, and 15 mg/L of extract, and for the micronucleus test in the peripheral blood, we used the dose of 15 mg/kg. | Chromosomal aberrations | (Rocha et al. 2016) | ||||||||||||||||
| Treatment | Damage Reduction Percentages of mitotic index |
Total (values compared with the control group) |
Damage Reduction Percentages | ||||||||||||||||||
| Mutagenicity AC – 5mg/L AC – 10mg/L AC – 15mg/L Control group (distilled water) Positive control MMS (Methyl methanesulfonate) Antimutagenicity AC – 5mg/L Pre-treatment Simple simultaneous Simultaneous with pre-incubation Post-treatment Continuous AC – 10mg/L Pre-treatment Simple simultaneous Simultaneous with pre-incubation |
−1.62 −11.35 −29.46 3.51 - −31.35 −44.86 −2.53 −58.38 −37.03 −64.05 −15.95 −35.68 |
31 27 34 238 40 - - - |
- - - - - −66.17 72.72 100.5 89.39 86.36 75.75 33.33 93.93 |
||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Annona crassiflora | The leaves | A. crassiflora (AC) | To evaluate the antimutagenic/ chemopreventive activity through the Allium cepa test, we used 5, 10, and 15 mg/L of extract, and for the micronucleus test in the peripheral blood, we used the dose of 15 mg/kg. | Chromosomal aberrations | (Rocha et al. 2016) | ||||||||||||||||
| Treatment | Damage Reduction Percentages of mitotic index |
Total (values compared with the control group) |
Damage Reduction Percentages | ||||||||||||||||||
| AC – 10mg/L Post-treatment Continuous AC – 10mg/L Pre-treatment Simple simultaneous Simultaneous with pre-incubation Post-treatment Continuous |
−21.35 −37.03 45.95 −23.78 −16.49 −36.76 −44.59 |
- |
79.79 81.31 69.19 22.22 102.52 84.34 93.43 |
||||||||||||||||||
| Annona hypoglauca | Stem |
Crude extract Fraction hexane Dichloromethane/Methanol Fraction ethyl acetate Fraction butanol Total alkaloid fraction |
Human tumor cell lines (MCF-7, breast adenocarcinoma; KM-12, colon adenocarcinoma; RPMI-8226, multiple myeloma; PC-3, prostate carcinoma; SF-268 glioblastoma and NCI-H460, non-small lung-cell carcinoma). | MCF-7 | PC-3 | NCI-H460 | KM-12 | SF-268 | RPMI-8226 | (Rinaldi et al. 2017) | |||||||||||
| −32.80 NI 2.90 Not tested 11.90 −8.90 |
11.4 77.4 59.6 67.1 46.6 17.04 |
1.3 48.1 44.9 38.6 65.6 71.7 |
−2.5 NI NI NI NI 32.4 |
58.5 NI 93.7 77.4 NI 73.3 |
17.3 53.5 53.4 NI 83.8 NI |
||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Annona hypoglauca | Stem |
Fraction alkaloid 4.4 Fraction alkaloid 5 Fraction alkaloid 9 Positive control Doxorubicin |
Human tumor cell lines (MCF-7, breast adenocarcinoma; KM-12, colon adenocarcinoma; RPMI-8226, multiple myeloma; PC-3, prostate carcinoma; SF-268 glioblastoma and NCI-H460, non-small lung-cell carcinoma). | MCF-7 | PC-3 | NCI-H460 | KM-12 | SF-268 | RPMI-8226 | (Rinaldi et al. 2017) | |||||||||||
|
Not tested -11.60 -3.10 -16.31 |
-3.8 Not tested Not tested -50.0 |
-26.0 Not tested Not tested -46.2 |
11.7 -14.4 -8.4 -5.1 |
45.4 Not tested Not tested -34.0 |
29.7 Not tested Not tested -14.3 |
||||||||||||||||
| Annona leptopetala | Leaves | Essential oil Annona leptopetala leaves (ALO) Doxurubicin (DOX) Positive control |
The tumor cell lines used were: U251 – glioma, MCF-7 – breast, NCI/ADR-RES - multidrug-resistant ovarian, 786-0 – kidney, NCI-H460 – non-small cell lung cancer, PC-3 – prostate, OVCAR – ovarian, HT29 – colon and K562 – leukemia, and HaCaT human keratinocytes served as the normal cell line. Sarcoma 180 tumor cells were maintained in the peritoneal cavity of Swiss mice. | Total inhibition of cancer cells proliferation (µg/ml) | (Brito et al. 2018) | ||||||||||||||||
| Cell Lines | DOX | ALO | |||||||||||||||||||
| Glioma (U251) Breast (MCF-7) Ovary Multidrug Resistance Phenotype (NCI-ADR/RES) Kidney (786-O) Lung (NCI-H460) Prostate (PC-3) Ovary (OVCAR) Colon (HT-29) |
0.06 0.21 1.35 0.04 0.01 0.27 0.26 0.22 |
47.23 49.91 >250 101.52 75.53 45.12 >250 75.26 |
|||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Annona leptopetala | Leaves | Essential oil Annona leptopetala leaves (ALO) Doxurubicin (DOX) Positive control |
The tumor cell lines used were: U251 – glioma, MCF-7 – breast, NCI/ADR-RES - multidrug-resistant ovarian, 786-0 – kidney, NCI-H460 – non-small cell lung cancer, PC-3 – prostate, OVCAR – ovarian, HT29 – colon and K562 – leukemia, and HaCaT human keratinocytes served as the normal cell line. Sarcoma 180 tumor cells were maintained in the peritoneal cavity of Swiss mice. | Total inhibition of cancer cells proliferation (µg/ml) | (Brito et al. 2018) | ||||||||||||||||
| Cell Lines | DOX | ALO | |||||||||||||||||||
|
Leukemia (K-562) Skin (line of non-tumor cells) (HaCat) |
0.40 0.23 |
0.64 >250 |
|||||||||||||||||||
| Annona muricata | Leaf, Seed, Fruit, Pericarp, Twing, Root |
Acetogenins, extracts and fractions | This current review demonstrates A. muricata’s anticancer potential and other health-related benefits by providing insights into its bioactive chemical constituents as well as the in vitro and in vivo studies that have been carried out to elucidate the molecular mechanisms of action of these constituents. |
Review Article Acetogenins or other A. muricata-derived compounds could be tested as monotherapy or as sensitizers in combination with standard cancer treatments for cancer patients. |
(Rady et al. 2018) | ||||||||||||||||
| Annona squamosa | Seeds |
Compound Squamostatiin A |
The antitumor activities of 1–5 and standard control taxol against the growth of S180 and HepS in mice were measured by methods reported previously. | Treatment effects of annonaceous acetogenins in the Heps and S180 xenograft tumor model | (Chen et al. 2012) | ||||||||||||||||
| Treatment group | Dose (µg/kg) | HepS | S180 | ||||||||||||||||||
| Tumor weight (g: mean±SD) | Inhibition ratio (%) | Tumor weight (g: mean±SD) | Inhibition ratio (%) | ||||||||||||||||||
| Squamostatin A | 15 60 |
0.96±0.19 0.79±0.12 |
15.0 31.2 |
0.36±0.09 0.34±0.10 |
52.7 54.2 |
||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Annona squamosa | Seeds |
Compounds Squamostatiin E 4-deoxyannoreticuin Desacetyluvaricin Bullatacin Taxol Positive control |
The antitumor activities of 1–5 and standard control taxol against the growth of S180 and HepS in mice were measured by methods reported previously. | Treatment effects of annonaceous acetogenins in the Heps and S180 xenograft tumor model | (Chen et al. 2012) | ||||||||||||||||
| Treatment group | Dose (µg/kg) | HepS | S180 | ||||||||||||||||||
| Tumor weight (g: mean±SD) | Inhibition ratio (%) | Tumor weight (g: mean±SD) | Inhibition ratio (%) | ||||||||||||||||||
| Control group Taxol Squamostatin E 4-deoxyannoreticuin Desacetyluvaricin Bullatacin |
40 15 60 15 60 15 60 15 60 |
1.13±0.46 0.43±0.15 0.75±0.16 0.55±0.18 0.43±0.12 0.37±0.14 0.77±0.18 0.33±0.22 0.38±0.18 - |
- 62.2 34.2 51.3 61.5 67.3 32.5 70.9 63.4 - |
0.75±0.16 0.38±0.12 0.55±0.10 0.37±0.08 0.42±0.10 0.39±0.11 0.43±0.12 0.27±0.08 0.26±0.06 - |
- 48.9 27.2 51.1 43.9 48.3 42.3 63.9 65.8 - |
||||||||||||||||
| Annona squamosa | Fruits |
Compounds (-)-entkaur-16-en-19-oic acid (1) 16-α,17- dihydroxy-ent-kauran-19-oic acid (2) |
Cytotoxic activity using in vitro cultures of Dalton’s lymphoma cells as well as HeLa cells. Cytotoxicity was detected by the Trypan blue exclusion test and induction of apoptosis was evaluated by [3-(4,5-Dimethylthiazol-2- yl)-2,5 diphenyltetrazolium bromide] assay (MTS assay) and DNA ladder assay. The inhibitory concentration required for 50% cytotoxicity (IC50) was also determined. | (%) of cytotoxicity |
(Joy and Remani 2008) |
||||||||||||||||
| There was an increase in the percentage cytotoxicity with increasing concentrations of the fraction containing the compounds. However a slight decrease in activity with increasing incubation time was noted, which implies that the compound has very high cytotoxicity even after 24 h of incubation. Even at a concentration of 1.65 lg/ml, the compounds exhibited more than 50% cytotoxicity after 24 h. The cytotoxic activity for different time intervals is compared. The highest activity was noted after 24 h, which implies that the efficacy of secondary metabolites as cytotoxic agents at a low dose and a short duration. | |||||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
|
Annona vepretorum |
Leaves |
Essential Oil Spathulenol α-Phellandrene σ- Cymene α- Pinene 5-Fluorouracil Positive control |
The in vivo antitumour effect was evaluated in C57BL/6 mice inoculated with B16-F10 melanoma. Tumour cells (2 9 106 cells per 500 lL) were implanted subcutaneously into the left hind groin of mice. Animals were euthanized by cervical dislocation, and tumours were excised and weighed. Drug effects are expressed as the per cent inhibition of control. | IC50 (µg/mL) | (Bomfim et al. 2016) | ||||||||||||||||
| B16-F10 | HepG2 | K562 | HL-60 | PBMCs | |||||||||||||||||
| 9.90 7.81-12.55 11.67 9.76-13.96 15.44 6.54-36.42 >25 11.46 5.46-24.04 0.68 0.21-1.45 |
10.60 8.55-13.26 11.19 9.58-13.07 17.30 13.89-21.55 >25 13.05 9.79-17.38 0.04 0.01-1.22 |
8.43 5.48-12.97 3.79 1.48-9.70 >25 >25 14.00 10.56-18.55 0.15 0.01-1.86 |
6.14 4.15-9.12 11.38 8.46-15.31 20.18 16.91-24.08 >25 14.96 12.25-18.26 0.29 0.21-0.38 |
22.82 19.18-27.15 15.59 13.12-18.53 >25 >25 >25 14.00 9.83-23.31 |
|||||||||||||||||
| Asimina triloba | Seeds | Compounds Asimitrin 4-Hydroxytrilobin Adriamicyn Positive control |
In vitro cytotoxicity tests against human tumor cell lines s for A-549 (human lung carcinoma),26 MCF-7 (human breast carcinoma),27 HT-29 (human colon adenocarcinoma),28 A-498 (human kidney carcinoma),26 PC-3 (human prostate adenocarcinoma),29 and MIA PaCa-2 (human pancreatic carcinoma) | Human cancer cell line ED50 (µg/mL) | (Kim et al. 2005) | ||||||||||||||||
| Cell lines | Asimitrin | 4-Hydroxytrilobin | Adriamicyn | ||||||||||||||||||
| BST A-549 MCF-7 HT-29 A-498 PC-3 MIA PaCa-2 |
2.07x10-2 1.19 2.12 1.19x10-4 7.50x10-1 1.72x10-6 2.11x10-4 |
7.00x10-2 1.54 3.79 1.54x10-6 3.62x10-2 2.01x10-4 2.01x10-4 |
NT 6.22x10-4 9.53x10-1 2.87x10-2 2.86x10-3 5.77x10-2 6.10x10-3 |
||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Anaxagorea dolichocarpa Sprague & Sandwith | The stem bark | Alkaloids Compounds Eupolauramine (1) Sampangine (2) |
The cytotoxity was evaluated through the MTT reduction assay, which determines the number of living cells able to reduce the yellow dye 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to formazan. The determination of the 50% inhibition concentration for cell growth (IC50) of The human leukemic strain K562. | IC50 (µg/mL) | (Lúcio et al. 2011) | ||||||||||||||||
| The in vitro effects of eupolauramine (1) and sampangine (2) against the K562 cell line were determined in three experiments and in quadruplicate. Both compounds exhibited concentration-dependent inhibitory effect on the proliferation of K562 cells. The IC50 values were 18.97 (17.06–21.10) μg/mL and 10.95 (10.15–11.80) μg/mL respectively. | |||||||||||||||||||||
|
Duguetia furfuracea |
Bark |
Duguetine Duguetine β-N-oxide Dicentrinone N-methyltetrahydropalmatine N-methylglaucine Alkaloid Extract Doxorrubicin Positive control |
The cytotoxic potential of alkaloids was evaluated by the MTT assay. Against three human tumor cell lines: SF-295 (glyoblastoma), HCT-8 (colon cancer) and MDA/MB-435 (melanoma). | Percentage of inhibition (%) | (da Silva et al. 2009) | ||||||||||||||||
| HCT-8 (%) | SF-295 (%) | MDA/MB-435 (%) | |||||||||||||||||||
| 91.1±0.8 92.0±1.0 68.0±0.7 57.6±0.7 45.2±2.1 90.2±1.1 93.3±1.7 |
86.0±1.1 87.1±0.7 50.3±1.5 39.8±0.7 25.1±2.3 85.1±0.6 92.3±2.2 |
98.1±0.1 84.5±1.6 37.5±4.0 31.1±0.8 15.1±6.4 99.6±0.4 97.0±1.1 |
|||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Fissistigma cavaleriei | The dried roots |
5-methoxy-2-methylisoindolin-1-yl, 4-methoxyphenyl Doxorubicin Positive control |
Evaluation of the antiproliferative effect of compound 1 under study against cells: K562, S-180, A549. | IC50 (mol/L) | (Yang et al. 2012) | ||||||||||||||||
| K562 | S-180 | A549 | |||||||||||||||||||
|
2.54±0.22x10-5 1.16±0.14x10-6 |
7.26±0.24x10-5 1.61±0.11x10-7 |
8.76±0.18x10-5 8.12±0.12x10-7 |
|||||||||||||||||||
|
Guatteria autralis Guatteria ferruginea Guatteria latifolia Guatteria sellowiana |
The aerial parts | Essential Oils (EO) Doxorubicin Positive control |
The antiproliferative activity of the materials tested was evaluated using eight human tumor cell lines: U251 (central nervous system, CNS, glioma), MCF-7 (breast cancer) NCI-ADR/RES (ovarian tumor with multidrug resistance phenotype), 786-0 (kidney cancer), NCI-H460 (non-small-cell lung cancer), OVCAR-03 (ovarian carcinoma), HT-29 (colorectal cancer), and K562 (leukemia). Using the concentration-response curve for each cell line. | Total growth inhibition (TGI) [µg/ml] | (Santos et al. 2017) | ||||||||||||||||
| Cell lines | EO G. australis | EO G. ferruginea | EO G. latifolia | EO G. sellowiana | Doxorubicin | ||||||||||||||||
| U251 MCF-7 NCI-ADR/RES 786-0 NCI-H460 OVCAR-3 HT-29 K562 HaCat |
40.4 37.8 15.2 45.6 49.5 3.2 38.6 86.2 48.0 |
36.3 37.6 34.6 50.9 69.4 1.8 52.7 18.6 63.4 |
36.6 47.2 10.0 63.9 44.9 1.1 39.7 15.6 41.0 |
89.1 95.5 250 107.8 82.8 4.1 143.1 1.1 75.6 |
2.7 0.88 >25 3.1 >25 11.7 3.6 0.031 1.0 |
||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Guatteria elliptica R. E. Fries | Leaves |
Essential oil Spathulenol |
The antitumor against the normal cell line derived from mouse fibroblasts (BALB/c 3T3, ATCC CCL163) was tested using the MTS method. The IC50 values were used to determine the median lethal dose (LD50) for cell lines MCF-7 (human breast cancer) and PC-3 (human prostate cancer. | IC50 ± SE (µg/mL) | (Rajca Ferreira et al. 2018) | ||||||||||||||||
| PC-3 | MCF-7 | ||||||||||||||||||||
| 5.32 ± 0.35 2.25 ± 0.28 |
7.01 ± 0.23 5,38 ± 0.20 |
||||||||||||||||||||
| Miliusa balansae | Leaves and branches |
Ombuine Crysosplenol B Pachypodol Crysosplenol C Eliptin Positive Control |
Cell culture: - KB (Human Epidermoide Carcinom); - Hep-G2 (Hepatoma G2); - RD (Rhabdosarcoma). |
IC50 (µg/mL) | (Huong et al. 2005) | ||||||||||||||||
| KB | Hep-G2 | RD | |||||||||||||||||||
|
˃ 5 4.6 0.7 4.3 0.002 |
1.5 0.93 0.55 0.57 0.001 |
˃ 5 ˃ 5 3.01 2.09 0.001 |
|||||||||||||||||||
| Mitrephora glabra | Stem Bark | 4-epi-kaurenoic acid (1) Mitrekaurenone (2) Methylmitrekaurenate (3) Oropheic acid (4) Methylloropheate (5) Octadeca-9,11,13-triynoic acid (6) Oropheolide (7) 9,10-Dehydrooropheolide (8) |
The cytotoxicity measurements against the KB human oral epidermoid carcinoma: MCF-7 human breast carcinoma, NCI-H460 human large cell lung carcinoma, and SF-268 human astrocytoma. | IC50 µM | (Li et al. 2009) | ||||||||||||||||
| The ent-kaurane diterpenoids (1-3), all were inactive (IC50 values >10 µM); the diterpenoids of the ent-trachylobane class, reported previously from the same plant, were more potent in this regard. The polyacetylenes (4 and 6-8) gave IC50 values ranging from 10 to 40 µM. However, compound 5 was completely inactive, suggesting that the methyl ester diminishes cytotoxicity. The known alkaloid liriodenine (9) was the most cytotoxic against all four of the cell lines in which it was evaluated (IC50 value ∼5 µM). | |||||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Mitrephora thorelii | The aerial parts | 6a,16,18-Trihydroxycleroda-3(4),13(14)-dien-15,16-olide (1) 16-Hydroxycleroda-3(4),13(14)-dien-15,16-olide (2) Cyclophosphamide Positive control |
In vivo evaluation female mice (5–6 weeks old) of Kunming strain were purchased from Shanghai SLAC Laboratory Animal Co. (Shanghai, China). Murine hepatoma H22 is maintained by serial intraperitoneal passage in Kunming mice. H22 cells were subcutaneously implanted into Kunming mice at Diterpenes from M. thorelii 683 1 £ 106 cells/mouse. | IC50 mM | (Meng et al. 2007) | ||||||||||||||||
| Compounds 1 and 2 inhibited the proliferation of BEL-7402 cells in vitro with the IC50 values 44.6 and 20.1 mM, respectively. In vivo anti-tumor effect of compound 2 was further evaluated in murine hepatoma H22 model. It was found that compound 2 significantly inhibited the growth of hepatoma H22 with the percentage inhibition of 30.7% (P, 0.05 vs control). The alkylation agent cyclophosphamide served as a positive control (63.8%, P, 0.01 vs control). The mice were well tolerated towards compound 2, and no significant loss of body weight was observed compared with control group (P. 0.05, data not shown). | |||||||||||||||||||||
| Polyalthia evecta | Leaves |
Hexane fraction (F1) 500 (µg/mL) Chloroform fraction (F2) 500 (µg/mL) Ethylacetate fraction (F3) 500 (µg/mL) Dichloromethane fraction (F4) 500 (µg/mL) Ethanol fraction (F5) 500 (µg/mL) |
Determination the cytotoxicity of the samples in the cell model, neutral red (NR) uptake assay was used for identification of vital cells. The cytotoxicity assays were performed with HepG2 and Vero cells. | % Cytotoxicity | % Apoptotic cells in HepG2 | (Machana et al. 2012) | |||||||||||||||
| HepG2 | Vero | ||||||||||||||||||||
|
74.6 ± 1.6 24.3 ± 9.3 29.6 ± 8.8 24.0 ± 7.4 22.7 ± 8.8 |
54.2 ± 15.4 2.2 ± 3.8 32.5 ± 8.1 25.5 ± 8.1 7.6 ± 8.3 |
46.4 ± 2.6 9.8 ± 4.7 3.2 ± 1.6 2.9 ± 1.1 2.4 ± 1.9 |
|||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Polyalthia evecta | Leaves |
Methanol fraction (F6) 500 (µg/mL) Water fraction (F7) 500 (µg/mL) Hexane: water (500:100) Hexane: water (500:250) Hexane: water (500:500) Hexane: methanol (500: 100) Hexane: methanol (500: 250) Hexane: methanol (500:500) P. evecta crude extract (EW-L) 140 (µg/mL) P. evecta crude extract (EW-L) 500 (µg/mL) |
Determination the cytotoxicity of the samples in the cell model, neutral red (NR) uptake assay was used for identification of vital cells. The cytotoxicity assays were performed with HepG2 and Vero cells. | % Cytotoxicity | % Apoptotic cells in HepG2 | (Machana et al. 2012) | |||||||||||||||
| HepG2 | Vero | ||||||||||||||||||||
|
9.9 ± 2.5 7.8 ± 2.5 98.9 ± 1.8 81.8 ± 10.6 100.0 ± 1.0 91.0 ± 6.2 100.0 ± 5.7 100.0 ± 8.9 60.0 ± 4.8 100.0 ± 4.1 |
12.3 ± 1.3 10.8 ± 0.8 4.0 ± 2.9 43.9 ± 3.3 45.1 ± 4.1 26.9 ± 5.6 36.1 ± 8.0 39.9 ± 3.2 30.2 ± 9.3 37.2 ± 4.2 |
4.6 ± 1.6 9.2 ± 2.1 n.d. n.d. 72.7 ± 13.6 n.d. n.d. 54.9 ± 10.8 46.4 ± 2.6 92.8 ± 10.8 |
|||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Polyalthia evecta | Leaves |
Melphalan 76 (µg/mL) |
Determination the cytotoxicity of the samples in the cell model, neutral red (NR) uptake assay was used for identification of vital cells. The cytotoxicity assays were performed with HepG2 and Vero cells. | % Cytotoxicity | % Apoptotic cells in HepG2 | (Machana et al. 2012) | |||||||||||||||
| HepG2 | Vero | ||||||||||||||||||||
| 67.2 ± 3.1 | 70.3 ± 3.1 | 41.6 ± 2.1 |
|||||||||||||||||||
| Polyalthia longifolia var. pendula | Stems | 15 compounds isolated were evaluated for cytotoxicity against MCF-7 (human breast carcinoma) and A549 (non-small cell lung cancer) cells with cell viabilities assessed using a MTT assay. | Cytotoxicity assessment was performed with Human breast carcinoma (MCF-7) cells and non-small cell lung cancer (A549) cells. | IC50 µM | (Lee et al. 2009) | ||||||||||||||||
| Compounds tested, only 16-oxo-cleroda3,13-dien-15-oic acid (8) was cytotoxic against both MCF-7 and A549 cell lines, with IC50 values of 3.7 (0.2 and 3.1 (0.3 µM, respectively. Under the same conditions, the IC50 values of the corresponding positive controls, paclitaxel, and doxorubicin, were 0.0020 (0.0001 and 0.837 (0.034 µM, respectively. | |||||||||||||||||||||
|
Xylopia frutescens Aubl. |
Leaves |
Essential oil Doxorubicin Positive Control |
The in vivo antitumor effect was evaluated using Sarcoma 180 ascites tumor cells. Ten-day-old Sarcoma 180 ascites tumor cells (2 106 cells per 500 ll) were implanted subcutaneously into the left hind groin of mice. | IC50 (µg/mL) | |||||||||||||||||
| OVCAR-8 | NCI-H358M | PC-3M |
(Ferraz et al. 2013) |
||||||||||||||||||
| 33.9 24.9-46.3 1.2 0.9-1.6 |
24.6 14.9-40.7 0.9 0.6-1.3 |
40.0 31.3-51.2 1.6 1.1-2.4 |
|||||||||||||||||||
|
Xylopia laevigata |
Leaves |
5% DMSO Essential oil 5-Fluororacil Positive control |
The in vivo antitumor effect was evaluated using sarcoma 180 ascites tumor cells. Ten-day-old sarcoma 180 ascites tumor cells were implanted subcutaneously into the left hind groin of mice. | Antitumor activity sarcoma 180 ascites tumor cells | (Quintans et al. 2013) | ||||||||||||||||
| Dose (mg/kg/day) | Tumor (g) | Inhibition (%) | |||||||||||||||||||
| - 50 100 25 |
1.97±0.14 1.24±0.09 1.13±0.27 0.63±0.16 |
- 37.3 42.5 67.8 |
|||||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
| Xylopia sericea | Dried roots |
Compounds kauren-19-oic acid (KA); (−)-kauran-19-oic acid (KAH). |
The human cancer cell-lines used in this work were HL60 and K562 (leukemias), MDA-MB435 (melanoma) and SF295 (glioblastoma). The growth of tumour cells and PBLs was quantified by the ability of living cells to reduce the yellow dye 3-(4,5-dimethyl-2-thiozolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a purple formazan product. | Mitotic index, frequency of chromosomal aberrations and numeric changes in human lymphocytes in culture after kaurenoic acid (KA) and their hydrogenated derivative (KAH) treatments. | (Cavalcanti et al. 2010) | ||||||||||||||||
| Substance | Treatment | Mitotic index | Aberrant cells | ||||||||||||||||||
| % | Mean±S.E.M | % | Mean±S.E.M | ||||||||||||||||||
| MMS (Positive control) DMSO (Vehicle) KA KAH |
4x10-5 M 0.1% 2.5µg/mL 5.0 µg/mL 10.0 µg/mL 30.0 µg/mL 60.0 µg/mL 2.5 µg/mL |
2.8 2.1 2.5 4.1 4.0 3.5 4.3 4.1 4.3 4.0 3.8 4.2 3.3 3.0 3.1 2.3 1.8 2.6 1.3 1.7 1.1 4.4 4.0 4.0 |
2.46±0.3 3.86±0.3 4.23±0.1 4.0±0.2 3.13±0.1 2.23±0.4 1.36±0.3 4.13±0.2 |
5.3 8.3 5.0 0.7 0.0 0.7 0.0 0.0 0.0 0.0 0.0 0.0 0.7 0.0 0.7 5.3 4.7 5.3 4.3 5.0 6.2 0.0 0.0 0.0 |
6.2±1.8 0.46±0.4 0.0 0.23±0.4 0.46±0.4 5.1±0.3 5.3±1.2 0.0 |
||||||||||||||||
| Annonaceae species | Used material |
Substances/ Extracts |
Methodology | Inhibition | Ref. | ||||||||||||||||
|
Xylopia sericea |
Dried roots |
(−)-kauran-19-oic acid (KAH). |
The human cancer cell-lines used in this work were HL60 and K562 (leukemias), MDA-MB435 (melanoma) and SF295 (glioblastoma). The growth of tumour cells and PBLs was quantified by the ability of living cells to reduce the yellow dye 3-(4,5-dimethyl-2-thiozolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a purple formazan product. | Mitotic index, frequency of chromosomal aberrations and numeric changes in human lymphocytes in culture after kaurenoic acid (KA) and their hydrogenated derivative (KAH) treatments. | (Cavalcanti et al. 2010) | ||||||||||||||||
| Substance | Treatment | Mitotic index | Aberrant cells | ||||||||||||||||||
| % | Mean±S.E.M | % | Mean±S.E.M | ||||||||||||||||||
|
KAH |
5.0 µg/mL 10.0 µg/mL 30.0 µg/mL 60.0 µg/mL |
4.2 4.1 3.7 3.5 3.9 3.1 3.2 3.7 3.4 3.0 3.1 3.6 |
4.0±0.2 3.5±0.4 3.43±0.2 3.43±0.3 |
0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.7 0.0 0.0 0.7 0.7 |
0.23±0.4 0.0 0.23±0.4 0.43±0.4 |
||||||||||||||||
3.7. Trypanocidal
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|---|---|---|---|---|---|---|---|
| IC50 µM |
IC50 µg/ml |
SI | |||||
| Annona amazonica | Stem bark | Acanthoic acid Benznidazole (Positive control) |
Epimastigote | 59 7 |
5.9 | (Pinheiro et al. 2009) | |
| Annona cornifolia | Seed | 4-Desoxylongimicin B + Folianin A Squamocin M Annofolin Annotacin Glaucanisin + Parviflorin Glaucanisin + Glaucanetin Benznidazole (Positive Control) |
Amastigote and Trypomastigote | 0.12 0.1 0.11 1.7 0.13 1.7 3.8 |
1 1 1 1 1 1 625 |
(Lima et al. 2014) | |
| Annona coriaceae | Leaves | Essential oil Benznidazole (Positive control) |
Trypomastigotes | 168.5 45.02 |
(Siqueira et al. 2011) | ||
| Annona crassiflora | Stem bark Stem wood Root Bark |
Ethanolic extract Ethanolic extract Hexanic extract Ethanolic extract Hexanic extract |
Amastigote | 14.9 ±2.3 20.5 ±1.1 45.9 ±3.1 5.9 ±1.3 18.6 ±6.8 |
(De Mesquita et al. 2005) | ||
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
| Annona crassiflora | Root wood | Ethanolic extract Benznidazole (Positive control) |
Amastigote | 9.9 ±0.5 1.0 ±0.1 |
(De Mesquita et al. 2005) | ||
| Annona foetida | Stem | Liriodenine O-methylmoschatoline Annomontine Benznidazole (Positive control) Crystal violet (Positive control) |
Epimastigote and Trypomastigote | 645.2 / 14.53 286.3 / 11.82 757.8 / 16.07 7.6 / - - / 31.37 |
(Costa et al. 2011a) | ||
| Annona muricataL. |
Leaves Stems |
Hexane extract Ethyl acetate extract Methanol extract Hexane extract Ethyl acetate extract Methanol extract Benznidazole (Positive control) |
Epimastigote | 100.0 25.0 40.2 ±11 >100.0 74.9 63.2 98.6 2.0 |
(Osorio et al. 2007) (Valencia et al. 2011) (Osorio et al. 2007) |
||
| Annona squamosa | Leaves | Essential oil Benznidazole (Positive control) |
Trypomastigote Epimastigote |
12.7 14.9 2.7 |
(Meira et al. 2015) | ||
| Annona vepretorum | Leaves | Essential oil Benznidazole (Positive control) |
Trypomastigote Epimastigote |
11.2 16.2 2.7 |
(Meira et al. 2015) | ||
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
| Bocageopsis multiflora(MART.) REFR. | Aerial parts | Essential oil Benznidazole (Positive control) |
Trypomastigote and Amastigote | 0.46 ± 0.07 1 |
2.9 625 |
(Bay et al. 2019a) | |
| Cardiopetalum calophyllum | Stem bark | Hexanic extract Benznidazole (Positive control) |
Amastigote | 60.4 ±0.1 1.0 ±0.1 |
(De Mesquita et al. 2005) | ||
| Desmos panamensis(B.L. Rob) Saff. |
Leaves Stems |
Hexane extract Ethyl acetate extract Methanol extract Hexane extract Ethyl acetate extract Methanol extract Benznidazole (Positive control) |
Epimastigote |
61.4 10.7 ±3.5 85.6 26 ±13.6 98.6 11.9 ±5.2 98.6 17.7 ±6.4 >100.0 14.5 ±2.7 87.5 18.2 ±3.6 2.0 |
1.77 1.02 3.36 1.35 2.4 2.86 |
(Osorio et al. 2007) (Valencia et al. 2011) |
|
| Duguetia furfuracea | Stem Root bark Root wood |
Hexanic extract Ethanolic extract Hexanic extract Ethanolic extract Benznidazole (Positive control) |
Amastigote | 50.0 ±1.6 30.4 ±1.3 6.6 ±0.6 25.6 ±1.5 ±0.1 |
(De Mesquita et al. 2005) | ||
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
| Duguetia furfuracea | Stem bark | Duguetine Duguetine β-N-oxide Dicentrinone N-methyltetrahydropalmatine N-methylglaucine Alkaloid extract Gentian violet (Positive control) |
Trypomastigote | 9.32 30.79 18.83 9072 4957 22.44 31 |
(da Silva et al. 2009) | ||
| Duguetia lanceolata | Branches Leaves |
Ethanolic extract Ethanolic extract Glaucine Oxoglaucine + liriodenine Oxoglaucine + lanuginosine + dehydroglaucine Norglaucine + Isocorydine + N-methyllaurotetanine Benznidazole (Positive control) |
Epimastigote Trypomastigote / Amastigote |
250.2 157.9 46.0/28.6 83/not active >100/not active >100/not active 4.6/1.3 11.77 (epimastigote) |
0.21 2.11 |
(Alves et al. 2012) (Dantas et al. 2020) |
|
| Duguetia quitarensisBENTH. | Aerial parts | Essential oil Benznidazole (Positive control) |
Trypomastigote and Amastigote | 0.26 ± 0.06 1 |
2.1 625 |
(Bay et al. 2019a) | |
| Fusaea longifolia(AUBL.) SAFF | Aerial parts | Essential oil Benznidazole (Positive control) |
Trypomastigote and Amastigote | 0.3 ± 0.11 1 |
3.1 625 |
(Bay et al. 2019a) | |
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
|
Greenwayodendron suaveolens (Engl. & Diels) Verdc. (sin. Polyalthia suaveolens Enlg. & Diels) |
Fruits Leaves Root bark |
Crude ethanol extract Dichloromethane fraction rich in alkaloids Alkaline aqueous rich in salts e hydrophilic substances Petroleum ether fraction rich in lipids and waxes 90% methanol fraction rich in steroids and terpenes Crude ethanol extract Dichloromethane fraction rich in alkaloids Alkaline aqueous rich in salts e hydrophilic substances Petroleum ether fraction rich in lipids and waxes 90% methanol fraction rich in steroids and terpenes Crude ethanol extract Dichloromethane fraction rich in alkaloids Alkaline aqueous rich in salts e hydrophilic substances Petroleum ether fraction rich in lipids and waxes 90% methanol fraction rich in steroids and terpenes |
Not determined |
34.27 14.79 >64.0 7.49 3.58 27.86 31.17 >64.0 8.33 8.06 7.38 0.25 >64.0 7.88 2.11 |
>1.87 2.34 - 4.07 >17.88 >2.30 >2.05 - 3.84 4.62 1.04 >256.0 - 2.90 13.92 |
(Muganza et al. 2016) |
|
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
|
Greenwayodendron suaveolens (Engl. & Diels) Verdc. (sin. Polyalthia suaveolens Enlg. & Diels) |
Stem bark | Crude ethanol extract Dichloromethane fraction rich in alkaloids Alkaline aqueous rich in salts e hydrophilic substances Petroleum ether fraction rich in lipids and waxes 90% methanol fraction rich in steroids and terpenes |
2.0 28.28 >64.0 2.0 2.05 |
17.94 >2.26 - 8.66 3.64 |
(Muganza et al. 2016) | ||
| Guatteria boliviana | Stem bark | Lanuginosine Pangkorimine Funiferine Tiliageine Antioquine Puertogaline A Puertogaline B Sepeerine Guatteboline |
Trypomastigote | >818.8 203.32 47.69 287.66 77.87 242.26 76.13 131.32 100.05 |
(Mahiou et al. 2000b) | ||
| Guatteria elliptica | Leaves | Ethanolic extract Benznidazole (Positive control) |
Epimastigote | 345.1 11.77 |
0.30 | (Alves et al. 2012) | |
| Guatteria friesiana | Leaves | Essential oil Benznidazole (Positive control) |
Epimastigote / Trypomastigote | 11.9 2.7 |
(Meira et al. 2017) | ||
| Guatteria pogonopus | Leaves | Essential oil Benznidazole (Positive control) |
Epimastigote / Trypomastigote | 28.0 2.7 |
(Meira et al. 2017) | ||
| Guatteria punctata(AUBL.) RAHOWARD. | Aerial parts | Essential oil Benznidazole (Positive control) |
Trypomastigote and Amastigote | 0.029 ± 0.014 1 |
32 625 |
(Bay et al. 2019a) | |
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
| Guatteria xf. tonduzii | Leaves Stem |
Hexane extract Hexane extract |
Epimastigote | 34.0 ±17 25.2 ±5.2 |
0.77 1.09 |
(Valencia et al. 2011) | |
| Isolona hexaloba |
Leaves Root bark Stem bark |
Aqueous Extract Dried crude extract 80% crude ethanol extract Dichloromethane fraction rich in alkaloids Alkaline aqueous fraction rich in salts and hydrophilic substances Petroleum ether fraction rich in lipids and waxes 90% methanol fraction rich in steroids and terpenes Aqueous Extract Dried crude extract 80% crude ethanol extract Dichloromethane fraction rich in alkaloids Alkaline aqueous fraction rich in salts and hydrophilic substances Petroleum ether fraction rich in lipids and waxes 90% methanol fraction rich in steroids and terpenes Aqueous Extract Dried crude extract 80% crude ethanol extract Dichloromethane fraction rich in alkaloids Alkaline aqueous fraction rich in salts and hydrophilic substances |
Not determined | 30.05 33.71 8.33 32.79 >64.00 8.50 16.54 33.07 21.30 34.56 10.34 >64.00 21.71 20.71 - 15.06 34.27 17.55 >64.00 |
>2.13 >1.90 3.92 >1.95 - 2.52 >3.87 >1.94 >3.00 >1.85 4.11 - 1.59 >3.09 - >4.25 >1.87 >3.65 - |
(Musuyu Muganza et al. 2015) | |
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
| Isolona hexaloba | Stem bark |
Petroleum ether fraction rich in lipids and waxes 90% methanol fraction rich in steroids and terpenes Benznidazole (Positive control) Suramine (Positive control) |
Not determined | >64.00 45.02 3.19 - |
- >1.42 |
(Musuyu Muganza et al. 2015) | |
| Oxandra sessiflora | Stem | 4α,10β-aromadendronediol 4β,10α -aromadendronediol 4α,10α-aromadendranediol 1β,6α-dihydroxy-4(15)-eudesmeno 4β,10α-dihydroxy-guai-6-eno 4β,6β,7β,10α-tetrahydroxy-guaiane Benznidazole (Positive control) |
Trypomastigote | 23.7 ±3.8 31.7 ±6.9 29.8 ±8.3 47.5 ±5.8 16.3 ±4.7 17.6 ±2.3 16.4 ±0.8 |
(Armenio et al. 2020) | ||
| Piptostigma preussi | - | Polycarpol | Trypomastigote | 5.114 | (Ngantchou et al. 2009) | ||
| Porcelia macrocarpa |
Flours Seed |
Stearolic acid Santalbic acid 8-hydroxyoctadec-9,11-diynoic Isanolic acid 12,14-octadecadiynoic acid/macrocarpic acid Benznidazole (Positive control) |
Trypomastigote | 27.6 59.9 57.3 Not active 38,77 16.4 534.2 |
>7.2 >3.3 >3.5 - 4.1 >12.2 0.9 |
(Londero et al. 2018) (Santos et al. 2015) |
|
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
| Pseudomalmea boyacana(J.F. Macbr.) L. W. Chatrou. |
Leaves Stems |
Hexane extract Ethyl acetate extract Methanol extract Hexane extract Ethyl acetate extract Methanol extract Benznidazole (Positive control) |
Epimastigote |
74.0 89.2 100.0 15.2 ±3.6 50.4 100.0 87.5 12.8 ±1.3 2.0 |
1.19 |
(Osorio et al. 2007) (Valencia et al. 2011) (Osorio et al. 2007) (Valencia et al. 2011) |
|
|
Rollinia exsucca (DC. Ex Dunal) A. DC. |
Leaves Stems |
Hexane extract Ethyl acetate extract Methanol extract Hexane extract Ethyl acetate extract Methanol extract Benznidazole (Positive control) |
Epimastigote |
74.4 98.6 61.4 26.1 18.1 ±7.2 58.3 41.7 ±12 >100.0 2.0 |
(Osorio et al. 2007) (Valencia et al. 2011) (Osorio et al. 2007) (Valencia et al. 2011) (Osorio et al. 2007) |
||
|
Rollinia pittieri Saff |
Leaves Stems |
Hexane extract Ethyl acetate extract Methanol extract Hexane extract Ethyl acetate extract Methanol extract Benznidazole (Positive control) |
Epimastigote |
16.5 46.4 ±3.1 20.8 39.8 16.4 20.8 >100.0 2.0 |
(Osorio et al. 2007) (Valencia et al. 2011) (Osorio et al. 2007) |
||
| Annonaceae species | Used material | Extracts/ Compounds | Parasite form | T. cruzi | Ref. | ||
|
IC50 µM |
IC50 µg/ml |
SI | |||||
| Xylopia aromatica(Lam.) Mart. |
Leaves Stems |
Hexane extract Ethyl acetate extract Methanol extract Hexane extract Ethyl acetate extract Methanol extract Benznidazole (Positive control) |
Epimastigote |
99.2 66.0 26.1 >100.0 58.3 >100.0 2.0 |
(Osorio et al. 2007) |
||
| Xylopia aromatica | Fruits Root wood Root bark |
Ethanolic extract Benznidazole (Positive control) Hexanic extract Hexanic extract Benznidazole (Positive control) |
Epimastigote Amastigote |
253.1 11.77 21.6 ±6.0 23.5 ±4.7 1.0 ±0.1 |
0.39 | (Alves et al. 2012) (De Mesquita et al. 2005) |
|
| Xylopia emarginata | Leaves | Hexanic extract Benznidazole (Positive control) |
Amastigote | 57.6 ±2.4 1.0 ±0.1 |
(De Mesquita et al. 2005) | ||
| Xylopia frutescens | Leaves | Essential oil Benznidazole (Positive control) |
Epimastigote / Trypomastigote | 20.2 / 11.9 2.8 / 2.8 |
(Da Silva et al. 2013a) | ||
| Xylopia laevigata | Leaves | Essential oil Benznidazole (Positive control) |
Epimastigote / Trypomastigote | 22.2 / 12.7 2.8 / 2.8 |
(Da Silva et al. 2013a) | ||
3.8. Antioxidant
| Annonaceae species | Used material | Substances/ Extracts |
IC50 | DPPH | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| %Interaction | %Interaction 20 min |
%Interaction 60 min |
%Free radical scavenging | |||||
|
Annona nutans |
Leaves |
Hydromthanolic fraction |
4.89 µg/mL |
- | - | - |
50.0 |
(Silva et al. 2019) |
|
Annona squamosa |
Seed Pulp |
Extract Extract Vitamin C (positive control) |
0.36 µg/mL 0.83 µg/mL 0.011 µg/mL |
- | - | - | - | (Leite et al. 2021) |
| Cananga Odorata | Essential oil | Essential oil | 75.50 | - | - | 63.80 | (Sacchetti et al. 2005) | |
| Cardiopetalum calophyllum | Leaf |
Essential oil Quercetin (standard compound) |
9.66 µg/mL 3.13 µg/mL |
- - |
- - |
- - |
- - |
(Xavier et al. 2016) |
| Duguetia furfuracea | Leaves | Methanolic extract Chloroform fraction Ethyl acetate fraction Hydromethanol fraction |
22.46 µg/mL 176.88 µg/mL 60.56 µg/mL 28.21 µg/mL |
50.0 50.0 50.0 50.0 |
- | - | - | (do Santos et al. 2018) |
| Polyalthia longifolia | Stem bark | 3-O-methyl Ellagic Acid | 2.5 µg/mL 5 µg/mL 10 µg/mL 20 µg/mL 40 µg/mL 24.28 μg/ml |
- | - | - | 10.36 28.17 34.83 45.80 69.81 50.00 |
(Jain et al. 2014) |
| Annonaceae species | Used material |
Substances/ Extracts |
IC50 | DPPH | Ref. | |||
| %Interaction |
%Interaction 20 min |
%Interaction 60 min |
%Free radical scavenging | |||||
| Sphaerocoryne affinis | Fruit |
Rumdul fruit water extract |
85.62 µg/mL | - | - | - | (Nghi et al. 2022) | |
| Xylopia aethiopica | Essential oil of leaf, stem bark, roots, and fruits | Leaf oil Leaf oil Root bark oil Stem bark oil Fresh fruit oil Dried fruit oil Dried fruit oil |
0.048 g/mL 4.9 mg/mL 0.033 g/mL 0.037 g/mL 0.045 g/mL 0.042 g/mL 4.1 mg/mL |
- 50.0 - - - - 50.0 |
43.8 - 36.5 32.4 85.6 21.2 - |
75.9 - 43.2 40.3 85.8 54.8 - |
86.8 - 68.4 73.9 66.5 57.2 - |
(Karioti et al. 2004) (Konan et al. 2009) (Karioti et al. 2004) (Karioti et al. 2004) (Karioti et al. 2004) (Karioti et al. 2004) (Konan et al. 2009) |
|
Xylopia langsdorffiana |
Leaf and stem bark | Discretamine | Concentrations Tested 240 µg/mL 120 µg/mL 60 µg/mL 30 µg/mL |
94.25 93.93 91.51 90.05 |
- | - | - | (Da Silva et al. 2009) |
3.9. Antimalarials
| Annonaceae species | Used material | Substances/Extracts |
P. falciparum (strain) (IC50) |
Ifβ-h (%) 2mg/ml | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| F32 (µg/ml) | W2 (µg/ml) | K1 (µg/ml) |
NF54 (µM) |
|||||
|
Annickia kummeriae |
Leaves |
Extract metanolic Lysicamine Trivalvone Palmatine Jatrorrhizine |
- |
- |
0.12 2.4 1.6 0.08 0.24 |
- |
- |
(Malebo et al. 2013a) |
| Cleistopholis patens | Steam bark Leaves | Essential oil | 9.19 15.19 |
(Boyom et al. 2011) | ||||
| Hexalobus crispiflorus | Stem bark | Essential oil | 2.0 | (Fekam Boyom et al. 2003) | ||||
| Mitrephora tomentosa | Leaves and twigs | (-)-(7R,8S)-mitrephentosin C (-)-(7R,8S)-mitrephentosin F Cyclogunil (Positive control) |
13.3 µM 18.7 µM 5.87 µM |
(Wongsomboon et al. 2021) | ||||
| Pachypodanthium confine | Steam bark | Essential oil | 16.6 | (Fekam Boyom et al. 2003) | ||||
| Polyalthia debilis | Roots | dichloromethane extract Bidebiline C Bidebiline D |
1.35 5.4 4.1 |
(Kanokmedhakul et al. 2003) | ||||
| Polyalthia longifólia | Steam bark | 16-hydroxycleroda-3,13-dien-16,15-olide Acid 16-oxocleroda-3,13E-dien-15-óico |
5.33 3.05 |
(Gbedema et al. 2015) | ||||
| Annonaceae species | Used material | Substances/Extracts |
P. falciparum (strain) (IC50) |
Ifβ-h (%) 2mg/ml | Ref. | |||
| F32 (µg/ml) | W2 (µg/ml) |
K1 (µg/ml) |
NF54 (µM) |
|||||
| Polyalthia longifolia | Steam bark | 3,16-dihydroxycleroda-4 (18),13 (14) Z-dien-15,16-olida β-Stigmasterol Darienine L-Stepholidine |
6.15 63.3 22.05 104.33 |
(Gbedema et al. 2015) | ||||
|
Polyalthia oliveri |
Steam bark |
N-acetil-8α-polivolinona N-acetil-polveolina Chloroquine (Positive Control) |
7.6 29.1 0.006 |
(Kouam et al. 2014) | ||||
| Pseudomalmea boyacana | Liriodenina Atherospermidina Isomoschatolina Chloroquine (Positive control) |
8 – 10 0.01 |
8.0 0.90 |
11.4 5.12 2.40 97.0 |
(Osorio D et al. 2006) | |||
| Rollinia pittieri | O-metilmoschatolina Melosmina Chloroquine (Positive control |
98.3 96.4 97.0 |
(Osorio D et al. 2006) | |||||
| Uvariastrum pierreanum | Steam bark Leaves | Essential oil | 6.08 13.96 |
(Boyom et al. 2011) | ||||
|
Xylopia phloiodara Xylopia aethiopica |
Steam bark | Essential oil | 17.9 17.8 |
(Fekam Boyom et al. 2003) | ||||
3.10. Gastroprotective
| Annonaceae species | Used material | Substances/Extracts | Methodology | Dose | Ulcer lesion index | %Lesion reduction (gastroprotection) |
Ref. |
|---|---|---|---|---|---|---|---|
|
Annona muricata |
Leaves |
EEAM Control Omeprazole |
Ethanol induced ulcer model |
200 mg/kg 400 mg/kg 20 mg/kg |
39.0 23.0 9.0 |
- |
(Moghadamtousi et al. 2014) |
|
Goniothalamus sp. |
- |
Rac-GNT Control Carbenoxolone 200 |
Ethanol induced ulcer model |
15 mg/kg 30 mg/kg 60 mg/kg 18 mg/kg (ED50) 200mg/kg |
- |
41.7 70.5 86.7 50.0 93.5 |
(Vendramini-Costa et al. 2014) |
|
Polyalthia macropoda |
Stem bark |
PMD Amides from PMD 1 10 11 Control Lansoprazole |
Ethanol induced ulcer model |
1 mg/kg 5 mg/kg 10 mg/kg 0.1 mg/kg 0.1 mg/kg 0.1 mg/kg 1 mg/kg |
20.4 17.7 9.4 10.4 20.8 14.7 13.8 |
68.3 72.5 85.4 76.7 67.7 77.2 78.6 |
(Olate et al. 2012) |
|
Xylopia langsdorffiana |
Leaves |
EtOHE HexPh Control Carbenoxolone |
Ethanol induced ulcer model |
500 mg/kg 250 mg/kg 500 mg/kg 100 mg/kg |
- |
83 81 84 40 |
(Montenegro et al. 2014) |
4. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- 2016 G (2017) GBD 2016 Disease Injury and Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systemic analysis for the Global Burden. Lancet 390:1211–1259.
- Abarikwu SO, Ogunlaja A, Otuechere CA, Gideon OB (2017) Effect of Ethanolic Extract from Seeds or Pods of Xylopia Aethiopica (Dunal) A. Rich (Annonaceae) on the Testicular Function of Adult Male Rats. Indian J Clin Biochem 32:420–428. [CrossRef]
- Acar JF, Moulin G (2012) Antimicrobial resistance: a complex issue Pre-existence of antimicrobial resistance determinants Origin of antimicrobial resistance determinants. Rev Sci Tech (Internationail Off Epizoot 31:23–31.
- Aciole SDG, Piccoli CF, COSTA E V, et al (2011) Insecticidal activity of three species of Guatteria (Annonaceae) against Aedes aegypti (Diptera: Culicidae). Rev Colomb Entomol 37:262–268.
- Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7:834–846.
- Ahalya B, Shankar Ravi K, Kiranmayi GVN (2014) Exploration of anti-hyperglycemic and hypolipidemic activities of ethanolic extract of Annona muricata bark in alloxan induced diabetic rats. Int J Pharm Sci Res 25:21–27.
- Aina SA, Banjo AD, Lawal OA, Jonathan K (2009) Efficacy of some plant extracts on Anopheles gambiae mosquito larvae. Acad J Entomol 2:31–35.
- Akah PA, Odoh UE, Ezike AC, et al (2012) Antimicrobial Effects of a Lipophilic Fraction and Kaurenoic Acid Isolated from the Root Bark Extracts of Annona senegalensis . Evidence-Based Complement. Altern. Med. 2012:1–10.
- Akendengue B, Roblot F, Loiseau PM, et al (2002) Klaivanolide, an antiprotozoal lactone from Uvaria klaineana. Phytochemistry 59:885–888. [CrossRef]
- Akinwumi FO, Fasakin EA, Adedire CO (2007) Toxic and repellence activities of four plant extracts to Dermestes maculatus Degeer on smoked african mud catfish, Clarias gariepinus Burchell. J Entomol 4:149–154.
- Aku AA, Ogunwolu EO, Attah JA (1998) Annona senegalensis L.(Annonaceae): Performance as a botanical insecticide for controlling cowpea seed bruchid, Callosobruchus maculatus (F.)(Coleoptera: Bruchidae) in Nigeria/Annona senegalensis L.(Annonaceae): Wirksamkeit als pflanzliches Insektizid zur. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/Journal Plant Dis Prot 513–519.
- Alali FQ, Kaakeh W, Bennett GW, McLaughlin JL (1998) Annonaceous Acetogenins as Natural Pesticides: Potent Toxicity Against Insecticide-Susceptible and -Resistant German Cockroaches (Dictyoptera: Blattellidae). J Econ Entomol 91:641–649. [CrossRef]
- Alberts B, Johnson A, Lewis J, et al (2010) Biologia molecular da célula. Artmed Editora.
- Alcântara JM, De Lucena JMVM, Facanali R, et al (2017) Chemical composition and bactericidal activity of the essential oils of four species of annonaceae growing in brazilian amazon. Nat Prod Commun 12:619–622. [CrossRef]
- Aller MA, Arias JL, Arias JI, et al (2007) The inflammatory response recapitulates phylogeny through trophic mechanisms to the injured tissue. Med Hypotheses 68:202–209. [CrossRef]
- Allison LN, Dike KS, Opara FN, et al (2013) Evaluation of larvicidal efficacy and phytochemical potential of some selected indigenous plant against Anopheles gambiense and Culex quinquefasciatus. Adv Biosci Biotechnol 2013:.
- Almeida JRG da S, Araújo C de S, Pessoa C do Ó, et al (2014) Antioxidant, cytotoxic and antimicrobial activity of Annona vepretorum MART. (Annonaceae). Rev Bras Frutic 36:258–264.
- Alolga RN, Chávez León MASC, Osei-Adjei G, Onoja V (2019) GC-MS-based metabolomics, antibacterial and anti-inflammatory investigations to characterize the quality of essential oil obtained from dried Xylopia aethiopica fruits from Ghana and Nigeria. J Pharm Pharmacol 71:1544–1552. [CrossRef]
- Alsenosy AA, El-Far AH, Sadek KM, et al (2019) Graviola (Annona muricata) attenuates behavioural alterations and testicular oxidative stress induced by streptozotocin in diabetic rats. PLoS One 14:e0222410. [CrossRef]
- Alvarez Colom O, Neske A, Popich S, Bardón A (2007) Toxic effects of annonaceous acetogenins from Annona cherimolia (Magnoliales: Annonaceae) on Spodoptera frugiperda (Lepidoptera: Noctuidae). J Pest Sci (2004) 80:63–67. [CrossRef]
- Alves RT, Regasini LO, Funari CS, et al (2012) Trypanocidal activity of Brazilian plants against epimastigote forms from Y and Bolivia strains of Trypanosoma cruzi. Rev Bras Farmacogn 22:528–533. [CrossRef]
- Aminimoghadamfarouj N, Nematollahi A, Wiart C, et al (2020) Secondary metabolismin in Annonaceae: potencial source of drugs. Rev Bras Frutic 36:120554. [CrossRef]
- Aminimoghadamfarouj N, Nematollahi A, Wiart C (2011) Annonaceae: Bio-resource for tomorrow’s drug discovery. J Asian Nat Prod Res 13:465–476. [CrossRef]
- Anadozie SO, Adewale OB, Meyer M, et al (2021) In vitro anti-oxidant and cytotoxic activities of gold nanoparticles synthesized from an aqueous extract of the Xylopia aethiopica fruit. Nanotechnology 32:. [CrossRef]
- Andrade AL (2018) Atividade Antibacteriana e Antibiofilme de Cmplexos de Rutênio do Tipo cis-[RuCl2(dppb)(NN-R)] 2+ Sobre Bactérias Resistentes e o Efeito destes Combinados aos Antibióticos Ampicilina e Tetraciclina. Universidade Federal do Ceará.
- Anza M, Endale M, Cardona L, et al (2021) Chemical composition, cytotoxicity and larvicidal activity of essential oils of three medicinal plants of Ethiopian flora against anisakis L3 larvae. Res J Pharmacogn 8:21–30. [CrossRef]
- Araújo C de S, Silva JC, Mendes RL, et al (2017) Phytochemical screening, cytotoxicity and acute toxicity of Annona vepretorum Mart (Annonaceae) leaf extracts. Trop J Pharm Res 16:597. [CrossRef]
- Araújo AMN de (2010) Bioatividade de espécies vegetais em relação a Zabrotes subfasciatus (Boheman, 1833)(Coleoptera: Chrysomelidae: Bruchinae) em feijão (Phaseolus vulharis L. 1753).
- Armenio AA, de Sousa EA, Alves Veras MD, et al (2020) In vitro anti-Trypanosoma cruzi evaluation of sesquiterpenes from the branches of Oxandra sessiliflora. Phytochem Lett 37:59–62. [CrossRef]
- Asase A, Akwetey GA, Achel DG (2010) Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J Ethnopharmacol 129:367–376. [CrossRef]
- Attiq A, Jalil J, Husain K (2017) Annonaceae: Breaking the wall of inflammation. Front Pharmacol 8:. [CrossRef]
- Attiq A, Jalil J, Husain K, et al (2021) A new prenylated benzoquinone from Cyathocalyx pruniferus abrogates LPS-induced inflammatory responses associated with PGE2, COX-2 and cytokines biosynthesis in human plasma. Inflammopharmacology 29:841–854. [CrossRef]
- Auddy B, Ferreira M, Blasina F, et al (2003) Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol 84:131–138. [CrossRef]
- Aziz RS, Siddiqua A, Shahzad M, et al (2019) Oxyresveratrol ameliorates ethanol-induced gastric ulcer via downregulation of IL-6, TNF-α NF-ĸB, and COX-2 levels, and upregulation of TFF-2 levels. Biomed Pharmacother 110:554–560. [CrossRef]
- Bakarnga-Via I, Hzounda JB, Fokou PVT, et al (2014) Composition and cytotoxic activity of essential oils from Xylopia aethiopica (Dunal) A. Rich, Xylopia parviflora (A. Rich) Benth.) and Monodora myristica (Gaertn) growing in Chad and Cameroon. BMC Complement Altern Med 14:1–8. [CrossRef]
- Bapela MJ, Kaiser M, Meyer JJM (2017) Antileishmanial activity of selected South African plant species. South African J Bot 108:342–345. [CrossRef]
- Barreiras DG, Ruiz FM, Gomes JEG, Souza BMS (2020) Eficácia da ação antimicrobiana do extrato de própolis de abelha jataí (Tetragonisca angustula) em bactérias Gram-positivas e Gram-negativas. Cad Ciências Agrárias 12:1–5. [CrossRef]
- Barros RPC (2014) Identificação de linhagens de Candida sp. e perfil de sensibilidade a antifúngicos convencionais e de origem vegetal. Universidade Estadual da paraíba.
- Basha SKH, Subramanian S (2011) Biochemical evaluation of antidiabetic and antioxidant potentials of Annona squamosa leaves extracts studied in STZ-induced diabetic rats. Int J Pharm Sci Res 2:643–655.
- Bay M, Souza de Oliveira JV, Sales Junior PA, et al (2019a) In Vitro Trypanocidal and Antibacterial Activities of Essential Oils from Four Species of the Family Annonaceae. Chem Biodivers 16:. [CrossRef]
- Bay M, Souza de Oliveira JV, Sales Junior PA, et al (2019b) In Vitro Trypanocidal and Antibacterial Activities of Essential Oils from Four Species of the Family Annonaceae. Chem Biodivers 16:. [CrossRef]
- Begum N, Sharma B, Pandey RS (2010) Evaluation of insecticidal efficacy of Calotropis procera and Annona squamosa ethanol extracts against Musca domestica. J Biofertil Biopestici 1:1–6.
- Bermejo A, Figadère B, Zafra-Polo MC, et al (2005) Acetogenins from annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 22:269–303.
- Betancur-Galvis LA, Saez J, Granados H, et al (1999) Antitumor and Antiviral Activity of Colombian Medicinal Plant Extracts. Mem Inst Oswaldo Cruz 94:531–535. [CrossRef]
- Bhalke RD, Chavan MJ (2011) Analgesic and CNS depressant activities of extracts of Annona reticulata Linn. bark. Phytopharmacology 1:160–165.
- Bobadilla M, Zavala F, Sisniegas M, et al (2005) Evaluación larvicida de suspensiones acuosas de Annona muricata Linnaeus “guanábana” sobre Aedes aegypti Linnaeus (Diptera, Culicidae). Rev Peru Biol 12:145–152. [CrossRef]
- Bomfim LM, Menezes LRA, Rodrigues ACBC, et al (2016) Antitumour Activity of the Microencapsulation of Annona vepretorum Essential Oil. Basic Clin Pharmacol Toxicol 118:208–213. [CrossRef]
- Boyom FF, Ngouana V, Kemgne EAM, et al (2011) Antiplasmodial volatile extracts from Cleistopholis patens Engler & Diels and Uvariastrum pierreanum Engl. (Engl. & Diels) (Annonaceae) growing in Cameroon. Parasitol Res 108:1211–1217. [CrossRef]
- Breiman A, Hand L, Mashall JE, et al (1990) Pneumococcal bacteremia in Charleston County, South Carolina. A decade later. Arch Intern Med 150:1401–05.
- Brígido HPC, Correa-Barbosa J, da Silva-Silva JV, et al (2020) Antileishmanial activity of Annona species (Annonaceae). SN Appl Sci 2:1–8. [CrossRef]
- Brindis F, González-Trujano ME, González-Andrade M, et al (2013) Aqueous Extract of Annona macroprophyllata : A Potential α -Glucosidase Inhibitor. Biomed Res Int 2013:1–6. [CrossRef]
- Brito IA, Oliveira EA, Chaves MH, et al (2021) Antileishmanial acetylene fatty acid and acetogenins from seeds of porcelia macrocarpa. J Braz Chem Soc 32:447–453. [CrossRef]
- Brito IA, Thevenard F, Costa-Silva TA, et al (2022) Antileishmanial Effects of Acetylene Acetogenins from Seeds of Porcelia macrocarpa (Warm.) R.E. Fries (Annonaceae) and Semisynthetic Derivatives. Molecules 27:893. [CrossRef]
- Brito MT, Ferreira RC, Beltrão DM, et al (2018) Antitumor activity and toxicity of volatile oil from the leaves of Annona leptopetala. Rev Bras Farmacogn 28:602–609. [CrossRef]
- Calzada F, Solares-Pascasio J, Ordoñez-Razo R, et al (2017) Antihyperglycemic activity of the leaves from Annona cherimola miller and rutin on alloxan-induced diabetic rats. Pharmacognosy Res 9:1. [CrossRef]
- Calzada F, Valdes M, Garcia-Hernandez N, et al (2019) Antihyperglycemic activity of the leaves from annona diversifolia Safford. and farnesol on normal and alloxan-induced diabetic mice*. Pharmacogn Mag 15:5. [CrossRef]
- Carmona D, Sáez J, Granados H, et al (2003) Antiprotozoal 6-substituted-5,6-dihydro-α-pyrones from Raimondia cf. Monoica. Nat Prod Res 17:275–280. [CrossRef]
- Carneiro ÂP (2010) Atividade biocida de Annona coriacea Mart 1841 sobre o vetor Rhodnius neglectus Lent 1954 (Hemiptera: Reduviidae). Universidade do Estado de Mato Grosso.
- Cascaes MM, Carneiro ODS, Do Nascimento LD, et al (2021) Essential oils from annonaceae species from brazil: A systematic review of their phytochemistry, and biological activities. Int J Mol Sci 22:. [CrossRef]
- Castillo L, Castillo LE, Jimenez JJ, Delgado MA (2010) Secondary metabolites of the Annonaceae, Solanaceae and Meliaceae families used as biological control of insects. Trop Subtrop Agroecosystems 12:445–462.
- Cavalcanti BC, Ferreira JRO, Moura DJ, et al (2010) Structure-mutagenicity relationship of kaurenoic acid from Xylopia sericeae (Annonaceae). Mutat Res - Genet Toxicol Environ Mutagen 701:153–163. [CrossRef]
- Cercato LM, White PAS, Nampo FK, et al (2015) A systematic review of medicinal plants used for weight loss in Brazil: Is there potential for obesity treatment? J Ethnopharmacol 176:286–296. [CrossRef]
- Chakraborty P (2018) Herbal genomics as tools for dissecting new metabolic pathways of unexplored medicinal plants and drug discovery. Biochim. Open 6:9–16.
- Chan H-H, Hwang T-L, Thang T, et al (2013) Isolation and Synthesis of Melodamide A, a New Anti-inflammatory Phenolic Amide from the Leaves of Melodorum fruticosum. Planta Med 79:288–294. [CrossRef]
- Chanakul W, Tuchinda P, Anantachoke N, et al (2011) Cytotoxic alkaloids from stems, leaves and twigs of Dasymaschalon blumei. Fitoterapia 82:964–968. [CrossRef]
- Chang FR, Hwang TL, Yang YL, et al (2006) Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med 72:1344–1347. [CrossRef]
- Chang FR, Yang P-Y, Lin J-Y, et al (1998) Bioactive Kaurane diterpenoids from Annona glabra. J Nat Prod 61:437–439. [CrossRef]
- Chatelain E (2015) Chagas Disease drug discovery: toward a new era. J Biomol Screen 20:.
- Chatelain E, Ioset JR (2011) Drug discovery and development for neglected diseases: the DNDi model. Drug Des Devel Ther 5:175. [CrossRef]
- Chavan MJ, Wakte PS, Shinde DB (2011) Analgesic and anti-inflammatory activities of 18-acetoxy-ent-kaur-16-ene from Annona squamosa L. bark. Inflammopharmacology 19:111–115. [CrossRef]
- Chavan MJ, Wakte PS, Shinde DB (2010a) Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 17:149–151. [CrossRef]
- Chavan MJ, Wakte PS, Shinde DB (2012) Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat Prod Res 26:1515–1518. [CrossRef]
- Chavan MJ, Wakte PS, Shinde DB (2010b) Analgesic and anti-inflammatory activities of saponified fraction from Annona reticulata L. Bark. Lat Am J Pharm 29:1246–1249.
- Chen CH, Hsieh TJ, Liu TZ, et al (2004) Annoglabayin, a novel dimeric kaurane diterpenoid, and apoptosis in Hep G2 cells of annomontacin from the fruits of Annona glabra. J Nat Prod 67:1942–1946. [CrossRef]
- Chen CY, Chang FR, Shih YC, et al (2000) Cytotoxic constituents of Polyalthia longifolia var. pendula. J Nat Prod 63:1475–1478. [CrossRef]
- Chen Y-C, Chia Y-C, Huang B-M (2021) Phytochemicals from Polyalthia Species: Potential and Implication on Anti-Oxidant, Anti-Inflammatory, Anti-Cancer, and Chemoprevention Activities. Molecules 26:5369. [CrossRef]
- Chen Y, Chen JW, Xu SS, et al (2012) Antitumor activity of annonaceous acetogenins in HepS and S180 xenografts bearing mice. Bioorganic Med Chem Lett 22:2717–2719. [CrossRef]
- Chowdhury SS, Tareq AM, Tareq SM, et al (2021) Screening of antidiabetic and antioxidant potential along with phytochemicals of Annona genus: a review. Futur J Pharm Sci 7:. [CrossRef]
- Christopher R, Mgani Q, Nyandoro S, et al (2018) Antitrypanosomal, antiplasmodial, and antibacterial activities of extracts from selected Diospyros and Annonaceae species. J Complement Med Res 7:161. [CrossRef]
- Coelho MB, Marangoni S, Macedo MLR (2007) Insecticidal action of Annona coriacea lectin against the flour moth Anagasta kuehniella and the rice moth Corcyra cephalonica (Lepidoptera: Pyralidae). Comp Biochem Physiol - C Toxicol Pharmacol 146:406–414. [CrossRef]
- Colom OA, Barrachina I, Mingol IA, et al (2008) Toxic effects of annonaceous acetogenins on Oncopeltus fasciatus. J Pest Sci (2004) 81:85–89. [CrossRef]
- Coria-Téllez A V., Montalvo-Gónzalez E, Yahia EM, Obledo-Vázquez EN (2018) Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem 11:662–691. [CrossRef]
- Cortes D, Moreno L, Párraga J, et al (2014) Nuevos fármacos inspirados en annonáceas. Rev Bras Frutic 36:22–31. [CrossRef]
- Costa, Marilza da Silva, Mônica Josene Barbosa Pereira, Simone Santos de Oliveira, Paulo Teixeira de Souza, Evandro Luiz Dall’oglio TCA (2013) ARTIGO Anonáceas provocam mortalidade em larvas de Aedes aegypti ( Linnaeus , 1762 ) ( Diptera : Culicidae ). Rev Bras Biociências v. 11, n 2:184–190.
- Costa EV, Pinheiro MLB, De Souza ADL, et al (2011a) Trypanocidal activity of oxoaporphine and pyrimidine-β-carboline alkaloids from the branches of annona foetida mart. (annonaceae). Molecules 16:9714–9720. [CrossRef]
- Costa EV, Pinheiro MLB, Silva JRDA, et al (2009) Antimicrobial and antileishmanial activity of essential oil from the leaves of Annona foetida (Annonaceae). Quim Nova 32:78–81. [CrossRef]
- Costa MS, Pinheiro DO, Serrão JE, Pereira MJB (2012) Morphological Changes in the Midgut of Aedes aegypti L. (Diptera: Culicidae) Larvae Following Exposure to an Annona coriacea (Magnoliales: Annonaceae) Extract. Neotrop Entomol 41:311–314. [CrossRef]
- Costa RAP, Romagna CD, Pereira JL, Souza-Pinto NC (2011b) The role of mitochondrial DNA damage in the citotoxicity of reactive oxygen species. J Bioenerg Biomembr 43:25–29.
- Costa RGA, Anunciação TA da, Araujo M de S, et al (2020) In vitro and in vivo growth inhibition of human acute promyelocytic leukemia HL-60 cells by Guatteria megalophylla Diels (Annonaceae) leaf essential oil. Biomed Pharmacother 122:109713. [CrossRef]
- Costa E V., Marques F de A, Pinheiro MLB, et al (2011c) Chemical constituents isolated from the bark of Guatteria blepharophylla (Annonaceae) and their antiproliferative and antimicrobial activities. J Braz Chem Soc 22:1111–1117. [CrossRef]
- Costa E V., Soares LDN, Chaar J da S, et al (2021) Benzylated dihydroflavones and isoquinoline-derived alkaloids from the bark of diclinanona calycina (Annonaceae) and their cytotoxicities. Molecules 26:1–13. [CrossRef]
- Costa E V., Teixeira SD, Marques FA, et al (2008) Chemical composition and antimicrobial activity of the essential oils of the Amazon Guatteriopsis species. Phytochemistry 69:1895–1899. [CrossRef]
- Cronquist A (1981) An integrated system of classification of flowering plants. Columbia University Press.
- Cruz-Estrada A, Gamboa-Angulo M, Borges-Argáez R, Ruiz-Sánchez E (2013) Insecticidal effects of plant extracts on immature whitefly Bemisia tabaci Genn.(Hemiptera: Aleyroideae). Electron J Biotechnol 16:6.
- Cruz PEO da (2011) Estudo fitoquímico e investigação das atividades antioxidante, antimicrobiana e larvicida das cascas de Annona salzmannii A. DC.(Annonaceae).
- Cunha LMA (2009) Estudo Fitoquímico e Biológico de Duguetia riparia ( Annonaceae ). Univerdidade Federal do Amazonas.
- da Silva APT, Pereira MJB, Bento LF (2007) Extrato metanólico da semente de Annona coriacea (MART.) sobre a mortalidade da traça-do-tomateiro. Rev Bras Agroecol 2:1150–1153.
- da Silva DB, Tulli ECO, Militão GCG, et al (2009) The antitumoral, trypanocidal and antileishmanial activities of extract and alkaloids isolated from Duguetia furfuracea. Phytomedicine 16:1059–1063. [CrossRef]
- da Silva FMA, de Lima BR, Soares ER, et al (2015) Polycarpol in unonopsis, Bocageopsis and Onychopetalum Amazonian species: Chemosystematical implications and antimicrobial evaluation. Rev Bras Farmacogn 25:11–15. [CrossRef]
- da Silva FMA, Koolen HHF, de Lima JPS, et al (2012) Leishmanicidal activity of fractions rich in aporphine alkaloids from Amazonian Unonopsis species. Rev Bras Farmacogn 22:1368–1371. [CrossRef]
- Da Silva MS, Tavares JF, Queiroga KF, et al (2009) Alcaloides e outros constituintes de Xylopia langsdorffiana (Annonaceae). Quim Nova 32:1566–1570.
- Da Silva TB, Menezes LRA, Sampaio MFC, et al (2013a) Chemical composition and anti-trypanosoma cruzi activity of essential oils obtained from leaves of Xylopia frutescens and X. laevigata (Annonaceae). Nat Prod Commun 8:403–406. [CrossRef]
- Da Silva VP, Pereira MJB, Turchen LM (2013b) Efeito de extratos vegetais no controle de Euschistus heros (F.) (Hemiptera: Pentatomidae) em lavoura de soja na região sudeste do estado de Mato Grosso. BRAZILIAN J Agric - Rev Agric 88:185. [CrossRef]
- Dadang EDF, Prijono D (2009) Effectiveness of two botanical insecticide formulations to two major cabbage insect pests on field application. J ISSAAS 15:42–51.
- Dantas EP, Monteiro J, Medeiros LS de, et al (2020) Dereplication of Aporphine Alkaloids by UHPLC-HR-ESI-MS/MS and NMR from. 31:1908–1916.
- Davies J, Davies D (2010) Origins and Evolution of Antibiotic resistance. Microbiol Mol Biol Rev 74:417–433.
- de Andrade FHD, de Araújo Batista RS, Lins TB, et al (2019) Thermal characterization and microbiology assay of Annona muricata L. leaves. J Therm Anal Calorim 138:3737–3745. [CrossRef]
- de Cássia Seffrin R, Shikano I, Akhtar Y, Isman MB (2010) Effects of crude seed extracts of Annona atemoya and Annona squamosa L. against the cabbage looper, Trichoplusia ni in the laboratory and greenhouse. Crop Prot 29:20–24. [CrossRef]
- De Lima JPS, Pinheiro MLB, Antonio AM, et al (2012) In Vitro Atileishmanial and Cytotoxic Activities of Annona mucosa (Annonaceae). Rev Virtual Quim 4:692–702. [CrossRef]
- De Mesquita ML, Desrivot J, Bories C, et al (2005) Antileishmanial and trypanocidal activity of Brazilian Cerrado plants. Mem Inst Oswaldo Cruz 100:783–787. [CrossRef]
- de Moraes JM, Pereira MJB, da Silva Costa M, et al (2011) Avaliação da atividade de Annona coriacea (Annonaceae) sobre pupas e adultos de Aedes aegypti (Diptera: Culicidae) em laboratório. BRAZILIAN J Agric Agric 86:115–121.
- de Moura F, Justino A, Ferreira B, et al (2019) Pro-Fibrogenic and Anti-Inflammatory Potential of a Polyphenol-Enriched Fraction from Annona crassiflora in Skin Repair. Planta Med 85:570–577. [CrossRef]
- de Omena MC, Navarro DMAF, de Paula JE, et al (2007) Larvicidal activities against Aedes aegypti of some Brazilian medicinal plants. Bioresour Technol 98:2549–2556. [CrossRef]
- Deshmukhe P V, Hooli AA, Holihosur SN (2010) Bioefficacy of cold ethyl alcohol extract of Annona squamosa against Spodoptera litura Fabricius. J Biopestic 3:271.
- Dikti Vildina J, Ndjonka D, Schmidt TJ, Liebau E (2021) Identification of anti-Caenorhabditis and anti-Onchocerca constituents from leaves of Annona senegalensis Pers. (Annonaceae). South African J Bot 138:84–93. [CrossRef]
- Dill EM, Pereira MJB, Costa MS (2012) Efeito residual do extrato de Annona coriacea sobre Aedes aegypti. Arq Inst Biol (Sao Paulo) 79:595–602. [CrossRef]
- Ding L, Münch J, Goerls H, et al (2010) Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorganic Med Chem Lett 20:6685–6687. [CrossRef]
- DNDi D for ND initiative (2022) Chagas Disease.
- do Santos RC, de Souza AV, Andrade-Silva M, et al (2018) Antioxidant, anti-rheumatic and anti-inflammatory investigation of extract and dicentrinone from Duguetia furfuracea (A. St.-Hil.) Benth. & Hook. f. J Ethnopharmacol 211:9–16. [CrossRef]
- Doyle JA, Sauquet H, Scharaschkin T, Le Thomas A (2004) Phylogeny, molecular and fossil dating, and biogeographic history of annonaceae and myristicaceae (Magnoliales). In: International Journal of Plant Sciences.
- Duarte-Almeida JM, Santos RJ dos, Genovese MI, Lajolo FM (2006) Avaliação da atividade antioxidante utilizando sistema beta-caroteno/ácido linoléico e método de seqüestro de radicais DPPH. Ciência e Tecnol Aliment 26:446–452. [CrossRef]
- Ducasse M, Brown MA (2006) Epigenetic aberrations and cancer. Mol Cancer 5:60.
- Dutra LM, Bomfim LM, Rocha SLA, et al (2014) Bioorganic & Medicinal Chemistry Letters ent -Kaurane diterpenes from the stem bark of Annona vepretorum ( Annonaceae ) and cytotoxic evaluation. 24:3315–3320. [CrossRef]
- Ellis L, Atadja PW, Johnstone RW (2009) Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther 8:1409–1420.
- Emerson F. Queiroz, François Roblot and, Cavé* A, et al (1996) Pessoine and Spinosine, Two Catecholic Berbines from Annona spinescens1. [CrossRef]
- Encina CL, Martin EC, Lopez AA, Padilla IMG (2014) Biotechnology applied to Annona species: a review. Rev Bras Frutic 36:17–21. [CrossRef]
- Epino PB, Chang F (1993) Insecticidal activity of Annona squamosa L. seed extracts against the Mediterranean fruit fly, Ceratitis capitata (Wiedemann)(Diptera: Tephritidae). Philipp Entomol.
- Esteller M (2008) Epigenetics in cancer. N Engl J Med 358:1148–1159.
- Etame RME, Mouokeu RS, Poundeu FSM, et al (2019) Effect of fractioning on antibacterial activity of n-butanol fraction from Enantia chlorantha stem bark methanol extract. BMC Complement Altern Med 19:1–7. [CrossRef]
- Ewete FK, Arnason JT, Larson J, Philogene BJR (1996) Biological activities of extracts from traditionally used Nigerian plants against the European corn borer, Ostrinia nubilalis. Entomol Exp Appl 80:531–537.
- Ezekwesili CN, Nwodo OFC, Eneh FU, Ogbunugafor HA (2010) Investigation of the chemical composition and biological activity of Xylopia aethiopica Dunal (Annonaceae). African J Biotechnol 9:7352–7356.
- Faizi S, Khan RA, Azher S, et al (2003) New Antimicrobial Alkaloids from the Roots of Polyalthia longifolia var. pendula. Planta Med 69:350–355. [CrossRef]
- Faizi S, Khan RA, Mughal NR, et al (2008) Antimicrobial activity of various parts of Polyalthia longifolia var. pendula: Isolation of active principles from the leaves and the berries. Phyther Res 22:907–912. [CrossRef]
- Fatope MO, Audu OT, Takeda Y, et al (1996) Bioactive ent-kaurene diterpenoids from Annona senegalensis. J Nat Prod 59:301–303. [CrossRef]
- Fekam Boyom F, Ngouana V, Amvam Zollo PH, et al (2003) Composition and anti-plasmodial activities of essential oils from some Cameroonian medicinal plants. Phytochemistry 64:1269–1275. [CrossRef]
- Fernandes AT (2000) Infecção Hospitalar e suas interfaces na área da saúde, 1st editio. São Paulo, Brasil.
- Ferraz RPC, Cardoso GMB, Da Silva TB, et al (2013) Antitumour properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem 141:542–547. [CrossRef]
- Ferreira C, Passos CLA, Soares DC, et al (2017) Leishmanicidal activity of the alkaloid-rich fraction from Guatteria latifolia. Exp Parasitol 172:51–60. [CrossRef]
- Ferreira G, De-La-Cruz-Chacón I, Fernandes Boaro CS, et al (2019) Propagation of Annonaceous plants. Rev Bras Frutic 41:1–14. [CrossRef]
- Ferreira J, Kurachi C, Melo CAS, et al (2004) Necrosis Characteristics of Photodynamics Therapy. Laser Phys 14:209–212.
- Février A, Ferreira ME, Fournet A, et al (1999) Acetogenins and other compounds from Rollinia emarginata and their antiprotozoal activities. Planta Med 65:47–49. [CrossRef]
- Figueroa-Miranda G, Wu C, Zhang Y, et al (2020) Polyethylene glycol-mediated blocking and monolayer morphology of anelectrochemical aptasensor for malaria biomarker detection in humanserum. Bioelectrochemistry 136:1–11.
- Florence NT, Benoit MZ, Jonas K, et al (2014) Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol 151:784–790. [CrossRef]
- Fontana JD, Passos M, Baron M, et al (1998) Selective polarity-and adsorption-guided extraction/purification of Annona sp. polar acetogenins and biological assay against agricultural pests. In: Biotechnology for Fuels and Chemicals. Springer, pp 67–76.
- Franke A, Teyssen S, Singer M V. (2005) Alcohol-Related Diseases of the Esophagus and Stomach. Dig Dis 23:204–213. [CrossRef]
- Fujiwara N, Kobayashi K (2005) Macrophages in Inflammation. Curr Drug Target -Inflammation Allergy 4:281–286. [CrossRef]
- Gajalakshmi S, Vijayalakshmi S, Davi Rajeswari V (2012) Phytochemical and pharmacological properties of Annona muricata: a review. Int J Pharm Sci Res 4:3–6.
- Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H (2014) Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med 7:S355–S363. [CrossRef]
- Gbedema SY, Bayor MT, Annan K, Wright CW (2015) Clerodane diterpenes from Polyalthia longifolia (Sonn) Thw. var. pendula: Potential antimalarial agents for drug resistant Plasmodium falciparum infection. J Ethnopharmacol 169:176–182. [CrossRef]
- Gomes CL, Cavalcante JE, Cunha FA, et al (2010) Identificação e perfil de sensibilidade de Candida spp. isoladas de urina de pacientes com candidúria em Iguatu-Ceará. Rev Bras Análises Cl;inicas 42:223–225.
- Gomes JVD, Borges AS, Athaydes BR, et al (2022) Anti-Helicobacter pylori potential, antioxidant capacity, and anti-inflammatory activity of Xylopia sericea A. St.-Hil. (Annonaceae) leaves. Phytomedicine Plus 2:100214. [CrossRef]
- González-Esquinca AR, Luna-Cazáres LM, Schlie Guzmán MA, et al (2012) In Vitro Larvicidal Evaluation of Annona muricata L., A. diversifolia Saff. and A. lutescens Saff. Extracts Against Anastrepha ludens Larvae (Diptera, Tephritidae). Interciencia 37:284–289.
- Guadaño A, Gutiérrez C, de la Peña E, et al (2000) Insecticidal and mutagenic evaluation of two annonaceous acetogenins. J Nat Prod 63:773–776. [CrossRef]
- Guarido MM (2009) Atividade inseticida de extratos de Annona foetida Mart.(Annonaceae) sobre imaturos de Aedes aegypti (Linnaeus, 1762)(Diptera: Culicidae).
- Guo H, Callaway JB, Ting JP-Y (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. [CrossRef]
- Guo X, Tang CC, Thomas DC, et al (2017) A mega-phylogeny of the Annonaceae: Taxonomic placement of five enigmatic genera and support for a new tribe, Phoenicantheae. Sci Rep 7:1–11. [CrossRef]
- Hadjichristou C, Koidis P, Bakopoulou A (2020) Advanced in Vitro Experimental Models for Tissue Engineering-based Reconstruction of a 3D Dentin/pulp Complex: a Literature Review. Stem Cell Rev Reports 1–18.
- Hammami F, Koubaa M, Chaari M, et al (2020) Haemophagocytic lymphohistiocytosis secondary to Plasmodium falciparum malaria: Case report and review of the literature. Asian Pac J Trop Med 13:467–471. [CrossRef]
- Hassan SS ul, Zhang W-D, Jin H, et al (2022) In-silico anti-inflammatory potential of guaiane dimers from Xylopia vielana targeting COX-2. J Biomol Struct Dyn 40:484–498. [CrossRef]
- Henao GJP, Pajón CMG, Torres JMC (2007) Actividad insecticida de extractos vegetales sobre Aedes aegypti (Diptera: Culicidae) vector del dengue en Colombia. Ces Med 21:.
- Hisham A, Rameshkumar KB, Sherwani N, et al (2012) The composition and antimicrobial activities of Cyperus conglomeratus, Desmos chinensis var. lawii and Cyathocalyx zeylanicus essential oils. Nat Prod Commun 7:663–666. [CrossRef]
- Hoes JN, Jacobs JWG, Buttgereit F, Bijlsma JWJ (2010) Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat Rev Rheumatol 6:693–702. [CrossRef]
- Hongthong S, Kuhakarn C, Jaipetch T, et al (2016) A new neolignan, and the cytotoxic and anti-HIV-1 activities of constituents from the roots of Dasymaschalon sootepense. Nat Prod Commun 11:809–813. [CrossRef]
- Horton NC, Mathew PA (2015) NKp44 and natural cytotoxicity receptors as damage-associated molecular pattern recognition receptors. Front Immunol 6:31.
- Hsu Y-M, Wu T-Y, Du Y-C, et al (2016) 3-Methyl-4,5-dihydro-oxepine, polyoxygenated seco-cyclohexenes and cyclohexenes from Uvaria flexuosa and their anti-inflammatory activity. Phytochemistry 122:184–192. [CrossRef]
- Hu JF, Garo E, Hough GW, et al (2006) Antibacterial, partially acetylated oligorhamnosides from Cleistopholis patens. J Nat Prod 69:585–590. [CrossRef]
- Huber LS, Rodriguez-Amaya DB (2008) Flavonóis e flavonas: fontes brasileiras e fatores que influenciam a composição em alimentos. Aliment e Nutr 18:97–108.
- Huong DT, Luong D V., Thao TTP, Sung T Van (2005) A new flavone and cytotoxic activity of flavonoid constituents isolated from Miliusa balansae (Annonaceae). Pharmazie 60:627–629. [CrossRef]
- Huscher D, Thiele K, Gromnica-Ihle E, et al (2009) Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis 68:1119–1124. [CrossRef]
- Ik-Soo Lee, Xianjian Ma, Hee-Byung Chai, et al (1995) Novel cytotoxic labdane diterpenoids from Neouvaria acuminatissima. Tetrahedron 51:21–28. [CrossRef]
- Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA) (2019) Estimativa 2020: incidência de Cancer no Brasil. p 120p.
- IOM I of M (2010) Antibiotic resistance: implications for global health and novel intervention strategies: workshop summary. Washington, DC: The National Academies Press.
- Ishola IO, Awodele O, Olusayero AM, Ochieng CO (2014) Mechanisms of Analgesic and Anti-Inflammatory Properties of Annona muricata Linn. (Annonaceae) Fruit Extract in Rodents. J Med Food 17:1375–1382. [CrossRef]
- Jachak S (2006) Cyclooxygenase Inhibitory Natural Products: Current Status. Curr Med Chem 13:659–678. [CrossRef]
- Jain P, Patra A, Jain S, et al (2014) Antibacterial and Antioxidant Activities of 3-O-methyl Ellagic Acid 4’-rhamnoside from Stem Bark of Polyalthia longifolia Thw. 45:b031. [CrossRef]
- Jossang A, Leboeuf M, Cavé A, et al (1984) Alcaloïdes des Annonacees, L: Alcaloïdes De Polyalthia cauliflora. J Nat Prod 47:504–513. [CrossRef]
- Joy B, Remani P (2008) Antitumor constituents from Annona squamosa fruit pericarp. Med Chem Res 17:345–355. [CrossRef]
- Kabir KE (2010) Larvicidal effect of an alkaloidal fraction of Artabotrys odoratissimus (Annonaceae) bark against the filarial mosquito Culex quinquefasciatus (Diptera: Culicidae). Int J Trop Insect Sci 30:167–169.
- Kabir S, Zahan R, Chowdhury AMS, et al (2019) Evaluation of Analgesic and Anti-inflammatory Activities of Polyalthia simiarum (Hook. F. &Thomson). J Natl Inst Neurosci Bangladesh 5:18–23. [CrossRef]
- Kaleem M, Medha P, Ahmed QU, et al (2008) Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med J 49:800–4.
- Kamaraj C, Bagavan A, Elango G, et al (2011) Larvicidal activity of medicinal plant extracts against Anopheles subpictus & Culex tritaeniorhynchus. Indian J Med Res 134:101.
- Kambiré DA, Boti JB, Kablan ACL, et al (2021) Chemical Variability and In Vitro Anti-Inflammatory Activity of Leaf Essential Oil from Ivorian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels. Molecules 26:6228. [CrossRef]
- Kanokmedhakul S, Kanokmedhakul K, Kantikeaw I, Phonkerd N (2006) 2-Substituted furans from the roots of Polyalthia evecta. J Nat Prod 69:68–72. [CrossRef]
- Kanokmedhakul S, Kanokmedhakul K, Yodbuddee D, Phonkerd N (2003) New antimalarial bis-dehydroaporphine alkaloids from Polyalthia debilis. J Nat Prod 66:616–619. [CrossRef]
- Karioti A, Hadjipavlou-Litina D, Mensah MLK, et al (2004) Composition and antioxidant activity of the essential oils of Xylopia aethiopica (Dun) A. Rich. (Annonaceae) leaves, stem bark, root bark, and fresh and dried fruits, growing in Ghana. J Agric Food Chem 52:8094–8098. [CrossRef]
- Karunaratne M, Arukwatta A (2009) Efficacy of three plant species on the mortality and food consumption of Epilachna vigintioctopunctata.
- Kawazu K, Alcantara JP, Kobayashi A (1989) Isolation and structure of neoannonin, a novel insecticidal compound from the seeds of Annona squamosa. Agric Biol Chem 53:2719–2722.
- Kempraj V, Bhat SK (2011) Acute and reproductive toxicity of Annona squamosa to Aedes albopictus. Pestic Biochem Physiol 100:82–86.
- Kesetyaningsih TW (2012) Efficacy of Annona squamosa leaf extract as an insecticide against Cockroach (Periplaneta americana).
- Khalequzzaman M, Sultana S (2006) Insecticidal activity of Annona squamosa L. seed extracts against the red flour beetle, Tribolium castaneum (Herbst). J Bio-Science 14:107–112.
- Kim EJ, Suh KM, Kim DH, et al (2005) Asimitrin and 4-hydroxytrilobin, new bioactive annonaceous acetogenins from the seeds of Asimina triloba possessing a bis-tetrahydrofuran ring. J Nat Prod 68:194–197. [CrossRef]
- Kithsiri Wijeratne EM, Leslie Gunatilaka AA, Kingston DGI, et al (1995) Artabotrine: A novel bioactive alkaloid from Artabotrys zeylanicus. Tetrahedron 51:7877–7882. [CrossRef]
- Klocke JA, Mei-Ying H, Shin-Foon C, Kubo I (1991) Grayanoid diterpene insect antifeedants and insecticides from Rhododendron molle. Phytochemistry 30:1797–1800. [CrossRef]
- Konan N, Kouame BA, Mamyrbekova-Bekro JA, et al (2009) Chemical composition and antioxidant activities of essential oils of xylopia aethiopica (dunal) a. rich. Eur J Sci Res 37:311–318.
- Kotkar HM, Mendki PS, Sadan SVGS, et al (2002) Antimicrobial and pesticidal activity of partially purified flavonoids of Annona squamosa. Pest Manag Sci Former Pestic Sci 58:33–37.
- Kouam SF, Ngouonpe AW, Lamshöft M, et al (2014) Indolosesquiterpene alkaloids from the Cameroonian medicinal plant Polyalthia oliveri (Annonaceae). Phytochemistry 105:52–59. [CrossRef]
- Krinski D, Massaroli A (2014) Nymphicidal effect of vegetal extracts of Annona mucosa and Anonna crassiflora (Magnoliales, Annonaceae) against rice stalk stink bug, Tibraca limbativentris (Hemiptera, Pentatomidae). Rev Bras Frutic 36:217–224. [CrossRef]
- Krinski D, Massaroli A, Machado M (2014) Potencial inseticida de plantas da família Annonaceae. Rev Bras Frutic 36:225–242. [CrossRef]
- Kumar JA, Rekha T, Devi SS, et al (2010) Insecticidal activity of ethanolic extract of leaves of Annona squamosa. J Chem Pharm Res 2:177–180.
- Lamidi M, DiGiorgio C, Delmas F, et al (2005) In vitro cytotoxic, antileishmanial and antifungal activities of ethnopharmacologically selected Gabonese plants. J Ethnopharmacol 102:185–190. [CrossRef]
- Leatemia JA, Isman MB (2004) Efficacy of crude seed extracts of Annona squamosa against diamondback moth, Plutella xylostella L. in the greenhouse. Int J Pest Manag 50:129–133.
- Leboeuf M, Cavé A, Bhaumik PK, et al (1980) The phytochemistry of the annonaceae. Phytochemistry 21:2783–2813. [CrossRef]
- Lee TH, Wang MJ, Chen PY, et al (2009) Constituents of Polyalthia longifolia var. pendula. J Nat Prod 72:1960–1963. [CrossRef]
- Leite DOD, Camilo CJ, Nonato C de FA, et al (2021) Chemical profile and evaluation of the antioxidant and anti-acetylcholinesterase activities of annona squamosa l. (annonaceae) extracts. Foods 10:. [CrossRef]
- Lemos DECV, Cavalcante-Silva LHA, de Almeida Lima É, et al (2017) Anti-inflammatory Effect of Discretamine, a Protoberberine Alkaloid Isolated from Duguetia moricandiana. Nat Prod Commun 12:1934578X1701201. [CrossRef]
- Lepper MH, Dowling HF (1951) Treatment of pneumococcic meningitis with penicillin compared with penicillin plus aureomycin: Studies including observations on an apparent antagonism between penicillin and aureomycin. AMA Arch Intern Med 88:489–494.
- Li C, Lee D, Graf TN, et al (2009) Bioactive Constituents of the Stem Bark of Mitrephora glabra. J Nat Prod 72:1949–1953. [CrossRef]
- Lima LAR dos S, Alves TMA, Zani CL, et al (2014) In vitro cytotoxic, antifungal, trypanocidal and leishmanicidal activities of acetogenins isolated from Annona cornifolia A. St. -Hil. (Annonaceae). An Acad Bras Cienc 86:829–839. [CrossRef]
- Link A, Balaguer F, Goel A (2010) Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol 80:1771–1792.
- Liu X, Wang J (2011) Anti-inflammatory effects of iridoid glycosides fraction of Folium syringae leaves on TNBS-induced colitis in rats. J Ethnopharmacol 133:780–787. [CrossRef]
- Llanos CAH, Arango DL, Giraldo MC (2008) Actividad insecticida de extractos de semilla de Annona muricata (Anonaceae) sobre Sitophilus zeamais (Coleoptera: Curculionidae). Rev Colomb Entomol 34:76–82.
- Londero VS, da Costa-Silva TA, Gomes KS, et al (2018) Acetylenic fatty acids from Porcelia macrocarpa (Annonaceae) against trypomastigotes of Trypanosoma cruzi: Effect of octadec-9-ynoic acid in plasma membrane electric potential. Bioorg Chem 78:307–311. [CrossRef]
- López-Rubalcava C, Piña-Medina B, Estrada-Reyes R, et al (2006) Anxiolytic-like actions of the hexane extract from leaves of Annona cherimolia in two anxiety paradigms: Possible involvement of the GABA/benzodiazepine receptor complex. Life Sci 78:730–737. [CrossRef]
- López R, Cuca LE, Delgado G (2009) Antileishmanial and immunomodulatory activity of Xylopia discreta. Parasite Immunol 31:623–630. [CrossRef]
- Lorenzo V, Lúcio A, Scotti L, et al (2016) Structure- and Ligand-Based Approaches to Evaluate Aporphynic Alkaloids from Annonaceae as Multi-Target Agent Against Leishmania donovani. Curr Pharm Des 22:5196–5203. [CrossRef]
- Lorenzo VP, Scotti L, da Silva Almeida JRG, Scotti MT (2020) Annonaceae Family Alkaloids as Agents Against Leishmaniasis: A Review and Molecular Docking Evaluation. Curr Drug Metab 21:482–492. [CrossRef]
- LS NT, Goudoum A, Ngassoum MB, et al (2004) Persistance of the insecticidal activity of five essential oils on the maize weevil Sitophilus zeamais (Motsch.)(Coleoptera: Curculionidae). Commun Agric Appl Biol Sci 69:145–147.
- Luciana BS, Jorlan C da S, Bruno EP, et al (2013) Insecticide irritability of plant extracts against Sitophilus zeamais. African J Agric Res 8:978–983.
- Lúcio ASSC, Da Silva Almeida JRG, Barbosa-Filho JM, et al (2011) Azaphenanthrene alkaloids with antitumoral activity from anaxagorea dolichocarpa sprague and sandwith (annonaceae). Molecules 16:7125–7131. [CrossRef]
- Ma C, Chen Y, Chen J, et al (2017) A Review on Annona squamosa L.: Phytochemicals and Biological Activities . Am J Chin Med 45:933–964. [CrossRef]
- Macedo T, Ribeiro V, Oliveira AP, et al (2020) Anti-inflammatory properties of Xylopia aethiopica leaves: Interference with pro-inflammatory cytokines in THP-1-derived macrophages and flavonoid profiling. J Ethnopharmacol 248:112312. [CrossRef]
- Machana S, Weerapreeyakul N, Barusrux S, et al (2012) Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG2) cells. Asian Pac J Trop Biomed 2:589–596. [CrossRef]
- Maeda G, Gilissen PJ, Bourgard C, et al (2022) Polyoxygenated cyclohexene derivatives and flavonoids from the leaves of Uvaria pandensis. Fitoterapia 158:. [CrossRef]
- Magadula JJ, Innocent E, Otieno JN (2009) Mosquito larvicidal and cytotoxic activities of 3 Annona species and isolation of active principles. J Med Plants Res 3:674–680.
- Mahiou V, Roblot F, Fournet A, Hocquemiller R (2000a) Bisbenzylisoquinoline alkaloids from Guatteria boliviana (Annonaceae). Phytochemistry 54:709–716. [CrossRef]
- Mahiou V, Roblot F, Fournet A, Hocquemiller R (2000b) Bisbenzylisoquinoline alkaloids from Guatteria boliviana (Annonaceae). Phytochemistry 54:709–716. [CrossRef]
- Mahmud PIAM, Yaacob WA, Ibrahim N, Bakar MA (2018) Antibacterial activity and major constituents of polyalthia cinnamomea basic fraction. Sains Malaysiana 47:2063–2071. [CrossRef]
- Malebo HM, Wenzler T, Cal M, et al (2013a) Anti-protozoal activity of aporphine and protoberberine alkaloids from Annickia kummeriae (Engl. & Diels) Setten & Maas (Annonaceae). BMC Complement Altern Med 13:1–10. [CrossRef]
- Malebo HM, Wenzler T, Cal M, et al (2013b) Anti-protozoal activity of aporphine and protoberberine alkaloids from Annickia kummeriae. BMC Complement Altern Med 13:1–10.
- Manoj Kumar S, Azamthulla M, Saravanan KS (2021) Pharmacognostical evaluation and anti-convulsant property of Annona reticulata Linn. (Annonaceae) root. Futur J Pharm Sci 7:. [CrossRef]
- Martínez-Solís J, Calzada F, Barbosa E, Valdés M (2021) Antihyperglycemic and Antilipidemic Properties of a Tea Infusion of the Leaves from Annona cherimola Miller on Streptozocin-Induced Type 2 Diabetic Mice. Molecules 26:2408. [CrossRef]
- Martínez-Vázquez M, Estrada-Reyes R, Araujo Escalona AG, et al (2012) Antidepressant-like effects of an alkaloid extract of the aerial parts of Annona cherimolia in mice. J Ethnopharmacol 139:164–170. [CrossRef]
- Massaroli A, Pereira MJB, Foerster LA (2013) Annona mucosa como fitoinseticida para o controle de Chrysodeixis includens (Lepidoptera: Noctuidae). SIMPÓSIO Control BIOLÓGICO 13:.
- Matchi JLT, Noungoue DT, Chaubet G, et al (2022) Antischistosomal evaluation of stem bark’s extract and chemiacl constituents from Anonidium mannii against schistosoma mansoni. Pharmacogn Mag 17:752–758.
- Mazumdar S, Ghosh AK, Dinda M, et al (2021) Evaluation of wound healing activity of ethanol extract of Annona reticulata L. leaf both in vitro and in diabetic mice model. J Tradit Complement Med 11:27–37. [CrossRef]
- Mbosso EJT, Ngouela S, Nguedia JCA, et al (2010) In vitro antimicrobial activity of extracts and compounds of some selected medicinal plants from Cameroon. J Ethnopharmacol 128:476–481. [CrossRef]
- Meira CS, Guimarães ET, MacEdo TS, et al (2015) Chemical composition of essential oils from Annona vepretorum Mart. and Annona squamosa L. (Annonaceae) leaves and their antimalarial and trypanocidal activities. J Essent Oil Res 27:160–168. [CrossRef]
- Meira CS, Menezes LRA, dos Santos TB, et al (2017) Chemical composition and antiparasitic activity of essential oils from leaves of Guatteria friesiana and Guatteria pogonopus (Annonaceae). J Essent Oil Res 29:156–162. [CrossRef]
- Mendes FLR (2019) Avaliação da atividade antibacteriana de extratos de Eugenia brasiliensis. Fundação Oswaldo Cruz.
- Mendes FLR, Carvalho EM de, Abrantes JA, Nogueira JM da R (2020) Buscando novos antimicrobianos: avaliação da atividade antibacteriana de extratos de Eugenia brasiliensis. Rev Bras Análises Clínicas 52:. [CrossRef]
- Mendes R de F, Pinto N de CC, da Silva JM, et al (2017) The essential oil from the fruits of the Brazilian spice Xylopia sericea A. St.-Hil. presents expressive in-vitro antibacterial and antioxidant activity. J Pharm Pharmacol 69:341–348. [CrossRef]
- Menezes L, Costa C, Rodrigues A, et al (2016) Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules 21:890. [CrossRef]
- Menezes R, Sessions Z, Muratov E, et al (2021) Secondary metabolites extracted from annonaceae and chemotaxonomy study of terpenoids. J Braz Chem Soc 00:1–10. [CrossRef]
- Meng DH, Xu YP, Chen WL, et al (2007) Anti-tumour clerodane-type diterpenes from Mitrephora thorelii. J Asian Nat Prod Res 9:679–684. [CrossRef]
- Mikolajczak KL, McLaughlin JL, Rupprecht JK (1988) Control of pests with annonaceous acetogenins.
- Moghadamtousi S, Fadaeinasab M, Nikzad S, et al (2015a) Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int J Mol Sci 16:15625–15658. [CrossRef]
- Moghadamtousi SZ, Fadaeinasab M, Nikzad S, et al (2015b) Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci 16:15625–15658. [CrossRef]
- Moghadamtousi SZ, Goh BH, Chan CK, et al (2013) Biological activities and phytochemicals of Swietenia macrophylla king. Molecules 18:10465–10483. [CrossRef]
- Moghadamtousi SZ, Rouhollahi E, Karimian H, et al (2014) Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther 8:2099–2111. [CrossRef]
- Mohammadi S, Jafari B, Asgharian P, et al (2020) Medicinal plants used in the treatment of Malaria: A key emphasis to Artemisia, Cinchona, Cryptolepis, and Tabebuia genera. Phyther Res 1–14. [CrossRef]
- Mohd M, Alam KS, Mohd A, et al (2009) Antidaiabetic activity of the aqueous extract o Annona squamosa in streptozotocin induced-hyperglycemic rats. Pharma Res 2:59–63.
- Montenegro C de A, Lima GR de M, Gomes IF, et al (2014) Gastroprotective effect of Xylopia langsdorffiana A. St.-Hil. & Tul. (Annonaceae): Involvement of endogenous sulfhydryls compounds and nitric oxide. Rec Nat Prod 8:165–183.
- Montenegro H, Gutiørrez M, Romero LI, et al (2003) - G. amplifolia e G. dumetorum (2003) - Montenegro - Alcaloides - atividade antileishmania. 677–679.
- Monzon RB, Alvior JP, Luczon LL, et al (1994) Larvicidal potential of five Philippine plants against Aedes aegypti (Linnaeus) and Culex quinquefasciatus (Say). Southeast Asian J Trop Med Public Health 25:755–759.
- Morales CA, González R, Aragón R (2004) Evaluación de la actividad larvicida de extractos polares y no polares de acetogeninas de annona muricata sobre larvas de Aedes aegypti y Anopheles albimanus (Diptera: Culicidae). Rev Colomb Entomol 30:187–192.
- Moreira JLB, Carvalho CBM de, Frota cristiane C (2015) Visualização bacteriana e colorações. Fortaleza.
- Moshi MJ, Joseph CC, Innocent E, Nkunya MHH (2004) In vitro antibacterial and antifungal activities of extracts and compounds from Uvaria scheffleri. Pharm Biol 42:269–273. [CrossRef]
- Muganza DM, Fruth B, Nzunzu JL, et al (2016) In vitro antiprotozoal activity and cytotoxicity of extracts and isolated constituents from Greenwayodendron suaveolens. J Ethnopharmacol 193:510–516. [CrossRef]
- Musuyu Muganza D, Fruth BI, Nzunzu Lami J, et al (2015) In vitro antiprotozoal activity and cytotoxicity of extracts and fractions from the leaves, root bark and stem bark of Isolona hexaloba. J Ethnopharmacol 174:187–194. [CrossRef]
- Nascimento TL do, Vasconcelos SP, Peres Y, et al (2019) Prevalence of malaria relapse: Systematic review with meta-analysis. Rev Lat Am Enferm 18:e3111.
- Nathan C, Ding A (2010) Nonresolving Inflammation. Cell 140:871–882. [CrossRef]
- Ndumu PA, George JBD, Choudhury MK (1999) Toxicity of neem seed oil (Azadiracta indica) against the larvae ofAmblyomma variegatum a three-host tick in cattle. Phyther Res 13:532–534. [CrossRef]
- Neill JO (2016) Nature reviews. Drug Discov 15:526.
- Newman DJ, Cragg GM (2016) Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 79:629–661.
- Newman DJ, Cragg GM (2020) Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. [CrossRef]
- Ngangoue MO, Ngameni B, Ambassa P, et al (2020) A phenanthridin-6(5H)-one derivative and a lanostane-type triterpene with antibacterial properties from Anonidium mannii (Oliv). Engl. & Diels (Annonaceae). Nat Prod Res 0:1–10. [CrossRef]
- Ngantchou I, Nkwengoua E, Nganso Y, et al (2009) Antitrypanosomal activity of polycarpol from Piptostigma preussi (Annonaceae). Fitoterapia 80:188–191. [CrossRef]
- Nghi NBT, Uyen TT, Anh HM, et al (2022) Rumdul (Sphaerocoryne affinis) Antioxidant Activity and Its Potential for Parkinson’s Disease Treatment. Oxid Med Cell Longev 2022:. [CrossRef]
- Ngouonpe AW, Mbobda ASW, Happi GM, et al (2019) Natural products from the medicinal plant Duguetia staudtii (Annonaceae). Biochem Syst Ecol 83:22–25. [CrossRef]
- Nguyen HT, Vu T-Y, Chandi V, et al (2020) Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci Rep 10:15965. [CrossRef]
- Nhiem NX, Hien NTT, Tai BH, et al (2015) New ent-kauranes from the fruits of Annona glabra and their inhibitory nitric oxide production in LPS-stimulated RAW264.7 macrophages. Bioorganic Med Chem Lett 25:254–258. [CrossRef]
- Nhiem NX, Tuong NT, Ky PT, et al (2017) Chemical Components from Phaeanthus vietnamensis and Their Inhibitory NO Production in BV2 Cells. Chem Biodivers 14:1–7. [CrossRef]
- Núcleo de estudos e Pesquisas de Produtos Naturais (2020) Atividade Antitumoral. Univ Fed Sergipe - UFS.
- Núñez-Sellés AJ (2005) Antioxidant therapy: Myth or reality? J Braz Chem Soc 16:699–710. [CrossRef]
- Nwokocha CR, Owu DU, Gordon A, et al (2012) Possible mechanisms of action of the hypotensive effect of Annona muricata (soursop) in normotensive Sprague–Dawley rats. Pharm Biol 50:1436–1441. [CrossRef]
- Nwosu RA, Suleiman MM, Makun HJ, et al (2022) Anthelmintic activity of methanol extract of Dennettia tripetala G. Baker (Annonaceae) fruits against Haemonchus contortus in red Sokoto goats. Trop Anim Health Prod 54:. [CrossRef]
- Obiri DD, Osafo N (2013) Aqueous ethanol extract of the fruit of Xylopia aethiopica (Annonaceae) exhibits anti-anaphylactic and anti-inflammatory actions in mice. J Ethnopharmacol 148:940–945. [CrossRef]
- Odalo JO, Omolo MO, Malebo H, et al (2005) Repellency of essential oils of some plants from the Kenyan coast against Anopheles gambiae. Acta Trop 95:210–218. [CrossRef]
- Ohashi M, Amoa-Bosompem M, Kwofie KD, et al (2018) In vitro antiprotozoan activity and mechanisms of action of selected G hanaian medicinal plants against Trypanosoma , Leishmania , and Plasmodium parasites. Phyther Res 32:1617–1630. [CrossRef]
- Okonkwo EU, Okoye WI (2001) Insecticidal activity of Dennettia tripetaia Baker f. and Piper guineense Schum. and Thonn. against Dermestes maculatus Degeer (Coleoptera: Dermestidae) and Necrobia rufipes Degeer (Coleoptera: Cleridae) on dried fish. Niger J Entomol 18:. [CrossRef]
- Okoye TC, Akah PA, Omeje EO, et al (2013) Anticonvulsant effect of kaurenoic acid isolated from the root bark of Annona senegalensis. Pharmacol Biochem Behav 109:38–43. [CrossRef]
- Okpekon TA, Dade JME, Say M V, et al (2015) Bingervone, an antiprotozoal beta-triketone derivative from the roots of Uvaria afzelii (Annonaceae). Int J Pharm Sci Res 6:4210–4215. [CrossRef]
- Olate VR, Pertino MW, Theoduloz C, et al (2012) New Gastroprotective Labdeneamides from. Planta Med 13:362–367.
- Oliveira ÂC de, Pereira MJB (2009) Efeito Antialimentar do Extrato Metanólico de Annona crassiflora Mart . sobre o Percevejo Marrom Euschistus heros ( Fabr . 1798 ) ( Heteroptera : Pentatomidae ). Rev Bras Agroecol 4:2633–2636.
- Oliveira de Souza LI, Bezzera-Silva PC, do Amaral Ferraz Navarro DM, et al (2017) The chemical composition and trypanocidal activity of volatile oils from Brazilian Caatinga plants. Biomed Pharmacother 96:1055–1064. [CrossRef]
- Oliveira ESC, Amaral ACF, Lima ES, Jefferson JR (2014) Chemical composition and biological activities of Bocageopsis multiflora essential oil. J Essent Oil Res 26:161–165. [CrossRef]
- Oliveira GLS (2015) Determinação da capacidade antioxidante de produtos naturais in vitro pelo método do dpph•: Estudo de revisão. Rev Bras Plantas Med 17:36–44. [CrossRef]
- Olivier DK, Van Vuuren SF, Moteetee AN (2015) Annickia affinis and A. chlorantha (Enantia chlorantha) - A review of two closely related medicinal plants from tropical Africa. J Ethnopharmacol 176:438–462. [CrossRef]
- Ortqvist A, Hedlund J, Kalin M (2005) Streptocuccus pneumonie: epidemiology, risk factors, and clinical features. Semin Respir Crit Care Med 26:563–574.
- Osafo N, Biney RP, Obiri DD (2016) Aqueous Ethanol Fruit Extract of Xylopia aethiopica and Xylopic Acid Exhibit Anti-inflammatory Activity Through Inhibition of the Arachidonic Acid Pathway. Pharm Biosci J 49–55. [CrossRef]
- Osafo N, Obiri DD, Danquah KO, et al (2019) Potential effects of xylopic acid on acetic acid-induced ulcerative colitis in rats. Turkish J Gastroenterol 30:732–744. [CrossRef]
- Osorio D EJ, Montoya P. GL, Muñoz D. K, Arango A. GJ (2006) Antiplasmodial Activity of Aporphinic Alkaloids from Rollinia pittieri and Pseudomalmea boyacana (Annonaceae). Vitae 13:49–54.
- Osorio E, Arango GJ, Jiménez N, et al (2007) Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J Ethnopharmacol 111:630–635. [CrossRef]
- Otręba M, Kośmider L (2020) In vitro anticancer activity of fluphenazine, perphenazine and prochlorperazine. A review. J Appl Toxicol.
- Owusu FWA, Boakye-Gyasi M El, Entsie P, et al (2021) Formulation and Pharmaceutical Assessment of Annona muricata Oral Capsules and Suspension as Antidiarrhea Dosage Forms. J Chem 2021:6–11. [CrossRef]
- Oyemitan IA, Elusiyan CA, Akinkunmi EO, et al (2019) Memory enhancing, anticholinesterase and antimicrobial activities of β-phenylnitroethane and essential oil of Dennettia tripetala Baker f. J Ethnopharmacol 229:256–261. [CrossRef]
- Paarakh PM, Chansouria JPN, Khosa RL (2009) Wound healing activity of Annona muricata extract. J Pharm Res 2:404–406.
- Palazzo MC, Wright HL, Agius BR, et al (2009) Chemical Compositions and Biological Activities of Leaf Essential Oils of Six Species of Annonaceae from Monteverde, Costa Rica. Rec Nat Prod 3:153–160.
- Pan S-Y, Zhou S-Z, Gao S-H, et al (2013) New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evidence-Based Complement Altern Med 2013:1–25. [CrossRef]
- Paredes A, Hasegawa M, Prieto F, et al (2001) Biological activity of Guatteria cardoniana fractions. J Ethnopharmacol 78:129–132. [CrossRef]
- Pecoul B, Batista C, Stobbaerts E, et al (2016) The BENEFIT Trial: Where Do We Go from Here? PLoS Negl Trop Dis 10:e0004343. [CrossRef]
- Peña-Hidalgo M, Furtado LC, Costa-Lotufo L V., et al (2021) Alkaloids from the Leaves of Annona crassiflora and Their Cytotoxic Activity. Rev Bras Farmacogn 31:244–248. [CrossRef]
- Pereira F, Madureira AM, Sancha S, et al (2016) Cleistochlamys kirkii chemical constituents: Antibacterial activity and synergistic effects against resistant Staphylococcus aureus strains. J Ethnopharmacol 178:180–187. [CrossRef]
- Pérez-Pacheco R, Rodríguez Hernández C, Lara-Reyna J, et al (2004) Toxicidad de aceites, esencias y extractos vegetales en larvas de mosquito Culex quinquefasciatus Say (Diptera: Culicidae). Acta zoológica Mex 20:141–152.
- Piacente S, Aquino R, Tommasi N, et al (1994) Constituents of Weneria ciliolata and their in vitro anti-HIV activity. Phytochemistry 36:4–9.
- Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042.
- Pinheiro MLB, Xavier CM, Souza ADL De, et al (2009) Acanthoic Acid and other Constituents from the Stem of. J Braz Chem Soc 20:1095–1102.
- Pinheiro R da S, Rabelo SV, de Oliveira AP, et al (2016) Phytochemical screening and evaluation of cytotoxicity of stem bark extracts of Anaxagorea dolichocarpa and Duguetia chrysocarpa (Annonaceae). Trop J Pharm Res 15:793–798. [CrossRef]
- Polbuppha I, Suthiphasilp V, Maneerat T, et al (2022) Nitric oxide production inhibitory activity of clerodane diterpenes from Monoon membranifolium. Nat Prod Res 36:2513–2517. [CrossRef]
- Popenoe W (1921) The native home of the cherimoya. J Hered 12:331–336.
- Pouofo Nguiam M, Njonte Wouamba SC, Longo F, et al (2021) Antibacterial and antishigellosis activity of Xylopia staudtii (engl. & diels), Annonaceae. J Ethnopharmacol 280:114406. [CrossRef]
- Prado A (2009) Composição fenólica e atividade antioxidante de frutas tropicais. Universidade de São Paulo.
- Prasad SK, Pradeep S, Shimavallu C, et al (2021) Evaluation of Annona muricata Acetogenins as Potential Anti-SARS-CoV-2 Agents Through Computational Approaches. Front Chem 8:1–7. [CrossRef]
- Pumiputavon K, Chaowasku T, Saenjum C, et al (2017) Cell cycle arrest and apoptosis induction by methanolic leaves extracts of four Annonaceae plants. BMC Complement Altern Med 17:1–11. [CrossRef]
- Qi L-W, Liu E-H, Chu C, et al (2010) Anti-Diabetic Agents from Natural Products — An Update from 2004 to 2009. Curr Top Med Chem 10:434–457. [CrossRef]
- Quintans JSS, Soares B, Ferraz RC, et al (2013) Chemical constituents and anticancer effects of the essential oil from leaves of xylopia laevigata. Planta Med 79:123–130. [CrossRef]
- Rabelo D de M, Pinheiro MLB, Barison A, et al (2014) Isoquinoline alkaloids and investigation of the antibacterial and antiplasmodial activities of Guatteria citriodora (Annonaceae). Quim Nova 37:1453–1458. [CrossRef]
- Rabêlo SV (2014) Revisão de alcalóides do gênero Annona, estudo fitoquímico e avaliação da atividade biológica de Atemoia (Annona cherimola x Annona squamosa ). Universidade Federal do Vale do São Francisco.
- Rabêlo SV, Da Costa MM, Libório RC, Almeida JRGDS (2014) Antioxidant and antimicrobial activity of extracts from atemoia (Annona cherimola Mill. x A. squamosa L.). Rev Bras Frutic 36:265–271. [CrossRef]
- Rady I, Bloch MB, Chamcheu RCN, et al (2018) Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxid Med Cell Longev 2018:. [CrossRef]
- Rajca Ferreira AK, Lourenço FR, Young MCM, et al (2018) Chemical composition and biological activities of Guatteria elliptica R. E. Fries (Annonaceae) essential oils. J Essent Oil Res 30:69–76. [CrossRef]
- Rao NS, Sharma K, Sharma RK (2005) Anti-feedant and growth inhibitory effects of seed extracts of custard apple, Annona squamosa against Khapra Beetle, Trogoderma granarium. J Agric Technol 1:43–54.
- Rejón-Orantes J, González-Esquinca A, de la Mora M, et al (2011) Annomontine, an Alkaloid Isolated from Annona purpurea , Has Anxiolytic-Like Effects in the Elevated Plus-Maze. Planta Med 77:322–327. [CrossRef]
- Rester U (2008) Fom virtually to reality - Virtual screening in lead discovery and lead optimization: a medicinal chemistry perspective. Curr Opin Drug Discov Devel 11:559–568.
- Ribeiro JEL da S, Hopkins MJG, Vicentini A, et al (1999) Flora da reserva Ducke - Guia de Identificação das plantas vasculares de uma floresta de terra-firme na Amazónia Central. INPA-DFID.
- Ribeiro L do P, Vendramim JD, Bicalho KU, et al (2013) Annona mucosa Jacq. (Annonaceae): A promising source of bioactive compounds against Sitophilus zeamais Mots. (Coleoptera: Curculionidae). J Stored Prod Res 55:6–14. [CrossRef]
- Richomme P, Godet MC, Foussard F, et al (1991) A novel leishmanicidal labdane from Polyalthia macropoda. Planta Med 57:552–554. [CrossRef]
- Rinaldi MVN, Díaz IEC, Suffredini IB, Moreno PRH (2017) Alkaloids and biological activity of beribá (Annona hypoglauca). Rev Bras Farmacogn 27:77–83. [CrossRef]
- Riss TL, Moravec RA (2004) Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev Technol 2:51–62.
- Rocha RS, Kassuya CAL, Formagio ASN, et al (2016) Analysis of the anti-inflammatory and chemopreventive potential and description of the antimutagenic mode of action of the Annona crassiflora methanolic extract. Pharm Biol 54:35–47. [CrossRef]
- Rodrigues AM, da Silva AA, de Freitas JCC, et al (2021) Larvicidal activity of Annona mucosa Jacq. extract and main constituents rolliniastatin 1 and rollinicin against Aedes aegypti and Aedes albopictus. Ind Crops Prod 169:. [CrossRef]
- Rodrigues AMS, De Paula JE, Degallier N, et al (2006) Larvicidal activity of some Cerrado plant extracts against Aedes aegypti. J Am Mosq Control Assoc 22:314–317. [CrossRef]
- Rogero SO, Lugão AB, Ikeda TI, Cruz ÁS (2003) Teste in vitro de citotoxicidade: estudo comparativo entre duas metodologias. Mater Res 6:317–320. [CrossRef]
- Rojano BA, GAVIRIA M CA, SÁEZ V JA, et al (2007) Berenjenol isolated from Oxandra cf xylopioides (Annonaceae) as insecticide. Vitae 14:95–100.
- Sacchetti G, Maietti S, Muzzoli M, et al (2005) Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem 91:621–632. [CrossRef]
- Saepou S, Pohmakotr M, Reutrakul V, et al (2010) Anti-HIV1 Diterpenoids from Leaves and Twigs of Polyalthia sclerophylla. Planta Med 76:721–725. [CrossRef]
- Sahu M, Sahoo NK, Alagarsamy V, Rath BP (2016) Comparative Evaluation of Antidiabetic and Antioxidant Activities of Aqueous Fruit Peel and Leaf Extracts of Annona Squamosa on High Fat Diet and Multiple Low Dose Streptozotocin Mouse Model of Diabetes. Austin J Pharmacol Ther 4:1081.
- Saito ML, Pott A, Ferraz JMG, Nascimento R dos S (2004) Avaliação de plantas com atividade deterrente alimentar em Spodoptera frugiperda (J.E.SMITH) E Anticarsia gemmatalis HUBNER. Pestic Rev Ecotoxicologia e Meio Ambient 14:1–10. [CrossRef]
- Saldanha AA, Vieira L, de Oliveira FM, et al (2020) Anti-inflammatory and central and peripheral anti-nociceptive activities of α-asarone through the inhibition of TNF-α production, leukocyte recruitment and iNOS expression, and participation of the adenosinergic and opioidergic systems. Inflammopharmacology 28:1039–1052. [CrossRef]
- Saldanha AA, Vieira L, Maia DS da S, et al (2021) Anti-inflammatory and antinociceptive activities of a phenylpropanoid-enriched fraction of Duguetia furfuracea. Inflammopharmacology 29:409–422. [CrossRef]
- Saldanha AA, Vieira L, Ribeiro RIM de A, et al (2019) Chemical composition and evaluation of the anti-inflammatory and antinociceptive activities of Duguetia furfuracea essential oil: Effect on edema, leukocyte recruitment, tumor necrosis factor alpha production, iNOS expression, and adenosinergic and opioid. J Ethnopharmacol 231:325–336. [CrossRef]
- Sales PA, Molina I, Murta SMF, et al (2017) Experimental and Clinical Treatment of Chagas Disease: A Review. Am J Trop Med Hyg 97:1289–1303. [CrossRef]
- Samwel S, Odalo JO, Nkunya MHH, et al (2011) Toussaintines A-E: Antimicrobial indolidinoids, a cinnamoylhydrobenzofuranoid and a cinnamoylcyclohexenoid from Toussaintia orientalis leaves. Phytochemistry 72:1826–1832. [CrossRef]
- Santos AR, Benghi TGS, Nepel A, et al (2017) In vitro Antiproliferative and Antibacterial Activities of Essential Oils from Four Species of Guatteria. Chem Biodivers 14:. [CrossRef]
- Santos F, Magalhães-Junior JT, Carneiro I de O, et al (2022) Eco-epidemiology of vectorial Trypanosoma cruzi transmission in a region of northeast Brazil. Acta Trop 225:106184. [CrossRef]
- Santos L de Á, Cavalheiro AJ, Tempone AG, et al (2015) Antitrypanosomal acetylene fatty acid derivatives from the seeds of Porcelia macrocarpa (Annonaceae). Molecules 20:8168–8180. [CrossRef]
- Santos MRA, Lima RA, Silva AG, et al (2010) Composição química e atividade inseticida do extrato acetônico de Piper alatabaccum Trel&Yuncker (Piperaceae) sobre Hypothenemus hampei Ferrari. Rev Bras Plantas Med 15:332–336.
- Santos SS, Araújo RV, Giarolla J, et al (2020) Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: a review. Int J Antimicrob Angents 55:105906. [CrossRef]
- Saxena RC, Harshan V, Saxena A, et al (1993) Larvicidal and chemosterilant activity of Annona squamosa alkaloids against Anopheles stephensi. Journal-American Mosq Control Assoc 9:84.
- Schmeda-Hirschmann G, de Arias AR (1992) A screening method for natural products on triatomine bugs. Phyther Res 6:68–73.
- Seangphakdee P, Pompimon W, Meepowpan P, et al (2013) Anti-inflammatory and anticancer activities of (−)-zeylenol from stems of Uvaria grandiflora. ScienceAsia 39:610. [CrossRef]
- Sedgwick AD, Lees P (1986) A comparison of air pouch, sponge and pleurisy models of acute carrageenan inflammation in the rat. Agents Actions 18:439–446. [CrossRef]
- Serhan CN (2010) Novel Lipid Mediators and Resolution Mechanisms in Acute Inflammation. Am J Pathol 177:1576–1591. [CrossRef]
- Sesang W, Punyanitya S, Pitchuanchom S, et al (2014) Cytotoxic Aporphine Alkaloids from Leaves and Twigs of Pseuduvaria trimera (Craib). Molecules 19:8762–8772. [CrossRef]
- Shanker KS, Kanjilal S, Rao BVSK, et al (2007) Isolation and antimicrobial evaluation of isomeric hydroxy ketones in leaf cuticular waxes of annona squamosa. Phytochem Anal 18:7–12. [CrossRef]
- Sharifi-Rad M, Roberts TH, Matthews KR, et al (2018) Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phyther. Res. 32:2131–2145.
- Sharma PP, Pardeshi AB, Vijigiri D (2011) Bioactivity of some medicinal plant extracts against Musca domestica L. J Ecobiotechnology.
- Sheen E, Triadafilopoulos G (2011) Adverse Effects of Long-Term Proton Pump Inhibitor Therapy. Dig Dis Sci 56:931–950. [CrossRef]
- Sherwood ER, Toliver-Kinsky T (2004) Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol 18:385–405. [CrossRef]
- Shirwaikar A, Rajendran K, Dinesh Kumar C, Bodla R (2004) Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J Ethnopharmacol 91:171–175. [CrossRef]
- Sidrim JJC, Rocha MFG (2004) Aspectos gerais de fungos filamentosos e dimórficos na apresentação filamentosa. In: Editora Guanabara Koogan (ed) Micologia médica à luz de autores com=ntemporâneos, 1st editio. Rio de Janeiro - RJ, pp 8–88.
- Silprakob W, Sukhamsri N, Kuhakarn C, et al (2018) A new oxoaporphine alkaloid from the root of dasymaschalon glaucum. Nat Prod Commun 13:1471–1474. [CrossRef]
- Silva AG, Rocha LC, Canniatti Brazaca SG (2009) Caracterização físico-química, digestibilidade proteica e atividade antioxidante de feijão (Phaseolus vulgaris L.). Aliment e Nutr 20:591–598.
- Silva CA, Domingues Neta AM (2011) Aspectos reprodutivos e visitantes florais de Duguetia marcgraviana Mart. (Annonaceae) na região sudoeste de Mato Grosso. Biotemas 23:69–76. [CrossRef]
- Silva LA da, Raposo JDA, Campos LPG, et al (2018) Atividade antioxidante do óleo essencial de Myrcia sylvatica (G. Mey.) DC. por diferentes métodos de análises antioxidantes (ABTS, DPPH, FRAP, β-caroteno/ácido linoleico). Rev Fitos 12:117–126. [CrossRef]
- Silva ITSS, Fernandes MJB, Oliveira RA, et al (2016) Annona squamosa L. (Annonaceae): Chemical bioprospection and biological activity in two phenological stages. Appl Ecol Environ Res 14:133–147. [CrossRef]
- Silva JC, Araújo C de S, de Lima-Saraiva SRG, et al (2015) Antinociceptive and anti-inflammatory activities of the ethanolic extract of Annona vepretorum Mart. (Annonaceae) in rodents. BMC Complement Altern Med 15:197. [CrossRef]
- Silva MO (2015) Atividade antioxidante e composição de oligossacarídeos em subproduto obtido do processamento industrial da goiaba (Psidium guajava). Universidade Estadual de Campinas.
- Silva NL, Saldanha AA, Silva DB, et al (2019) Anti-inflammatory, antinociceptive and antioxidant activities of the hydromethanolic fraction from Annona nutans leaves. Biosci J 35:1599–1613. [CrossRef]
- Simões CM de O, Schenkel EP, Gosmann G, et al (2003) Farmacognocia: da planta ao medicamento, Fifth edit. UFRGS editora.
- Simões S, Lopes R, Campos MCD, et al (2019) Animal models of acute gastric mucosal injury: Macroscopic and microscopic evaluation. Anim Model Exp Med 2:ame2.12060. [CrossRef]
- Sinchaisri N, Roongsook D, Chungsamarnyart N (1991) Insecticidal Activity of plant crude-extracts on Diamondback Moth Larvae. Kasetsart J. (Nat. Sci. Suppl.) 25:106–110.
- Siqueira CAT, Oliani J, Sartoratto A, et al (2011) Chemical constituents of the volatile oil from leaves of Annona coriacea and in vitro antiprotozoal activity. Rev Bras Farmacogn 21:33–40. [CrossRef]
- Siqueira CAT, Serain AF, Pascoal ACRF, et al (2015) Bioactivity and chemical composition of the essential oil from the leaves of Guatteria australis A.St.-Hil. Nat Prod Res 29:1966–1969. [CrossRef]
- Soerio M de NC (2022) Perspectives for a new drug candidate for Chagas disease therapy. Mem Inst Oswaldo Cruz 117:e220004. [CrossRef]
- Somdej Kanokmedhakul *, Kwanjai Kanokmedhakul, Daungrudee Yodbuddee and, Phonkerd N (2003) New Antimalarial Bis-dehydroaporphine Alkaloids from Polyalthia debilis†. [CrossRef]
- Son NT, Le TA, Thuy DTT, et al (2021) Essential Oils of the Leaf and Stem of Polyalthia viridis Craib and Their Biological Activities. Nat. Prod. Commun. 16.
- Song CM, Lim SJ, Tong JC (2009) Recnt advances in computer-aided drug design. Brief Bioinform 10:579–591.
- Sousa MSB, VIeira LM, Lima A (2011) Fenólicos totais e capacidade antioxidante in vitro de resíduos de polpas de frutas tropicais. Brazilian J Food Technol 14:202–210.
- Sousa O V., Del-Vechio-Vieira G, Alves MS, et al (2012) Chemical composition and biological activities of the essential oils from Duguetia lanceolata St. Hil. barks. Molecules 17:11056–11066. [CrossRef]
- Souza, E. M.; Cordeiro, J. R.; Pereira MJB (2007) Avaliação da atividade inseticida dos diferentes extratos das sementes de Annona coriaceae sobre Dichelops melacanthus (Dallas, 1851). In: Resumos do V CBA - Manejo de agroecossistemas sustentáveis. Rev Bras Agroecol 2:1107–1110.
- Spletozer AG, Santos CR dos, Sanches LA, Garlet J (2021) Plantas com potencial inseticida: enfoque em espécies amazônicas. Ciência Florest 31:974–997. [CrossRef]
- Sreeletha C, Geetha PR (2012) Pesticidal effects of Annona squamosa L. on male Oryctes rhinoceros Linn.(Coleoptera: Scarabaeidae) in relation to reproduction. Curr Biot 6:8–21.
- Suedee A, Mondranondra IO, Kijjoa A, et al (2007) Constituents of Polyalthia jucunda and their cytotoxic effect on human cancer cell lines. Pharm Biol 45:575–579. [CrossRef]
- Suthiphasilp V, Maneerat T, Andersen RJ, et al (2021) α-Glucosidase inhibitory activity of compounds isolated from the twig and leaf extracts of Desmos dumosus. Heliyon 7:e06180. [CrossRef]
- Taha H, Arya A, Khan AK, et al (2018) Effect of Pseuduvaria macrophylla in attenuating hyperglycemia mediated oxidative stress and inflammatory response in STZ-nicotinamide induced diabetic rats by upregulating insulin secretion and glucose transporter-1, 2 and 4 proteins expression. J Appl Biomed 16:263–273. [CrossRef]
- Talapko J, Škrlec I, Alebić T, et al (2019) Malaria: The past and the present. Microorganisms 7:. [CrossRef]
- Tamfu AN, Tagatsing Fotsing M, Talla E, et al (2021) Bioactive constituents from seeds of Annona Senegalensis Persoon (Annonaceae). Nat Prod Res 35:1746–1751. [CrossRef]
- Tan LTH, Lee LH, Yin WF, et al (2015) Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evidence-Based Complement Altern Med 2015:1–30. [CrossRef]
- Tavares JF, Queiroga KF, Silva MVB, et al (2006) ent-trachylobane diterpenoids from Xylopia langsdorffiana. J Nat Prod 69:960–962. [CrossRef]
- Tekuri S, Pasupuleti S, Konidala K, Pabbaraju N (2019) Pharmacological Effects of Polyalthia cerasoides (Roxb.) Bedd.: a brief Review. J Complement Med Res 10:38. [CrossRef]
- Teo SP, Bhakta S, Stapleton P, Gibbons S (2020) Bioactive compounds from the bornean endemic plant goniothalamus longistipetes. Antibiotics 9:1–11. [CrossRef]
- Tolosa D, Colom OA, Bardón A, Neske A (2012) Insecticidal effects of acetogenins from Rollinia occidentalis seed extract. Nat Prod Commun 7:1934578X1200701226.
- Tomasz A (1997) Antibiotic resistance in Streptococcus pneumonie. Clin Infect Dis 24:S85-88.
- Toto Blessing L Di, Colom OÁ, Popich S, et al (2010) Antifeedant and toxic effects of acetogenins from Annona montana on Spodoptera frugiperda. J Pest Sci (2004) 83:307–310. [CrossRef]
- Trieu QH, Pham VC, Retailleau P, et al (2021) Rare flavonoids and sesquiterpenoids isolated from the leaves of Goniothalamus gracilipes. Fitoterapia 155:105034. [CrossRef]
- Tsiang F, Li PT, Huong LT, et al (2022) Annonaceae Essential Oils : Antimicrobial and Compositions of the , Nguyen Thanh Chung Do Ngoc Dai. 4:387–392.
- Tuchinda P, Munyoo B, Pohmakotr M, et al (2006) Cytotoxic styryl-lactones from the leaves and twigs of Polyalthia crassa. J Nat Prod 69:1728–1733. [CrossRef]
- Tulassay Z, Herszényi L (2010) Gastric mucosal defense and cytoprotection. Best Pract Res Clin Gastroenterol 24:99–108. [CrossRef]
- Twilley D, Rademan S, Lall N (2020) A review on traditionally used South African medicinal plants, their secondary metabolites and their potential development into anticancer agents. J Ethnopharmacol 113101.
- Umeotok SBA, Isah MD, Ukeh JA, Udo IA (2013) Evaluation of the insecticidal and deterrence properties of pepper fruit, Dennettia tripetala (G. Baker) and Ginger Zingiber officinale Roscoe against maize weevil Sitophilus zeamais (Motsch.). J Biol Agric Healthc 3:76–82.
- Valencia L, Muñoz DL, Robledo SM, et al (2011) Trypanocidal and cytotoxic activity of extracts of Colombian plants. Biomedica 31:552–559. [CrossRef]
- Vannier-Santos MA, Brunoro G V., Soeiro M de NC, et al (2019) Parasite, Compartments, and Molecules: Trick versus Treatment on Chagas Disease. In: Biology of Trypanosoma cruzi , 1st edition. IntechOpen.
- Vendramini-Costa DB, Monteiro KM, Iwamoto LH, et al (2014) Gastroprotective effects of goniothalamin against ethanol and indomethacin-induced gastric lesions in rats: Role of prostaglandins, nitric oxide and sulfhydryl compounds. Chem Biol Interact 224:206–212. [CrossRef]
- Viegas C (2003) Terpenes with insecticidal activity: An alternative to chemical control of insects. Quim Nova 26:390–400. [CrossRef]
- Viegas Júnior C (2003) Terpenos com atividade inseticida: uma alternativa para o controle químico de insetos. Quim Nova 26:390–400.
- Vila-Nova NS, de Morais SM, Falcão MJC, et al (2011) Atividade leishmanicida e citotoxicidade de constituintes quimicos de duas especies de annonaceae cultivadas no Nordeste do Brasil. Rev Soc Bras Med Trop 44:567–571. [CrossRef]
- Volobuff CRF, Pederiva MMC, Benites RSR, et al (2019) Bioguided Fractionation, and Antioxidant, Antiproliferative, and Anti-Inflammatory Activity of Annona cacans Warm. J Med Food 22:1078–1086. [CrossRef]
- Vuk I, Rajic Z, Zorc B (2008) Malaria and antimalarial drugs. Farm Glas 64:51–60.
- Waechter AI, Cavé A, Hocquemiller R, et al (1999) Antiprotozoal activity of aporphine alkaloids isolated from Unonopsis buchtienii (Annonaceae). Phyther Res 13:175–177. [CrossRef]
- Waechter AI, Ferreira ME, Fournet A, et al (1997) Experimental treatment of cutaneous leishsmaniasis with argentilactone isolated from annona haematantha. Planta Med 63:433–435. [CrossRef]
- Waechter AI, Yaluff G, Inchausti A, et al (1998) Leishmanicidal and trypanocidal activities of acetogenins isolated from Annona glauca. Phyther Res 12:541–544. [CrossRef]
- Wafo P, Nyasse B, Fontaine C (1999) A 7,8-dihydro-8-hydroxypalmatine from Enantia chlorantha. Phytochemistry 50:279–281. [CrossRef]
- Walker N, Nadjm B, Whitty C (2017) Malaria. Medicine (Baltimore) 42:52–58.
- Wang B, Yang Z-F, Zhao Y-L, et al (2018) Anti-Inflammatory Isoquinoline with Bis- seco -aporphine Skeleton from Dactylicapnos scandens. Org Lett 20:1647–1650. [CrossRef]
- Wang B, Zhao Y-J, Zhao Y-L, et al (2020) Exploring Aporphine as Anti-inflammatory and Analgesic Lead from Dactylicapnos scandens. Org Lett 22:257–260. [CrossRef]
- Weber C, Noels H (2011) Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17:1410–1422. [CrossRef]
- White NJN, Pukrittayakamee S, Hien TTT, et al (2014) Malaria. Lancet 383:723–735.
- Williams RB, Hu JF, Olson KM, et al (2010) Antibiotic indole sesquiterpene alkaloid from Greenwayodendron suaveolens with a new natural product framework. J Nat Prod 73:1008–1011. [CrossRef]
- Wilson A, Trumpp A (2006) Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6:93–106. [CrossRef]
- Woguem V, Fogang HPD, Maggi F, et al (2014) Volatile oil from striped African pepper (Xylopia parviflora, Annonaceae) possesses notable chemopreventive, anti-inflammatory and antimicrobial potential. Food Chem 149:183–189. [CrossRef]
- Wongsomboon P, Rattanajak R, Kamchonwongpaisan S, et al (2021) Unique polyacetylenic ester-neolignan derivatives from Mitrephora tomentosa and their antimalarial activities. Phytochemistry 183:112615. [CrossRef]
- Woo MH, Kim DH, McLaughlin JL (1999) Asitrilobins A and B: Cytotoxic mono-THF annonaceous acetogenins from the seeds of Asimina triloba. Phytochemistry 50:1033–1040. [CrossRef]
- Woolf A, Rose R (2020) Gastric Ulcer - StatPearls - NCBI Bookshelf. In: StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK537128/. Accessed 22 Jul 2020.
- World Health Organization (2021) Chagas disease (also known as American trypanosomiasis). https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). Accessed 16 Feb 2022.
- World Health Organization (WHO) (2014) Antimicrobial resistance. Global Report on Surveillance. In: Bulletin of the World Health Organization. pp 383–384.
- World Health Organization (WHO) (2020) Lack of new antibiotics threatens global efforts to contain drug-resistant infections. Geneva.
- World Health Organization (WHO) (2019) World malaria report 2019.
- Wu JH, Wang XH, Yi YH, Lee KH (2003) Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorganic Med Chem Lett 13:1813–1815. [CrossRef]
- X W, Q H, N X, et al (2018) Antioxidative and Anti-Inflammatory Effects of Water Extract of Acrostichum aureum Linn. against Ethanol-Induced Gastric Ulcer in Rats. Evid Based Complement Alternat Med 2018:. [CrossRef]
- Xavier MN, Alves CCF, Cazal C de M, Santos NH (2016) Chemical composition of the volatile oil of Cardiopetalum calophyllum collected in the Cerrado area. Ciência Rural 46:937–942. [CrossRef]
- Yakubu MT, Fayemo HT (2021) Anti-hyperprolactinemic activities of aqueous extract of Uvaria chamae (P. Beauv) roots and associated biochemical changes in chlorpromazine-induced hyperprolactinemic female Wistar rats. J Ethnopharmacol 271:113863. [CrossRef]
- Yang-Chang Wu *,†, Yu-Chun Hung †, Fang-Rong Chang †, et al (1996) Identification of ent-16β,17-Dihydroxykauran-19-oic Acid as an Anti-HIV Principle and Isolation of the New Diterpenoids Annosquamosins A and B from Annona squamosa. [CrossRef]
- Yang YL, Chang FR, Wu CC, et al (2002) New ent-kaurane diterpenoids with anti-platelet aggregation activity from Annona squamosa. J Nat Prod 65:1462–1467. [CrossRef]
- Yang Z, Lu W, Ma X, Song D (2012) Bioassay-guided isolation of an alkaloid with antiangiogenic and antitumor activities from the extract of Fissistigma cavaleriei root. Phytomedicine 19:301–305. [CrossRef]
- Yao LJ, Jalil J, Attiq A, et al (2019) The medicinal uses, toxicities and anti-inflammatory activity of Polyalthia species (Annonaceae). J Ethnopharmacol 229:303–325. [CrossRef]
- Yasmen N, Aziz MA, Tajmim A, et al (2018) Analgesic and Anti-Inflammatory Activities of Diethyl Ether and n-Hexane Extract of Polyalthia suberosa Leaves. Evidence-Based Complement Altern Med 2018:1–8. [CrossRef]
- Yeung C, Mendoza I, Echeverria LE, Baranchuk A (2021) Chagas’ cardiomyopathy and Lyme carditis: Lessons learned from two infectious diseases affecting the heart. Trends Cardiovasc Med 31:233–239. [CrossRef]
- Yu Z, Han C, Song X, et al (2019) Bioactive aporphine alkaloids from the stems of Dasymaschalon rostratum. Bioorg Chem 90:103069. [CrossRef]
- Zaridah MZ, Azah MAN, Rohani A (2006) Mosquitocidal activities of Malaysian plants. J Trop For Sci 74–80.
- Zgoda-Pols JR, Freyer AJ, Killmer LB, Porter JR (2002) Antimicrobial diterpenes from the stem bark of Mitrephora celebica. Fitoterapia 73:434–438. [CrossRef]
- Zhang Y, Li H, Zhang J, et al (2019) The combinatory effects of natural products and chemotherapy drugs and their mechanisms in breast cancer treatment. Phytochem Rev 1–19.
- Zhou D, Yang Q, Tian T, et al (2020) Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: Involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed Pharmacother 126:110075. [CrossRef]
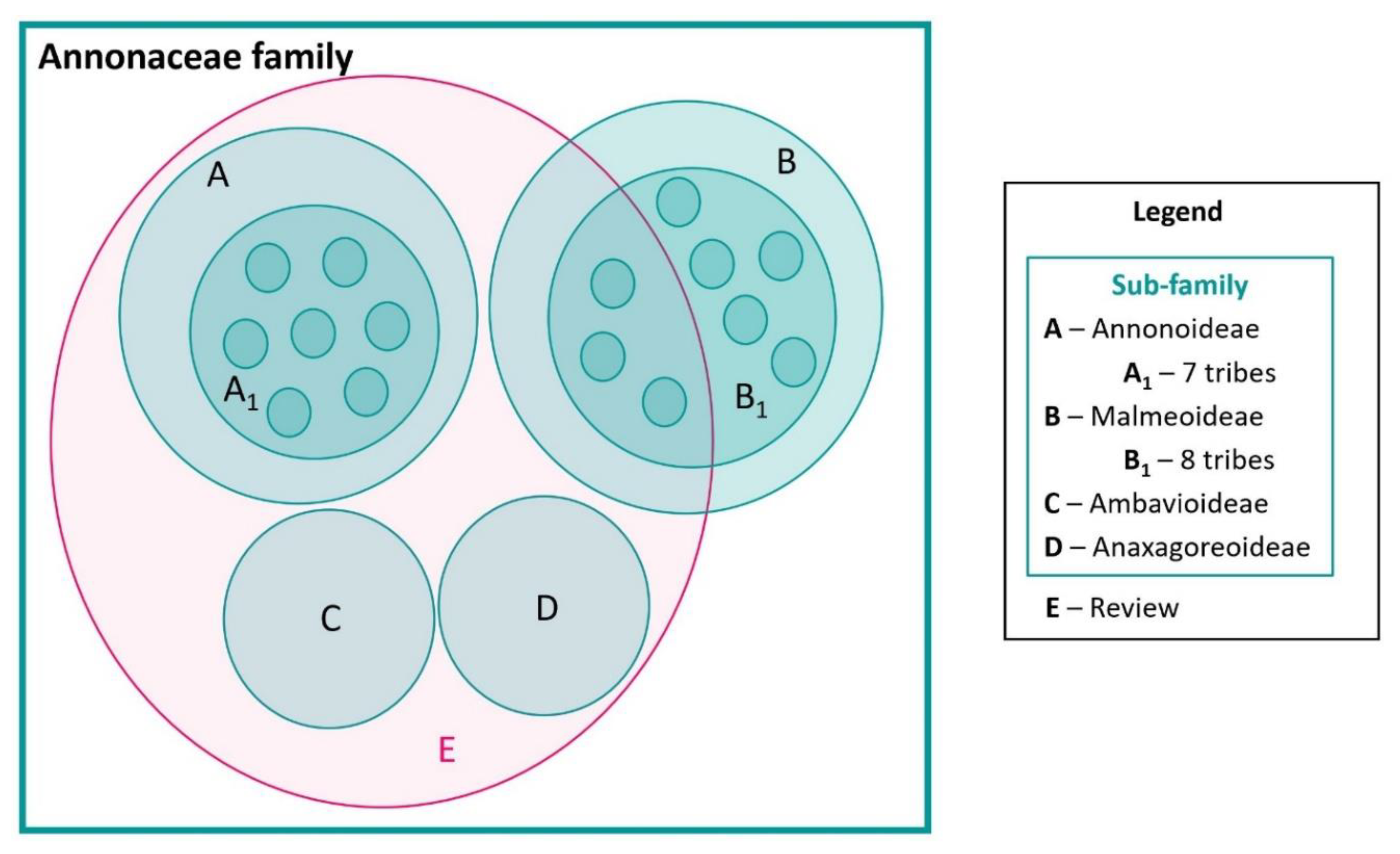
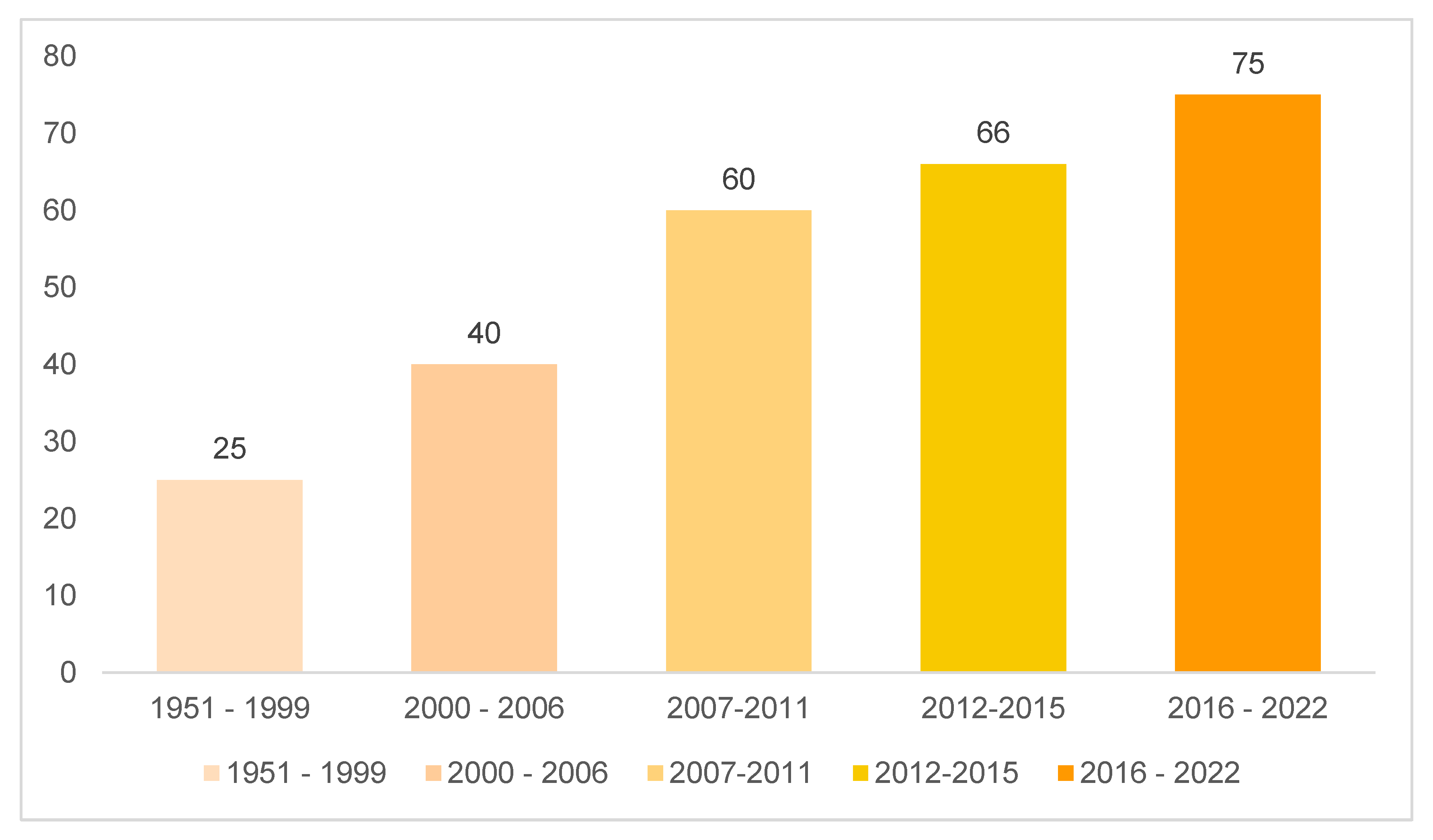
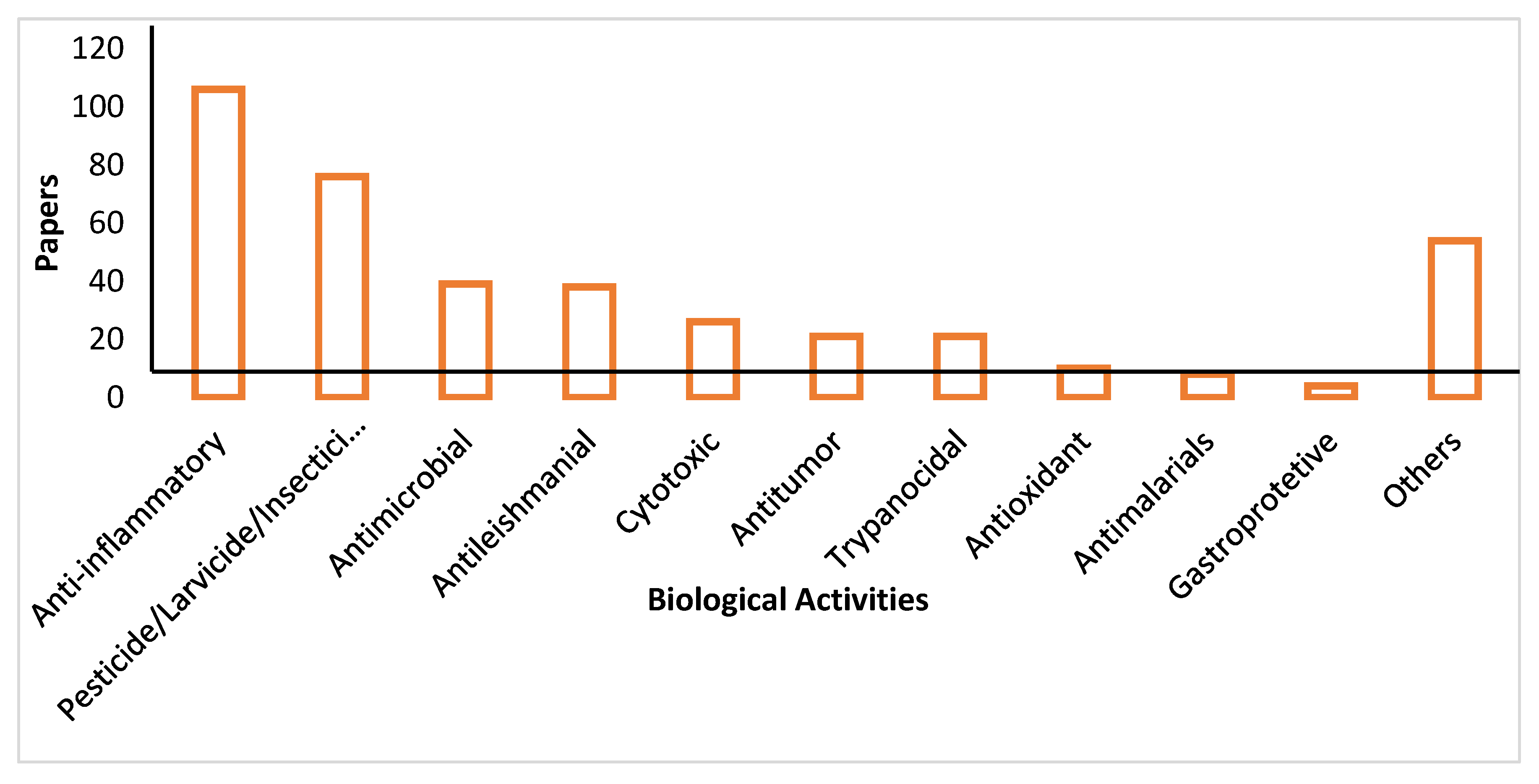
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





