Submitted:
05 November 2025
Posted:
06 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
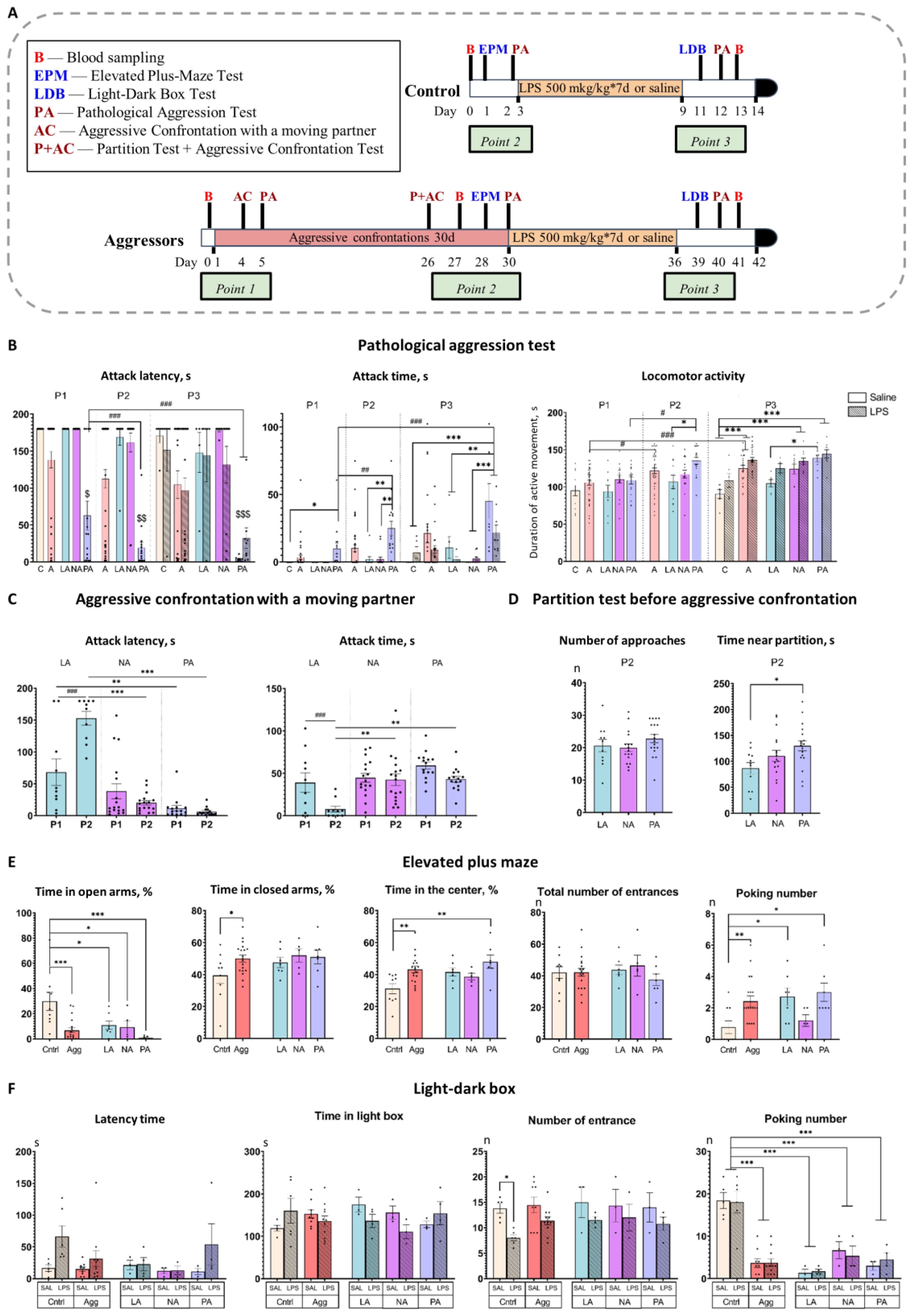
2.1. Prolonged Experience of Aggression Leads to the Development of Three Distinct Patterns of Aggressive Behavior
2.2. Mice Demonstrate Increased Impulsivity, Impaired Decision-Making, And Risk Assessment After Prolonged Experience of Aggression
2.3. Chronic LPS Treatment Fails to Reduce Aggression and Has no Effect on Anxious Behavior
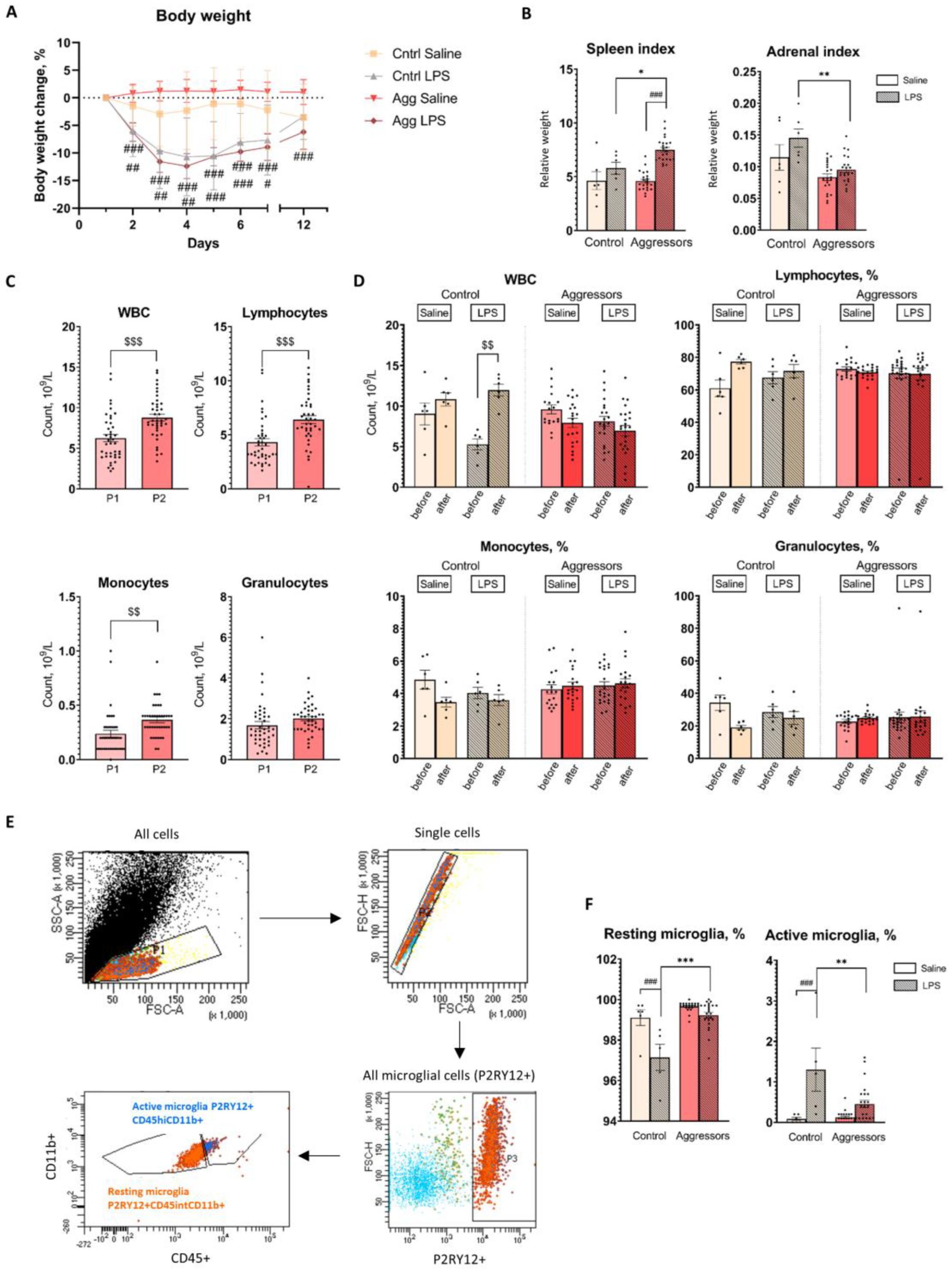
2.4. Aggressive Mice Show Faster Resolution of Inflammation Five Days after Chronic LPS treatment
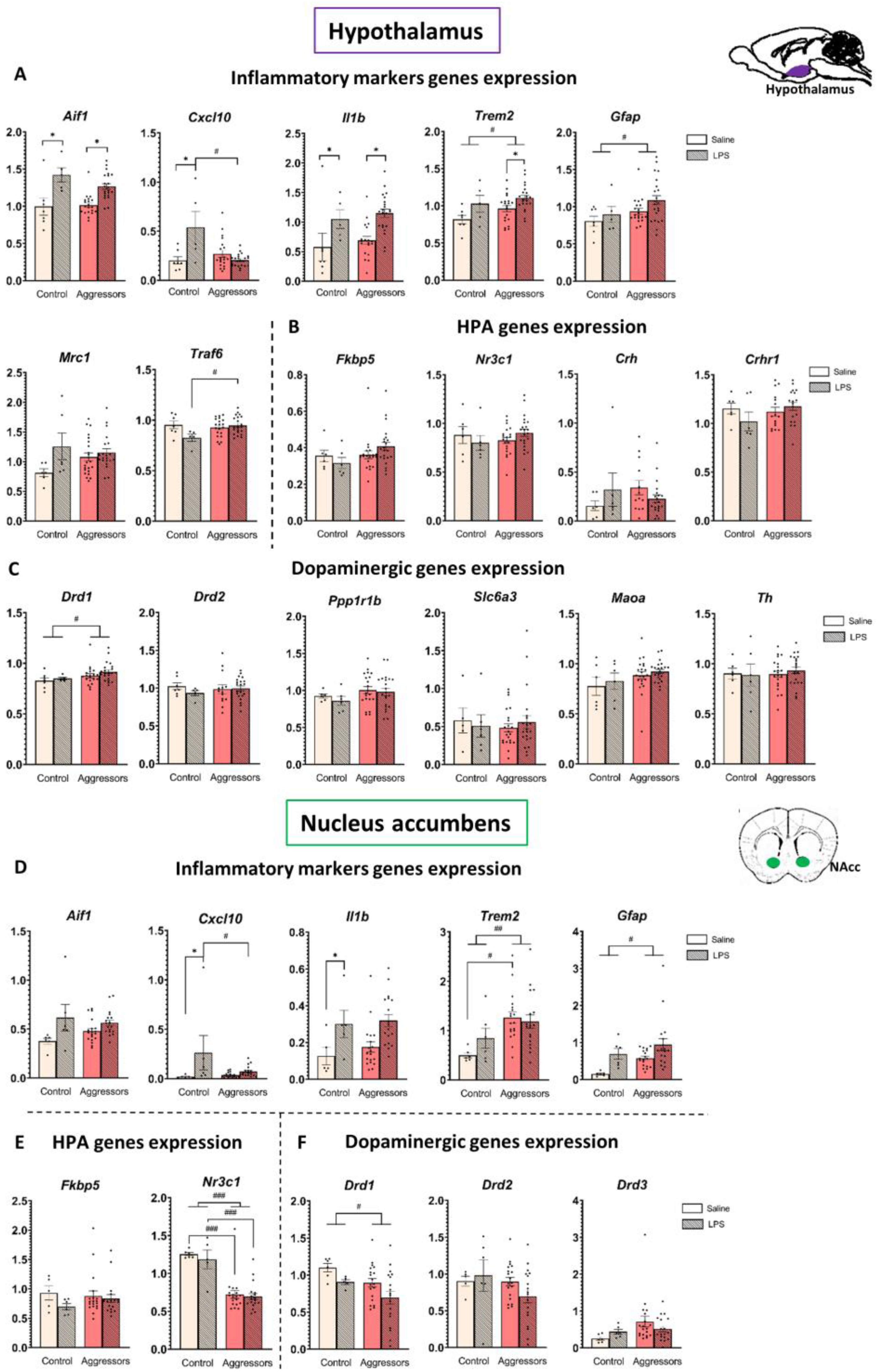
2.5. Expression of Inflammatory Genes After LPS Treatment Is More Pronounced in the Hypothalamus than in the NAc
2.6. Expression of Genes Related to the HPA and Dopaminergic System Is Modulated Differently in Hypothalamus and Nucleus Accumbens and Is Affected by Aggressive Experience, but Not LPS Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Prolonged Aggressive Experience Model
4.3. Experimental Design
4.4. Tissue Collection
4.5. Behavioral Tests
4.5.1. Pathological Aggression Test
4.5.2. Aggressive Confrontation with a Moving Partner
4.5.3. Partition Test
4.5.4. Elevated Plus-Maze Test
4.5.5. Light-Dark Box Test
4.6. Complete Blood count
4.7. Isolation of Microglia and Flow Cytometry Analysis
4.8. Isolation of the Nucleus Accumbens
4.9. RNA Extraction and Real-Time PCR
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| NAc | nucleus accumbens |
| VTA | ventral tegmental area |
| C/Cntrl LA NA PA |
Control Low Aggression mice Non-pathological Aggressors Pathological Aggressors |
References
- Haller, J.; van de Schraaf, J.; Kruk, M. R. Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for 'pathological' aggression? Journal of neuroendocrinology 2001, 13.1, 102–107. [Google Scholar] [CrossRef]
- Haller, J.; Kruk, M.R. Normal and Abnormal Aggression: Human Disorders and Novel Laboratory Models. Neurosci. Biobehav. Rev. 2006, 30, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; De Boer, S.F.; Haller, J. Excessive Aggression as Model of Violence: A Critical Evaluation of Current Preclinical Methods. Psychopharmacology 2013, 226, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Haller, J. The Role of the Lateral Hypothalamus in Violent Intraspecific Aggression—The Glucocorticoid Deficit Hypothesis. Front. Syst. Neurosci. 2017, 12, 26. [Google Scholar] [CrossRef]
- Wrangham, R.W. Two Types of Aggression in Human Evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 245–253. [Google Scholar] [CrossRef]
- Porges, E.C.; Decety, J. Violence as a Source of Pleasure or Displeasure Is Associated with Specific Functional Connectivity with the Nucleus Accumbens. Front. Hum. Neurosci. 2013, 7, 447. [Google Scholar] [CrossRef]
- Chester, D.S.; DeWall, C.N. Combating the Sting of Rejection with the Pleasure of Revenge: A New Look at How Emotion Shapes Aggression. J. Pers. Soc. Psychol. 2017, 112, 413–430. [Google Scholar] [CrossRef]
- Golden, S.A.; Jin, M.; Heins, C.; Venniro, M.; Michaelides, M.; Shaham, Y. Nucleus Accumbens Drd1-Expressing Neurons Control Aggression Self-Administration and Aggression Seeking in Mice. J. Neurosci. 2019, 39, 2482–2496. [Google Scholar] [CrossRef]
- Van Erp, A.M.M.; Miczek, K.A. Aggressive Behavior, Increased Accumbal Dopamine, and Decreased Cortical Serotonin in Rats. J. Neurosci. 2000, 20, 9320–9325. [Google Scholar] [CrossRef]
- Lischinsky, J.E.; Lin, D. Neural Mechanisms of Aggression Across Species. Nat. Neurosci. 2020, 23, 1317–1328. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N. Positive Fighting Experience, Addiction-like State, and Relapse: Retrospective Analysis of Experimental Studies. Aggress. Violent Behav. 2020, 52, 101403. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Lipina, T.V.; Koryakina, L.A. Effects of Haloperidol on Communicative and Aggressive Behavior in Male Mice with Different Experiences of Aggression. Pharmacol. Biochem. Behav. 1999, 63, 229–236. [Google Scholar] [CrossRef]
- Bondar, N.P.; Kudryavtseva, N.N. The Effects of the D1 Receptor Antagonist SCH-23390 on Individual and Aggressive Behavior in Male Mice with Different Experience of Aggression. Neurosci. Behav. Physiol. 2005, 35, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N.; Smagin, D.A.; Bondar, N.P. Modeling Fighting Deprivation Effect in Mouse Repeated Aggression Paradigm. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1472–1478. [Google Scholar] [CrossRef]
- Hsu, Y.; Lee, I.; Lu, C. Prior Contest Information: Mechanisms Underlying Winner and Loser Effects. Behav. Ecol. Sociobiol. 2009, 63, 1247–1257. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N. An Experimental Approach to the Study of Learned Aggression. Aggress. Behav. 2000, 26, 241–256. [Google Scholar] [CrossRef]
- Bondar, N.P.; Kudriavtseva, N.N. Impaired Social Recognition in Male Mice with Repeated Experience of Aggression. Zh. Vyssh. Nerv. Deiat. Im. I. P. Pavlova 2005, 55, 378–384. [Google Scholar]
- Haller, J. Aggression, Aggression-Related Psychopathologies and Their Models. Front. Behav. Neurosci. 2022, 16, 936105. [Google Scholar] [CrossRef]
- Covington, H. E. , 3rd, Newman, E. L., Leonard, M. Z., & Miczek, K. A. Translational models of adaptive and excessive fighting: an emerging role for neural circuits in pathological aggression. F1000Research. 2019, 8, F1000. [Google Scholar] [CrossRef]
- Natarajan, D. , de Vries, H., Saaltink, D.J. et al. Delineation of violence from functional aggression in mice: an ethological approach. Behav Genet 2009, 39, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Bondar, N.P.; Kudryavtseva, N.N. Repeated Experience of Aggression and Consequences of Deprivation in Male Mice. Psikhofarmakol. Biol. Narkol. 2010, 10, 2636–2648. [Google Scholar]
- Mutovina, A.; Sapronova, A.A.; Mezhevalova, P.S. Airiyants, K.A.; Ryabushkina, Y.A.; Salman, R.; Bondar, N.P. “The development of pathological aggression in mice during prolonged experience of aggression: behavioral and molecular changes”. Research gate, 2025; [preprint]. [Google Scholar] [CrossRef]
- Felger, J.C.; Treadway, M.T. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 2017, 42, 216–241. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.W.; de Sá-Rocha, L.C. Differential Effects of Lipopolysaccharide in the Social Behavior of Dominant and Submissive Mice. Physiol. Behav. 2006, 87, 932–937. [Google Scholar] [CrossRef]
- Couch, Y.; Trofimov, A.; Markova, N.; Nikolenko, V.; Steinbusch, H.W.; Chekhonin, V.; Schroeter, C.; Lesch, K.P.; Anthony, D.C.; Strekalova, T. Low-Dose Lipopolysaccharide (LPS) Inhibits Aggressive and Augments Depressive Behaviours in a Chronic Mild Stress Model in Mice. J. Neuroinflammation 2016, 13, 108. [Google Scholar] [CrossRef]
- Granger, D.A.; Hood, K.E.; Ikeda, S.C.; Reed, C.L.; Block, M.L. Effects of Peripheral Immune Activation on Social Behavior and Adrenocortical Activity in Aggressive Mice: Genotype-Environment Interactions. Aggress. Behav. 1997, 23, 93–105. [Google Scholar] [CrossRef]
- Weil, Z.M.; Bowers, S.L.; Dow, E.R.; Nelson, R.J. Maternal Aggression Persists Following Lipopolysaccharide-Induced Activation of the Immune System. Physiol. Behav. 2006, 87, 694–699. [Google Scholar] [CrossRef]
- Alperina, E.; Idova, G.; Zhukova, E.; Gevorgyan, M.; Cheido, M. Cytokine Variations Within Brain Structures in Rats Selected for Differences in Aggression. Neurosci. Lett. 2019, 692, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, F.A.; Cohn, D.W.H.; Sa-Rocha, V.M.; Sa-Rocha, L.C.; Palermo-Neto, J. Behavior: A Relevant Tool for Brain–Immune System Interaction Studies. Ann. N. Y. Acad. Sci. 2009, 1153, 107–119. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Tenditnik, M.V.; Nikolin, V.P.; Popova, N.A.; Kaledin, V.I. The Influence of Psychoemotional Status on Metastasis of Lewis Lung Carcinoma and Hepatocarcinoma-29 in Mice of C57BL/6J and CBA/Lac Strains. Exp. Oncol. 2007, 29, 35–38. [Google Scholar]
- Idova, G.V.; Markova, E.V.; Gevorgyan, M.M.; Alperina, E.L.; Cheido, M.A. Changes in Production of Cytokines by C57Bl/6J Mouse Spleen during Aggression Provoked by Social Stress. Bull. Exp. Biol. Med. 2016, 160, 679–682. [Google Scholar] [CrossRef]
- Petitto, J.M.; Lysle, D.T.; Gariepy, J.L.; Lewis, M.H. Association of Genetic Differences in Social Behavior and Cellular Immune Responsiveness: Effects of Social Experience. Brain Behav. Immun. 1994, 8, 111–122. [Google Scholar] [CrossRef]
- Kopec, A.M.; Smith, C.J.; Bilbo, S.D. Neuro-Immune Mechanisms Regulating Social Behavior: Dopamine as Mediator? Trends Neurosci. 2019, 42, 337–348. [Google Scholar] [CrossRef]
- Takahashi, A.; Flanigan, M.E.; McEwen, B.S.; Russo, S.J. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front. Behav. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Danver, J.; Ji, K.; Miyauchi, J.T.; Chen, D.; Anderson, M.E.; West, B.L.; Kofman, O.; Sasaki, Y. Dynamic Microglial Modulation of Spatial Learning and Social Behavior. Brain Behav. Immun. 2016, 55, 6–16. [Google Scholar] [CrossRef]
- Nelson, L.H.; Lenz, K.M. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav Brain Res. 2017, 316, 279–293. [Google Scholar] [CrossRef]
- Takahashi, A. Associations of the immune system in aggression traits and the role of microglia as mediators. Neuropharmacology. 2024, 256, 110021. [Google Scholar] [CrossRef]
- Chaibi, I.; Ait-Mansour, I.; Tari, M.; Bennis, M.; Ba-M'hamed, S. Effects of Topiramate on Morphological and Structural Alterations of the Anterior Cingulate Cortex in Aggressive Socially Isolated Mice. Psychopharmacology 2023, 240, 1473–1492. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N. , Smagin, D.A., Kovalenko, I.L., Vishnivetskaya, G.B. Repeated positive fighting experience in male inbred mice. Nat Protoc. 2014, 9(11), 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N. Use of the "Partition" Test in Behavioral and Pharmacological Experiments. Neurosci. Behav. Physiol. 2003, 33, 461–471. [Google Scholar] [CrossRef]
- Rico, J.L.; Hurtado-Parrado, C.; Cardona, Á.; et al. Time in the Central Area of the Elevated Plus Maze Correlates with Impulsivity-Related Measures During an Operant Task. Univ. Psychol. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Johnson, N.J. Factor Analysis of Spatiotemporal and Ethological Measures in the Murine Elevated Plus-Maze Test of Anxiety. Pharmacol. Biochem. Behav. 1995, 52, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hascoët, M.; Bourin, M. A New Approach to the Light/Dark Test Procedure in Mice. Pharmacol. Biochem. Behav. 1998, 60, 645–653. [Google Scholar] [CrossRef]
- Soto-Tinoco, E.; Guerrero-Vargas, N.N.; Buijs, R.M. Interaction Between the Hypothalamus and the Immune System. Exp. Physiol. 2016, 101, 1463–1471. [Google Scholar] [CrossRef]
- Walker, S.E.; Papilloud, A.; Huzard, D.; Sandi, C. The Link Between Aberrant Hypothalamic-Pituitary-Adrenal Axis Activity During Development and the Emergence of Aggression—Animal Studies. Neurosci. Biobehav. Rev. 2018, 91, 138–152. [Google Scholar] [CrossRef]
- Bondar, N.P.; Boyarskikh, U.A.; Kovalenko, I.L.; Filipenko, M.L.; Kudryavtseva, N.N. Molecular Implications of Repeated Aggression: Th, Dat1, Snca and Bdnf Gene Expression in the VTA of Victorious Male Mice. PLoS ONE 2009, 4, e4190. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Sugino, Y.; Shibagaki, F.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Dopamine Attenuates Lipopolysaccharide-Induced Expression of Proinflammatory Cytokines by Inhibiting the Nuclear Translocation of NF-κB p65 Through the Formation of Dopamine Quinone in Microglia. Eur. J. Pharmacol. 2020, 866, 172826. [Google Scholar] [CrossRef]
- Liu, A.; Ding, S. Anti-Inflammatory Effects of Dopamine in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells via Inhibiting NLRP3 Inflammasome Activation. Ann. Clin. Lab. Sci. 2019, 49, 353–360. [Google Scholar] [PubMed]
- Bellavance, M.A.; Rivest, S. The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Bondar, N.P.; Avgustinovich, D.F. Association between Experience of Aggression and Anxiety in Male Mice. Behav. Pharmacol. 2001, 12, S30. [Google Scholar] [CrossRef]
- Kovalenko, I.L.; Galyamina, A.G.; Smagin, D.A.; Kudryavtseva, N.N. Hyperactivity and Abnormal Exploratory Activity Developing in CD-1 Male Mice under Chronic Experience of Aggression and Social Defeats. J. Behav. Brain Sci. 2015, 5, 478–490. [Google Scholar] [CrossRef]
- Parmigiani, S.; Palanza, P.; Rogers, J.; Ferrari, P.F. Selection, Evolution of Behavior and Animal Models in Behavioral Neuroscience. Neurosci. Biobehav. Rev. 1999, 23, 957–969. [Google Scholar] [CrossRef]
- Sapronova, A.A.; Kisaretova, P.E.; Salman, R.; Bondar, N.P. Repeated Experience of Aggression Changes in Gene Expression in the Hypothalamus in Male Mice of Two Strains. Neurochem. J. 2023, 17, 359–368. [Google Scholar] [CrossRef]
- Natarajan, D. , Caramaschi, D. Animal violence demystified. Front Behav Neurosci. 2010, 4, 9. [Google Scholar] [CrossRef]
- Grigoleit, J.S.; Kullmann, J.S.; Wolf, O.T.; Hammes, F.; Wegner, A.; Jablonowski, S.; Engler, H.; Gizewski, E.; Oberbeck, R.; Schedlowski, M. Dose-Dependent Effects of Endotoxin on Neurobehavioral Functions in Humans. PLoS ONE 2011, 6, e28330. [Google Scholar] [CrossRef]
- Ji, M.H.; Zhang, L.; Mao, M.J.; Zhang, H.; Yang, J.J.; Qiu, L.L. Overinhibition Mediated by Parvalbumin Interneurons Might Contribute to Depression-like Behavior and Working Memory Impairment Induced by Lipopolysaccharide Challenge. Behav. Brain Res. 2020, 383, 112509. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, K.; Li, Y.; Tang, Z.; Zheng, R.; Ma, Y.; Chen, Z.; Lei, N.; Xiong, L.; Guo, P.; Li, G.; Xie, Y. Lipopolysaccharide-Induced Depression-like Model in Mice: Meta-Analysis and Systematic Evaluation. Front. Immunol. 2023, 14, 1181973. [Google Scholar] [CrossRef]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health Evaluation of Experimental Laboratory Mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef]
- Kim, J.; Sullivan, O.; Lee, K.; Jao, J.; Tamayo, J.; Madany, A.M.; Wong, B.; Ashwood, P.; Ciernia, A.V. Repeated LPS Induces Training and Tolerance of Microglial Responses Across Brain Regions. J. Neuroinflammation 2024, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Devoino, L.; Alperina, E.; Kudryavtseva, N.; Popova, N. Immune Responses in Male Mice with Aggressive and Submissive Behavior Patterns: Strain Differences. Brain Behav. Immun. 1993, 7, 91–96. [Google Scholar] [CrossRef]
- Devoino, L.V.; Idova, G.V.; Alperina, E.L.; Cheido, M.A. Neurochemical Set-up of the Brain - An Extra-Immune Mechanism of Psychoneuroimmunomodulation. Vestn. Ross. Akad. Med. Nauk 1998, 9, 19–24. [Google Scholar]
- Idova, G.V.; Pavina, T.A.; Alperina, E.L.; Devoino, L.V. Influence of Submissive and Aggressive Behavior Patterns on Changes in the Number of T-Lymphocytes CD4+ and CD8+ in Bone Marrow. Immunologiya 2000, 1, 24–26. [Google Scholar]
- Kavelaars, A.; Heijnen, C.J.; Tennekes, R.; Bruggink, J.E.; Koolhaas, J.M. Individual Behavioral Characteristics of Wild-Type Rats Predict Susceptibility to Experimental Autoimmune Encephalomyelitis. Brain Behav. Immun. 1999, 13, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Corr, E.M.; van Solingen, C.; et al. Chronic Stress Primes Innate Immune Responses in Mice and Humans. Cell Rep. 2021, 36, 109595. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Fite, P.J.; DiPierro, M.; Bortolato, M. Links Between Stressful Life Events and Proactive and Reactive Functions of Aggression. J. Aggress. Maltreat. Trauma 2017, 26, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Amkraut, A.; Solomon, G.F. Stress and Murine Sarcoma Virus (Moloney)-Induced Tumors. Cancer Res. 1972, 32, 1428–1433. [Google Scholar]
- van Heukelum, S.; Tulva, K.; Geers, F.E.; van Dulm, S.; Ruisch, I.H.; Mill, J.; Viana, J.F.; Beckmann, C.F.; Buitelaar, J.K.; Poelmans, G.; Glennon, J.C.; Vogt, B.A.; Havenith, M.N.; França, A.S.C. A Central Role for Anterior Cingulate Cortex in the Control of Pathological Aggression. Curr. Biol. 2021, 31, 2321–2333.e5. [Google Scholar] [CrossRef]
- Du Preez, A.; Law, T.; Onorato, D.; Lim, Y.M.; Eum, J.; Marino, M.; Mian, F.; Kouser, L.; Rizzo, D.; Tse, A.C.K.; et al. The Type of Stress Matters: Repeated Injection and Permanent Social Isolation Stress in Male Mice Have a Differential Effect on Anxiety- and Depressive-like Behaviours, and Associated Biological Alterations. Transl. Psychiatry 2020, 10, 325. [Google Scholar] [CrossRef]
- Pavlou, A.; Mulenge, F.; Gern, O.L.; Busker, L.M.; Greimel, E.; Waltl, I.; Kalinke, U. Orchestration of Antiviral Responses Within the Infected Central Nervous System. Cell. Mol. Immunol. 2024, 21, 943–958. [Google Scholar] [CrossRef]
- Shimada, A.; Murata, M.; Aoyagi, S.; Asano, H.; Obara, A.; Hasegawa-Ishii, S. Delayed Microglial Activation Associated with the Resolution of Neuroinflammation in a Mouse Model of Sublethal Endotoxemia-Induced Systemic Inflammation. Toxicol. Rep. 2022, 9, 1380–1390. [Google Scholar] [CrossRef]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W.G.M. Neuronal 'On' and 'Off' Signals Control Microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Takahashi, K.; Rochford, C.D.P.; Neumann, H. Clearance of Apoptotic Neurons Without Inflammation by Microglial Triggering Receptor Expressed on Myeloid Cells-2. J. Exp. Med. 2005, 201, 647–657. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Jarjoura, J.R.; Humphrey, M.B.; Nakamura, M.C.; Seaman, W.E.; Lanier, L.L. Cutting Edge: Inhibition of TLR and FcR Responses in Macrophages by Triggering Receptor Expressed on Myeloid Cells (TREM)-2 and DAP12. J. Immunol. 2006, 177, 2051–2055. [Google Scholar] [CrossRef]
- Takahashi, A.; Aleyasin, H.; Stavarache, M.A.; et al. Neuromodulatory Effect of Interleukin 1β in the Dorsal Raphe Nucleus on Individual Differences in Aggression. Mol. Psychiatry 2022, 27, 2563–2579. [Google Scholar] [CrossRef]
- Caramaschi, D.; de Boer, S.F.; de Vries, H.; Koolhaas, J.M. Development of Violence in Mice Through Repeated Victory Along with Changes in Prefrontal Cortex Neurochemistry. Behav. Brain Res. 2008, 189, 263–272. [Google Scholar] [CrossRef]
- Veenema, A.H.; Meijer, O.C.; de Kloet, E.R.; Koolhaas, J.M. Differences in Basal and Stress-Induced HPA Regulation of Wild House Mice Selected for High and Low Aggression. Horm. Behav. 2003, 43, 197–204. [Google Scholar] [CrossRef]
- Neumann, I.D.; Veenema, A.H.; Beiderbeck, D.I. Aggression and Anxiety: Social Context and Neurobiological Links. Front. Behav. Neurosci. 2010, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Galigniana, M.D.; Morishima, Y.; Murphy, P.J. Role of Molecular Chaperones in Steroid Receptor Action. Essays Biochem. 2004, 40, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Sapronova, A.A.; Ryabushkina, Y.A.; Kisaretova, P.E.; Bondar, N.P. Mechanisms of Adaptation of the Hypothalamic-Pituitary-Adrenal Axis in Male Mice in Chronic Social Defeat Stress. Neurosci. Behav. Physiol. 2024, 54, 1289–1297. [Google Scholar] [CrossRef]
- Scotti, M.A.L.; Rendon, N.M.; Greives, T.J.; Romeo, R.D.; Demas, G.E. Short-Day Aggression Is Independent of Changes in Cortisol or Glucocorticoid Receptors in Male Siberian Hamsters (Phodopus sungorus). J. Exp. Zool. A Ecol. Genet. Physiol. 2015, 323, 331–341. [Google Scholar] [CrossRef]
- Grinevich, V.; Ma, X.M.; et al. Effect of Repeated Lipopolysaccharide Administration on Tissue Cytokine Expression and Hypothalamic-Pituitary-Adrenal Axis Activity in Rats. J. Neuroendocrinol. 2001, 13, 711–723. [Google Scholar] [CrossRef]
- Ferrari, P.F.; van Erp, A.M.M.; Tornatzky, W.; Miczek, K.A. Accumbal Dopamine and Serotonin in Anticipation of the Next Aggressive Episode in Rats. Eur. J. Neurosci. 2003, 17, 371–378. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Smagin, D.A.; Redina, O.E.; Kovalenko, I.L.; Galyamina, A.G.; Babenko, V.N. Neurotransmitter Genes in the Nucleus Accumbens That Are Involved in the Development of a Behavioral Pathology After Positive Fighting Experiences and Their Deprivation: A Conceptual Paradigm for Data Analysis. Int. J. Mol. Sci. 2025, 26, 8580. [Google Scholar] [CrossRef]
- Smagin, D.A.; Galyamina, A.G.; Kovalenko, I.L.; Kudryavtseva, N.N. Altered Expression of Genes Associated with Major Neurotransmitter Systems in the Reward-Related Brain Regions of Mice with Positive Fighting Experience. Int. J. Mol. Sci. 2022, 23, 13644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; van den Pol, A.N. Hypothalamic Arcuate Nucleus Tyrosine Hydroxylase Neurons Play Orexigenic Role in Energy Homeostasis. Nat. Neurosci. 2016, 19, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine Controls Systemic Inflammation Through Inhibition of NLRP3 Inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef]
- Winland, C.D.; Welsh, N.; Sepulveda-Rodriguez, A.; Vicini, S.; Maguire-Zeiss, K.A. Inflammation Alters AMPA-Stimulated Calcium Responses in Dorsal Striatal D2 but Not D1 Spiny Projection Neurons. Eur. J. Neurosci. 2017, 46, 2519–2533. [Google Scholar] [CrossRef]
- Du, R.H.; Zhou, Y.; Xia, M.L.; Lu, M.; Ding, J.H.; Hu, G. α-Synuclein Disrupts the Anti-Inflammatory Role of Drd2 via Interfering β-Arrestin2-TAB1 Interaction in Astrocytes. J. Neuroinflammation 2018, 15, 258. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, S.Z.; Tang, M.; Zhang, X.H.; Zhou, Z.; Yin, Y.Q.; Zhou, Q.B.; Huang, Y.Y.; Liu, Y.J.; Wawrousek, E.; Chen, T.; Li, S.B.; Xu, M.; Zhou, J.N.; Hu, G.; Zhou, J.W. Suppression of Neuroinflammation by Astrocytic Dopamine D2 Receptors via αB-Crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef]
- van Heesch, F.; Prins, J.; Konsman, J.P.; Korte-Bouws, G.A.H.; Westphal, K.G.C.; Rybka, J.; Olivier, B.; Kraneveld, A.D.; Korte, S.M. Lipopolysaccharide Increases Degradation of Central Monoamines: An In Vivo Microdialysis Study in the Nucleus Accumbens and Medial Prefrontal Cortex of Mice. Eur. J. Pharmacol. 2014, 725, 55–63. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Shou, S.S.; Lin, Y.X.; Chen, C.C.; Chiang, C.Y.; Yeh, C.Y. Effect of Ginkgo Biloba Extract on Lipopolysaccharide-Induced Anhedonic Depressive-like Behavior in Male Rats. Phytother. Res. 2015, 29, 260–266. [Google Scholar] [CrossRef]
- Felger, J.C.; Mun, J.; Kimmel, H.L.; Nye, J.A.; Drake, D.F.; Hernandez, C.R.; Freeman, A.A.; Rye, D.B.; Goodman, M.M.; Howell, L.L.; Miller, A.H. Chronic Interferon-α Decreases Dopamine 2 Receptor Binding and Striatal Dopamine Release in Association with Anhedonia-Like Behavior in Nonhuman Primates. Neuropsychopharmacology 2013, 38, 2179–2187. [Google Scholar] [CrossRef]
- Perreault, M.L.; Hasbi, A.; O'Dowd, B.F.; George, S.R. Heteromeric Dopamine Receptor Signaling Complexes: Emerging Neurobiology and Disease Relevance. Neuropsychopharmacology 2014, 39, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Hasbi, A.; Sivasubramanian, M.; Milenkovic, M.; Komarek, K.; Madras, B.K.; George, S.R. Dopamine D1-D2 Receptor Heteromer Expression in Key Brain Regions of Rat and Higher Species: Upregulation in Rat Striatum After Cocaine Administration. Neurobiol. Dis. 2020, 143, 105017. [Google Scholar] [CrossRef] [PubMed]
- Vekshina, N.L.; Anokhin, P.K.; Veretinskaya, A.G.; Shamakina, I.Y. Heterodimeric D1-D2 Dopamine Receptors: A Review. Biomed. Khim. 2017, 63, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Herron, S.; Delpech, J.C.; Madore, C.; Ikezu, T. Using mechanical homogenization to isolate microglia from mouse brain tissue to preserve transcriptomic integrity. STAR protocols, 2022, 3(4), 101670. [CrossRef]
- Friard, O.; Gamba, M. BORIS: A Free, Versatile Open-Source Event-Logging Software for Video/Audio Coding and Live Observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




