1. Introduction
For developing sustainable construction materials and optimizing the mechanical performance of composite systems, understanding the interaction between components and their structural behavior is essential. Earlier studies demonstrated the importance of material structure and micro-interactions for achieving reliable performance in civil engineering applications, including foundations and geotechnical systems. The rapid expansion of the construction industry has intensified the demand for advanced thermal insulation materials[
1,
2]. Among these, mineral-based insulators synthesized through the thermal treatment of volcanic basalt rock have attracted increasing attention due to their high thermal stability, environmental compatibility, and durability. This type of insulation is mineral in connection with what it is much more effective and safer in contrast to its analogs of organic origin as it is not flammable is not subject to aging does not form condensate in the space between the insulation and the insulated structure. The main problematic aspect of production of mineral insulating materials is its waste. Therefore, the development of sustainable technologies for recycling such wastes into valuable secondary materials has become an urgent task[
3,
4]. This study aims to develop and optimize a chemical recycling process converting basalt insulation waste into active mineral powder applicable in asphalt concrete production.
The review of sources of world researches in the field of utilization of wastes of production of mineral insulators has shown that there are serious ecological problems connected with storage in landfills and leaching of stone wool wastes [
5,
6,
7]. The term “mineral wool” includes a variety of inorganic materials used as insulating agents. Predominantly used in construction, mineral wool serves to provide thermal insulation, cold and fire protection, and sound insulation. Despite the fact that mineral wool waste accounts for only a small proportion of the total construction waste by mass, it requires large capacities for transportation and disposal due to its low bulk density and its utilization remains low compared to other types of waste[
8].
According to statistical studies on waste disposal by industrial enterprises, about 13,000 tons of waste thermal insulation materials based on basalt fiber, 4,350 tons of waste basalt fiber and materials based on it are generated annually in Kazakhstan. To date, the main producers of mineral wool are the companies “Izoterm-Oskemen” (Oskemen), ‘MakWool’ (MZTI) (Akmola region, Makinsk), “EcoTERM.KZ” (Temirtau), “Technonikol-Kazakhstan LLP” (Almaty)[
9].
There are examples of processing of basalt slab residues in the world in countries with developed economies. For example, the German company FAS for many years quite effectively processes basalt residues as a result of sawing basalt slabs at the plant[
10,
11]. There is a separate technological line that fully utilizes and re-produces finished basalt materials from secondary raw materials using reverse technology[
12,
13].
During the production process of mineral wool, in addition to carpet edge trimming, which accounts for 6 to 8 %, additional substandard product residues and non-marketable products are generated, which account for approximately 5 to 8 % of the total output. Thus, between 5 and 15 % of the products of the total production volume are not suitable for sale due to their condition. Many mineral wool manufacturers remove these substandard products or a significant part of them, having to pay not only for their removal but also for the cost of transportation. This generates a large amount of waste, which many manufacturers in Kazakhstan and construction companies simply throw away. Often they are not properly processed in landfills, and consequently pollute the environment. Therefore, recycling of basalt slabs and proper utilization with further effective use of the secondary product is of great importance.
To date, developed a lot of methods of utilization of mineral insulation materials, one of which is the thermal insulation of the goresontal surfaces of buildings and structures, but these technologies are ineffective, as they have many disadvantages. Not uniform structure significantly reduces thermal efficiency and over time changes density increases water absorption that leads to high humidity and the formation of fungi, mold on the surface. Also waste mineral insulators are stored on the established palegons, however, and this method of utilization is not effective because the waste has low density and with strong gusts of wind flies around the territory not only of the landfill, but also for its divisions and since the waste has a mineral structure it is not subject to rotting and decomposition.
The article considers new effective technologies of utilization of waste mineral insulating materials waste technllogy implies both chemical and mechanical method.
The aim of the article is to develop the technology of utilization of mineral insulation waste by chemical method for the production of mineral powder.
Objectives:
Development of the technology of waste mineral insulators by chemical method;
Selection and optimization of the process of utilization of waste mineral insulators;
Analysis of the obtained raw materials from waste mineral insulators for effective utilization.
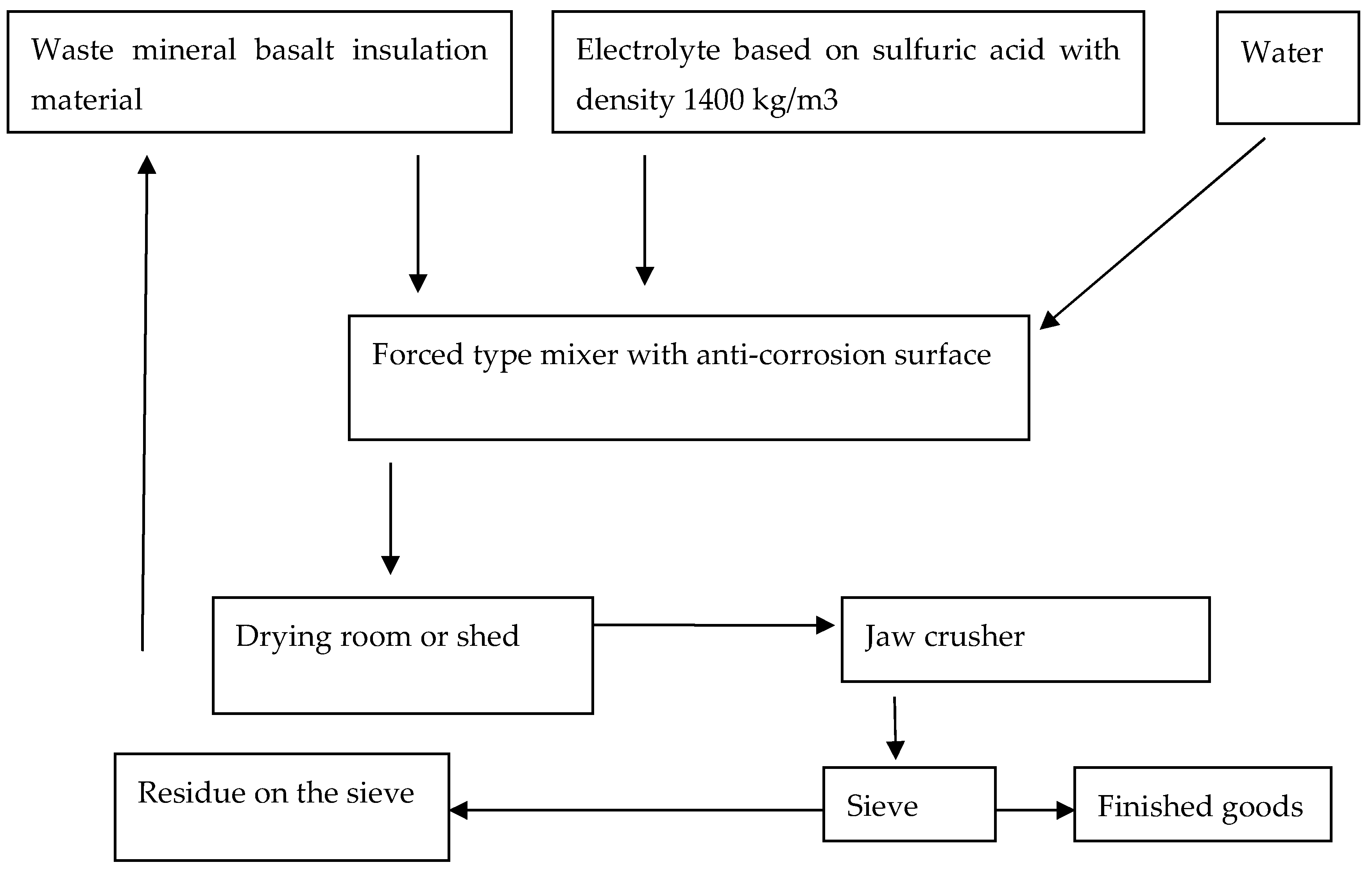
The technological process for chemical activation and mechanical processing of waste basalt insulation materials into mineral powder is illustrated in
Figure 1.
In industrial conditions, the mixture can be dried under a canopy in the summer season, after which the obtained material is crushed in a jaw crusher for its further use in the production of raw materials for construction production as effective fillers and mineral powders. And also the obtained raw material is reduced in volume by 10 times after leaching, so the occupied area for the produced waste is reduced by 10 times, and the waste will be utilized by complete reuse.
2. Materials and Methods
2.1. Materials characterization
In the process of oxidation was selected the optimal composition of the amount of electrolyte water and waste mineral insulation to obtain active powder for its further use in the construction industry. Selection of the optimum composition of mineral insulator waste by chemical method is presented in
Table 1.
The main indicator of qualitative and effective composition of ratios of all components was accepted hydrogen index and the structure of the powder after milling that shows to determine the maximum suitability of the obtained powder as a raw material. To optimize the composition was taken into account high adsorption property of mineral powder, as the powder absorbs a large amount of moisture was calculated maximum saturation of moisture mineral insulation that showed 50% of the mass of mineral insulation in connection with which was taken a constant unit of water exactly half of the mass of mineral powder. The main chemical reagent Electrolyte was selected in relation to the qualitative characteristics of hydrogen index and the residue on the sieve after grinding.
According to the obtained results, presented in Table 4, we see that the most optimal amount of electrolyte is 20% of the total mass of all components at this amount is achieved neutral environment 7 pH of the obtained mixture that allows to use it in different materials as active fillers, and the residue on the sieve 5.7% is the most optimal providing a high yield of finished raw materials for production.
The technology of mineral powder production implies obtaining an effective additive in asphalt concrete pavement, and is also considered as a way of utilization of basalt mineral insulation waste, solving the environmental problem.
For obtaining mineral powder on the basis of wastes of basalt mineral insulators production, the composition presented in
Table 2 isdetermined.
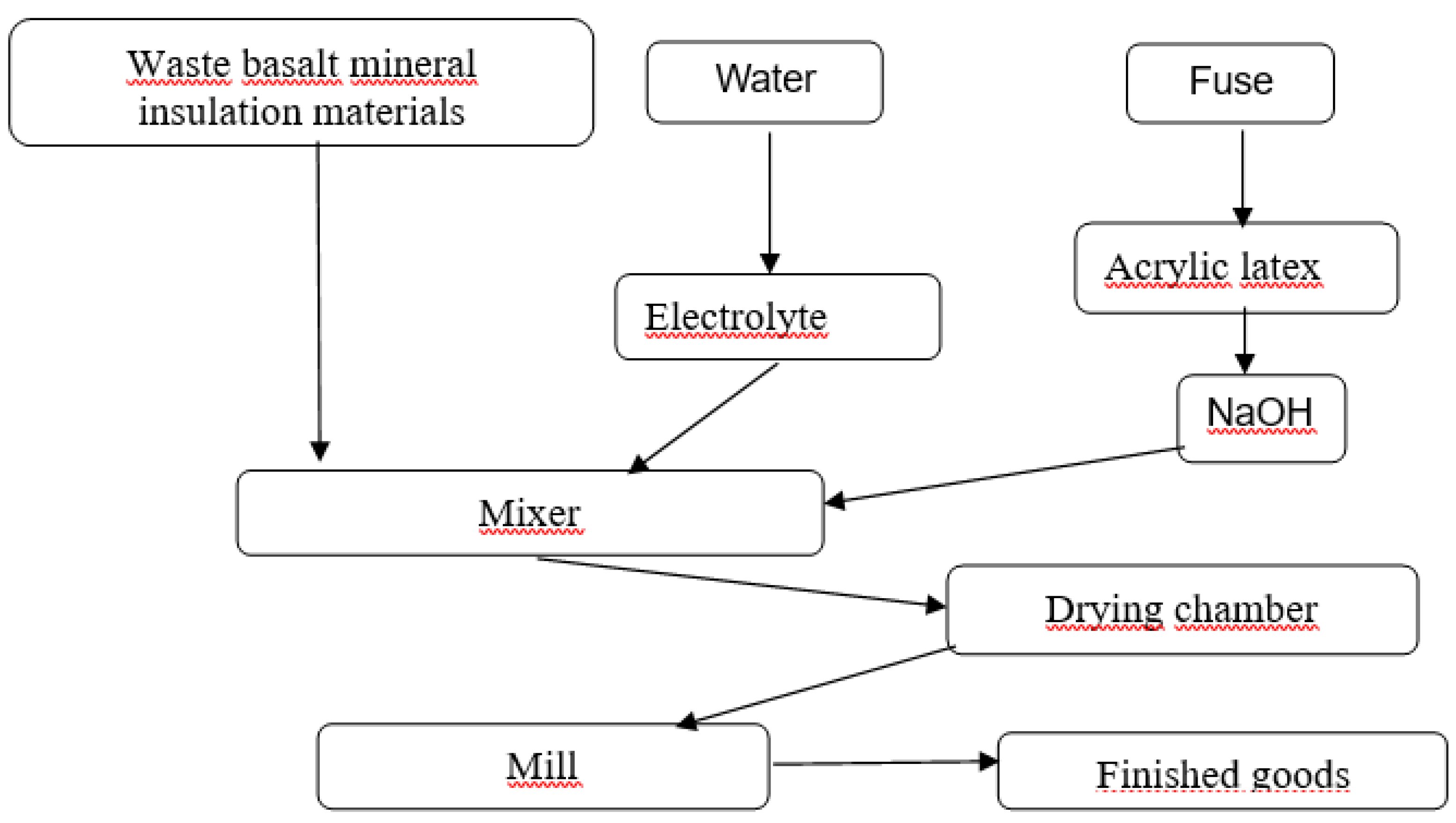
2.2. Chemical treatment process
In the production of mineral powder it is necessary to soak mineral insulation waste with water, temperature not lower than 18°C. The modifying component (hydrophobiser) is prepared separately: the fuse is mixed with acrylic latex and combined with NaOH alkali, the temperature of combination is not lower than 22°С. The mixture is then passed through an RPA (rotary pulsation apparatus) to form a water-soluble emulsion. After the obtained mixture is combined with water-soaked mineral insulation waste and electrolyte and mixed thoroughly. The obtained mixture is placed in a drying chamber and then in a mill for grinding. The obtained powder is sieved and packed.
The technological scheme of mineral powder production for asphalt concrete is presented in
Figure 2.
Research of chemical composition of waste mineral insulation on the basis of basalt fibre has shown that its composition is predominantly silica (SiO2) and has an alkaline environment in connection with what to change its structure by chemical method it was accepted to carry out oxidation with the use of acids, in our case electrolyte based on sulphuric acid density 1400 kg/m3. In the process of oxidation was obtained reaction with the release of heat average reaction temperature of 80 C° in the process of oxidation mixture of waste basalt mineral insulation was thoroughly stirred as a result was obtained pasty mixture, which was placed in the drying chamber at a temperature of 100 C°.
2.3. Analytical methods
In the article the technology of utilisation of mineral insulation waste by chemical method for the development of the technology the composition of mineral insulation waste is studied by spectrometer. A comprehensive set of analytical methods is employed to characterize both the initial basalt fiber–based insulation waste and the mineral powder obtained after chemical treatment. The analyses aimed to determine the physical, chemical, and structural parameters governing the suitability of the recycled material for use in construction composites and asphalt mixtures. To determine the elemental composition of the mineral insulation waste, EDS analysis was performed using a benchtop SEM system, as shown in
Figure 3
The chemical composition of the raw insulation waste and of the mineral powder produced after oxidation are determined using energy-dispersive X-ray spectroscopy (EDS) coupled with a scanning electron microscope (SEM, JEOL JSM-6610LV). The analysis identified major oxide components such as SiO₂, Al₂O₃, CaO, MgO, Na₂O, and Fe₂O₃. The EDS detector is calibrated using certified reference materials, and both the mass and atomic fractions of elements are computed. Measurement errors is not exceed ±5 %, which ensured high analytical reliability. The results allowed assessing changes in elemental composition caused by the chemical treatment.
The porosity and particle size distribution of the processed mineral insulation waste are evaluated using a wet sieving method in accordance with Interstate Standard (GOST) 32764-2014 in
Figure 4.
The true particle density of the processed mineral insulation waste was measured according to Interstate Standard (GOST) 32763-2014 using a volumetric compaction apparatus in
Figure 5.
The swelling of bitumen–mineral powder briquettes is determined following the procedure specified in Interstate Standard (GOST) 32707-2014 (
Figure 6).
The bitumen capacity of mineral powder was evaluated following Interstate Standard (GOST) 32766-2017 using a plunger-type testing device in
Figure 7.
The moisture content of the processed mineral powder was determined following Interstate Standard (GOST) 32762-2014, using volumetric flasks and a controlled-pressure drying apparatus (
Figure 8).
3. Results
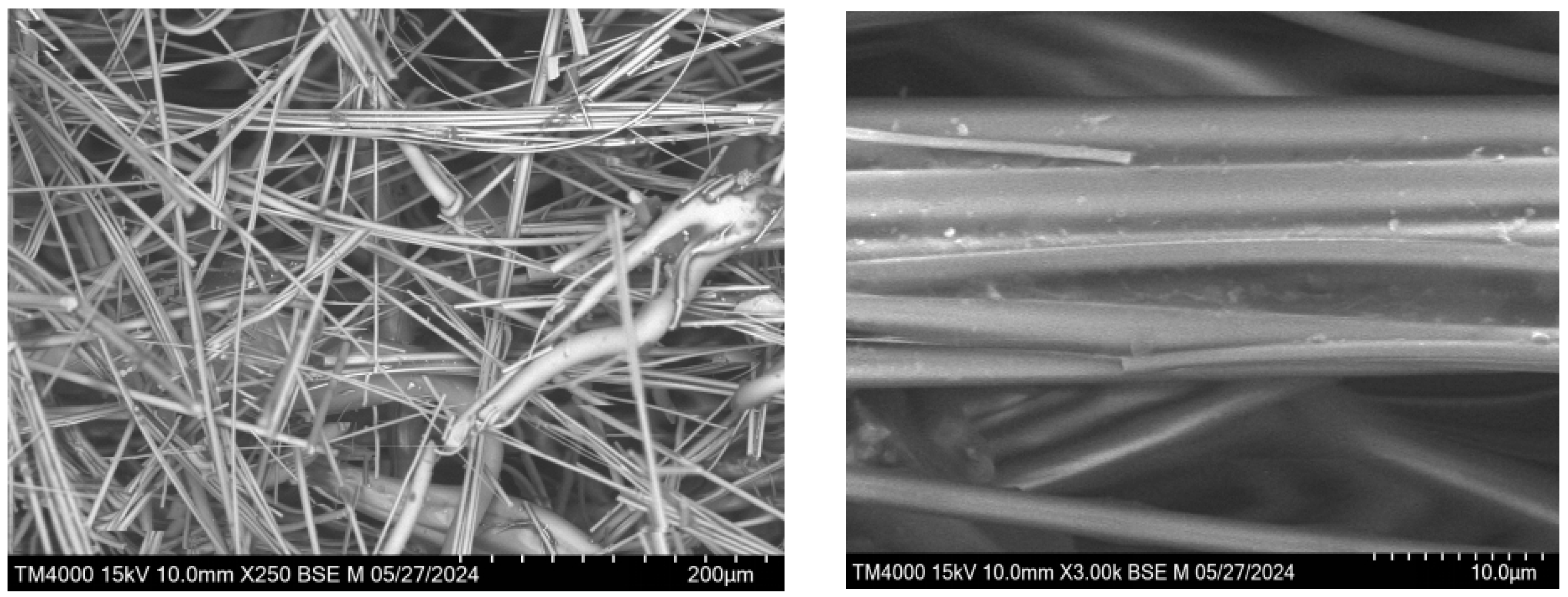
The image shows thin fibres of basalt, their structure is chaotic, which is typical for natural minerals. The fibres intersect in different directions, creating a mesh structure. The morphology and structure of the recovered basalt mineral fibers are examined using SEM at different magnifications to evaluate their surface texture and integrity in
Figure 9.
At high magnification, finer details of the fibre structure are visible. The fibres have a smooth surface and small inclusions, which may be defects or other mineral phases.
Magnification allows a better view of the individual fibres of basalt. The fibres have a smooth surface and varying thicknesses. The image shows the dimensions of some of the fibres. It can be seen that the fibres have different thicknesses: from 1.24 mcm to 9.3 mcm.
The image shows large inclusions among the fibres in
Figure 10.
Measurements show that the inclusions range in size from 154 µm to 385 µm. These inclusions may be other mineral phases or contaminants.
Spherical inclusions are seen in the fibres. These inclusions have a smooth surface and can be of various origins such as glass droplets or other minerals.
Overall conclusions: the images show that basalt fibres have a chaotic mesh structure with varying fibre thicknesses. Inclusions of different sizes and shapes are present in the fibres, indicating the presence of other mineral phases or contaminants. High magnification allows detailed examination of the surface of the fibres and reveals small defects or inclusions.
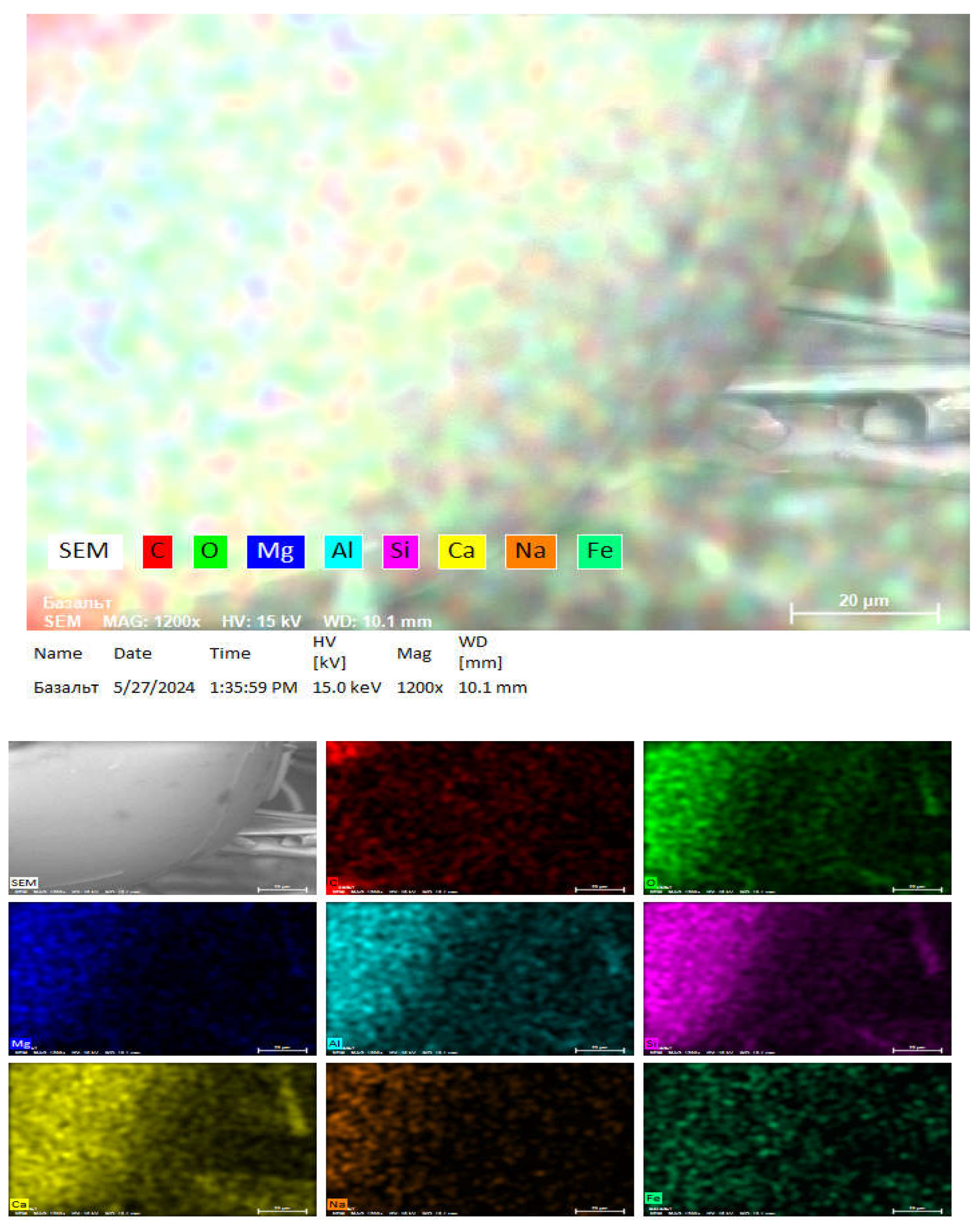
Next, energy dispersive X-ray spectroscopy is performed using a scanning electron microscope (SEM) with an EDS detector installed and is presented in
Figure 11.
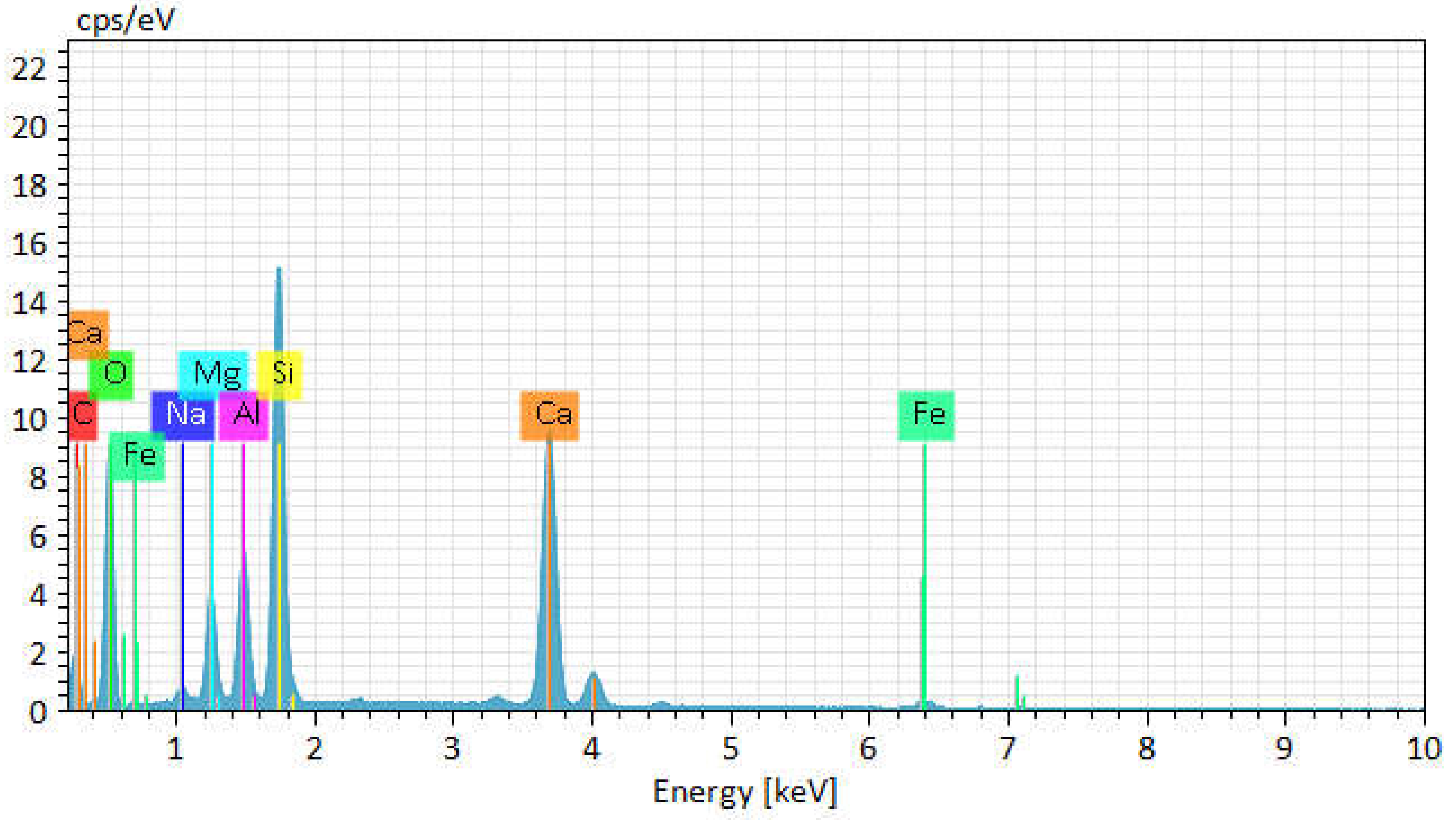
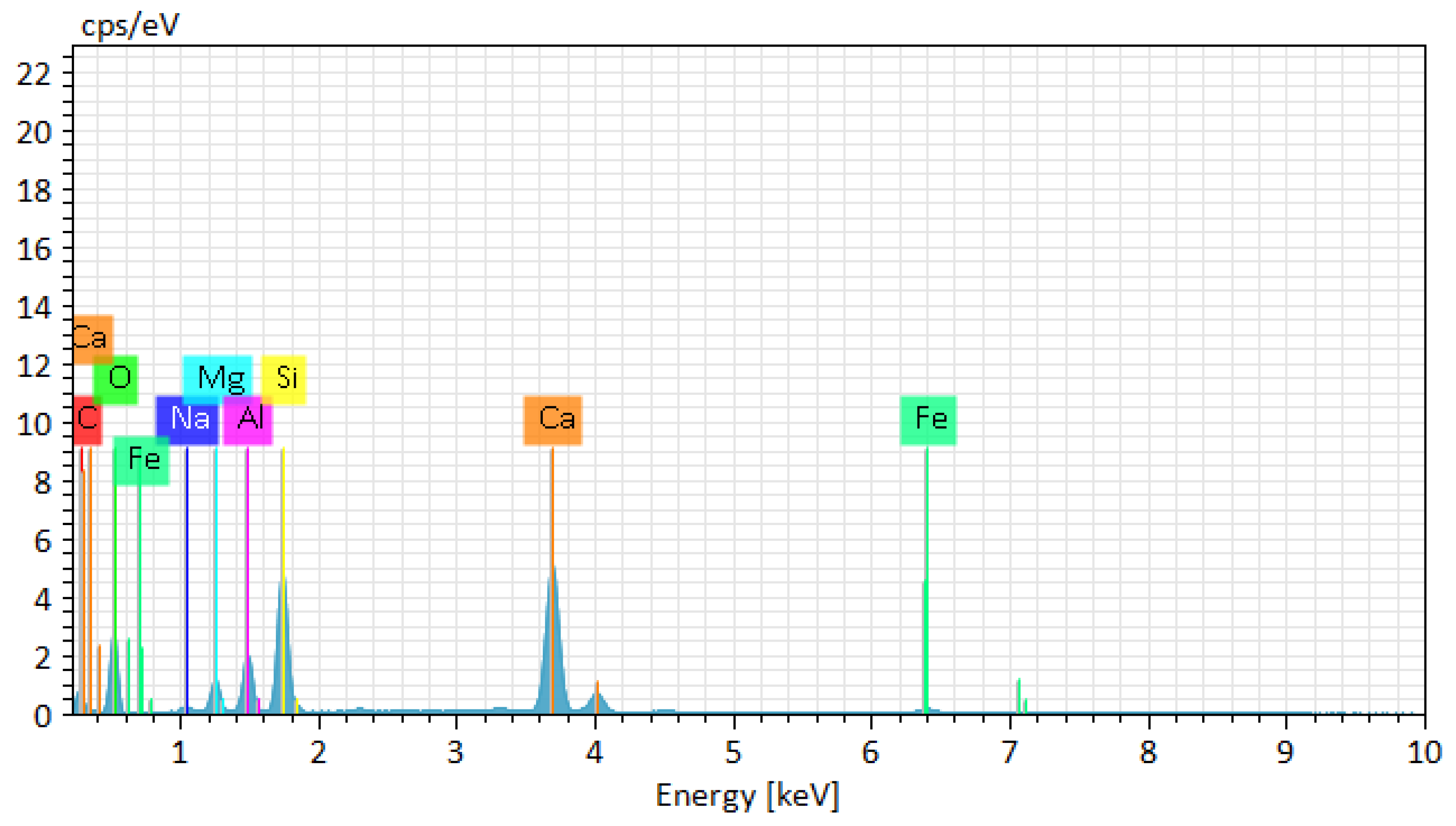
The image shows various elements such as C, O, Mg, Al, Si, Ca, Na, Fe. This is indicated on the colored element map which shows the distribution of these elements in the sample 1 in
Figure 12.
Figure 12.
Chemical element activity of basalt insulation sample 1.
Figure 12.
Chemical element activity of basalt insulation sample 1.
Figure 13.
Chemical element activity of basalt insulation sample 2.
Figure 13.
Chemical element activity of basalt insulation sample 2.
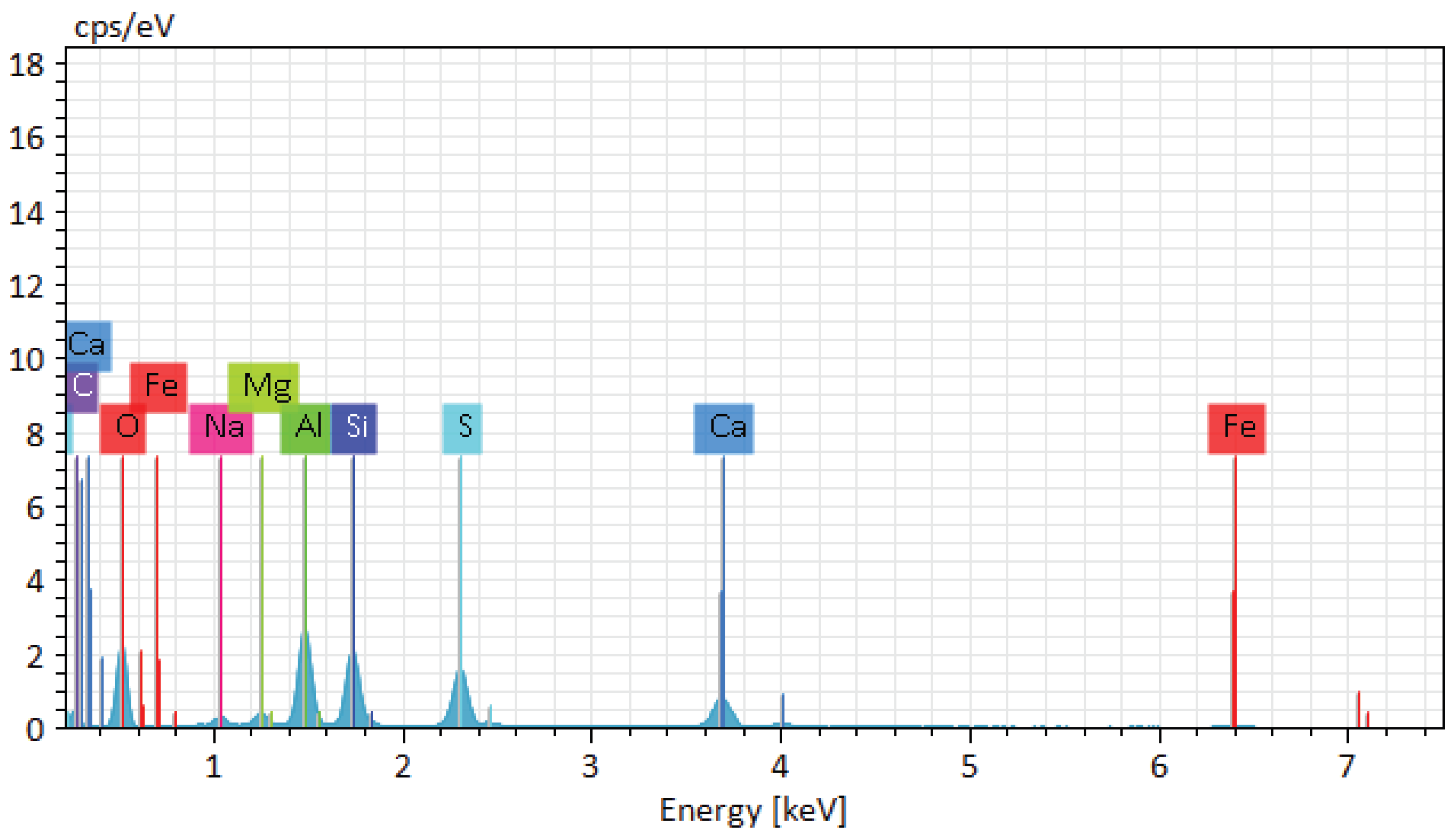
According to the figures of chemical detailed analysis results, the following results were obtained:
Oxygen (O)
-
Table 1: Mass fraction - 41.14%, atomic fraction - 41.47%, absolute error - 0.28%, relative error - 0.69%
-
Table 2: Mass fraction - 41.33%, atomic fraction - 41.61%, absolute error - 0.34%, relative error - 0.83%
High oxygen content is typical for basalt, as the bulk of the material consists of silicates and oxides. Low absolute and relative errors indicate high accuracy of measurements.
Silicon (Si)
-
Table 1: Mass fraction - 18.96%, atomic fraction - 15.44%, absolute error - 0.77%, relative error - 4.05%.
-
Table 2: Mass fraction - 18.76%, atomic fraction - 15.22%, absolute error - 0.82%, relative error - 4.36%
Silicon is the main element that makes up silicate minerals such as plagioclases and pyroxenes. High accuracy of measurements is confirmed by low errors.
Aluminium (Al)
-
Table 1: Mass fraction - 6.08%, atomic fraction - 5.15%, absolute error - 0.28%, relative error - 2.43%
-
Table 2: Mass fraction - 7.02%, atomic fraction - 7.07%, absolute error - 0.35%, relative error - 5.06%
Aluminium is part of plagioclases and contributes to the thermal stability and strength of basalt. The low measurement errors indicate high accuracy of the data.
Magnesium (Mg)
-
Table 1: Mass fraction - 4.66%, atomic fraction - 4.38%, absolute error - 0.25%, relative error - 5.44%
-
Table 2: Mass fraction - 4.13%, atomic fraction - 4.16%, absolute error - 0.25%, relative error - 6.03%
Magnesium is present in the form of olivines and pyroxenes, which improves the heat resistance and strength of the material. The relatively low errors indicate the accuracy of the measurements.
Sodium (Na)
-
Table 1: Mass fraction - 0.79%, atomic fraction - 0.78%, absolute error - 0.07%, relative error - 8.59%.
-
Table 2: Mass fraction - 0.94%, atomic fraction - 0.95%, absolute error - 0.08%, relative error - 8.70%
Sodium is found in small amounts and is mainly a part of plagioclases. High relative errors are associated with low concentration of the element.
Calcium (Ca)
-
Table 1: Mass fraction - 25.63%, atomic fraction - 25.84%, absolute error - 0.82%, relative error - 3.21%.
-
Table 2: Mass fraction - 25.62%, atomic fraction - 25.80%, absolute error - 0.79%, relative error - 3.09%
Calcium is a constituent of plagioclases and pyroxenes, improving the mechanical properties and thermal stability of basalt. Low errors indicate high accuracy of measurements.
Iron (Fe)
-
Table 1: Mass fraction - 1.94%, atomic fraction - 0.79%, absolute error - 0.10%, relative error - 5.37%.
-
Table 2: Mass fraction - 1.52%, atomic fraction - 1.53%, absolute error - 0.07%, relative error - 4.83%.
Iron is present in the form of oxides and silicates, affecting the colour and magnetic properties of basalt. Low errors confirm the high accuracy of measurements.
Analyses of basalt samples showed similar results, confirming the stable composition of the material. The main elements are oxygen, silicon, aluminium, magnesium, sodium, calcium and iron. High accuracy of measurements, confirmed by low values of absolute and relative errors, provides reliability of the obtained data. These elements and their combinations form the main minerals of basalt, such as plagioclases, pyroxenes and olivines, which determine its physical and chemical properties.
Studies of the chemical composition of waste mineral insulation on the basis of basalt fibres showed that its composition is predominantly silica (SiO2) and has an alkaline environment in connection with what to change its structure by chemical method it was accepted to carry out oxidation with the use of acids, in our case electrolyte based on sulfuric acid density of 1400 kg/m3. In the process of oxidation was obtained reaction with the release of heat average reaction temperature of 80 C ° in the process of oxidation mixture of waste basalt mineral insulation was thoroughly mixed as a result was obtained pasty mixture, which was placed in the drying chamber at a temperature of 100 C °.
Complete chemical qualitative and quantitative analyses of the obtained mineral powder from waste basalt mineral insulation material showed the changes from the initial results of the analysis are presented in Table 5 and
Figure 14 and
Figure 15.
4. Discussion
The results of the obtained chemical analysis confirm the change of chemical composition of waste mineral basalt insulation after chemical treatment also in figure ????. mycorostructure of powder particles has oblong shape that will positively reflect on micro-reinforcing abilities of any material where this powder will be used as an aggregate. One of the ways of utilisation of waste mineral insulation is production of mineral powder for road construction.
To determine the qualitative indicators of mineral powder Type 2 (developed composition) in comparison with the indicators of Type 1 (analogue) were tested in accordance with the current regulations. The results of determining the grain composition of mineral powder Type 1 in comparison with Type 2 allow us to objectively assess the quality of the structure of materials according to GOST. The obtained results showed a denser structure of mineral powder Type 2 in comparison with Type 1, with a minimum content of dusty particles, affecting the quality of the production process. The results of tests to determine the grain composition of Type 1 and Type 2 mineral powders are shown in
Table 3.
The moisture content and swelling of Type 1 and Type 2 mineral powder samples with bitumen was determined to evaluate the interaction between mineral powder and bitumen in asphalt concrete. This parameter is important to prevent undesirable phenomena such as bitumen separation from the mineral aggregate. Tests were also carried out to determine the bituminous capacity of Type 1 and Type 2 mineral powder to characterise the interaction with bitumen in
Table 4.
The test results showed that Type 2 mineral powder has a higher capacity than Type 1 due to the adhesion of bitumen to the surface of mineral particles.
Thus, the quality structure of asphalt concrete provided by the effective interaction between bitumen and mineral filler can improve the material's resistance to moisture, temperature changes and chemical attack.
Processing of construction waste into mineral powder and its use in asphalt pavement is effective from the point of view of environmental protection and resource saving. Our proposed technology of obtaining mineral powder on the basis of processing of waste production of basalt insulators will make it possible to obtain high-quality road asphalt concrete with maximum Kazakhstani content and quality indicators, in accordance with the norms of GOST 16557-2005.
The offered technology of obtaining mineral powder on the basis of processing of wastes of basalt insulators production as a filler for asphalt concrete is considered for the first time and assumes production of material for road pavements with effective connection ‘binder-mineral filler’ for stabilisation of main quality indicators of asphalt concrete under the influence of temperatures.
5. Conclusions
The conducted researches have shown that the development of technology of utilisation of waste mineral insulators by chemical method is the most optimal and effective. One of the advantages of this technology is its low cost and the possibility of using it as a quality raw material opens up great opportunities for its complete insulation by using it as mineral powders.
The results of researches carried out within the limits of the given article have shown that the reaction proceeds with temperature release, and also in the article the optimum composition of a ratio of electrolyte on the basis of sulphuric acid and waste of bosalt payer has been received. From the obtained data it is observed that the process contributes to non-alkalise the alkaline environment of basalt insulation waste by oxidation of the optimum amount of waste 40%, water 40%, electrolyte 20%. This ratio allows to obtain a powder with non-alkaline medium Ph7, this hydrogen carrier allows to use the obtained raw material in many building materials of civil and industrial as well as road construction.
This conclusion is justified by the fact that after chemical treatment of wastes the volumes of wastes are reduced 10 times as they are compacted ten times and thus do not occupy huge areas for storages. Also the analysis of the finished powder has in its composition the compound SO3 25 % that at interaction with various mineral components promotes formation of sulphates, and the need in catalysts promoting formation of sulphates at manufacture of building materials by calcination and hydrotation is very high.
World experience has shown that the most effective and environmentally friendly method for utilisation of mineral insulation waste is the reverse re-firing of waste, which is a very expensive process. Chemical method of recycling of basalt insulation waste does not require high temperatures and special equipment for firing. And the simplicity of processing opens the possibility to get effective raw materials without large financial investments.
Thus, the obtained mineral powder has a wide range of application both in chemical and construction industries. Also this powder in the ungrinded state has a dense structure is not subject to dusting, does not occupy large areas, which is very convenient and safe with respect to the harmful effects on the environment and humans.
Advantages of using mineral powder from mineral insulation waste in asphalt concrete:
− increased stability and durability;
− the introduction of mineral powder derived from mineral insulation waste strengthens the asphalt mix, increasing its stability and overall durability;
− the unique composition of these mineral powders can improve critical asphalt properties, including resistance to rutting, cracking and moisture.
Sustainable resource utilisation: by recycling waste basalt insulation products into valuable mineral powder, this approach promotes sustainability by reducing waste and reducing demand for virgin materials in the construction industry.
The main focus of this study is the possibility of utilising industrial waste and achieving high environmental performance.
Supplementary Materials
Not applicable.
Author Contributions
Conceptualization, D.D.; validation, R.L.; investigation, A.A.; writing—original draft preparation, Z.S.; writing—review and editing, Z.S.; supervision, D.D.; project administration, A.J.; funding acquisition, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been/was/is funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP 22683302 Modernisation of driven piles based on high-strength concrete with the use of modified additive from industrial wastes). This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant no. BR21882278 “Establishment of a construction and technical engineering centre to provide a full cycle of accredited services to the construction, road-building sector of the Republic of Kazakhstan”).
Data Availability Statement
The data supporting the findings of this study were generated in-house through laboratory experiments. The datasets are not publicly available due to internal research storage policy, but they are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to express thanks of gratitude to the research and production centre "ENU-Lab" of L.N. Gumilyov Eurasian National University for providing base of the experimental section.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zhussupbekov, A.Zh.; Utepov, Ye.B.; Shakhmov, Zh.A.; Ling, H.I. Model Testing of Piles in a Centrifuge for Prediction of Their In-Situ Performance. Soil Mech Found Eng 2013, 50, 92–96. [Google Scholar] [CrossRef]
- Awwad, T.; Shakhmov, Zh.A.; Lukpanov, R.E.; Yenkebayev, S.B. Experimental Study on the Behavior of Pile and Soil at the Frost Condition. In Proceedings of the Sustain. Civil Infrastruct.; Springer Science and Business Media B.V. 2019; pp. 69–76. [Google Scholar]
- Mukhambetkaliev, K.; Shakhmov, Z.; Alizhanov, D.; Utepov, Y. Utilization of Modified Ash-Based Mineral Powders in Asphalt Concrete Mixtures for Enhanced Performance and Sustainability. In Proceedings of the E3S Web Conf.; EDP Sciences, 2025; Vol. 648. [Google Scholar]
- Mkilima, T.; Sabitov, Y.; Shakhmov, Z.; Abilmazhenov, T.; Tlegenov, A.; Jumabayev, A.; Turashev, A.; Kaliyeva, Z. Exploring the Synergistic Effect of Recycled Glass Fibres and Agricultural Waste Ash on Concrete Strength and Environmental Sustainability. Clean. Eng. Technol. 2024, 20. [Google Scholar] [CrossRef]
- Yap, Z.S.; Khalid, N.H.A.; Haron, Z.; Mohamed, A.; Tahir, M.M.; Hasyim, S.; Saggaff, A. Waste Mineral Wool and Its Opportunities—A Review. Materials 2021, 14, 5777. [Google Scholar] [CrossRef] [PubMed]
- Väntsi, O.; Kärki, T. Utilization of Recycled Mineral Wool as Filler in Wood–Polypropylene Composites. Construction and Building Materials 2014, 55, 220–226. [Google Scholar] [CrossRef]
- Yliniemi, J.; Ramaswamy, R.; Luukkonen, T.; Laitinen, O.; de Sousa, Á.N.; Huuhtanen, M.; Illikainen, M. Characterization of Mineral Wool Waste Chemical Composition, Organic Resin Content and Fiber Dimensions: Aspects for Valorization. Waste Management 2021, 131, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Väntsi, O.; Kärki, T. Mineral Wool Waste in Europe: A Review of Mineral Wool Waste Quantity, Quality, and Current Recycling Methods. J Mater Cycles Waste Manag 2014, 16, 62–72. [Google Scholar] [CrossRef]
- Militký, J.; Mishra, R.; Jamshaid, H. 20 - Basalt Fibers. In Handbook of Properties of Textile and Technical Fibres (Second Edition); Bunsell, A.R., Ed.; The Textile Institute Book Series; Woodhead Publishing, 2018; Pp. 805–840 ISBN 978-0-08-101272-7.
- Persico, L.; Giacalone, G.; Cristalli, B.; Tufano, C.; Saccorotti, E.; Casalone, P.; Mattiazzo, G. Recycling Process of a Basalt Fiber-Epoxy Laminate by Solvolysis: Mechanical and Optical Tests. Fibers 2022, 10, 55. [Google Scholar] [CrossRef]
- Lilli, M.; Sarasini, F.; Fausto, L.D.; González, C.; Fernández, A.; Lopes, C.S.; Tirillò, J. Chemical Regeneration of Thermally Conditioned Basalt Fibres. Applied Sciences 2020, 10, 6674. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, W.; Zhang, Y.; Du, C.; Li, Y.; Wang, C.; Bi, J. Evaluating Fracture Resistance of Basalt Fiber Reinforced Mortar to Mode I/III Load Using Edge Notched Disc Bend (ENDB) Specimen: Insights from Acoustic Emission and Morphological Analysis. Construction and Building Materials 2024, 411, 134421. [Google Scholar] [CrossRef]
- Tiozzo, S.; Sanchetti, S.; Picicco, M.; Zanforlin, M.; Bemporad, E.; Zacco, A.; Depero, L.E. Basaltic Glass Fibers from Industrial Wastes: A Laboratory-Scale Technical Feasibility Study. Crystals 2022, 12, 359. [Google Scholar] [CrossRef]
Figure 1.
Technological scheme of mineral basalt insulation waste utilization by processing into mineral powder.
Figure 1.
Technological scheme of mineral basalt insulation waste utilization by processing into mineral powder.
Figure 2.
Scheme of production of mineral powder for asphalt concrete.
Figure 2.
Scheme of production of mineral powder for asphalt concrete.
Figure 3.
Chemical composition determination by X-ray spectroscopy (EDS).
Figure 3.
Chemical composition determination by X-ray spectroscopy (EDS).
Figure 4.
Determination of porosity and particle separation of processed basalt insulation waste.
Figure 4.
Determination of porosity and particle separation of processed basalt insulation waste.
Figure 5.
Determination of true density of mineral insulation waste particles using a volumetric compaction device.
Figure 5.
Determination of true density of mineral insulation waste particles using a volumetric compaction device.
Figure 6.
Swelling of samples from the mixture of powder with bitumen.
Figure 6.
Swelling of samples from the mixture of powder with bitumen.
Figure 7.
Manual mixing of mineral powder with bitumen and subsequent determination of bitumen capacity using a standardized plunger device.
Figure 7.
Manual mixing of mineral powder with bitumen and subsequent determination of bitumen capacity using a standardized plunger device.
Figure 8.
Determination of moisture content of mineral powder.
Figure 8.
Determination of moisture content of mineral powder.
Figure 9.
SEM images of waste basalt mineral fibers at different magnifications (×250 and ×3000).
Figure 9.
SEM images of waste basalt mineral fibers at different magnifications (×250 and ×3000).
Figure 10.
SEM images of waste basalt mineral fibers showing spherical melt inclusions and fiber surface morphology at magnifications ×40 (left) and ×120 (right), with measured particle sizes.
Figure 10.
SEM images of waste basalt mineral fibers showing spherical melt inclusions and fiber surface morphology at magnifications ×40 (left) and ×120 (right), with measured particle sizes.
Figure 11.
SEM–EDS elemental mapping of waste basalt mineral insulation fibers showing the spatial distribution of major elements (O, Mg, Al, Si, Ca, Na, Fe).
Figure 11.
SEM–EDS elemental mapping of waste basalt mineral insulation fibers showing the spatial distribution of major elements (O, Mg, Al, Si, Ca, Na, Fe).
Figure 14.
Sample of mineral powder from mineral insulating material.
Figure 14.
Sample of mineral powder from mineral insulating material.
Figure 15.
Activity of chemical elements of basalt insulation after electrolyte treatment.
Figure 15.
Activity of chemical elements of basalt insulation after electrolyte treatment.
Table 1.
Selection of the optimal amount of components for utilization of mineral insulation waste by chemical method.
Table 1.
Selection of the optimal amount of components for utilization of mineral insulation waste by chemical method.
| Raw materials in % |
Quality parameters of the product |
| mineral basalt insulation waste |
Electrolyte density 1400 kg/m3 |
Water
|
pH of mixture |
Residue after sieving (%) 0,08% |
| 47,5 |
5 |
47,5 |
10 |
30 |
| 45 |
10 |
45 |
9,2 |
12 |
| 42,5 |
15 |
42,5 |
8 |
7 |
| 40 |
20 |
40 |
7 |
5,7 |
| 37,5 |
25 |
37,5 |
6 |
4,2 |
| 35 |
30 |
35 |
5,1 |
3,8 |
Table 2.
Composition of mineral powder on the basis of waste production of basalt insulators for 0.5 m3.
Table 2.
Composition of mineral powder on the basis of waste production of basalt insulators for 0.5 m3.
| № |
Type of materials |
Waste mineral insulation, kg |
Water, kg |
NaOH, кг |
Fuse, kg |
Acryllatex, kg
|
Elektrolyte, kg |
| 1 |
Mineral powder based on waste from basalt heat insulation production |
1000 |
1000 |
15 |
10 |
20 |
200 |
Table 3.
Grain composition of Type 1 and Type 2 mineral powders.
Table 3.
Grain composition of Type 1 and Type 2 mineral powders.
| № |
Sample |
Grain composition according to GOST 32719-2014, not less than |
Porosity according to GOST 32764-2014, %, no more than |
True density according to GOST 32763-2014, g/cm3 |
smaller
2,0 mm
|
smaller
0,125 mm
|
smaller
0,063 mm
|
35 |
It's not OK. |
| 1 |
type 1 |
100,0 |
94,2 |
78,1 |
29,0 |
2,45 |
| 2 |
type 2 |
100,0 |
91,4 |
82,2 |
28,1 |
2,49 |
Table 4.
Bitumen interaction test for Type 1 and Type 2 mineral powder.
Table 4.
Bitumen interaction test for Type 1 and Type 2 mineral powder.
| № |
Sample |
Swelling of samples from the mixture of powder and bitumen according to GOST 32707-2014, % by volume, not more than |
Bitumen capacity index according to GOST 32766-2017, g, not more |
Humidity according to GOST 32762-2014, % by weight, no more than |
| 2,5 |
65 |
1,0 |
| 1 |
type 1 |
2,1 |
54 |
less than 0,1 |
| 2 |
type 2 |
2,0 |
52 |
less than 0,1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).