1. Introduction
Over the past two decades, substantial progress has been achieved in malaria control, especially through the large-scale implementation of preventive and therapeutic interventions [
1,
2]. Vector control strategies such as insecticide-treated nets (ITNs), and indoor residual spraying (IRS), have played a central role in malaria control. These interventions are credited with over 70% of the substantial decline in malaria cases during this period [
1]. Despite these achievements, malaria remains a major public health concern in sub-Saharan Africa [
3], accounting for over 263 million reported cases and nearly 600,000 deaths in 2023 according to the WHO. This persistence is driven by the emergence of drug resistance in
Plasmodium species, insecticide resistance in mosquito populations, and behavioural changes that undermine the effectiveness of existing control tools [
4,
5]. The disease is caused by five
Plasmodium species, with
P. falciparum being the most prevalent and virulent in Africa [
6]. Transmission occurs through female
Anopheles mosquitoes, a genus comprising nearly 500 species worldwide, of which about 100 species are recognised as malaria vectors [
7].
Mosquitoes (
Culicidae) are widely distributed across tropical and temperate regions, comprising more than 3,500 species grouped into three subfamilies. Among these,
Anopheles species are of major medical importance as malaria vectors [
8,
9]. Africa hosts a wide diversity of
Anopheles mosquitoes, with hundreds of species described across the continent [
10]. However, only a subset is responsible for most malaria parasites transmission, and their distribution varies across ecological zones. In sub–Saharan Africa, the dominant vectors are
An. gambiae s.s.,
An. coluzzii,
An. arabiensis, and
An. funestus [
11]. Other species such as
An. nili,
An. rufipes,
An. pharoensis, and
An. coustani typically occur at lower densities and act as secondary or potential vectors depending on local ecological conditions. The importance of these species varies across regions, reflecting differences in climate, vegetation, and human activities [
12].
In Burkina Faso, the main malaria vectors are
An. gambiae s.s.,
An. coluzzii,
An. arabiensis, and
An. funestus [
13,
14]. Ecological and climatic factors influence their distribution.
Anopheles gambiae s.s. is typically associated with temporary, rain-dependent breeding sites, whereas
An. coluzzii predominates in irrigated and semi-permanent habitats such as rice fields;
An. arabiensis is now present in urban areas, probably reflecting its adaptation to human-modified environments [
15].
Human-driven environmental changes, such as urbanisation, deforestation, and agricultural expansion, influence mosquito ecology [
16]. These alterations modify habitat availability, species composition, and population abundance, which can increase in vector species adapted to those altered ecosystems. Consequently, human vector contact may increase, thereby affecting the transmission dynamics of mosquito-borne diseases, including malaria [
16]. To better understand how these environmental changes influence vector populations and transmission risk, biodiversity indices were used to provide reliable quantitative tools for describing and comparing mosquito populations across spatial and temporal gradients. Species richness (S) indicates the number of species present, the Shannon index (H′) integrates both richness and evenness, while the Simpson index (D) reflects dominance patterns within the populations. By combining information on species richness and relative abundance, these indices offer valuable insights into community structure and help elucidate the ecological complexity underlying malaria transmission systems. Applying such metrics to
Anopheles populations enhances understanding of vector diversity and informs adaptive, evidence-based control strategies [
17]. Several studies have examined the diversity, distribution, and ecology of mosquitoes in different regions of Burkina Faso; however, most have focused primarily on malaria vectors and were geographically limited [
9].
To address these limitations, the present study provides an updated, nationwide assessment of Anopheles species diversity across the climatic zones of the country, combining morphological and molecular identification methods with biodiversity indices. Understanding mosquito biodiversity within these changing environments is essential for designing effective surveillance and control strategies, which rely on accurate identification and characterisation of vector species across ecological contexts. By exploring spatial and ecological variations in vector composition, this work aims to enhance understanding of Anopheles biodiversity and support the development of more effective, locally adapted malaria control strategies.
2. Materials and Methods
2.1. Study Sites and Ecological Zones
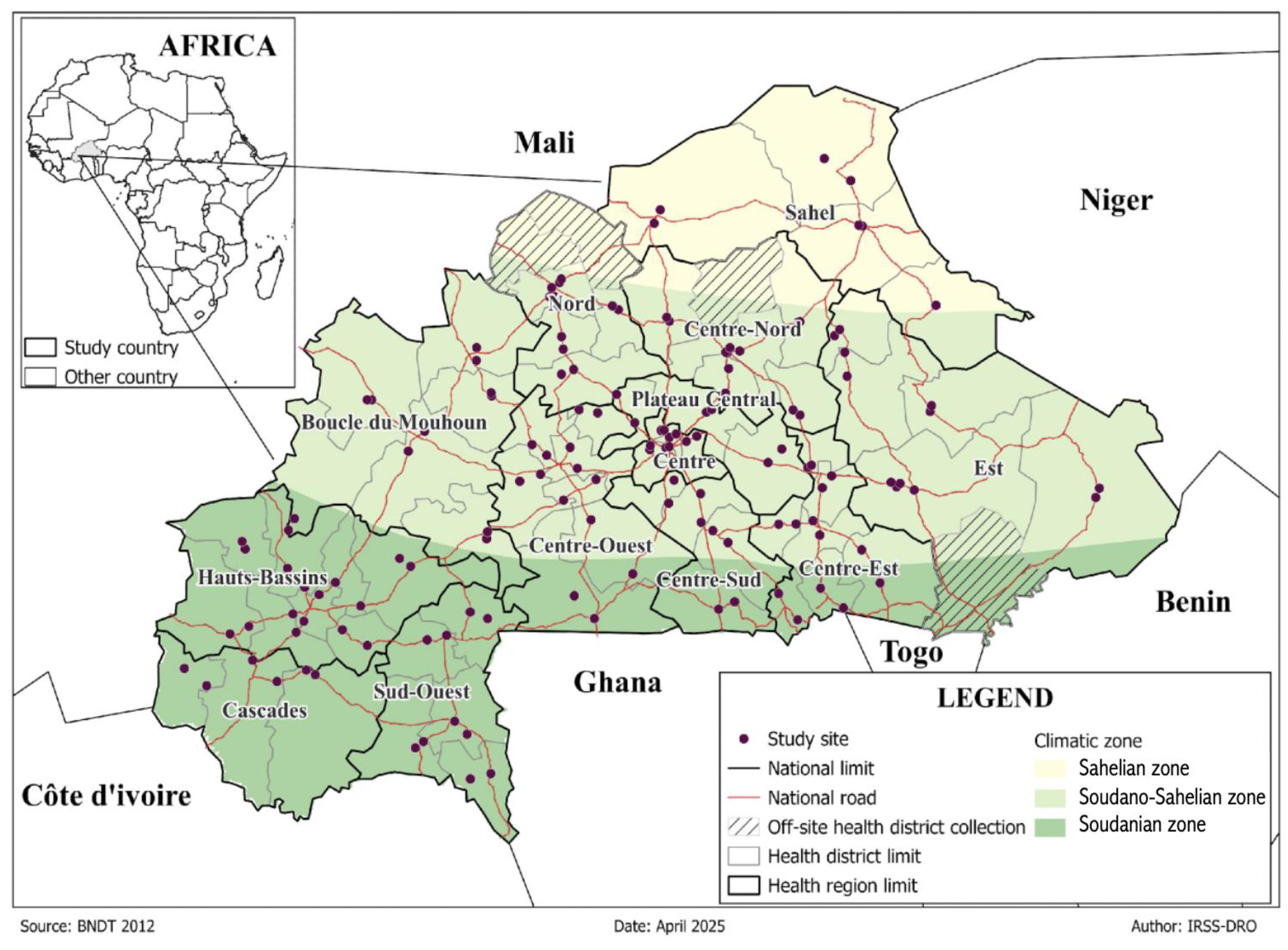
Entomological surveys were conducted in 67 health districts across the regions of Burkina Faso from September to December 2022. The country has a tropical climate with two distinct seasons: a rainy season lasting 3–6 months (May–October) and a dry season of about 6 months (November–April). These 13 regions are located in three main ecological zones: the Sahelian, Sudano-Sahelian, and Soudanian (
Figure 1). Each is characterised by distinct climatic and environmental features that influence mosquito vector distribution. The Sahelian zone, located in the northern part of the country, represents the driest ecological region. It has a mean annual temperature of 29.1 °C and rainfall ranging from 300 - 600 mm per year, mostly falling between June and September. It is characterised by sparse vegetation, consisting mainly of grasslands, thorny shrubs, and drought-tolerant tree species. Agricultural activity is limited, with sorghum and millet as the dominant crops, typically cultivated near seasonal water bodies that may also serve as mosquito breeding habitats. The Sudano-Sahelian zone has a dry season from November to April, with average annual rainfall ranging between 600 and 900 mm and a mean annual temperature of 28.4 °C. Dry forests, savannas, and scattered trees dominate vegetation. Agricultural production is mainly based on cereal cultivation, particularly millet and maize, while vegetable farming is also carried out on a smaller scale. Furthermore, human activities such as vegetable cultivation and unregulated urban expansion contribute to the development of mosquito breeding habitats. The Soudanian zone, which receives the highest rainfall, 900–1200 mm annually, mainly from May to October, is rich in vegetation and constitutes the most humid ecological region in the country [
18]. Gallery forests, dense savannas, and wooded landscapes dominate. Cotton and cereal crops are widely cultivated, and the combination of abundant rainfall, lush vegetation, and standing water creates favourable ecological conditions for mosquito proliferation [
19,
20]. Malaria control in these regions is based on the use of antimalarial drugs combined with preventive measures, including insecticide-based interventions and seasonal malaria chemoprevention for children under five years of age [
21].
2.2. Mosquito and Metadata Collection
Indoor-resting mosquitoes were collected early in the morning (06:00–09:00 a.m.) using pyrethroid spray catches (PSC) from ten randomly selected houses per site across 67 health districts in Burkina Faso. [
22]. Each collection site was georeferenced, and mosquito specimens were identified to the genus level using standard morphological keys (Gillies and Coetzee, 1987). All specimens were counted, identified, and preserved in 80% ethanol for subsequent analyses. PCR assays were performed on
An. gambiae complex (s.l.) specimens from 33 districts, selected based on the three main climatic zones, to determine species composition, specifically distinguishing
An. gambiae s.s.,
An. coluzzii, and
An. arabiensis.
2.3. Molecular Identification of Anopheles gambiae s.l.
Molecular analyses were conducted to identify
An. gambiae complex species. Genomic DNA was extracted from a single mosquito using a 2% CTAB protocol [
23]. The extracted DNA served as the template for PCR amplification targeting the SINE200 region to identify species within the
An. gambiae complex (s.l.) (Scott et al., 1993; Santolamazza et al., 2008). A single pair of primers, S200 X6.1 F (5′-TCGCCTTAGACCTTGCGTTA-3′) and S200 X6.1 R (5′-CGCTTCAAGAATTCGAGATAC-3′), was used with identification based on the size of the amplified fragment: approximately 479 bp for
An. coluzzii, 249 bp for
An. gambiae s.s., and 223 bp for
An. arabiensis. PCR reactions were carried out in a 20 μl reaction, which contained 3 µL of template DNA, 0.4 µL of each primer, 4 µL of 5× Hot Firepol Blend Master Mix, and 12.2 µL of molecular-grade water. Thermocycler conditions were 95°C for 15 minutes followed by 35 cycles of denaturation at 95°C for 1 minute, annealing at 62°C for 1 minute, extension at 72°C for 1 minute, final extension step at 72°C for 5 minutes, and a 4°C hold. PCR products were visualized on 2% agarose gels stained with ethidium bromide.
2.4. Data Management and Metadata
We managed our data in accordance with our approved data management plan (DMP), an approach was designed to uphold the FAIR principles, making data Findable, Accessible, Interoperable and Reusable, thereby guaranteeing its integrity and supporting reproducible results [
24]. Field data were collected using the KoBoToolbox platform [
25] on ruggedized tablets, with customized electronic forms incorporating validation rules to minimize entry errors. A two-step verification process [
26] was applied, and the two resulting datasets were programmatically cross validated using the arsenal package [
27], with discrepancies resolved by reference to original filed records. Missing data were handled to predefined outlined in our DMP. For sporadic missing environmental covariates (<5%), multiple imputation by chained equation (MICE) was performed using the nice package [
28] and results from five imputed datasets were pooled. The final validated dataset was archived in non-proprietary CSV format on secure, access-controlled servers with automated daily backups. In line with our commitment to data sharing, the de-identified dataset will be deposited in the Dryad Repository [
29] upon manuscript acceptance, assigned a DOI and released under CCO waiver to ensure global accessibility and reusability.
2.5. Statistical Analyses
Statistical analyses were conducted in R version 4.5.1 to investigate mosquito species abundance, diversity patterns and their association with climatic zones and seasons. Species abundance and relative abundance were computed by aggregating counts of each
Anopheles species by district and period. Relative abundance for each species was calculated as the proportion of its abundance to the total abundance within each district/period combination according to the following formula:
where Ni represents the abundance of species i and S denotes the total number of species in the population, which allows comparisons of species composition across spatial and temporal scales while accounting for variations in sampling effort [
30].
Biodiversity metrics (species richness, Shannon diversity index and Simpson diversity index) were calculated to characterise population structure. Species richness (S) was quantified as the number of species with non-zero abundance in each district/period combination. The Shanon index (H’) was computed as:
where pi represents the proportion of species
i in the population. This index quantifies species diversity by combining richness and evenness with greater emphasis on rare species (Shannon, 1948). The Simpson index (D) was calculated as:
which represents the probability that two randomly selected individuals belong to the same species (range 0 < D ≤ 1), was calculated. For the primary analysis, D was transformed into a component, D’ = 1 – D, representing the probability that two randomly selected individuals range [0, 1). This bounded metric (D) was selected for subsequent beta regression modeling. All diversity indices were computed using the “vegan version 2.7.1” package in R with appropriate handling of zero-inflated data through boundary adjustment (0.001 for zeros and 0.999 for ones) [
32].
PCR-confirmed species data from An. gambiae s.l. specimens were used to validate morphological identifications and to assess species distribution patterns across climatic zones.
A contingency table of species counts by zone was built and chi-square tests of independence were performed to evaluate the association between climatic zone and species composition. The strength was quantified using Cramer’s V:
where chi
2 denotes chi-square statistic,
n represents the total sample size, and
r and
c correspond to dimensions of the contingency table [
33]. Species proportions with 95% confidence intervals were calculated using the Clopper-Pearson exact method for binomial proportions [
34] and visualised to highlight compositional differences among zones.
To investigate the effects of climatic zone and season on biodiversity patterns, we used beta regression models with the Simpson index as the response variable. Given the bounded nature of diversity indices (0-1) and the presence of zero values, we implemented both standard beta regression and zero-inflated beta regression approaches using the “glmmTMB version 1.1.12” package [
35]. Models were fitted with climatic zone and season as fixed effects and district as a random. Model selection was performed using the Akaike Information Criterion (AIC) to identify the most parsimonious model [
24,
36]. Separate models were fitted for each climatic zone to account for zone-specific dynamics, followed by a combined model including all zones to assess overall patterns.
In cases where frequentist models exhibited convergence issues or poor fit, we employed Bayesian beta regression models with the “brms version 2.23.0” package as an alternative [
37]. These models were implemented with four chains, 2000 iterations (1000 warmup) and default weakly informative priors. Convergence was assessed using the R-hat statistic (target <1.01) and effective sample sizes (target > 400) [
38]. Posterior predictive checks were conducted to validate model fit, and summaries were reported with 95% credible intervals.
3. Results
3.1. Species Composition of Anopheles Mosquitoes Across Burkina Faso
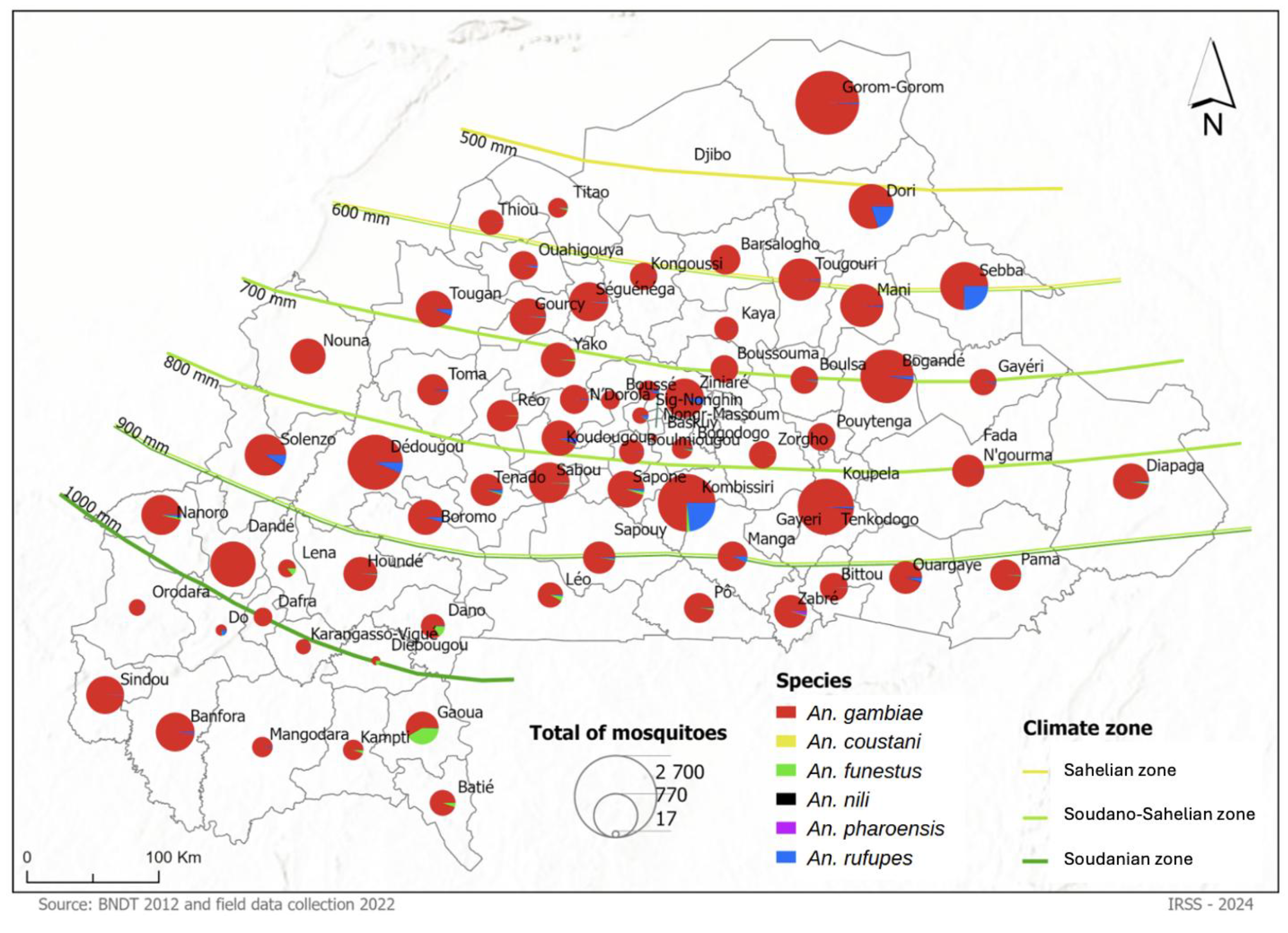
A total of 30,521
Anopheles specimens were collected between September and December 2022 across 67 districts in three climatic zones of Burkina Faso: Sahelian, Sudano-Sahelian, and Soudanian.
Anopheles gambiae s.l. was the most dominant species, representing 94.38% (28,820/30,521) of the overall specimens collected (
Figure 2). Other species included
An. rufipes (4.06%; 1,239/30,521),
An. funestus (1.36%; 415/30,521),
An. pharoensis (0.11%; 33/30,521),
An. nili (0.04%; 12/30,521), and
An. coustani (0.01%; 2/30,521) (
Table 1).
The Sahelian Zone was shown to be dominated by
An. gambiae s.l., accounting for 91.85% (4,743/5,164) of the total collected mosquitoes.
Anopheles rufipes was the secondary (8.03% (415/5,164)) dominant species after
An. gambiae complex. The other Anopheles species were composed of
An.funestus,
An.pharoenis,
An.coustani collected at frequencies less than 1% each. These results confirm the predominance of
An.gambiae s.l. as the main malaria vector in this climatic area. The mosquito composition remained relatively consistent during the sampling period across the Sahelian region, with
An. gambiae being the predominant species from September to December, with frequencies exceeding 85%. We observed clear geographical variability at the district level over the course of the trial. For example, Gorom-Gorom district reported steady counts from September (420) through December (224), whereas Kongoussi recorded no mosquitoes in September and December, but reported 229
An. gambiae s.l. in October and only 2
An. rufipes in November. In October,
An. rufipes was found at 18.98% (141/743) in the central district of Dori and at 24.41% (208/852) in Sebba, both within the same region (
Table S1). The presence of this species suggests that local environmental factors, such as specific larval habitat characteristics, favour
An. rufipes in these areas, even within landscapes where
An. gambiae s.l. remains the dominant species.
In the Sudano-Sahelian zone,
An. gambiae was the predominant
Anopheles species, accounting for 95.19% (17,602/18,492) of the collected mosquitoes. The other species included
An. rufipes at 4.1% (751/18,492), along with
An. coustani and
An. funestus, which were collected at low frequencies. Regarding the spatiotemporal distribution, the dominance of
An. gambiae was consistent across most districts and months, particularly during the peak abundance observed in October, which represented 95.89% (10,586/11,040) of all collections. However, significant temporal shifts were noted in certain districts as the season progressed into November and December. For example, in Kombissiri, the proportion of
An. gambiae s.
l. decreased from 84% (420/500) in October to 37.37% (37/99) in November, coinciding with an increase in the proportion of
An. rufipes from 15% (75/500) to 47.47% (47/99). Similarity, in Solenzo a notable shift was observed between October and December, with
An. rufipes accounting for 44.66% (46/103) of the catches in October prior to
An. gambiae s.l. Re-establishment dominance at 96.87 % (433/447) in December (
Table S1). These spatiotemporal dynamics suggest a complex ecological; succession of anopheline species which may have implications for seasonal malaria transmission dynamics and vector control strategies
The anopheline species in the Soudanian zone was overwhelmingly dominated by the
An. gambiae complex, which represented 94,32% (6,457/6,865) of the total specimens collected. The following secondary species was
An. funestus with 4.32% (301/865), while other species such as
An. rufipes (1.06%),
An. pharoensis (0.22%) and
An.nili (0.01%) were significantly rarer. This indicated that
An. gambiae complex is the primary vector in this ecological zone with
An. funestus emerging as a significant secondary vector of interest. About the space-time pattern, the dominance of the
An. gambiae complex was highly dominant across most districts and throughout the sampling period from September to December, consistently accounting for over 90% of the monthly total catches (e.g. up to 94.85% in October). However,
An. funestus reached significant levels in certain districts. For instance, in Gaoua district,
An. funestus accounted for 38.64% (153/396) of the catches in October and became the most prevalent
Anopheles species in December at 66.67% (26/39). Similar proportions of this species were observed in Dano in November, at 46% (23/50), and in Léo during December, at 57.14% (4/7) (
Table S1). These findings confirm the overall dominance of the
An. gambiae complex, but in some districts and periods,
An. funestus may play a relatively larger role in malaria parasite transmission and monitoring.
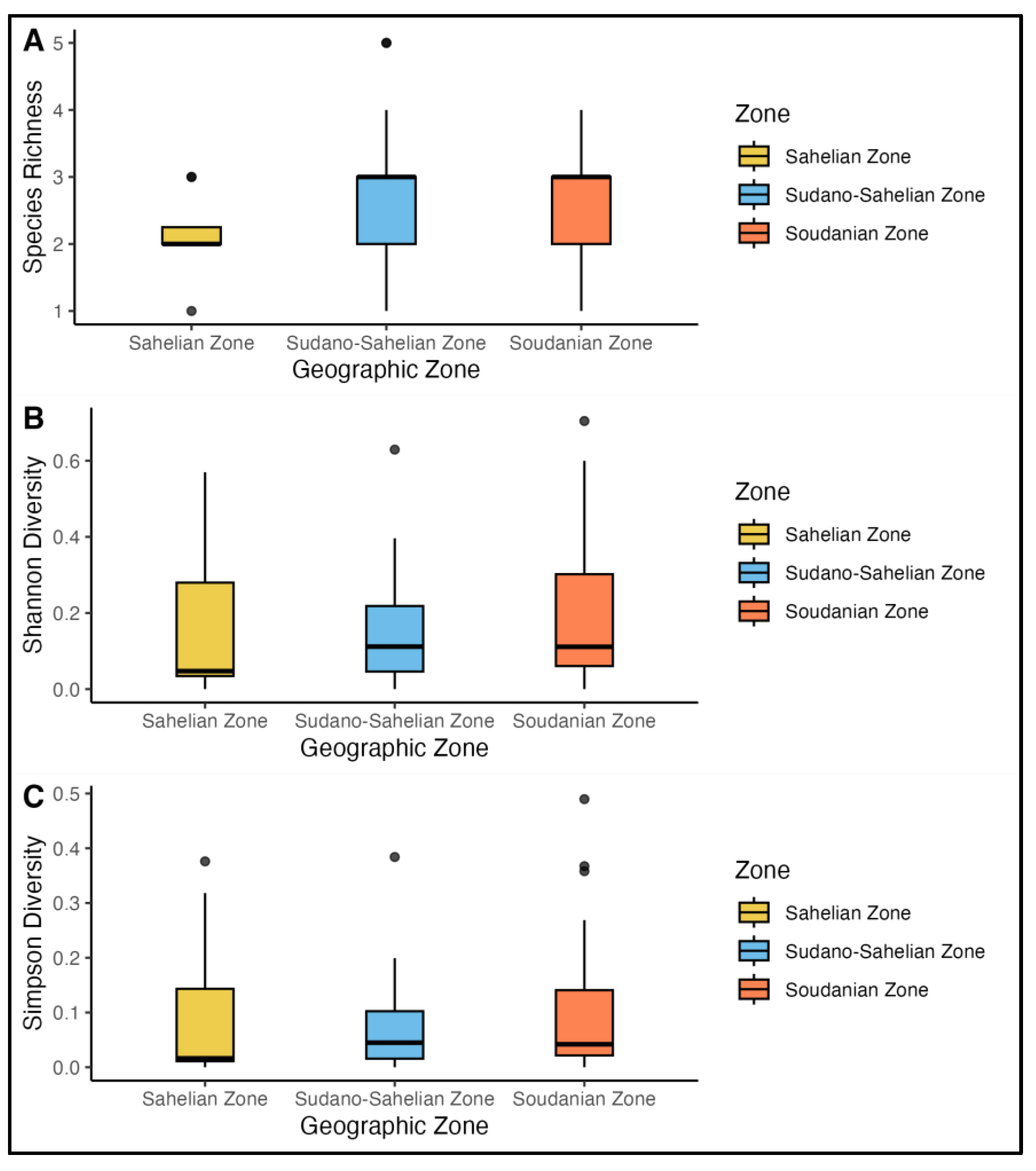
3.2. Biodiversity Indices Across Climatic Zones and Collection Periods
Biodiversity patterns of
Anopheles mosquitoes were assessed across 67 districts in three climatic zones of Burkina Faso (September–December 2022) using species richness, Shannon (H′), and Simpson (D) diversity indices. Species richness quantified the number of
Anopheles species per zone, while the Shannon and Simpson indices integrated both species abundance and evenness to describe population diversity and dominance patterns, revealing marked spatiotemporal variation driven by seasonal and environmental factors (
Figure 3).
The arid sahelian Zone exhibited the lowest biodiversity with richness ranging from 1-3 species per district-period (Figure3). This low diversity was most observed in Barsalogho, Kongoussi and Thiou where only one species was collected, resulting in Shannon and Simpson diversity indices of zero across all sampling periods. The highest diversity was recorded in Sebba during October (Richness = 3, Shannon = 0.85 and Simpson = 0.46), reflecting temporary habitat suitability during peak of rainfall (
Table S2). Dori and Titao showed moderate diversity in October to November (Shannon = 0.78 – 0.53, Simpson = 0.41-0.51), but diversity reduced to zero by December as water dried up. This temporal decline reveals the significant environmental constraints in the region. Only
An. gambiae s.l. (
An. coluzzii) appears to survive in the few remaining permanent water sources, while other species cannot withstand the dry conditions.
The transitional Sudano Sahelian Zone displayed intermediate biodiversity with greater spatiotemporal heterogeneity. Richness varied widely (0-5 species) with Solenzo recording the highest exceptional diversity in October (richness = 5, Shannon = 1.14 and Simpson = 0.56) in this zone was comparable to Soudanian diversity (
Table S2). In Kombissiri, species diversity remained relatively high across sampling periods. A notable peak in November (richness =3, Shannon = 1.76, and Simpson = 0.77) confirms that the area’s variety of larval breeding sites provides a stable foundation for a multi-species mosquito population. The zone’s biodiversity reflects the interaction between seasonal water availability and competitive dynamics among
An. gambiae s.l.
The humid Soudanian Zone exhibited the highest and most stable biodiversity, with richness spanning 0 – 4 species but fewer zero diversity periods (
Table S2). During the peak diversity period of October-November, several districts, notably Dano, Do, Gaoua and Kampti, consistently showed high species richness. This was accompanied by elevated Shannon diversity indices, surpassing 1.07 in Dano, 1.42 in Do and Kampti 0.50 during November, indicating a more balanced species distribution as reflected in their respective Simpson indices (0.53 and 0.51). Banfora and Zabré showed the widest temporal variation, with October peaks (richness = 3-4) collapsing to monospecific or absent collection by December, reflecting seasonal breeding site dynamics in floodplain areas. Gaoua’s sustained diversity into December (richness = 3, Shannon = 0.97, Simpson = 0.56) and suggested permanent water bodies supporting year-round multi-species communities, potentially including
An. funestus alongsides
An.gambiaes s.l. (
Table S2). The higher Simpson values in the zone indicate more equitable species proportions, consistent with greater habitat heterogeneity and reduced environmental stress compared to northern zones.
3.3. Modeling Simpson Diversity Across Climatic Zones
Simpson’s diversity index was calculated for the Anopheles population across 67 districts in Burkina Faso, spanning three climatic zones: Sahelian (n = 21 samples), Sudano-Sahelian (n= 96), and Soudanian (n=57), for a total of 174 sampling observations representing site-level Anopheles assemblages from dry (November to December) and wet (September to October) seasons. Due to the high prevalence of structural zeros (37.4% overall, ranging 36.5-42.9% by zone) reflecting single species dominance, we applied beta regression on epsilon transformed values (ε = 5.8×10⁻⁴ to 1.1×10⁻³) using the glmmTMB package. For each zone, we compared null models against season effects via Akaike Information Criterion (AIC), with null models consistently preferred (Δ AIC = 1.2-2.0), indicating negligible seasonal influence on diversity. Globally, the null model outperformed the full zone × season interaction (AIC = -639 vs. -631, ΔAIC = 7.7), providing moderate evidence against climatic effects. The mean Simpson’s index was uniformly low across zones (0.094-0.132), with a global estimate of 0.110 (95% CI: 0.087-0.134), suggesting persistent dominance of few Anopheles species. Model diagnostics using the DHARMa package confirmed adequate fit at the zone level (dispersion p > 0.75; uniformity p > 0.01), though global uniformity showed minor deviation (p < 0.001), likely attributable to structural zero-inflation rather than model misspecification. This pattern underscores ecological homogeneity in Anopheles assemblages across Burkina Faso’s climatic gradients, with single species dominance (often An.gambiae s.l.) persisting irrespective of aridity of seasonality. Such low diversity may constrain malaria transmission dynamics and vector control efficacy, warranting species specific intervention over zone tailored strategies.
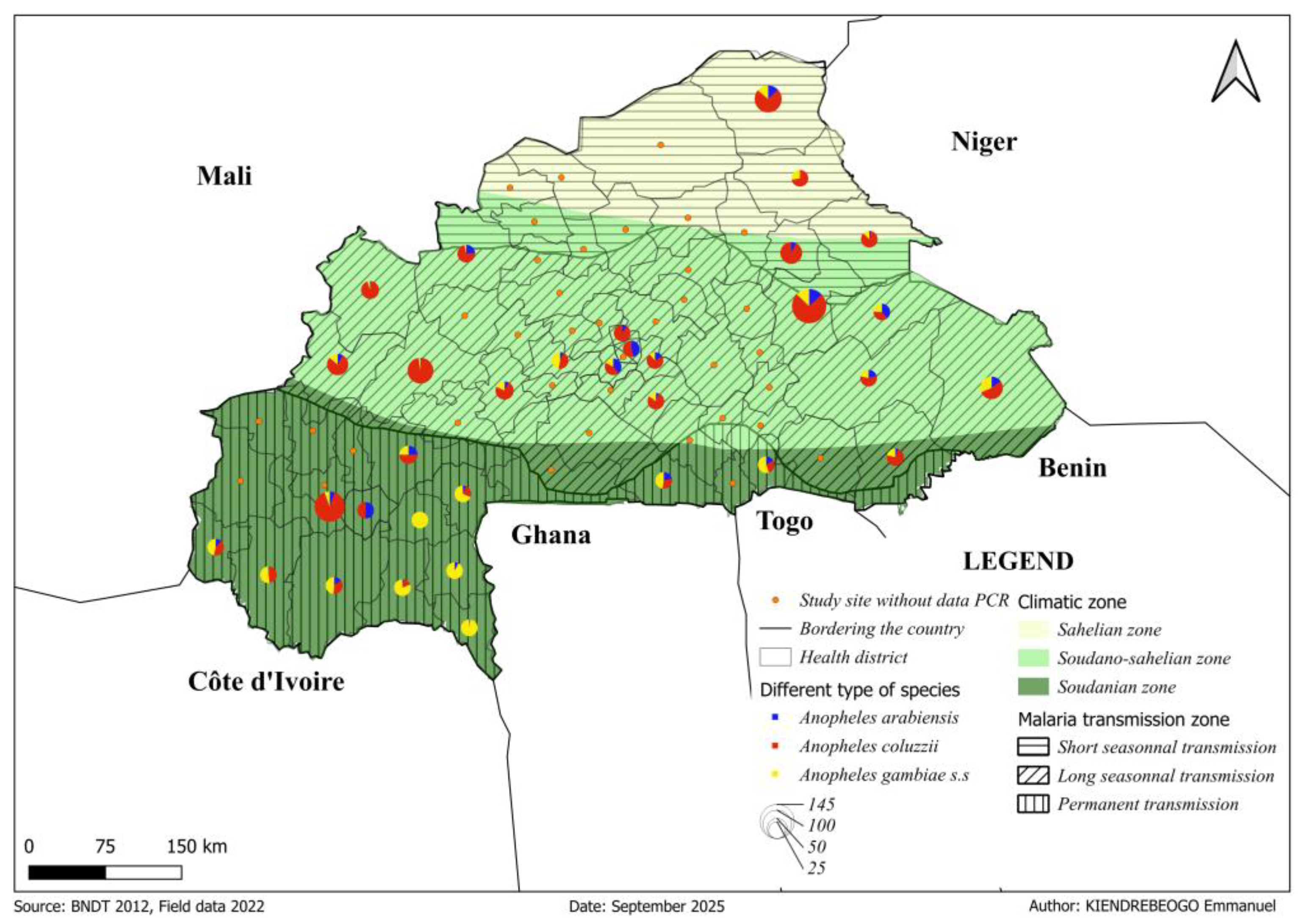
3.4. Composition of Anopheles gambiae s.l. Species Across Climatic Zones
Polymerase chain reaction (PCR) analysis was conducted on 2,026
An. gambiae s.l specimens collected between September and December across three climatic zones in Burkina Faso (Table1). The molecular identification revealed clear climatic structure of sibling species within the complex:
An. coluzzi, An. gambiae s.s., and
An. arabenssis (
Figure 4). The analyses revealed distinct species distributions across the climatic gradient.
Anopheles coluzzii was the predominant species in both the Sahelian (74.9%, 143/191), and Sudano-Sahelian (71.0%, 632/890) zones (
Table S4). In the Soudanian zone,
An. gambiae s.s. accounted for the majority of specimens (53.7%, 508/945), while
An. coluzzii was less frequent (36.1%, 341/945). Only a small proportion of mosquitoes collected were
An. arabiensis, which varied from 8.9% (17/191) in the Sahelian zone to 14.3% (127/890) in the Sudano-Sahelian zone (
Table 1). A chi-square test showed that species distribution patterns were strongly dependent on climatic zone (χ² = 353.86, df = 4, p < 0.00001), with a moderate effect size (Cramer’s V = 0.3). The analysis of residuals provided clear detail, showing that
An. gambiae s.s. was disproportionately abundant in the Soudanian Zone (residual = 11.06) while being significantly underrepresented further north (residual = - 4.05 in Sahelian, - 9.52 in Sudano-Sahelian). PCR-based diversity indices showed all zones had richness = 3 species, but diversity increased southward. Analysis of diversity indices revealed a pronounced major latitudinal cline. Population evenness was highest in the Soudanian zone (Shannon = 0.93, Simpson = 0.57) due to the influential presence of
An. gambiae s.s., decreased in the transitional Sudano-Sahelian Zone (Shannon = 0.80, Simpson = 0.45), and was most suppressed in the arid Sahelian zone (Shannon = 0.72, Simpson = 0.40). This pattern results from the increasing monopolization of the mosquito population by
An. coluzzii as conditions become drier.
3. Discussion
An entomological survey has been carried out for
Anopheles specimen’s collection across Burkina Faso’s three major climatic zones. This study showed that a distinct ecological pattern dominated by
Anopheles gambiae complex. Despite the country’s high climatic gradient from the arid Sahel in the north to the humid Soudanian zone in the south, mosquito populations exhibited remarkable structural uniformity.
Anopheles gambiae s.l. accounted for over 94% of all specimens, confirming its overwhelming predominance as the primary malaria vector in Burkina Faso. Molecular analyses further revealed clear geographic structure within the complex, with
An. coluzzii dominating northern and central regions, while
An. gambiae s.s. was more frequent in the humid southern areas.
Anopheles arabiensis, though less common, was detected in all zones, suggesting broad ecological adaptability. These patterns are consistent with findings from Burkina Faso, Cameroon, Mali, and Niger [
39,
40,
41] and align with previous national surveys [
14,
42].
Our results confirmed previous studies indicating that malaria transmission in Burkina Faso is primarily driven by members of the
An. gambiae complex and
An. funestus [
14,
43] Across sub-Saharan Africa,
An. gambiae s.l. remains the principal malaria vector, with population peaks typically occurring during and immediately after the rainy season, when breeding sites are most abundant [
44,
45]. Seasonal shifts often involve increases in
An. funestus populations in areas with permanent or vegetated water bodies, whereas mosquito densities in the Sahel decline sharply during the dry season due to the loss of larval habitats [
45,
46].
In contrast, in the East and Central African contexts, where species composition and diversity vary substantially along climatic and altitudinal gradients [
47,
48]. The ecological uniformity observed in Burkina Faso likely reflects the extensive anthropogenic transformation of landscapes. Agricultural expansion, irrigation schemes, and urban growth have produced stable aquatic habitats enabling ecologically flexible breeding sites for
An. coluzzii. Moreover, the extended use of insecticide-based interventions may have selectively reduced populations of less resilient, habitat-specialist species, thereby promoting dominance of a few highly adaptable taxa [
49].
A distinct biodiversity gradient was observed across Burkina Faso. The southern Soudanian zone showed the highest species richness and evenness, with the coexistence of
An. funestus,
An. rufipes,
An. pharoensis,
An. nili, and
An. coustani alongside
An. gambiae s.l.. These species, although less abundant, may sustain residual malaria transmission during periods of low
An. gambiae density, as reported in Ghana and Tanzania [
50,
51]. In contrast, the Sudano-Sahelian and Sahelian zones exhibited very low diversity (mean richness 1.0; Shannon and Simpson indices ≈ 0), consistent with the dominance of
An. gambiae s.l. (91.8–95.2%). Similar declines in biodiversity with increasing aridity have been reported across West Africa [
52]. These findings highlight the ecological and epidemiological implications of simplified vector populations. Low diversity may facilitate targeted control interventions but also increases vulnerability to environmental changes. Irrigation and peri-urban expansion could create new habitats favouring secondary species such as
An. rufipes, which, although historically considered zoophilic, has been detected carrying
Plasmodium in Burkina Faso [
53]
The results showed that, despite the observed biodiversity gradient across climatic zones, mosquito population structure remains remarkably uniform. This pattern suggests a dominance constraint mechanism, where strong environmental pressures and competition favour a few highly adaptable species. Members of the
An. gambiae complex, with traits such as desiccation-resistant eggs and behavioural plasticity, thrive in both natural and human-modified habitats [
43,
54]. Human activities such as irrigation and agriculture strongly influence vector distribution and reinforce this ecological dominance [
49]. These dynamics highlight the need for adapted control strategies, combining timely interventions before seasonal peaks with zone-specific approaches targeting dominant and emerging secondary vectors.
From a public health perspective, the uniformity of
Anopheles populations across Burkina Faso offers both advantages and risks. The dominance of
An. gambiae s.l. supports continued use of LLINs and IRS, but also intensifies insecticide selection pressure, accelerating resistance. Given the higher frequency of pyrethroid resistance in
An. gambiae s.s. from southern regions [
43] a dual strategy is recommended: maintaining universal coverage while strengthening molecular surveillance. Integrating next-generation tools such as dual-active LLINs, larvicide, genetic control approaches, and environmental management will be essential to mitigate resistance and sustain long-term control effectiveness.
This study offers a broad overview of Anopheles diversity in Burkina Faso but is limited by its four-month sampling period, which did not capture full annual dynamics. Morphological identification may have underestimated cryptic species, and insecticide resistance was not evaluated. Future research should include year-round molecular monitoring and resistance testing, coupled with fine-scale environmental data to better understand local drivers of vector distribution. Experimental studies on larval ecology and competition are also needed to clarify mechanisms sustaining An. gambiae dominance.
3. Conclusions
This study assessed Anopheles diversity and distribution across Burkina Faso, providing important ecological insights along the country’s major climatic zones. The persistence of Anopheles dominance across environmental gradients suggests that human activities such as irrigation, agriculture, and land-use change exert a stronger influence than climate alone on vector composition. A clear biodiversity gradient was detected, with secondary species occurring mainly in the southern zone. Although less abundant, these species may sustain residual transmission during periods of low An. gambiae density. Such ecological simplification challenges expectations of strong species turnover along environmental gradients and indicates a vector population that is highly adapted and relatively stable. The predominance of An. gambiae s.l. supports continued deployment of LLINs and IRS, but also underscores the need for enhanced molecular surveillance, development of genetic-control tools, and adaptive management to mitigate insecticide resistance and environmental change. Overall, this work emphasizes the need to monitor and design locally tailored, evidence-based interventions across the country’s ecological zones.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org
Funding
This study is supported by Wellcome Trust (UNS1285360), Bill and Melinda Gates Foundation (INV037164) and Pan-African Mosquito Control Association (OPP1214408).
Authors' contributions
O.N. Z, M. K, N.T, H.M and AD conceived the study. O.N.Z, G. S, G.Y and S.I performed the field collection. O.N.Z, S.O.G.Y, G.S, B.N, H.K, I.T, A.A.M.A performed laboratory experiments. O.N.Z, S.B performed the data analyses. E.K and A.A.M established the sampling map. O.N.Z and S.B wrote the early version of the manuscript. A.D secured the funding acquisition. H.K, M.K, N.T, R.S, M.N, A.M, A.L, T.N, I.T, H.M, and AD edited and validated the manuscript. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Ethics approval
This study was approved by the Institutional Ethics Committee of the Institut de Recherche en Sciences de la Santé (32-2022/CEIRES, 27 May 2022). The protocol was reviewed and approved by the same Ethics Committee. All volunteers involved in the study were informed about the project's activities before giving their informed consent to participate.
Availability of data and materials
Data are provided within the manuscript or supplementary information files
Acknowledgements
The authors would like to acknowledge with appreciation the valuable collaboration and technical support provided by the Institut de Recherche en Sciences de la Santé, Direction Régionale de l’Ouest (IRSS-DRO, Burkina Faso).
Competing interests
All the authors have read and accepted this version of the manuscript. The authors also declare there are no conflicts of interest.
References
- S Bhatt, D J Weiss, E Cameron, D Bisanzio, B Mappin, U Dalrymple, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. 2015. [CrossRef]
- Janet Hemingway, Rima Shretta, Timothy N C Wells, David Bell, Abdoulaye A Djimdé, Nicole Achee, et al. Tools and Strategies for Malaria Control and Elimination: What Do We Need to Achieve a Grand Convergence in Malaria? PLoS Biol. 2016. [CrossRef]
- Sempungu JK, Choi M, Lee EH, Lee YH. The burden of malaria in sub-Saharan Africa and its association with development assistance for health and governance: An exploration of the GBD 2019 study. Glob Public Health. 2023;18 1:2229892; https://www.ncbi.nlm.nih.gov/pubmed/37438859. [CrossRef]
- Tanya L Russell, Nigel W Beebe, Cooper RD, Burkot NFLaTR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malaria Journal. 2013. [CrossRef]
- Matowo NS, Martin J, Kulkarni MA, Mosha JF, Lukole E, Isaya G, et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone, Tanzania. Sci Rep. 2021;11 1:13457; https://www.ncbi.nlm.nih.gov/pubmed/34188090. [CrossRef]
- Mwesigwa A, Ocan M, Musinguzi B, Nante RW, Nankabirwa JI, Kiwuwa SM, et al. Plasmodium falciparum genetic diversity and multiplicity of infection based on msp-1, msp-2, glurp and microsatellite genetic markers in sub-Saharan Africa: a systematic review and meta-analysis. Malar J. 2024;23 1:97; https://www.ncbi.nlm.nih.gov/pubmed/38589874. [CrossRef]
- Laurent BS. Mosquito vector diversity and malaria transmission. 2025. [CrossRef]
- Syed Ali Haider Shah, Maria Amjad, Nimra Sagheer, Minahil Mehmood sheikh, Muhammad Qasim, Sadia Ashraf, et al. Diversity, relative abundance and ecology of the mosquito(Diptera: Culicidae) fauna in district Okara, Pakistan. Azerbaijan Pharmaceutical and Pharmacotherapy. 2024. [CrossRef]
- Srisuka W, Sulin C, Sommitr W, Rattanarithikul R, Aupalee K, Saeung A, et al. Mosquito (Diptera: Culicidae) Diversity and Community Structure in Doi Inthanon National Park, Northern Thailand. Insects. 2022;13 9; https://www.ncbi.nlm.nih.gov/pubmed/36135515. [CrossRef]
- Kiam BC, Bouopda-Tuedom AG, Mbida Mbida JA, Ibrahima I, White SJ, Tchuenkam PVK, et al. Diversity of anopheline species and malaria transmission dynamics in high-altitude areas of western Cameroon. Malar J. 2025;24 1:251; https://www.ncbi.nlm.nih.gov/pubmed/40770357. [CrossRef]
- Takken W, Charlwood D, Lindsay SW. The behaviour of adult Anopheles gambiae, sub-Saharan Africa's principal malaria vector, and its relevance to malaria control: a review. Malar J. 2024;23 1:161; https://www.ncbi.nlm.nih.gov/pubmed/38783348. [CrossRef]
- Tsegaye A, Demissew A, Hawaria D, Getachew H, Habtamu K, Asale A, et al. Susceptibility of primary, secondary and suspected vectors to Plasmodium vivax and Plasmodium falciparum infection in Ethiopia. Parasit Vectors. 2022;15 1:384; https://www.ncbi.nlm.nih.gov/pubmed/36271436. [CrossRef]
- Soma DD, Poda SB, Hien AS, Namountougou M, Sangare I, Sawadogo JME, et al. Malaria vectors diversity, insecticide resistance and transmission during the rainy season in peri-urban villages of south-western Burkina Faso. Malar J. 2021;20 1:63; https://www.ncbi.nlm.nih.gov/pubmed/33494779. [CrossRef]
- Traore A, Badolo A, Guelbeogo MW, Sanou A, Viana M, Nelli L, et al. Anopheline species composition and the 1014F-genotype in different ecological settings of Burkina Faso in relation to malaria transmission. Malar J. 2019;18 1:165; https://www.ncbi.nlm.nih.gov/pubmed/31068189. [CrossRef]
- Roch K Dabiré, Moussa Namountougou, Simon P Sawadogo, Lassina B Yaro, Hyacinthe K Toé, Ali Ouari, et al. Population dynamics of Anopheles gambiae s.l. in Bobo-Dioulasso city: bionomics, infection rate and susceptibility to insecticides. Parasites & Vectors. 2012. [CrossRef]
- Patindé Didier Alexandre KABORE, Dieudonné Diloma Soma, Patricia Gil, Mahamadi Kientega, Simon P. Sawadogo, Georges Anicet Ouédraogo, et al. Mosquito (Diptera: Culicidae) communities in contrasting areas from the Western regions of Burkina Faso: species diversity, abundance and implication in pathogen transmission. Parasites & Vectors. 2023. [CrossRef]
- J Guillermo Bond, Mauricio Casas-Martínez, Humberto Quiroz-Martínez, Rodolfo Novelo-Gutiérrez, Carlos F Marina, Armando Ulloa, et al. Diversity of mosquitoes and the aquatic insects associated with their oviposition sites along the Pacific coast of Mexico. Parasites & Vectors. 2014. [CrossRef]
- Basson; F, Zougmoré; F, Somda; J, Dipama; J-M. Analyse Du Plan National d’Adaptation Aux Changements Climatiques (PNA) Du Burkina Faso Et De Sa Capacité A Atteindre Ses Objectifs. 2020. [CrossRef]
- Namountougou M, Soma DD, Kientega M, Balbone M, Kabore DPA, Drabo SF, et al. Insecticide resistance mechanisms in Anopheles gambiae complex populations from Burkina Faso, West Africa. Acta Trop. 2019;197:105054; https://www.ncbi.nlm.nih.gov/pubmed/31175862. [CrossRef]
- Cédric B, Mady C, Abdoulaye K, Bry S, Ambroise O, Jean Baptiste O, et al. Malaria in Burkina Faso: A comprehensive analysis of spatiotemporal distribution of incidence and environmental drivers, and implications for control strategies. PLoS One. 2023; doi: https://doi.org/10.1371/.
- Paul S, Marc CT, Toussaint R, Karim D, Bérenger K, Cheick SC, et al. Assessment of a combined strategy of seasonal malaria chemoprevention and supplementation with vitamin A, zinc and Plumpy'Doz™ to prevent malaria and malnutrition in children under 5 years old in Burkina Faso: a randomized open-label trial (SMC-NUT). 2021. [CrossRef]
- Yovogan B, Adoha CJ, Akinro B, Accrombessi M, Dangbenon E, Sidick A, et al. Field performance of three mosquito collection methods for assessing the entomological efficacy of dual-active ingredient long-lasting insecticidal nets. Sci Rep. 2023;13 1:12263; https://www.ncbi.nlm.nih.gov/pubmed/37507478. [CrossRef]
- Sam Mugford, Roland Wouters, Thomas C Mathers, Saskia Hogenhout, Innes. J. High quality DNA extraction from very small individual insects. 2020. [CrossRef]
- William M. Giuliano, Lauren N. Watine, John M. Olson, Mitchell Blake, Ober H. Florida Turkey Nest Site Selection and Success. Natural Resources. 2016. [CrossRef]
- KoBoToolbox. Intuitive and adaptable data tools to maximize your impact. 2022. https://www.kobotoolbox.org/.
- Miyuki Kawado, Shiro Hinotsu, Yutaka Matsuyama, Takuhiro Yamaguchi, Shuji Hashimoto, Ohashi Y. A comparison of error detection rates between the reading aloud method and the double data entry method. 2003. [CrossRef]
- Ethan Heinzen, Jason Sinnwell, Elizabeth Atkinson, Tina Gunderson, Gregory Dougherty, Patrick Votruba, et al. An Arsenal of 'R' Functions for Large-Scale Statistical Summaries. 2021. [CrossRef]
- Stef van Buuren, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. 2011. [CrossRef]
- Švarcová M, Kolařík M, Li Y, Tsui CKM, Hubka V. Resolving phylogenetic relationships within the Trichophyton mentagrophytes complex: A RADseq genomic approach challenges status of ‘terbinafine-resistant’ Trichophyton indotineae as distinct species. 2025. [CrossRef]
- Jetty. Measuring biological diversity. Blackwell Science Ltd. 2004.
- SIMPSON EH. Measurement of Diversity. 1949.
- Jari Oksanen, Gavin Leslie Simpson, F. Guillaume Blanchet, Gavin Leslie Simpson, Kindt. R. vegan community ecology package version 2.6-2 April 2022. 2022.
- Umakantha N. A New Approach to Probability Theory with Reference to Statistics and Statistical Physics. Modern Physics 2016. [CrossRef]
- C. J. Clopper, B.Sc., E. S. Pearson, D.Sc. The use of confidence or fiducial limits illustrated in the case of the binomial. 1934. [CrossRef]
- Mollie Elizabeth Brooks, Kasper Kristensen, Koen Johannes van Benthem, Magnusson. A. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. ResearchGate. 2017. [CrossRef]
- Akaike H. A New Look at the Statistical Model Identification. IEEE Transactions on Automatic Control. 1974. [CrossRef]
- Bürkner. An R Package for Bayesian Multilevel Models Using Stan. Journal of Statistical. 2017. [CrossRef]
- Gelman A, Rubin DB. Inference from Iterative Simulation Using Multiple Sequences. Statistical Science. 1992. [CrossRef]
- Ayala D, Costantini C, Ose K, Kamdem GC, Antonio-Nkondjio C, Agbor JP, et al. Habitat suitability and ecological niche profile of major malaria vectors in Cameroon. Malar J. 2009;8:307; https://www.ncbi.nlm.nih.gov/pubmed/20028559. [CrossRef]
- Sanata Coulibaly, Simon Péguédwindé Sawadogo, Aristide Sawdetuo Hien, Achille Sindimbasba Nikièma, Ibrahim Sangaré, Bamogo Rabila, et al. Malaria and Lymphatic Filariasis Co-Transmission in Endemic Health Districts in Burkina Faso. Advances in Entomology. 2021. [CrossRef]
- Sougoufara S, Doucoure S, Backe Sembene PM, Harry M, Sokhna C. Challenges for malaria vector control in sub-Saharan Africa: Resistance and behavioral adaptations in Anopheles populations. J Vector Borne Dis. 2017;54 1:4-15. https://www.ncbi.nlm.nih.gov/pubmed/28352041.
- Soma; DD, Zogo; BM, Somé; A, Tchiekoi; BNC, Hien; DFdS, Pooda; HS, et al. Anopheles bionomics, insecticide resistance and malaria transmission in southwest Burkina Faso: A pre-intervention study. Plos one 2020. [CrossRef]
- Abdoulaye Diabaté, Adama Dao, Alpha S. Yaro, Abdoulaye Adamou, Rodrigo Gonzalez, Nicholas C. Manoukis, et al. Spatial swarm segregation and reproductive isolation between the molecular forms of Anopheles gambiae. 2009. [CrossRef]
- Mwima R, Hui TJ, Nanteza A, Burt A, Kayondo JK. Potential persistence mechanisms of the major Anopheles gambiae species complex malaria vectors in sub-Saharan Africa: a narrative review. Malar J. 2023;22 1:336; https://www.ncbi.nlm.nih.gov/pubmed/37936194. [CrossRef]
- Epopa PS, Collins CM, North A, Millogo AA, Benedict MQ, Tripet F, et al. Seasonal malaria vector and transmission dynamics in western Burkina Faso. Malar J. 2019;18 1:113; https://www.ncbi.nlm.nih.gov/pubmed/30940141. [CrossRef]
- Adamou A, Dao A, Timbine S, Kassogue Y, Yaro AS, Diallo M, et al. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar J. 2011;10:151; https://www.ncbi.nlm.nih.gov/pubmed/21645385. [CrossRef]
- Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, et al. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science. 2015;347 6217:1258522; https://www.ncbi.nlm.nih.gov/pubmed/25554792. [CrossRef]
- Emmanuel W. Kaindoa, Nancy S. Matowo, Halfan S. Ngowo, Gustav Mkandawile, Arnold Mmbando, Marcelina Finda, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south–eastern Tanzania. PLoS One. 2017. [CrossRef]
- Ryan SJ, Lippi CA, Villena OC, Singh A, Murdock CC, Johnson LR. Mapping current and future thermal limits to suitability for malaria transmission by the invasive mosquito Anopheles stephensi. Malar J. 2023;22 1:104;https://www.ncbi.nlm.nih.gov/pubmed/36945014. [CrossRef]
- Mayagaya VS, Nkwengulila G, Lyimo IN, Kihonda J, Mtambala H, Ngonyani H, et al. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17; https://www.ncbi.nlm.nih.gov/pubmed/25604150. [CrossRef]
- Coetzee M, van Wyk P, Booman M, Koekemoer LL, Hunt RH. Insecticide resistance in malaria vector mosquitoes in a gold mining town in Ghana and implications for malaria control. Bull Soc Pathol Exot. 2006;99 5:400-3. https://www.ncbi.nlm.nih.gov/pubmed/17253060.
- A. Djènontin, BZ, J. Ahlonsou, A. Bouraima, M. Ibikounle, D. Courtin, et al. Mosquitoes fauna diversity, Plasmodium falciparum infection and insecticide resistance status in malaria vectors in a lagoon area in Southern Benin, West Africa. 2017. [CrossRef]
- Lefevre T, Vantaux A, Dabire KR, Mouline K, Cohuet A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013;9 6:e1003365; https://www.ncbi.nlm.nih.gov/pubmed/23818841. [CrossRef]
- Gimonneau G, Tchioffo MT, Abate L, Boissiere A, Awono-Ambene PH, Nsango SE, et al. Composition of Anopheles coluzzii and Anopheles gambiae microbiota from larval to adult stages. Infect Genet Evol. 2014;28:715-24; https://www.ncbi.nlm.nih.gov/pubmed/25283802. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).