Submitted:
03 November 2025
Posted:
04 November 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.2.3. Search Strategies and Information Source
2.2.4. Selection of Sources of Evidence
2.2.5. Methodological and Reporting Quality Assessment
2.2.6. Analysis of Included Studies
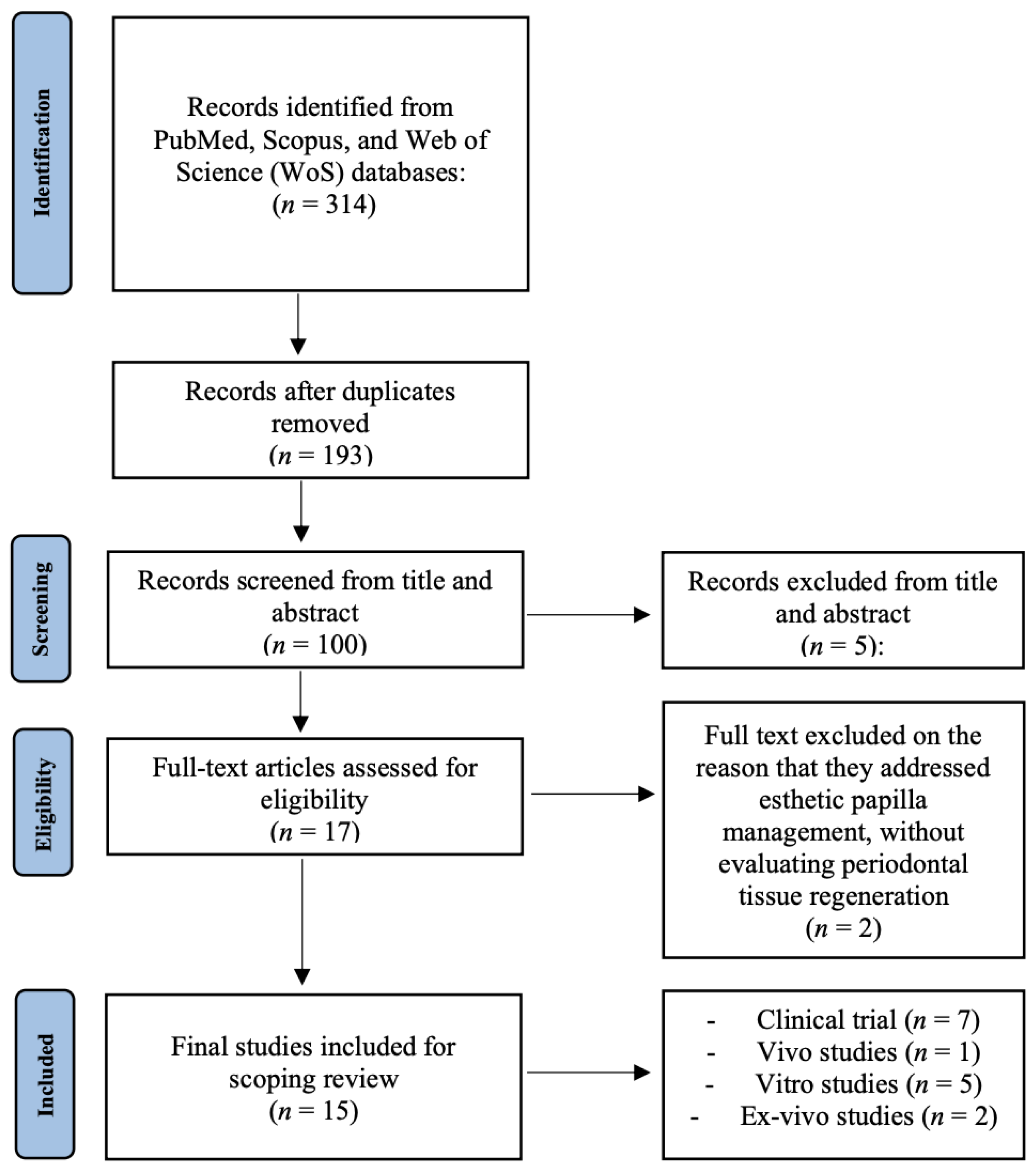
3. Results
Results of Individual Sources of Evidence
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-PRF | Advanced Platelet-Rich Fibrin |
| A-PRF+ | Advanced Platelet-Rich Fibrin Plus |
| ALP | Alkaline Phosphatase |
| aPDT | Antimicrobial Photodynamic Therapy |
| BMP-2 | Bone Morphogenetic Protein-2 |
| CAL | Clinical Attachment Level |
| CCK-8 | Cell Counting Kit-8 |
| DBM | Demineralized Bone Matrix |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ERK | Extracellular Signal-Regulated Kinase |
| GMSCs | Gingival Mesenchymal Stem Cells |
| GTR | Guided Tissue Regeneration |
| HLLT | High-Level Laser Therapy |
| LANAP | Laser-Assisted New Attachment Procedure |
| LED | Light-Emitting Diode |
| LLLT | Low-Level Laser Therapy |
| MAPK | Mitogen-Activated Protein Kinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide assay |
| OCN | Osteocalcin |
| OPN | Osteopontin |
| PBM | Photobiomodulation |
| PD | Probing Depth |
| PDGF-BB | Platelet-Derived Growth Factor-BB |
| PDLSCs | Periodontal Ligament Stem Cells |
| PRF | Platelet-Rich Fibrin |
| RCT | Randomized Controlled Trial |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| RUNX2 | Runt-Related Transcription Factor 2 |
| SEM | Scanning Electron Microscopy |
| SDF-1 | Stromal Cell-Derived Factor-1 |
| SRP | Scaling and Root Planing |
| TGF-β | Transforming Growth Factor-Beta |
| VEGF | Vascular Endothelial Growth Factor |
| Wnt | Wingless/Integrated signaling pathway |
References
- Santonocito S, Polizzi A, Cavalcanti R, Lo Giudice A, Indelicato F, Isola G. Impact of laser therapy on periodontal and peri-implant diseases: A review. Photobiomodul Photomed Laser Surg. 2022;40(7):454-462. [CrossRef]
- Sanz M, Teughels W; Group A of the F. European Workshop on Periodontology. Innovations in non-surgical periodontal therapy: Consensus report of Group A. J Clin Periodontol. 2012;39 Suppl 12:133-138. [CrossRef]
- Behdin S, Monje A, Lin GH, Edwards B, Othman A, Romanos GE. Effectiveness of laser application for periodontal surgical therapy: Systematic review and meta-analysis. J Periodontol. 2015;86(12):1352-1363. [CrossRef]
- Aoki A, Mizutani K, Schwarz F, Sculean A, Yukna RA, Takasaki AA, Romanos GE, Taniguchi Y, Sasaki KM, Zeredo JL, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68(1):217-269. [CrossRef]
- Dukić W, Bago I. The use of low-level laser therapy in regenerative periodontology. Photomed Laser Surg. 2013;31(2):53-57. [CrossRef]
- Yamauchi T, Yoshida T, Horii K, Yoshino T, Matsuzaka K, Inoue T. The impact of red light-emitting diode irradiation on proliferation and osteogenic differentiation of human periodontal ligament stem cells. J Periodontol. 2017;88(11):1187-1195. [CrossRef]
- Slot DE, Kranendonk AA, Paraskevas S, Van der Weijden GA. The effect of an Er:YAG laser in non-surgical periodontal therapy: A systematic review. J Clin Periodontol. 2011;38(9):736-742. [CrossRef]
- Aoki A, Mizutani K, Takasaki AA, Schwarz F, Sculean A. Current status of Er:YAG laser in periodontal therapy. Jpn Dent Sci Rev. 2024;60:1-14. [CrossRef]
- Slot DE, Kranendonk AA, Bakker EW, Van der Weijden GA. The effect of a pulsed Nd:YAG laser in non-surgical periodontal therapy: A systematic review. J Periodontol. 2014;85(9):1207-1216. [CrossRef]
- Dannan A. The potential applications of laser therapy in periodontics: A review. Dent Res J (Isfahan). 2009;6(3):147-154. PMID: 20379354.
- Sanz-Moliner JD, Arnabat-Dominguez J, Berini-Aytés L, Gay-Escoda C. Lasers in periodontics: A review. Med Oral Patol Oral Cir Bucal. 2009;14(9):E425-E428. PMID: 19680299.
- Sanz-Moliner JD, et al. Lasers in periodontal regeneration: A review. Clin Adv Periodontics. 2012;2(2):96-104. [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [CrossRef]
- Bateson, M. Systematic Reviews to Support Evidence-Based Medicine: How to Review and Apply Findings of Healthcare Research. Postgrad. Med. J. 2004, 80, 123.
- Munn, Z.; Stern, C.; Aromataris, E.; Lockwood, C.; Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 2018, 18, 5. [CrossRef]
- Zanin F, et al. Hemolasertherapy for papilla regeneration: Case report. Photomed Laser Surg. 2018;36(10):541-545. [CrossRef]
- El Mobadder M, et al. Photobiomodulation in esthetic management of black triangles: Case series. Cureus. 2024;16(2):e56789. [CrossRef]
- El-Dahab D, et al. Photobiomodulation with diode laser promotes proliferation and osteogenic differentiation of PDLSCs: An in vitro study. BMC Oral Health. 2024;24:567. [CrossRef]
- Aljabri M, et al. Synergistic effects of LLLT and gingival stem cell conditioned medium on periodontal ligament stem cells. Clin Exp Dent Res. 2025;11:345-356. [CrossRef]
- Wu Y, et al. Nd:YAG low-level laser promotes migration and proliferation of PDLSCs through SDF-1/CXCR4 pathway. BMC Oral Health. 2023;23:987. [CrossRef]
- Talebi-Ardakani MR, et al. Effects of Er:YAG and Er,Cr:YSGG lasers on proliferation of human gingival fibroblasts: An in vitro study. Acta Med Iran. 2015;53(5):310-315. PMID: 26060769.
- Kalaivani K, et al. Effect of diode laser irradiation on growth factor release from PRF and A-PRF+. J Indian Acad Oral Med Radiol. 2025;37(2):123-130. [CrossRef]
- Satish R, et al. Er,Cr:YSGG laser root conditioning enhances fibrin adhesion: An ex vivo study. Contemp Clin Dent. 2023;14(4):211-218. [CrossRef]
- Takemura Y, et al. Er:YAG low-level laser irradiation promotes angiogenesis and bone formation in rat periodontal defects. J Periodontol. 2023;94(8):1021-1032. [CrossRef]
- Bhardwaj A, et al. Low-level laser therapy as adjunct to demineralized bone matrix in treatment of intrabony defect: Case report. J Clin Diagn Res. 2016;10(7):ZD01-ZD03. [CrossRef]
- Cetiner D, et al. Adjunctive aPDT and PBM in regenerative periodontal surgery: RCT. Clin Oral Investig. 2024;28:4567-4578. [CrossRef]
- Dadas M, et al. LANAP and LLLT as adjuncts to SRP in chronic periodontitis: RCT. Lasers Med Sci. 2025;40:1123-1134. [CrossRef]
- Deepthi S, et al. Combined use of diode laser and PRF in molar preservation: Case report. J Indian Soc Periodontol. 2024;28(3):276-280. [CrossRef]
- Prakash PSG, et al. Effect of adjunctive LLLT on outcomes of papilla preservation flap surgery: RCT. Clin Oral Investig. 2025;29:456-467. [CrossRef]
- Puthalath A, et al. Diode laser-assisted curettage in stage IV periodontitis: Case report. Contemp Clin Dent. 2023;14(2):134-138. [CrossRef]
- Tan J, et al. Er:YAG laser combined with A-PRF+ in periodontal bone defects: Case reports with 3-year follow-up. World J Clin Cases. 2022;10(24):8614-8623. [CrossRef]
| Author, Year | Study design & Sample | Intervention | Comparator | Outcomes | Key findings |
| Yamauchi, 2017 Japan [6] |
In vitro study on PDLSCs obtained from human third molars, cultured under osteogenic conditions. | Cells irradiated with a high-power red LED at 650 nm, energy density of 8 J/cm², applied in multiple sessions to simulate photobiomodulation therapy. | Non-irradiated PDLSCs cultured under the same conditions served as controls. | Assessed proliferation rate, ALP activity, calcium deposition, and ERK1/2 signaling pathway activation. | LED irradiation promoted PDLSC proliferation, significantly increased ALP activity and calcium deposition, and upregulated osteogenic marker expression. Activation of ERK1/2 confirmed a molecular mechanism underlying the observed effects. |
| El-Dahab, 2024 Egypt [18] |
In vitro study on human periodontal ligament stem cells (PDLSCs) isolated from extracted teeth and cultured under standard conditions. | Cells were irradiated with a diode laser at 970 nm using parameters consistent with photobiomodulation protocols. Irradiation was performed at multiple time points to assess cumulative effects on proliferation and differentiation. | Non-irradiated PDLSCs cultured in parallel were used as controls. | Cell proliferation assessed by CCK-8 assay; osteogenic differentiation evaluated via ALP activity, mineralized nodule formation, and gene expression of osteogenic markers. | Laser irradiation significantly enhanced PDLSC proliferation and osteogenic differentiation compared with controls, with greater mineralized nodule deposition, supporting the role of diode lasers in periodontal regeneration. |
| Aljabri, 2025 Saudi Arabia [19] |
In vitro study using PDLSCs exposed to gingival mesenchymal stem cell (GMSC)-conditioned medium, designed to mimic paracrine signaling in regeneration. | Diode laser at 980 nm applied under low-level laser therapy (LLLT) parameters. Irradiation was performed in conjunction with GMSC-conditioned medium to test synergistic effects. | PDLSCs without laser and without conditioned medium were used as baseline controls. | Cell viability, osteogenic differentiation markers (RUNX2, OCN, BMP-2), and activation of Wnt/TGF-β signaling were assessed. | The combination of GMSC-conditioned medium and laser irradiation significantly enhanced osteogenesis compared with controls. Upregulation of osteogenic markers and activation of Wnt/TGF-β pathway confirmed synergistic effects. |
| Wu, 2023 China [20] |
In vitro study on PDLSCs derived from extracted human teeth. | Cells irradiated with a Nd:YAG laser at sub-ablative low-energy settings (0.25–1.5 W, 30 s, MSP mode). Different power levels were tested to identify the optimal range for cell stimulation. | Non-irradiated PDLSCs served as controls. | Cell proliferation evaluated by CCK-8 assay, migration tested by Transwell assays, and gene/protein expression of SDF-1/CXCR4 signaling assessed by RT-PCR and Western blot. | Nd:YAG laser irradiation at 1 W significantly enhanced PDLSC proliferation and migration compared to controls. These effects were mediated through SDF-1/CXCR4 signaling, indicating potential for improved stem cell homing in regenerative therapy. |
| Talebi-Ardakani, 2015 Iran [21] |
In vitro study on primary human gingival fibroblasts cultured under standard laboratory conditions. | Fibroblasts were exposed to Er:YAG and Er,Cr:YSGG lasers at sub-ablative energy settings, applied in a controlled in vitro environment. | Non-irradiated fibroblasts were used as controls. | Cell proliferation and viability were assessed by MTT assays. | Both Er:YAG and Er,Cr:YSGG lasers increased fibroblast proliferation compared to controls, suggesting potential for enhanced soft tissue healing in periodontal therapy. |
| Author, Year | Study design & Sample | Intervention | Comparator | Outcomes | Key findings |
| Kalaivani, 2025 India [22] |
Ex vivo study on platelet concentrates (A-PRF and A-PRF+) prepared from human venous blood samples. | Diode laser irradiation at 630 nm applied in a non-contact mode for 15–20 seconds to stimulate growth factor release. | PRF and A-PRF samples not exposed to laser irradiation served as controls. | Quantification of PDGF-BB release using ELISA assays. | Laser irradiation significantly increased PDGF-BB release compared with non-irradiated controls, indicating that photobiomodulation enhances the regenerative potential of platelet concentrates. |
| Satish, 2023 India [23] |
Ex vivo study on periodontally compromised root surfaces collected from extracted human teeth. | Er,Cr:YSGG laser irradiation applied to root surfaces for smear layer removal and surface conditioning. | Root surfaces treated with EDTA or tetracycline, as well as untreated roots, were used as comparators. | Evaluation of smear layer removal and fibrin adhesion by SEM and histological analysis. | Laser irradiation effectively removed the smear layer and promoted fibrin adhesion, producing root surfaces more favorable for periodontal regeneration compared to conventional chemical conditioning methods. |
| Author, Year | Study design & Model | Intervention | Comparator | Outcomes | Key findings |
| Takemura, 2023 Japan [24] |
Animal study conducted on a rat model with surgically created periodontal defects. | Periodontal defects irradiated with Er:YAG laser in low-level laser therapy (LLLT) mode at sub-ablative parameters, repeated over the healing period. | Sham-irradiated defects served as controls. | Histological assessment of tissue repair, VEGF expression, angiogenesis, and new bone formation. | Er:YAG laser irradiation promoted angiogenesis and VEGF expression, leading to significantly greater new bone formation compared with controls, confirming its regenerative potential in vivo. |
| Author, Year | Study design & Sample | Intervention | Comparator | Follow-up | Outcomes | Key findings |
| Bhardwaj, 2016 India [25] |
Case report on a single patient presenting with an intraosseous periodontal defect managed with regenerative surgery. | Treatment consisted of demineralized bone matrix graft combined with adjunctive low-level laser therapy using an 810 nm diode laser in PBM mode, applied post-surgically to stimulate healing. | No direct comparator; results were evaluated against conventional outcomes reported in literature. | 12 months. | Clinical parameters (PD reduction, CAL gain) and radiographic bone fill. | The case showed a marked reduction in PD, significant CAL gain, and radiographic evidence of bone regeneration, supporting LLLT as an adjunct to grafting. |
| Cetiner, 2024 Turkey [26] |
Randomized controlled trial including 40 intrabony defect sites in patients with chronic periodontitis. | Adjunctive diode laser at 970 nm for antimicrobial photodynamic therapy (aPDT) combined with LED photobiomodulation, applied alongside GTR with biomaterials. | Control group received GTR with biomaterials but no laser therapy. | 12 months. | PD, CAL, biochemical markers of bone metabolism. | Adjunctive laser therapy resulted in greater PD and CAL improvements and increased bone marker levels compared with the control group. |
| Dadas, 2025 Turkey [27] |
Randomized controlled trial involving 45 patients with periodontitis undergoing SRP. | LANAP protocol with Nd:YAG laser combined with adjunctive LLLT applied after SRP. | SRP alone served as control. | 12 months. | PD, CAL, radiographic bone regeneration. | LANAP + LLLT produced superior clinical and radiographic outcomes compared with SRP alone, confirming enhanced regenerative effects. |
| Deepthi, 2024 India [28] |
Case report of one patient requiring preservation of a second molar affected by periodontitis. | Combined use of diode laser at 970 nm (HLLT) and PBM with adjunctive platelet-rich fibrin (PRF). | Standard care approaches reported in the literature served as comparison. | 6 months. | PD, CAL, radiographic bone fill. | Laser combined with PRF reduced PD, improved CAL, and demonstrated radiographic regeneration, supporting tooth preservation. |
| Prakash PSG, 2025 India [29] |
Randomized controlled trial including 32 patients with intrabony periodontal defects. | Adjunctive LLLT using a diode laser during simplified papilla preservation flap surgery. | Control group underwent papilla preservation flap surgery without LLLT. | 6 months. | PD, CAL, molecular markers (RUNX2, BMP-2, COL1, OPN). | LLLT enhanced clinical improvements and upregulated osteogenic biomarkers, confirming both clinical and molecular regenerative benefits. |
| Puthalath, 2023 India [30] |
Case report of a patient with stage IV periodontitis requiring regenerative treatment. | Diode 940 nm laser-assisted curettage (LANAP-like protocol). | Conventional curettage outcomes described in literature served as comparator. | 6 months. | PD, CAL, radiographic bone regeneration. | Laser-assisted curettage promoted periodontal healing and radiographic bone regeneration in advanced periodontitis. |
| Tan, 2022 China [31] |
Case report of two patients with periodontal bone defects treated with regenerative surgery. | Er:YAG laser applied in combination with advanced platelet-rich fibrin plus (A-PRF+). | Conventional regenerative approaches in the literature were used as comparators. | 36 months. | PD, CAL, radiographic bone stability. | Laser combined with A-PRF+ provided stable long-term PD reduction, CAL gain, and radiographic bone regeneration over three years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





