1. Introduction
The unprecedented health crisis in 2019 caused by coronavirus disease 2019 (COVID-19) became a global pandemic, spreading across many countries, causing respiratory illness and deaths linked to SARS-CoV-2 [
1,
2]. Symptoms, severity and recovery times during COVID-19 vary, and SARS-CoV-2 infection can lead to post-acute sequelae, with most recovering in a month, but some report prolonged health issues [
3]. Various names have been used to refer to this condition; however, the preferred term for this condition is “long COVID” (LC) [
4].
LC can impact and elevate the risk of disorders across multiple organ systems, leading to a reduction in quality of life, with symptoms varying significantly in severity and duration [
5,
6,
7]. With over 200 reported symptoms associated with LC, the most frequently reported are fatigue, cognitive impairment, joint pain, anxiety, and depression [
5,
8]. Reports highlight diverse MSK symptoms linked to LC, mainly fatigue and widespread joint and muscle pain, with many experiencing back or neck pain, complicating the condition. [
9,
10,
11,
12,
13,
14,
15,
16].
Reports also indicate that patients with LC frequently exhibit vitamin D insufficiency, with deficiency associated with an increased number of symptoms [
17,
18,
19]. LC systemic inflammation may affect the MSK system, with some studies suggesting that acute COVID-19 markers influence bone metabolism [
20]. Evidence shows that LC patients have elevated inflammatory markers and cytokines [
21,
22]. Joint pain post-COVID-19 can resemble other inflammatory conditions, with 2% to 65% experiencing symptoms lasting one month to a year [
23].
This paper aims to assess HRQoL and circulating markers of inflammation, as well as BTM and vitamin D in LC individuals. However, additional data, such as imaging, is planned as part of a broader effort to explore the underlying mechanisms in greater depth.
2. Materials and Methods
Study Design and Ethics Approval
This prospective longitudinal feasibility study received ethical approval from the Yorkshire & The Humber - Bradford Leeds Research Ethics Committee (Ref: 23/YH/0031), 12 April 2023. All participants provided written informed consent.
Study Population
Participants were recruited from LC clinics, ongoing studies focused on LC participants, and through advertisements using leaflets, posters, and social media platforms. A total of 85 participants were recruited from a cohort of individuals who had previously been diagnosed with COVID-19. Participants were divided into two groups: the LC group and the WR group. The inclusion/exclusion criteria included further imaging investigation [
Table 1]. As mentioned above, while the primary aim was to assess changes in HRQoL and blood analysis, additional imaging data were planned to explore significant LC effects in more depth. Thus, pregnant women were excluded due to potential risks associated with radiation exposure from imaging, in accordance with standard safety guidelines.
The LC group (n=45) includes individuals with persistent symptoms lasting ≥12 weeks after acute COVID-19, self-reported based on clinical diagnosis by a physician or self-reported symptoms aligned with NICE criteria [
24]. The WR group (n=40) comprises individuals who had confirmed SARS-CoV-2 infection but recovered fully without persistent symptoms. Recruitment began in June 2023. Because the date of acute infection was rarely verifiable, “time since infection” could not be determined for most participants so it has not been considered in the analysis.
Table 1.
Study Inclusion/Exclusion Criteria.
Table 1.
Study Inclusion/Exclusion Criteria.
| Inclusion Criteria |
Exclusion Criteria |
| Adults aged ≥18 years of any gender and ethnicity. |
Participants who had hospitalisation due to COVID-19 requiring intubation, ICU admission, or ventilatory support (to exclude post-intensive care syndrome). |
| Participants with a history of SARS-CoV-2 infection confirmed via RT-PCR or antigen testing. |
Individuals with pre-existing osteoporosis or metabolic bone diseases (e.g., primary hyperparathyroidism, osteogenesis imperfecta). |
| LC participants met the WHO and NICE definitions of long COVID or had a confirmed diagnosis of LC. |
Those undergoing long-term corticosteroid therapy (≥5 mg prednisolone daily) or taking bisphosphonates, denosumab, or teriparatide. |
| |
Pregnant or breastfeeding women due to the use of ionising radiation in DXA scans. |
| |
Participants with recent fractures (<12 months) or conditions affecting joint health, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE). |
Data Collection and Assessments:
Weight (kg) and height (cm) were measured using a calibrated Marsden M-530 bariatric scale and wall-mounted stadiometer. Body mass index (BMI) is calculated as weight (kg)/height (m²).
Blood Tests
To determine the presence of the COVID-19 virus within the bloodstream, the subgenomic RNA (sgRNA) test was used [
25]. The sgRNA is a proxy for the virus with infective potential, as it is only present in an actively replicating virus. In contrast, conventional COVID PCR picks up viral fragments, whether active or inactive. The latter can give false positives due to the persistence of viral fragments in the absence of an active viral reservoir. In the absence of nasal or nose swabs, this is an acceptable proxy and has been used in other peer-reviewed studies [
26]. The kinetics of the assay are given in the paper describing the design of the assay [
25]. The limit of detection (LOD) for quantification is >100 viral copies, and the reproducibility r
2 was 0.996.
Cytokine analysis offers insight into LC’s circulatory mechanisms, possibly involving prolonged SARS-CoV-2 presence [
27]. Reports link systemic inflammation to pro-inflammatory cytokines[
28,
29,
30], which are crucial for bone homeostasis [
31]. During COVID-19’s acute phase, markers like IL-6, IFN-γ, and TNF-α affect bone mechanisms [
20]. Two studies systematically found elevated CRP and cytokines/chemokines, including TNF-α, IFN-γ, and IL-6, in LC patients [
21,
22]. Regarding the BTM, IOF, and IFCC, they recommend using one bone formation marker (s-PINP) and one resorption marker (s-CTX), measured by standardised assays, in studies[
32].
Blood samples were collected to assess inflammatory markers, BTM, vitamin D level, and SARS-CoV-2 sgRNA. To maintain the integrity of BTM, participants were required to fast for 10 hours and avoid intense exercise for 48 hours before the test [
33]. Additionally, participants were asked to refrain from taking multivitamin supplements on the day of the appointment, as these may affect BTM levels [
34,
35]. Due to these requirements, blood collection was scheduled as the first procedure of the appointment.
Blood samples were obtained through a phlebotomy using a butterfly needle BD Vacutainer Safety-Lok 21g Blood Collection Set. BD Vacutainer® serum separator tube (SST)™ (Clot activator/polymer gel) and PAXgene® Blood RNA Tube.
The SST, for analysis of BTM (PINP ng/mL), CTX ng/mL, and vitamin D (25 OH D ng/mL) by an external laboratory, Sheffield Biochemical Laboratory, using Cobas e411, Roche Diagnostics (Mannheim, Germany) analyser. After ensuring clotting, a centrifuge at 2000 xG for 10 min in a swinging bucket centrifuge [
36] was used to isolate serum. The serum was then transferred to small aliquots in anticoagulated vacutainer bottles and stored at -80 °C.
The PINP/CTX ratio serves as a relative BTM balance indicator because it offers a broader view of bone remodelling and is more reliable than absolute PINP and CTX levels. It minimises confounding effects, such as circadian and physiological fluctuations. Research indicates that this ratio gives better insight into overall bone health in older adults than individual markers [
37,
38].
The second tube is the PAXgene RNA tube, filled with stabilising reagent to prevent backflow. After stabilisation, the sample is stored at -80°c. The analysis was conducted by the Royal Devon University Healthcare NHS Foundation Trust at the Research, Innovation, Learning, and Development (RILD) building. SARS-CoV-2 (sgRNA) was tested [
39], along with quantitative PCR (qPCR) inflammatory markers ( CRP, IL-6, IFN-γ, and TNF-α).
cDNA Synthesis
RNA was converted to cDNA using the PrimeScript™ RT Master Mix (Perfect Real Time) kit (RR036A, Takara, Japan). Each reaction contained 1000 ng RNA, 4 µL 5X PrimeScript RT Master Mix, and sufficient RNase Free dH2O to reach a total reaction volume of 20 µL. Reactions were mixed gently and incubated at 37 °C for 15 min, followed by 85 °C for 5 s. Samples were stored at -20 °C.
qPCR Analysis
cDNA was diluted 1:3 in RNase Free dH2O to ensure sufficient volume for all assays. qPCR was performed using TaqMan™ Universal Master Mix II, no UNG (4440048, Thermo Fisher Scientific) and TaqMan probes (Thermo Fisher Scientific). Genes of interest included COL1A1 (Hs00164004_m1), COL1A2 (Hs01028956_m1), CRP (Hs00265044_m1), IL-6 (Hs00174131_m1), IFN-γ (Hs00989291_m1), and TNF-α (Hs00174128_m1). Housekeepers included GUSB (Hs00939627_m1), HPRT (Hs02800695_m1), PPIA (Hs04194521_s1), and UBC (Hs05002522_g1). Individual qPCR reactions contained 2.5 µL TaqMan™ Universal Master Mix II, 0.25 µL TaqMan probe, 1 µL cDNA, and 1.25 µL H2O. Each sample was run in triplicate in a 384-well plate. qPCR was performed using the QuantStudio™ 12K (Thermo Fisher Scientific) with the following cycling conditions: 50 °C for 2 min, 95 °C for 10 min, 40–45 cycles of 95 °C for 15 s and 60 °C for 1 min. qPCR results were analysed using the 2-ΔΔCt method.
Quality of Life and Joint Pain Assessment
Subjective measures of HRQoL, which provide descriptive health state profiles through the EQ-5D-5L, and joint pain assessed via the VAS, provide critical patient-reported outcomes. HRQoL was evaluated using EQ-5D-5L, which assesses mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each of these has five levels, coded from 1 to 5, corresponding to responses (no=1, slight=2, moderate=3, severe=4, and extreme problems=5). The second part contains a rating scale ranging from 0, indicating the worst imaginable health, to 100, indicating the best imaginable health, as a VAS [
40]. EQ-5D utility index (UI) reflects average public preferences, which the UK standard was adapted from the EUROQOL [
41,
42], which has a maximum value of 1 (for domains’ health state 11111), indicating the best possible health, and a minimum value of -0.594 (for 55555) indicating the worst possible health.
The EQ-5D-5L is a simple, validated screening questionnaire recommended by NICE for managing the long-term effects of COVID-19 as a control measure for confining LC symptoms and monitoring their condition [
43]. Research indicates high test-retest reliability, making it appropriate for monitoring changes over time and for comparative analysis among groups [
44,
45]. Various studies have shown that EQ-5D-5L demonstrates good to excellent test-retest reliability for the index score, with substantial agreement at the dimension level. Recent online administration also reported strong agreement across dimensions [
46,
47].
Joint pain severity was recorded using a VAS. It indicates the presence of pain in the hand or knee joint, along with its intensity. The scale ranged from 0 to 10, which correlates with visual and verbal scales as follows: 0 no pain, 1-3 indicates mild pain, 4-6 denotes moderate pain, and 7-10 signifies severe pain and its impact on daily activities [
48]. Studies have demonstrated excellent test-retest reliability in musculoskeletal populations, such as osteoarthritic knee pain, with an ICC of approximately 0.97, and have also shown good reliability, with ICC values exceeding 0.80, across an inflammatory rheumatic cohort [
49,
50].
Statistical Analysis
Data were analysed using Stata v18.0 (StataCorp, College Station, TX, USA). Descriptive statistics, including means and standard deviations (SD), were used for normally distributed continuous variables. Categorical variables were presented as counts and percentages. Associations between categorical variables were assessed as appropriate using the Chi-square test when the groups were independent, provided that the expected value in at least 80% of the cells was ≥ 5, or Fisher’s exact test was used when more than 20% of cells had expected frequencies < 5 [
51,
52,
53].
Given the exploratory and feasibility nature of this study, as well as the small sample size, statistical analyses were limited to independent t-tests (or Mann-Whitney U tests for non-parametric data) for between-group comparisons and Wilcoxon signed-rank tests for within-group changes over 12 months. Only completed records were used. While this method allowed for the initial investigation of differences, it does not fully account for repeated measures or interactions between groups and time.
All tests were two-tailed, and a p-value cutoff of <0.01 was considered statistically significant for essential differences. Statistical significance was set at p<0.01 beforehand, a more stringent threshold than the usual p<0.05, based on advice from a consulting statistician to reduce the likelihood of Type I errors due to multiple univariate comparisons. No formal correction for multiple comparisons raises potential biases and limitations; however, the analyses were mainly exploratory and aimed to identify patterns for future research. Nevertheless, the risk of false-positive results from multiple testing persists, and all findings should be interpreted with this in mind. When relevant, effect sizes and 95% confidence intervals are also provided to offer insights into the magnitude and reliability of the observed differences.
3. Results
Demographics and Characteristics
The full characteristics of the participants are summarised in
Table 2. A total of 85 participants were included, comprising 45 in the LC group and 40 in the WR group. The average age was 52.22±9.94 years for the LC group and 51±15.2 years for the WR group, showing no significant differences in age between the groups (
p=0.658). The LC group had a significantly higher percentage of female participants compared to the WR group (84.45% vs. 47%,
p<0.001). Most participants ethnicity was White (94.12%), with a small proportion of individuals from Indian, Pakistani, Black African, and Chinese backgrounds.
No significant differences in BMI between the LC and WR groups (
p=0.2142). However, both groups were similar in terms of BMI, socio-economic status, smoking, and daily alcohol consumption, as shown in
Table 2. At baseline, 17.8% of LC groups and 7.5% of WR participants reported using hormonal replacement therapy (HRT). Regarding vitamin D supplements, 38% of participants in the LC group reported taking supplements, compared to 20% in the WR group (
p=0.096).
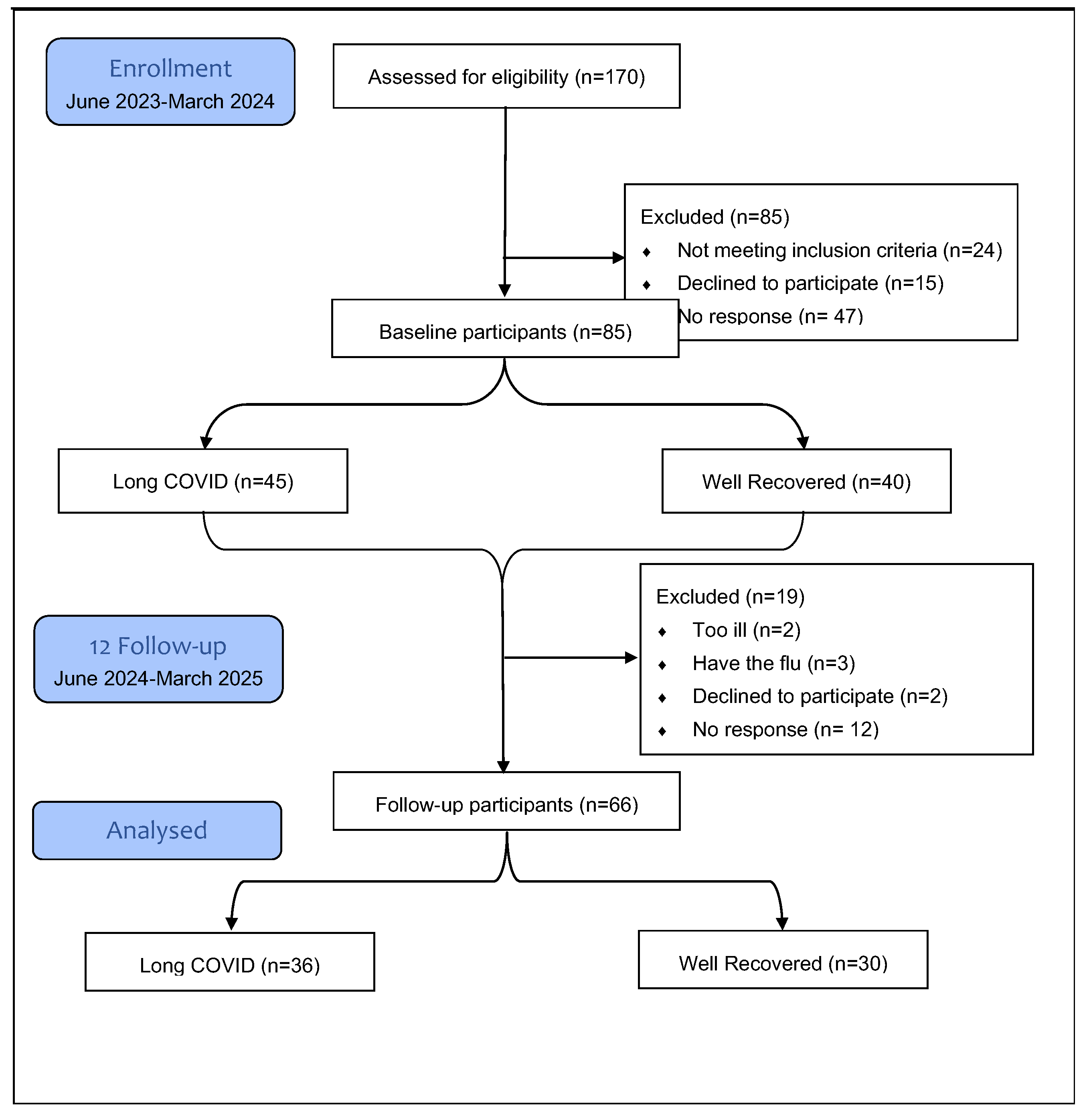
Of the 85 participants who completed the baseline assessment, 19 (22.4%) were lost to follow-up. Of these, 12 (63%) did not respond to the follow-up invitation, 5 (26.3%) were too ill due to ongoing disease, and 2 (10.5%) were not interested in continuing with the study [
Figure 1]. At follow-up, conducted 12±2 months later, 66 participants were re-evaluated. Of these, 44 (66.67%) were female (LC=29 vs. WR=15),
p=0.017. The mean age was similar across the groups (
p=0.8928). LC still had an insignificantly higher BMI at follow-up compared to WR (
p=0.09). Over 12 months, the median BMI in the LC group increased from 27.6 kg/m
2 (IQR 24.7-33.5) to 28.8kg/m
2 (23.8-34.45), but this change was not statistically significant (
p=0.037) vs WR (
p=0.52).
Socioeconomic levels were similar across groups (p=0.450). The ethnic makeup of the LC group comprises 91.66% White, 2.78% Indian, 2.78% Pakistani, and 2.78% Chinese individuals. In comparison, the WR group is entirely composed of Whites. Smoking and alcohol status show no significant differences (p=0.74 and p=0.396), respectively.
After the baseline visit, 5 WR and 1 LC participants started taking medication for bone health.
Table 2.
BMI: Body Mass Index (kg/m2); yr: years; WR: Well-recovered; LC: long COVID; (n): participants number at each timepoints; p-values using appropriate tests (Mann–Whitney U or independent t-tests) for continues data based on data normality and X2 or Fisher’s exact test for categorical data; Data are presented as median with interquartile range (IQR) or mean ± standard deviation (SD) or n (%): number and percentages as appropriate; (₽): t-tests; (‡): Mann–Whitney U; (¥): Fisher’s exact test; (X): X2 test; * Statistically significant at p<0.01.
Table 2.
BMI: Body Mass Index (kg/m2); yr: years; WR: Well-recovered; LC: long COVID; (n): participants number at each timepoints; p-values using appropriate tests (Mann–Whitney U or independent t-tests) for continues data based on data normality and X2 or Fisher’s exact test for categorical data; Data are presented as median with interquartile range (IQR) or mean ± standard deviation (SD) or n (%): number and percentages as appropriate; (₽): t-tests; (‡): Mann–Whitney U; (¥): Fisher’s exact test; (X): X2 test; * Statistically significant at p<0.01.
| Variables |
Baseline |
Follow-up |
| WR (n=40) |
LC (n=45) |
Test value |
p |
WR (n=30) |
LC (n=36) |
Test value |
p |
|
Age (yr)(₽)
|
51±15.17 |
52.22±9.94 |
t(83)= -0.444 |
0.658 |
52.83±14.85 |
53.28±10.08 |
t(64)= -0.144 |
0.885 |
|
Gender (Female), n (%)(X)
|
19 (47) |
38 (84.45) |
X2(1)= 13.084 |
< 0.001*
|
15 (50) |
29 (80.56) |
X2(1)= 6.875 |
0.009* |
|
‡BMI (kg/m2)(‡)
|
26.6 (23.8;30.65) |
27.9 (24.7;33) |
z= -1.242 |
0.214 |
25.55 (23.4;29.3) |
28.8 (23.8;34.45) |
z= -1.707 |
0.087 |
|
Ethnicity, n (%)(¥)
|
|
0.459 |
|
|
1.00 |
| White or not stated |
39 (97.5) |
41 (91.1) |
|
|
30 (100) |
33 (91.7) |
|
|
| Indian |
0 (0.0) |
2 (4.4) |
|
|
0 (0.0) |
1 (2.8) |
|
|
| Pakistani |
0 (0.0) |
1 (2.2) |
|
|
0 (0.0) |
1 (2.8) |
|
|
| Black African |
1 (2.5) |
0 (0.0) |
|
|
0 (0.0) |
0 (0.0) |
|
|
| Chinese |
0 (0.0) |
1 (2.22) |
|
|
0 (0.0) |
1 (2.8) |
|
|
|
Socio-economic, n (%)(X)
|
X2(2)= 2.649 |
0.266 |
|
X2(2)= 1.602 |
0.449 |
| Upper |
20 (50) |
23 (51.1) |
|
|
15 (50) |
18 (50) |
|
|
| Upper Middle |
13 (32.5) |
19 (42.2) |
|
|
12 (40) |
17 (47.2) |
|
|
| Lower Middle |
7 (17.5) |
3 (6.7) |
|
|
3 (10) |
1 (1.8) |
|
|
|
Smoking status, n (%)(¥)
|
|
0.298 |
|
|
0.742 |
| Non-smoker |
26 (65) |
35 (77.8) |
|
|
21 (70) |
26 (72.2) |
|
|
| Ex-smoker |
8 (20) |
8 (17.8) |
|
|
4 (13.3) |
6 (16.7) |
|
|
| Light smoker (less than 10) |
2 (5) |
2 (4.44) |
|
|
2 (6.67) |
3 (8.3) |
|
|
| Moderate smoker (10 to 19) |
3 (7.5) |
0 (0.0) |
|
|
1 (3.33) |
1 (2.8) |
|
|
| Heavy smoker (20 or over) |
1 (2.5) |
0 (0.0) |
|
|
2 (6.67) |
0 (0.0) |
|
|
|
Alcohol status, n (%)(¥)
|
|
0.526 |
|
|
0.396 |
| Non |
15 (37.5) |
22 (48.89) |
|
|
16 (53.33) |
21 (58.3) |
|
|
| < 1 unit per day |
12 (11.3) |
10 (22.2) |
|
|
7 (23.33) |
9 (25) |
|
|
| 1-2 units per day |
9 (22.5) |
8 (17.8) |
|
|
4 (13.33) |
4 (11.1) |
|
|
| 3-6 units per day |
1 (2.5) |
4 (8.9) |
|
|
3 (10) |
0 (0.0) |
|
|
| 7-9 units per day |
1 (2.5) |
0 (0.0) |
|
|
0 (0.0) |
1 (2.8) |
|
|
| >9 units per day |
2 (5) |
1 (2.22) |
|
|
0 (0.0) |
1 (2.8) |
|
|
|
Hormonal Replacement Therapy,n(%)(X)
|
3 (7.5) |
8 (17.8) |
X2(1)= 1.985 |
0.159 |
|
|
|
|
|
Supplementation of Vitamin D,n(%)(X)
|
6 (20) |
14 (38.9) |
X2(1)= 3.054 |
0.080 |
|
|
|
|
|
Bone Health Medication,n(%)(X)
|
- |
- |
|
- |
5 (16.7) |
1 (2.8) |
X2(1)= 1.985 |
0.084 |
Figure 1.
Flow chart recruitment numbers at each study phase. The overall dropout in the 12-month was 22.4%.
Figure 1.
Flow chart recruitment numbers at each study phase. The overall dropout in the 12-month was 22.4%.
Joint Pain
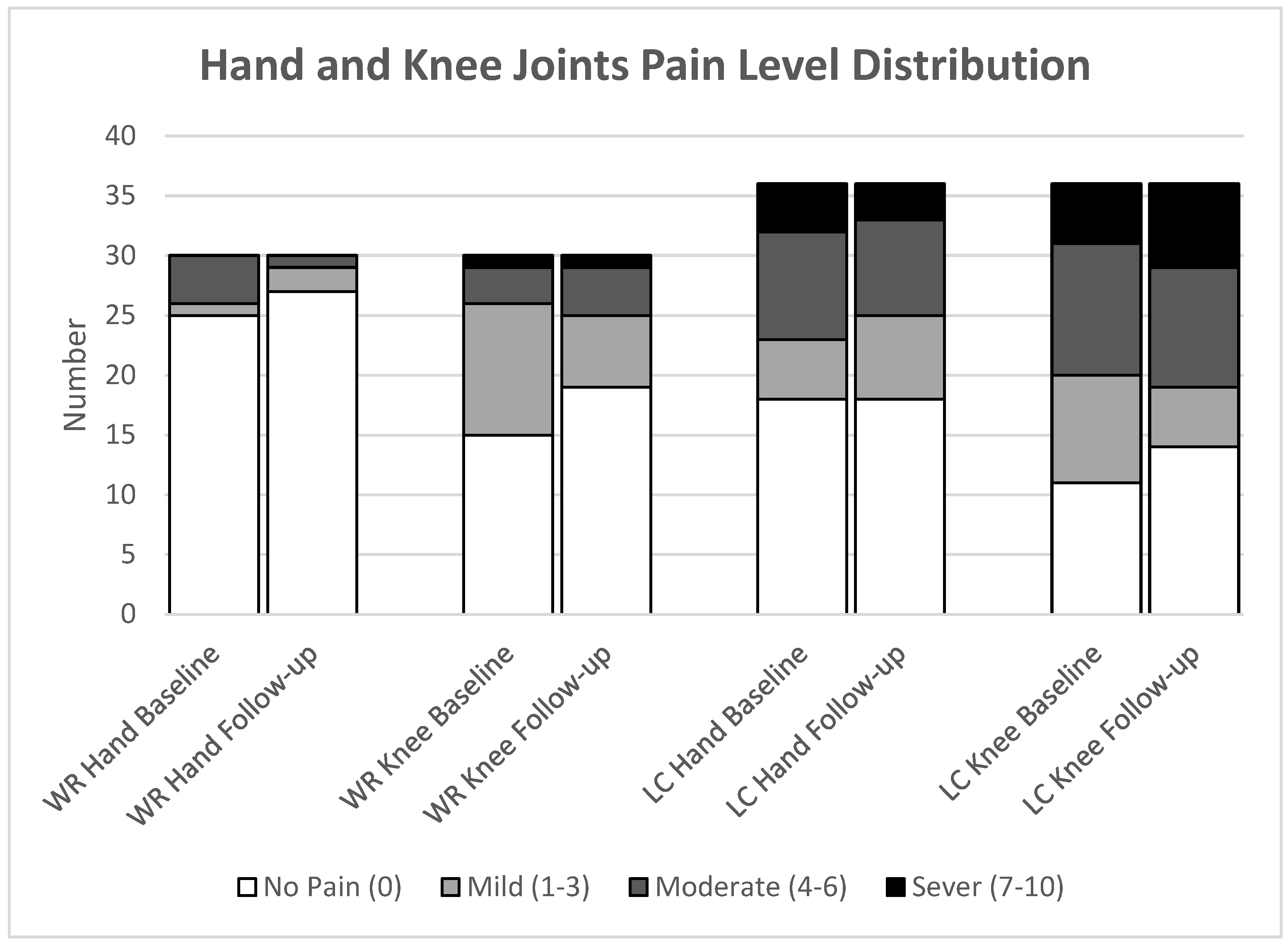
Notably, at baseline, the median hand joint pain was significantly higher in the LC group compared to the WR group, 1 (IQR 0;5) vs 0 (IQR 0;0); 95% CI 0.208;0.526, (
p=0.003). However, in the follow-up, there was no significant difference in hand joint pain between the groups, LC median 0.5 (IQR 0;4) vs WR median 0 (IQR 0;0); 95% CI 0.244;0.571, (
p=0.039). Additionally, the LC group reported a marginally higher pain level than the WR group at each time point, at baseline median 3 (IQR 0;6) vs 0 (IQR 0;2); 95% CI for change 0.176;0.434, (
p=0.024), at follow-up showed similar medians and IQR, with a weaker, non-significant difference (95% CI 0.087;0.411, (
p=0.093), as summarised in
Table 3. Within-group comparisons reveal that participants in the LC group continued to face persistent hand and knee joint pain after 12 months, with a median hand pain level from 0.5 (IQR 0;5) to 0.5 (IQR 0;4); 95% CI -0.782;0.393, (
p=0.624), and from 3 (IQR 0;5) to 3 (IQR 0;6); 95% CI -1.164;0.553, (
p=0.573) knee pain level, as summarised in
Table 4.
Participants in the LC group consistently reported higher levels of joint pain in the hand and knee compared to those in the WR group at each time point. At baseline, participants reported 11% hand and 14% knee severe pain, while 8% and 19% reported severe pain during the follow-up, respectively, as shown in
Figure 4.
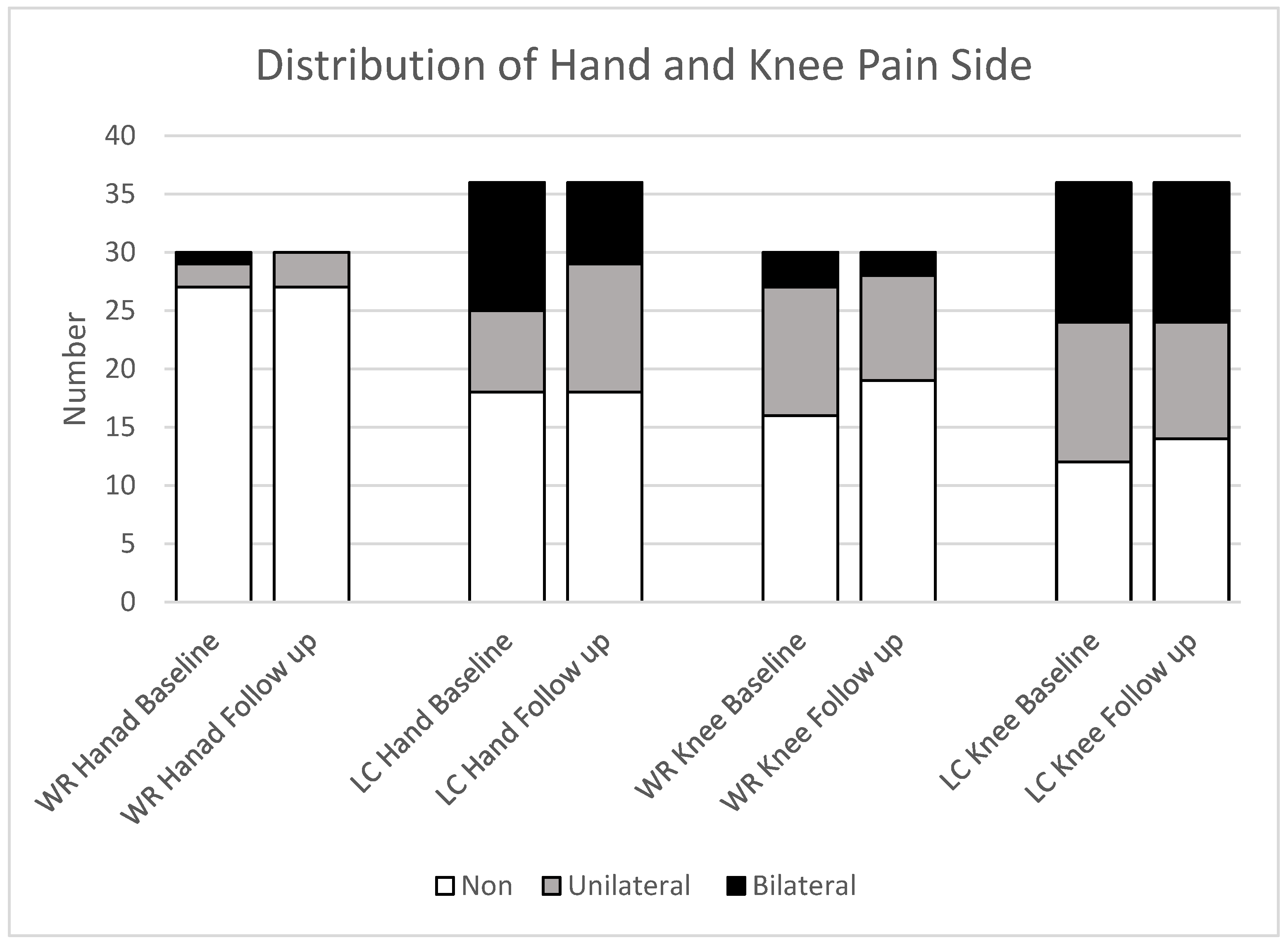
The distribution of hand and knee pain among completed participant data at baseline shows that the LC group had a higher prevalence of pain in the bilateral hand (31%), with 19% experiencing unilateral pain. Knee joint pain was present in 33% of participants, both bilateral and unilateral, at baseline, with similar rates observed at follow-up. The WR group experienced less hand pain, with 3% having bilateral pain and 7% having unilateral pain, with no bilateral cases at follow-up and a slight increase to 19% in unilateral pain. Unilateral knee joint pain decreased from 37% to 30%, and unilateral pain decreased from 10% to 7% [
Figure 5].
Figure 4.
The figure illustrates the frequency of different pain levels in the hand and knee for both groups: Well-recovered (WR) and Long COVID (LC). The groups consist of WR (n=30) and LC (n=36).
Figure 4.
The figure illustrates the frequency of different pain levels in the hand and knee for both groups: Well-recovered (WR) and Long COVID (LC). The groups consist of WR (n=30) and LC (n=36).
Figure 5.
Side joint pain in the hand and knee distribution among completed study groups; LC: Long COVID; WR: Well recovered, (WR: n=30; LC: n=36). Pain is categorised as absent (Non), unilateral (one side), or bilateral (both sides).
Figure 5.
Side joint pain in the hand and knee distribution among completed study groups; LC: Long COVID; WR: Well recovered, (WR: n=30; LC: n=36). Pain is categorised as absent (Non), unilateral (one side), or bilateral (both sides).
Blood Analysis
The blood analysis assessed SARS-CoV-2 RNA, cytokines, BTMs, and vitamin D. Neither the LC nor the WR groups had detectable SARS-CoV-2 sgRNA, and CRP levels were too low to be measured reliably. Cytokines were quantified at the level of the mRNA transcript by RTqPCR and expressed as a ratio of CRP relative to the average of a set of 3 housekeeping genes. Individual crossing points were in the range of 36.1 to 37.5 for 10 replicates, with the remaining 455 replicates reading no crossing point, meaning that no transcript was detected. In comparison, the crossing points of the housekeeping genes were 23.9-29.3, 26.5-31.0, and 20.6-25.8 out of a total of 45 cycles. qPCR is a well-accepted means of quantifying gene expression.
In addition, sensitivity analyses were conducted to evaluate how hormone replacement therapy (HRT) might affect the BTM ratio. The findings showed that HRT had no significant effect on the BTM ratio in both the overall cohort and the LC group [Supplementary S.1]. Similarly, additional analyses investigating the impact of supplementation on serum 25(OH)D levels found no significant relationship, indicating that supplementation did not alter vitamin D levels in this cohort or specifically within the LC group [Supplementary S.2].
Cytokine levels at each study point showed no statistically significant differences between the two groups, as summarised in
Table 3. Notably, LC participants showed lower baseline levels of TNF-α compared to WR participants, but this difference did not reach the predetermined level of significance (LC median 0.771, IQR 0.605-0.983 vs WR 1.028, IQR 0.678-1.284; 95% CI (-0.0276;-0.032),
p=0.0151). By follow-up, the difference was no longer observed (
p=0.1628). Gender stratification revealed that males primarily contributed to the overall reduction: LC males had a median TNF-α of 0.7 (IQR 0.53-0.86), compared to 1.12 (IQR 0.88-1.28) in WR males, with an insignificant difference (
p=0.0182). For females, the difference was less pronounced, with LC females at a median of 0.8 (IQR 0.61-0.98) and WR females at 0.94 (IQR 0.66-1.28;
p=0.18). Within-group comparisons showed no significant changes in TNF-α, IL-6, or IFN-γ levels from baseline to follow-up in either group [
Table 4].
Results of BTM ratio comparison between LC and WR groups at the baseline and follow-up are summarised in
Table 3. At both assessment points, there were no statistically significant differences in the PINP/CTX ratio between the two groups. Over the course of 12 months, within-group comparisons revealed an increase in the BTM ratio in both the LC and WR groups. The increase was more pronounced in the LC group than the WR group; however, the difference was not significant between the two groups. The median for the LC group increased from 149.2 (IQR: 113.2-190.1) to 169.4 (IQR: 143.6-206.2); 95% CI (3.583;31.629), (
p=0.0111), while in the WR group it increased from 143.2 (IQR: 124.2-182.6) to 168.151 (IQR: 132.6-194.7), 95% CI (8.227;38.819), (
p=0.192) [
Table 4].
Vitamin D comparison between the LC and WR groups at the baseline and follow-up showed that vitamin D levels differed significantly at baseline, with the LC group exhibiting a higher median 25 OH D concentration of 29.46 ng/mL (IQR: 23.75-35.06) compared to 20.36 ng/mL (IQR: 15.99-27.65) in the WR group, 95% CI (0.072;0.318), (
p=0.0021). By follow-up, the difference in vitamin D levels between the groups was no longer statistically significant (
p=0.099). Within-group analyses revealed significant increases in vitamin D levels over 12 months in both groups: from 32.70 ng/mL (IQR: 23.66;35.1) to 35.89 ng/mL (IQR: 30.1;41.2), 95% CI (2.752;9.259), (
p=0.0023) in LC, and from 21.36 ng/mL (IQR: 16.34;27.89) to 29.58 ng/mL (IQR: 25.33;41.74), 95% CI (4.889;11.603), (
p=0.0001) in WR [
Table 4].
Table 3.
UI: Utility Index; WR: Well-recovered; LC: long COVID; VAS: visual analogue scale; TNF-α:Tumor Necrosis Factor alpha, IL-6: Interleukin-6, IFN-γ: interferon gamma; BTM: Bone Turnover Markers; CTX: C-terminal telopeptide of type I collagen; PINP: procollagen type I N-propeptide, 25 OH D: 25-hydroxyvitamin D; (n): participants number at each timepoints; CI: confidence interval; p-values using appropriate tests (Mann–Whitney) for continuous data based on data normality X2 test for categorical data; Data are presented as median with interquartile range (IQR); (‡): Mann–Whitney U; (¥): Fisher’s exact test; (X): X2 test; * Statistically significant at p<0.01.
Table 3.
UI: Utility Index; WR: Well-recovered; LC: long COVID; VAS: visual analogue scale; TNF-α:Tumor Necrosis Factor alpha, IL-6: Interleukin-6, IFN-γ: interferon gamma; BTM: Bone Turnover Markers; CTX: C-terminal telopeptide of type I collagen; PINP: procollagen type I N-propeptide, 25 OH D: 25-hydroxyvitamin D; (n): participants number at each timepoints; CI: confidence interval; p-values using appropriate tests (Mann–Whitney) for continuous data based on data normality X2 test for categorical data; Data are presented as median with interquartile range (IQR); (‡): Mann–Whitney U; (¥): Fisher’s exact test; (X): X2 test; * Statistically significant at p<0.01.
| Systemic Results Compared Between the LC and WR Groups at Baseline and Follow-Up. |
|---|
| (EQ-5D-5L) |
Variables |
n |
Baseline |
n |
Follow-up |
| WR |
LC |
Test value |
p |
95% CI |
WR |
LC |
Test value |
p |
95% CI |
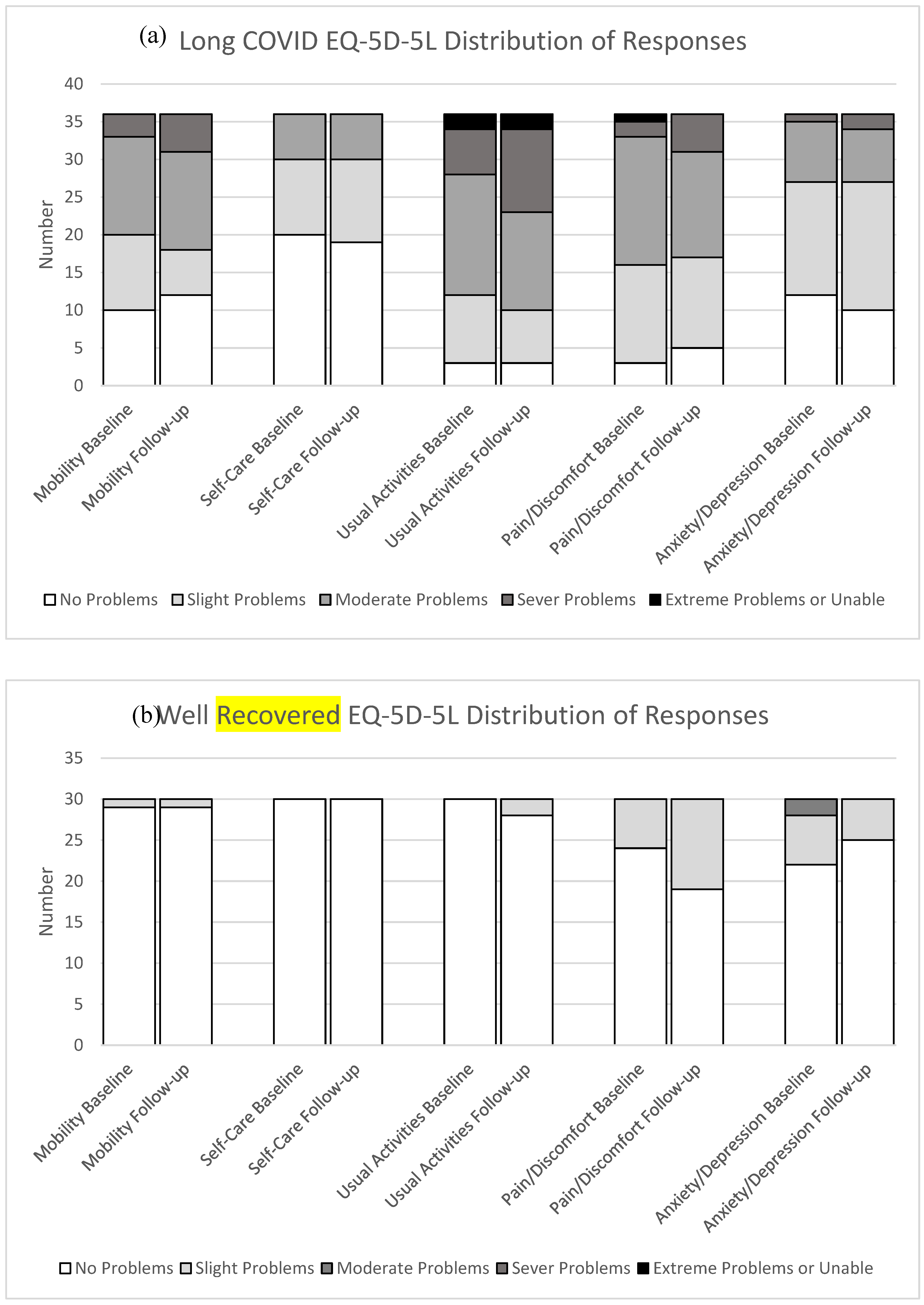
| Mobility(X)
|
40/45 |
0 (0;0) |
0.058 (0;0.076) |
X2(3) = 38.995 |
< 0.001* |
|
30/36 |
0 (0;0) |
0.067 (0;0.076) |
X2(3) = 28.308 |
< 0.001* |
|
| Self-Care(X)
|
0 (0;0) |
0 (0;0.05) |
X2(2) = 20.465 |
< 0.001* |
|
0 (0;0) |
0 (0;0.05) |
X2(2) = 19.081 |
< 0.001* |
|
| Usual Activities(X)
|
0 (0;0) |
0.063 (0.05;0.063) |
X2(4) = 59.792 |
< 0.001* |
|
0 (0;0) |
0.063 (0.05;0.162) |
X2(4) = 48.796 |
< 0.001* |
|
| Pain/Discomfort(X)
|
0 (0;0.032) |
0.084 (0.063;0.084) |
X2(4) = 42.561 |
< 0.001* |
|
0 (0;0.063) |
0.084 (0.063;0.084) |
X2(3) = 26.886 |
< 0.001* |
|
| Anxiety/Depression(X)
|
0 (0;0.078) |
0.075 (0;0.104) |
X2(3) = 17.111 |
0.001* |
|
0 (0;0) |
0.078 (0;0.091) |
X2(3) = 18.172 |
< 0.001* |
|
| UI(‡)
|
1 (0.922;1) |
0.72 (0.55;0.808) |
z = 7.041 |
< 0.001* |
(-0.564;-0.415) |
1 (0.937;1) |
0.697 (0.53;0.809) |
z = 6.632 |
< 0.001* |
(-0.584;-0.452) |
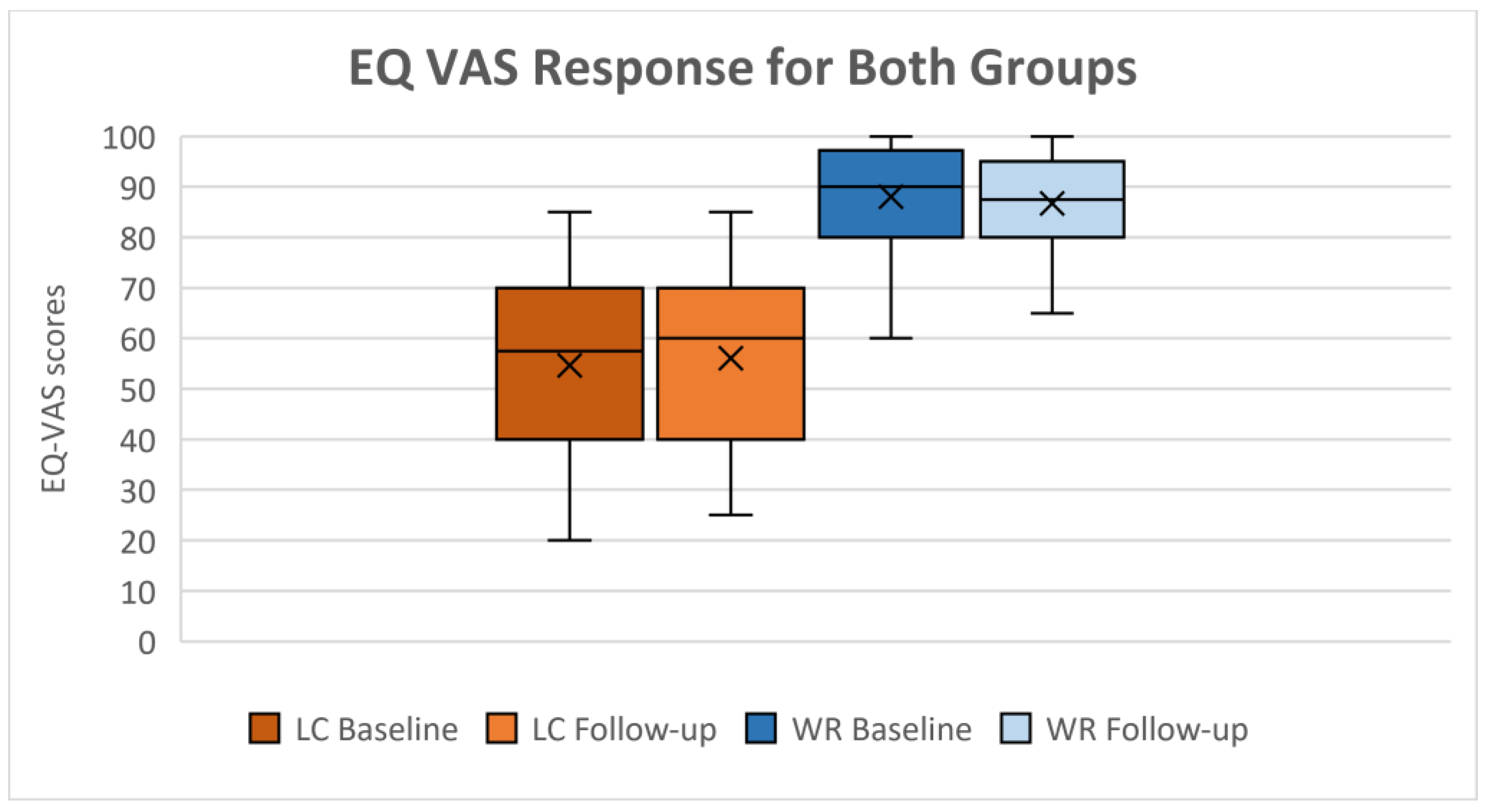
| VAS(‡)
|
87.5 (80;95) |
55 (40;70) |
z = 6.993 |
< 0.001* |
(-0.519;-0.423) |
90 (80;97) |
60 (40;70) |
z = 6.362 |
< 0.001* |
(-0.532;-0.430) |
| Joint Pain |
Hand Pain (¥)
|
0 (0;0) |
1 (0;5) |
|
0.003* |
(0.208;0.526) |
0(0;0) |
0.5 (0;4) |
|
0.039 |
(0.244;0.571) |
| Knee Pain (¥)
|
0 (0;2) |
3 (0;6) |
|
0.024 |
(0.176;0.434) |
0 (0;2) |
3 (0;6) |
|
0.093 |
(0.087;0.411) |
| Biomarkers |
TNF-α(‡)
|
40/45 |
1.03 (0.68;1.28) |
0.77 (0.61;0.98) |
z = 2.430 |
0.015 |
(-0.0276;-0.032) |
29/35 |
0.96 (0.68;1.39) |
0.86 (0.76;0.98) |
z = 1.396 |
0.162 |
(-0.258;0.053) |
| IL-6(‡)
|
1.03 (0.63;1.41) |
1.08 (0.72;1.66) |
z = -0.977 |
0.328 |
-(0,063;0.0187) |
0.81 (0.48;1.35) |
1.06 (0.62;1.44) |
z = -0.816 |
0.414 |
(-0.085;0.205) |
| IFN-γ(‡)
|
1.03 (0.55;1.53) |
0.73 (0.52;1.44) |
z = 1.083 |
0.278 |
(-0.0195;0.057) |
0.82 (0.48;1.23) |
0.95 (0.51;1.40) |
z = -0.573 |
0.566 |
(-0.103;0.188) |
| PINP/CTX ratio(‡)
|
40/44 |
150.6 (124.5;184.6) |
152.91 (125;200.3) |
z = -0.448 |
0.654 |
(-0.098;0.156) |
30/36 |
168.2 (132.6;194.7) |
171.5 (144.6;208.9) |
z = -0.541 |
0.588 |
(-0.106;0.1844) |
| 25 OH D ng/mL(‡)
|
40/45 |
20.36 (15.995;27.65) |
29.46 (23.75;35.06) |
z = -3.073 |
0.0021* |
(0.072;0.318) |
30/36 |
29.58 (25.33;41.74) |
35.89 (30.095;41.16) |
z = -1.648 |
0.099 |
(-0.028;0.267) |
Table 4.
UI: Utility Index; WR: Well-recovered; LC: long COVID; EQ VAS: visual analogue scale; TNF-α: Tumor Necrosis Factor alpha, IL-6: Interleukin-6, IFN-γ: Interferon gamma; (n): participants number at both timepoints; CI: confidence interval; p-values based on Wilcoxon signed-rank; values reported as median with interquartile range (IQR) at baseline and follow-up; * Statistically significant at p<0.01.
Table 4.
UI: Utility Index; WR: Well-recovered; LC: long COVID; EQ VAS: visual analogue scale; TNF-α: Tumor Necrosis Factor alpha, IL-6: Interleukin-6, IFN-γ: Interferon gamma; (n): participants number at both timepoints; CI: confidence interval; p-values based on Wilcoxon signed-rank; values reported as median with interquartile range (IQR) at baseline and follow-up; * Statistically significant at p<0.01.
| Paired analysis Within Groups from Baseline to Follow-Up. |
|---|
| (EQ-5D-5L) |
Variables |
|
WR (n=30) |
|
LC (n=36) |
| n |
Baseline |
Follow up |
CI |
p |
n |
Baseline |
Follow up |
CI |
p |
| Mobility |
30 |
0 (0;0) |
0 (0;0) |
|
1.000 |
36 |
0.058 (0;0.076) |
0.067 (0;0.076) |
|
0.528 |
| Self-Care |
0 (0;0) |
0 (0;0) |
|
1.000 |
0 (0;0.05) |
0 (0;0.05) |
|
0.948 |
| Usual Activities |
0 (0;0) |
0 (0;0) |
|
0.157 |
0.063 (0.05;0.063) |
0.063 (0.05;0.162) |
|
0.060 |
| Pain/Discomfort |
0 (0;0) |
0 (0;0.063) |
|
0.058 |
0.084 (0.063;0.084) |
0.084 (0.063;0.084) |
|
0.867 |
| Anxiety/Depression |
0 (0;0.078) |
0 (0;0) |
|
0.282 |
0.078 (0;0.091) |
0.078 (0;0.091) |
|
0.362 |
| UI |
1 (0.922;1) |
1 (0.937;1) |
-0.028;0.019 |
0.946 |
0.725 (0.587;0.809) |
0.697 (0.53;0.809) |
-0.057;0.024 |
0.398 |
| VAS |
87.5 (80;95) |
90 (80;97) |
-1.133;3.666 |
0.264 |
57.5 (40;70) |
60 (40;70) |
-5.653;8.486 |
0.868 |
| Joint Pain |
Hand Pain |
0 (0;0) |
0 (0;0) |
-0.122;0.755 |
0.917 |
0.5 (0;5) |
0.5 (0;4) |
-0.782;0.393 |
0.624 |
| Knee Pain |
0.5 (0;2) |
0 (0;2) |
-0.805;1.005 |
0.371 |
3 (0;5) |
3 (0;6) |
-1.164;0.553 |
0.573 |
| Biomarkers |
TNF-α |
29 |
0.98 (0.67;1.28) |
0.96 (0.68;1.39) |
-0.122;0.262 |
0.991 |
35 |
0.77 (0.61;0.98) |
0.86 (0.76;0.98) |
-0.107;0.133 |
0.412 |
| IL-6 |
0.85 (0.58;1.35) |
0.81 (0.48;1.35) |
-0.248;0.190 |
0.566 |
1.25 (0.72;1.88) |
1.06 (0.62;1.44) |
-0.489;0.012 |
0.076 |
| IFN-γ |
0.91 (0.43;1.52) |
0.82 (0.48;1.23) |
-0.862;0.210 |
0.112 |
0.84 (0.55;1.63) |
0.95 (0.51;1.4) |
-0.444;0.210 |
0.831 |
| PINP/CTX ratio |
30 |
143.2 (124.2;182.6) |
168.151 (132.6;194.7) |
-8.227;38.819 |
0.192 |
149.2 (113.2;190.1) |
169.4 (143.6;206.2) |
3.583;31.629 |
0.011 |
| 25 OH D ng/mL |
24 |
21.4 (16.34;27.89) |
29.58 (25.33;41.74) |
4.889;11.603 |
<0.001* |
36 |
32.695 (23.665;35.1) |
35.89 (30.1;41.2) |
2.752;9.259 |
0.0023* |
4. Discussion
LC is an often-debilitating condition that affects at least 10% of SARS-CoV-2 infections. This study aims to evaluate HRQoL and circulating markers of inflammation, as well as BTM and vitamin D in LC individuals. The study found significant differences in two strands: between the two groups, LC had reduced HRQoL and reported more pain in the hand joints. Additionally, they had, on average, a higher level of vitamin D at baseline compared to the WR group. Within 12 months, there was an improvement in vitamin D levels for both groups. Furthermore, no significant differences were observed in BTM ratio or inflammatory markers. This suggests that factors beyond visible inflammation, such as deconditioning, metabolic changes, autonomic dysfunction, or central pain mechanisms, might contribute to ongoing symptoms. Notably, the study offers a 12-month longitudinal perspective, which is scarce in the current literature and was identified as a gap by our previous systematic review [
54].
No Association of Bone Turnover Markers in Long COVID:
No significant differences were observed between LC and WR in BTMs, with no notable decline within either group over the one year. This indicates that, in this cohort, LC did not lead to detectable disrupted bone remodelling during the study period. Our systematic review concluded that COVID-19 (both acute and post-acute) may adversely affect bone health [
54]. It noted increases in bone regulatory markers, reductions in bone formation and resorption, and lower BMD. Some studies have found that non-human models infected with SARS-CoV-2 experience acute harm to trabecular bone, resulting in alterations in bone structure and an increase in osteoclast numbers [
62,
63,
64,
65,
66]. While this study did not find a significant difference in BTM between LC and WR individuals, the observed trend of lower values in LC participants merits further exploration in future research. The proposed mechanisms encompass viral persistence, or reactivation, induction of autoimmunity, tissue damage, and the development of microclots [
67].
It is known that changes in bone volume occur over time after the acute infection has resolved, whereas BTMs can change during the active phase of the disease. The results did not reveal any significant differences in BTM between participants with LC and WR throughout the study period. This indicates that approximately one year following infection, LC is not associated with markedly abnormal ongoing bone resorption or formation. A decreased level of circulating osteocalcin (OC) had been observed in critically ill ICU COVID-19 patients in comparison to non-COVID-19 patients [
68]. Additionally, individuals with non-severe COVID-19 had lower levels of total P1NP and osteocalcin N-terminal in the middle (N-MID OC) compared to healthy individuals [
69]. Another study found that the COVID-19 patient group had a lower level of serum OPG compared to the matched control group [
66].
No Association of Inflammatory Markers in Long COVID:
No viral persistence was observed in the study population. Additionally, the inflammatory marker profiles within the LC cohort showed no statistically significant differences when compared to the WR group. Specifically, CRP and other commonly measured inflammatory markers primarily remained within normal ranges and demonstrated a similar pattern across both groups. These findings do not indicate a notable viral persistence or elevation in inflammation within the LC cohort.
This finding is somewhat unexpected, as previous studies have identified residual inflammation in LC cases. The discrepancy may be due to differences in cohort characteristics or timing; the participants were evaluated at a median of less than two years post-infection, whereas many had experienced symptoms for over two years.
Conversely, systematic review reports elevated cytokine levels frequently concentrate on patients in the early stages or those exhibiting more severe LC symptoms [
21]. This systematic review compared cytokines in LC versus recovered, healthy controls, and often active infection; symptom subgroups were also analysed. Key findings were higher IL-6 and TNF-α (plus CRP), with broader panels (e.g., chemokines, IFNs, IL-17) differing versus controls and symptom clusters. Sampling windows post-infection varied: <3 months (28.6%), 3–6 months (32.1%), ≥6 months (10.7%), mixed (3.6%), or unspecified (25%), with most cytokines measured after symptoms appeared, limiting prognostic conclusions. Yet, our findings are consistent with recent observations indicating that systemic inflammation may decrease in numerous LC patients within one year or, at the very least, may not reach levels that differentiate them from fully WR individuals [
70,
71].
Other explanations focus on ongoing changes in the MSK in LC patients, which can lead to reduced HRQoL even when inflammatory markers appear normal. Recent research suggests that patients with LC may experience mitochondrial and endothelial dysfunction in skeletal muscles, capillary loss, and post-exertional myopathy, sometimes accompanied by amyloid-like deposits that worsen after activity[
72,
73]. Additionally, cardiovascular autonomic problems, such as decreased heart rate variability and chronotropic incompetence, can further restrict physical activity despite normal inflammatory biomarkers like CRP or ESR levels [
74,
75]. Overall, these findings imply that fatigue in LC is mainly driven by neuromuscular and autonomic dysfunction rather than ongoing systemic inflammation[
76,
77,
78].
Vitamin D Level Initially High in Long COVID and Improved in Both Groups During 12 Months:
Interestingly, vitamin D levels in both groups increased significantly over the 12 months, possibly due to supplementation or seasonal influences. LC participants may have proactively begun supplements more frequently following their illness, possibly influenced by advice or personal choice, which could have elevated their vitamin D levels. Meanwhile, WR started with lower baseline vitamin D levels, with a median of ~21 ng/mL, and “caught up” by the follow-up. Participants and their clinicians took action during the year. Vitamin D exhibits immunomodulatory characteristics that help regulate the immune system. It plays a crucial role in adjusting both the adaptive and innate immune systems by influencing cytokines and cell signalling pathways [
79,
80]. Studies have linked its deficiency to dysregulated immune responses and heightened vulnerability to respiratory infections [
81].
Long COVID Linked to Persistent Joints Pain With 12-Month:
Joint pain scores remained elevated in LC participants, with no resolution observed after one year. This was an unexpected observation, likely resulting from incidental or transient findings in individuals who are otherwise recovered. It is acknowledged that viral infections may affect joints, including hepatitis B or C, Epstein-Barr virus, and HIV, among others [
82,
83,
84,
85]. It had been suggested that the widespread joint and muscle pain is likely due to the acute COVID-19 [
86]. Similarly, half of the patients 180 days post SARS-CoV-1, with considerable joint pain in multiple joints observed with a negative MRI scan, leading to the assumption of neurogenic pain or low-grade synovitis that couldn’t be detected via MRI, lasted up to 4 years [
87].
There are two theories regarding the relationship between COVID-19 and articular manifestations. One suggests that COVID-19, accompanied by viremia or a cytokine storm, may cause viral arthritis, or it may be a non-specific consequence of the cytokine storm associated with symptomatic forms of the disease. However, no confirmed cases have been reported so far [
88]. Another theory suggests that arthritis may be triggered by an inflammatory response to the systemic condition caused by COVID-19, which may lead to reactive arthritis [
89].
This longitudinal study found that individuals with LC showed a consistent decline in HRQoL and ongoing joint pain over 12 months, despite normal inflammatory profiles and stable bone turnover markers. Baseline vitamin D was higher in LC and increased in both groups, likely due to supplementation or seasonal effects. Mechanisms beyond inflammation or bone remodelling, such as deconditioning, autonomic dysregulation, metabolic issues, or central pain mechanisms, may drive persistent symptoms. The findings support multidisciplinary care focused on pain management or functional restoration. Future research should involve larger cohorts with longer follow-up, mechanistic studies using advanced assessments, and targeted interventions such as rehabilitation. These efforts aim to refine personalised care and improve outcomes for people with LC. LC patients with declining HRQoL, a multidisciplinary rehabilitation approach is advised. This includes personalised, graded physical activity with pacing to prevent post-exertional symptoms, physiotherapy, psychological support, and managing comorbidities. Evidence also supports adding autonomic rehab, nutritional optimisation, and gradual return-to-work plans to enhance function and reduce fatigue [
90,
91]. Regular monitoring of musculoskeletal health, instead of only inflammatory markers, is crucial for guiding recovery and avoiding relapse.
Limitations
The study has a few limitations worth noting. Internal validity is affected by the statistical approach using paired and independent tests because it ignores repeated measures and interactions. Repeated measures or linear mixed models are better for capturing longitudinal effects and handling missing data. This study conducted many univariate statistical tests without using formal corrections for multiple comparisons, raising the risk of false positives, although a more conservative p<0.01 threshold was used instead. Future research should consider implementing formal multiplicity adjustments or focusing analyses on pre-specified hypotheses. Causal inferences are also inherently limited by the observational cohort design, where confounding factors may influence the results. For example, any observed improvements or declines might be due to natural recovery or external factors rather than being directly caused by LC. Some participants started new treatments, including osteoporosis medications and vitamin D, during the study which may have influenced BTM. The increase in vitamin D levels, likely due to supplementation, may have improved bone outcomes and masked differences attributed to other LC factors. The potential impact of seasonal recruitment; could have influenced the vitamin D levels between groups at baseline. However, most baseline assessments occurred in winter. Additionally, vitamin D’s seasonality means baseline differences and within-year changes in 25(OH)D might reflect timing effects rather than true group differences, without adjusting for calendar month or supplementation. We also lacked standardised data on analgesic use and physical activity, which could affect pain scores and bone markers. External validity is reduced as most participants were from semi-urban areas in the Southwest UK, primarily Caucasian, with females being more dominant in the LC group. Thus, findings may not generalise to more diverse populations and could over-represent females. Participation was also voluntary, which may have led to more motivated individuals with more severe symptoms joining and participating in follow-up, potentially introducing recruitment bias. Approximately 22% of participants dropped out during the year, potentially causing attrition bias, which should be considered in future studies.
5. Conclusions
We evaluated HRQoL, joint pain scores, serum vitamin D, BTM, and standard inflammatory markers in LC participants. LC was associated with poorer HRQoL, particularly in physical health domains, and with greater baseline hand joint pain, suggesting that physical therapy should focus on symptom-driven reconditioning and rehabilitation, and raising important questions regarding the physiological mechanisms underpinning the observed changes in HRQoL. We found no differences between the LC and WR groups concerning inflammatory markers or bone profiles, underscoring the promise of imaging-derived phenotyping as a complementary or alternative approach to blood-based markers. This highlights the need for further research into the MSK health of individuals with LC, as MSK sequelae may not be readily captured through blood markers alone, potentially requiring imaging data to unravel the mechanisms underpinning the reduced HRQoL in LC.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Investigation, methodology, formal analysis, data curation, writing – original draft preparation, visualisation, FA; supervision, methodology KK, WDS, RM and OAD; conceptualisation, project Administration KK, WDS; blood analysis and methodology, LH and SA; writing, review and editing KM, RM. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is funded by a PhD scholarship from Qassim University, Saudi Arabia.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BTM |
Bone Turnover Markers |
| CTX |
C-Terminal Telopeptide of Type 1 Collagen |
| EQ-5D-5L |
EuroQol 5-Dimension 5-Level |
| HRQoL |
Health-Related Quality of Life |
| HRT |
Hormone Replacement Therapy |
| IL |
Interleukins |
| INF-γ |
Interferon-Gamma |
| MSK |
Musculoskeletal |
| PINP |
Procollagen Type I N Propeptide |
| TNF-α |
Tumor Necrosis Factor-Alpha |
| VAS |
Visual Analogue Scale |
References
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: progress and lessons learned. Nature Reviews Drug Discovery 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Coronavirus disease (COVID-19). Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 27 October).
- Mahase, E. Covid-19: What do we know about “long covid”? BMJ 2020, 370, m2815. [Google Scholar] [CrossRef] [PubMed]
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc Sci Med 2021, 268, 113426. [Google Scholar] [CrossRef] [PubMed]
- Prevention, C.f.D.C.a. Signs and Symptoms of Long COVID. Available online: https://www.cdc.gov/covid/long-term-effects/long-covid-signs-symptoms.html (accessed on 02/12).
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Picone, P.; Sanfilippo, T.; Guggino, R.; Scalisi, L.; Monastero, R.; Baschi, R.; Mandalà, V.; San Biagio, L.; Rizzo, M.; Giacomazza, D. Neurological consequences, mental health, physical care, and appropriate nutrition in long-COVID-19. Cellular and Molecular Neurobiology 2023, 43, 1685–1695. [Google Scholar] [CrossRef]
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q. A systematic review and meta-analysis of long COVID symptoms. Systematic reviews 2023, 12, 88. [Google Scholar] [CrossRef]
- Nabavi, N. Long covid: How to define it and how to manage it. BMJ 2020, 370, m3489. [Google Scholar] [CrossRef]
- Bakılan, F.; Gökmen, İ.G.; Ortanca, B.; Uçan, A.; Eker Güvenç, Ş.; Şahin Mutlu, F.; Gökmen, H.M.; Ekim, A. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. International journal of clinical practice 2021, 75, e14734. [Google Scholar] [CrossRef]
- Karaarslan, F.; Demircioğlu Güneri, F.; Kardeş, S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: prospective follow-up by phone interviews. Rheumatology international 2021, 41, 1263–1271. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Karaarslan, F.; Guneri, F.D.; Kardes, S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol 2022, 41, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clinical Microbiology and Infection 2021, 27, 1507–1513. [Google Scholar] [CrossRef]
- Ghosn, J.; Piroth, L.; Epaulard, O.; Le Turnier, P.; Mentré, F.; Bachelet, D.; Laouénan, C. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clinical Microbiology and Infection 2021, 27, 1041–e1041. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Jain, V.K.; Iyengar, K.P. Musculoskeletal manifestations of COVID-19. Journal of Clinical Orthopaedics & Trauma 2021, 17, 280–281. [Google Scholar]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Rovere Querini, P.; Giustina, A. Low Vitamin D Levels Are Associated With Long COVID Syndrome in COVID-19 Survivors. The Journal of Clinical Endocrinology & Metabolism 2023, 108, e1106–e1116. [Google Scholar] [CrossRef]
- Charoenporn, V.; Charernboon, T. Prevalence of vitamin D deficiency in patients with fatigue and neuropsychiatric symptoms of long COVID and its correlation with symptom severity. Current Nutrition & Food Science 2025, 21, 501–507. [Google Scholar] [CrossRef]
- Matangkha, K.; Punyahotara, V.; Rintra, J.; Sittiprapaporn, P. Association Between Vitamin D Levels and Long COVID Signs and Symptoms. Med Sci (Basel) 2025, 13. [Google Scholar] [CrossRef]
- Gulzar, R.; Rasheed, A.; Riaz, S.; Adnan, W.A.; Hafeez, U.; Malik, A.M. Musculoskeletal Symptoms in Patients Recovering from COVID-19. Muscles, Ligaments & Tendons Journal (MLTJ) 2022, 12. [Google Scholar]
- Lai, Y.-J.; Liu, S.-H.; Manachevakul, S.; Lee, T.-A.; Kuo, C.-T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Frontiers in Medicine 2023, 10. [Google Scholar] [CrossRef]
- Espín, E.; Yang, C.; Shannon, C.P.; Assadian, S.; He, D.; Tebbutt, S.J. Cellular and molecular biomarkers of long COVID: a scoping review. Ebiomedicine 2023, 91. [Google Scholar] [CrossRef]
- Ciaffi, J.; Vanni, E.; Mancarella, L.; Brusi, V.; Lisi, L.; Pignatti, F.; Naldi, S.; Assirelli, E.; Neri, S.; Reta, M. Post-acute COVID-19 joint pain and new onset of rheumatic musculoskeletal diseases: a systematic review. Diagnostics 2023, 13, 1850. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Long-term effects of coronavirus (long COVID): What is it? Available online: https://cks.nice.org.uk/topics/long-term-effects-of-coronavirus-long-covid/background-information/definition/ (accessed on 09 September).
- Arostegui, D.; Castro, K.; Schwarz, S.; Vaidy, K.; Rabinowitz, S.; Wallach, T. Persistent SARS-CoV-2 Nucleocapsid Protein Presence in the Intestinal Epithelium of a Pediatric Patient 3 Months After Acute Infection. JPGN Reports 2022, 3, e152. [Google Scholar] [CrossRef]
- Lin, S. Using genomic approaches to characterise the immune responses to biologicals; University of Exeter (United Kingdom): 2023.
- Yang, C.; Zhao, H.; Espín, E.; Tebbutt, S.J. Association of SARS-CoV-2 infection and persistence with long COVID. The Lancet Respiratory Medicine 2023, 11, 504–506. [Google Scholar] [CrossRef]
- Beck Jr, G.R.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomedicine: Nanotechnology, Biology and Medicine 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nature medicine 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Guidarelli, G.; Ostan, R.; Giampieri, E.; Fabbri, C.; Bertarelli, C.; Nicoletti, C.; Kadi, F.; De Groot, L.C.; Feskens, E. Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. European Radiology 2019, 29, 4968–4979. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y. The role of the immune microenvironment in bone regeneration. International Journal of Medical Sciences 2021, 18, 3697. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.; Cooper, C.; Eastell, R.; Griesmacher, A.; Morris, H.A.; Trenti, T.; Kanis, J.A. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med 2011, 49, 1271–1274. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. The lancet Diabetes & endocrinology 2017, 5, 908–923. [Google Scholar]
- Kruger, M.C.; Schollum, L.M.; Kuhn-Sherlock, B.; Hestiantoro, A.; Wijanto, P.; Li-Yu, J.; Agdeppa, I.; Todd, J.M.; Eastell, R. The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone 2010, 46, 759–767. [Google Scholar] [CrossRef]
- Ferrar, L.; Van Der Hee, R.; Berry, M.; Watson, C.; Miret, S.; Wilkinson, J.; Bradburn, M.; Eastell, R. Effects of calcium-fortified ice cream on markers of bone health. Osteoporosis international 2011, 22, 2721–2731. [Google Scholar] [CrossRef]
- Becton, D.a.C. General BD Vacutainer® blood collection tubes FAQ. Available online: https://www.bd.com/en-ca/offerings/capabilities/specimen-collection/blood-specimen-collection/venous-collection/bd-vacutainer-blood-collection-tubes/vacutainer-blood-collection-tube-faq/general-tubes-faq (accessed on 19 September).
- Fisher, A.; Srikusalanukul, W.; Fisher, L.; Smith, P.N. Lower serum P1NP/βCTX ratio and hypoalbuminemia are independently associated with osteoporotic nonvertebral fractures in older adults. Clinical interventions in aging 2017, 1131–1140. [Google Scholar] [CrossRef]
- Fisher, A.; Fisher, L.; Srikusalanukul, W.; Smith, P.N. Bone turnover status: classification model and clinical implications. International Journal of Medical Sciences 2018, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Bramwell, L.R.; Jeffery, N.; Bunce, B.; Lee, B.P.; Knight, B.; Auckland, C.; Masoli, J.A.; Harries, L.W. Persistence of clinically relevant levels of SARS-CoV2 envelope gene subgenomic RNAs in non-immunocompromised individuals. International journal of infectious diseases 2022, 116, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Foundation, E.R. EQ-5D-3L User Guide; EQ-5D-3L User Guide. 2018. Accessed December 18, 2018. https://euroqol. org …: 2018.
- Devlin, N.J.; Shah, K.K.; Feng, Y.; Mulhern, B.; Van Hout, B. Valuing health-related quality of life: An EQ-5 D-5 L value set for E ngland. Health economics 2018, 27, 7–22. [Google Scholar] [CrossRef]
- EuroQol. Generic Health-Related Quality of Life (HRQOL), Available online: https://euroqol.org/information-and-support/resources/value-sets/ (accessed on 19/05).
- National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the longterm effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (accessed on March, 15).
- Lam, C.L.K.; Tse, E.T.Y.; Wong, C.K.H.; Lam, J.S.M.; Chen, S.S.; Bedford, L.E.; Cheung, J.P.Y.; Or, C.K.; Kind, P. A pilot study on the validity and psychometric properties of the electronic EQ-5D-5L in routine clinical practice. Health Qual Life Outcomes 2021, 19, 266. [Google Scholar] [CrossRef]
- Tran, B.X.; Ohinmaa, A.; Nguyen, L.T. Quality of life profile and psychometric properties of the EQ-5D-5L in HIV/AIDS patients. Health Qual Life Outcomes 2012, 10, 132. [Google Scholar] [CrossRef]
- Feng, Y.-S.; Kohlmann, T.; Janssen, M.F.; Buchholz, I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual. Life Res. 2021, 30, 647–673. [Google Scholar] [CrossRef]
- Long, D.; Polinder, S.; Bonsel, G.J.; Haagsma, J.A. Test-retest reliability of the EQ-5D-5L and the reworded QOLIBRI-OS in the general population of Italy, the Netherlands, and the United Kingdom. Qual Life Res 2021, 30, 2961–2971. [Google Scholar] [CrossRef]
- The British Pain Society. Outcome Measures. Available online: https://www.britishpainsociety.org/static/uploads/resources/files/Outcome_Measures_January_2019.pdf (accessed on 25 Oct).
- Alghadir, A.H.; Anwer, S.; Iqbal, A.; Iqbal, Z.A. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res 2018, 11, 851–856. [Google Scholar] [CrossRef]
- Leung, J.L.; Twohig, H.; Muller, S.; Maxwell, L.; Mackie, S.L.; Neill, L.M.; Owen, C.E. Test-retest reliability of pain VAS/NRS, stiffness VAS/NRS, HAQ-DI and mHAQ in polymyalgia rheumatica: An OMERACT study. Seminars in Arthritis and Rheumatism 2023, 62, 152239. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Berry, G.; Matthews, J. Statistical Methods in Medical Research. JohnWiley & Sons. Inc., New York 1971, 362-365.
- McHugh, M.L. The chi-square test of independence. Biochemia medica 2013, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restorative dentistry & endodontics 2017, 42, 152. [Google Scholar]
- Alghamdi, F.; Mokbel, K.; Meertens, R.; Obotiba, A.D.; Alharbi, M.; Knapp, K.M.; Strain, W.D. Bone Mineral Density, Bone Biomarkers, and Joints in Acute, Post, and Long COVID-19: A Systematic Review. Viruses 2024, 16, 1694. [Google Scholar] [CrossRef]
- Hay, J.W.; Gong, C.L.; Jiao, X.; Zawadzki, N.K.; Zawadzki, R.S.; Pickard, A.S.; Xie, F.; Crawford, S.A.; Gu, N.Y. A US population health survey on the impact of COVID-19 using the EQ-5D-5L. J. Gen. Intern. Med. 2021, 36, 1292–1301. [Google Scholar] [CrossRef]
- Wright, J.; Astill, S.L.; Sivan, M. The Relationship between Physical Activity and Long COVID: A Cross-Sectional Study. International Journal of Environmental Research and Public Health 2022, 19, 5093. [Google Scholar] [CrossRef]
- Carlile, O.; Briggs, A.; Henderson, A.D.; Butler-Cole, B.F.; Tazare, J.; Tomlinson, L.A.; Marks, M.; Jit, M.; Lin, L.-Y.; Bates, C. Impact of long COVID on health-related quality-of-life: an OpenSAFELY population cohort study using patient-reported outcome measures (OpenPROMPT). The Lancet Regional Health–Europe 2024, 40. [Google Scholar] [CrossRef]
- Moens, M.; Duarte, R.V.; De Smedt, A.; Putman, K.; Callens, J.; Billot, M.; Roulaud, M.; Rigoard, P.; Goudman, L. Health-related quality of life in persons post-COVID-19 infection in comparison to normative controls and chronic pain patients. Frontiers in Public Health 2022, 10, 991572. [Google Scholar] [CrossRef]
- Shanbehzadeh, S.; Zanjari, N.; Yassin, M.; Yassin, Z.; Tavahomi, M. Association between long COVID, functional activity, and health-related quality of life in older adults. BMC Geriatr 2023, 23, 40. [Google Scholar] [CrossRef]
- Oliver-Mas, S.; Matias-Guiu, J.A.; Delgado-Alonso, C.; Cuevas, C.; Alcalá Ramírez del Puerto, J.M.; López-Carbonero, J.I.; Matias-Guiu, J.; Diez-Cirarda, M. Differential Fatigue Profile in Patients with Post-COVID Condition, Fibromyalgia, and Multiple Sclerosis. Journal of Clinical Medicine 2025, 14, 952. [Google Scholar] [CrossRef]
- Diez-Cirarda, M.; Yus-Fuertes, M.; Polidura, C.; Gil-Martinez, L.; Delgado-Alonso, C.; Delgado-Alvarez, A.; Gomez-Ruiz, N.; Gil-Moreno, M.J.; Jorquera, M.; Oliver-Mas, S. Neural basis of fatigue in post-COVID syndrome and relationships with cognitive complaints and cognition. Psychiatry Research 2024, 340, 116113. [Google Scholar] [CrossRef]
- Haudenschild, A.K.; Christiansen, B.A.; Orr, S.; Ball, E.E.; Weiss, C.M.; Liu, H.; Fyhrie, D.P.; Yik, J.H.; Coffey, L.L.; Haudenschild, D.R. Acute bone loss following SARS-CoV-2 infection in mice. Journal of Orthopaedic Research® 2023, 41, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Lau, H.E.; Xie, H.; Poon, V.K.-M.; Chan, C.C.-S.; Chu, H.; Yuan, S.; Yuen, T.T.-T.; Chik, K.K.-H.; Tsang, J.O.-L. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nature communications 2022, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Awosanya, O.D.; Dalloul, C.E.; Blosser, R.J.; Dadwal, U.C.; Carozza, M.; Boschen, K.; Klemsz, M.J.; Johnston, N.A.; Bruzzaniti, A.; Robinson, C.M. Osteoclast-mediated bone loss observed in a COVID-19 mouse model. Bone 2022, 154, 116227. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Lau, H.E.; Xie, H.; Poon, V.K.-M.; Chan, C.C.-S.; Chu, H.; Yuan, S.; Yuen, T.T.-T.; Chik, K.K.-H.; Tsang, J.O.-L. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nature communications 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Queiroz-Junior, C.M.; Santos, A.C.; Gonçalves, M.R.; Brito, C.B.; Barrioni, B.; Almeida, P.J.; Gonçalves-Pereira, M.H.; Silva, T.; Oliveira, S.R.; Pereira, M.M. Acute coronavirus infection triggers a TNF-dependent osteoporotic phenotype in mice. Life Sci. 2023, 324, 121750. [Google Scholar] [CrossRef]
- Mantovani, A.; Morrone, M.C.; Patrono, C.; Santoro, M.G.; Schiaffino, S.; Remuzzi, G.; Bussolati, G.; 20 Vineis Paolo 21, C.-C.o.t.A.N.d.L.C.P.F.G.B.M.L.M.G.P.G.R.R.R.G. Long Covid: where we stand and challenges ahead. Cell Death & Differentiation 2022, 29, 1891–1900. [Google Scholar] [CrossRef]
- Arrieta, F.; Martinez-Vaello, V.; Bengoa, N.; Rosillo, M.; de Pablo, A.; Voguel, C.; Pintor, R.; Belanger-Quintana, A.; Mateo-Lobo, R.; Candela, A. Stress hyperglycemia and osteocalcin in COVID-19 critically ill patients on artificial nutrition. Nutrients 2021, 13, 3010. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Wang, H.; Gao, Y.; Hu, X.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Characteristics of laboratory indexes in COVID-19 patients with non-severe symptoms in Hefei City, China: diagnostic value in organ injuries. European Journal of Clinical Microbiology & Infectious Diseases 2020, 39, 2447–2455. [Google Scholar] [CrossRef]
- Hu, M.; Song, T.; Gong, Z.; Che, Q.; Guo, J.; Chen, L.; Zhang, H.; Li, H.; Liang, N.; Zhao, G. Symptom Trajectories and Clinical Subtypes in Post–COVID-19 Condition: Systematic Review and Clustering Analysis. JMIR Public Health and Surveillance 2025, 11, e72221. [Google Scholar] [CrossRef]
- Demko, Z.O.; Yu, T.; Mullapudi, S.K.; Varela Heslin, M.G.; Dorsey, C.A.; Payton, C.B.; Tornheim, J.A.; Blair, P.W.; Mehta, S.H.; Thomas, D.L. Two-year longitudinal study reveals that long COVID symptoms peak and quality of life nadirs at 6–12 months postinfection. In Proceedings of the Open forum infectious diseases, 2024; p. ofae027. [Google Scholar]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nature Communications 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Charlton, B.T.; Goulding, R.P.; Jaspers, R.T.; Appelman, B.; van Vugt, M.; Wüst, R.C.I. Skeletal muscle adaptations and post-exertional malaise in long COVID. Trends in Endocrinology & Metabolism 2025, 36, 614–622. [Google Scholar] [CrossRef]
- Marques, K.C.; Quaresma, J.A.S.; Falcão, L.F.M. Cardiovascular autonomic dysfunction in “Long COVID”: pathophysiology, heart rate variability, and inflammatory markers. Front Cardiovasc Med 2023, 10, 1256512. [Google Scholar] [CrossRef]
- Mustonen, T.; Kanerva, M.; Luukkonen, R.; Lantto, H.; Uusitalo, A.; Piirilä, P. Cardiopulmonary exercise testing in long covid shows the presence of dysautonomia or chronotropic incompetence independent of subjective exercise intolerance and fatigue. BMC Cardiovascular Disorders 2024, 24, 413. [Google Scholar] [CrossRef]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O. Long COVID: mechanisms, risk factors and recovery. Experimental physiology 2023, 108, 12–27. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Christodoulatos, G.S.; Papavasileiou, G.; Petropoulou, D.; Magkos, F.; Dalamaga, M. Laboratory Findings and Biomarkers in Long COVID: What Do We Know So Far? Insights into Epidemiology, Pathogenesis, Therapeutic Perspectives and Challenges. International Journal of Molecular Sciences 2023, 24, 10458. [Google Scholar] [CrossRef]
- Pasculli, P.; Zingaropoli, M.A.; Dominelli, F.; Solimini, A.G.; Masci, G.M.; Birtolo, L.I.; Pasquariello, L.; Paribeni, F.; Iafrate, F.; Panebianco, V.; et al. Insights into Long COVID: Unraveling Risk Factors, Clinical Features, Radiological Findings, Functional Sequelae and Correlations: A Retrospective Cohort Study. The American Journal of Medicine 2025, 138, 721–731. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: a helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J 2020, 113, 81. [Google Scholar]
- Selmi, C.; Gershwin, M.E. Diagnosis and classification of reactive arthritis. Autoimmunity reviews 2014, 13, 546–549. [Google Scholar] [CrossRef]
- Siva, C.; Velazquez, C.; Mody, A.; Brasington, R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. American family physician 2003, 68, 83–90. [Google Scholar]
- Parisi, S.; Borrelli, R.; Bianchi, S.; Fusaro, E. Viral arthritis and COVID-19. The Lancet Rheumatology 2020, 2, e655–e657. [Google Scholar] [CrossRef]
- Vassilopoulos, D.; Calabrese, L.H. Virally associated arthritis 2008: clinical, epidemiologic, and pathophysiologic considerations. Arthritis research & therapy 2008, 10, 1–8. [Google Scholar]
- Baimukhamedov, C.; Barskova, T.; Matucci-Cerinic, M. Arthritis after SARS-CoV-2 infection. The Lancet Rheumatology 2021, 3, e324–e325. [Google Scholar] [CrossRef]
- Griffith, J.F. Musculoskeletal complications of severe acute respiratory syndrome. In Proceedings of the Seminars in musculoskeletal radiology; 2011; pp. 554–560. [Google Scholar]
- Yokogawa, N.; Minematsu, N.; Katano, H.; Suzuki, T. Case of acute arthritis following SARS-CoV-2 infection. Annals of the Rheumatic Diseases 2021, 80, e101–e101. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Kishimoto, M.; Shimasaki, T.; Uchida, H.; Kurai, D.; Deshpande, G.A.; Komagata, Y.; Kaname, S. Reactive arthritis after COVID-19 infection. RMD Open 2020, 6, e001350. [Google Scholar] [CrossRef] [PubMed]
- (NICE), N.I.f.H.a.C.E. COVID-19 rapid guideline: managing the long-term effects of COVID-19 (NG188). Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 30 Oct).
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).