Submitted:
24 October 2025
Posted:
03 November 2025
You are already at the latest version
Abstract
Keywords:
Abbreviations
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Data Charting
2.3. Additional Non-Systematic Searches for Subtopics and Preprints
3. Part One- Findings from the Systematic Scoping Review
- Observational human studies (excluding case series, case reports, and conference abstracts) (n=60) [2,10,16,48,49,52,61,64,65,67,68,69,75,77,81,85,88,91,93,99,100,101,104,105,108,111,112,113,114,115,118,121,127,128,129,130,132,133,134,135,136,137,140,141,144,145,146,148,154,155,163,167,170,173,177,178,179,182,183,185]
- Official clinical guidelines (n=1) [186]
3.1. Definitions, Research Inclusion/Exclusion Criteria in LC studies, and Measurement Tools
3.1.1. Definitions & Research Inclusion/Exclusion Criteria
-
COVID-19 individuals: COVID-19 or SARS-CoV-2 infected individuals were seen defined differently across studies and involved different inclusion and exclusion criteria. A “COVID-19” individual was established either by self-report of the participant (e.g., based on symptoms consistent with COVID-19, a self-reported physician diagnosis of COVID-19, or self reported positive COVID-19 test), positive immunoglobulin response, a documented positive test in healthcare databases or COVID registries (e.g., PCR test), or by logical combinations of these conditions, for example. To give a specific example, in a study by Peterson et al. [75], symptomatic COVID-19 individuals were those who self-reported the symptoms that they had experienced during active COVID-19 infection from a list modified from the CDC and provided evidence of a previous positive PCR or antibody ELISA test indicating infection. On the other hand, the asymptomatic COVID-19 group consisted of those participants who self-reported no symptoms and had a previous positive PCR and/or positive antibody test (or self-reported that they had no symptoms but had a positive antibody test).In case of a non-LC (i.e., recovered COVID-19) infected individual, absence of persistent symptoms beyond a certain timeframe or the absence of symptoms altogether defined the convalescent group [91,185]. Such participants may have undergone a brief verbal screening to confirm no active symptomatology.Severity of acute illness: few studies stratified the COVID-19 group based on the severity of their acute COVID-19 illness (e.g., hospitalized vs. non-hospitalized).Additionally, unclear onset of symptoms was sometimes used as an exclusion criteria for self-reported COVID-19 [153].
- Controls and Healthy controls: were also seen defined differently across studies, depending on the study [81,91,104,115]. The heterogeneity in defining these crucial comparator groups has significant implications for interpreting research findings and understanding the true impact of SARS-CoV-2 infection. Healthy uninfected controls were often defined as individuals who have no prior history of COVID-19 infection, often confirmed through PCR and antibody testing, while other studies defined control groups as those with no active symptomatology or implementing both conditions [91]. Naturally, the longer the duration into the pandemic the more difficult it would have been for investigators to find non-infected individuals. Damasceno et al. (2023) [115] chose adults who had COVID-19 for at least 3 months prior to the data collection and without a chronic pain syndrome. Examples of inclusion criteria are (i) individuals that reported they did not have a confirmed objective COVID-19 test (e.g., PCR or home kit) [127], (ii) individuals that do not have a previous history of medical conditions as self-reported, (iii) no previous symptoms self-reportedly and negative result on the PCR and antibody test [75,118], and (iv) based on (absence of) diagnoses in medical records or healthcare registries. The non-infection healthy control group in Peterson et al., for example, were those who self-reported no previous symptoms and were negative on the PCR and antibody test administered immediately prior to carrying out the study’s investigation [75].
-
Long COVID, Post-COVID condition, Persistent COVID symptoms, and other parallel terms: A fundamental aspect of LC research is the definition used to identify affected individuals. Various studies employ different criteria, often aligning with guidelines from organizations like NICE and WHO, or developing their own definitions.A consistent inclusion criterion across many studies was, naturally, the persistence of symptoms for a defined duration following the acute phase of SARS-CoV-2 infection (e.g., 4, 6, 12 weeks). Another additional inclusion condition often used for LC was confirmation of prior SARS-CoV-2 infection (by positive PCR, serology, self-reportedly, independent clinician, rapid antigen test with documentary proof from a health authority [159], or documentation in electronic health records). Some LC studies included only previously healthy individuals. Self-reported history of confirmed or probable COVID-19 infection according to WHO guidelines was also seen integrated into the inclusion criteria [91]. Certain studies focused on individuals experiencing a particular set of new persistent symptoms after acute COVID-19 [133], such as musculoskeletal pain [114] or neurological symptoms [182] whereas others focused on evident reduction in the level of functioning and activity or participation in daily life compared to before the infection [16]. Exclusion of alternative etiologies: some studies incorporated a process to rule out alternative medical etiologies for persistent symptoms, such as medical evaluations by physicians, or self-reportedly. Pre-existing chronic pain prior to COVID-19 infection, pre-existing chronic fatigue syndrome or fibromyalgia were sometimes part of the exclusion criteria [155].Examples of inclusion criteria for Post COVID/Long COVID/Chronic COVID/Subacute COVID/“persistent symptoms” or “non-recovery from COVID”: (i) participant self-reporting not to have been fully recovered after COVID-19 [146], (ii) participant self-reported physician-made diagnosis of LC [150], (iii) self-reported physician made diagnosis combined with a previous positive COVID-19 test [128], (iv) persistent symptoms beyond a specified interval of time (e.g., 12 weeks) [141,165], (v) presence of any persistent symptom since SARS-CoV-2 infection (or any persistent symptom among a predetermined list of symptoms) [93,99,185], (vi) persistent symptoms and negative Covid test for excluding active infection [75,154], (vii) based on the world health organization’s consensus definition [101], (viii) Bierle et al.’s (2021) criteria [144], (ix) fulfilling the official 2015 diagnostic criteria for ME/CFS [155], (x) persistent post-exertional malaise for 3 or more months verified by the DePaul Symptom Questionnaire [89], (xi) referral to- or diagnosis by- a post-Covid clinic, and more [137,156].A reader would notice correctly that some of the above examples can conflate “LC syndrome” and “persistent COVID symptoms,” which are not necessarily the same. Noteworthy, as opposed to simply including persistent symptoms, in case a syndrome is what investigators are aiming to investigate, defining LC for the purpose of a study as at least one persistent symptom, any symptom, even hyposmia, does not necessarily reflect a syndrome, in agreement with Phillips and Williams [8]. Lau et al. (2024) [159], for example, included individuals fulfilling the Centers for Disease Control and Prevention criteria for post-acute COVID condition and at least one of 14 symptoms included in their post-acute COVID-19 syndrome 14-item improvement questionnaire (PACSQ-14) for four weeks or more after SARS-CoV-2 infection. Matta et al. (2022) [185], in their widely cited publication of whether belief in having had COVID-19 and actually having had the infection (when verified by SARS-CoV-2 serology testing) were associated with persistent physical symptoms after COVID-19, in the context of LC, included individuals with at least one persistent symptom among a list of symptoms present for the past 4 weeks and lasting more than 8 weeks. That list consisted of headache, back pain, joint pain, muscular pain, sore muscles, sleep problems, fatigue, sensory symptoms such as pins and needles, tingling or burning sensation, skin problems, poor attention or concentration, hearing impairment, stomach pain, constipation, breathing difficulties, palpitations, chest pain, dizziness, cough, diarrhea, anosmia, and other symptoms.Eccles et al. (2024) [170] determined non-recovery from COVID-19 from a dichotomous self-reported response to the question “Thinking about the last or only episode of COVID-19 you have had, have you now recovered and are back to normal?” while Amsterdam et al. (2024) [133] recruited outpatients from a post-COVID clinic who, subsequent to non-hospitalized COVID-19, developed a prolonged illness, leading to a diagnosis of LC syndrome characterized by the persistence of one or more symptoms for over a month: dyspnea, cough, cognitive decline, brain fog, or fatigue, going by reference to the 2020 published NICE guidelines. Azcue et al. [141] took a similar approach and explicitly excluded respiratory symptoms persisting for 12 weeks post-infection, severe bilateral pneumonia, admission to an intensive care unit, or other manifestations necessitating hospitalization.
3.1.2. Assessment and Measurement Tools
- The visual analogue scale: for multiple measures such as pain and fatigue.
- Fatigue Severity Scale [128]: for assessing fatigue.
- Insomnia Severity Index: for the evaluation of insomnia [16].
- Fibromyalgia Symptom Scale (FSS) including the widespread pain index (WPI) and symptom severity scale (SSS) based on the ACR fibromyalgia diagnostic criteria and/or modified for self-administration [10,128], Fibromyalgia Rapid Screening (FIRST) questionnaire [135], and the central sensitization inventory (CSI) [146]: for assessment of fibromyalgia-type features, screening, or diagnosis. It is worth noting here that the CSI has not been validated to assess or measure central sensitization or neural activity [29], despite several studies using it for this purpose.
- Post-COVID-19 Functional Status (PCFS) self-reporting version: for assessing functional status post-COVID-19 infection.
- Yorkshire Rehabilitation Scale (C19-YRS) questionnaire: for assessing LC impact and need for rehabilitation in LC patients.
- Versions of the Patient Health Questionnaire (PHQ-2, 8, 9): for depression assessment.
- Patient Health Questionnaire 15 (PHQ-15) for assessing somatic symptoms.
- Hospital Anxiety and Depression Scale [16] and Generalised Anxiety Disorder-7 scale: for anxiety assessment.
3.2. Long COVID-19 Mechanisms
3.3. Observational Studies on Widespread Musculoskeletal Pain and Fibromyalgia After SARS-CoV-2 Infection
3.3.1. Cross-Sectional and Cohort Studies on Post-Covid Fibromyalgia Prevalence and Incidence
3.3.2. Observational Studies on LC, Chronic Fatigue Syndrome, and Overlapping Fibromyalgia (Molecular Mechanisms, Laboratory Investigations, and Others)
| Topic/Context | Study | Description of Study | Main Findings |
|---|---|---|---|
| Pain after COVID-19 | Amsterdam et al. [133] | A cross-sectional survey via self-reported questionnaires explored the association between distinctive personality profiles, particularly type D personality, and LC among convalescent asymptomatic to mild acute-COVID-19 cases without a need for hospitalization or oxygen supplementation. Adult participants were recruited from a pool of 750 individuals undergoing follow-up at the Tel Aviv Sourasky Medical Center post-COVID-19 clinic as outpatients. | 31% completion rate yielded 114 respondents (74.6% women), mean age was 44.5 years. 68.4% were healthy prior to contracting COVID-19 and developing LC. 37 of 114 met diagnostic criteria for fibromyalgia, and in 28 (24.5%) it was a new diagnosis after COVID-19. None of the patients reported experiencing prior mental health issues, nor did they have previous psychiatric diagnoses. Clustering into two groups showed an association between more pronounced fibromyalgia features, a higher burden of depression and anxiety, diffuse pain, attention deficit, memory problems, headaches, perception of lower quality of life, and type D personality, as well as a trend towards poor sleep quality. |
| Pain after COVID-19 | Damasceno et al. [115] | A case control study form Brazil that aimed to establish etiological factors associated with chronic pain syndromes in adult patients with post-COVID-19 conditions during 2021. Participants were adults who had COVID-19 at least 3 months prior to data collection with and without chronic pain syndromes. CSI was used to assess “central sensitivity” (i.e., fibromyalgia-type manifestations) | In total, 120 individuals were recruited (51 patients and 69 controls, average age ~30 years). CSI scores differed significantly between the groups with average scores of 50.51 in patients vs. 24 in controls (p < 0.001). |
| Pain after COVID-19 | Ebbesen et al. [105] | A nationwide cross-sectional study to investigate the prevalence and risk factors of de novo widespread musculoskeletal pain after COVID-19 in non-hospitalized COVID-19 survivors. Demographic and medical data were collected through an online questionnaire from Danish adults with a confirmed SARS-CoV-2 infection at least 6 months prior to the study, between March 2020 and December 2021. Widespread pain was defined as participants experiencing pain in at least 2 sites of the body, in the upper part of the body and 1 site on the lower part. | Among 130,443 nonhospitalized respondents (58.2% women, mean age was 50.2 years), 5.3 percent (n=6,875) of nonhospitalized COVID-19 survivors had new-onset widespread musculoskeletal pain at approximately 14 ± 6.0 months after infection, which was rated as moderate to severe in its intensity in 75.6% of cases. In a multivariate analysis, female sex, age, higher BMI, and previous history of migraine, whiplash, stress, type-2 diabetes, and comorbid chronic neurological disorders, were found as risk factors for de novo widespread LC pain, with adjusted odds ratio of 1.549, 1.003, 1.043, 1.554, 1.562, 1.47, 1.56, and 1.532, respectively. Also, among a few other factors found to be significant, higher income was associated with less development of widespread pain. Time elapsed since infection was also significantly positively correlated. Rates differed according to stratification by SARS-CoV-2 variant. |
| Pain after COVID-19 | Ketenci et al. [111] | A multicenter cross-sectional survey that was conducted during 2021 in physical and rehabilitative medicine outpatient clinics in Turkey categorized chronic pain after COVID-19 into predetermined categories. Diagnosis of pain phenotypes (nociceptive, neuropathic, or nociplastic/central sensitization) was made by physicians according to data from outcome measures including Pain Numerical Rating Scale, CSI, BDI, and HADS, Self-Report Leeds Assessment of Neuropathic Symptoms and Signs, clinical examination, and their experience in musculoskeletal diseases. Patients with overlapping phenotypes were excluded. | In total, 437 patients were grouped by diagnosis into predetermined chronic pain phenotypes, and subjects with overlapping clinical features were excluded. 66.13% of the patients were diagnosed with nociceptive pain, 11.67% with neuropathic pain, and 22.20% with central sensitization based on the CSI (i.e., fibromyalgia-type features). According to the authors, central sensitization was associated with females, hypertension, physical activity, and pre-existing chronic disease prior to COVID-19. |
| Pain after COVID-19 | Khoja et al. [112] | As part of a larger UK longitudinal study on musculoskeletal pain in LC (MUSLOC), cross-sectional data was reported on 30 adults with a history of COVID-19 with a diagnosis of LC and new onset musculoskeletal pain. The COVID-19 Yorkshire Rehabilitation Scale (C19-YRS) was used to capture the overall impact and health state before and after COVID-19 infection and the symptoms and their effect on individuals functioning. Other outcomes measures and self-assessment tools included QST and time up and go test, PHQ-9, GAD-7, PCS, EuroQol, and additional other tools. Central sensitization in participants was recognized if there was one of the three specific criteria: abnormally increased mechanical pain sensitivity, a reduced mechanical pain threshold, or presence of dynamic mechanical allodynia. | 30 participants in total (19 female) were included. The mean duration from the onset of musculoskeletal pain to evaluation in the study was 519.1 days (± 231.7). Only three participants were hospitalized due to COVID-19. Forty percent had no pre-existing medical condition. New-onset chronic musculoskeletal pain was mostly reported by the participants as generalized widespread pain (90%), characterized predominantly as joint pain. Ninety percent of the participants experienced continuous pain that always remains present, though its intensity may vary. 82.8% reported a high interference score, 19 participants stated that their employment status was affected by the health consequences associated with LC. QST indicated mechanical hyperalgesia and gain of function for wind up ratio, suggesting enhanced temporal summation of pain. In total, 25 participants (83%) showed central sensitization signs. There was a variability in the cytokine profiles. Investigation into individual cytokine levels using univariate analysis revealed no association between pain scores and any individual cytokines or C-reactive protein (CRP). The authors conclude that chronic new-onset musculoskeletal pain in LC tends to be generalized, widespread, continuous and is associated with central sensitization, elevated pro-inflammatory cytokines, weakness, reduced function and physical activity, depression, anxiety, and reduced quality of life. |
| Pain after COVID-19 | Khoja et al. [114] | An observational study that was conducted as part of a larger Musculoskeletal Pain in Long COVID (MUSLOC) UK study. Participants were adults (18 years or older) that tested positive for COVID-19 or had COVID-19 symptoms confirmed by an independent clinician, received a clinical diagnosis of post-COVID-19 syndrome according to the NICE guidelines, and experienced new-onset musculoskeletal pain since COVID-19. LC-associated symptoms were assessed using the COVID-19 Yorkshire Rehabilitation Scale (C19-YRS) questionnaire. The assessment of fibromyalgia was conducted as part of the standard clinical examination and using the American College of Rheumatology (ACR) 2010 criteria. | In total 18 patients were recruited, mean age was 49.6 (± 11.8) years, comprising 12 females (66.7%), mean duration since the onset of COVID-19 infection to the data collection point was 27.9 (± 6.97) months. Fourteen (77.8%) patients reported experiencing generalized widespread pain, while the remaining patients, who did not report widespread pain, still experienced pain in at least four distinct body areas. LC symptoms interfered with daily living activities for 17 (94.4%) patients, 13 (72.2%) of the evaluated patients met the diagnostic criteria for fibromyalgia as defined by the ACR. The average WPI score among the patients was 8.8, indicating a high level of pain spread across multiple body regions. Additionally, the average SS score was 8.2, reflecting significant symptom severity related to fatigue, waking unrefreshed, cognitive symptoms, and the extent of other somatic symptoms. Patients that did not meet the high cut-off of the ACR criteria for diagnosis still had fibromyalgia features of widespread pain. |
| Pain after COVID-19 | Kim et al. [127] | A 2022 cohort study that used data from electronic medical records from a nationwide population of all persons with COVID-19 in South Korea. Included were only individuals who had been diagnosed with COVID-19 during the first four months of the pandemic (February to May 2020) by means of real-time reverse-transcription polymerase chain reaction (PCR). Individuals in the control group were chosen as those who did not receive PCR testing. The authors investigated incidence rates of pain diagnoses of unspecified or idiopathic pain (e.g., fibromyalgia, headache, etc.), using diagnostic codes of the international classification, as well as prescription of medication as the outcome measures. | The diagnoses of fibromyalgia, temporomandibular joint disorders, and atypical facial pain did not occur at any time during 90 days from the index date. Opioid prescribed medications were higher in the COVID-19 group. When performing subgroup analysis the results were reversed, indicating higher rates of idiopathic pain in the control group. |
| Pain after COVID-19 | Kim et al. [163] | A population-based cohort study to determine changes in the level of incidence of musculoskeletal disorders among the Korean population in pre-pandemic and during the pandemic (through the periods of 2018-2021), using electronic medical record registries of the Korean National Health Insurance Service. The incidence of orthopedic diseases was evaluated based on diagnostic codes of the international classification of diseases. | The incidence of myofascial pain had decreased during the pandemic compared to pre-pandemic levels, while gout and frozen shoulder increased.. |
| Pain after COVID-19 | Patel and Javed [19] | Case series from a pain clinic | Medical records of individuals who developed myofascial pain after a diagnosis of COVID-19 between March 2020 and December 2020 were obtained. Three patients with considerable pre-existing chronic pain conditions experienced worsening musculoskeletal symptoms after SARS-CoV-2 infection. The first patient, a 68-year-old female, developed post-COVID-19 myalgia and muscle spasms, which improved by 75% following trigger point injections and physical therapy. The second patient, a 35-year-old female with congenital scoliosis, developed bilateral shoulder pain post-COVID-19, with taut bands in the infraspinatus muscle, showing moderate improvement with conservative treatment but refusing intervention. The third patient, a 71-year-old male with a substantial orthopedic lumbar medical history, developed new-onset neck pain and headaches post-COVID-19, with palpable taut bands in the trapezius bilaterally (a total of six) referring pain to the occipital region, achieving 40–50% immediate pain relief after myofascial trigger point injections, resulting in a numerical pain rating scale rating of 3/10 (compared to 6/10 pre-intervention) during the 4 week follow-up. |
| Pain after COVID-19 | Zha et al. [162] | Case report | A 59-year-old previously healthy Hispanic male developed persistent myalgia following COVID-19, with pain localized to trigger points in the neck, shoulders, upper back, arms, and legs, consistent with myofascial pain syndrome. Wet needling with lidocaine provided immediate but temporary relief, requiring multiple sessions over months. After experiencing a relapse associated with psychological stress, dry needling was introduced, leading to rapid and sustained pain reduction. |
| Pain after COVID-19 | Gouraud et al. [68] | A retrospective observational study from France investigated the characteristics, medical conclusions, and satisfaction of 286 patients with persistent symptoms after COVID-19 who attended a multidisciplinary day-hospital program. Evaluation was done by medical workup as recommended by official guidelines. | A total of 286 patients (of which 12.7% were hospitalized) were included in the study. The most common symptoms were fatigue, breathlessness, and joint/muscle pain. Cognitive and behavioral features that may contribute to the maintenance of physical symptoms were identified in 75.5% of patients after clinical evaluation and were considered as positive arguments in favor of a diagnosis of functional somatic disorder. Among these patients, 95.6% did not present any abnormal clinical findings or test results that could potentially explain the symptoms, and a diagnosis of functional somatic disorder was established for 72.2% of the patients after the multidisciplinary assessment. Patients with a diagnosis of functional somatic disorder had similar rates of major depression (32.8%) and anxiety disorders (25.0%) than in the whole sample, with no significant difference compared to those without (χ2 = 0.24, p = 0.63 and χ2 = 2.22, p = 0.14). |
| Pain after COVID-19 | Bakilan et al. [121] | A retrospective cross-sectional study aiming to evaluate frequency of musculoskeletal problems in post-acute COVID-19 patients. The study used medical records of LC adult patients who were admitted to the physical medicine and rehabilitation outpatient clinic in Tukey between December 2020 and May and who reported musculoskeletal symptoms. | 280 LC patients were included in the study (65% women, mean age 47.45±13.92, 70% not hospitalized). At admission to the outpatient clinic the frequency of symptoms of widespread myalgia was 3.9%, back pain 28.6%, and fatigue was 12.1%. Muscle pain in more than one site that was initiated or aggravated with COVID-19 was 51.1%. |
| Somatic symptoms in LC | Kachaner et al. [182] | A single-centre observational study from France that assessed the diagnosis of somatic symptom disorder (SSD) in patients with unexplained long-lasting neurological symptoms after mild COVID-19. Consecutive patients referred to a neurologist for post-COVID-19 consultation were reviewed. Main outcome was positive diagnosis of SSD according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders- 5 (DSM- 5). Brain MRI findings were extracted from patient records | 32 of 50 patients (64%) met the DSM-5 criteria for SSD. In the remaining 36%, SSD was considered possible given the high scores on diagnostic scales. Physical examinations were normal for all patients. Brain MRI showed unspecific minor white matter hyperintensities in 17% (8/46) of patients, considered non-specific findings, consistent with prevalence in the general population of that age range. Neuropsychological assessment (in 15 patients) showed exclusively mild impairment of attention in 93% (14/15), in discrepancy with their major subjective complaint. A high proportion of patients (90%, n=45/50) met criteria for chronic fatigue syndrome. A significant number of patients screened positive for mood-anxiety disorders (32%, n=17/50), had a history of prior SSD (38%, n=19/50), and reported past trauma (54%, n=27/50). Self-survey results highlighted post-traumatic stress disorder in 28% (12/43), high levels of alexithymia traits (42%, n=18/43), and high levels of self-oriented perfectionism (79%, n=33/42) |
| Chronic fatigue syndrome | Das et al. [61] | A study that investigated genetic risk factors associated with ME/CFS using combinatorial analysis on genotype data from 2,382 ME/CFS patients reporting a diagnosis in the UK Biobank Pain Questionnaire, matching them against 4,764 controls. | The study stratifies ME/CFS patients genetically and correlates this stratification with clinical criteria. Biological analysis of identified genes reveals links to key cellular mechanisms hypothesized to underpin ME/CFS, such as vulnerabilities to stress and infection, mitochondrial dysfunction, sleep disturbance, and autoimmune development. |
| Fibromyalgia after COVID-19 | Akel et al. [132] | A 2022 web-based cross-sectional study “to investigate the prevalence and predictors” (associated factors) of fibromyalgia in individuals recuperating from COVID-19, based on the study of Ursini et al. (2021). The ACR survey criteria were used with a cutoff score ≥ 13. | Out of 404 respondents (75% women, mean BMI 26.6, mean duration of COVID-19 infection was 12.8 ± 5.3 days) 89% were treated at home, while only six (1.5%) patients needed a ward admission and one (0.2%) an intensive care unit admission. 80 individuals (19.8%) satisfied the ACR survey criteria for fibromyalgia (out of them 93.8% were women). Females (OR: 6.557, 95% CI: 2.376 - 18.093, p = 0.001) and dyspnea (OR: 1.980, 95% CI: 1.146 - 3.420, p = 0.014) were associated with post-COVID-19 fibromyalgia. The fibromyalgia group had more pre-existing comorbidities. In bivariate correlation analysis age (r = 0.200, p = 0.001) and duration of COVID-19 infection (r = 0.121, p = 0.015) were said to be weakly correlated with fibromyalgia symptom score. |
| Fibromyalgia after COVID-19 | Bileviciute-Ljungar et al. [16] | A Swedish web-based survey combined with face-to-face interviews, using several questionnaires inquiring into mood, pain, fibromyalgia criteria, functional status, and quality of life. The study included adults who had COVID-19 according to anamnesis or a positive test, and self-reported a significantly reduced level of functioning and persistent symptoms for more than 12 weeks. | A total of 100 individuals (82% female), at a mean of 47 weeks after SARS-CoV-2 infection, 90% were not hospitalized for COVID-19. Irritable stomach, pain in varied sites, and widespread pain were reported by 75%, 15%, and 50%, respectively (30 out of the 50 with widespread pain were healthy prior to their infection) with a mean pain intensity of 5.16 in the latter. 40 out of 100 fulfilled fibromyalgia criteria, of them 22 indicated being healthy before their infection. Previous comorbidities were found to be associated with generalized pain and with fibromyalgia. Health-related quality of life was decreased in more than 80 percent of individuals, not surprising considering the study’s inclusion criteria. |
| Fibromyalgia after COVID-19 | Ganesh et al. [52] | Descriptive paper of 108 patients seen at a Post-COVID clinic at Mayo Clinic during 2021. Clinical symptoms were analyzed and assigned to one of six phenotypes: dyspnea, chest pain, myalgia, orthostasis, fatigue, and headache predominant. Patients with no evidence of tissue damage on testing were determined as likely to have a central sensitization phenotype, which was treated with a virtual treatment program aimed at patient education with elements of cognitive-behavioral therapy, health coaching, and paced rehabilitation. The fatigue-predominant, myalgia-predominant, and orthostasis-predominant phenotypes were considered together as central sensitization phenotypes. | 108 fibromyalgia-type patients were seen (75% female, median age of 46 years, 16% were admitted for acute COVID-19). Patients were evaluated on average 148.5 days after the onset of symptoms (interquartile range, 111.5 to 179.3 days). The most common comorbidities were obesity (39%), anxiety (33%), depression (28%), and gastrointestinal disease (25%), while only 5% had irritable bowel syndrome. At the time of evaluation, the most common symptoms were fatigue, shortness of breath, brain fog, anxiety, and unrefreshing sleep. Patients were classified into six phenotypes: fatigue predominant (n=69), dyspnea predominant (n=23), myalgia predominant (n=6), orthostasis predominant (n=6), chest pain predominant (n=3), and headache predominant (n=1), with more women being predominant for fatigue, orthostasis, and chest pain. The “central sensitization” phenotype (n=82) had statistically significantly higher IL-6 levels (P=.01), and higher proportion of women (82%) compared to other phenotypes of post-covid symptoms (54%; P<.0001). Age was not significantly different. |
| Fibromyalgia after COVID-19 | Jennifer et al. [129] | A 2022 population based retrospective cohort study using data from electronic healthcare database and a COVID registry, during March 2020 and May 2021, investigating incidence rates of several medical conditions following COVID-19 (including deep vein thrombosis, lung disease, fibromyalgia, diabetes, cerebrovascular accident, myocardial infarction, ischemic heart disease, acute kidney disease, hypertension, use of antidepressants/anxiolytics as an indication of depression/anxiety, and use of benzodiazepines as an indication of sleep disturbance). Records for out-patient and community-based physician or other health profession visits were used. Diagnosis of fibromyalgia was based on hospital and community-based physician visit diagnoses. | Slightly higher crude incidence rates were found for depression/anxiety, sleep disturbance, fibromyalgia (0.28% new cases compared to 0.24% in controls p = 0.034 in non-hospitalized cases), deep vein thrombosis, lung disease, and diabetes among convalescent persons after COVID-19. |
| Fibromyalgia after COVID-19 | Martin et al. [125] | Patients recruited from a post-COVID-19 infection clinic were assessed at 6 months using the widespread pain index (WPI), symptom severity scale (SSS), 10 point visual analogue scale for fatigue severity (VAS-F) and a 9-item, 7-point fatigue severity scale. (congress abstract) | At six months following infection, five patients out of 25 recruited in total met criteria for fibromyalgia based on the WPI and SSS. Female patients and patients younger than 60-years-old had higher scores. |
| Fibromyalgia after COVID-19 | Miladi et al. [131] | A web-based cross sectional survey during February 2022 to estimate the prevalence of fibromyalgia in patients who recovered from COVID-19 and to identify associated factors. ACR Survey Criteria and the Fibromyalgia Rapid screening Tool (FIRST) questionnaire were used. (meeting abstract) |
A total of 150 respondents (66% women) at an average of 6 ± 3 months after the COVID-19 diagnosis, majority in the age group of 21-30, 31% of responders had comorbidities, median BMI was 24.7. Median COVID-19 duration was 7 days with 0,7% of patients requiring hospital admission. ~19% screened positive with the FIRST questionnaire for fibromyalgia. Seven of the 29 subjects with fibromyalgia had seen a physician after the occurrence of widespread pain. Post-COVID-19 fibromyalgia was significantly associated with females (p = 0.003), comorbidities (p = 0.01) and obesity (0.03). |
| Fibromyalgia after COVID-19 | Scherlinger et al. [118] | A prospective observational study from France that aimed to describe the clinical and biologic characteristics of post-acute COVID-19 syndrome. Consecutive patients seeking medical help for persistent symptoms self-attributed to COVID-19 during the first wave (February to April 2020) of the pandemic underwent a multimodal evaluation. Results were compared to convalescent COVID-19 individuals without persistent symptoms. The study also aimed to investigate the potential underlying mechanisms, including autoimmunity and psychological distress. | 30 patients (60% women) were included in total (7 visited the emergency department, 1 was hospitalized for COVID-19). Patients were clinically evaluated after a median of 152 days following the reported onset of initial symptoms (symptom persistence was median of 6 months duration). Seventeen (56.7%) reported a resolution of initial symptoms after a median of 21 days (IQR 15–33), followed by a resurgence at a median of 21 days later (IQR 15–44). Persistent symptoms had a cyclical pattern in 28 (93.3%) patients and were mostly represented by fatigue, myalgia and thoracic oppression Fatigue was severe for most patients and rated at a median of 7 (IQR 5–8) on a 10-point scale, with pain rated at 5 (IQR 2–6). The DN4 questionnaire screening neuropathic pain was positive (≥ 4/10) for 50% (15/30) of patients, and the FiRST questionnaire screening for fibromyalgia-like symptoms was positive (≥ 5/6) for 56.7%. For most clinical features there was no significant difference between immunized and non-immunized individuals. A clinical examination, including neurologic examination, was unremarkable. Nasopharyngeal and stool samples for SARS-CoV-2 RT-PCR tests were negative. Routine biologic test results were within normal limits for all but one patient (iron-deficiency anemia). Screening for autoimmunity revealed low (1/160) and medium (1/320 to 1/640) titers of anti-nuclear antibodies in 12 and 3 patients, respectively. Low to medium anti-nuclear antibody titers were numerically more prevalent in SARS-CoV-2 immunized than non-immunized patients (66.7% vs. 33.3%, p = 0.067). 10% (3/30) and 26.7% (8/30) of patients had a previous history of depression and anxiety disorders, respectively. HADS screening for anxiety and depression was positive for 11 (36.7%) and 13 (43.3%) of patients, respectively. Only half of post-acute COVID-19 syndrome patients had cellular and humoral immunity for SARS-CoV-2. |
| Fibromyalgia after COVID-19 | Shani et al. [134] | A retrospective cohort study that investigated the associations between the BNT162b2 vaccine, infection with coronavirus, and the incidence of new registered diagnosis of autoimmune disease within ~1 year follow-up using electronic medical records of a large healthcare database. Vaccinated and unvaccinated individuals were compared as a cohort, and infected and uninfected as another cohort. The minimum follow-up time was four months, during the first phase of the pandemic. Included were individuals 12 years of age and above. Findings were reported in hazard ratios (HR) and incidence rates per 100,00 person years. Statistical analysis included incidence rate ratio tests (univariate), and multivariate Cox proportional hazards models with time-dependent exposure status. Correction for multiple comparisons was applied using the False Discovery Rate (FDR) method to account for the investigation of multiple clinical outcomes. | More than 3 million were included with considerable differences between group characteristics. Vaccination did not influence rates of new registered diagnosis of fibromyalgia in any age group within the timeframe of the study. Infection with COVID-19 increased the risk for fibromyalgia (HR=1.72 95 % CI: 1.36–2.19 in individuals aged 18–44, HR = 1.71, 95 % CI: 1.31–2.22 in individuals aged 45–64) and hypothyroidism (in individuals aged 65 or older). The authors note that the process of reaching diagnoses in the primary care setting in many circumstances is not immediate, therefore, the results should be interpreted with caution. |
| Fibromyalgia after COVID-19 | Sørensen et al. [65] | A nation-wide cross-sectional survey collecting data on self-reported symptoms using web-based questionnaires in Denmark via national e-Boks system (with access to 92% of all residents aged 15 years and above). Cases were chosen based on RT-PCR positive tests that were recorded in the national COVID-19 tracking system. Data were collected from August 1, 2021 to December 11, 2021. Questionnaires evaluated post-COVID symptoms and register-based information supplemented data on risk differences in new onset diagnoses of anxiety, chronic fatigue syndrome, depression, fibromyalgia and post-traumatic stress disorder (PTSD) confirmed by a medical doctor since the test (onset between time of testing and questionnaire completion). | 153,412 individuals fully completed the questionnaire, 61,002 participants tested positive for sars-cov-2. There were significant differences in population characteristics between the study groups (in age, sex, physical activity, and more). Based on the statistical analysis of the findings, at least one diagnosis of depression, anxiety, chronic fatigue symptom (CFS), fibromyalgia, or post-traumatic stress disorder (PTSD) of new onset within the first 6, 9, or 12 months after the test was reported by 7.2% of individuals with a positive COVID test, compared to 3.3% of negatives. Risk for fibromyalgia was not found to be significantly different between the groups, amounting at 1% in COVID positive compared to 1.1% in COVID negatives (risk difference 0.02 95% CI: -0.09-0.14). |
| Fibromyalgia after COVID-19 | Ursini et al. [10] | A cross-sectional online survey via social network among Italian speaking adult individuals (≥18 years) who developed COVID-19 three or more months prior to the study, with the objective of estimating fibromyalgia prevalence after COVID-19. The Fibromyalgia Symptom Scale, based on the ACR 2016 criteria, was used to identify fibromyalgia by a cutoff score of 13. | 616 eligible individuals completed the survey, (77.4% women). In total, 30.7% fulfilled criteria appropriate for classifying fibromyalgia, 6 months on average after contracting COVID-19. Fibromyalgia was associated with a more severe acute infection, obesity, and with males. |
| Fibromyalgia and LC | Calvache-Mateo et al. [104] | A cross-sectional study from Spain aiming to assess clinical and psychological variables among non-hospitalized adult patients with LC, compared to recovered patients after COVID-19 and healthy controls. Outcomes were assessed using questionnaires such as the brief pain inventory, CSI, insomnia severity index, Tampa Scale for Kinesiophobia, Pain Catastrophizing Scale, Depression, Anxiety and Stress Scale, and Fear Avoidance Beliefs Questionnaire, and analyzed using chi-squared and ANOVA tests to identify group differences. | 170 participants in total (healthy control group n = 58, successfully recovered group n = 57, and LC group n = 55, mean age ~45 years for all groups). Mean CSI (indicating fibromyalgia-type features with a conventional cutoff score of 40) for the LC group was 54.53 ± 17.10 at 105 ± 12 weeks since infection on average, which was significantly higher compared to recovered patients (18.81 ± 16.24) and healthy controls (17.69 ± 14.30). Brief pain inventory and insomnia severity index were also significantly higher in the LC group, as well as rates of pharmacological treatment. Scores for the pain catastrophizing scale components relating to helplessness were 9.93 ± 5.70 compared to 2.16 ± 3.09 in the COVID-19 recovered group. No statistically significant differences were found between the healthy control group and the successfully recovered COVID-19 patients in any variable. |
| Fibromyalgia and LC | Hackshaw et al. [135] | A pilot study aiming to develop a metabolic fingerprint approach for diagnosing clinically similar LC and fibromyalgia using Fourier-transform mid-infrared spectroscopic techniques for analysis. Fifty fibromyalgia and 50 LC patients were recruited. Spectral data were split into two sets randomly to train and externally-validate a predictive algorithm for making diagnosis. The relative percentage area of each IR band in the region of 1500 to 1700 cm−1 was taken. Chemometric analysis was then done to analyze the spectral data. | 50 subjects in the LC group (64% female) and 50 in the fibromyalgia group (100% female), and 6 controls. The deconvolution analysis of spectral data identified a unique spectral band at 1565 cm−1, linked to glutamate which was present only in fibromyalgia patients. The Orthogonal Signal Correction Partial Least Squares Discriminant Analysis classified spectra with high accuracy and specificity in the subgroup of external validation. Differences in the population demographic characteristics and medication history potentially introduced confounding factors. |
| Fibromyalgia and LC | Haider et al. [128] | A cross-sectional study to characterize pain, fatigue, and function in individuals reporting post-COVID-19 and compare the clinical phenotype to those with fibromyalgia and ME/CFS. Included were adult individuals that self-reported a physician diagnosis of LC, fibromyalgia, and/or CFS, and were given self-report outcome measures to assess fatigue, dyspnea, pain, quality of sleep, catastrophizing, kinesiophobia, depression, anxiety, cognitive function, and physical function. The study’s survey was distributed by e-mail and research websites targeting patients, faculty, and staff through the University of Iowa. | 707 respondents included in the final analysis: 203 had LC, 99 fibromyalgia, and 87 ME/CFS, while the rest had more than one of these diagnoses in combination. Individuals with post-COVID-19 reported mild to moderate fibromyalgia symptom severity, with 8.4% scoring 13 or higher on the fibromyalgia severity score. The fibromyalgia severity score was significantly lower in the post-COVID-19 group when compared to all groups with fibromyalgia and was similar to those with ME/CFS. Individuals with post-COVID-19 reported lower multisensory sensitivity compared to fibromyalgia and fibromyalgia+ME/CFS (p<.001). Overall LC respondents reported multiple symptoms that overlap with fibromyalgia and ME/CFS, but with less severe fatigue compared to ME/CFS and less severe pain compared to fibromyalgia. |
| Fibromyalgia and LC | Hyland et al. [64] | A study that conducted a symptom network analysis of LC, ME/CFS irritable bowel syndrome (IBS), fibromyalgia, severe asthma, and a healthy control group to provide insight into the etiology of medically unexplained symptoms. Participants completed a 65-item questionnaire assessing psychological and somatic symptoms, and a network analysis was conducted taking the 22 symptoms that best discriminated between the six groups. Connectivity, fragmentation, and the number of symptom clusters were then assessed to determine relationships between symptoms and underlying causes. | Among 2,164 subjects, the symptom networks of LC and ME/CFS differed. When compared to LC, there was significantly lower connectivity, greater fragmentation and more symptom clusters in ME/CFS, IBS, and fibromyalgia. Although the symptom networks of LC and ME/CFS differed, the variation of cluster content across the groups was inconsistent with a modular causal structure but rather consistent with a connectionist biological basis of medically unexplained symptoms. |
| Fibromyalgia and LC | Nuguri et al. [136] | A follow-up study of the 2023 Hackshaw et al. [135] study to differentiate fibromyalgia from LC based on a metabolic signature. Venous blood samples were collected from LC and fibromyalgia patients using both dried bloodspot cards and volumetric absorptive micro-sampling tips. Data were acquired using surface-enhanced Raman spectroscopy (SERS). | Amide groups, aromatic and acidic amino acids patterns could help discriminate between fibromyalgia and LC. The study demonstrates the potential of SERS in identifying unique metabolites that can be used as spectral biomarkers to differentiate fibromyalgia from “LC”. |
| Fibromyalgia and LC | Saito et al. [81] | A study of immunological dysregulation, chronic inflammation, and impaired erythropoiesis in LC individuals compared to recovered and healthy individuals, that used two independent cohorts of LC patients. Cohort 1 was from a previous study, while participants in cohort 2 were chosen if they had LC with ME/CFS symptoms. Clinical evaluation involved serial assessments using questionnaires such as the De Paul Symptom Questionnaire (DSQ), FACIT Fatigue scale, and Cognitive Failure Questionnaire (CFQ) Fibromyalgia (FM) diagnosis was based on ACR criteria, using the Widespread Pain Index (WPI) and Symptom Severity (SS) scale. The study used peripheral blood mononuclear cells (PBMCs) isolated for flow cytometry analysis, clinical tests (CBC, CRP, autoantibodies), cytokine and chemokine multiplex analysis, and ELISA assays. Statistical analysis included Wilks-Shapiro test, Mann-Whitney U test, and Kruskal–Wallis ANOVA | The LC and recovered (R) groups were well-matched in age. Hospitalization rates during acute phase were 22.6% LC vs.16.6% R in cohort 1 and 11.8% LC vs. 11.7 % R in cohort 2. Comorbidities in cohort 1 were 15.9% and 16.6 % for LC and R, respectively. In cohort 2, co-morbidities were 8.8% for LC and 5.8% for R. The odds of females having LC were 3 times higher than males in both cohorts. The study noted that the majority of patients with LC suffered from comorbid fibromyalgia (72.7% in cohort 1 and 67.6% in cohort 2) and cognitive dysfunction. The LC group showed a relative increase in absolute neutrophils and monocytes but a decrease in lymphocyte counts. There was a significant reduction in the absolute number of naïve T cells in the LC cohorts, indicating selective T cell exhaustion with reduced naïve but increased terminal effector T cells. Pro-inflammatory cytokines/chemokines were significantly elevated in both LC cohorts. LC was associated with elevated levels of plasma pro-inflammatory cytokines, chemokines, Galectin-9 (Gal-9), and artemin (ARTN). The presence of autoantibodies was found in 54.5% of the first LC cohort and approximately 55.8% of the second LC cohort. Multiple regression model revealed an increase in CD4TE, ARTN, CEC, Gal-9, CD8TE, and MCP1, and a decrease in TGF-β1 and MAIT cells that distinguished LC from the recovered group. |
| Fibromyalgia and LC | Zhang et al. [77] | Using microarray data of blood transcriptome from 75 fibromyalgia patients (GSE67311 dataset) and 29 covid-19 patients (GSE177477 dataset), authors sought to identify differential expression among the groups and potential drug targets. Machine learning was used for identifying key diagnostic genes for COVID-related fibromyalgia (LASSO algorithm and random forest in packages of R software) | Pathways of neuroactive ligand receptor interaction, ECM receptor interaction, and calcium signalling were significantly activated in the fibromyalgia group. The authors of the study concluded that these findings provide new evidence for the central sensitization hypothesis. Further analysis linked the differentially expressed genes to multiple various signalling pathways. Some commonality in differentially expressed genes between fibromyalgia and covid-19 was found. A diagnostic nomogram was developed (area under the curve of the receiver operating characteristic was 0.746) but was not validated on a new external sample of patients. |
| Fibromyalgia in rheumatoid arthritis during pandemic | Foti et al. [122,183] | A population of Italian patients diagnosed with pre-existing rheumatological disease (rheumatoid arthritis (RA) or psoriatic arthritis) was screened for fibromyalgia via The Fibromyalgia Rapid Screening Tool questionnaire and assessed for pain, depression, anxiety, and disease impact using questionnaires, during the lockdown period of the COVID-19 pandemic via telemedicine. | High rates of fibromyalgia (21.1% in RA and 24% psoriatic arthritis) were found. Patients with a fibromyalgia positive screening had a higher median RA impact of disease. These figures are similar to those known in literature regarding fibromyalgia’s increased prevalence in RA populations. |
| Fibromyalgia in rheumatoid arthritis patients during pandemic | Upadhyaya et al. [130] | A cross-sectional study aimed to evaluate the rates of depression, anxiety, and fibromyalgia using questionnaires, among a group of RA adult patients in New Delhi, India, between June 2020 and June 2021. Fibromyalgia was assessed using the Polysymptomatic Distress Scale (range 0–31) (as the sum of the WPI and SSS). | 200 patients were included. Comorbid fibromyalgia with RA was associated with more disease activity, less remission, more functional disability, and poorer quality of life. Although it is known that fibromyalgia is more common in populations with rheumatological diseases, the study found that 31% of RA patients had fibromyalgia compared to 4% of the control group, which is higher than the 15-21% prevalence reported in pre-pandemic literature for RA [130]. |
| LC in rheumatoid arthritis patients | Michaud et al. [120] | A study of RA patients with physician-diagnosed RA and self-reported COVID infections evaluated at 6-month intervals. Database used for medical and demographic data. Questionnaires were used to assess depression, anxiety, fibromyalgia, fatigue, pain, and sleep problems. (Congress abstract) | LC in the RA population was found to be associated with severe acute COVID-19, use of antibiotics, more severe RA disease, fibromyalgia prior to infection, other comorbidities, and hospitalization for COVID-19. Pre-existing fibromyalgia was not found to be statistically significant in the multivariate regression model while age, number of infections, pain, and depression were significantly associated with LC. |
| Vaccination | Di Stefano et al. [167] | Due to reports that vaccination might trigger harmful effects on the somatosensory nervous system, the authors investigated the relationship between adverse effects of coronavirus vaccination, quantitative sensory testing (QST), autonomic symptoms, and small fiber pathology on skin biopsy. They recruited adult female patients between January and June of 2022 that experienced generalized sensory symptoms and pain as long-term complications after COVID-19 vaccination (for more than 6 months). Skin biopsy was taken from the distal leg to calculate intraepidermal nerve fibre density according to the European Federation of Neurological Societies and Peripheral Nerve Society guidelines. | 15 female individuals (mean age 48.5 years), most of them had received mRNA vaccine and did not have a previous diagnosis of chronic pain or fibromyalgia, or concomitant peripheral or central nervous system diseases, experienced generalized sensory symptoms and pain and fibromyalgia-type features after vaccination. eleven met diagnostic criteria for fibromyalgia. Orthostatic intolerance was found in the majority. Nerve conduction studies were unremarkable, and most participants had normal QST. They were also found to have a normal skin biopsy. |
3.4. Central and Peripheral Nervous System Abnormalities
3.5. Generalized Joint Hypermobility
3.6. Studies on Interventions
3.7. Reviews (Systematic Reviews, Meta-Analyses, and Narrative Reviews)
- Fowler-Davis et al. (2021) conducted a systematic review of studies of interventions for post-viral fatigue [59]. They found a range of treatment modalities that have been studied so far but conclude that more research involving heterogenous populations is needed to properly assess their effectiveness in the context of post-viral fatigue syndromes.
- Cohen and colleagues (2022) published a comprehensive review on the relationship between chronic pain and infections, elaborating on mechanisms that could be relevant to LC-associated pain [92].
- Rao et al. (2022) conducted a systematic review and meta-analysis (41 studies, 9,362 patients in total) to evaluate the prevalence and prognosis of post-COVID-19 fatigue [60]. They found that fatigue prevalence was 44.9% (95% CI 0.329 - 0.575, I2 = 70.57%) within the first 3 months post-recovery according to a small number of relevant studies, but substantial differences existed among studies. Female patients, inpatient setting, and individuals recruited through social media and in Europe had a higher prevalence of fatigue.
- A systematic review and meta-analysis by Kerzhner et al. (2024) [124] sought to evaluate rates of LC’s persistent pain manifestations, as well as the impairment to health-related quality of life and data on laboratory inflammatory markers in LC. In their analysis, a substantial level of heterogeneity was found and funnel plots demonstrated considerable asymmetry. The pooled proportion of individuals experiencing general body pain symptoms up to one year after COVID-19 acute phase resolution was found to be higher in the nonhospitalized compared to hospitalized individuals (0.306 vs. 0.089, respectively, I2 = 95%, p(subgroup) = 0.009). They also discuss the increased associations related to young age, females, and less severe acute COVID-19, as well as a progressive temporal-proportional trend instead of the usual subsiding nature of most other symptoms [124]. On that note, Ebbesen et al. witnessed a similar trend in their findings from a nationwide cross-sectional study [105].
- A systematic review and meta-analysis by Hwang et al. (2023) [62] appraised viral infections as an etiology of ME/CFS.
3.8. Findings from Preprints and the Narrative Literature Search of Subtopics
4. Summary and Conclusions
- (1)
-
Etiopathogenesis and theoretical framework: unraveling the underlying pathobiology and disease mechanisms. These crucial knowledge gaps revolve around the fundamental mechanisms that drive fibromyalgia-type clinical features, the role of viral triggering, contribution of dysregulated immune pathways, genetic and epigenetic predisposition, environmental and lifestyle factors, neural mechanisms and their temporal dynamics, and the supposed role of emotional stress. Also, the current evidence concerning the impact of hospitalization history on the incidence of post-COVID fibromyalgia is inconclusive. This ambiguity is likely attributable to methodological inconsistencies, disparate definitions of fibromyalgia and LC, and variations in the selection criteria of study populations.A comprehensive theoretical framework for fibromyalgia (and LC) is needed that goes beyond explanations focused solely on pain and hyperalgesia or fatigue. It should enable robust theory-based predictions and potentially lead to the development of disease-modifying treatments. Common symptoms that are documented as associated with LC include fatigue, low mood, shortness of breath, persistent cough, autonomic symptoms such as postural orthostatic intolerance, cognitive dysfunction, brain fog, sleep difficulties, low grade fever, and joint pain [212,226,252,253,254]. Additional multiorgan manifestations as described in literature are myalgia, headache, chest pain or chest tightness, poor appetite, sicca, diarrhea, dizziness, sweating, alopecia, insomnia, restless legs, nightmares, and lucid dreams [252,255,256,257]. An online survey conducted across multiple countries found that approximately 85% of respondents with persistent illness reported relapses, primarily due to exercise, physical or mental activity, and stress [252].Besides chronic pain, other manifestations of fibromyalgia include easy bruising [27,258], urinary urgency [259], functional gastrointestinal disturbances, sleep disturbances, autonomic symptoms, wheezing, brain fog [27], a reportedly distinct brain pattern on functional magnetic resonance imaging [28,139,260], tingling, creeping or crawling sensations [259], reduced skin innervation [261], close association with gastroesophageal reflux disease [262], various autoantibodies among subgroups of patients [263,264], dry mouth, dry eyes, blurred vision, restless legs, multiple chemical sensitivity, fluid retention, and more [27,44,265]. In a 2023 meta-analysis that included 188,751 patients, an increased standardized mortality (SMR) ratio in fibromyalgia was found for mortality from infections (SMR 1.66, 95% CI 1.15 to 2.38), accidents (SMR 1.95, 95% CI 0.97 to 3.92), and suicide (SMR 3.37, 95% CI 1.52 to 7.50) [266].
- (2)
- Diagnosis and assessment: bridging the gap to objective measures. This includes the need for biomarkers, the standardization of diagnostic criteria, phenotyping, correlation between objective findings and symptom severity, and addressing symptomatic overlap of the related syndromes.
- (3)
- Phenotyping: can help clarify the varied underlying biological mechanisms and facilitate the development of subtype-specific therapies.
- (4)
- Patient experience and coping. This includes, among other things, the consequences of clinician dismissal of symptoms [8], factors associated with late diagnosis, addressing the problem of medical stigma, and factors associated with over/under-diagnosis.
- (5)
- Treatment/rehabilitation and interventions: moving towards effective strategies for treatment, including treatments for addressing specific symptoms (neurological, fatigue, musculoskeletal pain, mood, etc.), non-pharmacological treatments, personalized medicine approaches, understanding mechanisms of treatments and how they relate to the pathophysiology, integration of digital therapeutics, and striving for more patient education. Future research into LC interventions shouldn’t neglect the role of a rehabilitative approach for treating LC and fibromyalgia.
- (6)
- Prevention: Need for better knowledge on evidence-based prevention strategies besides the obvious effort of avoiding infection.
- (7)
- Prognosis: Need for better knowledge regarding fibromyalgia and post-COVID fibromyalgia-type syndrome prognosis.
- (8)
- Methodological synchronization and harmonization in the field. As an evolving relatively amorphous field of research there is heterogeneity and inconsistency in fundamental aspects such as definitions, methods, and instruments used. Also, there are still limited systematic reviews, and there is a need for longitudinal studies.
- (9)
- Bridging basic science and clinical research.
- (10)
- Another significant knowledge gap concerns the extent to which clinicians are equipped with contemporary evidence-based knowledge regarding the evolving understanding of LC.
5. Recommendations for Future Research
-
First, accurate assessment of post-COVID symptom trajectories necessitates future research that stratifies analyses by acute phase severity and hospitalization status. An analysis of all patients without making a distinction between severities of COVID-19 can add confounding factors related to hospitalization, antibiotic use, intensive care admission, and cases with well-defined organ damage, which could make it difficult to draw meaning from their results in terms of “LC.” The number of infection episodes, immunization status, behaviour and environmental factors during the initial recovery from the acute phase, and variant type, are also variables that could potentially be relevant to further investigate in the future. During the review process, patient surveys were found that did not corroborate the presence of the outcome being measured prior to acute covid-19, which makes it difficult to infer anything about new-onset or worsening of symptoms, or other self-reported measures.Also, hypermobility syndrome appears to be another confounding factor that should be taken into account in future epidemiological studies of LC, as this seems to be an important variable for the phenomenon. It is important to emphasize that undiagnosed fibromyalgia and/or GJH may contribute to the development of LC but are frequently overlooked in the clinical setting. This can add confounding to studies that make use of official diagnostic codes and criteria for fibromyalgia. Due to the high cut-off set by the ACR criteria, the absence of fibromyalgia diagnosis, taken as an indicator for absence of fibromyalgia syndrome, may not suffice for choosing controls. For example, an individual with chronic widespread pain and somatic symptom severity score of 4 (that is, not eligible for official fibromyalgia diagnosis) in the control group could confound the results, as the cut-off chosen for fibromyalgia diagnosis by the ACR seems to be biologically arbitrary.
- Secondly, in studies using diagnostic criteria or diagnostic codes that distinguish functional psychosomatic syndromes, the investigator should recognize that making a distinction between chronic widespread pain and fibromyalgia diagnosis (and even ME/CFS), or ignoring their overlap, may confound results if the mechanism is shared, as has been suggested by some authors [43,64]. Moreover, researchers conducting a correlation analysis between variables or outcome measures that represent overlapping constructs such as stress and fibromyalgia diagnosis or chronic widespread pain and fibromyalgia-type symptoms, or CSI score and depression [108,120,243,267], will end up with results that seem redundant unless that is what the study was designed to do.
- Third, it’s worth noting that studies that recognize central sensitization as a phenomenon simply based on hypersensitivity in the palmar side of the participants’ dominant hand, for example [112], do not necessarily relate to a mechanism of nociplastic generalized central pain augmentation and sensory hypersensitivity. If the authors of a study conclude that generalized central hypersensitivity and allodynia were found, then they might like to demonstrate that it is, indeed, both central and generalized. A methodological issue [29]was evident in literature in relation to the “central sensitization inventory” questionnaire, which tries to capture the impact of chronic pain conditions such as fibromyalgia [268] or “fibromyalgia-type features.” Authors have mistaken the CSI for central sensitization [111,115,117,139,184].
- A multifaceted etiology.
- Overlap between LC, chronic fatigue syndrome, related functional somatic syndromes and fibromyalgia symptomatology (including multiple medically unexplained multisystemic refractory symptoms, widespread myofascial discomfort and myofascial pain, hyperalgesia, itching, fatigue, post-exertional malaise, POT syndrome and autonomic symptoms, morning stiffness, spasms, irritable bowel, multiple chemical sensitivity, and more)
- Multisystem non-specific clinical findings (subclinical inflammation and immune dysregulation, metabolic abnormalities, low-grade hypoxia, muscle histopathological abnormalities, intraepidermal small fibre pathology, etc.)
- Unremarkable results on routine medical tests.
- The risk factors.
- Significant association with both hypermobility syndrome and low vitamin D.
- A relatively high prevalence of LC in mild and subclinical acute disease cases among previously healthy individuals.
- Insidious and heterogenous nature of the condition.
- Pain varying in anatomical location, and neuroanatomically illogical distributions.
- Other anomalies and counterinstances such as discrepancies between empirical findings and expected findings in nerve conduction studies and pressure pain threshold measurements, dissociation between measures of sensitization and subjective burden [249], low correlations between disease burden and conditioned pain modulation [269], autoantibodies and inconsistent findings regarding them [263,270], evidence of peripheral neuropathy in subgroups, disappointing and poor response to theory-based pharmacotherapies, symptomatic response to weather change [271], discordance between autonomic small fiber pathology and autonomic symptoms [272], and more [248,249,250].
6. Part Two– Synthesis of Data and Formulating a Mechanism for “Fibromyalgia Syndrome” Pathophysiology
6.1. Fascial Armouring: A Conceptual Framework for the Etiopathogenesis and Cellular Pathway of ‘Primary Fibromyalgia Syndrome’
- (i)
- Normal mechanobiology of myofibroblasts
- (ii)
- Tensegrity qualities when superimposed on the interconnectedness of the fascio-musculo-skeletal system
- (iii)
- Myofascial chains
- (iv)
- Innervation and sensory functions of fascia
- (v)
- Substrate stiffness & rigidity of ECM
6.2.“. Fascial Armoring” as a Fascio-Musculoskeletal Medical Entity of Continuum Biomechanics
6.3. Soft Tissue Myofibroblasts in the Context of COVID-19
7. Interpreting LC Manifestations and Drawing Theory-Based Predictions
7.1. Mechanistic Predictions of 'Long COVID-19' Manifestations Based on the Suggested Biomechanical Model
- Hypermobility syndrome/Ehlers-Danlos syndrome: Collagen microarchitecture affects mechanosensitive signaling in cells followed by an induction of myofibroblasts and secretion of proangiogenic factors (vascular endothelial growth factor and IL-8) when studied in human adipose-derived stem cell culture [435]. Hypermobile Ehlers-Danlos syndrome is associated with ECM disarray and increased myofibroblast phenotype when studied in vitro [436]. Hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders are probably not separate entities but rather appear to be both on a continuum characterized by altered ECM homeostasis and a chronic inflammatory state [436]. If ECM microarchitecture augments myofibroblast activity in patients with hypermobility syndrome, it is mainly for this reason that GJH is expected to increase their risk for fibromyalgia-type symptoms, a relationship that should be weakly explained statistically by psychological stress levels alone [290].
- Mechanical tension on the skin has been shown to enhance myofibroblast activity [437]. The application of mechanical forces such as by use of splints further corroborates this finding [438]. The development of myofascial pain is linked to tight-fitting clothes [290,439,440]. Tight-fitting clothes and accessories are expected to predispose individuals to fibromyalgia due to input into the integrin-mediated yes-associated protein cascade of myofibroblast mechano-activity.
- Lifestyle and exercise (movement): Though not performed on myofascial tissue in vivo, a study showed that cyclical mechanical stretch reduces myofibroblast differentiation of primary lung fibroblasts [441]. Tissue stretch reduces TGF-β1 and type-1 procollagen in mouse subcutaneous connective tissue [442]. Immobility leads to fibrosis and an increase in myofibroblasts in knee joint capsule when studied in vivo [443]. Immobility allows for the development of abnormal cross linking between connective tissue fibres [444]. These findings provide, in general, the biological rationales for the role of exercise (movement) versus sedentarism according to the suggested myofascial-based mechanism. It is interesting in this respect that yoga involves cyclical stretching of almost all body parts as an integral aspect of the practice.
- The effect of taking hot showers during the acute induction phase of myofibroblasts following infection, and the long-term effect of a possible activation of heat shock proteins in a subcutaneous population of myofibroblast requires further investigation. It is not necessarily expected to be inert.
- The effect of weather changes on symptoms will be facilitated by the biophysical effects of temperature, electromagnetics and humidity on myofascial tissue and hyaluronic acid.
- If factors such as tattoo ink or smoking induce subcutaneous myofibroblasts, sedentary people with whole-body tattoos and smokers are expected to have worse and more prolonged psychosomatic symptoms after covid-19. A similar link is expected for those using cosmetics and topical creams containing substances that upregulate pathways of subcutaneous fibroblast-to-myofibroblast differentiation.
- Diet and the gut brain axis [446] fit in the mechanism of LC from multiple angles and not only in relation to connective tissue.
- Obesity: in obesity, connective-tissue fibrosis is induced and mediated by mechano-transducing signaling pathways [447]. Fibrotic processes mediated by myofibroblasts can transform the mechanical properties of subcutaneous tissue, increasing its rigidity and connective tissue stiffness [447]. For this reason, obesity is predicted to be a significant risk factor for LC.
- Hair loss: adipocyte to myofibroblast transition is a possible cause of alopecia [448]. The connective tissue sheath and follicular papilla can use gap junctions to form a communicating network. During hair cycling, this network plays a part in the control of hair follicle dynamic structural changes [449]. Hair loss is therefore expected to occur and be weakly explained by psychological stress levels.
- Pallor: might be an overlooked manifestation, reflecting impaired peripheral perfusion due to autonomic and non-autonomic or hydrostatic causes.
- Explaining Morning stiffness: Tomasek et al. (2002) [291] describe the dynamics of fibroblast populations in three-dimensional collagen lattices and the process of generating traction and tension in their surrounding matrix of collagen fibrils. Over several hours the forces increase until a plateau is reached. If a similar process occurs in fascia in vivo, then a period of immobility would be comparable to this process of allowing the cells to reach the plateau of a higher tension state uninterrupted.
- (Fascio)Musculoskeletal: altered pendulousness of the legs. If the physician is searching for an objective mechanism-based sign of the disease to test bedside, this might be a relatively good one.
- Cardiovascular: A mild chronic compartment-like syndrome affecting multiple muscles should, by a chronic contraction of skeletal muscles, impair perfusion and lymph flow and alter starling forces which could exacerbate pre-existing subclinical cardiovascular issues. The typical presentation would, by reasoning, include changes in blood pressure regulation, fatigue on exertion or after a heavy meal, palpitations, higher resting heart rate, cold feet and palms, sub/clinical impairment of sexual function, and absence/impairment of morning erection in males, due to impaired blood flow to various organs. Chronic compressive forces in the periorbital fascia would lead to subclinical reduced optic disc perfusion. Idiopathic fluid retention might also be derived mechanistically.
- Active loci: possibly due to mechanical stress on the muscle spindles as well as sympathetic overactivity. Tonic slow adapting receptors in nuclear chain fibers of the muscle spindle would activate gamma motoneurons via the stretch reflex in prestressed myofascial tissue. Also, afferent input from gastrocnemius-soleus muscle C-fibres produces long-lasting excitability of the biceps femoris/semitendinosus α-motoneuron efferent fibers through the flexion reflex in an animal model [450]. A mechanistic discussion of myofascial pain syndrome and active loci in the context of this framework is available in a recent study [290].
- Immune system: based on a finding that substrate stiffness affects immune cell function [398]. Fibroblasts and inflammatory myofibroblasts secrete cytokines as part of their natural activity [295]. An overactive (or “irritable”) state of immune cells due to paracrine proinflammatory cytokine secretion, chronic low-grade inflammation, and increased substrate rigidity of the ECM would likely predispose the immune system to over-reacting in intolerance to “irritant” antigens. Such immune hyperirritability could be evident in the form of predisposition to gluten intolerance, multiple chemical sensitivity, association with autoinflammatory reactions, or other clinical or subclinical immune dysregulation. Fibromyalgia and LC are associated with mast cell dysregulation [451]. A mechanistic explanation, among several, can be related to findings [452] that tissue stiffness affects mast cell behavior and function.
- Metabolism: Myofibroblasts secrete IL-6, IL-8, and IL-11 [295,401]. The cytokine IL-6, besides its effect on CD4+ T lymphocytes, can activate indoleamine 2,3-dioxygenase, as shown in different cell types [84,453,454], and therefore is potentially intimately related to the metabolic balance of the tryptophan-indoleamine 2,3-dioxygenase 1-kynurenine and serotonin pathway. Metabolites of this pathway (e.g., the neurotoxic metabolite quinolinic acid) [455], some of which can cross the blood brain barrier [456], were observed in altered systemic levels in fibromyalgia [457,458], and are linked to cognitive impairment and depression [84]. Besides cytokines, the gut microbiome has the capacity to modulate indoleamine 2,3-dioxygenase 1 too, for example via butyrate production [84,459].
- Mood and psychosomatic disorders: post-traumatic stress disorder, anxiety, and depression are known manifestations of 'long COVID-19' [460]. “Post-traumatic stress disorder” in this framework (not only in the context of LC) is expected to have a bio-mechanical aspect involving the (fascio)musculoskeletal system. Any acute sympathetic or inflammatory reaction which leads to a simultaneous abrupt contraction of multiple muscles and of the osteomyofascial tensegrity structure would cause a sudden shift in its biomechanical and energetic elastic state. The energetic shift and the mechanical tension locked in the ECM by contracting cells would lead to an increase in widespread tension in the body irrespective of alpha motoneurons. Sympathetic nerve fibers embedded in fascia would also be affected, which is a relevant interface with emotion and cognition. If the musculoskeletal tension is not released after this acute event, overtime fascia and ECM will be remodeled in this higher-tension state which initially was supposed to be a temporary sympathetic defensive reaction. This is followed by myo/fibroblasts remodeling the ECM and stress shielding themselves to mask the tension while, importantly, they form “supermature” focal adhesions and upregulated expression of α-SMA. In their resolution phase, the balance of proliferative and apoptotic signals is crucial for the outcome of myofibroblast cells [296]. They can either undergo apoptosis (mediated by fibroblast growth factor 1, prostaglandin E2, and IL-1beta), evade apoptosis and persist in the tissue, or enter senescence (mediated by CCN1 with upregulated intracellular p16 and p21, and characterized by the acquisition of a senescence-associated secretory phenotype, specifically the secretion of TGF-β1 and pro-inflammatory cytokines and chemokines such as IL-6, CCL2, IL-1α, IL-1β, IL-8, PDGF, and ECM proteins), or other possible fates [296]. Myofibroblasts become much more active above a certain threshold of matrix rigidity [461]. Higher ECM pre-stress in the tensegrity-like structure crosses the threshold for myofibroblast activity and propels their cascade of mechanobiology and stress shielding, but once fascia is remodeled this way, it is much more difficult to resolve. Fibromyalgia does not typically erupt in patients overnight. The systemic implications aren't limited to myofascial tissue, and include changes in metabolism and secretory profile of myofascial cells, changes in vasculature, effects on the immune system, and more. Interestingly, circulating systemic fibroblast growth factors can deeply affect brain physiology [462]. Also, the intracranial ECM is suggested to be implicated in the pathophysiology of stress-induced depression [463].
- Overlap with “myofascial pain syndromes”: The clinical overlap of myofascial pain and associated psychosomatic and "non-specific" pain conditions (or “central sensitizations symptoms”) is likely to be evident in relation to LC. Figure 7 illustrates in general the clinical overlap reflected by the mechanistic overlap, as suggested by this conceptual framework (not all relationships are depicted in this scheme).
7.2. Predictions of Results on Investigations and Means for Testing the Hypotheses
- A preliminary non-invasive straightforward approach would be to measure muscle damping [350] which should reflect increased muscle tension. Pendulousness of the legs or arms of fibromyalgia-type LC patients compared to controls could be a relatively simple clinical test to start with, after controlling for age, sex, and body mass index. The inclusion of subjects would focus more on persistent "fibromyalgia-ness" patients who had mild acute infection, rather than dyspneic patients who had severe acute pulmonary covid-19. Shear wave elastography/strain elastography or magnetic resonance elastography can help measure the stiffness of tissue for comparing fibromyalgia-like LC patients and healthy controls. Again- focusing on those with multiple multiorgan psychosomatic complaints after mild/asymptomatic infection and excluding subjects who were hospitalized during the acute phases. The resonance of tissue and its response to internal organ oscillations might also be found to be altered.
- Sub/clinical decreased joint range of motion should be seen on careful examination when taking into consideration hypermobility syndrome. Demonstrating an inappropriately normal joint range of motion in a hypermobile individual is false-normal and pathological.
- Biopsies can be used to measure myofibroblast density, or to examine if fascial cells express elevated levels of α-SMA. But since myofibroblast can de-differentiate and leave behind a remodeled dysfunctional fascia, testing only by this method might actually be deceptive. Needle biopsy may be sufficient for this [464]. Smokers are expected to have a higher density of myofibroblasts in myofascial tissue compared to non-smokers.
- Overall, pharmacological agents that enhance myofascial fibroblast-to-myofibroblast transdifferentiating are expected to predispose to LC and fibromyalgia.
- Microdialysis of muscle, for example of the trapezius muscle [368] or vastus lateralis [340]- not all patients necessarily have increased concentrations of algesic substances and signs of anerobic metabolism in the same muscles because not all patients necessarily have the trapezius (or vastus lateralis) deeply affected. The clinical variability is derived from a mechanistic variability regarding which anatomical structures and layers are more involved. Laser doppler fluxmetry and isotope washout methods can also be used [465].
- Quality of radial pulse on palpation could be a fairly useful clinical sign for the condition.
- Severe cases might have abnormally elevated serum and urinary creatine as a result of muscle breakdown and oxidative stress, if muscle cells fail to compensate.
- Increased physiological response to the Valsalva maneuver and exertional dizziness would be characteristic of a mild-to-moderate global chronic compartment-like syndrome.
- Activation of the stretch reflex due to diffuse involvement of the muscle spindles or tendon would manifest as increased muscle tone not mediated by alpha motoneurons.
- Biophysical tests- strain elastography, atomic force microscopy, optical coherence elastography, dynamic mechanical analysis, etc., of fascial/myofascial tissue might be insightful, although these would have to take into account the complexity of the model and possible confounding factors. Age, sex, pH, temperature, hydration, hyaluronic acid composition, elastin and collagen polymorphisms, adipocytes, cell phenotype and density, are all variables that may affect the properties of fascia in vivo.
- Heterogenous clinical complaints are derived from the mechanistic variability. When the transformation of fascial ECM reinforces the cycle of myofibroblast force generation, myofascial degree of stiffness increases and muscles are then subjected to low-grade chronic ischaemia. Over time, in the absence of full muscle relaxation and due to insufficient nutrients and oxygen, muscle mass and muscle cells experiencing longstanding low-grade hypoxia will undergo long term structural, metabolic, and genetic/epigenetic level adaptations and compensations, while the immune system is continuously drawn into the process due to ongoing tissue injury. Sedentarism reinforces atrophy of skeletal muscle. Afterwards, matrix material such as collagen replaces atrophic skeletal muscle mass and at this stage fatigue and weakness become more prominent. Meanwhile, fascia can either continue in its positive feedback, or break the cycle and proceed to a stress-relaxation failure stage, where it experiences mechanical creep and has lower shear modulus. A higher myofascial Young’s modulus is expected if pain, tension, and stiffness are the main complaint, and lower fascial stiffness is expected if fatigue, weakness, and pain are the predominant complaint.
8. Conclusions
9. Limitations
References
- Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021 Jul 26;374:n1648. Erratum in: BMJ. 2021 Aug 3;374:n1944. doi: 10.1136/bmj.n1944. PMID: 34312178. [CrossRef]
- Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, Horn C, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021 Jul;6:100122. Epub 2021 May 18. PMID: 34027514; PMCID: PMC8129613. [CrossRef]
- Datta SD, Talwar A, Lee JT. A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness beyond Acute Infection and Public Health Implications. Vol. 324, JAMA - Journal of the American Medical Association. American Medical Association; 2020. p. 2251–2.
- Wang S, Li Y, Yue Y, Yuan C, Kang JH, Chavarro JE, Bhupathiraju SN, Roberts AL. Adherence to Healthy Lifestyle Prior to Infection and Risk of Post-COVID-19 Condition. JAMA Intern Med. 2023 Mar 1;183(3):232-241. PMID: 36745445; PMCID: PMC9989904. [CrossRef]
- https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-adds-funds-long-covid-19-research-advances-work-new-clinical-trials.
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch Med Res. 2021 Aug;52(6):575-581. Epub 2021 May 4. PMID: 33962805; PMCID: PMC8093949. [CrossRef]
- Greenhalgh T, Sivan M, Perlowski A, Nikolich JŽ. Long COVID: a clinical update. Lancet. 2024 Aug 17;404(10453):707-724. Epub 2024 Jul 31. PMID: 39096925. [CrossRef]
- Phillips S, Williams MA. Confronting Our Next National Health Disaster - Long-Haul Covid. N Engl J Med. 2021 Aug 12;385(7):577-579. Epub 2021 Jun 30. PMID: 34192429. [CrossRef]
- Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Vol. 27, Trends in Molecular Medicine. Elsevier Ltd; 2021. p. 895–906.
- Ursini F, Ciaffi J, Mancarella L, Lisi L, Brusi V, Cavallari C, et al. Fibromyalgia: A new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open. 2021 Aug 23;7(3).
- Yong SJ, Liu S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev Med Virol. 2022 Jul;32(4):e2315. Epub 2021 Dec 9. PMID: 34888989. [CrossRef]
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Vol. 21, Nature Reviews Microbiology. Nature Research; 2023. p. 133–46.
- Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022 May;28(5):911-923. doi: 10.1038/s41591-022-01810-6. Epub 2022 May 18. Erratum in: Nat Med. 2022 Aug;28(8):1723. PMID: 35585196. [CrossRef]
- Dotan A, David P, Arnheim D, Shoenfeld Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun Rev. 2022 May;21(5):103071. Epub 2022 Feb 16. PMID: 35182777; PMCID: PMC8848724. [CrossRef]
- Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006 Sep 16;333(7568):575. Epub 2006 Sep 1. PMID: 16950834; PMCID: PMC1569956. [CrossRef]
- Bileviciute-Ljungar I, Norrefalk JR, Borg K. Pain Burden in Post-COVID-19 Syndrome following Mild COVID-19 Infection. J Clin Med. 2022 Feb 1;11(3).
- Savin E, Rosenn G, Tsur AM, Hen O, Ehrenberg S, Gendelman O, Buskila D, Halpert G, Amital D, Amital H. The possible onset of fibromyalgia following acute COVID-19 infection. PLoS One. 2023 Feb 10;18(2):e0281593. PMID: 36763625; PMCID: PMC9916594. [CrossRef]
- Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Vol. 12, Frontiers in Microbiology. Frontiers Media S.A.; 2021.
- Patel J, Javed S. Myofascial pain syndrome and SARS-CoV-2: A case series. Pain Manag. 2022 Apr 1;12(3):255–60.
- Sarzi-Puttini P, Giorgi V, Marotto D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Vol. 16, Nature Reviews Rheumatology. Nature Research; 2020. p. 645–60.
- Martínez-Lavín M, Miguel-Álvarez A. Hypothetical framework for post-COVID 19 condition based on a fibromyalgia pathogenetic model. Clin Rheumatol. 2023 Nov;42(11):3167-3171. Epub 2023 Sep 14. PMID: 37707639. [CrossRef]
- Silverwood V, Chew-Graham CA, Raybould I, Thomas B, Peters S. “If it’s a medical issue I would have covered it by now”: learning about fibromyalgia through the hidden curriculum: a qualitative study. BMC Med Educ. 2017 Sep 12;17(1):160. PMID: 28899390; PMCID: PMC5596866. [CrossRef]
- Simms RW. Fibromyalgia is not a muscle disorder. Am J Med Sci. 1998 Jun;315(6):346-50. PMID: 9638890. [CrossRef]
- Silva-Passadouro B, Tamasauskas A, Khoja O, Casson AJ, Delis I, Brown C, Sivan M. A systematic review of quantitative EEG findings in Fibromyalgia, Chronic Fatigue Syndrome and Long COVID. Clin Neurophysiol. 2024 Jul;163:209-222. Epub 2024 May 6. PMID: 38772083. [CrossRef]
- Anita Holdcroft, Sian Jaggar. Core topics in pain. 2005. UK: cambridge university press. G. Carli & G. Biasi chapter 19: myofascial/musculoskeletal pain pp 132-135.
- D’Onghia M, Ciaffi J, Ruscitti P, Cipriani P, Giacomelli R, Ablin JN, Ursini F. The economic burden of fibromyalgia: A systematic literature review. Semin Arthritis Rheum. 2022 Oct;56:152060. Epub 2022 Jul 3. PMID: 35849890. [CrossRef]
- Firestein GS, Kelley WN. Kelley’s textbook of rheumatology. 9th ed. Philadelphia, PA: Elsevier/Saunders (2013). pp. 351, 730, 733-750.
- Clauw DJ. From fibrositis to fibromyalgia to nociplastic pain: how rheumatology helped get us here and where do we go from here? Ann Rheum Dis. 2024 Oct 21;83(11):1421-1427. PMID: 39107083; PMCID: PMC11503076. [CrossRef]
- Velasco E, Flores-Cortés M, Guerra-Armas J, Flix-Díez L, Gurdiel-Álvarez F, Donado-Bermejo A, van den Broeke EN, Pérez-Cervera L, Delicado-Miralles M. Is chronic pain caused by central sensitization? A review and critical point of view. Neurosci Biobehav Rev. 2024 Dec;167:105886. Epub 2024 Sep 13. PMID: 39278607. [CrossRef]
- Landewé RBM. Correspondence on “Long COVID: a new word for naming fibromyalgia?” by Mariette. Ann Rheum Dis. 2024 Jun 12;83(7):e15. PMID: 38171597. [CrossRef]
- Wolfe F, Walitt B. Culture, science and the changing nature of fibromyalgia. Nat Rev Rheumatol. 2013 Dec;9(12):751-5. Epub 2013 Jul 2. PMID: 23820862. [CrossRef]
- Katz RS, Leavitt F, Small AK, Small BJ. Intramuscular pressure is almost three times higher in fibromyalgia patients: A possible mechanism for understanding the muscle pain and tenderness. Journal of Rheumatology. 2021 Apr 1;48(4):598–602.
- Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, Sandor K, Vastani N, Maurer M, Cuhadar U, Sensi S, Nomura Y, Menezes J, Baharpoor A, Brieskorn L, Sandström A, Tour J, Kadetoff D, Haglund L, Kosek E, Bevan S, Svensson CI, Andersson DA. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 2021 Jul 1;131(13):e144201. PMID: 34196305; PMCID: PMC8245181. [CrossRef]
- Garrison RL, Breeding PC. A metabolic basis for fibromyalgia and its related disorders: the possible role of resistance to thyroid hormone. Med Hypotheses. 2003 Aug;61(2):182-9. PMID: 12888300. [CrossRef]
- Staud R. Is it all central sensitization? Role of peripheral tissue nociception in chronic musculoskeletal pain. Curr Rheumatol Rep. 2010 Dec;12(6):448-54. PMID: 20882373. [CrossRef]
- Goebel A, Andersson D, Shoenfeld Y. The biology of symptom-based disorders - time to act. Autoimmun Rev. 2023 Jan;22(1):103218. Epub 2022 Oct 22. PMID: 36280093. [CrossRef]
- Clauw D, Sarzi-Puttini P, Pellegrino G, Shoenfeld Y. Is fibromyalgia an autoimmune disorder?. Autoimmunity Reviews. 2024 Jan 1;23(1):103424.
- Kosek E, Clauw D, Nijs J, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. 2021;162:2629–2634. [CrossRef]
- Agarwal A, Oparin Y, Glick L, Fitzcharles MA, Adachi JD, Cooper MD, Gallo L, Wong L, Busse JW. Attitudes Toward and Management of Fibromyalgia: A National Survey of Canadian Rheumatologists and Critical Appraisal of Guidelines. J Clin Rheumatol. 2018 Aug;24(5):243-249. PMID: 29280818. [CrossRef]
- Bass C, Henderson M. Fibromyalgia: an unhelpful diagnosis for patients and doctors. BMJ. 2014 Mar 19;348:g2168. PMID: 24647170. [CrossRef]
- Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007 Jun;21(3):481-97. PMID: 17602995. [CrossRef]
- Bradley LA. Pathophysiology of fibromyalgia. Am J Med. 2009 Dec;122(12 Suppl):S22-30. PMID: 19962493; PMCID: PMC2821819. [CrossRef]
- Calabrese LH, Mease PJ. Improving the nosology of Long COVID: it is not so simple. Ann Rheum Dis. 2024 Jan 2;83(1):9-11. PMID: 37989548. [CrossRef]
- Kaplan, C.M., Kelleher, E., Irani, A. et al. Deciphering nociplastic pain: clinical features, risk factors and potential mechanisms. Nat Rev Neurol 20, 347–363 (2024). [CrossRef]
- Mascarenhas RO, Souza MB, Oliveira MX, Lacerda AC, Mendonça VA, Henschke N, Oliveira VC. Association of Therapies With Reduced Pain and Improved Quality of Life in Patients With Fibromyalgia: A Systematic Review and Meta-analysis. JAMA Intern Med. 2021 Jan 1;181(1):104-112. PMID: 33104162; PMCID: PMC7589080. [CrossRef]
- Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015 Jun 5;2015(6):CD011735. PMID: 26046493; PMCID: PMC4755337. [CrossRef]
- Gilron I, Chaparro LE, Tu D, Holden RR, Milev R, Towheed T, DuMerton-Shore D, Walker S. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016 Jul;157(7):1532-40. PMID: 26982602. [CrossRef]
- Hurt RT, Yadav S, Schroeder DR, Croghan IT, Mueller MR, Grach SL, Aakre CA, Gilman EA, Stephenson CR, Overgaard J, Collins NM, Lawson DK, Thompson AM, Natividad LT, Mohamed Elfadil O, Ganesh R. Longitudinal Progression of Patients with Long COVID Treated in a Post-COVID Clinic: A Cross-Sectional Survey. J Prim Care Community Health. 2024 Jan-Dec;15:21501319241258671. PMID: 38813984; PMCID: PMC11141226. [CrossRef]
- Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, Raza H, Okeke O, Dewar RL, Higgins BP, Tolstenko K, Kwan RW, Gittens KR, Seamon CA, McCormack G, Shaw JS, Okpali GM, Law M, Trihemasava K, Kennedy BD, Shi V, Justement JS, Buckner CM, Blazkova J, Moir S, Chun TW, Lane HC. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann Intern Med. 2022 Jul;175(7):969-979. Epub 2022 May 24. PMID: 35605238; PMCID: PMC9128805. [CrossRef]
- Kersten J, Baumhardt M, Hartveg P, Hoyo L, Hüll E, Imhof A, et al. Long COVID: Distinction between organ damage and deconditioning. J Clin Med. 2021 Sep 1;10(17).
- Goldenberg DL. Applying Lessons From Rheumatology to Better Understand Long COVID. Arthritis Care Res (Hoboken). 2024 Jan;76(1):49-56. [CrossRef]
- Ganesh R, Grach SL, Ghosh AK, Bierle DM, Salonen BR, Collins NM, Joshi AY, Boeder ND Jr, Anstine CV, Mueller MR, Wight EC, Croghan IT, Badley AD, Carter RE, Hurt RT. The Female-Predominant Persistent Immune Dysregulation of the Post-COVID Syndrome. Mayo Clin Proc. 2022 Mar;97(3):454-464. Epub 2022 Feb 5. PMID: 35135695; PMCID: PMC8817110. [CrossRef]
- Mariette X. Long COVID: a new word for naming fibromyalgia? Ann Rheum Dis. 2024 Jan 2;83(1):12-14. PMID: 37923365. [CrossRef]
- https://www.prisma-statement.org/scoping.
- Goldenberg DL. How to understand the overlap of long COVID, chronic fatigue syndrome/myalgic encephalomyelitis, fibromyalgia and irritable bowel syndromes. Semin Arthritis Rheum. 2024 Aug;67:152455. Epub 2024 May 7. PMID: 38761526. [CrossRef]
- Ostojic SM. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients. 2021 Feb 4;13(2):503. PMID: 33557013; PMCID: PMC7913646. [CrossRef]
- Deumer US, Varesi A, Floris V, Savioli G, Mantovani E, López-Carrasco P, Rosati GM, Prasad S, Ricevuti G. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J Clin Med. 2021 Oct 19;10(20):4786. PMID: 34682909; PMCID: PMC8538807. [CrossRef]
- Cardinali DP, Brown GM, Pandi-Perumal SR. Possible Application of Melatonin in Long COVID. Biomolecules. 2022 Nov 7;12(11):1646. PMID: 36358996; PMCID: PMC9687267. [CrossRef]
- Fowler-Davis S, Platts K, Thelwell M, Woodward A, Harrop D. A mixed-methods systematic review of post-viral fatigue interventions: Are there lessons for long Covid? PLoS One. 2021 Nov 9;16(11):e0259533. PMID: 34752489; PMCID: PMC8577752. [CrossRef]
- Rao S, Benzouak T, Gunpat S, Burns RJ, Tahir TA, Jolles S, Kisely S. Fatigue Symptoms Associated With COVID-19 in Convalescent or Recovered COVID-19 Patients; a Systematic Review and Meta-Analysis. Ann Behav Med. 2022 Mar 1;56(3):219-234. PMID: 34665858; PMCID: PMC8574547. [CrossRef]
- Das S, Taylor K, Kozubek J, Sardell J, Gardner S. Genetic risk factors for ME/CFS identified using combinatorial analysis. J Transl Med. 2022 Dec 14;20(1):598. PMID: 36517845; PMCID: PMC9749644. [CrossRef]
- Hwang JH, Lee JS, Oh HM, Lee EJ, Lim EJ, Son CG. Evaluation of viral infection as an etiology of ME/CFS: a systematic review and meta-analysis. J Transl Med. 2023 Oct 28;21(1):763. PMID: 37898798; PMCID: PMC10612276. [CrossRef]
- Rovigatti U. Viruses in fibromyalgia aetiology-new wisdom after the COVID-19 pandemic?. P-16 at the 4th international congress on controversies in fibromyalgia. clinical and experimental rheumatology. 2023. 41: 1351-1376.
- Hyland ME, Antonacci Y, Bacon AM. Comparison of the symptom networks of long-COVID and chronic fatigue syndrome: From modularity to connectionism. Scand J Psychol. 2024 Dec;65(6):1132-1140. Epub 2024 Jul 21. PMID: 39034480. [CrossRef]
- Sørensen AIV, Spiliopoulos L, Bager P, Nielsen NM, Hansen JV, Koch A, Meder IK, Ethelberg S, Hviid A. A nationwide questionnaire study of post-acute symptoms and health problems after SARS-CoV-2 infection in Denmark. Nat Commun. 2022 Jul 21;13(1):4213. PMID: 35864108; PMCID: PMC9302226. [CrossRef]
- Versace V, Tankisi H. Long-COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Potential neurophysiological biomarkers for these enigmatic entities. Clin Neurophysiol. 2023 Mar;147:58-59. Epub 2023 Jan 13. PMID: 36657309; PMCID: PMC9838078. [CrossRef]
- Appelman B, Charlton BT, Goulding RP, Kerkhoff TJ, Breedveld EA, Noort W, Offringa C, Bloemers FW, van Weeghel M, Schomakers BV, Coelho P, Posthuma JJ, Aronica E, Joost Wiersinga W, van Vugt M, Wüst RCI. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. 2024 Jan 4;15(1):17. PMID: 38177128; PMCID: PMC10766651. [CrossRef]
- Gouraud C, Thoreux P, Ouazana-Vedrines C, Pitron V, Betouche S, Bolloch K, Caumes E, Guemouni S, Xiang K, Lemogne C, Ranque B; CASPer-COVID Study Group. Patients with persistent symptoms after COVID-19 attending a multidisciplinary evaluation: Characteristics, medical conclusions, and satisfaction. J Psychosom Res. 2023 Nov;174:111475. Epub 2023 Aug 23. PMID: 37741114. [CrossRef]
- Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, O’Connor L, Leavy D, O’Brien K, Dowds J, Sugrue JA, Hopkins D, Martin-Loeches I, Ni Cheallaigh C, Nadarajan P, McLaughlin AM, Bourke NM, Bergin C, O’Farrelly C, Bannan C, Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020 Nov 9;15(11):e0240784. PMID: 33166287; PMCID: PMC7652254. [CrossRef]
- Arienti C, Cordani C, Lazzarini SG, Del Furia MJ, Negrini S, Kiekens C. Fatigue, post-exertional malaise and orthostatic intolerance: a map of Cochrane evidence relevant to rehabilitation for people with post COVID-19 condition. Eur J Phys Rehabil Med. 2022 Dec;58(6):857-863. Epub 2022 Dec 6. PMID: 36472558; PMCID: PMC10077961. [CrossRef]
- Ablin J. Long-COVID, chronic fatigue and everything in between - what have we learned and where may it impact on fibromyalgia. Meeting abstract IS-16. The 4th International Virtual Congress on Controversies in Fibromyalgia (2022). 40(6):1225-46. [CrossRef]
- Mohamed MS, Johansson A, Jonsson J, Schiöth HB. Dissecting the Molecular Mechanisms Surrounding Post-COVID-19 Syndrome and Neurological Features. Int J Mol Sci. 2022 Apr 12;23(8):4275. PMID: 35457093; PMCID: PMC9028501. [CrossRef]
- Goldman M. Long Covid, a great imitator of the 21th century. Front Med (Lausanne). 2022 Sep 15;9:1026425. PMID: 36186771; PMCID: PMC9519984. [CrossRef]
- Mahroum N, Shoenfeld Y. Autoimmune Autonomic Dysfunction Syndromes: Potential Involvement and Pathophysiology Related to Complex Regional Pain Syndrome, Fibromyalgia, Chronic Fatigue Syndrome, Silicone Breast Implant-Related Symptoms and Post-COVID Syndrome. Pathophysiology. 2022 Jul 28;29(3):414-425. PMID: 35997389; PMCID: PMC9396987. [CrossRef]
- Peterson JA, Bemben MG, Larson RD, Pereira H, Crowson HM, Black CD. Symptomatic but not Asymptomatic COVID-19 Impairs Conditioned Pain Modulation in Young Adults. J Pain. 2022 Nov;23(11):1923-1932. Epub 2022 Jul 22. PMID: 35872293; PMCID: PMC9303070. [CrossRef]
- Malkova AM, Shoenfeld Y. Autoimmune autonomic nervous system imbalance and conditions: Chronic fatigue syndrome, fibromyalgia, silicone breast implants, COVID and post-COVID syndrome, sick building syndrome, post-orthostatic tachycardia syndrome, autoimmune diseases and autoimmune/inflammatory syndrome induced by adjuvants. Autoimmunity reviews. 2023 Jan 1;22(1):103230.
- Zhang Z, Zhu Z, Liu D, Mi Z, Tao H, Fan H. Blood transcriptome and machine learning identified the crosstalk between COVID-19 and fibromyalgia: a preliminary study. Clin Exp Rheumatol. 2023 Jun;41(6):1262-1274. Epub 2023 Feb 8. PMID: 36762746. [CrossRef]
- Dotan A, Shoenfeld Y. Post-COVID syndrome: the aftershock of SARS-CoV-2. Int J Infect Dis. 2022 Jan;114:233-235. Epub 2021 Nov 14. PMID: 34785367; PMCID: PMC8590600. [CrossRef]
- Paroli M, Gioia C, Accapezzato D, Caccavale R. Inflammation, Autoimmunity, and Infection in Fibromyalgia: A Narrative Review. Int J Mol Sci. 2024 May 29;25(11):5922. PMID: 38892110; PMCID: PMC11172859. [CrossRef]
- Stefanou MI, Panagiotopoulos E, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Moschovos C, Paraskevas GP, Rizos E, Boutati E, Tzavellas E, Gatzonis S, Mengel A, Giannopoulos S, Tsiodras S, Kimiskidis VK, Tsivgoulis G. Current update on the neurological manifestations of long COVID: more questions than answers. EXCLI J. 2024 Nov 27;23:1463-1486. PMID: 39850323; PMCID: PMC11755773. [CrossRef]
- Saito S, Shahbaz S, Osman M, Redmond D, Bozorgmehr N, Rosychuk RJ, Lam G, Sligl W, Cohen Tervaert JW, Elahi S. Diverse immunological dysregulation, chronic inflammation, and impaired erythropoiesis in long COVID patients with chronic fatigue syndrome. J Autoimmun. 2024 Jul;147:103267. Epub 2024 May 25. PMID: 38797051. [CrossRef]
- Bustamante C, Pinilla Bonilla LB, Restrepo JC. Neurological symphony: post-acute COVID-19 syndrome, an innovative pathophysiological exploration from neuraltherapeutic medicine. Front Integr Neurosci. 2024 Jul 12;18:1417856. PMID: 39070159; PMCID: PMC11275269. [CrossRef]
- Balzanelli MG, Rastmanesh R, Distratis P, Lazzaro R, Inchingolo F, Del Prete R, Pham VH, Aityan SK, Cong TT, Nguyen KCD, Isacco CG. The Role of SARS-CoV-2 Spike Protein in Long-term Damage of Tissues and Organs, the Underestimated Role of Retrotransposons and Stem Cells, a Working Hypothesis. Endocr Metab Immune Disord Drug Targets. 2025;25(2):85-98. PMID: 38468535. [CrossRef]
- Dehhaghi M, Heydari M, Panahi HKS, Lewin SR, Heng B, Brew BJ, Guillemin GJ. The roles of the kynurenine pathway in COVID-19 neuropathogenesis. Infection. 2024 Oct;52(5):2043-2059. Epub 2024 May 27. PMID: 38802702; PMCID: PMC11499433. [CrossRef]
- Bitirgen G, Korkmaz C, Zamani A, Ozkagnici A, Zengin N, Ponirakis G, Malik RA. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. 2022 Dec;106(12):1635-1641. Epub 2021 Jul 26. PMID: 34312122; PMCID: PMC8359871. [CrossRef]
- Salvato M, Doria A, Giollo A. The overlooked epidemic: Fibromyalgia in the shadows of long COVID. Semin Arthritis Rheum. 2025 Feb;70:152596. Epub 2024 Nov 17. PMID: 39580341. [CrossRef]
- Plaut S (2023) “Long COVID-19” and viral “fibromyalgia-ness”: Suggesting a mechanistic role for fascial myofibroblasts (Nineveh, the shadow is in the fascia). Front. Med. 10:952278. [CrossRef]
- Sepic A, Tryfonos A, Rundqvist H, Lundberg TR, Gustafsson T, Pourhamidi K. Non-Hospitalized Patients With Post-COVID Condition and Myopathic Electromyography Findings Show no Difference in Symptom Severity and Clinical Manifestations Compared to Those Without Myopathic Findings. Muscle Nerve. 2025 Feb;71(2):223-228. Epub 2024 Dec 13. PMID: 39673190; PMCID: PMC11708447. [CrossRef]
- Tryfonos A, Pourhamidi K, Jörnåker G, Engvall M, Eriksson L, Elhallos S, Asplund N, Mandic M, Sundblad P, Sepic A, Rullman E, Hyllienmark L, Rundqvist H, Lundberg TR, Gustafsson T. Functional Limitations and Exercise Intolerance in Patients With Post-COVID Condition: A Randomized Crossover Clinical Trial. JAMA Netw Open. 2024 Apr 1;7(4):e244386. PMID: 38573638; PMCID: PMC11192186. [CrossRef]
- Monje M, Iwasaki A. The neurobiology of long COVID. Neuron. 2022 Nov 2;110(21):3484-3496. Epub 2022 Oct 7. PMID: 36288726; PMCID: PMC9537254. [CrossRef]
- Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, Tabachnikova A, Greene K, Tabacof L. Distinguishing features of long COVID identified through immune profiling. Nature. 2023 Nov;623(7985):139-148. [CrossRef]
- Cohen SP, Wang EJ, Doshi TL, Vase L, Cawcutt KA, Tontisirin N. Chronic pain and infection: mechanisms, causes, conditions, treatments, and controversies. BMJ Med. 2022 Mar 31;1(1):e000108. PMID: 36936554; PMCID: PMC10012866. [CrossRef]
- Bodansky A, Wang CY, Saxena A, Mitchell A, Kung AF, Takahashi S, Anglin K, Huang B, Hoh R, Lu S, Goldberg SA, Romero J, Tran B, Kirtikar R, Grebe H, So M, Greenhouse B, Durstenfeld MS, Hsue PY, Hellmuth J, Kelly JD, Martin JN, Anderson MS, Deeks SG, Henrich TJ, DeRisi JL, Peluso MJ. Autoantigen profiling reveals a shared post-COVID signature in fully recovered and long COVID patients. JCI Insight. 2023 Jun 8;8(11):e169515. PMID: 37288661; PMCID: PMC10393220. [CrossRef]
- Skare TL, de Carvalho JF, de Medeiros IRT, Shoenfeld Y. Ear abnormalities in chronic fatigue syndrome (CFS), fibromyalgia (FM), Coronavirus-19 infectious disease (COVID) and long-COVID syndrome (PCS), sick-building syndrome (SBS), post-orthostatic tachycardia syndrome (PoTS), and autoimmune/inflammatory syndrome induced by adjuvants (ASIA): A systematic review. Autoimmun Rev. 2024 Oct;23(10):103606. [CrossRef]
- Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, Dos Santos Freitas A, Ribeiro da Silveira P, Tiwari S, Alzahrani KJ, Góes-Neto A, Azevedo V, Ghosh P, Barh D. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses. 2021 Apr 18;13(4):700. PMID: 33919537; PMCID: PMC8072585. [CrossRef]
- Ablin JN, Shoenfeld Y, Buskila D. Fibromyalgia, infection and vaccination: two more parts in the etiological puzzle. J Autoimmun. 2006 Nov;27(3):145-52. Epub 2006 Oct 30. PMID: 17071055. [CrossRef]
- Saunders C, Sperling S, Bendstrup E. A new paradigm is needed to explain long COVID. Lancet Respir Med. 2023 Feb;11(2):e12-e13. Epub 2023 Jan 5. PMID: 36620963. [CrossRef]
- Lemogne C, Gouraud C, Pitron V, Ranque B. Why the hypothesis of psychological mechanisms in long COVID is worth considering. J Psychosom Res. 2023 Feb;165:111135. Epub 2023 Jan 4. PMID: 36623391; PMCID: PMC9825049. [CrossRef]
- Yin K, Peluso MJ, Luo X, Thomas R, Shin MG, Neidleman J, Andrew A, Young KC, Ma T, Hoh R, Anglin K, Huang B, Argueta U, Lopez M, Valdivieso D, Asare K, Deveau TM, Munter SE, Ibrahim R, Ständker L, Lu S, Goldberg SA, Lee SA, Lynch KL, Kelly JD, Martin JN, Münch J, Deeks SG, Henrich TJ, Roan NR. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat Immunol. 2024 Feb;25(2):218-225. [CrossRef]
- Wallukat G, Hohberger B, Wenzel K, Fürst J, Schulze-Rothe S, Wallukat A, Hönicke AS, Müller J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. Epub 2021 Apr 16. PMID: 33880442; PMCID: PMC8049853. [CrossRef]
- Fleischer M, Szepanowski F, Tovar M, Herchert K, Dinse H, Schweda A, Mausberg AK, Holle-Lee D, Köhrmann M, Stögbauer J, Jokisch D, Jokisch M, Deuschl C, Skoda EM, Teufel M, Stettner M, Kleinschnitz C. Post-COVID-19 Syndrome is Rarely Associated with Damage of the Nervous System: Findings from a Prospective Observational Cohort Study in 171 Patients. Neurol Ther. 2022 Dec;11(4):1637-1657. Epub 2022 Aug 26. PMID: 36028604; PMCID: PMC9417089. [CrossRef]
- Clauw DJ, Häuser W, Cohen SP, Fitzcharles MA. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain. 2020 Aug;161(8):1694-1697. PMID: 32701829; PMCID: PMC7302093. [CrossRef]
- Fernández-de-Las-Peñas C, Nijs J, Neblett R, Polli A, Moens M, Goudman L, Shekhar Patil M, Knaggs RD, Pickering G, Arendt-Nielsen L. Phenotyping Post-COVID Pain as a Nociceptive, Neuropathic, or Nociplastic Pain Condition. Biomedicines. 2022 Oct 13;10(10):2562. PMID: 36289827; PMCID: PMC9599440. [CrossRef]
- Calvache-Mateo A, Navas-Otero A, Heredia-Ciuró A, Matín-Núñez J, Torres-Sánchez I, López-López L, Valenza MC. Post-COVID Patients With New-Onset Chronic Pain 2 Years After Infection: Cross-Sectional Study. Pain Manag Nurs. 2023 Oct;24(5):528-534. Epub 2023 May 22. PMID: 37225540; PMCID: PMC10201348. [CrossRef]
- Ebbesen BD, Giordano R, Valera-Calero JA, Hedegaard JN, Fernández-de-Las-Peñas C, Arendt-Nielsen L. Prevalence and Risk Factors of De Novo Widespread Post-COVID Pain in Nonhospitalized COVID-19 Survivors: A Nationwide Exploratory Population-Based Survey. J Pain. 2024 Jan;25(1):1-11. Epub 2023 Aug 24. PMID: 37633573. [CrossRef]
- Pires RE, Reis IGN, Waldolato GS, Pires DD, Bidolegui F, Giordano V. What Do We Need to Know About Musculoskeletal Manifestations of COVID-19?: A Systematic Review. JBJS Rev. 2022 Jun 3;10(6). PMID: 35658089. [CrossRef]
- Ciaffi J, Vanni E, Mancarella L, Brusi V, Lisi L, Pignatti F, Naldi S, Assirelli E, Neri S, Reta M, Faldini C, Ursini F. Post-Acute COVID-19 Joint Pain and New Onset of Rheumatic Musculoskeletal Diseases: A Systematic Review. Diagnostics (Basel). 2023 May 25;13(11):1850. PMID: 37296705; PMCID: PMC10252492. [CrossRef]
- Liu YD, Noga H, Allaire C, Bedaiwy MA, Lee CE, Williams C, Booth A, Galea LAM, Kaida A, Ogilvie GS, Brotto LA, Yong PJ. Mental Health Outcomes of Endometriosis Patients during the COVID-19 Pandemic: Impact of Pre-pandemic Central Nervous System Sensitization. J Pain. 2024 Jul;25(7):104481. Epub 2024 Jan 19. PMID: 38246253. [CrossRef]
- Mantle D, Hargreaves IP, Domingo JC, Castro-Marrero J. Mitochondrial Dysfunction and Coenzyme Q10 Supplementation in Post-Viral Fatigue Syndrome: An Overview. Int J Mol Sci. 2024 Jan 1;25(1):574. PMID: 38203745; PMCID: PMC10779395. [CrossRef]
- Fernández-de-Las-Peñas C, Nijs J, Giordano R, Arendt-Nielsen L. Precision management of post-COVID pain: An evidence and clinical-based approach. Eur J Pain. 2023 Oct;27(9):1107-1125. Epub 2023 Feb 28. PMID: 36852606. [CrossRef]
- Ketenci A, Zure M, Akpınar FM, Soluk Özdemir Y, Balbaloğlu Ö, Akaltun MS, Erden E, Çağlıyan Türk A, Korkmaz MD, Metin Ökmen B, Altındağ Ö, Soyupek F, Yakşi E, Sindel D, Sezgin N, Ustaömer K, Kesiktaş FN, Dere D, Güneş Ş, Medin Ceylan C, Sonel Tur B, Evcik D. Pain types and risk factors in post-COVID-19. Turk J Phys Med Rehabil. 2024 Feb 1;70(1):30-38. PMID: 38549834; PMCID: PMC10966756. [CrossRef]
- Khoja O, Silva-Passadouro B, Cristescu E, McEwan K, Doherty D, O’Connell F, Ponchel F, Mulvey M, Astill S, Tan AL, Sivan M. Clinical Characterization of New-Onset Chronic Musculoskeletal Pain in Long COVID: A Cross-Sectional Study. J Pain Res. 2024 Jul 31;17:2531-2550. PMID: 39100135; PMCID: PMC11298172. [CrossRef]
- Pinho H, Neves M, Costa F, Silva AG. Associations between pain intensity, pain sensitivity, demographics, psychological factors, disability, physical activity, pain phenotype and COVID-19 history in low back pain: An observational study. Physiother Res Int. 2024 Jul;29(3):e2094. PMID: 38741292. [CrossRef]
- Khoja O, Mulvey M, Astill S, Tan AL, Sivan M. New-Onset Chronic Musculoskeletal Pain Following COVID-19 Infection Fulfils the Fibromyalgia Clinical Syndrome Criteria: A Preliminary Study. Biomedicines. 2024 Aug 23;12(9):1940. PMID: 39335454; PMCID: PMC11429044. [CrossRef]
- Damasceno DFO, Cavalcante TF, Andrade LKA, de Oliveira FBB, de Oliveira Lopes MV, Moreira RP, Morais HCC. Etiological factors of chronic pain syndrome in young adults with post-coronavirus disease 2019 condition. Int J Nurs Knowl. 2024 Apr;35(2):152-162. Epub 2023 May 26. PMID: 37243313. [CrossRef]
- Fernández-de-Las-Peñas C, Navarro-Santana M, Plaza-Manzano G, Palacios-Ceña D, Arendt-Nielsen L. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived severe acute respiratory syndrome coronavirus 2 infection: a systematic review and meta-analysis. Pain. 2022 Jul 1;163(7):1220-1231. Epub 2021 Sep 23. PMID: 34561390. [CrossRef]
- Calabrese C, Kirchner E, Calabrese LH. Long COVID and rheumatology: Clinical, diagnostic, and therapeutic implications. Best Pract Res Clin Rheumatol. 2022 Dec;36(4):101794. Epub 2022 Nov 8. PMID: 36369208; PMCID: PMC9641578. [CrossRef]
- Scherlinger M, Felten R, Gallais F, Nazon C, Chatelus E, Pijnenburg L, Mengin A, Gras A, Vidailhet P, Arnould-Michel R, Bibi-Triki S, Carapito R, Trouillet-Assant S, Perret M, Belot A, Bahram S, Arnaud L, Gottenberg JE, Fafi-Kremer S, Sibilia J. Refining “Long-COVID” by a Prospective Multimodal Evaluation of Patients with Long-Term Symptoms Attributed to SARS-CoV-2 Infection. Infect Dis Ther. 2021 Sep;10(3):1747-1763. [CrossRef]
- Mariette X. Response to: Correspondence on 'Long COVID: a new word for naming fibromyalgia?" by Mariette. Ann Rheum Dis. 2024 Jun 12;83(7):e16. PMID: 38171599. [CrossRef]
- Michaud K, Pedro S, Gandhi S, Wolfe F. Persons with Rheumatoid Arthritis and Long COVID Had Worse Pre-COVID RA Symptoms and Worse Non-RA Symptoms, as Well as Higher Rates of Fibromyalgia Compared with COVID Infected Long COVID Negative. Abstract 1629 at the 2023 ACR convergence. Arthritis Rheumatol. 2023; 75 (suppl 9).
- Bakılan F, Gökmen İG, Ortanca B, Uçan A, Eker Güvenç Ş, Şahin Mutlu F, Gökmen HM, Ekim A. Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Int J Clin Pract. 2021 Nov;75(11):e14734. Epub 2021 Aug 18. PMID: 34387911; PMCID: PMC8420386. [CrossRef]
- Foti R, Amato G, Dal Bosco Y, Gagliano C, Longo A, Falsaperla R, et al. INCIDENCE OF PSYCHIATRIC DISORDERS AND FIBROMYALGIA IN PATIENTS WITH RHEUMATOID ARTHRITIS AND PSORIATIC ARTHRITIS DURING COVID 19 PANDEMIC: THE ROLE OF TELEMEDICINE. Meeting abstract at Annual European Congress of Rheumatology (EULAR) 2022. ANNALS OF THE RHEUMATIC DISEASES. 2022; 81:1092-1092.
- Ablin J. From long COVID to fibromyalgia: insights from an evolving trajectory. Clin exp rheumatology. 2023; 41(6):1357-1358. meeting abstract in The 5th International Congress on Controversies in Fibromyalgia. [CrossRef]
- Kerzhner O, Berla E, Har-Even M, Ratmansky M, Goor-Aryeh I. Consistency of inconsistency in long-COVID-19 pain symptoms persistency: A systematic review and meta-analysis. Pain Pract. 2024 Jan;24(1):120-159. Epub 2023 Jul 21. PMID: 37475709. [CrossRef]
- Martin E, Wimaleswaran H, McMaster C, Shivakumar S, Howard M, Buchanan R, et al. FIBROMYALGIA, “FIBROMYALGIANESS”, AND FATIGUE ARE COMMON SIX MONTHS FOLLOWING COVID-19 INFECTION. Poster presentation at the Australian Rheumatology Association 61st Annual Scientific Meeting 21–23 May 2021. Internal medicine journal 2021. 51 (Suppl. 2):5-52. [CrossRef]
- Gavrilova N, Soprun L, Lukashenko M, Ryabkova V, Fedotkina TV, Churilov LP, Shoenfeld Y. New Clinical Phenotype of the Post-Covid Syndrome: Fibromyalgia and Joint Hypermobility Condition. Pathophysiology. 2022 Jan 19;29(1):24-29. PMID: 35366287; PMCID: PMC8954589. [CrossRef]
- Kim N, Kim J, Yang BR, Hahm BJ. Associations of unspecified pain, idiopathic pain and COVID-19 in South Korea: a nationwide cohort study. Korean J Pain. 2022 Oct 1;35(4):458-467. PMID: 36175345; PMCID: PMC9530679. [CrossRef]
- Haider S, Janowski AJ, Lesnak JB, Hayashi K, Dailey DL, Chimenti R, Frey-Law LA, Sluka KA, Berardi G. A comparison of pain, fatigue, and function between post-COVID-19 condition, fibromyalgia, and chronic fatigue syndrome: a survey study. Pain. 2023 Feb 1;164(2):385-401. Epub 2022 Jun 29. PMID: 36006296; PMCID: PMC9797623. [CrossRef]
- Jennifer K, Shirley SBD, Avi P, Daniella RC, Naama SS, Anat EZ, Miri MR. Post-acute sequelae of COVID-19 infection. Prev Med Rep. 2023 Feb;31:102097. Epub 2022 Dec 21. PMID: 36567743; PMCID: PMC9767882. [CrossRef]
- Upadhyaya SK, Malgutte DR, Handa R, Gupta S, Kumar A, Budumuru S. Fibromyalgia and mental health in rheumatoid arthritis: a cross-sectional prevalence study from the COVID-19 pandemic. BMJ Open. 2023 Jun 15;13(6):e069014. PMID: 37321814; PMCID: PMC10276963. [CrossRef]
- Miladi S, Ketata M, Fazaa A, Boussaa H, Makhlouf Y, Ben Abdelghani K, Laatar A. PREVALENCE OF FIBROMYALGIA OCCURRING AFTER A COVID-19 INFECTION World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (WCO-IOF-ESCEO 2023). Aging Clin Exp Res 35 (Suppl 1), 37–613 (2023). Aging Clin Exp Res (2023). page 198. [CrossRef]
- Akel A, Almanasyeh B, Abo Kobaa A, Aljabali A, Al-Abadleh A, Alkhalaileh A, Alwardat AR, Sarhan MY, Abu-Jeyyab M. A Cross-Sectional Study of Fibromyalgia and Post-acute COVID-19 Syndrome (PACS): Could There Be a Relationship? Cureus. 2023 Jul 29;15(7):e42663. PMID: 37644924; PMCID: PMC10462402. [CrossRef]
- Amsterdam D, Kupershmidt A, Avinir A, Matalon R, Ohana O, Feder O, Shtrozberg S, Choshen G, Ablin JN, Elkana O. Long COVID-19 Enigma: Unmasking the Role of Distinctive Personality Profiles as Risk Factors. J Clin Med. 2024 May 14;13(10):2886. PMID: 38792428; PMCID: PMC11122355. [CrossRef]
- Shani M, Hermesh I, Feldhamer I, Reges O, Lavie G, Arbel R, Sagy YW. The association between BNT162b2 vaccinations and incidence of immune-mediated comorbidities. Vaccine. 2024 Jul 11;42(18):3830-3837. Epub 2024 May 10. PMID: 38729910. [CrossRef]
- Hackshaw KV, Yao S, Bao H, de Lamo Castellvi S, Aziz R, Nuguri SM, Yu L, Osuna-Diaz MM, Brode WM, Sebastian KR, Giusti MM, Rodriguez-Saona L. Metabolic Fingerprinting for the Diagnosis of Clinically Similar Long COVID and Fibromyalgia Using a Portable FT-MIR Spectroscopic Combined with Chemometrics. Biomedicines. 2023 Oct 5;11(10):2704. PMID: 37893078; PMCID: PMC10604557. [CrossRef]
- Nuguri SM, Hackshaw KV, Castellvi SL, Wu Y, Gonzalez CM, Goetzman CM, Schultz ZD, Yu L, Aziz R, Osuna-Diaz MM, Sebastian KR, Brode WM, Giusti MM, Rodriguez-Saona L. Surface-Enhanced Raman Spectroscopy Combined with Multivariate Analysis for Fingerprinting Clinically Similar Fibromyalgia and Long COVID Syndromes. Biomedicines. 2024 Jun 28;12(7):1447. PMID: 39062021; PMCID: PMC11275161. [CrossRef]
- Munipalli B, Smith A, Baird AR, Dobrowolski CS, Allman ME, Thomas LG, Bruce BK. A description of the development of an innovative multi-component long COVID treatment program based on central sensitization with preliminary patient satisfaction data. J Psychosom Res. 2024 Oct;185:111884. Epub 2024 Aug 12. PMID: 39163793. [CrossRef]
- Mirofsky M, Catalano H. Long COVID: a new disease? Medicina (B Aires). 2024;84(5):937-945. English. PMID: 39399934.
- Clauw DJ, Calabrese L. Rheumatology and Long COVID: lessons from the study of fibromyalgia. Ann Rheum Dis. 2024 Jan 11;83(2):136-138. PMID: 37230736; PMCID: PMC10850638. [CrossRef]
- Grach SL, Dudenkov DV, Pollack B, Fairweather D, Aakre CA, Munipalli B, Croghan IT, Mueller MR, Overgaard JD, Bruno KA, Collins NM, Li Z, Hurt RT, Tal MC, Ganesh R, Knight DTR. Overlapping conditions in Long COVID at a multisite academic center. Front Neurol. 2024 Oct 25;15:1482917. PMID: 39524912; PMCID: PMC11543549. [CrossRef]
- Azcue N, Teijeira-Portas S, Tijero-Merino B, Acera M, Fernández-Valle T, Ayala U, Barrenechea M, Murueta-Goyena A, Lafuente JV, de Munain AL, Ruiz-Irastorza G, Martín-Iglesias D, Gabilondo I, Gómez-Esteban JC, Del Pino R. Small fiber neuropathy in the post-COVID condition and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical significance and diagnostic challenges. Eur J Neurol. 2025 Feb;32(2):e70016. [CrossRef]
- Fialho MFP, Brum ES, Oliveira SM. Could the fibromyalgia syndrome be triggered or enhanced by COVID-19? Inflammopharmacology. 2023 Apr;31(2):633-651. Epub 2023 Feb 27. PMID: 36849853; PMCID: PMC9970139. [CrossRef]
- Kocyigit BF, Akyol A. The relationship between COVID-19 and fibromyalgia syndrome: prevalence, pandemic effects, symptom mechanisms, and COVID-19 vaccines. Clin Rheumatol. 2022 Oct;41(10):3245-3252. Epub 2022 Jul 8. PMID: 35804273. [CrossRef]
- Bierle DM, Aakre CA, Grach SL, Salonen BR, Croghan IT, Hurt RT, Ganesh R. Central Sensitization Phenotypes in Post Acute Sequelae of SARS-CoV-2 Infection (PASC): Defining the Post COVID Syndrome. J Prim Care Community Health. 2021 Jan-Dec;12:21501327211030826. PMID: 34231404; PMCID: PMC8267019. [CrossRef]
- Asquini G, Bianchi AE, Borromeo G, Locatelli M, Falla D. The impact of Covid-19-related distress on general health, oral behaviour, psychosocial features, disability and pain intensity in a cohort of Italian patients with temporomandibular disorders. PLoS One. 2021 Feb 2;16(2):e0245999. PMID: 33529226; PMCID: PMC7853459. [CrossRef]
- Goudman L, De Smedt A, Noppen M, Moens M. Is Central Sensitisation the Missing Link of Persisting Symptoms after COVID-19 Infection? J Clin Med. 2021 Nov 28;10(23):5594. PMID: 34884296; PMCID: PMC8658135. [CrossRef]
- Halili A. Temporal model for central sensitization: A hypothesis for mechanism and treatment using systemic manual therapy, a focused review. MethodsX. 2022 Nov 28;10:101942. PMID: 36570602; PMCID: PMC9772546. [CrossRef]
- Goudman L, De Smedt A, Roggeman S, Fernández-de-Las-Peñas C, Hatem SM, Schiltz M, Billot M, Roulaud M, Rigoard P, Moens M. Association between Experimental Pain Measurements and the Central Sensitization Inventory in Patients at Least 3 Months after COVID-19 Infection: A Cross-Sectional Pilot Study. J Clin Med. 2023 Jan 13;12(2):661. PMID: 36675590; PMCID: PMC9862134. [CrossRef]
- Fioravanti A, Antonelli M, Vitale M. Advances in modern Balneology: new evidence-based indications from recent studies. Int J Biometeorol. 2024 Nov;68(11):2447-2452. Epub 2024 Jul 31. PMID: 39085662. [CrossRef]
- Bramante CT, Buse JB, Liebovitz DM, Nicklas JM, Puskarich MA, Cohen K, Belani HK, et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023 Oct;23(10):1119-1129. Epub 2023 Jun 8. Erratum in: Lancet Infect Dis. 2023 Oct;23(10):e400. doi: 10.1016/S1473-3099(23)00562-5. [CrossRef]
- Teitelbaum J, Goudie S. An Open-Label, Pilot Trial of HRG80TM Red Ginseng in Chronic Fatigue Syndrome, Fibromyalgia, and Post-Viral Fatigue. Pharmaceuticals (Basel). 2021 Dec 29;15(1):43. PMID: 35056100; PMCID: PMC8777686. [CrossRef]
- Bileviciute-Ljungar I, Norrefalk JR, Borg K. Improved Functioning and Activity According to the International Classification of Functioning and Disability after Multidisciplinary Telerehabilitation for Post-COVID-19 Condition-A Randomized Control Study. J Clin Med. 2024 Feb 8;13(4):970. PMID: 38398284; PMCID: PMC10889504. [CrossRef]
- Bileviciute-Ljungar I, Apelman A, Braconier L, Östhols S, Norrefalk JR, Borg K. A First Randomized Eight-Week Multidisciplinary Telerehabilitation Study for the Post-COVID-19 Condition: Improvements in Health- and Pain-Related Parameters. J Clin Med. 2025 Jan 14;14(2):486. PMID: 39860492; PMCID: PMC11766284. [CrossRef]
- Scaturro D, Vitagliani F, Di Bella VE, Falco V, Tomasello S, Lauricella L, Letizia Mauro G. The Role of Acetyl-Carnitine and Rehabilitation in the Management of Patients with Post-COVID Syndrome: Case-Control Study. Applied Sciences. 2022; 12(8):4084. [CrossRef]
- Barletta MA, Marino G, Spagnolo B, Bianchi FP, Falappone PCF, Spagnolo L, Gatti P. Coenzyme Q10 + alpha lipoic acid for chronic COVID syndrome. Clin Exp Med. 2023 Jul;23(3):667-678. Epub 2022 Aug 22. PMID: 35994177; PMCID: PMC9395797. [CrossRef]
- Zulbaran-Rojas A, Bara RO, Lee M, Bargas-Ochoa M, Phan T, Pacheco M, Camargo AF, Kazmi SM, Rouzi MD, Modi D, Shaib F, Najafi B. Transcutaneous electrical nerve stimulation for fibromyalgia-like syndrome in patients with Long-COVID: a pilot randomized clinical trial. Sci Rep. 2024 Nov 8;14(1):27224. PMID: 39516528; PMCID: PMC11549448. [CrossRef]
- Zilberman-Itskovich S, Catalogna M, Sasson E, Elman-Shina K, Hadanny A, Lang E, Finci S, Polak N, Fishlev G, Korin C, Shorer R, Parag Y, Sova M, Efrati S. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci Rep. 2022 Jul 12;12(1):11252. PMID: 35821512; PMCID: PMC9276805. [CrossRef]
- Lewthwaite H, Byrne A, Brew B, Gibson PG. Treatable traits for long COVID. Respirology. 2023 Nov;28(11):1005-1022. Epub 2023 Sep 16. PMID: 37715729. [CrossRef]
- Lau RI, Su Q, Lau ISF, Ching JYL, Wong MCS, Lau LHS, Tun HM, Mok CKP, Chau SWH, Tse YK, Cheung CP, Li MKT, Yeung GTY, Cheong PK, Chan FKL, Ng SC. A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2024 Mar;24(3):256-265. Epub 2023 Dec 7. PMID: 38071990. [CrossRef]
- Petracek LS, Broussard CA, Swope RL, Rowe PC. A Case Study of Successful Application of the Principles of ME/CFS Care to an Individual with Long COVID. Healthcare (Basel). 2023 Mar 16;11(6):865. PMID: 36981522; PMCID: PMC10048325. [CrossRef]
- Wagner B, Steiner M, Markovic L, Crevenna R. Successful application of pulsed electromagnetic fields in a patient with post-COVID-19 fatigue: a case report. Wien Med Wochenschr. 2022 Jun;172(9-10):227-232. Epub 2022 Jan 10. PMID: 35006516; PMCID: PMC8743351. [CrossRef]
- Zha M, Chaffee K, Alsarraj J. Trigger point injections and dry needling can be effective in treating long COVID syndrome-related myalgia: a case report. J Med Case Rep. 2022 Dec 1;16(1).
- Kim JH, Kwon MJ, Choi HG, Lee SJ, Hwang S, Lee J, Lee SH, Lee JW. Changes in the mean incidence and variance of orthopedic diseases before and during the COVID-19 pandemic in Korea: a retrospective study. BMC Musculoskelet Disord. 2023 Jul 1;24(1):540. PMID: 37393227; PMCID: PMC10314473. [CrossRef]
- Blanchard M, Backhaus L, Ming Azevedo P, Hügle T. An mHealth App for Fibromyalgia-like Post-COVID-19 Syndrome: Protocol for the Analysis of User Experience and Clinical Data. JMIR Res Protoc. 2022 Feb 4;11(2):e32193. PMID: 34982039; PMCID: PMC8820761. [CrossRef]
- Kjellberg A, Abdel-Halim L, Hassler A, El Gharbi S, Al-Ezerjawi S, Boström E, Sundberg CJ, Pernow J, Medson K, Kowalski JH, Rodriguez-Wallberg KA, Zheng X, Catrina S, Runold M, Ståhlberg M, Bruchfeld J, Nygren-Bonnier M, Lindholm P. Hyperbaric oxygen for treatment of long COVID-19 syndrome (HOT-LoCO): protocol for a randomised, placebo-controlled, double-blind, phase II clinical trial. BMJ Open. 2022 Nov 2;12(11):e061870. PMID: 36323462; PMCID: PMC9638753. [CrossRef]
- Cohen Tervaert JW, Martinez-Lavin M, Jara LJ, Halpert G, Watad A, Amital H, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun Rev. 2023 May;22(5):103287. Epub 2023 Feb 3. PMID: 36738954. [CrossRef]
- Di Stefano G, Falco P, Galosi E, De Stefano G, Di Pietro G, Leone C, Litewczuk D, Tramontana L, Strano S, Truini A. Pain associated with COVID-19 vaccination is unrelated to skin biopsy abnormalities. Pain Rep. 2023 Aug 10;8(5):e1089. PMID: 38225959; PMCID: PMC10789449. [CrossRef]
- Naik H, Cooke E, Boulter T, Dyer R, Bone JN, Tsai M, Cristobal J, McKay RJ, Song X, Nacul L. Low-dose naltrexone for post-COVID fatigue syndrome: a study protocol for a double-blind, randomised trial in British Columbia. BMJ Open. 2024 May 13;14(5):e085272. PMID: 38740499; PMCID: PMC11097836. [CrossRef]
- Ganesh R, Munipalli B. Long COVID and hypermobility spectrum disorders have shared pathophysiology. Front Neurol. 2024 Sep 5;15:1455498. PMID: 39301475; PMCID: PMC11410636. [CrossRef]
- Jessica A Eccles, Dorina Cadar, Lisa Quadt, Alan J Hakim, Nicholas Gall, , Vicky Bowyer, Nathan Cheetham, Claire J Steves, Hugo D Critchley, Kevin A Davies - Is joint hypermobility linked to self-reported non-recovery from COVID-19? Case–control evidence from the British COVID Symptom Study Biobank: BMJ Public Health 2024;2:e000478.
- Logarbo BP, Yang M, Longo MT, Kingry C, Courseault J. Long COVID and the diagnosis of underlying hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. PM R. 2024 Aug;16(8):935-937. Epub 2024 Feb 14. PMID: 38116712. [CrossRef]
- Gasión V, Barceló-Soler A, Beltrán-Ruiz M, Hijar-Aguinaga R, Camarero-Grados L, López-Del-Hoyo Y, García-Campayo J, Montero-Marin J. Effectiveness of an amygdala and insula retraining program combined with mindfulness training to improve the quality of life in patients with long COVID: a randomized controlled trial protocol. BMC Complement Med Ther. 2023 Nov 9;23(1):403. PMID: 37946190; PMCID: PMC10634181. [CrossRef]
- Blanchard M, Koller CN, Azevedo PM, Prétat T, Hügle T. Development of a Management App for Postviral Fibromyalgia-Like Symptoms: Patient Preference-Guided Approach. JMIR Form Res. 2024 Apr 19;8:e50832. PMID: 38639986; PMCID: PMC11069091. [CrossRef]
- Blanchard M. User experience research in the development of digital health products: Research letter. Health Policy and Technology. 2023; 12(2):100753. [CrossRef]
- Blanchard M, Hügle T, Ming Azevedo P. DEVELOPMENT OF A PATIENT-CENTERED MULTIMODAL DISEASE MANAGEMENT PLATFORM FOR THE FIBROMYALGIA-LIKE POST-COVID19 SYNDROME. Abstract POS0803-HP in the 2023 European Congress of Rheumatology (EULAR). Annals of the rheumatic diseases. 2023; 82(supp 1):696. [CrossRef]
- Byrne, E. A. (2022). Affective scaffolding and chronic illness. Philosophical Psychology, 37(4), 921–946.
- Blanchard M, Venerito V, Ming Azevedo P, Hügle T. Generative AI-based knowledge graphs for the illustration and development of mHealth self-management content. Front Digit Health. 2024 Oct 7;6:1466211. PMID: 39434919; PMCID: PMC11491428. [CrossRef]
- Hejbøl EK, Harbo T, Agergaard J, Madsen LB, Pedersen TH, Østergaard LJ, Andersen H, Schrøder HD, Tankisi H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle histopathology. Eur J Neurol. 2022 Sep;29(9):2832-2841. Epub 2022 Jun 23. PMID: 35661354; PMCID: PMC9348124. [CrossRef]
- Agergaard J, Leth S, Pedersen TH, Harbo T, Blicher JU, Karlsson P, Østergaard L, Andersen H, Tankisi H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin Neurophysiol. 2021 Aug;132(8):1974-1981. Epub 2021 May 7. PMID: 34020890; PMCID: PMC8102077. [CrossRef]
- Buskila D, Atzeni F, Sarzi-Puttini P. Etiology of fibromyalgia: the possible role of infection and vaccination. Autoimmun Rev. 2008 Oct;8(1):41-3. Epub 2008 Aug 13. PMID: 18706528. [CrossRef]
- Giorgi V, Sirotti S, Romano ME, Marotto D, Ablin JN, Salaffi F, Sarzi-Puttini P. Fibromyalgia: one year in review 2022. Clin Exp Rheumatol. 2022 Jun;40(6):1065-1072. Epub 2022 Jun 22. PMID: 35748720. [CrossRef]
- Kachaner A, Lemogne C, Dave J, Ranque B, de Broucker T, Meppiel E. Somatic symptom disorder in patients with post-COVID-19 neurological symptoms: a preliminary report from the somatic study (Somatic Symptom Disorder Triggered by COVID-19). J Neurol Neurosurg Psychiatry. 2022 Aug 25:jnnp-2021-327899. Epub ahead of print. PMID: 36008115. [CrossRef]
- Foti R, Amato G, Dal Bosco Y, Longo A, Gagliano C, Falsaperla R, Foti R, Speranza S, De Lucia F, Visalli E. Telemedicine in the Management of Patients with Rheumatic Disease during COVID-19 Pandemic: Incidence of Psychiatric Disorders and Fibromyalgia in Patients with Rheumatoid Arthritis and Psoriatic Arthritis. Int J Environ Res Public Health. 2022 Mar 8;19(6):3161. PMID: 35328849; PMCID: PMC8956021. [CrossRef]
- Di Carlo M, Bianchi B, Salaffi F, Pellegrino G, Iannuccelli C, Giorgi V, Sarzi-Puttini P. Fibromyalgia: one year in review 2024. Clin Exp Rheumatol. 2024 Jun;42(6):1141-1149. Epub 2024 Apr 10. PMID: 38607678. [CrossRef]
- Matta J, Wiernik E, Robineau O, Carrat F, Touvier M, Severi G, de Lamballerie X, et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern Med. 2022 Jan 1;182(1):19-25.
- COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence (NICE); 2024 Jan 25. PMID: 33555768.
- Cavalli G, Cariddi A, Ferrari J, Suzzi B, Tomelleri A, Campochiaro C, De Luca G, Baldissera E, Dagna L. Living with fibromyalgia during the COVID-19 pandemic: mixed effects of prolonged lockdown on the well-being of patients. Rheumatology (Oxford). 2021 Jan 5;60(1):465-467. PMID: 33188686; PMCID: PMC7717382. [CrossRef]
- Hruschak V, Flowers KM, Azizoddin DR, Jamison RN, Edwards RR, Schreiber KL. Cross-sectional study of psychosocial and pain-related variables among patients with chronic pain during a time of social distancing imposed by the coronavirus disease 2019 pandemic. Pain. 2021 Feb 1;162(2):619-629. PMID: 33230007; PMCID: PMC7808279. [CrossRef]
- Salaffi F, Giorgi V, Sirotti S, Bongiovanni S, Farah S, Bazzichi L, Marotto D, Atzeni F, Rizzi M, Batticciotto A, Lombardi G, Galli M, Sarzi-Puttini P. The effect of novel coronavirus disease-2019 (COVID-19) on fibromyalgia syndrome. Clin Exp Rheumatol. 2021 May-Jun;39 Suppl 130(3):72-77. Epub 2020 Nov 16. PMID: 33200740. [CrossRef]
- Rivera J, Castrejón I, Vallejo-Slocker L, Offenbächer M, Molina-Collada J, Trives L, López K, Caballero L, Hirsch JK, Toussaint L, Nieto JC, Alvaro-Gracia JM, Vallejo MA. Clinical impact of confinement due to the COVID-19 pandemic on patients with fibromyalgia: a cohort study. Clin Exp Rheumatol. 2021 May-Jun;39 Suppl 130(3):78-81. Epub 2021 Mar 16. PMID: 33734969. [CrossRef]
- Macfarlane GJ, Hollick RJ, Morton L, Heddle M, Bachmair EM, Anderson RS, Whibley D, Keenan KF, Murchie P, Stelfox K, Beasley MJ, Jones GT. The effect of COVID-19 public health restrictions on the health of people with musculoskeletal conditions and symptoms: the CONTAIN study. Rheumatology (Oxford). 2021 Oct 9;60(SI):SI13-SI24. PMID: 34009314; PMCID: PMC8244573. [CrossRef]
- Amital M, Ben-Shabat N, Amital H, Buskila D, Cohen AD, Amital D. COVID-19 associated hospitalization in 571 patients with fibromyalgia-A population-based study. PLoS One. 2021 Dec 30;16(12):e0261772. PMID: 34968398; PMCID: PMC8717981. [CrossRef]
- Koppert TY, Jacobs JWG, Lumley MA, Geenen R. The impact of COVID-19 stress on pain and fatigue in people with and without a central sensitivity syndrome. J Psychosom Res. 2021 Dec;151:110655. Epub 2021 Oct 29. PMID: 34739944; PMCID: PMC8553422. [CrossRef]
- Aloush V, Gurfinkel A, Shachar N, Ablin JN, Elkana O. Physical and mental impact of COVID-19 outbreak on fibromyalgia patients. Clin Exp Rheumatol. 2021 May-Jun;39 Suppl 130(3):108-114. Epub 2021 Mar 11. PMID: 33734970. [CrossRef]
- Vieira Rezende RP, Braz AS, Guimarães et al. Characteristics associated with COVID-19 vaccine hesitancy: A nationwide survey of 1000 patients with immune-mediated inflammatory diseases. Vaccine. 2021 Oct 22;39(44):6454-6459.
- Rivera J, Rodríguez T, Pallarés M, Castrejón I, González T, Vallejo-Slocker L, Molina-Collada J, Montero F, Arias A, Vallejo MA, Alvaro-Gracia JM, Collado A. Prevalence of post-COVID-19 in patients with fibromyalgia: a comparative study with other inflammatory and autoimmune rheumatic diseases. BMC Musculoskelet Disord. 2022 May 19;23(1):471. PMID: 35590317; PMCID: PMC9117853. [CrossRef]
- Aloush V, Gurfinkel A, Shachar N, Ablin J, Elkana O. Pain in the Time of Corona: Impact of COVID 19 Outbreak on Fibromyalgia Patients. Meeting abstract at the 2020 ACR convergence.. Arthritis Rheumatol. 2020; 72 (suppl 10).
- Pérez Catalán I, Roig Martí C, Fabra Juana S, Domínguez Bajo E, Herrero Rodríguez G, Segura Fábrega A, Varea Villanueva M, Folgado Escudero S, Esteve Gimeno MJ, Palomo de la Sota D, Cardenal Álvarez A, Mateu Campos ML, Usó Blasco J, Ramos Rincón JM. One-year quality of life among post-hospitalization COVID-19 patients. Front Public Health. 2023 Oct 6;11:1236527. PMID: 37869178; PMCID: PMC10588695. [CrossRef]
- Savarraj JPJ, Burkett AB, Hinds SN, Paz AS, Assing A, Juneja S, Colpo GD, Torres LF, Cho SM, Gusdon AM, McCullough LD, Choi HA. Pain and Other Neurological Symptoms Are Present at 3 Months After Hospitalization in COVID-19 Patients. Front Pain Res (Lausanne). 2021 Nov 16;2:737961. PMID: 35295410; PMCID: PMC8915679. [CrossRef]
- Giménez-Orenga K, Pierquin J, Brunel J, Charvet B, Martín-Martínez E, Perron H, Oltra E. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front Immunol. 2022 Oct 27;13:1020064. PMID: 36389746; PMCID: PMC9647063. [CrossRef]
- Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011 Mar 24;11:37. PMID: 21435231; PMCID: PMC3071317. [CrossRef]
- Fernández-de-Las-Peñas C, Giordano R, Díaz-Gil G, Gil-Crujera A, Gómez-Sánchez SM, Ambite-Quesada S, Arendt-Nielsen L. Are Pain Polymorphisms Associated with the Risk and Phenotype of Post-COVID Pain in Previously Hospitalized COVID-19 Survivors? Genes (Basel). 2022 Jul 26;13(8):1336. PMID: 35893072; PMCID: PMC9394327. [CrossRef]
- Fernández-de-Las-Peñas C, Fuensalida-Novo S, Ortega-Santiago R, Valera-Calero JA, Cescon C, Derboni M, Giuffrida V, Barbero M. Pain Extent Is Not Associated with Sensory-Associated Symptoms, Cognitive or Psychological Variables in COVID-19 Survivors Suffering from Post-COVID Pain. J Clin Med. 2022 Aug 8;11(15):4633. PMID: 35956247; PMCID: PMC9369807. [CrossRef]
- Fernández-de-Las-Peñas C, Herrero-Montes M, Ferrer-Pargada D, Izquierdo-Cuervo S, Arendt-Nielsen L, Nijs J, Parás-Bravo P. Sensitization-Associated Post-COVID-19 Symptoms at 6 Months Are Not Associated with Serological Biomarkers at Hospital Admission in COVID-19 Survivors: A Secondary Analysis of a Cohort Study. J Clin Med. 2022 Jun 18;11(12):3512. [CrossRef]
- Fernández-de-Las-Peñas C, Parás-Bravo P, Ferrer-Pargada D, Cancela-Cilleruelo I, Rodríguez-Jiménez J, Nijs J, Arendt-Nielsen L, Herrero-Montes M. Sensitization symptoms are associated with psychological and cognitive variables in COVID-19 survivors exhibiting post-COVID pain. Pain Pract. 2023 Jan;23(1):23-31. Epub 2022 Jul 5. PMID: 35757896; PMCID: PMC9350126. [CrossRef]
- Baroni A, Fregna G, Lamberti N, Manfredini F, Straudi S. Fatigue can influence the development of late-onset pain in post-COVID-19 syndrome: An observational study. Eur J Pain. 2024 Jul;28(6):901-912. Epub 2023 Dec 28. PMID: 38155562. [CrossRef]
- https://nap.nationalacademies.org/read/27768/chapter/3#28.
- https://nap.nationalacademies.org/resource/27768/Long_COVID_Definition_infographic.pdf.
- https://www.cdc.gov/covid/long-term-effects/?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.
- https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition.
- https://www.covid.gov/sites/default/files/documents/National-Research-Action-Plan-on-Long-COVID-08012022.pdf.
- Soriano JB, Murthy S, Marshall JC, Relan P, Diaz J V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Vol. 22, The Lancet Infectious Diseases. Elsevier Ltd; 2022. p. e102–7.
- https://www.england.nhs.uk/coronavirus/post-covid-syndrome-long-covid/ https://www.nice.org.uk/guidance/ng188/chapter/1-Identification#case-definition (Last updated: 25 January 2024).
- https://www.nice.org.uk/guidance/ng188/chapter/1-Identification#case-definition.
- https://covid19dashboard.mohfw.gov.in/pdf/NationalComprehensiveGuidelinesforManagementofPostCovidSequelae.pdf.
- Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, McCorkell L, et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA. 2023 Jun 13;329(22):1934-1946. PMID: 37278994; PMCID: PMC10214179. [CrossRef]
- https://recovercovid.org/long-covid.
- Carrasco-Vega E, Martínez-Moya M, Barni L, Guiducci S, Nacci F, Gonzalez-Sanchez M. Questionnaires for the subjective evaluation of patients with fibromyalgia: a systematic review. Eur J Phys Rehabil Med. 2023 Jun;59(3):353-363. Epub 2023 May 15. PMID: 37184415; PMCID: PMC10272930. [CrossRef]
- Shevlin M, Nolan E, Owczarek M, McBride O, Murphy J, Gibson Miller J, Hartman TK, Levita L, Mason L, Martinez AP, McKay R, Stocks TVA, Bennett KM, Hyland P, Bentall RP. COVID-19-related anxiety predicts somatic symptoms in the UK population. Br J Health Psychol. 2020 Nov;25(4):875-882. Epub 2020 May 27. PMID: 32458550; PMCID: PMC7283836. [CrossRef]
- Falco P, Litewczuk D, Di Stefano G, Galosi E, Leone C, De Stefano G, Di Pietro G, Tramontana L, Ciardi MR, Pasculli P, Zingaropoli MA, Arendt-Nielsen L, Truini A. Small fibre neuropathy frequently underlies the painful long-COVID syndrome. Pain. 2024 Sep 1;165(9):2002-2010. Epub 2024 May 7. PMID: 38723183. [CrossRef]
- Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022 Feb;23(2):194-202. Epub 2022 Feb 1. PMID: 35105985; PMCID: PMC9127978. [CrossRef]
- Gendelman O, Amital H, Bar-On Y, Ben-Ami Shor D, Amital D, Tiosano S, Shalev V, Chodick G, Weitzman D. Time to diagnosis of fibromyalgia and factors associated with delayed diagnosis in primary care. Best Pract Res Clin Rheumatol. 2018 Aug;32(4):489-499. Epub 2019 Mar 4. PMID: 31174818. [CrossRef]
- Lee JY, Park SY, Kim WH, Cho HR. Nationwide-incidence and trends of fibromyalgia in South Korea: a population-based study. Rheumatol Int. 2023 Nov;43(11):2049-2056. Epub 2023 Aug 25. PMID: 37624398. [CrossRef]
- Ora J, Calzetta L, Frugoni C, Puxeddu E, Rogliani P. Expert guidance on the management and challenges of long-COVID syndrome: a systematic review. Expert Opin Pharmacother. 2023 Feb;24(3):315-330. Epub 2023 Jan 1. PMID: 36542805. [CrossRef]
- Chee YJ, Fan BE, Young BE, Dalan R, Lye DC. Clinical trials on the pharmacological treatment of long COVID: A systematic review. J Med Virol. 2023 Jan;95(1):e28289. Epub 2022 Nov 18. PMID: 36349400; PMCID: PMC9878018. [CrossRef]
- Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020 Aug 11;370:m3026. PMID: 32784198. [CrossRef]
- Torok RA, Lubell J, Rudy RM, Eccles JA, Quadt L. Variant connective tissue as a risk factor for Long COVID: a case-control study. Preprint (medRxiv). March 1, 2025. Available from https://doi.org/10.1101/2025.02.27.25323047. [CrossRef]
- Eckey M, Li P, Morrison B, Davis RW, Xiao W. Patient-Reported Treatment Outcomes in ME/CFS and Long COVID. Preprint (medRxiv) 2024. Available from: https://doi.org/10.1101/2024.11.27.24317656. [CrossRef]
- Eastina EF, Machnik JV, Larsen NW, Seliger J, Geng LN, Bonilla H, et al. Evaluating Long-Term Autonomic Dysfunction and Functional Impacts of Long COVID: A Follow-Up Study. Preprint (medRxiv). 2024. Available from: https://doi.org/10.1101/2024.10.11.24315277. [CrossRef]
- Eastin EF, Machnik JV, Stiles LE, Larsen NW, Seliger J, Geng LN, Bonilla H, Yang PC, Miglis MG. Chronic autonomic symptom burden in long-COVID: a follow-up cohort study. Clin Auton Res. 2025 Feb 5. Epub ahead of print. PMID: 39907931. [CrossRef]
- Wilson GN. A gene network implicated in the joint-muscle pain, brain fog, chronic fatigue, and bowel irregularity of Ehlers-Danlos and “long” COVID19 syndromes. Preprint (medRxiv). 2023:2023.03.24.23287706. Available from: https://doi.org/10.1101/2023.03.24.23287706. [CrossRef]
- Liu S, Liu Y, Liu Y. Somatic symptoms and concern regarding COVID-19 among Chinese college and primary school students: A cross-sectional survey. Psychiatry Res. 2020 Jul;289:113070. Epub 2020 May 15. PMID: 32422501; PMCID: PMC7227526. [CrossRef]
- Peter RS, Nieters A, Göpel S, Merle U, Steinacker JM, Deibert P, et al. Persistent symptoms and clinical findings in adults with post-acute sequelae of COVID-19/post-COVID-19 syndrome in the second year after acute infection: A population-based, nested case-control study. PLoS Med. 2025 Jan 23;22(1):e1004511.
- Chasco EE, Dukes K, Jones D, Comellas AP, Hoffman RM, Garg A. Brain Fog and Fatigue following COVID-19 Infection: An Exploratory Study of Patient Experiences of Long COVID. Int J Environ Res Public Health. 2022 Nov 23;19(23):15499. PMID: 36497573; PMCID: PMC9737348. [CrossRef]
- Wurz A, Culos-Reed SN, Franklin K, DeMars J, Wrightson JG, Twomey R. “I feel like my body is broken”: exploring the experiences of people living with long COVID. Qual Life Res. 2022 Dec;31(12):3339-3354. Epub 2022 Jul 11. PMID: 35816258; PMCID: PMC9272651. [CrossRef]
- Grayston R, Czanner G, Elhadd K, Goebel A, Frank B, Üçeyler N, Malik RA, Alam U. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019 Apr;48(5):933-940. Epub 2018 Aug 23. PMID: 30314675. [CrossRef]
- Santos Guedes de Sa K, Silva J, Bayarri-Olmos R, Brinda R, Alec Rath Constable R, Colom Diaz PA, Kwon DI, Rodrigues G, Wenxue L, Baker C, Bhattacharjee B, Wood J, Tabacof L, Liu Y, Putrino D, Horvath TL, Iwasaki A. A causal link between autoantibodies and neurological symptoms in long COVID. medRxiv [Preprint]. 2024 Jun 19:2024.06.18.24309100. PMID: 38947091; PMCID: PMC11213106. [CrossRef]
- Goldenberg DL. Fibromyalgia and other chronic fatigue syndromes: is there evidence for chronic viral disease? Semin Arthritis Rheum. 1988 Nov;18(2):111-20. PMID: 3064302. [CrossRef]
- Neblett R, Sanabria-Mazo JP, Luciano JV, Mirčić M, Čolović P, Bojanić M, Jeremić-Knežević M, Aleksandrić T, Knežević A. Is the Central Sensitization Inventory (CSI) associated with quantitative sensory testing (QST)? A systematic review and meta-analysis. Neurosci Biobehav Rev. 2024 Jun;161:105612. Epub 2024 Apr 10. PMID: 38604015. [CrossRef]
- Bergmans RS, Clauw DJ, Flint C, Harris H, Lederman S, Schrepf A. Chronic overlapping pain conditions increase the risk of long COVID features, regardless of acute COVID status. Pain. 2024 May 1;165(5):1112-1120. Epub 2023 Nov 9. PMID: 38112577; PMCID: PMC11017744. [CrossRef]
- Lugassy-Galper BE, Amital M, Amital H, Buskila D, Amital D. The Role of Obsessive Compulsive Traits in Fibromyalgia: Is Pain-Related Obsessive Ideation Involved in Pathogenesis? Medicina (Kaunas). 2024 Jun 23;60(7):1027. PMID: 39064456; PMCID: PMC11279314. [CrossRef]
- Ghavidel-Parsa B, Bidari A, Atrkarroushan Z, Khosousi MJ. Implication of the Nociplastic Features for Clinical Diagnosis of Fibromyalgia: Development of the Preliminary Nociplastic-Based Fibromyalgia Features (NFF) Tool. ACR Open Rheumatol. 2022 Mar;4(3):260-268. Epub 2021 Dec 22. PMID: 34936234; PMCID: PMC8916565. [CrossRef]
- Cliton Bezerra M, Valentim Bittencourt J, Reis FJJ, de Almeida RS, Meziat-Filho NAM, Nogueira LAC. Central Sensitization Inventory is a useless instrument for detection of the impairment of the conditioned pain modulation in patients with chronic musculoskeletal pain. Joint Bone Spine. 2021 May;88(3):105127. Epub 2021 Jan 30. PMID: 33359767. [CrossRef]
- Clauw DJ, Choy EHS, Napadow V, Soni A, Boehnke KF, Naliboff B, Hassett AL, Arewasikporn A, Schrepf A, Kaplan CM, Williams D, Basu N, Bergmans RS, Harris RE, Harte SE, Chadwick A, Macfarlane GJ. Hypothetical model ignores many important pathophysiologic mechanisms in fibromyalgia. Nat Rev Rheumatol. 2023 May;19(5):321. PMID: 36964334; PMCID: PMC10878028. [CrossRef]
- Gil-Ugidos A, Vázquez-Millán A, Samartin-Veiga N, Carrillo-de-la-Peña MT. Conditioned pain modulation (CPM) paradigm type affects its sensitivity as a biomarker of fibromyalgia. Sci Rep. 2024 Apr 2;14(1):7798. PMID: 38565572; PMCID: PMC10987675. [CrossRef]
- Rost S, Van Ryckeghem DM, Schulz A, Crombez G, Vögele C. Generalized hypervigilance in fibromyalgia: Normal interoceptive accuracy, but reduced self-regulatory capacity. J Psychosom Res. 2017 Feb;93:48-54. Epub 2016 Dec 6. PMID: 28107892. [CrossRef]
- Zetterman T, Markkula R, Partanen JV, Miettinen T, Estlander AM, Kalso E. Muscle activity and acute stress in fibromyalgia. BMC Musculoskelet Disord. 2021 Feb 14;22(1):183. PMID: 33583408; PMCID: PMC7883576. [CrossRef]
- Frangos E, Čeko M, Wang B, Richards EA, Gracely JL, Colloca L, Schweinhardt P, Bushnell MC. Neural effects of placebo analgesia in fibromyalgia patients and healthy individuals. Pain. 2021 Feb 1;162(2):641-652. PMID: 32925593; PMCID: PMC7808362. [CrossRef]
- Pettersen PS, Haugmark T, Berg IJ, Hammer HB, Neogi T, Zangi H, Haugen IK, Provan SA. Pain sensitization in fibromyalgia. Cross-sectional associations between quantitative sensory testing of pain sensitization and fibromyalgia disease burden. Eur J Pain. 2025 Jan;29(1):e4771. PMID: 39670546; PMCID: PMC11639049. [CrossRef]
- Müller M, Wüthrich F, Federspiel A, Wiest R, Egloff N, Reichenbach S, Exadaktylos A, Jüni P, Curatolo M, Walther S. Altered central pain processing in fibromyalgia-A multimodal neuroimaging case-control study using arterial spin labelling. PLoS One. 2021 Feb 2;16(2):e0235879. PMID: 33529254; PMCID: PMC7853499. [CrossRef]
- CROHN BB. Peptic ulcer as a psychosomatic disease. Surg Clin North Am. 1947 Apr;27:309-14. PMID: 20293907. [CrossRef]
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021 Aug 1;38.
- Lam GY, Damant RW, Ferrara G, Lim RK, Stickland MK, Ogando NS, Power C, Smith MP. Characterizing long-COVID brain fog: a retrospective cohort study. J Neurol. 2023 Oct;270(10):4640-4646. Epub 2023 Aug 9. PMID: 37555926. [CrossRef]
- Zhou F, Tao M, Shang L, Liu Y, Pan G, Jin Y, et al. Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis. Front Med (Lausanne). 2021 Nov 23;8.
- www.uptodate.com search “COVID-19: Evaluation and management of adults following acute viral illness” section: Persistent symptoms, by Mark E Mikkelsen & Benjamin Abramoff. Accessed February 2022.
- Lauwers M, Au M, Yuan S, Wen C. COVID-19 in Joint Ageing and Osteoarthritis: Current Status and Perspectives. Vol. 23, International Journal of Molecular Sciences. MDPI; 2022.
- Willi S, Lüthold R, Hunt A, Hänggi NV, Sejdiu D, Scaff C, et al. COVID-19 sequelae in adults aged less than 50 years: A systematic review. Vol. 40, Travel Medicine and Infectious Disease. Elsevier Inc.; 2021.
- Wolfe F, Rasker JJ, Häuser W. Hearing loss in fibromyalgia? Somatic sensory and non-sensory symptoms in patients with fibromyalgia and other rheumatic disorders. Clin Exp Rheumatol. 2012;30(6 Suppl 74):88–93.
- https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-fibromyalgia-in-adults?search=fibrommyalgia&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 By Don L Goldenberg, accessed March 2021.
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010 Aug;62(8):2545-55. PMID: 20506181; PMCID: PMC2921024. [CrossRef]
- Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol. 2019 Oct 1;86(4):504–16.
- Wang JC, Sung FC, Men M, Wang KA, Lin CL, Kao CH. Bidirectional association between fibromyalgia and gastroesophageal reflux disease: two population-based retrospective cohort analysis. Pain. 2017 Oct;158(10):1971-1978. PMID: 28683023. [CrossRef]
- Seefried S, Barcic A, Grijalva Yepez MF, Reinhardt L, Appeltshauser L, Doppler K, Üçeyler N, Sommer C. Autoantibodies in patients with fibromyalgia syndrome. Pain. 2025 Feb 5. Epub ahead of print. PMID: 39907533. [CrossRef]
- Nishikai M, Tomomatsu S, Hankins RW, Takagi S, Miyachi K, Kosaka S, Akiya K. Autoantibodies to a 68/48 kDa protein in chronic fatigue syndrome and primary fibromyalgia: a possible marker for hypersomnia and cognitive disorders. Rheumatology (Oxford). 2001 Jul;40(7):806-10. PMID: 11477286. [CrossRef]
- D.J. Wallace, D.J. Clauw (Eds.), Fibromyalgia and other central syndromes, Lippincott Williams & Wilkins, Philadelphia (2005). Chapter 4: The Concept of Central Sensitivity Syndromes by Yunus MB. pp 29-44.
- Treister-Goltzman Y, Peleg R. Fibromyalgia and mortality: a systematic review and meta-analysis. RMD Open. 2023 Jul;9(3):e003005. PMID: 37429737; PMCID: PMC10335452. [CrossRef]
- Adams GR, Gandhi W, Harrison R, van Reekum CM, Wood-Anderson D, Gilron I, Salomons TV. Do “central sensitization” questionnaires reflect measures of nociceptive sensitization or psychological constructs? A systematic review and meta-analyses. Pain. 2023 Jun 1;164(6):1222-1239. Epub 2022 Nov 29. PMID: 36729810. [CrossRef]
- Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, Perez Y, Gatchel RJ. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012 Apr;12(4):276-85. Epub 2011 Sep 27. PMID: 21951710; PMCID: PMC3248986. [CrossRef]
- Salbego RS, Conti PCR, Soares FFC, Ferreira DMAO, Herreira-Ferreira M, de Lima-Netto BA, Costa YM, Bonjardim LR. Central sensitization inventory is associated with psychological functioning but not with psychophysical assessment of pain amplification. Eur J Pain. 2025 Feb;29(2):e4713. Epub 2024 Aug 9. PMID: 39120067. [CrossRef]
- Berwick RJ, Sahbaie P, Kenny G, Guo TZ, Neiland H, Andersson DA, Clark JD, Mallon P, Goebel A. Postacute COVID-19 syndrome and fibromyalgia syndrome are associated with anti-satellite glial cell IgG serum autoantibodies but only fibromyalgia syndrome serum-IgG is pronociceptive. Pain. 2025 May 6. Epub ahead of print. PMID: 40408228. [CrossRef]
- Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8.
- Falco P, Galosi E, Di Stefano G, Leone C, Di Pietro G, Tramontana L, De Stefano G, Litewczuk D, Esposito N, Truini A. Autonomic Small-Fiber Pathology in Patients With Fibromyalgia. J Pain. 2024 Jan;25(1):64-72. Epub 2023 Jul 29. PMID: 37524221. [CrossRef]
- Fang H, Hou Q, Zhang W, Su Z, Zhang J, Li J, Lin J, Wang Z, Yu X, Yang Y, Wang Q, Li X, Li Y, Hu L, Li S, Wang X, Liao L. Fecal Microbiota Transplantation Improves Clinical Symptoms of Fibromyalgia: An Open-Label, Randomized, Nonplacebo-Controlled Study. J Pain. 2024 Sep;25(9):104535. Epub 2024 Apr 24. PMID: 38663650. [CrossRef]
- Pinto AM, Luís M, Geenen R, Palavra F, Lumley MA, Ablin JN, Amris K, Branco J, Buskila D, Castelhano J, Castelo-Branco M. Neurophysiological and psychosocial mechanisms of fibromyalgia: a comprehensive review and call for an integrative model. Neuroscience & Biobehavioral Reviews. 2023 Aug 1;151:105235.
- Schrepf A, Moser S, Harte SE, Basu N, Kaplan C, Kolarik E, Tsodikov A, Brummett CM, Clauw DJ. Top down or bottom up? An observational investigation of improvement in fibromyalgia symptoms following hip and knee replacement. Rheumatology (Oxford). 2020 Mar 1;59(3):594-602. PMID: 31411333; PMCID: PMC7998337. [CrossRef]
- Liptan GL. Fascia: A missing link in our understanding of the pathology of fibromyalgia. J Bodyw Mov Ther. 2010 Jan;14(1):3–12.
- Pinto AM, Geenen R, Wager TD, Lumley MA, Häuser W, Kosek E, Ablin JN, Amris K, Branco J, Buskila D, Castelhano J, Castelo-Branco M, Crofford LJ, Fitzcharles MA, López-Solà M, Luís M, Marques TR, Mease PJ, Palavra F, Rhudy JL, Uddin LQ, Castilho P, Jacobs JWG, da Silva JAP. Emotion regulation and the salience network: a hypothetical integrative model of fibromyalgia. Nat Rev Rheumatol. 2023 Jan;19(1):44-60. Epub 2022 Dec 5. PMID: 36471023. [CrossRef]
- Wallace DJ. To fibromyalgia nihilists: stop pontificating and test your hypothesis. J Rheumatol. 2004 Apr;31(4):632. PMID: 15088283.
- Fülöp B, Borbély É, Helyes Z. How does chronic psychosocial distress induce pain? Focus on neuroinflammation and neuroplasticity changes. Brain Behav Immun Health. 2025 Feb 10;44:100964. PMID: 40034488; PMCID: PMC11875130. [CrossRef]
- Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J Pain. 2016 Sep;17(9 Suppl):T93-T107. PMID: 27586833; PMCID: PMC6193199. [CrossRef]
- Bruti G, Foggetti P. Insecure Attachment, Oxytocinergic System and C-Tactile Fibers: An Integrative and Translational Pathophysiological Model of Fibromyalgia and Central Sensitivity Syndromes. Biomedicines. 2024 Aug 2;12(8):1744. PMID: 39200209; PMCID: PMC11351601. [CrossRef]
- Mercado F, Almanza A, Martínez-Martínez LA, Martínez-Lavín M. Fibromyalgia: a satellite gliopathy? Clin Exp Rheumatol. 2025 Jan;43(1):1-3. Epub 2024 Dec 11. PMID: 39661565. [CrossRef]
- Pearce JM. Myofascial pain, fibromyalgia or fibrositis? Eur Neurol. 2004;52(2):67-72. Epub 2004 Jul 13. PMID: 15256826. [CrossRef]
- Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999 Sep 11;354(9182):936-9. PMID: 10489969. [CrossRef]
- Chen G, Olver JS, Kanaan RA. Functional somatic syndromes and joint hypermobility: A systematic review and meta-analysis. J Psychosom Res. 2021 Sep;148:110556. Epub 2021 Jun 24. PMID: 34237584. [CrossRef]
- Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Drewes AM. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain. 2018 Feb;22(2):216-241. Epub 2017 Nov 5. PMID: 29105941. [CrossRef]
- van der Meulen ML, Bos M, Bakker SJL, Gans ROB, Rosmalen JGM. Validity and diagnostic overlap of functional somatic syndrome diagnoses. J Psychosom Res. 2024 Jun;181:111673. Epub 2024 Apr 15. PMID: 38678828. [CrossRef]
- Luís M, Pinto AM, Häuser W, Jacobs JW, Saraiva A, Giorgi V, Sarzi-Puttini P, Castelo-Branco M, Geenen R, Pereira da Silva JA. Fibromyalgia and post-traumatic stress disorder: different parts of an elephant? Clin Exp Rheumatol. 2025 Jun;43(6):1146-1160. [CrossRef]
- Ohlsson B. Extraintestinal manifestations in irritable bowel syndrome: A systematic review. Therap Adv Gastroenterol. 2022 Aug 9;15:17562848221114558. [CrossRef]
- Plaut S. Scoping review and interpretation of myofascial pain/fibromyalgia syndrome: An attempt to assemble a medical puzzle. PLoS One. 2022 Feb 1;17(2 February).
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano: Regulation of connective tissue remodelling. Vol. 3, Nature Reviews Molecular Cell Biology. 2002. p. 349–63.
- Jendzjowsky NG, Kelly MM. The Role of Airway Myofibroblasts in Asthma. Chest. 2019 Dec;156(6):1254-1267. Epub 2019 Aug 28. PMID: 31472157. [CrossRef]
- Johnson RD, Lei M, McVey JH, Camelliti P. Human myofibroblasts increase the arrhythmogenic potential of human induced pluripotent stem cell-derived cardiomyocytes. Cell Mol Life Sci. 2023 Sep 5;80(9):276. Erratum in: Cell Mol Life Sci. 2024 Dec 27;82(1):20. doi: 10.1007/s00018-024-05492-w. PMID: 37668685; PMCID: PMC10480244. [CrossRef]
- Kruglikov IL, Scherer PE. The Role of Adipocytes and Adipocyte-Like Cells in the Severity of COVID-19 Infections. Obesity. 2020 Jul 1;28(7):1187–90.
- Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Vol. 587, Nature. Nature Research; 2020. p. 555–66.
- Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020 Jan;16(1):11-31. Epub 2019 Dec 2. PMID: 31792399; PMCID: PMC7913072. [CrossRef]
- Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005 Mar;288(3):H1131-8. Epub 2004 Oct 21. PMID: 15498824. [CrossRef]
- Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thüringer A, Leski K, Fickert P, Karpen SJ, Trauner M. Curcumin improves sclerosing cholangitis in Mdr2-/- mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010 Apr;59(4):521-30. PMID: 20332524; PMCID: PMC3756478. [CrossRef]
- Lee SA, Yang HW, Um JY, Shin JM, Park IH, Lee HM. Vitamin D attenuates myofibroblast differentiation and extracellular matrix accumulation in nasal polyp-derived fibroblasts through smad2/3 signaling pathway. Sci Rep. 2017 Aug 4;7(1):7299. PMID: 28779150; PMCID: PMC5544725. [CrossRef]
- Wu M, Han M, Li J, Xu X, Li T, Que L, Ha T, Li C, Chen Q, Li Y. 17beta-estradiol inhibits angiotensin II-induced cardiac myofibroblast differentiation. Eur J Pharmacol. 2009 Aug 15;616(1-3):155-9. Epub 2009 May 24. PMID: 19470381. [CrossRef]
- Yang W, Zhang S, Zhu J, Jiang H, Jia D, Ou T, Qi Z, Zou Y, Qian J, Sun A, Ge J. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J Mol Cell Cardiol. 2019 Sep;134:119-130. [CrossRef]
- Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J, Zhang JS, Kwapiszewska G, Herold S, Schermuly RT, Mari B, Li X, Seeger W, Günther A, Bellusci S, El Agha E. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019 Jul 5;10(1):2987. PMID: 31278260; PMCID: PMC6611870. [CrossRef]
- Schleip R, Klingler W. Active contractile properties of fascia. Vol. 32, Clinical Anatomy. John Wiley and Sons Inc.; 2019. p. 891–5.
- Chaitow L, Schleip R, Huijing P, Findley TW. Fascia: The tensional network of the human body - the science and clinical applications in manual and movement therapy. Churchill Livingstone / Elsevier Ltd. (2012). Chapter 3.5 - Biotensegrity: The mechanics of fascia, by Stephen M Levin and Danièle-Claude Martin. [CrossRef]
- Fede C, Pirri C, Fan C, Petrelli L, Guidolin D, De Caro R, et al. A closer look at the cellular and molecular components of the deep/muscular fasciae. Vol. 22, International Journal of Molecular Sciences. MDPI AG; 2021. p. 1–13.
- Chiu PE, Fu Z, Sun J, Jian GW, Li TM, Chou LW. Efficacy of Fu’s Subcutaneous Needling in Treating Soft Tissue Pain of Knee Osteoarthritis: A Randomized Clinical Trial. J Clin Med. 2022 Dec 1;11(23).
- Wilke J, Schleip R, Yucesoy CA, Banzer W. Not merely a protective packing organ? A review of fascia and its force transmission capacity. J Appl Physiol [Internet]. 2018;124:234–44. Available from: http://www.jappl.org.
- Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971 Aug 6;173(3996):548-50. [CrossRef]
- Hansson E, Skiöldebrand E. Coupled cell networks are target cells of inflammation, which can spread between different body organs and develop into systemic chronic inflammation. Vol. 12, Journal of Inflammation (United Kingdom). BioMed Central Ltd.; 2015.
- Langevin HM, Cornbrooks CJ, Taatjes DJ. Fibroblasts form a body-wide cellular network. Histochem Cell Biol. 2004;122(1):7–15.
- Bisson MA, Mudera V, McGrouther DA, Grobbelaar AO. The contractile properties and responses to tensional loading of Dupuytren’s disease--derived fibroblasts are altered: a cause of the contracture? Plast Reconstr Surg. 2004 Feb;113(2):611-21; discussion 622-4. PMID: 14758224. [CrossRef]
- Moyer KE, Banducci DR, Graham WP 3rd, Ehrlich HP. Dupuytren’s disease: physiologic changes in nodule and cord fibroblasts through aging in vitro. Plast Reconstr Surg. 2002 Jul;110(1):187-93; discussion 194-6. PMID: 12087251. [CrossRef]
- Novotny GE, Gnoth C. Variability of fibroblast morphology in vivo: a silver impregnation study on human digital dermis and subcutis. J Anat. 1991 Aug;177:195-207. PMID: 1769894; PMCID: PMC1260427.
- Ko K, Arora P, Lee W, McCulloch C. Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am J Physiol Cell Physiol. 2000 Jul;279(1):C147-57. PMID: 10898726. [CrossRef]
- Lembong J, Sabass B, Sun B, Rogers ME, Stone HA. Mechanics regulates ATP-stimulated collective calcium response in fibroblast cells. J R Soc Interface. 2015 Jul 6;12(108):20150140. PMID: 26063818; PMCID: PMC4528580. [CrossRef]
- Tadeo I, Berbegall AP, Escudero LM, Álvaro T, Noguera R. Biotensegrity of the extracellular matrix: Physiology, dynamic mechanical balance, and implications in oncology and mechanotherapy. Frontiers in Oncology. Frontiers Research Foundation; 2014.
- Micheletti A, Podio-Guidugli P. Seventy years of tensegrities (and counting). Vol. 92, Archive of Applied Mechanics. Springer Science and Business Media Deutschland GmbH; 2022. p. 2525–48.
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124-7. PMID: 7684161. [CrossRef]
- López-Carrasco A, Martín-Vañó S, Burgos-Panadero R, Monferrer E, Berbegall AP, Fernández-Blanco B, Navarro S, Noguera R. Impact of extracellular matrix stiffness on genomic heterogeneity in MYCN-amplified neuroblastoma cell line. J Exp Clin Cancer Res. 2020 Oct 28;39(1):226. PMID: 33109237; PMCID: PMC7592549. [CrossRef]
- Scarr G. Biotensegrity: What is the big deal? Vol. 24, Journal of Bodywork and Movement Therapies. Churchill Livingstone; 2020. p. 134–7.
- Dischiavi SL, Wright AA, Hegedus EJ, Bleakley CM. Biotensegrity and myofascial chains: A global approach to an integrated kinetic chain. Med Hypotheses. 2018 Jan;110:90-96. Epub 2017 Nov 20. PMID: 29317079. [CrossRef]
- Chen, Y. H., Chai, H. M., Shau, Y. W., Wang, C. L., Wang, S. F. (2016). Increased sliding of transverse abdominis during contraction after myofascial release in patients with chronic low back pain. Man Ther, 23, 69-75. [CrossRef]
- Chen B, Cui S, Xu M, Zhang Z, Liu C. Effects of Isometric Plantar-Flexion on the Lower Limb Muscle and Lumbar Tissue Stiffness. Front Bioeng Biotechnol. 2022 Feb 11;9.
- Ingber, D. E. (2006). Cellular mechanotransduction: putting all the pieces together again. FASEB J, 20(7), 811-27. [CrossRef]
- Ingber, D. E. (2008). Tensegrity and mechanotransduction. J Bodyw Mov Ther, 12(3), 198-200. [CrossRef]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003 Apr 1;116(Pt 7):1157-73. PMID: 12615960. [CrossRef]
- Wilke J, Krause F, Vogt L, Banzer W. What is evidence-based about myofascial chains: A systematic review. Vol. 97, Archives of Physical Medicine and Rehabilitation. W.B. Saunders; 2016. p. 454–61.
- Nordez A, Gross R, Andrade R, le Sant G, Freitas S, Ellis R, et al. Non-Muscular Structures Can Limit the Maximal Joint Range of Motion during Stretching. Sports Medicine. 2017 Oct 1;47(10):1925–9.
- Wilke J, Krause F. Myofascial chains of the upper limb: A systematic review of anatomical studies. Clin Anat. 2019 Oct;32(7):934-940. Epub 2019 Jul 2. PMID: 31226229. [CrossRef]
- Behm DG, Cavanaugh T, Quigley P, Reid JC, Nardi PSM, Marchetti PH. Acute bouts of upper and lower body static and dynamic stretching increase non-local joint range of motion. Eur J Appl Physiol. 2016 Jan 1;116(1):241–9.
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, van Wingerden JP, Snijders CJ. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine (Phila Pa 1976). 1995 Apr 1;20(7):753-8. PMID: 7701385.
- Fede C, Porzionato A, Petrelli L, Fan C, Pirri C, Biz C, et al. Fascia and soft tissues innervation in the human hip and their possible role in post-surgical pain. Journal of Orthopaedic Research. 2020 Jul 1;38(7):1646–54.
- Sinhorim L, Amorim MDS, Ortiz ME, Bittencourt EB, Bianco G, da Silva FC, et al. Potential nociceptive role of the thoracolumbar fascia: A scope review involving in vivo and ex vivo studies. Vol. 10, Journal of Clinical Medicine. MDPI; 2021.
- Suarez-rodriguez V, Fede C, Pirri C, Petrelli L, Loro-ferrer JF, Rodriguez-ruiz D, et al. Fascial Innervation: A Systematic Review of the Literature. Vol. 23, International Journal of Molecular Sciences. MDPI; 2022.
- Stacey MJ. Free nerve endings in skeletal muscle of the cat. J Anat. 1969 Sep;105(Pt 2):231-54. PMID: 5802932; PMCID: PMC1232131.
- Fede C, Petrelli L, Guidolin D, Porzionato A, Pirri C, Fan C, et al. Evidence of a new hidden neural network into deep fasciae. Sci Rep. 2021 Dec 1;11(1).
- Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977 Dec;273(1):179-94. PMID: 599419; PMCID: PMC1353733. [CrossRef]
- Marchettini P, Simone DA, Caputi G, Ochoa JL. Pain from excitation of identified muscle nociceptors in humans. Brain Res. 1996 Nov 18;740(1-2):109-16. PMID: 8973804. [CrossRef]
- Laursen RJ, Graven-Nielsen T, Jensen TS, Arendt-Nielsen L. The effect of differential and complete nerve block on experimental muscle pain in humans. Muscle Nerve. 1999 Nov;22(11):1564-70. PMID: 10514235. [CrossRef]
- Gerdle B., Ernberg M., Mannerkorpi K., Larsson B., Kosek E., Christidis N., Ghafouri B. Increased Interstitial Concentrations of Glutamate and Pyruvate in Vastus Lateralis of Women with Fibromyalgia Syndrome Are Normalized after an Exercise Intervention—A Case-Control Study. PLoS ONE. 2016;11:e0162010. [CrossRef]
- Stecco A, Gesi M, Stecco C, Stern R. Fascial components of the myofascial pain syndrome topical collection on myofascial pain. Curr Pain Headache Rep. 2013 Aug 1;17(8).
- Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, et al. Mammalian transient receptor potential TRPA1 channels: From structure to disease. Physiol Rev. 2020 Apr 1;100(2 725):803.
- Richter, F., Segond von Banchet, G. & Schaible, HG. Transient Receptor Potential vanilloid 4 ion channel in C-fibres is involved in mechanonociception of the normal and inflamed joint. Sci Rep 9, 10928 (2019). [CrossRef]
- Di X, Gao X, Peng L, Ai J, Jin X, Qi S, Li H, Wang K, Luo D. Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets. Signal Transduct Target Ther. 2023 Jul 31;8(1):282. PMID: 37518181; PMCID: PMC10387486. [CrossRef]
- Kayal C, Moeendarbary E, Shipley RJ, Phillips JB. Mechanical Response of Neural Cells to Physiologically Relevant Stiffness Gradients. Adv Healthc Mater. 2020 Apr;9(8):e1901036. Epub 2019 Dec 2. PMID: 31793251; PMCID: PMC8407326. [CrossRef]
- Lantoine J, Grevesse T, Villers A, Delhaye G, Mestdagh C, Versaevel M, Mohammed D, Bruyère C, Alaimo L, Lacour SP, Ris L, Gabriele S. Matrix stiffness modulates formation and activity of neuronal networks of controlled architectures. Biomaterials. 2016 May;89:14-24. Epub 2016 Feb 26. PMID: 26946402. [CrossRef]
- Gu Y, Ji Y, Zhao Y, Liu Y, Ding F, Gu X, Yang Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials. 2012 Oct;33(28):6672-81. Epub 2012 Jun 25. PMID: 22738780. [CrossRef]
- Abdo H, Calvo-Enrique L, Lopez JM, Song J, Zhang MD, Usoskin D, El Manira A, Adameyko I, Hjerling-Leffler J, Ernfors P. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019 Aug 16;365(6454):695-699. PMID: 31416963. [CrossRef]
- www.uptodate.com search “chronic exertional compartment syndrome” (section Performance and interpretation of testing) by William P Meehan, III & Michael J O’Brien. Accessed August 2021.
- Wachter KC, Kaeser HE, Gühring H, Ettlin TM, Mennet P, Müller W. Muscle damping measured with a modified pendulum test in patients with fibromyalgia, lumbago, and cervical syndrome. Spine (Phila Pa 1976). 1996;21(18):2137-42.
- Wolfe F, Simons DG, Fricton J, Bennett RM, Goldenberg DL, Gerwin R, Hathaway D, McCain GA, Russell IJ, Sanders HO, et al. The fibromyalgia and myofascial pain syndromes: a preliminary study of tender points and trigger points in persons with fibromyalgia, myofascial pain syndrome and no disease. J Rheumatol. 1992 Jun;19(6):944-51. PMID: 1404132.
- Navarro-Ledesma S, Aguilar-García M, González-Muñoz A, Pruimboom L, Aguilar-Ferrándiz ME. Do Psychological Factors Influence the Elastic Properties of Soft Tissue in Subjects with Fibromyalgia? A Cross-Sectional Observational Study. Biomedicines. 2022 Nov 30;10(12):3077. PMID: 36551833; PMCID: PMC9775315. [CrossRef]
- Lim H, Lee Y, Cha Y, et al. Investigating the Association Between Central Sensitization and Breathing Pattern Disorders: A STROBE-Compliant Cross-Sectional Study. Preprints database December 30, 2024. https://www.preprints.org/manuscript/202412.2517/v1. [CrossRef]
- Staud R. Peripheral pain mechanisms in chronic widespread pain. Best Pract Res Clin Rheumatol. (2011) 25(2):155-64. PMID: 22094192; PMCID: PMC3220877. [CrossRef]
- Ge HY, Wang Y, Danneskiold-Samsøe B, Graven-Nielsen T, Arendt-Nielsen L. The predetermined sites of examination for tender points in fibromyalgia syndrome are frequently associated with myofascial trigger points. J Pain. 2010 Jul;11(7):644-51. Epub 2009 Nov 14. PMID: 19914876. [CrossRef]
- Kawakita K, Miura T, Iwase Y. Deep pain measurement at tender points by pulse algometry with insulated needle electrodes. Pain. 1991 Mar;44(3):235-239. PMID: 2052391. [CrossRef]
- Jespersen K. Fibrositis of Muscles. Ann Rheum Dis. 1950 Mar;9(1):66-70. PMID: 18623836; PMCID: PMC1011658. [CrossRef]
- Kalyan-Raman et al. Muscle pathology in primary fibromyalgia syndrome: a light microscopic, histochemical and ultrastructural study. J Rheumatol. 1984 Dec;11(22):808.
- Sprott H, Salemi S, Gay RE, Bradley LA, Alarcón GS, Oh SJ, et al. Increased DNA fragmentation and ultrastructural changes in fibromyalgic muscle fibres. Ann Rheum Dis. 2004 Mar;63(3):245–51.
- Hénriksson KG, Bengtsson A, Larsson J, Lindström F, Thornell LE. Muscle biopsy findings of possible diagnostic importance in primary fibromyalgia (fibrositis, myofascial syndrome). Lancet. 1982 Dec 18;2(8312):1395. PMID: 6129478. [CrossRef]
- Dolcino M, Tinazzi E, Puccetti A, Lunardi C. Gene Expression Profiling in Fibromyalgia Indicates an Autoimmune Origin of the Disease and Opens New Avenues for Targeted Therapy. J Clin Med. 2020 Jun 10;9(6):1814. PMID: 32532082; PMCID: PMC7356177. [CrossRef]
- Evdokimov D, Kreß L, Dinkel P, Frank J, Sommer C, Uceyler N. Pain-associated mediators and axon pathfinders in fibromyalgia skin cells. Journal of Rheumatology. 2020;47(1):140–8.
- Ramírez-Tejero JA, Martínez-Lara E, Peinado MÁ, Moral ML Del, Siles E. Hydroxytyrosol as a promising ally in the treatment of fibromyalgia. Nutrients. 2020 Aug 1;12(8):1–21.
- Salemi S, Rethage J, Wollina U, Michel BA, Gay RE, Gay S, Sprott H. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003 Jan;30(1):146-50. PMID: 12508404.
- Gronemann ST, Ribel-Madsen S, Bartels EM, Danneskiold-Samsøe B, Bliddal H. Collagen and muscle pathology in fibromyalgia patients. Rheumatology. 2004 Jan;43(1):27–31.
- Gerdle B, Söderberg K, Puigvert LS, Rosendal L, Larsson B. Increased interstitial concentrations of pyruvate and lactate in the trapezius muscle of patients with fibromyalgia: A microdialysis study. J Rehabil Med. 2010 Jul;42(7):679–87.
- McIver KL, Evans C, Kraus RM, Ispas L, Sciotti VM, Hickner RC. NO-mediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain. 2006 Jan;120(1–2):161–9.
- Gerdle B, Ghafouri B, Lund E, Bengtsson A, Lundberg P, Ettinger-Veenstra HV, Leinhard OD, Forsgren MF. Evidence of Mitochondrial Dysfunction in Fibromyalgia: Deviating Muscle Energy Metabolism Detected Using Microdialysis and Magnetic Resonance. J Clin Med. 2020 Oct 31;9(11):3527. PMID: 33142767; PMCID: PMC7693920. [CrossRef]
- Gerdle B., Larsson B., Forsberg F., Ghafouri N., Karlsson L., Stensson N., Ghafouri B. Chronic Widespread Pain: Increased Glutamate and Lactate Concentrations in the Trapezius Muscle and Plasma. Clin. J. Pain. 2014;30:409–420. [CrossRef]
- Israel L, Furer V, Levin-Zaidman S, Dezorella N, Brontvein O, Ablin JN, Gross A. Mitochondrial structural alterations in fibromyalgia: a pilot electron microscopy study. Clin Exp Rheumatol. 2024 Jun;42(6):1215-1223. Epub 2024 Jun 28. PMID: 38966946. [CrossRef]
- Ciampi de Andrade D, Maschietto M, Galhardoni R, Gouveia G, Chile T, Victorino Krepischi AC, et al. Epigenetics insights into chronic pain: DNA hypomethylation in fibromyalgia-a controlled pilot-study. Pain. 2017; 158(8):1473–1480. PMID: 28621701. [CrossRef]
- van Tilburg MAL, Parisien M, Boles RG, Drury GL, Smith-Voudouris J, Verma V, et al. A genetic polymorphism that is associated with mitochondrial energy metabolism increases risk of fibromyalgia. Pain. 2020; 161(12):2860–2871. PMID: 32658146. [CrossRef]
- Rus A, Robles-Fernandez I, Martinez-Gonzalez LJ, Carmona R, Alvarez-Cubero MJ. Influence of Oxidative Stress-Related Genes on Susceptibility to Fibromyalgia. Nurs Res. 2021 Jan/Feb;70(1):44-50. PMID: 32991532. [CrossRef]
- La Rubia M, Rus A, Molina F, Del Moral ML. Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status? Clin Exp Rheumatol. 2013 Nov-Dec;31(6 Suppl 79):S121-7. Epub 2013 Dec 16. Erratum in: Clin Exp Rheumatol. 2015 Nov-Dec;33(6):950. PMID: 24373370.
- Efrati S, Golan H, Bechor Y, Faran Y, Daphna-Tekoah S, Sekler G, et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome - Prospective clinical trial. PLoS One. 2015 May 1;10(5).
- Morf S, Amann-Vesti B, Forster A, Franzeck UK, Koppensteiner R, Uebelhart D, et al. Open Access Microcirculation abnormalities in patients with fibromyalgia-measured by capillary microscopy and laser fluxmetry. 2004; Available from: http://arthritis-research.com/content/7/2/R209.
- Grassi W, Core P, Carlino G, Salaffi F, Cervini C. Capillary permeability in fibromyalgia. J Rheumatol. 1994 Jul;21(7):1328-31. PMID: 7966078.
- Triantafyllias K, Stortz M, de Blasi M, Leistner C, Weinmann-Menke J, Schwarting A. Increased aortic stiffness in patients with fibromyalgia: results of a prospective study on carotid-femoral pulse wave velocity. Clin Exp Rheumatol. 2019 Jan-Feb;37 Suppl 116(1):114-115. Epub 2017 Nov 20. PMID: 29185967.
- Costantini R, Affaitati G, Massimini F, Tana C, Innocenti P, Giamberardino MA. Laparoscopic cholecystectomy for gallbladder calculosis in fibromyalgia patients: Impact on musculoskeletal pain, somatic hyperalgesia and central sensitization. PLoS One. 2016 Apr 15;11(4).
- Raftopoulos Y, Papasavas P, Landreneau R, Hayetian F, Santucci T, Gagné D, Caushaj P, Keenan R. Clinical outcome of laparoscopic antireflux surgery for patients with irritable bowel syndrome. Surg Endosc. 2004 Apr;18(4):655-9. Epub 2004 Mar 19. PMID: 15026924. [CrossRef]
- Disdier P, Harle JR, Brue T, Jaquet P, Chambourlier P, Grisoli F, Weiller PJ. Severe fibromyalgia after hypophysectomy for Cushing’s disease. Arthritis Rheum. 1991 Apr;34(4):493-5. PMID: 2012629. [CrossRef]
- Bhatti MI, Hollingworth P, Leach P. Significant improvement of fibromyalgia symptoms after excision of large meningioma--a case report. Br J Neurosurg. 2014 Jan;28(1):131-2. Epub 2013 Jun 14. PMID: 23767682. [CrossRef]
- D’Onghia M, Ciaffi J, McVeigh JG, Di Martino A, Faldini C, Ablin JN, et al. Fibromyalgia syndrome – a risk factor for poor outcomes following orthopaedic surgery: A systematic review. Semin Arthritis Rheum. 2021 Aug 1;51(4):793–803.
- Saber AA, Boros MJ, Mancl T, Elgamal MH, Song S, Wisadrattanapong T. The effect of laparoscopic Roux-en-Y gastric bypass on fibromyalgia. Obes Surg. 2008 Jun;18(6):652–5.
- Adkisson CD, Yip L, Armstrong MJ, Stang MT, Carty SE, McCoy KL. Fibromyalgia symptoms and medication requirements respond to parathyroidectomy. Surgery (United States). 2014;156(6):1614–21.
- Yang CY, Wu MC, Lin MC, Wei JCC. Risk of irritable bowel syndrome in patients who underwent appendectomy: A nationwide population-based cohort study. EClinicalMedicine. 2020 Jun 1;23.
- Janda AM, As-Sanie S, Rajala B, Tsodikov A, Moser SE, Clauw DJ, Brummett CM. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015 May;122(5):1103-11. PMID: 25768860. [CrossRef]
- Vincent A, Whipple MO, Luedtke CA, Oh TH, Sood R, Smith RL, et al. Pain and other symptom severity in women with fibromyalgia and a previous hysterectomy. J Pain Res. 2011;4:325–9.
- Perez-Ruiz F, Calabozo M, Alonso-Ruiz A, Herrero A, Ruiz-Lucea E, Otermin I. High prevalence of undetected carpal tunnel syndrome in patients with fibromyalgia syndrome. J Rheumatol. 1995 Mar;22(3):501-4. PMID: 7783070.
- Zdebik N, Zdebik A, Bogusławska J, Przeździecka-Dołyk J, Turno-Kręcicka A. Fibromyalgia syndrome and the eye-A review. Surv Ophthalmol. 2021 Jan-Feb;66(1):132-137. Epub 2020 Jun 5. Erratum in: Surv Ophthalmol. 2021 Nov-Dec;66(6):1079. PMID: 32512032. [CrossRef]
- Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol. 1983 Sep;342:383-97. PMID: 6631740; PMCID: PMC1193965. [CrossRef]
- Sugawara O, Atsuta Y, Iwahara T, Muramoto T, Watakabe M, Takemitsu Y. The effects of mechanical compression and hypoxia on nerve root and dorsal root ganglia. An analysis of ectopic firing using an in vitro model. Spine (Phila Pa 1976). 1996;21(18):2089–94. [CrossRef]
- Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999 Dec;82(6):3347-58. PMID: 10601466. [CrossRef]
- Konno S, Kikuchi S, Nagaosa Y. The relationship between intramuscular pressure of the paraspinal muscles and low back pain. Spine (Phila Pa 1976). 1994; 19(19): 2186–9. [CrossRef]
- Palazzo E, Marconi A, Truzzi F, Dallaglio K, Petrachi T, Humbert P, Schnebert S, Perrier E, Dumas M, Pincelli C. Role of neurotrophins on dermal fibroblast survival and differentiation. J Cell Physiol. 2012 Mar;227(3):1017-25. PMID: 21503896. [CrossRef]
- Dolivo DM, Larson SA, Dominko T. Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell Mol Life Sci. 2018 Oct;75(20):3663-3681. Epub 2018 Jul 20. PMID: 30027295; PMCID: PMC11105268. [CrossRef]
- Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019 Aug;9(8):1102-1123. Epub 2019 Jun 13. PMID: 31197017; PMCID: PMC6727976. [CrossRef]
- Mennens SFB, Bolomini-Vittori M, Weiden J, Joosten B, Cambi A, Van Den Dries K. Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci Rep. 2017 Dec 1;7(1).
- Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14292-7. Epub 2010 Jul 26. PMID: 20660725; PMCID: PMC2922571. [CrossRef]
- Ye C, Li WY, Zheng MH, Chen YP. T-helper 17 cell: A distinctive cell in liver diseases. Hepatol Res. 2011 Jan;41(1):22-9. Epub 2010 Nov 25. PMID: 21108703. [CrossRef]
- Zhang S, Howarth PH, Roche WR. Cytokine production by cell cultures from bronchial subepithelial myofibroblasts. J Pathol. 1996 Sep;180(1):95-101. PMID: 8943823. [CrossRef]
- Zhang J, Wang D, Wang L, Wang S, Roden AC, Zhao H, Li X, Prakash YS, Matteson EL, Tschumperlin DJ, Vassallo R. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2019 Mar 1;316(3):L487-L497. Epub 2019 Jan 3. PMID: 30604628. [CrossRef]
- Melms JC, Biermann J, Huang H, Wang Y, Nair A, Tagore S, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021 Jul 1;595(7865):114–9.
- Dinicolantonio JJ, McCarty MF, Barroso-Aranda J, Assanga S, Lujan LML, O’Keefe JH. A nutraceutical strategy for downregulating TGFβ signalling: Prospects for prevention of fibrotic disorders, including post-COVID-19 pulmonary fibrosis. Vol. 8, Open Heart. BMJ Publishing Group; 2021.
- Hartmann C, Miggiolaro AFR dos S, Motta J da S, Baena Carstens L, Busatta Vaz De Paula C, Fagundes Grobe S, et al. The Pathogenesis of COVID-19 Myocardial Injury: An Immunohistochemical Study of Postmortem Biopsies. Front Immunol. 2021 Nov 5;12.
- Yao XH, Luo T, Shi Y, He ZC, Tang R, Zhang PP, et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021 Aug 1;31(8):836–46.
- Omer D, Pleniceanu O, Gnatek Y, Namestnikov M, Cohen-Zontag O, Goldberg S, Friedman YE, Friedman N, Mandelboim M, Vitner EB, Achdout H, Avraham R, Zahavy E, Israely T, Mayan H, Dekel B. Human Kidney Spheroids and Monolayers Provide Insights into SARS-CoV-2 Renal Interactions. J Am Soc Nephrol. 2021 Sep;32(9):2242-2254. Epub 2021 Jun 10. PMID: 34112705; PMCID: PMC8729846. [CrossRef]
- Deshmukh V, Motwani R, Kumar A, Kumari C, Raza K. Histopathological observations in COVID-19: a systematic review. J Clin Pathol. 2021 Feb;74(2):76-83. Epub 2020 Aug 18. PMID: 32817204. [CrossRef]
- Beydon M, Chevalier K, Al Tabaa O, Hamroun S, Delettre AS, Thomas M, et al. Myositis as a manifestation of SARS-CoV-2. Vol. 80, Annals of the Rheumatic Diseases. BMJ Publishing Group; 2021.
- Smelcerovic A, Kocic G, Gajic M, Tomovic K, Djordjevic V, Stankovic-Djordjevic D, et al. DPP-4 Inhibitors in the Prevention/Treatment of Pulmonary Fibrosis, Heart and Kidney Injury Caused by COVID-19—A Therapeutic Approach of Choice in Type 2 Diabetic Patients? Front Pharmacol. 2020 Aug 5;11.
- Cenko E, Badimon L, Bugiardini R, Claeys MJ, De Luca G, De Wit C, et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Vol. 117, Cardiovascular Research. Oxford University Press; 2021. p. 2705–29.
- Unudurthi SD, Luthra P, Bose RJC, McCarthy J, Kontaridis MI. Cardiac inflammation in COVID-19: Lessons from heart failure. Vol. 260, Life Sciences. Elsevier Inc.; 2020.
- Zickler M, Stanelle-Bertram S, Ehret S, Heinrich F, Lange P, Schaumburg B, et al. Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans. Vol. 34, Cell Metabolism. Cell Press; 2022. p. 1–2.
- Kolesova O, Vanaga I, Laivacuma S, Derovs A, Kolesovs A, Radzina M, et al. Intriguing findings of liver fibrosis following COVID-19. BMC Gastroenterol. 2021 Dec 1;21(1).
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16;181(2):271-280.e8.
- Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S, et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Vol. 131, Biomedicine and Pharmacotherapy. Elsevier Masson SAS; 2020.
- Saba L, Gerosa C, Fanni D, Marongiu F, Nasa G La, Caocci G, et al. Molecular pathways triggered by COVID-19 in different organs: ACE2 receptor-expressing cells under attack? A review.
- Queiroz-Junior CM, Santos ACPM, Galvão I, Souto GR, Mesquita RA, Sá MA, et al. The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone. 2019 Nov 1;128.
- Sapra L, Saini C, Garg B, Gupta R, Verma B, Mishra PK, et al. Long-term implications of COVID-19 on bone health: pathophysiology and therapeutics. Vol. 71, Inflammation Research. Springer Science and Business Media Deutschland GmbH; 2022. p. 1025–40.
- Mokuda S, Tokunaga T, Masumoto J, Sugiyama E. Angiotensin-converting enzyme 2, a SARS-CoV-2 receptor, is upregulated by interleukin 6 through STAT3 signaling in synovial tissues. Vol. 47, Journal of Rheumatology. Journal of Rheumatology; 2020. p. 1593–5.
- Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, et al. Musculoskeletal Consequences of COVID-19. Vol. 102, Journal of Bone and Joint Surgery - American Volume. Lippincott Williams and Wilkins; 2020. p. 1197–204.
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology. 2004 Jun;203(2):631–7.
- Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Katoyich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci. 2007 Oct;113(7–8):357–64.
- Jiang HS, Zhu LL, Zhang Z, Chen H, Chen Y, Dai YT. Estradiol attenuates the TGF-β1-induced conversion of primary TAFs into myofibroblasts and inhibits collagen production and myofibroblast contraction by modulating the Smad and Rho/ROCK signaling pathways. Int J Mol Med. 2015 Sep 1;36(3):801–7.
- Wu M, Han M, Li J, Xu X, Li T, Que L, et al. 17β-estradiol inhibits angiotensin II-induced cardiac myofibroblast differentiation. Eur J Pharmacol. 2009 Aug 15;616(1–3):155–9.
- Mongelli A, Barbi V, Zamperla MG, Atlante S, Forleo L, Nesta M, et al. Evidence for biological age acceleration and telomere shortening in covid-19 survivors. Int J Mol Sci. 2021 Jun 1;22(11).
- Neurath MF, Uberla K, Ng SC. Gut as viral reservoir: Lessons from gut viromes, HIV and COVID-19. Gut. 2021 Sep 1;70(9):1605–8.
- Opening remarks at the media briefing on COVID-19. 2020 March 11. In: WHO Director- General. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed December 12, 2024.
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA et al (2021) Post-acute COVID-19 syndrome. Nat Med 27(4):601–615.
- Voruz P, Assal F, Péron JA. The economic burden of the post-COVID-19 condition: Underestimated long-term consequences of neuropsychological deficits. J Glob Health. 2023 May 5;13:03019. PMID: 37141527; PMCID: PMC10159592. [CrossRef]
- McNarry MA, Berg RMG, Shelley J, Hudson J, Saynor ZL, Duckers J, Lewis K, Davies GA, Mackintosh KA. Inspiratory muscle training enhances recovery post-COVID-19: a randomised controlled trial. Eur Respir J. 2022 Oct 6;60(4):2103101. PMID: 35236727; PMCID: PMC8900538. [CrossRef]
- Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021 Oct;53(10):737-754. Epub 2021 May 22. PMID: 34024217; PMCID: PMC8146298. [CrossRef]
- Enck P, Mazurak N. The “Biology-First” Hypothesis: Functional disorders may begin and end with biology-A scoping review. Neurogastroenterol Motil. 2018 Oct;30(10):e13394. Epub 2018 Jun 28. PMID: 29956418. [CrossRef]
- Jorge MSG, Nepomuceno P, Schneider RH, Wibelinger LM. Eight weeks of Pilates Method improves physical fitness and sleep quality of individuals with post-COVID-19 syndrome: A randomized clinical trial blinded. J Bodyw Mov Ther. 2025 Mar;41:238-245. Epub 2024 Nov 23. PMID: 39663092. [CrossRef]
- Seo BR, Chen X, Ling L, Song YH, Shimpi AA, Choi S, et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc Natl Acad Sci U S A. 2020;117(21):11387–11398. [CrossRef]
- Zoppi N, Chiarelli N, Binetti S, Ritelli M, Colombi M. Dermal fibroblast-to-myofibroblast transition sustained by αvß3 integrin-ILK-Snail1/Slug signaling is a common feature for hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Biochim Biophys Acta Mol Basis Dis. 2018 Apr 1;1864(4):1010–23.
- Squier CA. Cell and Tissue Research The effect of stretching on formation of myofibroblasts in mouse skin. Vol. 220, Cell Tissue Res. 1981.
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. American Journal of Pathology. 2001;159(3):1009–20.
- Ramirez-Moreno JM, Ceberino D, Gonzalez Plata A, Rebollo B, Macias Sedas P, Hariramani R, et al. Mask-associated ‘de novo’ headache in healthcare workers during the COVID-19 pandemic. Occup Environ Med. 2021 Aug 1;78(8):541–7.
- Lim ECH, Ong BKC, Seet RCS. Headaches and the N95 face-mask amongst healthcare providers [2]. Vol. 116, Acta Neurologica Scandinavica. 2007. p. 73.
- Blaauboer ME, Smit TH, Hanemaaijer R, Stoop R, Everts V. Cyclic mechanical stretch reduces myofibroblast differentiation of primary lung fibroblasts. Biochem Biophys Res Commun. 2011 Jan 7;404(1):23-7. Epub 2010 Nov 20. PMID: 21094632. [CrossRef]
- Bouffard NA, Cutroneo KR, Badger GJ, White SL, Buttolph TR, Ehrlich HP, Stevens-Tuttle D, Langevin HM. Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: evidence from ex vivo and in vivo models. J Cell Physiol. 2008 Feb;214(2):389-95. PMID: 17654495; PMCID: PMC3065715. [CrossRef]
- Sasabe R, Sakamoto J, Goto K, Honda Y, Kataoka H, Nakano J, et al. Effects of joint immobilization on changes in myofibroblasts and collagen in the rat knee contracture model. Journal of Orthopaedic Research. 2017 Sep 1;35(9):1998–2006.
- Farmer‹ SE, Jamesoe M. Contractures in orthopaedic and neurological conditions: a review of causes and treatment. Disabil Rehabil. 2001;23(13):549-58.
- Ko UH, Choi J, Choung J, Moon S, Shin JH. Physicochemically Tuned Myofibroblasts for Wound Healing Strategy. Sci Rep. 2019 Nov 5;9(1):16070. PMID: 31690789; PMCID: PMC6831678. [CrossRef]
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of gastroenterology: quarterly publication of the Hellenic Society of Gastroenterology. 2015 Apr;28(2):203.
- Marcelin G, Silveira ALM, Martins LB, Ferreira AVM, Clément K. Deciphering the cellular interplays underlying obesityinduced adipose tissue fibrosis. Vol. 129, Journal of Clinical Investigation. American Society for Clinical Investigation; 2019. p. 4032–40.
- Kruglikov IL, Scherer PE. Adipocyte-myofibroblast transition as a possible pathophysiological step in androgenetic alopecia. Vol. 26, Experimental Dermatology. Blackwell Publishing Ltd; 2017. p. 522–3.
- Makiko Iguchi AS, Hara M, Manome H, Kobayasi H. EXPERIMENTAL DERMATOLOGY Communication network in the follicular papilla and connective tissue sheath through gap junctions in human hair follicles Communication network in the follicular papilla and connective tissue Hachiro Tagami and Setsuya Aiba controlling the dynamic structural changes of hair follicles during hair cycling. Exp Dermatol. 2003;12:283–8.
- Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984 Nov;356:443-58. PMID: 6520794; PMCID: PMC1193174. [CrossRef]
- Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. 2021 Nov;112:217-226. Epub 2021 Sep 23. PMID: 34563706; PMCID: PMC8459548. [CrossRef]
- Yu Y, Ren LJ, Liu XY, Gong XB, Yao W. Effects of substrate stiffness on mast cell migration. Eur J Cell Biol. 2021 Sep-Nov;100(7-8):151178. Epub 2021 Sep 17. PMID: 34555639. [CrossRef]
- Belle L, Zhou V, Stuhr KL, Beatka M, Siebers EM, Knight JM, Lawlor MW, Weaver C, Hashizume M, Hillard CJ, Drobyski WR. Host interleukin 6 production regulates inflammation but not tryptophan metabolism in the brain during murine GVHD. JCI Insight. 2017 Jul 20;2(14):e93726. PMID: 28724796; PMCID: PMC5518565. [CrossRef]
- Xie T, Lv T, Zhang T, Feng D, Zhu F, Xu Y, Zhang L, Gu L, Guo Z, Ding C, Gong J. Interleukin-6 promotes skeletal muscle catabolism by activating tryptophan-indoleamine 2,3-dioxygenase 1-kynurenine pathway during intra-abdominal sepsis. J Cachexia Sarcopenia Muscle. 2023 Apr;14(2):1046-1059. Epub 2023 Mar 7. PMID: 36880228; PMCID: PMC10067504. [CrossRef]
- Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012 Apr;279(8):1356-65. Epub 2012 Mar 27. PMID: 22248144. [CrossRef]
- Walker AK, Wing EE, Banks WA, Dantzer R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol Psychiatry. 2019 Oct;24(10):1523-1532. Epub 2018 Jul 9. PMID: 29988087; PMCID: PMC6326900. [CrossRef]
- Groven N, Reitan SK, Fors EA, Guzey IC. Kynurenine metabolites and ratios differ between Chronic Fatigue Syndrome, Fibromyalgia, and healthy controls. Psychoneuroendocrinology. 2021 Sep;131:105287. Epub 2021 May 27. PMID: 34090138. [CrossRef]
- Schwarz MJ, Offenbaecher M, Neumeister A, Ewert T, Willeit M, Praschak-Rieder N, Zach J, Zacherl M, Lossau K, Weisser R, Stucki G, Ackenheil M. Evidence for an altered tryptophan metabolism in fibromyalgia. Neurobiol Dis. 2002 Dec;11(3):434-42. PMID: 12586552. [CrossRef]
- Martin-Gallausiaux C, Larraufie P, Jarry A, Béguet-Crespel F, Marinelli L, Ledue F, Reimann F, Blottière HM, Lapaque N. Butyrate produced by commensal bacteria down-regulates indolamine 2, 3-dioxygenase 1 (IDO-1) expression via a dual mechanism in human intestinal epithelial cells. Front Immunol. 2018;9:2838.
- Andrade BS, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, Dos Santos Freitas A, et al. Long-covid and post-covid health complications: An up-to-date review on clinical conditions and their possible molecular mechanisms. Vol. 13, Viruses. MDPI AG; 2021.
- Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. (2006) 172(2):259-68. Epub 2006 Jan 9. PMID: 16401722; PMCID: PMC2063555. [CrossRef]
- Katsouri L, Ashraf A, Birch AM, Lee KKL, Mirzaei N, Sastre M. Systemic administration of fibroblast growth factor-2 (FGF2) reduces BACE1 expression and amyloid pathology in APP23 mice. Neurobiol Aging. 2015 Feb 1;36(2):821–31.
- Koskinen MK, van Mourik Y, Smit AB, Riga D, Spijker S. From stress to depression: development of extracellular matrix-dependent cognitive impairment following social stress. Sci Rep. 2020 Dec 1;10(1).
- Schleip R, Wilke J, Schreiner S, Wetterslev M, Klingler W. Needle biopsy-derived myofascial tissue samples are sufficient for quantification of myofibroblast density. Clin Anat. 2018 Apr;31(3):368-372. Epub 2018 Jan 30. PMID: 29314236. [CrossRef]
- Langevin HM, Churchill DL, Cipolla MJ. Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. FASEB J. (2001) 15(12):2275-82. PMID: 11641255. [CrossRef]
- Greenhalgh T, Sivan M, Delaney B, Evans R, Milne R. Long covid - an update for primary care. The BMJ. 2022;
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015 Sep;13(3):141-6. PMID: 26134548. [CrossRef]
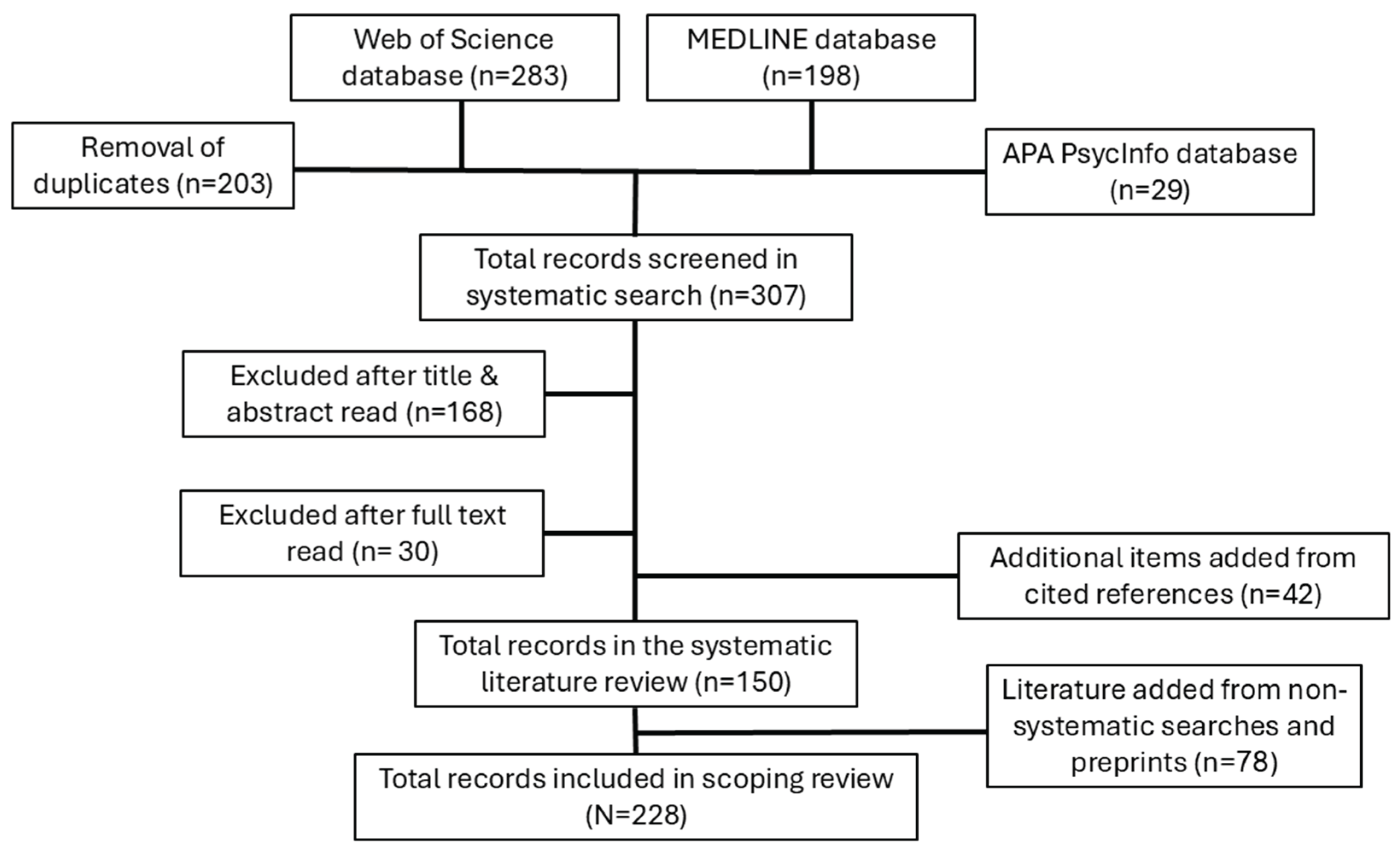
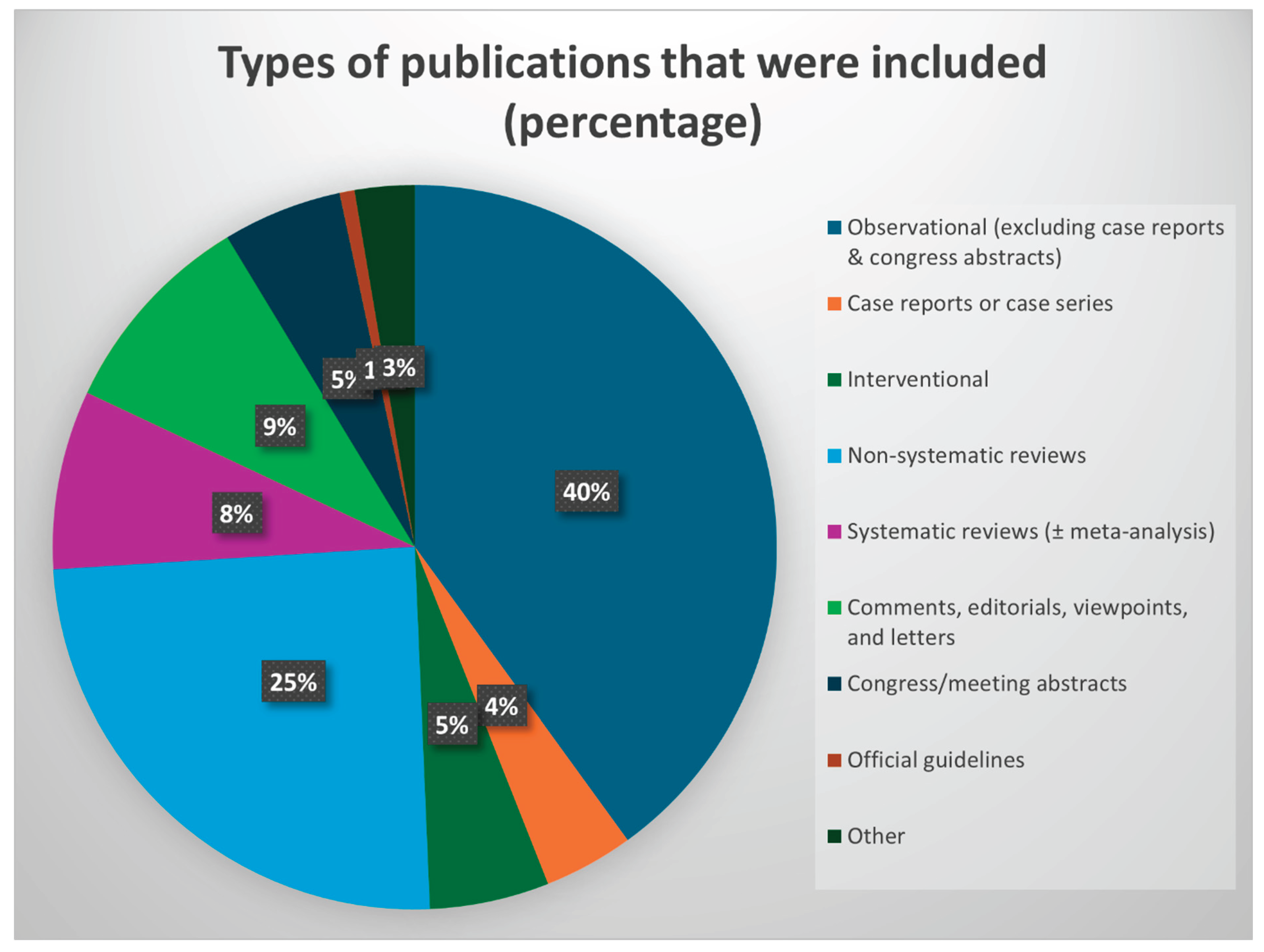

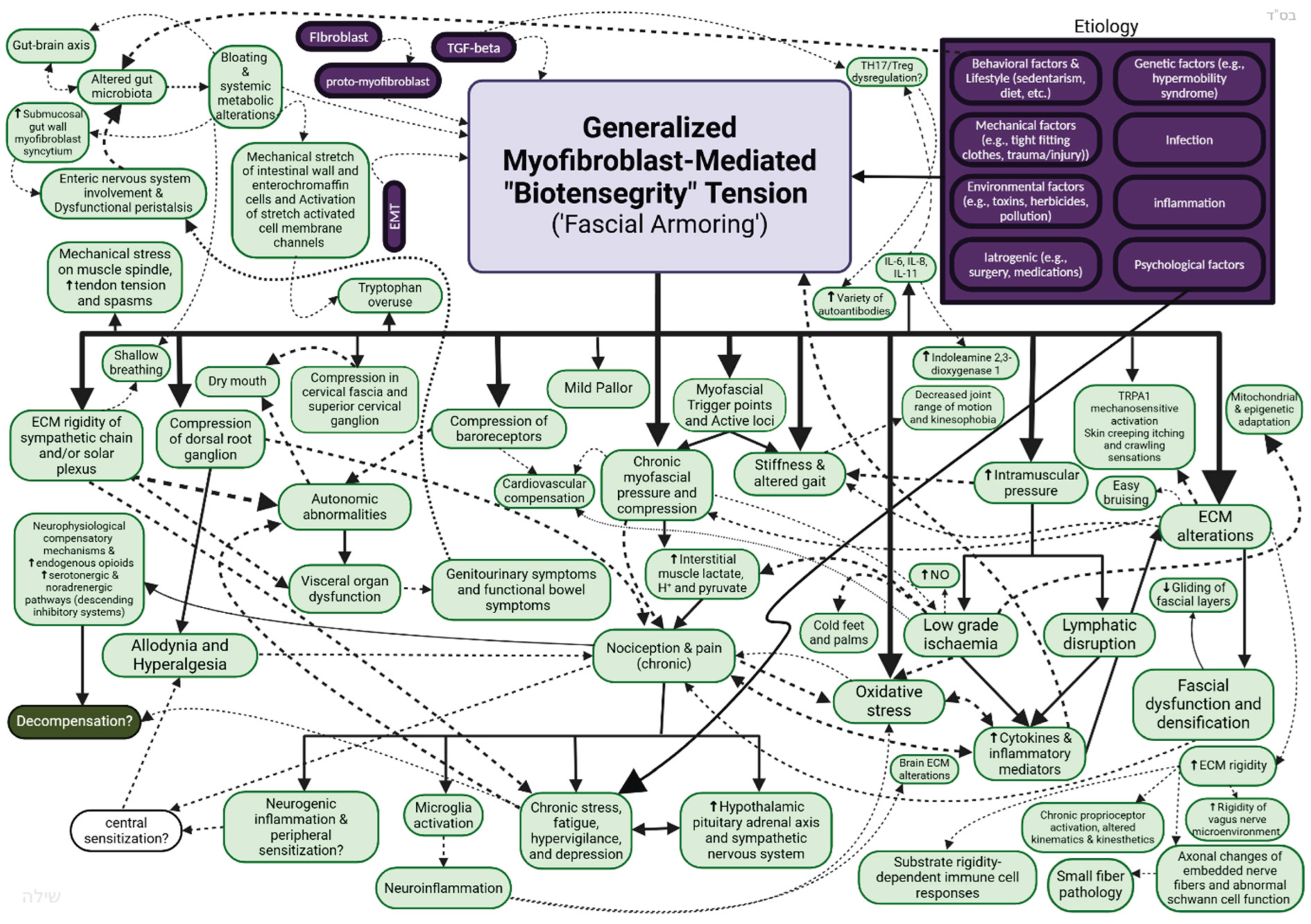
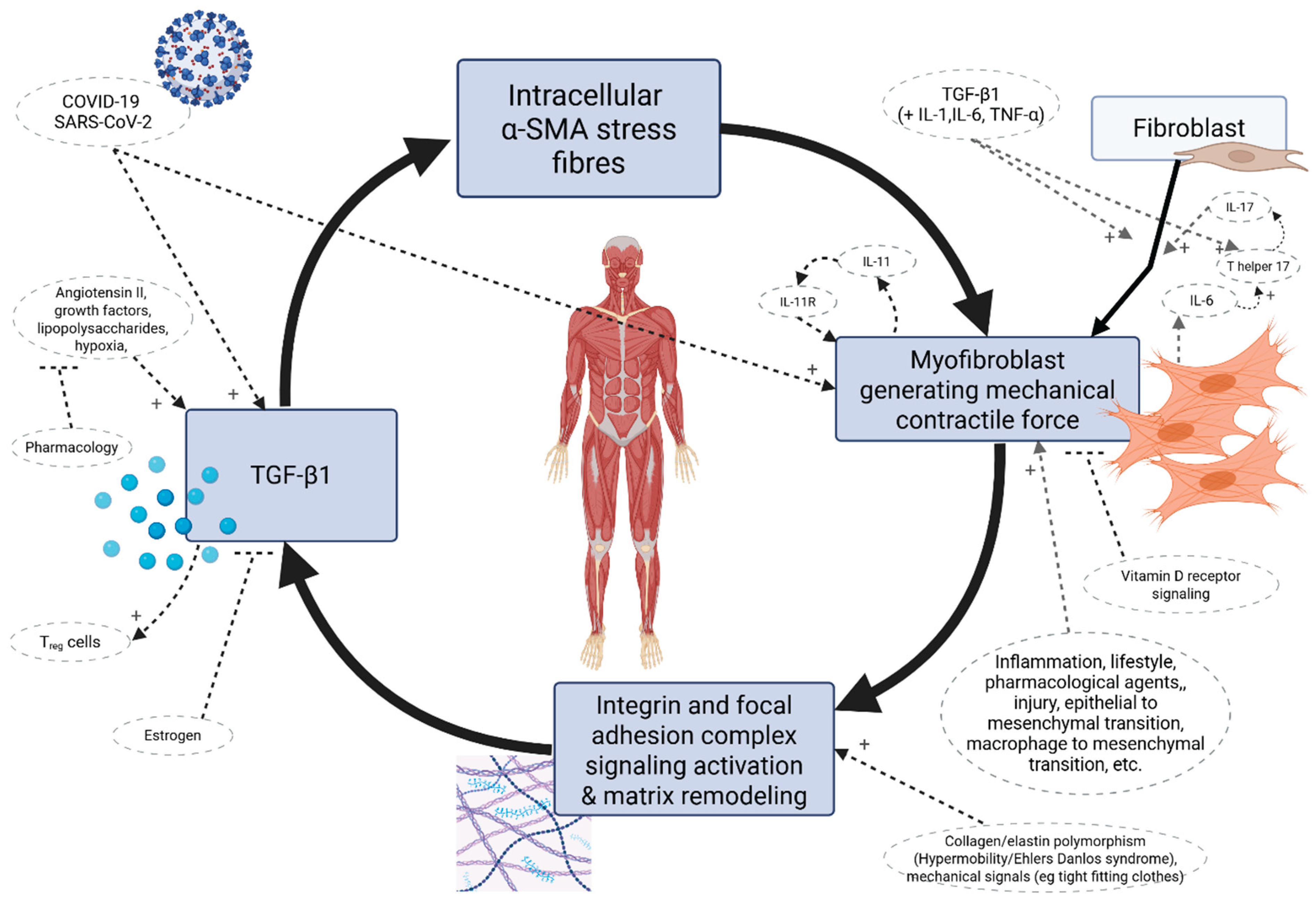
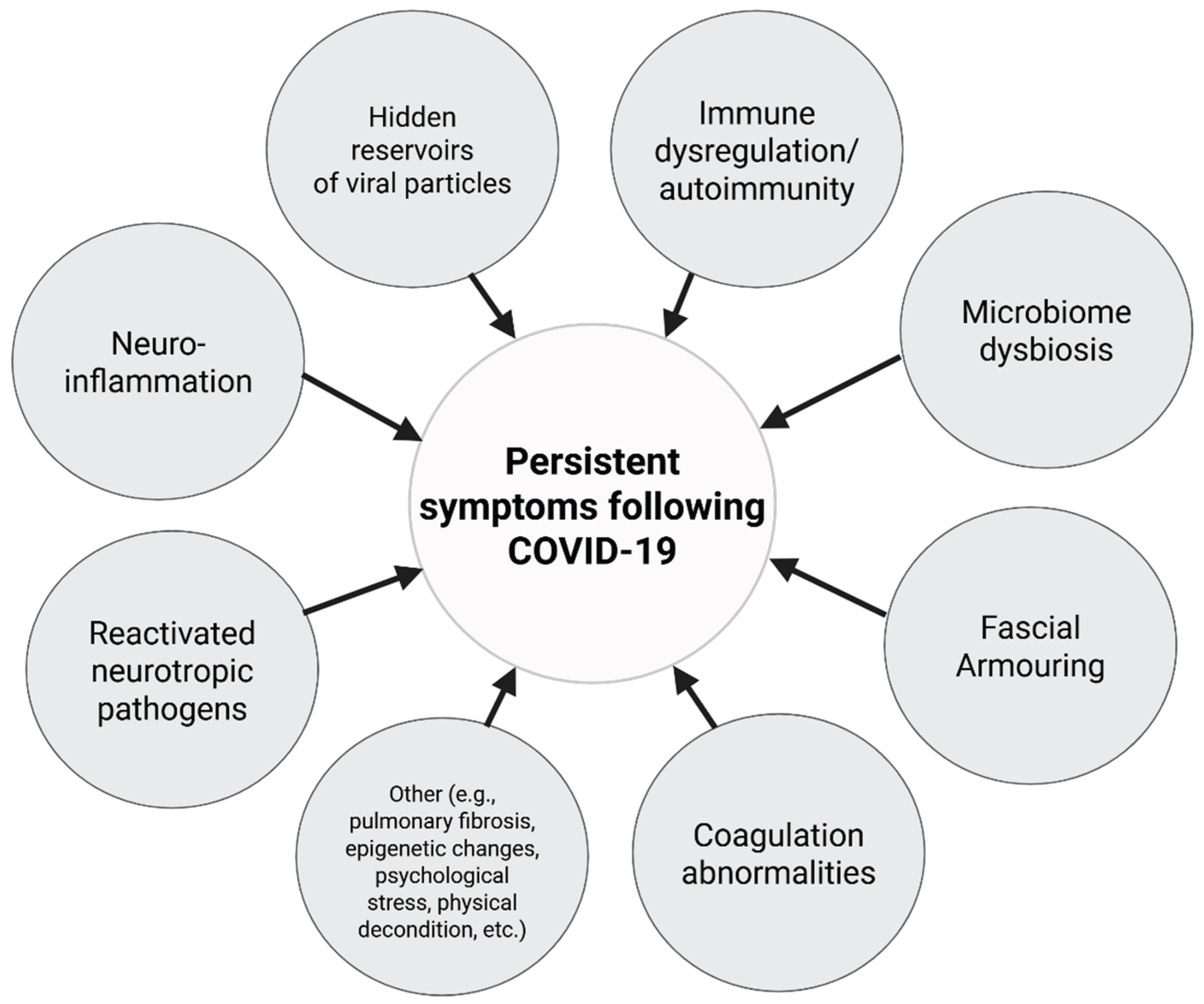
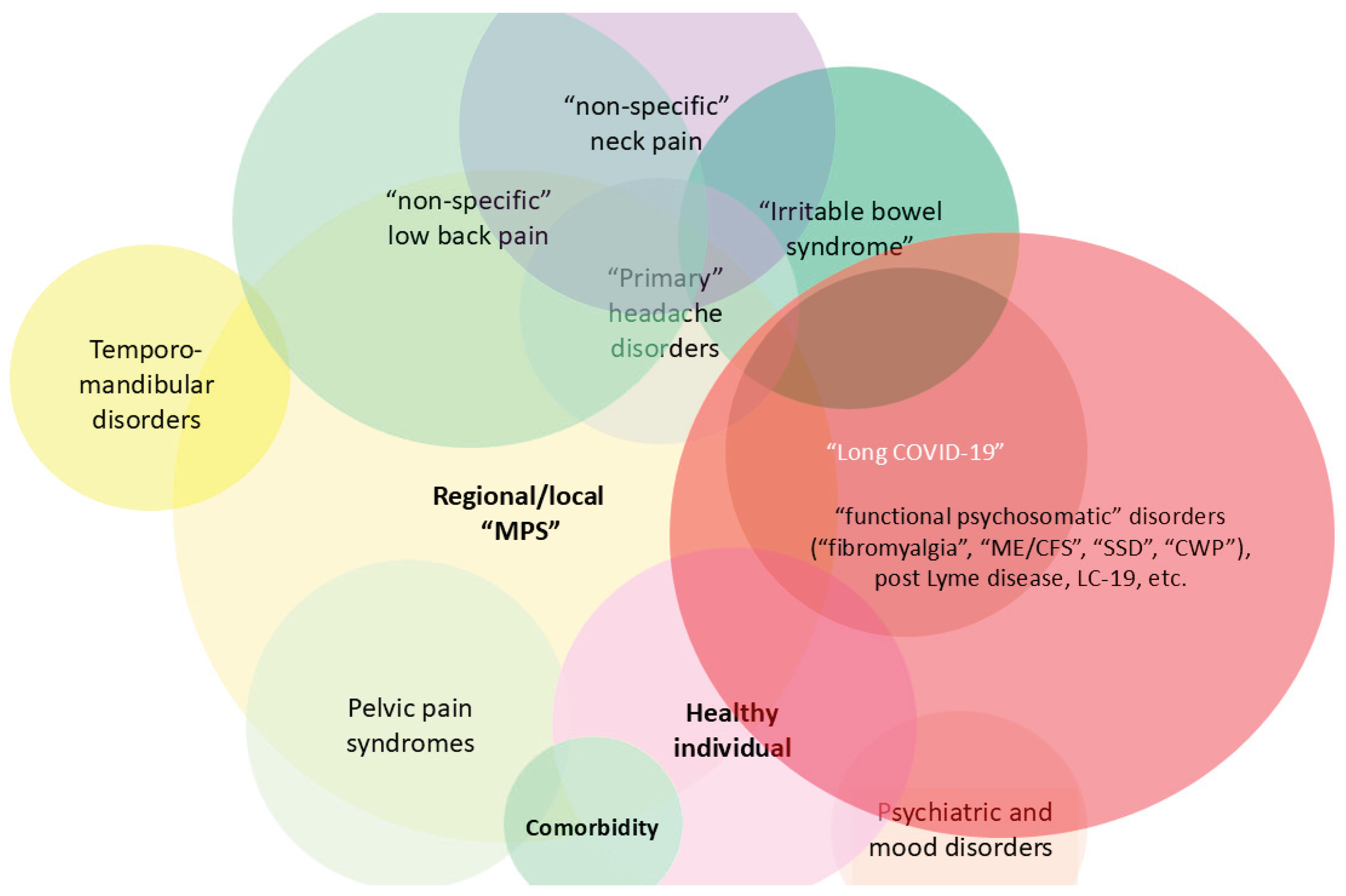
| Source | Term | Definition |
|---|---|---|
| United States National Academies of Science Engineering and Medicine (NASEM) 2024 [207,208] |
Long Covid | An infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems. |
| Centers for Disease Control and Prevention (CDC) [209] | Long Covid | (Based on the NASEM 2024 definition) a chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months. Long COVID includes a wide range of symptoms or conditions that may improve, worsen, or be ongoing. |
| World Health Organization (WHO) [210] (7 December 2022) |
Post Covid-19 condition (Long Covid) |
The continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation. |
| Soriano et al. (2022) WHO clinical case definition based on Delphi consensus [211,212] | Post-Covid-19 Condition | Post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms include, but are not limited to, fatigue, shortness of breath, and cognitive dysfunction (and other symptoms) and generally have an impact on everyday functioning. Symptoms might be new onset after initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms might also fluctuate or relapse over time. A separate definition might be applicable for children. |
| National Institutes of Health (NIH) | Post-acute Sequelae of SARS CoV-2 infection | Ongoing, relapsing, or new symptoms, or other health effects occurring after the acute phase of SARS-CoV-2 infection (i.e., present four or more weeks after the acute infection). The definition will be revised in an iterative manner based on existing and new data, medical literature, and feedback from the scientific community |
| British National Institute for Health and Care Excellence (NICE) [186,213,214] | Post Covid-19 syndrome | Signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis. It usually presents with clusters of symptoms, often overlapping, which can fluctuate and change over time and can affect any system in the body. Post-COVID-19 syndrome may be considered before 12 weeks while the possibility of an alternative underlying disease is also being assessed. In addition to the clinical case definitions, the term 'long COVID' is commonly used to describe signs and symptoms that continue or develop after acute COVID-19. It includes both ongoing symptomatic COVID-19 (from 4 to 12 weeks) and post-COVID-19 syndrome (12 weeks or more). (Last updated: 25 January 2024 |
| British National Institute for Health and Care Excellence (NICE) [214] | Ongoing symptomatic Covid-19 (contrary to “Post Covid-19 syndrome”) | Signs and symptoms of COVID-19 from 4 weeks up to 12 weeks. |
| National Comprehensive guidelines for management of post COVID sequelae -Ministry of Health and Family Welfare Government of India [215] | Post-Covid Sequelae | (Reference to consensus definition) “Post-COVID Syndrome by consensus is defined as signs and symptoms that develop during or after an infection consistent with COVID-19 which continue for more than 12 weeks and are not explained by alternative diagnosis.” |
| Thaweethai et al. (2023) [216] RECOVER Initiative (in collaboration with NIH) |
Post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long COVID | Symptom based definition using a score consisting of postexertional malaise, fatigue, brain fog, dizziness, gastrointestinal symptoms, palpitations, changes in sexual desire or capacity, loss of or change in smell or taste, thirst, chronic cough, chest pain, and abnormal movements. |
| RECOVER initiative (in collaboration with NIH) (2025) [217] | Long Covid |
Reference to the NASEM definition |
| Patient-Filled Questionnaires and Scores | Other Measurement Tools and Methods | |
|---|---|---|
| The visual analogue scale for multiple measures such as pain and fatigue | The brief pain inventory (BPI) [104,128], short form BPI (BPI-sf) [112] and numeric pain rating scale for assessing pain [148] | Physical exam [68,130] |
| Chronic Pain Grading Scale (CPGS) for intensity of chronic pain [172] | Patient-Reported Outcomes Information System (PROMIS) Pain Interference v1.1 (PROMIS-PI) computerized adaptive test were used to evaluate pain and pain interference [128] | Diagnostic codes of the international classification of diseases [134] |
| Fatigue Severity Scale (FSS) [128] and Multidimensional Fatigue Inventory (MFI-20) [16,67] for assessing fatigue | Multi-dimensional assessment of fatigue questionnaire and global fatigue index [156], Modified Fatigue Impact Scale (MFIS) [172], Chalder Fatigue Scale [69,124], Pichot scale [68] for fatigue | The world health organization’s definition of post-COVID-19 [104,128] |
| PROMIS v1.0 Fatigue (PROMIS-Fatigue) computerized adaptive test questionnaire was used to evaluate self-reported daily fatigue symptoms [128] | The FACIT Fatigue Scale [81] for assessing severity of fatigue | LC definition according to the British NICE guidelines [112] |
| dePaul Symptom Questionnaire for post-exertional malaise [67] or for assessing the symptomatology and case definition fulfillment of ME/CFS [81] | General Symptom Questionnaire (GSQ) [64] to assess symptoms of people with irritable bowel syndrome, ME/CFS and fibromyalgia. | Rome III criteria [108] |
| Insomnia Severity Index (ISI) was used for the evaluation of insomnia [16] |
The PROMIS Sleep Disturbance (PROMIS-SD) computerized adaptive test survey was used to measure self-reported quality of sleep for a 7-day period [128], Pittsburgh Sleep Quality Index (PSQI) for sleep quality [81]. single-item sleep quality scale [91] | Bierle et al. (2021) criteria for indicating LC, developed by a modified Delphi process consisting of scores based on necessary conditions and major and minor criteria [144] |
| Fibromyalgia Symptom Scale including the widespread pain index and symptom severity scale based on the ACR criteria [81,130] and modified for self-administration [10,128], the central sensitization inventory (CSI) [146], Fibromyalgia Rapid Screening Tool [135], and at home fibromyalgia questionnaire [85], for assessment of fibromyalgia-type features, screening, or diagnosis | Fibromyalgia impact questionnaire (or revised version) for classifying fibromyalgia severity, and the Symptom Impact Questionnaire Revised for a fibromyalgia-neutral version [135] | The 2015 Institute of Medicine ME/CFS Diagnostic Criteria [128], and Fukuda et al. criteria for CFS [141] |
| Post-COVID-19 Functional Status self-reporting version, for assessing functional status post-COVID-19 infection [89,148] | Lau et al. (2024) [159] post-acute COVID-19 syndrome 14-item improvement questionnaire (PACSQ-14) | Algometer for pressure pain threshold in each limb, or trapezius, and/or center of rectus femoris, or other [75,148] |
| COVID-19 Yorkshire Rehabilitation Scale [112,114] for capturing the impact of LC and for the assessment of overall health status before and after infection. It is also used for assessment of rehabilitation needs in LC | Long COVID questionnaire [133] | Nerve conduction studies, quantitative sensory testing (e.g., cold detection threshold, warm detection threshold, thermal sensory limen to assess paradoxical heat sensation, heat pain threshold, mechanical detection threshold, mechanical pain threshold, mechanical pain sensitivity, dynamic mechanical allodynia, wind-up ratio, vibration detection threshold, and pressure pain threshold.) [112], cold pressor test for conditioned pain modulation [75], and other neurological tests [101] |
| EuroQol with five dimensions and three or five levels [16,104,112,146], and 36-item Short Form health survey (SF-36) and its components [16,68,89] and SF-12 [133] for evaluating quality of life and/or health-related quality of life. | WHOQOL-BREF for assessing quality of life [130] | Exercise-Induced Hypoalgesia (EIH) protocol [75] |
| Patient Health Questionnaire 9 (PHQ-9), PHQ-8 and PHQ-2 were used for depression screening/assessment [16,112,128], Beck Depression Inventory [135], BDI-II [183], Center for Epidemiologic Studies of Depression (CES–D) scale for assessing depression [137] |
Hospital Anxiety and Depression Scale for anxiety and Generalised Anxiety Disorder-7 scale (GAD-7) [16,112]. The State-Trait Anxiety Inventory [183] | Peripheral venous blood sampling (for hormone concentrations, circulating mitochondrial DNA, and more) [67] |
| Depression, Anxiety and Stress Scale (DASS-21) for assessing stress, anxiety, and depression [104] and perceived stress scale [161] for assessing stress. | The Patient Health Questionnaire 15 (PHQ-15) for assessing somatic symptoms [93,219] | RT-PCR, ELISA, or serology for testing for SARS-CoV-2 (active or past infection) |
| Somatic Symptom Disorder-B criteria Scale (SSD-12) for psychological burden associated with the persistent symptoms [68] | The Symptom Impact Questionnaire-Revised (SIQR) [137] to assess symptom severity and functional ability in individuals with chronic pain symptoms [128] | Timed Up and Go test [112] for balance and mobility, Assessment of gait using wearable device [156] |
| Pain catastrophizing scale (PCS) [75,137] for assessing catastrophic thinking related to pain in adults | Pain attitudes questionnaire-revised (PAQ-R) [75] | Handgrip strength test using dynamometry [89,112] |
| Emotion Regulation Questionnaire (ERQ) [172] | Acceptance and Action Questionnaire-II (AAQ-II) [172] | Head-up tilt test for orthostatic intolerance testing (based on consensus criteria) [89] |
| Fear Avoidance Beliefs Questionnaire for assessing fear avoidance and beliefs [104] | The Coping Strategies Questionnaire-Catastrophizing subscale (CSQ-CAT) queried catastrophic thinking related to pain by quantifying a person’s pain experience and analyzing thoughts and feelings pertaining to their pain experience [128] |
Maximal incremental ramp exercise test [67] |
| Polysymptomatic Distress Scale [130] | Pain Self-Efficacy Questionnaire [112] for assessment of individual's confidence in their ability to perform activities despite pain. | 7-day step count [168], Accelerometer for physical activity monitoring [67,89] |
| Brief resilience scale [161] | Short form of Profile of Mood States [75] | Near-infrared spectroscopy [67] |
| DS-14 questionnaire for assessing Type D personality [133]. | The Multi-dimensional Perceived Social Support scale for assessing a person’s subjective perception of the extent of his or her social support [133] | Cardio-pulmonary tests such as spirometry, echocardiography, continuous gas analyzer [89] |
| Memory Failures of Everyday (MEF-30) [172] | Five Facet Mindfulness Questionnaire (FFMQ-24) [172] | Aortic pulse wave velocity using arteriography [89] |
| The Multidimensional Inventory of Subjective Cognitive Impairment (MISCI) was used to assess self-reported cognitive abilities related to memory, verbal language ability, general mental clarity, attention/concentration, and executive functioning [128], and NeuroTrax MindStreams Computerized Battery [157] for cognitive assessment. | Montreal Cognitive Assessment (MoCA) [80] and Mini-Mental State Examination (MMSE) [80] | Flow cytometry [81,99] |
| Cognitive Failure Questionnaire [81] |
Subjective Cognitive Decline (SCD) questionnaire for assessing cognitive complaints [133] | Gene expression assay, proliferation assay, Elispot Assay [[81], microarray and blood transcriptome, combining machine learning [77], RNA-sequencing and more [99] |
| The Tampa Scale of Kinesiophobia – 11 (TSK-11) assessed fear-related beliefs pertaining to physical movement and re-injury in individuals with chronic pain [128] | Godin-Shephard Leisure-Time Physical Activity [89] |
Genomic studies including genome wide association study and a combinatorial analysis platform [61] |
| International Physical Activity Questionnaire-short form [112] | Physical activity readiness questionnaire (PAR-Q) [75], International Physical Activity Questionnaire (IPAQ) [75,104], The PROMIS Physical Function (PROMIS-PF) computerized adaptive test was used to measure self-reported physical function [128] |
Metabolomics [67], Spectroscopy and chemometrics [135] |
| Physical activity scale of Ricci & Gagnon [68] previously used in studies of elderly population | Functional Impairment Checklist [124]. | Immunoassay [81,112] |
| Work Ability Index [161] | Roland Morris Low-Back pain Disability Questionnaire [113] | Interviews [93] |
| BORG Rating of Perceived Exertion (RPE) [112] for perceived effort during Timed Up and Go test |
The Multisensory Amplification Scale (MSAS) was used to assess heightened sensitivity to vision, hearing, tactile, smell, and internal bodily perceptions [128] | Clinical evaluation by medical work-up (laboratory, imaging, referral to specialist, etc.) [118] according to guidelines [68] |
| Medical Research Council breathlessness scale [91]. Modified Medical Research Council Dyspnea Scale [89]. FACIT-Dyspnea [81], London chest activity of daily living (LCADL) scale was assessed to evaluate the level of dyspnea during activities of daily living [148] | The PROMIS Dyspnea Severity (PROMIS-DS) Computerized Adaptive Test questionnaire was used to assess the severity of shortness of breath in response to activity [128] | Skin biopsy for investigating small fibre pathology [220] |
| RA Health Assessment Questionnaire Disability Index [130] | The RA Disease Activity Score-28, Clinical Disease Activity Index, and Simplified Disease Activity Index for rheumatoid arthritis disease evaluation [130]. | Muscle biopsy from vastus lateralis for investigating skeletal muscle (e.g., respirometry, immune-histochemistry, immunofluorescence, etc.) [67] |
| Patient Phenotyping Questionnaire Short Form [168], | PainDETECT questionnaire [113] | Neuroimaging, fMRI, Diffusion Tensor Imaging [157] |
| Topic/Context | Study | Description of Study | Main Findings |
|---|---|---|---|
| Neurological assessment in LC | Fleischer et al. [101] | A prospective observational cohort study from Germany to better define and validate subjective neurological disturbances in patients with LC syndrome. Patients fulfilling the post-COVID-19 WHO Delphi consensus criteria underwent a neurological diagnostic work-up. | 171 patients predominantly female, middle-aged, and had incurred mostly mild-to-moderate acute COVID-19 (94% did not require any professional medical care). 81% had no previous psychiatric condition. In 97.7% of the cases, either no diagnosis other than LC, or no diagnosis likely related to preceding acute COVID-19 could be established. In most of the patients (85.8%), neurological examination did not yield any abnormal findings. Electrophysiological assessment of all 171, including extensive nerve conduction studies of sensory and motor nerves, sensory and motor evoked potentials and brainstem reflexes, revealed no pathological findings in 89.2% of study participants. High somatization scores correlated with cognitive deficits and the extent of fatigue. |
| Central sensitization in LC | Goudman et al. [146] | A cross-sectional study via online survey aiming to explore the presence of central sensitisation as an underlying factor for long-term secondary effects in LC patients, during June 2021 - August 2021 among Dutch population in Belgium. The study used three questionnaires: CSI, Post-COVID-19 Functional Status, and EuroQol with five dimensions and three levels. Included individuals who self-reported confirmed or presumptive COVID-19. | A total of 491 respondents to the CSI questionnaire, 70% of them had CSI scores ≥ 40, and 95% could be classified as medium to high level of severity. 45% in total reported moderate to severe functional limitations. |
| PPT, temporal summation, CPM, and central sensitization in LC | Goudman et al. [148] | A cross-sectional study in Belgium seeking to investigating central sensitization and impaired nociceptive processing in LC. The study included subjects if at least 3 months had passed since testing positive for COVID. Assessment with self-reported patient questionnaires. In addition, PPT, temporal summation, and CPM were assessed with algometer and verbal numeric pain rating scale. | 30 out of 42 females, 41/42 not hospitalized for COVID-19, “Central sensitization symptoms” as captured by the CSI questionnaire, were present in 64% (by using a cutoff score of 40/100). Approximately 80% were classified as medium to high severity. The majority had slight or medium functional impairment assessed in interview and by the post COVID functional scale. 34 and 35 patients were not found to have an abnormality on CPM testing in the trapezius and quadriceps muscle respectively. Total scores on the London chest activity of daily living scale and CSI were positively correlated (r = 0.8, p < 0.001). Correlations between temporal summation and the CSI were low and insignificant. |
| Conditioned pain modulation and exercise induced hypoalgesia after COVID-19 | Peterson et al. [75] | A study that aimed to investigate CPM and exercise-induced hypoalgesia (EIH) after coronavirus infection included 59 American participants who had COVID-19 (symptomatic or asymptomatic) and no active coronavirus infection. Pressure-pain thresholds (PPT) were measured with a pressure algometer. | The three groups analyzed were: infected individuals with symptomatic COVID-19 (n = 26, 61% female) asymptomatic COVID-19 (n = 13, 66% female) and healthy controls (n = 20, 44% female). None of the participants presented with reported symptoms of Long COVID. No differences were found in baseline PPT between patients and controls and the magnitude of EIH did not differ between the groups. Significant differences in CPM (by cold pressor test) were observed between symptomatic group and controls, but not between the asymptomatic group and controls. The authors conclude that symptomatic COVID-19 might be associated with impaired CPM. |
| Electromyography, QST, and nerve conduction studies in LC | Sepic et al. [88] | A cross-sectional study from Sweden that assessed myopathic findings in non-hospitalized “post COVID condition” patients using needle electromyography (EMG). Individuals with and without myopathic EMG findings were compared according to clinical outcomes of symptom severity, quality of life, physical function, and nerve conduction studies, quantitative sensory testing (QST). Inclusion criteria for defining post COVID condition were persistent post-exertional malaise for 3 or more months, verified by the DePaul Symptom Questionnaire. | Among 29 post covid condition patients (all of them non-hospitalized during acute COVID-19) myopathic EMG findings were present in 62% across multiple muscles. Severity of symptoms (muscle pain and fatigue), and quality of life (by SF-36 and the Multidimensional Fatigue Inventory questionnaire scores) did not differ between post covid condition patients with myopathic EMG findings than those without (p > 0.05). Nerve conduction studies showed no signs of peripheral nerve dysfunction (sensory or motor) in either group. QST results were similar between post covid condition patients with and without myopathic EMG findings. |
| Electromyography, QST, and nerve conduction studies in LC | Tryfonos et al. [89] | A Swedish randomized crossover clinical trial that aimed to investigate exercise intolerance in patients with well-defined “post covid condition” without prior comorbidities compared with age- and sex-matched healthy controls. Nonhospitalized patients with persistent symptoms after SARS-CoV-2 infection such as post-exertional malaise were recruited. Several outcome measures were assessed using questionnaires, physiological characterization, cardiopulmonary exercise testing, and inflammatory markers. Inclusion criteria for defining “post-covid condition” were mainly persistent post-exertional malaise for three or more months, verified by the DePaul Symptom Questionnaire. | Among 62 nonhospitalized participants (76% women, mean age: 47.0±9.4 years), 31 were “post-covid condition” patients with symptom duration of 21.6±9.2 months. After strength training, post covid condition individuals reported a greater increase in muscle soreness and lymph node discomfort. Myopathic findings were observed in 18 patients with post covid condition (62%), 13 (45%) had myopathic findings in ≥2 muscles and 5 (17%) in 1 muscle, compared with only 1 control participant (4%), who had myopathic indices in 1 muscle. Nerve conduction studies were not significantly different in terms of mean sensory nerve action potential, sensory or motor conduction velocity, F-wave, and compound muscle action potential. In all participants the sympathetic skin response was normally elicited. Arterial stiffness was also shown to be increased in the patients, with an 8.3% higher aortic pulse wave velocity. At baseline, patients with post covid condition showed preserved lung and heart function but peak volume of oxygen consumption was 21% lower. |
| Small fiber pathology and QST in LC | Azcue et al. (2025) [141] | A study from Spain that investigated dysautonomia and small fiber neuropathy in both post-COVID condition and chronic fatigue syndrome. Neuropathic, autonomic, and fatigue symptoms were evaluated. In Vivo Corneal Confocal Microscopy assessed corneal small fiber morphology. Quantitative sensory testing (QST) was used to determine sensory thresholds, including heat and cold detection. | 30 participants with LC (average disease duration of 20.4±8.9 months), 30 with ME/CFS, and 30 age- and sex-matched healthy controls were included in the study. Both LC and ME/CFS patients demonstrated sensory SFN, characterized by impaired heat detection and increased tortuosity of small fibers in the central cornea subbasal plexus. QST demonstrated significant differences in heat detection thresholds between LC patients and healthy controls, indicating impaired heat detection in the LC group. While traditional measures of small fiber density did not show significant differences between the groups, a more detailed analysis of corneal subbasal plexus small fibers using corneal confocal microscopy revealed statistically significant differences in corneal small fiber tortuosity between patients (both LC and ME/CFS) and healthy controls, suggesting the presence of sensory small fiber neuropathy in the patient groups. Furthermore, while both LC and ME/CFS patients demonstrated sensory small fiber neuropathy characterized by impaired heat detection and increased tortuosity of corneal small fibers, there were no significant differences in fatigue, autonomic, and neuropathic symptoms between the LC and ME/CFS groups, highlighting a clinical overlap between these conditions |
| Corneal analysis after COVID-19 | Bitirgen et al. [85] | A cross-sectional study in which corneal sub-basal nerve plexus morphology and dendritic cell density were quantified in patients with and without LC. Patients recruited were within 1–6 months of COVID-19. LC was determined by a questionnaire in accord with national guidelines. | In a total of 40 patients and 30 controls mean time after the diagnosis of COVID-19 was 3.7±1.5 months. LC patients showed corneal small nerve fiber loss and increased dendritic cells. with significant inverse correlations between corneal nerve fiber density and length and the total score on the NICE long COVID questionnaire. Significant inverse correlations were also found between corneal nerve fiber length and the FM-Q score (ρ=−0.419; p=0.007). There were significant inverse correlations between corneal nerve branch density and the score for neuropathic pain (ρ=−0.347; p=0.028), CRP (ρ=−0.365; p=0.043), total dendritic cell density (ρ=−0.365; p=0.020), mature dendritic cell density (ρ=−0.419; p=0.007), and immature dendritic cell density (ρ=−0.353; p=0.025). |
| Central sensitization in endometriosis patients during COVID-19 pandemic | Liu et al. [108] | A study from Vancouver, Canada, using data from EPPIC registry and the RESPPONSE study attempted to correlate pain-related phenotyping for central nervous system sensitization in endometriosis-associated pain with mental health outcomes during the coronavirus pandemic. PHQ-9 was used for the assessment of depression, GAD-7 for anxiety. Other experimental measures were central sensitization inventory, endometriosis-associated chronic pain, Pain Catastrophizing Scale score, Rome III criteria, American Urological Association or International Continence Society’s criteria. Myofascial pelvic pain syndrome was diagnosed by palpation of the pelvic floor musculature for tenderness and abdominal wall pain was diagnosed by the Carnett's test | A total of 278 individuals (mean age 38, mean BMI 26.5). Baseline CSI score was 43.8 ± 17.3. The findings showed a previously known correlation between CSI scores and psychological aspects such as depression, stress and anxiety (p < .0001). The authors conclude that “endometriosis patients with clinical evidence of central sensitization pre-pandemic had higher scores for depression and anxiety symptoms during the COVID-19 pandemic.” |
| Topic/Context | Study | Description of Study | Main Findings |
|---|---|---|---|
| GJH | Eccles et al. [170] | A 2024 UK case-control study to explore whether generalized joint hypermobility (GJH) was a risk factor for self-reported non-recovery from COVID-19 infection. Data was collected through a mobile health application developed with input from physicians and scientists at King’s College London, Lund and Uppsala Universities, and Massachusetts General Hospital. The 5-part Hakim and Grahame self-report questionnaire were used to determine GJH by a cut-off score of ≥2 indicating GJH. Based on regression analysis they determined if the presence of GJH was a predictor of non-recovery. | Among individuals reporting incomplete recovery from COVID-19 infection (n=914), 269 patients (254 (29.4%) female) had GJH. In the fully recovered group, 439 of 1940 patients (22.6%, 400 female) had GJH. While the presence of GJH did not show a specific association with COVID-19 infection risk itself, it was significantly associated with non-recovery from COVID-19 (OR 1.43 (95% CI 1.20 to 1.70). GJH significantly predicted higher fatigue levels, the latter which mediated the link between GJH and non-recovery from COVID-19 [170]. |
| GJH | Gavrilova et al. [126] | Case report | Young female patients with joint hypermobility that develop new manifestations after discharge from hospital for COVID-19, including myalgia, postural orthostatic tachycardia (POT), antinuclear antibodies, a noticeable thickened/swollen tendon, and new onset fibromyalgia-type features. |
| GJH | Grach et al. [140] | The objective of this study was to ascertain if individuals diagnosed with LC exhibited a higher incidence of new or exacerbated health conditions. The cohort comprised patients diagnosed with LC at Mayo Clinic facilities in Rochester, Minnesota, and Jacksonville, Florida. Controls had a confirmed history of SARS-CoV-2 infection but had not received a diagnosis of LC. Questionnaires were sent to LC patients and controls to assess new or worsening comorbidities following COVID-19. The questions screened for ME/CFS, GJH, and orthostatic intolerance. The self-assessment 5-part hypermobility questionnaire was used for assessing GJH. | 247 respondents of LC group and 40 controls. The mean age of controls was higher (p = 0.021). 94.4% of LC individuals reported that they had a pain condition that worsened or started after COVID-19 compared to 0% of non-LC controls. 58.3% of LC patients met the criteria for ME/CFS, compared to 0% of controls. 27.0% of LC patients had GJH (out of them 90.5% female), compared to 10.3% of controls. LC patients showed significantly higher scores for orthostatic intolerance compared to controls. LC respondents were also found to be more likely to report sensitivity to medications (22.5% vs. 4.9%, p = 0.007). Other reported findings in LC group included neurological (92.4%), sleep (82.8%), skin (69.8%), genitourinary (60.6%), allergies/sensitivities/intolerances (31.8%), mood disorders (31.6%), gastrointestinal disorders (21.9%), viral reactivation (e.g., Epstein–Barr virus, herpesvirus, or other viruses) (18.7%), autoimmune diagnoses (16.2%), pulmonary disorders (15.8%), and cardiac disorders (12.1%). |
| GJH | Logarbo et al. [171] | Case report | Five female patients, aged 33 to 51 years, with no known history of hypermobility, presented with persistent and debilitating fatigue, cognitive dysfunction, dysautonomia, and diffuse joint pain, among other neuromusculoskeletal symptoms, over a period of 3 to 15 months following acute SARS-CoV-2 infection. Clinical evaluation confirmed generalized joint hypermobility using the Beighton Score, receiving scores at or above the age-appropriate diagnostic threshold. Notably, all patients exhibited C677T or A1298C polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene, which was previously shown to be linked to the development of hypermobile Ehlers-Danlos Syndrome and hypermobility spectrum disorder. Given that up to 35% of the general population carries one of these MTHFR polymorphisms and hypermobility of any etiology may affect up to 57%, the findings suggest a possible predisposition unmasked by SARS-CoV-2 infection [171]. Pathophysiological mechanisms suggested may include immune dysregulation, mast cell activation, and connective tissue inflammation. Patients were managed with methylated folate and B12 supplementation, physical therapy, and mast cell stabilization strategies. |
| Study (Year) | Databases Used | Topic |
|---|---|---|
| Fowler-Davis et al. (2021) [59] | CENTRAL, CINAHL, MEDLINE (EBSCO), ProQuest (APA PsycINFO), SCOPUS, SportDISCUS, the International Clinical Trials Registry Platform (World Health Organization) the UK Clinical Trials Gateway (NHS, National Institute for Health Research). | Interventions for fatigue and post-viral fatigue |
| Arienti et al. (2022) [70] | Cochrane Systematic Reviews | Fatigue, post-exertional malaise and orthostatic intolerance |
| Cohen et al. (2022) [92] | MEDLINE, Embase, OVID, and Google Scholar | Comprehensive review on the relationship between chronic pain and infection (mechanisms, causes, treatments, and more) |
| Fernández-de-Las-Peñas et al. (2022) [116] | MEDLINE, CINAHL, EMBASE, Web of Science, medRxiv and bioRxiv | Prevalence and time-trends of musculoskeletal pain (e.g., myalgia, joint pain, and chest pain) after COVID-19, in hospitalized and non-hospitalized cases (systematic review with meta-analysis) |
| Kocyigit & Akyol (2022) [143] | Web of Science, Scopus, and MEDLINE | The COVID-19 pandemic and fibromyalgia - the review focuses on prevalences, the suggested relevance of psychological and psychosocial factors, the role of inflammation, and vaccination. |
| Pires et al. (2022) [106] | MEDLINE (via PubMed) and Google Scholar | Musculoskeletal manifestations of acute and long COVID-19 and the effect of COVID-19 on bone, joints, muscle, rheumatological diseases, and susceptibility to musculoskeletal infection |
| Rao et al. (2022) [60] | MEDLINE, Embase, PsycINFO, CINAHL, Web of Science, Scopus, trial registries (i.e., NIH clinical trials registry, Cochrane Central Register of Controlled Trials, and ISRCTN registry), and Google Scholar. Pre-print servers (MedRxiv and Psycharxiv) were also included and a manual search of OpenGrey. | Post-COVID-19 fatigue (a systematic review and meta-analysis) |
| Yong et al. (2022) [11] | PubMed, SCOPUS and Web of Science | Proposed subtypes of LC: non-severe COVID-19 multi-organ sequelae, pulmonary fibrosis sequelae, ME/CFS, POT syndrome, post-intensive care syndrome, and medical or clinical sequelae |
| Ciaffi et al. (2023) [107] | MEDLINE and Web of Science | Post-acute COVID-19 musculoskeletal manifestations review aimed for rheumatologists |
| Hwang et al. (2023) [62] | MEDLINE and Cochrane Library | Viral infections as an etiology of ME/CFS (systematic review and meta-analysis) |
| Lewthwaite et al. (2023) [158] | MEDLINE, Embase, CINAHL, Scopus and Cochrane | Two reviews: the first is an umbrella review of systematic reviews of LC symptoms, prevalence, and complications grouped into eight treatable trait clusters. The second is an umbrella systematic review of randomized control trials of interventions for LC prevention or management. |
| Kerzhner et al. (2024) [124] | MEDLINE and EMBASE | Pain in LC after hospitalized and non-hospitalized COVID-19 (a systematic review and meta-analysis) |
| Silva-Passadouro et al. (2024) [24] | MEDLINE, Embase, CINHAL, PsycINFO and Web of Science | Resting-state surface quantitative EEG findings in fibromyalgia, ME/CFS and LC compared to healthy controls. |
| Skare et al. (2024) [94] | Pubmed, Scielo and Embase | Ear abnormalities in fibromyalgia, ME/CFS, COVID-19, LC, POT syndrome, and more related syndromes |
| Topic | Database and Search Phrase | Study (Year of Upload/Publication) | Description of Study |
|---|---|---|---|
| GJH | medRxiv (“hypermobility long COVID”) | Torok et al. (2025) [227] | A 2025 preprint by Torok et al. further reveals a link between GJH and LC in their cross-sectional online survey among U.S. and U.K. populations to compare those with self-reported LC to those without, assessing generalized and extreme joint hypermobility via questionnaires. Logistic regressions were then employed, controlling for covariates, to examine the predictive relationship between hypermobility and LC. Among 1,816 respondents, 352 (19.4%) self reported LC. LC individuals reported a higher number of coronavirus infection episodes and more severe infections. In logistic regression analysis it was found that in mild-asymptomatic COVID-19 subgroup, GJH was associated with LC with OR 1.54 (95% CI: 1.05-2.25 p=0.027) compared to OR 0.90 (0.64-1.28) in severe infection. Extreme joint hypermobility was associated with LC, with OR 2.59 (95% CI 1.37-4.89 p=0.003) in mild-to-asymptomatic COVID-19, whereas in the severe infection there was no statistically significant association between extreme joint hypermobility and LC. Also, 29.6% of LC individuals reported a parent, child or sibling with LC, compared to 11.7% in non-LC individuals (p<0.001). Overall, their analysis suggests that hypermobility influences the odds of LC by two pathways: (i) GJH increases the risk that individuals with no or moderate initial symptoms from a COVID-19 infection experience LC, (ii) GJH is a predictor of developing severe initial symptoms from COVID-19, which is independently associated with increased LC risk. |
| GJH | medRxiv (“hypermobility long COVID”) | Eckey et al. (2025) [228] | A retrospective online survey of 3,925 patients with either ME/CFS or LC. The study aimed to identify treatments with the greatest perceived benefits for these conditions and to understand how these treatments affected different core symptoms. Ehlers-Danlos Syndrome/joint hypermobility was reported as a comorbidity in both ME/CFS (40.2%) and LC (27.2%) patient groups. When the patients were clustered into four distinct subgroups based on their symptoms and comorbidities, Cluster 2 was characterized by a "POT syndrome-Dominant Presentation". Patients in this cluster reported the highest rates of POT syndrome as a primary symptom (75.0%) and comorbidity (95.1%). Compared to Cluster 3, patients in Cluster 2 reported higher rates of certain conditions, including EDS (33.5%), dysautonomia, mast-cell activation syndrome, and craniocervical instability. This suggests an association between POT syndrome-dominant presentation and joint hypermobility within this cohort. |
| GJH | medRxiv (“hypermobility long COVID”) | Eastina et al. (2025) [229,230] | A follow-up online survey of 526 adults aged 20-65 with a history of LC to assess the duration and severity of autonomic dysfunction and its impact on function and quality of life. Data were collected via the REDCap platform using questionnaires such as the COMPASS-31 and SF-36, and multivariable logistic regression was used for statistical analysis. This study revealed significant associations between GJH and LC-related autonomic dysfunction. A substantial portion of the LC cohort, 29.7%, self-reported joint hypermobility. Moderate to severe autonomic dysfunction (defined as a COMPASS-31 score ≥ 20) in LC was associated with female sex (p = 0.012) and joint hypermobility (p = 0.019). Multivariable logistic regression analysis demonstrated that those with joint hypermobility had a 1.68-fold increased risk of moderate-to-severe autonomic dysfunction compared with those without joint hypermobility (OR 1.68, p = 0.044). Notably, joint hypermobility emerged as the most robust risk factor for developing POT syndrome following SARS-CoV-2 infection, conferring a 200% increased odds (OR = 2.05, 95% CI: 1.34-3.14, p = 0.001). The researchers suggest that the underlying mechanism for this association might involve connective tissue laxity in individuals with GJH, potentially leading to increased venous pooling and reduced venous return, thus contributing to postural tachycardia |
| GJH | medRxiv (“hypermobility long COVID”) | Wilson (2023) [231] | An investigation into the overlapping clinical and genetic features of Ehlers-Danlos syndrome (EDS) and LC. This study assessed 1,261 EDS outpatients, utilizing a database of 120 history and physical findings, including genetic data from genetic sequencing in 568 of these patients. These data were then compared to 15 cases of LC from an extensive review and to 104 genes previously associated with COVID-19 severity in previous molecular studies. The study identified six identical genes (F2, LIFR, NLRP3, STAT1, TICAM1, TNFRSF13B) and 18 similar genes (including POLG-POLD4, SLC6A2-SLC6A20, and NFKB1-NFKB2) relevant to both EDS and COVID-19 severity. |
| Somatic symptoms and COVID-19 | PubMed (“somatic symptoms COVID”) | Shevlin et al. (2020) [219] | An investigation of the association between COVID-19-related anxiety and somatic symptoms in adult UK population. Among a representative sample of 2,025 individuals, moderate to high levels of COVID-19-related anxiety were significantly associated with general somatic symptoms, particularly gastrointestinal and fatigue symptoms. This remained significant after controlling for factors such as generalized anxiety disorder. |
| Somatic symptoms and COVID-19 | PubMed (“somatic symptoms COVID”) | Liu et al. (2020) [232] | A cross-sectional survey by Liu et al. of China found that concern regarding COVID-19 was positively correlated with the occurrence of somatic symptoms in college students and primary school children [232]. The incidence of somatic symptoms among college students was 34.85 (out of them 26.26% mild and 8.59% moderate). While the incidence of somatic symptoms in primary school respondents was 2.39% (all mild). |
| Fibromyalgia incidence trends 2014-2021 | PubMed (“fibromyalgia incidence nationwide”) | Lee et al. (2023) [223] | Lee et al. (2023) [223] report on nationwide data of fibromyalgia incidence from 2012 to 2021 in South Korea population. The data showed a peak in the annual incidence in the year 2019 reaching 109.20 per 100,000 persons compared to 88.07 in the year 2014. |
| Non-recovery after COVID-19 | PubMed (“long COVID”) | Peter et al. (2025) [233] | A prospective longitudinal cohort study followed LC patients into the second year since contracting COVID-19 and found that the majority of working age patients with LC, who demonstrated no major pathology in laboratory investigations, did not recover. Many remained with a non-specific ME/CFS-type clinical picture despite no obvious clinical finding and no viral persistence in blood and stool testing. |
| LC patient experiences and perspective | PubMed (“long COVID”) | Chasco et al. (2022) [234] | A 2022 study by Chasco and colleagues conducted by interviews offers valuable insight into patient perspective on the impact of the fatigue and brain-fog in LC capturing not only physical descriptions but the social, occupational, and interpersonal aspects as well. |
| LC patient experiences and perspective | PubMed (“long COVID”) | Wurz et al. (2022) [235] | Wurz et al. (2022) evaluated LC experiences, noticing that patients had a deep sense of loss over one's prior identity. In their inquiries they found four main themes (numerous and exhausting LC symptoms, pervasive LC effects, physical activity is difficult or too demanding, and asking for help when few are listening, and little is working) commonly reported by 213 participants (88.2% woman, 33.1% aged 40–49, 74% experienced LC symptoms for ≥ 6 months). |
| Skin biopsy and QST in LC | PubMed (“conditioned pain modulation”, “quantitative sensory testing” and “COVID”) | Flaco et al. (2024) [220] | A case-control study that evaluated possible small fibre neuropathy in patients experiencing painful LC. Skin biopsies were taken from 26 patients with painful LC and quantitative sensory testing was done. Both outcomes were compared to individuals with past COVID-19 infection with painless LC, which was characterized mainly by symptoms such as brain fog and fatigue, and compared also to asymptomatic post-COVID-19 non-LC controls. Among the 26 patients with painful LC, twelve had skin biopsy and/or quantitative sensory testing abnormalities compatible with small fibre neuropathy, while the rest did not. Interestingly, approximately 50% of patients experiencing painful LC had small fibre neuropathy, similar to the rates of small fiber pathology among fibromyalgia patients according to a 2018 meta-analysis [236]. |
| Immunology in LC | medRxiv (“long COVID”) | Santos Guedes de Sa et al. (2024) [237] | In a preprint by a research group from Connecticut mechanisms related to autoimmunity continue to receive attention in LC research. The study investigates whether autoantibodies are causally linked to neurological symptoms in LC. Total IgG was purified from plasma of LC participants and its reactivity against human and mouse tissues was analyzed. The study found that IgG from LC patients showed increased reactivity against neural tissues such as the human pons and mouse sciatic nerve, meninges, and cerebellum. This suggests that autoantibodies targeting neural tissues may contribute to the development of neurological symptoms in LC. |
| # | Abnormality | Findings |
|---|---|---|
| 1 | Increased intramuscular pressure | High intramuscular pressures in trapezius muscle of fibromyalgia patients were documented in a study by Katz et al. (2021). The patient group had a mean pressure value of 33.48 mmHg compared to 12.23 mmHg in controls [32]. The authors of that study assert that such muscular pressure abnormality might be a critical finding that contributes to diffuse muscle pain in fibromyalgia and may be an intrinsic feature of the disease. They conclude that the idea of fibromyalgia as a disorder driven exclusively by central mechanisms without any peripheral organic causes should be reevaluated [32]. This musculoskeletal finding is interesting because in common practice [349] chronic exertional compartment syndrome usually exhibits silent routine medical investigations and is diagnosed if at least one of the following intramuscular compartment pressure measurements is made: (a) pre-exercise intramuscular pressure of more than 15 mmHg (b) one minute post-exercise pressure of more than 30 mmHg, or (c) five-min post-exercise pressure of greater or equal to 20 mmHg. Studies measuring intramuscular pressure of other muscles besides trapezius in fibromyalgia were not found |
| 2 | Muscle tension and myofascial taut bands | Significantly higher measurements of muscle damping were found in fibromyalgia patients which reflects increased muscle tension [350]. Myofascial taut bands (i.e., palpable hardened and contracted muscle fibers) are very common in fibromyalgia patients [351]. According to a patient survey, morning stiffness was ranked as being bothersome to a similar degree as pain in individuals suffering from fibromyalgia [271]. A 2022 observational study from Spain found a weak but significant association between elastic properties of soft tissue measured by ultrasound elastography at the tender point areas, and psychological factors (Pain Catastrophizing Scale scores) in 42 female patients with fibromyalgia [352]. Zetterman and colleagues (2021) report that 51 female fibromyalgia patients in their study had significantly higher mean %EMG and shorter EMG rest time values than the controls [247]. Fibromyalgia disease burden, as evaluated via patient self-reported questionnaire (CSI), is significantly correlated (r=0.546) with muscle stiffness in patients rating pain 4 or higher on a numeric rating scale [353]. |
| 3 | Imaging | A study by Müller et al. [250] found no evidence for functional or structural alterations in brain areas typically involved in acute pain processing that could reflect chronic stimulus-independent pain in FM patients. |
| 4 | Trigger points | The local pain of chronic widespread pain and fibromyalgia patients is often related to the presence of myofascial trigger points [354]. For the most part, myofascial trigger points make up the topography of fibromyalgia tender spots (r = 0.78, p-value <0.001) [355]. It is recognized that in a substantial amount of cases symptoms attributed to “central sensitization” are actually maintained by bottom-up sensory signals originating in peripheral tissue such as soft tissue and the musculoskeletal system [275]. |
| 5 | Fascial/epimysium stiffness | Kawakita and colleagues from Japan in their 1991 observational study [356] investigated deep pain measurements in fibromyalgia tender spots by using needle electrodes. They describe that the needle was inserted to a depth where the experimenter felt some physical resistance or stiffness against the needle insertion (what they have termed “needling stiffness”). The minimum pain thresholds under the tender point occurred at the depth of the needling stiffness. Observations via ultrasound scan suggested that the needle tip at this depth was located on or near the fascia. |
| 6 | Chronic ischemia [357] and muscle histopathological findings | Histological analysis of fibromyalgia patient biopsies reveals nonspecific muscle changes, such as segmental fiber necrosis, accumulation of lipids and glycogen, and subsarcolemmal mitochondrial clustering, which were hypothesized to stem from sustained muscle contraction and ischemia of unknown etiology [358]. In muscles of fibromyalgia patients, chronic contraction appears to contribute to increased DNA fragmentation and structural changes within the muscle tissue [359]. Hénriksson et al. (1982) investigated biopsy specimens from muscle of fibromyalgia patients. They described finding moth-eaten fibres that were evenly distributed over the whole cross-section and only type I fibres were affected. Electronmicroscopy of specimens revealed mitochondrial abnormalities (such as electron-dense inclusions and lack of inner membrane), myofibrillar Z-streaming, and cytoplasmic bodies, and glycogen concentrations were below normal. There was an abnormal relation between mitochondria and myofibrils. The authors stated that the biopsy findings described may be of diagnostic importance [360]. |
| 7 | Serum cytokines, elevated levels of fibroblast TGF-β expression and ECM abnormalities | A 2020 gene expression analysis study of peripheral blood samples from ten fibromyalgia patients identified a transcriptional profile related to the immune system [361]. It also found significantly higher levels of two long non-coding RNAs and higher serum levels of IL-17, TGF-β, IL-6, IL-21, and IL-23 in the fibromyalgia group using ELISA method. The results of the study were validated on a group of 50 fibromyalgia patients (48 females, mean age: 49 ± 20.5 years) [361]. A biopsy study of fibromyalgia patients indicated a significant increase in TGF-β gene expression in peripheral fibroblasts compared to controls [362]. The expression of proteins associated with ECM remodeling and oxidative processes appears to differ in fibromyalgia fibroblasts, potentially explaining a heightened inflammatory state [363]. Findings from a 2002 study indicate significantly elevated levels of interleukin 1-beta, interleukin 6, and tumor necrosis factor alpha in skin of a subset of fibromyalgia patients, detected in biopsy samples taken from the deltoid region of female patients, by using RT-PCR and immunohistochemistry [364]. Immunoglobulin G deposits in dermis were also previously demonstrated and could be related to the patho-mechanism or merely an epiphenomenon [364]. The observed decrease in intramuscular collagen levels could predispose fibromyalgia patients to muscle micro-injuries and result in non-specific signs of muscle pathology [365]. |
| 8 | Metabolic alterations | The interstitial levels of metabolic substances in individuals with fibromyalgia were evaluated through microdialysis [366]. Concentrations of glutamate, lactate, and pyruvate were found to be significantly elevated. The response to acetylcholine stimulation was shown to be associated with increased dialysate lactate in fibromyalgia [367]. Increased pyruvate levels and lower adenosine triphosphate and creatine phosphate are seen in muscle interstitium in fibromyalgia patients [368]. More studies have revealed findings in line with this result [340,369]. |
| 9 | Oxidative stress | An abnormality of mitochondrial function is suspected to occur in fibromyalgia [368], and may help explain the pain of the syndrome. A transmission electron microscopy study revealed morphological alterations in mitochondria of peripheral blood mononuclear cells from fibromyalgia patients. This finding is suggestive of mitochondrial dysfunction and consequential inefficient oxidative phosphorylation, metabolic and redox disorders, and increased reactive oxygen species levels, which may play a pathogenetic role in fibromyalgia [370]. Also, fibromyalgia is characterized by a hypomethylated DNA profile with an overrepresentation of genes linked to stress response and DNA repair/free radical clearance [371]. Cellular energy metabolism abnormalities seem to promote fibromyalgia and possibly other chronic pain conditions, indicative of a role of oxidative phosphorylation in pathophysiology of chronic pain [372]. Oxidative stress seems to have an important role in fibromyalgia's clinical picture [373,374]. A clinical study demonstrated that hyperbaric oxygen therapy significantly alleviates fibromyalgia symptoms and improves patient reported quality of life significantly [375]. |
| 10 | Cardiovascular system | Evidence of decreased peripheral blood flow in individuals with fibromyalgia suggests underlying functional impairments in their cardiovascular system [376]. There is evidence indicative of decreased transcapillary permeability in fibromyalgia patients [377] as well as abnormalities in peripheral arteries [378]. |
| 11 | Differential modulation of disease course by invasive surgery | Surgery deeply affects fibromyalgia manifestations in a differential manner: whereas some surgeries improve the course of the disease, other operations are associated with triggering or worsening fibromyalgia [379,380,381,382]. For example, while fibromyalgia is a risk factor for less satisfaction, more postoperative pain and opioid prescription, worse functional outcome, and higher rate of medical and surgical complications following orthopaedic surgery [383], a 2008 study found that laparoscopic Roux-en-Y surgery is associated with resolution or improvement of fibromyalgia [384]. A 2014 study found that following parathyroidectomy, fibromyalgia medication use decreases remarkably, and quality of life is significantly improved [385]. In hip and knee arthroplasty, fibromyalgia patients that had pre-operative lower body symptoms experienced general improvement, while patients with upper body pain reported a worsening of symptoms, when studied at one and six-months postoperatively [275]. Irritable bowel syndrome (IBS) [286], a functional gastrointestinal disorder which has much clinical overlap with fibromyalgia [55], was shown to be relieved to below Rome II criteria in 80 percent of patients after laparoscopic anti-reflux surgery [380]. Meanwhile, fibromyalgia patients have a higher incidence of IBS after appendectomies [386]. Laparoscopic cholecystectomy has been shown to deeply influence fibromyalgia symptoms [379], and hysterectomy, with or without oophorectomy, seems to worsen fibromyalgia [387,388]. |
| 12 | Correlations between disease burden and other variables misalign with theory-based expectations | A systematic review and meta-analysis investigated the association between the CSI questionnaire scores (evaluating fibromyalgia-type disease burden) and quantitative sensory testing (QST) in different chronic pain populations and found that for conditioned pain modulation, the best correlation found with the CSI score was r=-0.13. Temporal summation, pressure pain threshold, heat pain threshold, and cold pain threshold, showed correlation coefficients of r=0.16. r=-0.36, r=-0.17, and r=-0.27, respectively. |
| 13 | Other | Studies suggest that fibromyalgia patients have higher prevalence of carpal tunnel syndrome [389], tension type headache [27], gastroesophageal reflux disease [262], and reduced optic disc perfusion [390]. Additional anomalies and counterinstances are also found [249]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





