Submitted:
14 October 2025
Posted:
31 October 2025
You are already at the latest version
Abstract
Keywords:
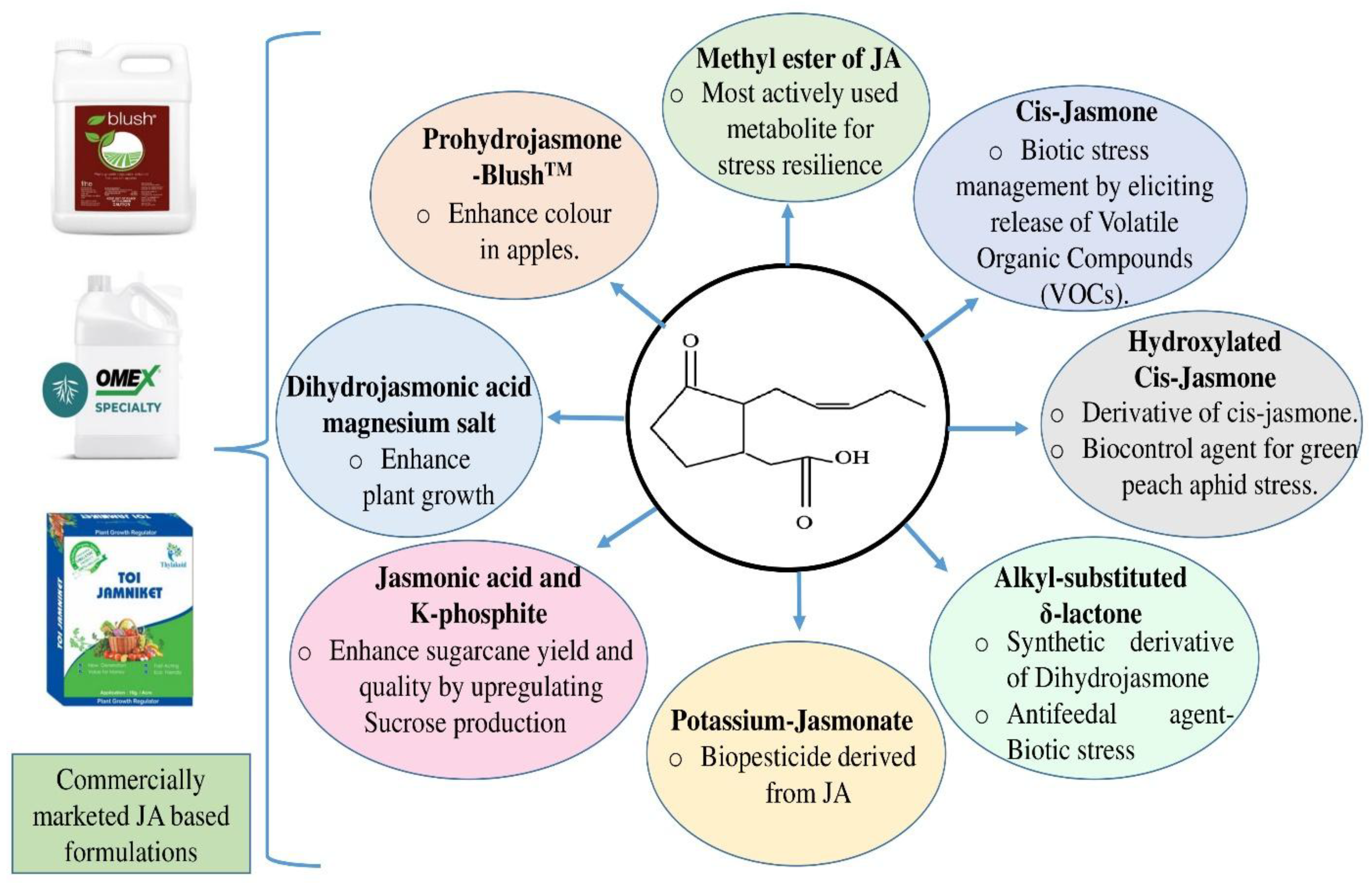
Conclusion
References
- Aldridge, D.C., Galt, S., Giles, D., Turner, W.B., 1971. Metabolites of Lasiodiplodia theobromae. Journal of the chemical society, C: Organic. 1623-1627.
- Bell, E., Creelman, R.A., Mullet, J.E., 1995. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA. 92, 8675-8679.
- Cohen, S., and Flescher, E., (2009). Methyl jasmonate: a plant stress hormone as an anti-cancer drug. Phytochemistry. 70: 1600-1609.
- David Marks, 2012. Agricultural composition. Patent no. US8137429B2.
- Demole, E., Lederer, E., Mercier, D., 1962. lsolement et determination de Ia structure du jasmonate methyl, constituent odorant characteristique de I’essence de jasmin. Helv. Chim. Acta. 45, 675-685.
- Farmer, E.E., Ryan, C.A., 1990. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proceedings of the National Academy of Sciences. 87(19), 7713-7716.
- Gewehr, M., 2014. Pesticidal Mixtures comprising Jasmonic Acid or a derivative thereof. Patent no. PCT/EP2013/070149.
- Ghasemi Pirbalouti, A., Sajjadi, S.E. and Parang, K., 2014. A review (research and patents) on jasmonic acid and its derivatives. Archiv der Pharmazie, 347(4), pp.229-239.
- Gunjegaonkar, S.M., Shanmugarajan, T.S., and Wankhede S.B. (2018). Plant Stress Hormone Methyl Jasmonate: Potential Candidate for Human Therapeutic Applications. International Journal of Pharmacognosy and Chinese Medicine. 2576-4772.
- He, Y., Fukushige, H., Hildebrand, D.F. and Gan, S., 2002. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant physiology, 128(3), pp.876-884.
- Hesse, A. and Müller, F., 1899. Ueber ätherisches Jasminblüthenöl. I. Berichte der deutschen chemischen Gesellschaft, 32(1), pp.565-574.
- Kaushik, S., Ranjan, A., Sidhu, A., Singh, A.K. and Sirhindi, G., 2024. Cadmium toxicity: its’ uptake and retaliation by plant defence system and ja signaling. BioMetals, 37(4), pp.755-772.
- Kaushik, S., Sidhu, A., Singh, A.K. and Sirhindi, G., 2022. Bioscience of Jasmonates in Harmonizing Plant Stress Conditions. In Jasmonates and Brassinosteroids in Plants (pp. 99-118). CRC Press.
- Kondo, S., Tomiyama, A. and Seto, H., 2000. Changes of endogenous jasmonic acid and methyl jasmonate in apples and sweet cherries during fruit development. Journal of the American Society for Horticultural Science, 125(3), pp.282-287.
- Mason, H.S., DeWald, D.B., Creelman, R.A., Mullet, J.E., 1992. Coregulation of Soybean Vegetative Storage Protein Gene Expression by Methyl Jasmonate and Soluble Sugars. Plant Physiol. 98, 859-867.
- Moreira, B.D.A., Viana, R.D.S., Lisboa, L.A.M., Lopes, P.R.M., Figueiredo, P.A.M., Ramos, S.B., Bonini, C.S.B., Trindade, V.D.R., ANDRADE, M.D.O. and May, A., 2019. Jasmonic acid and K-phosphite enhance productivity and technological quality of sugarcane crop.
- Omelianski, V.L., 1923. Aroma-producing microörganisms. Journal of bacteriology, 8(4), pp.393-419.
- Paprocka, M., Gliszczyńska, A., Dancewicz, K. and Gabryś, B., 2018. Novel hydroxy-and epoxy-cis-jasmone and dihydrojasmone derivatives affect the foraging activity of the peach potato aphid Myzus persicae (Sulzer)(Homoptera: Aphididae). Molecules, 23(9), p.2362.
- Power, F.B., 1921. The detection of methyl anthranilate in fruit juices. Journal of the American Chemical Society, 43(2), pp.377-381.
- Raviv, Z., Cohen, S., and Reischer-Pelech, D. (2013). The anti-cancer activities of jasmonates. Cancer Chemother Pharmacol. 71:275-285.
- Sahil, Das, A., Mehta, S., Abdelmotelb, K.F., Lavale, S.A., Aggarwal, S.K., Jat, B.S., Tripathi, A. and Garg, S., 2021. Jasmonic acid for sustainable plant growth and production under adverse environmental conditions. Plant Performance Under Environmental Stress: Hormones, Biostimulants and Sustainable Plant Growth Management, pp.71-98.
- Schubert, R., Dobritzsch, S., Gruber, C., Hause, G., Athmer, B., Schreiber, T., Marillonnet, S., Okabe, Y., Ezura, H., Acosta, I.F. and Tarkowska, D., 2019. Tomato MYB21 acts in ovules to mediate jasmonate-regulated fertility. The Plant Cell, 31(5), pp.1043-1062.
- Sembdner, G., Parthier, B., 1993. The biochemistry and the physiological and molecular actions of jasmonates. Annu. Rev. Plant Physiol. Plant MOI. Biol. 44, 569-589.
- Sidhu, A., Kaushik, S., Singh, A.K. and Sirhindi, G., 2022. Application of Jasmonates in the Sustainable Development of Agriculture and Horticulture Crops. In Jasmonates and Brassinosteroids in Plants (pp. 187-198). CRC Press.
- Sirhindi, G., Kaushik, S., Mushtaq, R., Sharma, P. and Dogra, N., 2020. Jasmonates induce growth and modulation in pigments and vitamins of Brassica oleracea var. capitata, italica and botrytis edible heads (foliage/inflorescence). Brazilian Journal of Botany, 43, pp.705-719.
- Sirhindi, G., Mir, M.A., Abd-Allah, E.F., Ahmad, P. and Gucel, S., 2016. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Frontiers in plant science, 7, p.591.
- Sobhy, I.S., Woodcock, C.M., Powers, S.J., Caulfield, J.C., Pickett, J.A. and Birkett, M.A., 2017. cis-Jasmone elicits aphid-induced stress signalling in potatoes. Journal of Chemical Ecology, 43, pp.39-52.
- Sood, M., 2023. Jasmonates: “The master switch” for regulation of developmental and stress responses in plants. Journal of Plant Growth Regulation, 42(8), pp.5247-5265.
- Vick, B.A., Zimmerman, D.C., 1987. “Oxidative systems for modification of fatty acids: the lipoxygenase pathway.” Lipids: structure and function. Academic Press. Pp. 53-90.
- Vincent, S.A., Kim, J.M., Pérez-Salamó, I., To, T.K., Torii, C., Ishida, J., Tanaka, M., Endo, T.A., Bhat, P., Devlin, P.F. and Seki, M., 2022. Jasmonates and Histone deacetylase 6 activate Arabidopsis genome-wide histone acetylation and methylation during the early acute stress response. BMC biology, 20(1), p.83.
- Wasternack, C. and Hause, B., 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of botany, 111(6), pp.1021-1058.
- Wasternack, C. and Song, S., 2017. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. Journal of Experimental Botany, 68(6), pp.1303-1321.
- Wasternack, C., 2015. How jasmonates earned their laurels: past and present. Journal of plant growth regulation, 34, pp.761-794.
- Wasternack, C., 2020. Determination of sex by jasmonate. Journal of Integrative Plant Biology, 62(2), pp.162-164.
- Xu, P., Zhao, P.X., Cai, X.T., Mao, J.L., Miao, Z.Q. and Xiang, C.B., 2020. Integration of jasmonic acid and ethylene into auxin signaling in root development. Frontiers in Plant Science, 11, p.271.
- Yoshida, Y., Sano, R., Wada, T., Takabayashi, J. and Okada, K., 2009. Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



