Submitted:
27 October 2025
Posted:
05 November 2025
You are already at the latest version
Abstract
Keywords:
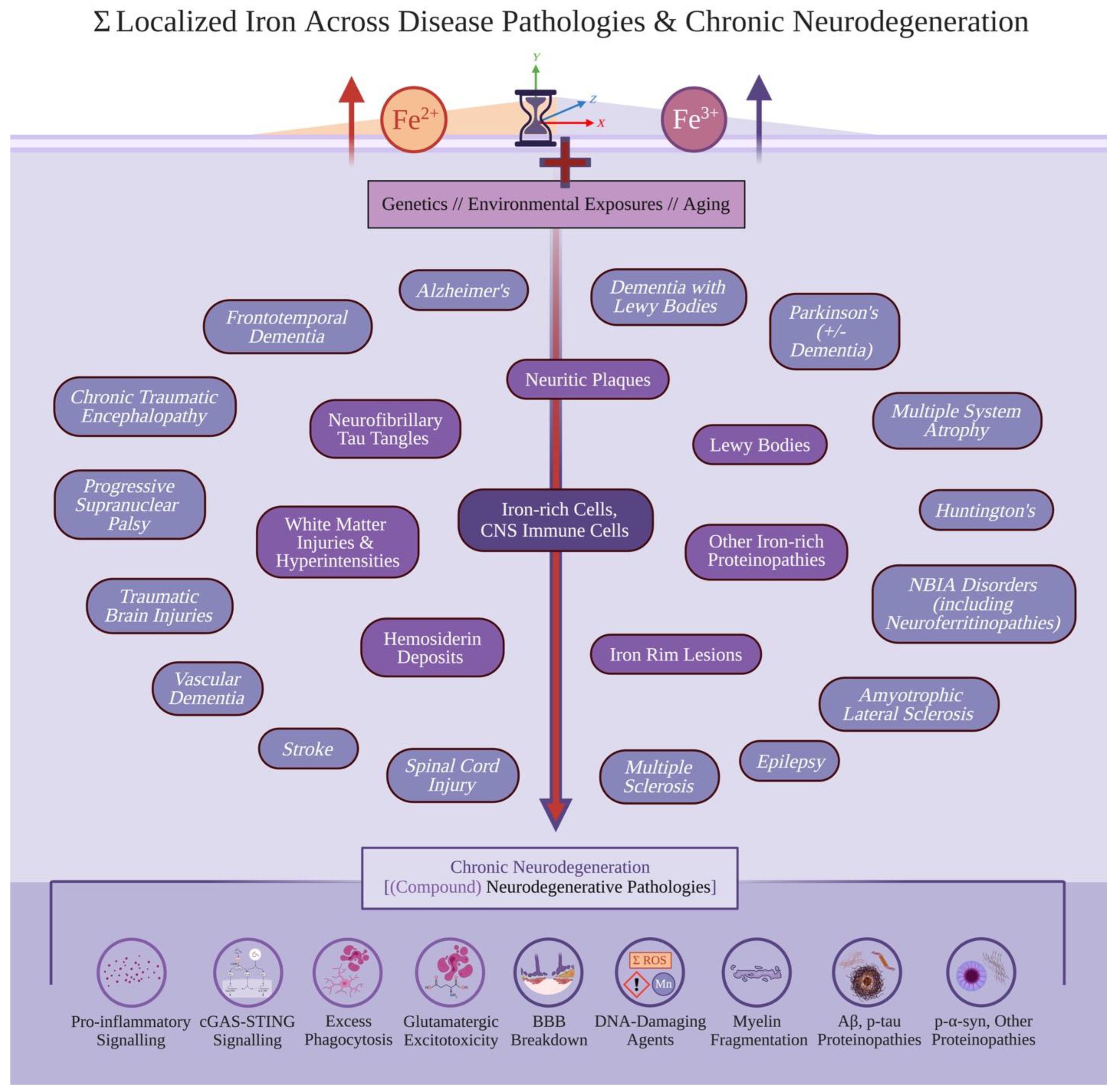
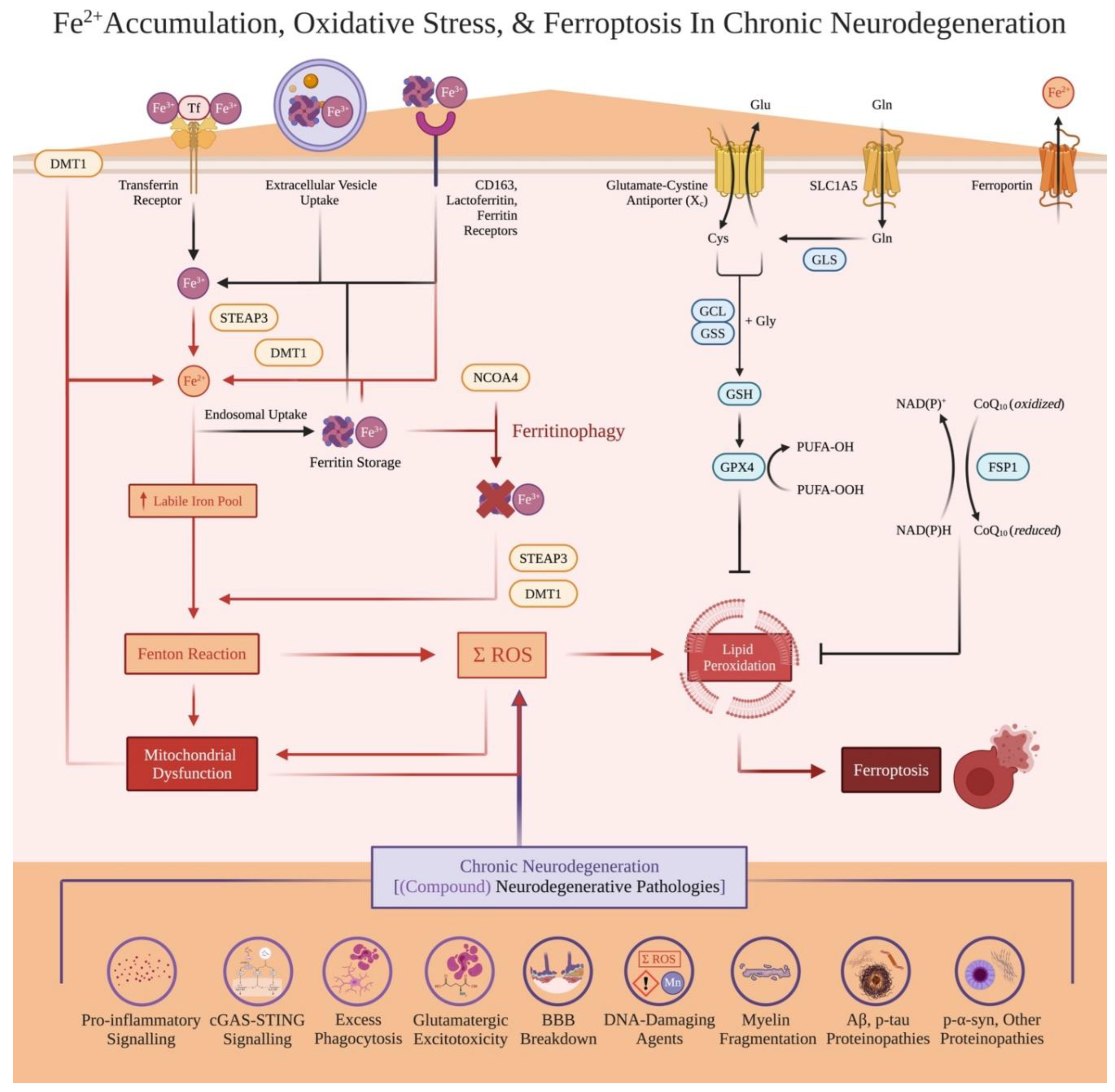
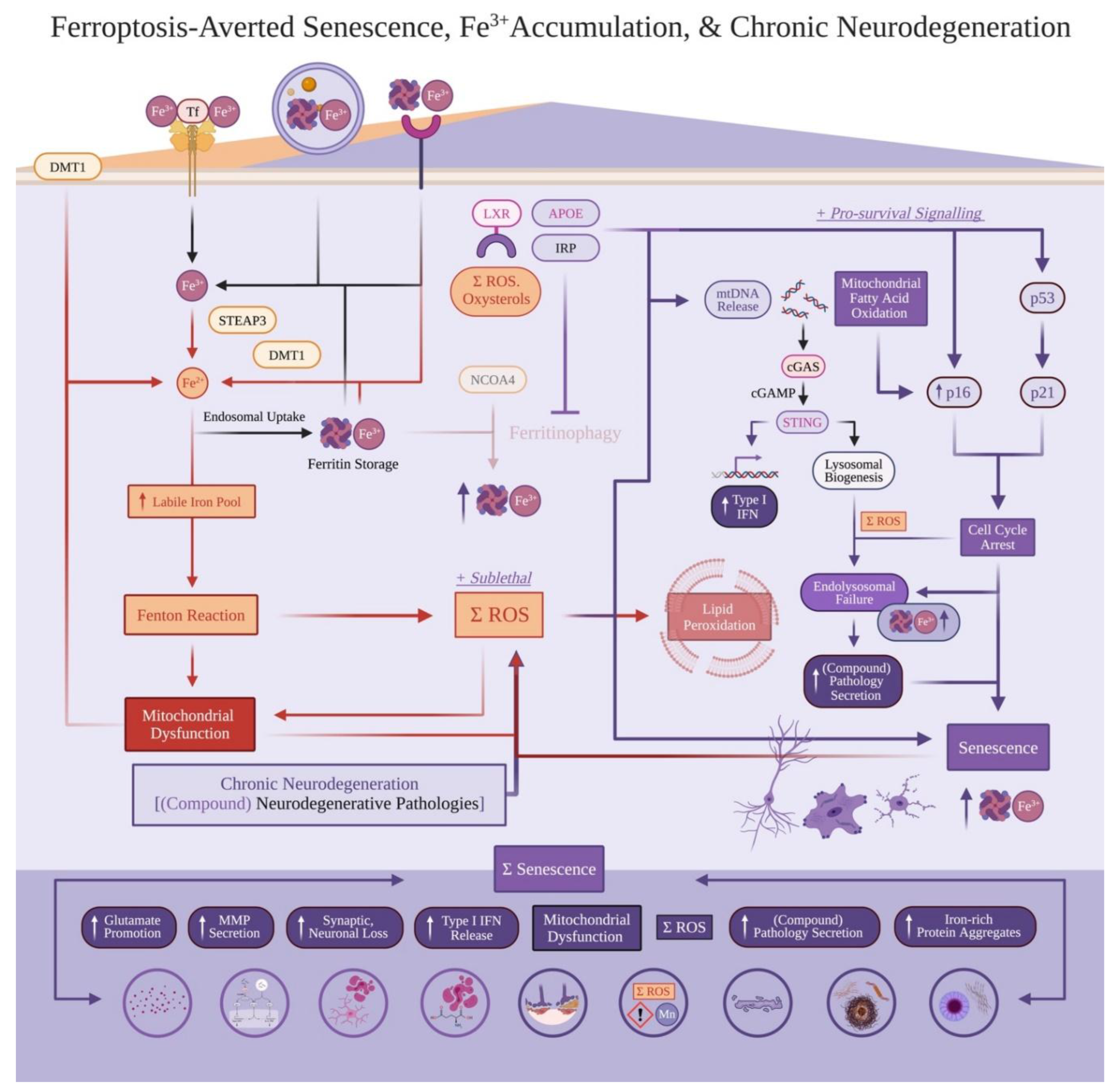
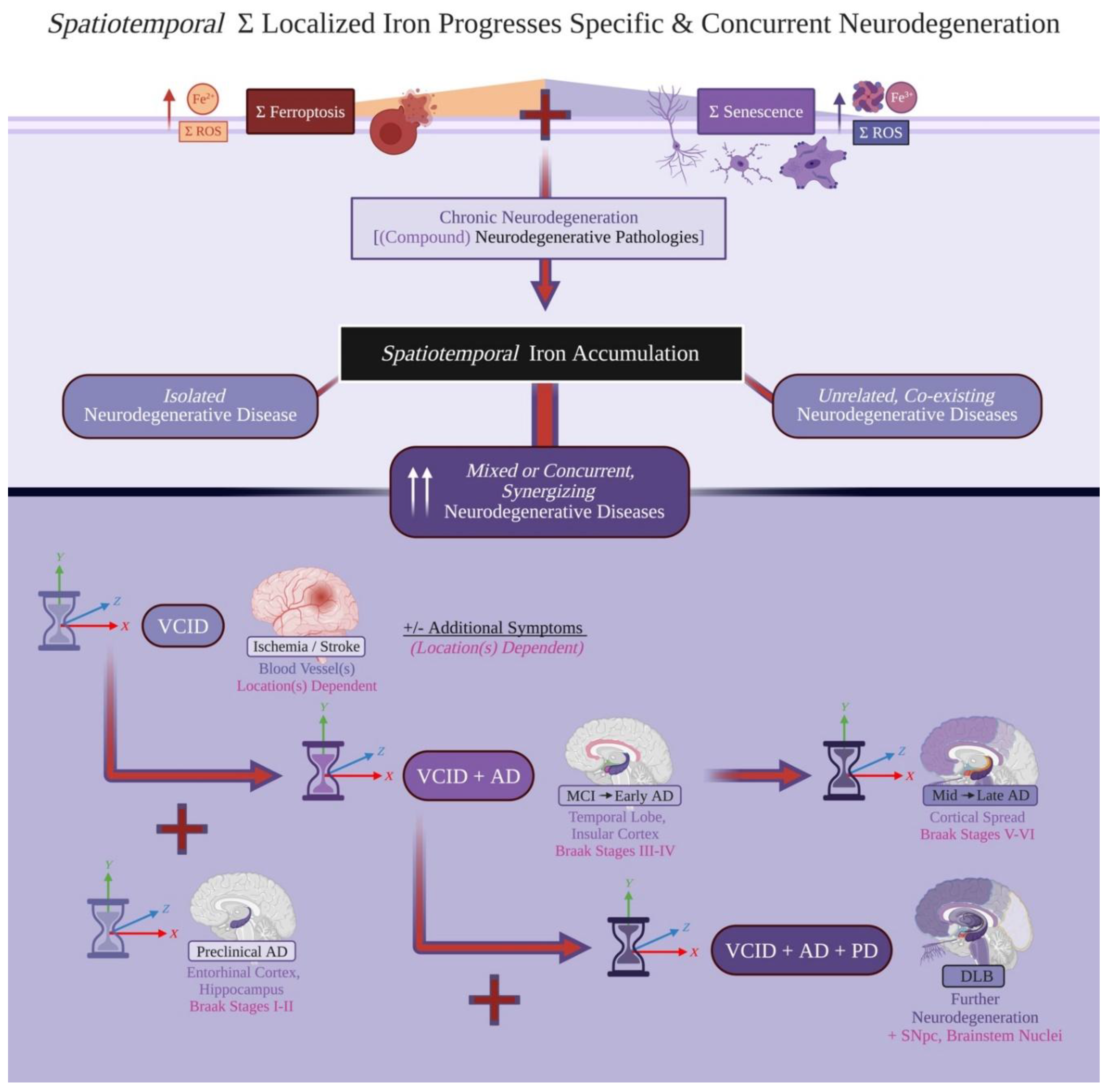
Main
Discussion
Author Contributions
Acknowledgements
Competing Interests
List of Abbreviations
References
- Kovacs, G.G. Molecular pathology of neurodegenerative diseases: principles and practice. BMJ 2019, 72. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; et al. Pathological combinations in neurodegenerative disease are heterogeneous and disease-associated. Brain 2023, 146, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.; Ramer, L.; Tremblay, M.-È. An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer’s disease. Nat. Commun. 2023, 14, 1670. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease - lessons from pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef]
- Paolini Paoletti, F.; Simoni, S.; Parnetti, L.; Gaetani, L. The Contribution of Small Vessel Disease to Neurodegeneration: Focus on Alzheimer’s Disease, Parkinson’s Disease and Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 4958. [Google Scholar] [CrossRef]
- Lerma-Martin, C. Cell type mapping reveals tissue niches and interactions in subcortical multiple sclerosis lesions. Nat. Neurosci. 2024, 27. [Google Scholar] [CrossRef]
- Alsema, A.M.; et al. Spatially resolved gene signatures of white matter lesion progression in multiple sclerosis. Nat. Neurosci. 2024, 27, 1–13. [Google Scholar] [CrossRef]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxid. Med. Cell. Longev. 2015, 2015, 151979. [Google Scholar] [CrossRef]
- Paulsen, J.S. Cognitive Impairment in Huntington Disease: Diagnosis and Treatment. Curr. Neurol. Neurosci. Rep. 2011, 11, 474–483. [Google Scholar] [CrossRef]
- Stankovic, I.; et al. Cognitive impairment in multiple system atrophy. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 857–867. [Google Scholar] [CrossRef]
- DeLuca, G.C.; Yates, R.L.; Beale, H.; Morrow, S.A. Cognitive Impairment in Multiple Sclerosis: Clinical, Radiologic and Pathologic Insights. Brain Pathol. 2014, 25, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron imbalance in neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Berggren, K.L.; Marks, E.; Fox, J.H. Impact of high iron intake on cognition and neurodegeneration in humans and in animal models: a systematic review. Nutr. Rev. 2017, 75, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; et al. Iron retardation in lysosomes protects senescent cells from ferroptosis. Aging 2024, 16, 7683–7703. [Google Scholar] [CrossRef]
- Andersen, H.H.; Johnsen, K.B.; Moos, T. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell. Mol. Life Sci. 2014, 71, 1607–1622. [Google Scholar] [CrossRef]
- Leon-Oliva, D.D.; et al. Improving understanding of ferroptosis: molecular mechanisms, connection with cellular senescence and implications for aging. 2024, 10.
- Ravanfar, P.; et al. Systematic Review: Quantitative Susceptibility Mapping (QSM) of Brain Iron Profile in Neurodegenerative Diseases. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Dusek, P.; Hofer, T.; Alexander, J.; Roos, P.M.; Aaseth, J.O. Cerebral Iron Deposition in Neurodegeneration. Biomolecules 2022, 12, 714. [Google Scholar] [CrossRef]
- Guo, J.; Tuo, Q.; Lei, P. Iron, Ferroptosis, and Ischemic Stroke. J. Neurochem. 2023, 165. [Google Scholar] [CrossRef]
- Riederer, P.; et al. Lewy bodies, iron, inflammation and neuromelanin: pathological aspects underlying Parkinson’s disease. J. Neural Transm. 2023, 130, 627–646. [Google Scholar] [CrossRef]
- Spence, H.; McNeil, C.J.; Waiter, G.D. The impact of brain iron accumulation on cognition: A systematic review. 2020, 15. [CrossRef]
- Chen, L.; et al. Quantitative Susceptibility Mapping of Brain Iron and β-Amyloid in MRI and PET Relating to Cognitive Performance in Cognitively Normal Older Adults. Radiology 2021, 298, 353–362. [Google Scholar] [CrossRef]
- Zachariou, V.; Pappas, C.; Bauer, C.E.; Seago, E.R.; Gold, B.T. Exploring the links among brain iron accumulation, cognitive performance, and dietary intake in older adults: A longitudinal MRI study. Neurobiol. Aging 2024, 145. [Google Scholar] [CrossRef]
- Li, Z.; et al. Paramagnetic susceptibility measured by magnetic resonance imaging as an in vivo biomarker for iron pathology in epilepsy. Sci. Adv. 2025, 11, eads8149. [Google Scholar] [CrossRef]
- Lu, W.; et al. Relaxometry network based on MRI R2* mapping revealing brain iron accumulation patterns in Parkinson’s disease. NeuroImage 2024, 303, 120943. [Google Scholar] [CrossRef]
- Alushaj, E.; et al. Increased iron in the substantia nigra pars compacta identifies patients with early Parkinson’s disease: A 3T and 7T MRI study. NeuroImage Clin. 2024, 41, 103577. [Google Scholar] [CrossRef]
- Yao, S.; et al. Quantitative Susceptibility Mapping Reveals an Association between Brain Iron Load and Depression Severity. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Yan, S.; Sun, J.; Chen, Y.; Selim, M.; Lou, M. Brain iron deposition in white matter hyperintensities: a 3-T MRI study. AGE 2013, 35, 1927–1936. [Google Scholar] [CrossRef]
- Valdés Hernández, M.; et al. Do white matter hyperintensities mediate the association between brain iron deposition and cognitive abilities in older people? Eur. J. Neurol. 2016, 23, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, M.-S. Brain Iron Accumulation in Atypical Parkinsonian Syndromes: in vivo MRI Evidences for Distinctive Patterns. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; et al. Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia. Acta Neuropathol. (Berl.) 1990, 81, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, W.; Wang, X.; Peng, D.; Jiang, Z. Exploring the Causal Relationship Between Inflammatory Cytokines and MRI-Derived Brain Iron: A Mendelian Randomization Study. Brain Behav. 2024, 14, e70181. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; et al. Ferroptosis: past, present and future. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Joppe, K.; Roser, A.-E.; Maass, F.; Lingor, P. The Contribution of Iron to Protein Aggregation Disorders in the Central Nervous System. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Jhelum, P.; et al. Ferroptosis induces detrimental effects in chronic EAE and its implications for progressive MS. Acta Neuropathol. Commun. 2023, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.K.; et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2023, 26, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxid. Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Stys, P. White Matter Injury Mechanisms. Curr. Mol. Med. 2004, 4, 113–130. [Google Scholar] [CrossRef]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef]
- Goldman, S.M. Environmental Toxins and Parkinson’s Disease. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef]
- Javadov, S. Mitochondria and ferroptosis. Curr. Opin. Physiol. 2022, 25, 100483. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2020, 99, 151058. [Google Scholar] [CrossRef]
- Tisdall, M.D.; et al. Ex vivo MRI and histopathology detect novel iron-rich cortical inflammation in frontotemporal lobar degeneration with tau versus TDP-43 pathology. NeuroImage Clin. 2022, 33, 102913. [Google Scholar] [CrossRef] [PubMed]
- Giannini, L.A.A.; et al. Cortical iron accumulation in MAPT- and C9orf 72-associated frontotemporal lobar degeneration. Brain Pathol. 2023, 33, e13158. [Google Scholar] [CrossRef] [PubMed]
- Waldron, F.M.; et al. Brain Iron as a Surrogate Biomarker of Pathological TDP-43 Identifies Brain Region-Specific Signatures in Ageing, Alzheimer’s Disease and Amyotrophic Lateral Sclerosis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Seo, A.Y.; et al. Mitochondrial iron accumulation with age and functional consequences. Aging Cell 2008, 7, 706–716. [Google Scholar] [CrossRef]
- Chen, Y.; et al. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Tan, E.-K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 75. [Google Scholar] [CrossRef]
- Truman-Rosentsvit, M.; et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 2018, 131, 342–352. [Google Scholar] [CrossRef]
- Yanatori, I.; Richardson, D.R.; Dhekne, H.S.; Toyokuni, S.; Kishi, F. CD63 is regulated by iron via the IRE-IRP system and is important for ferritin secretion by extracellular vesicles. Blood 2021, 138, 1490–1503. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Hou, W.; et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Masaldan, S.; et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, P.A.; et al. Ferroptosis of Microglia in Aging Human White Matter Injury. Ann. Neurol. 2023, 94, 1048–1066. [Google Scholar] [CrossRef] [PubMed]
- da Costa Caiado, M.J.; Dolga, A.M.; den Dunnen, W.F.A. Iron(ing) out parkinsonisms: The interplay of proteinopathy and ferroptosis in Parkinson’s disease and tau-related parkinsonisms. Redox Biol. 2025, 79, 103478. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; et al. The Emerging Roles of Ferroptosis in Huntington’s Disease. NeuroMolecular Med. 2019, 21, 110–119. [Google Scholar] [CrossRef]
- Cozzi, A.; et al. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem Cell Rep. 2019, 13, 832–846. [Google Scholar] [CrossRef]
- Good, P.F.; Perl, D.P.; Bierer, L.M.; Schmeidler, J. Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer’s disease: A laser microprobe (LAMMA) study. Ann. Neurol. 1992, 31, 286–292. [Google Scholar] [CrossRef]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. 1997, 94, 9866–9868. [Google Scholar] [CrossRef]
- Killilea, D.W.; Wong, S.L.; Cahaya, H.S.; Atamna, H.; Ames, B.N. Iron accumulation during cellular senescence. Ann. N. Y. Acad. Sci. 2004, 1019, 365–367. [Google Scholar] [CrossRef]
- Maus, M.; et al. Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype. Nat. Metab. 2023, 5, 1–20. [Google Scholar] [CrossRef]
- Admasu, T.D.; et al. Selective ablation of primary and paracrine senescent cells by targeting iron dyshomeostasis. Cell Rep. 2023, 42. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, G.G.; et al. In vivo DNA replication dynamics unveil aging-dependent replication stress. Cell 2024, 187. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell senescence, rapamycin and hyperfunction theory of aging. Cell Cycle 2022, 21, 1456–1467. [Google Scholar] [CrossRef]
- Rovira, M.; et al. The lysosomal proteome of senescent cells contributes to the senescence secretome. Aging Cell 2022, 21, e13707. [Google Scholar] [CrossRef]
- Victorelli, S.; et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature 2023, 622, 627–636. [Google Scholar] [CrossRef]
- Xu, M.; et al. Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 2018, 24, 1246. [Google Scholar] [CrossRef]
- Zhang, X.; et al. Rejuvenation of the aged brain immune cell landscape in mice through p16-positive senescent cell clearance. Nat. Commun. 2022, 13, 5671. [Google Scholar] [CrossRef]
- Jin, M.; et al. Type I interferon signaling drives microglial dysfunction and senescence in human iPSC models of Down syndrome and Alzheimer’s disease. Cell Stem Cell 2022, 29, 1135–1153.e8. [Google Scholar] [CrossRef]
- Brelstaff, J.H.; et al. Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Shen, Q.; et al. Cell senescence induced by toxic interaction between α-synuclein and iron precedes nigral dopaminergic neuron loss in a mouse model of Parkinson’s disease. Acta Pharmacol. Sin. 2024, 45, 268–281. [Google Scholar] [CrossRef]
- Hong, B.; Ohtake, Y.; Itokazu, T.; Yamashita, T. Glial senescence enhances α-synuclein pathology owing to its insufficient clearance caused by autophagy dysfunction. Cell Death Discov. 2024, 10, 1–12. [Google Scholar] [CrossRef]
- Safaiyan, S.; et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef]
- Matsudaira, T.; et al. Cellular senescence in white matter microglia is induced during ageing in mice and exacerbates the neuroinflammatory phenotype. Commun. Biol. 2023, 6, 665. [Google Scholar] [CrossRef]
- Zhang, X.; et al. Exposure to Manganese Induces Autophagy–Lysosomal Pathway Dysfunction-Mediated Tauopathy by Activating the cGAS–STING Pathway in the Brain. Environ. Health 2024, 3. [Google Scholar] [CrossRef] [PubMed]
- López-Polo, V.; et al. Release of mitochondrial dsRNA into the cytosol is a key driver of the inflammatory phenotype of senescent cells. Nat. Commun. 2024, 15, 7378. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; et al. Mitochondrial fatty acid oxidation drives senescence. Sci. Adv. 2024, 10, eado5887. [Google Scholar] [CrossRef] [PubMed]
- Torres-Querol, C.; et al. Acute ischemic stroke triggers a cellular senescence-associated secretory phenotype. Sci. Rep. 2021, 11, 15752. [Google Scholar] [CrossRef]
- Baixauli-Martín, J.; et al. Spatio-Temporal Characterization of Cellular Senescence Hallmarks in Experimental Ischemic Stroke. Int. J. Mol. Sci. 2025, 26, 2364. [Google Scholar] [CrossRef]
- Gross, P.S.; et al. Senescent-like microglia limit remyelination through the senescence associated secretory phenotype. Nat. Commun. 2025, 16, 2283. [Google Scholar] [CrossRef]
- Belaidi, A.A.; et al. Apolipoprotein E potently inhibits ferroptosis by blocking ferritinophagy. Mol. Psychiatry 2024, 29, 211–220. [Google Scholar] [CrossRef]
- Lee, H.; et al. Cell cycle arrest induces lipid droplet formation and confers ferroptosis resistance. Nat. Commun. 2024, 15, 79. [Google Scholar] [CrossRef]
- Courtney, R.; Landreth, G.E. LXR regulation of brain cholesterol: from development to disease. Trends Endocrinol. Metab. TEM 2016, 27, 404–414. [Google Scholar] [CrossRef]
- Jiang, Q.; et al. ApoE promotes the proteolytic degradation of Aβ. Neuron 2008, 58, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; et al. Destabilizing heterochromatin by APOE mediates senescence. Nat. Aging 2022, 2, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; et al. Microglial apolipoprotein E particles contribute to neuronal senescence and synaptotoxicity. iScience 2024, 27, 110006. [Google Scholar] [CrossRef] [PubMed]
- Bancaro, N.; et al. Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer. Cancer Cell 2023, 41, 602–619.e11. [Google Scholar] [CrossRef]
- Windham, I.A.; Cohen, S. The cell biology of APOE in the brain. Trends Cell Biol. 2024, 34, 338–348. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; An, S.S.A.; Kim, S.Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015, 11, 1723–1737. [Google Scholar] [CrossRef]
- Höhn, A.; Jung, T.; Grimm, S.; Grune, T. Lipofuscin-bound iron is a major intracellular source of oxidants: Role in senescent cells. Free Radic. Biol. Med. 2010, 48, 1100–1108. [Google Scholar] [CrossRef]
- Reinert, A.; Morawski, M.; Seeger, J.; Arendt, T.; Reinert, T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019, 20, 25. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Ren, J.; Chen, Q.; Chen, Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4612. [Google Scholar] [CrossRef]
- Karabag, D.; et al. Characterizing microglial senescence: Tau as a key player. J. Neurochem. 2023, 166. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; et al. Nuclear alpha-synuclein accelerates cell senescence and neurodegeneration. Immun. Ageing 2024, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.M.; et al. Senescent and disease-associated microglia are modifiable features of aged brain white matter. Res. Sq. rs.3.rs-3467812 (2023). [CrossRef]
- Yoon, Y.-S.; et al. Senescence and impaired DNA damage responses in alpha-synucleinopathy models. Exp. Mol. Med. 2022, 54, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Carlsson, F.; Laskar, A.; Yuan, X.-M.; Li, W. Lysosomal membrane permeabilization causes oxidative stress and ferritin induction in macrophages. FEBS Lett. 2011, 585, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Kaji, S.; et al. Apolipoprotein E aggregation in microglia initiates Alzheimer’s disease pathology by seeding β-amyloidosis. Immunity 2024, 57. [Google Scholar] [CrossRef]
- Quick, J.D.; et al. Lysosomal acidification dysfunction in microglia: an emerging pathogenic mechanism of neuroinflammation and neurodegeneration. J. Neuroinflammation 2023, 20, 185. [Google Scholar] [CrossRef]
- Jin, M.; et al. Tau activates microglia via the PQBP1-cGAS-STING pathway to promote brain inflammation. Nat. Commun. 2021, 12, 6565. [Google Scholar] [CrossRef]
- Gulen, M.F.; et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 1–7. [Google Scholar] [CrossRef]
- Lv, B.; et al. A TBK1-independent primordial function of STING in lysosomal biogenesis. Mol. Cell 2024, 84. [Google Scholar] [CrossRef] [PubMed]
- Curnock, R.; et al. TFEB-dependent lysosome biogenesis is required for senescence. EMBO J. 2023, 42, e111241. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Martinez-Valbuena, I.; Lang, A.E.; Kovacs, G.G. Cellular iron deposition patterns predict clinical subtypes of multiple system atrophy. Neurobiol. Dis. 2024, 197, 106535. [Google Scholar] [CrossRef]
- Simmons, D.A.; et al. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia 2007, 55, 1074–1084. [Google Scholar] [CrossRef]
- Kwan, J.Y.; et al. Iron Accumulation in Deep Cortical Layers Accounts for MRI Signal Abnormalities in ALS: Correlating 7 Tesla MRI and Pathology. PLOS ONE 2012, 7, e35241. [Google Scholar] [CrossRef]
- Shahidehpour, R.K.; et al. Dystrophic microglia are associated with neurodegenerative disease and not healthy aging in the human brain. Neurobiol. Aging 2021, 99, 19–27. [Google Scholar] [CrossRef]
- Shahidehpour, R.K.; Nelson, P.T.; Katsumata, Y.; Bachstetter, A.D. Exploring the link between dystrophic microglia and the spread of Alzheimer’s neuropathology. Brain 2024, awae258. [Google Scholar] [CrossRef]
- Swanson, M.E.V.; et al. Quantitative immunohistochemical analysis of myeloid cell marker expression in human cortex captures microglia heterogeneity with anatomical context. Sci. Rep. 2020, 10, 11693. [Google Scholar] [CrossRef]
- Kenkhuis, B.; et al. Iron loading is a prominent feature of activated microglia in Alzheimer’s disease patients. Acta Neuropathol. Commun. 2021, 9, 27. [Google Scholar] [CrossRef]
- Zeineh, M.M.; et al. Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol. Aging 2015, 36, 2483–2500. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, S.; et al. Cortical Iron Reflects Severity of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Pisa, M.; et al. Aberrant iron deposition in the multiple sclerosis spinal cord relates to neurodegeneration. Preprint 2024. [Google Scholar] [CrossRef]
- Tsering, W.; et al. Preferential clustering of microglia and astrocytes around neuritic plaques during progression of Alzheimer’s disease neuropathological changes. J. Neurochem. 2025, 169, e16275. [Google Scholar] [CrossRef]
- Swanson, M.E.V.; et al. Identification of a dysfunctional microglial population in human Alzheimer’s disease cortex using novel single-cell histology image analysis. Acta Neuropathol. Commun. 2020, 8, 170. [Google Scholar] [CrossRef]
- Thrupp, N.; et al. Single-Nucleus RNA-Seq Is Not Suitable for Detection of Microglial Activation Genes in Humans. Cell Rep. 2020, 32, 108189. [Google Scholar] [CrossRef]
- Fancy, N.N.; et al. Characterisation of premature cell senescence in Alzheimer’s disease using single nuclear transcriptomics. Acta Neuropathol. (Berl.) 2024, 147, 78. [Google Scholar] [CrossRef]
- Wang, Q.; et al. Single cell transcriptomes and multiscale networks from persons with and without Alzheimer’s disease. Nat. Commun. 2024, 15, 5815. [Google Scholar] [CrossRef]
- Fagiani, F.; et al. Spatially-restricted inflammation-induced senescent-like glia in multiple sclerosis and patient-derived organoids. Nat. Commun. 2025, 16, 8477. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Wang, J.; Pan, X. Arsenic exposure induces neural cells senescence and abnormal lipid droplet accumulation leading to social memory impairment in mice. Environ. Pollut. 2025, 368, 125779. [Google Scholar] [CrossRef]
- Zhao, X.; et al. Up-regulated succinylation modifications induce a senescence phenotype in microglia by altering mitochondrial energy metabolism. J. Neuroinflammation 2024, 21, 296. [Google Scholar] [CrossRef] [PubMed]
- Angelova, D.M.; Brown, D.R. Model Senescent Microglia Induce Disease Related Changes in α-Synuclein Expression and Activity. Biomolecules 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Angelova, D.M.; Brown, D.R. Altered Processing of β-Amyloid in SH-SY5Y Cells Induced by Model Senescent Microglia. ACS Chem. Neurosci. 2018, 9, 3137–3152. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.; et al. Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol. 2019, 29, 606–621. [Google Scholar] [CrossRef]
- Wei, L.; et al. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway. J. Neuroinflammation 2023, 20, 208. [Google Scholar] [CrossRef]
- Kenkhuis, B.; et al. Iron accumulation induces oxidative stress, while depressing inflammatory polarization in human iPSC-derived microglia. Stem Cell Rep. 2022. [Google Scholar] [CrossRef]
- Choi, I.; et al. Autophagy enables microglia to engage amyloid plaques and prevents microglial senescence. Nat. Cell Biol. 2023, 1–12. [Google Scholar] [CrossRef]
- Malvaso, A.; et al. Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring. Cells 2023, 12, 2824. [Google Scholar] [CrossRef]
- Jiao, L.; et al. Iron metabolism mediates microglia susceptibility in ferroptosis. Front. Cell. Neurosci. 2022, 16, 995084. [Google Scholar] [CrossRef]
- Roe, M.T.; Dawson, D.V.; Hulette, C.M.; Einstein, G.; Crain, B.J. Microglia Are Not Exclusively Associated with Plaque-rich Regions of the Dentate Gyrus in Alzheimer’s Disease. J. Neuropathol. Exp. Neurol. 1996, 55, 366–371. [Google Scholar] [CrossRef]
- Langhi Prata, L.G.P.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; et al. Soluble iron accumulation makes microglia to overproduce and release glutamate via aconitase 1, tace and glutaminase c in ALS spinal cords. J. Neurol. Sci. 2017, 381, 710. [Google Scholar] [CrossRef]
- Ogrodnik, M.; et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 2021, 20, e13296. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J.R.; et al. Microglial ferroptotic stress causes non-cell autonomous neuronal death. Mol. Neurodegener. 2024, 19, 14. [Google Scholar] [CrossRef]
- Gan, L.; et al. Soluble DLK1 secreted by telomere-shortening-induced senescent microglia impairs myelination and alters neuronal activity. Preprint 2024. [Google Scholar] [CrossRef]
- Shin, H.J.; et al. Rejuvenating aged microglia by p16ink4a-siRNA-loaded nanoparticles increases amyloid-β clearance in animal models of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 25. [Google Scholar] [CrossRef]
- Carling, G.K.; et al. Alzheimer’s disease-linked risk alleles elevate microglial cGAS-associated senescence and neurodegeneration in a tauopathy model. Neuron 2024, 112, 3877–3896.e8. [Google Scholar] [CrossRef]
- Lee, S.H.; et al. Amyloid-β-activated microglia can induce compound proteinopathies. Brain 2024, 147. [Google Scholar] [CrossRef]
- Chung, S.; et al. Blockade of STING activation alleviates microglial dysfunction and a broad spectrum of Alzheimer’s disease pathologies. Exp. Mol. Med. 2024, 1–16. [Google Scholar] [CrossRef]
- Hu, Y.; et al. Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology. Cell Rep. 2021, 35, 109228. [Google Scholar] [CrossRef]
- Caldeira, C.; et al. Key Aging-Associated Alterations in Primary Microglia Response to Beta-Amyloid Stimulation. Front. Aging Neurosci. 2017, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.M.; et al. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol. Aging 2019, 77, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Brkic, M.; Balusu, S.; Libert, C.; Vandenbroucke, R.E. Friends or Foes: Matrix Metalloproteinases and Their Multifaceted Roles in Neurodegenerative Diseases. Mediators Inflamm. 2015, 2015, 620581. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.R.; et al. Concerted type I interferon signaling in microglia and neural cells promotes memory impairment associated with amyloid β plaques. Immunity 2022, 55, 879–894.e6. [Google Scholar] [CrossRef]
- Roy, E.R.; et al. Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J. Clin. Invest. 2020, 130, 1912. [Google Scholar] [CrossRef]
- He, Z.; et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 2018, 24, 29–38. [Google Scholar] [CrossRef]
- Li, T.; et al. The neuritic plaque facilitates pathological conversion of tau in an Alzheimer’s disease mouse model. Nat. Commun. 2016, 7, 12082. [Google Scholar] [CrossRef]
- Baligács, N.; et al. Homeostatic microglia initially seed and activated microglia later reshape amyloid plaques in Alzheimer’s Disease. Nat. Commun. 2024, 15, 10634. [Google Scholar] [CrossRef]
- Bassil, F.; et al. Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology. Neuron 2020, 111. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. (Berl.) 2006, 112, 389–404. [Google Scholar] [CrossRef]
- Borghammer, P. The α-Synuclein Origin and Connectome Model (SOC Model) of Parkinson’s Disease: Explaining Motor Asymmetry, Non-Motor Phenotypes, and Cognitive Decline. J. Park. Dis. 2021, 11, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; et al. Unified Staging System for Lewy Body Disorders: Clinicopathologic Correlations and Comparison to Braak Staging. J. Neuropathol. Exp. Neurol. 2019, 78, 891. [Google Scholar] [CrossRef] [PubMed]
- Reinert, A.; Morawski, M.; Seeger, J.; Arendt, T.; Reinert, T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.L.; Martin, J.A.; Lee, V.M.-Y.; Trojanowski, J.Q. Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer’s disease and Lewy body disorders. Acta Neuropathol. (Berl.) 1996, 91, 475–481. [Google Scholar] [CrossRef]
- Wang, C.; et al. Methods for quantitative susceptibility and R2* mapping in whole post-mortem brains at 7T applied to amyotrophic lateral sclerosis. Neuroimage 2020, 222, 117216. [Google Scholar] [CrossRef]
- Bagnato, F.; et al. Untangling the R2* contrast in multiple sclerosis: A combined MRI-histology study at 7.0 Tesla. PLoS ONE 2018, 13, e0193839. [Google Scholar] [CrossRef]
- Zhang, W.; et al. Brain Iron Deposits in Thalamus Is an Independent Factor for Depressive Symptoms Based on Quantitative Susceptibility Mapping in an Older Adults Community Population. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




