Submitted:
29 October 2025
Posted:
30 October 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
2.1. Population
2.2. Risk Factors for IFD and Statistical Analysis
Results
3.1. Characteristics of the Population
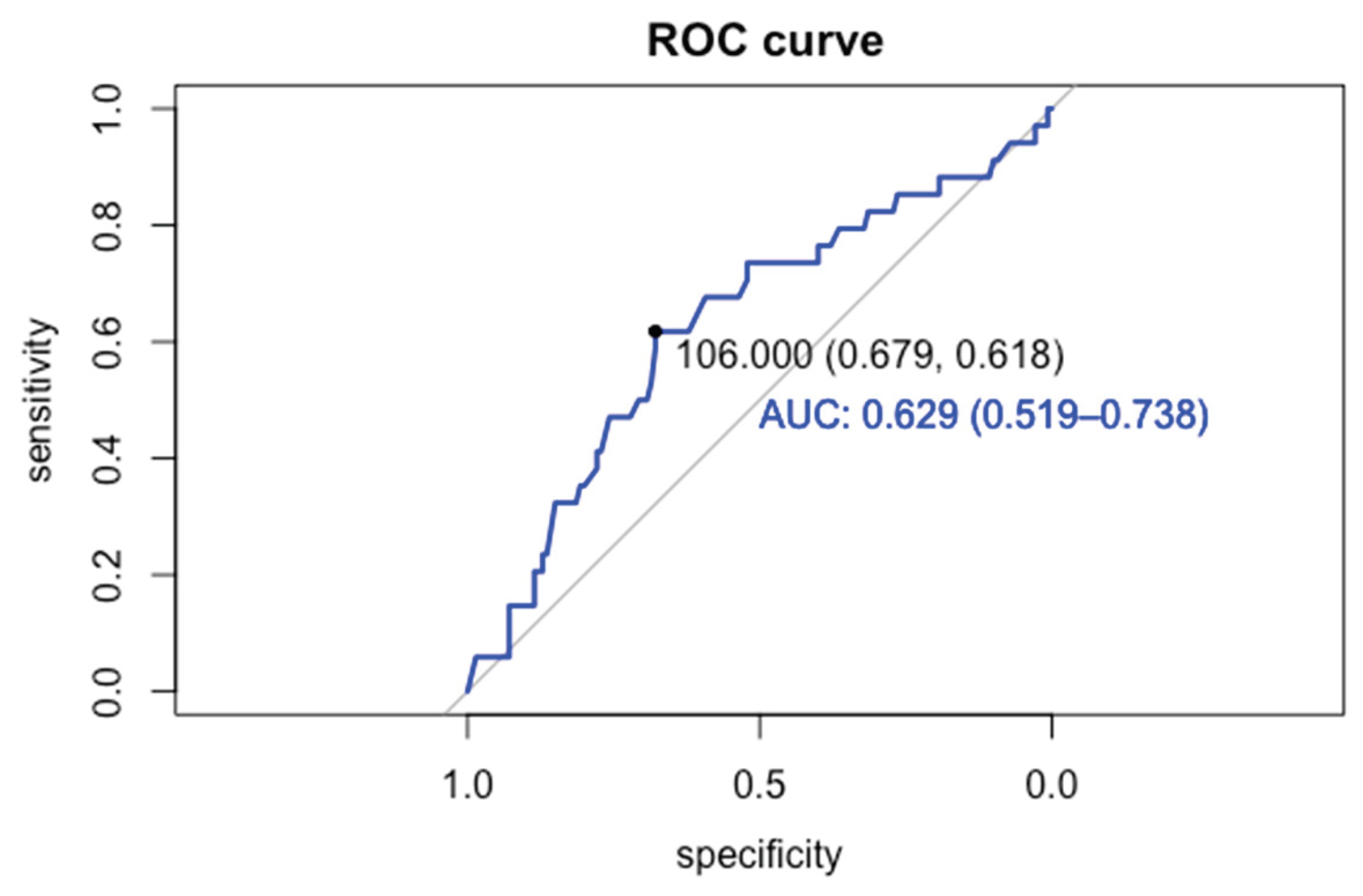
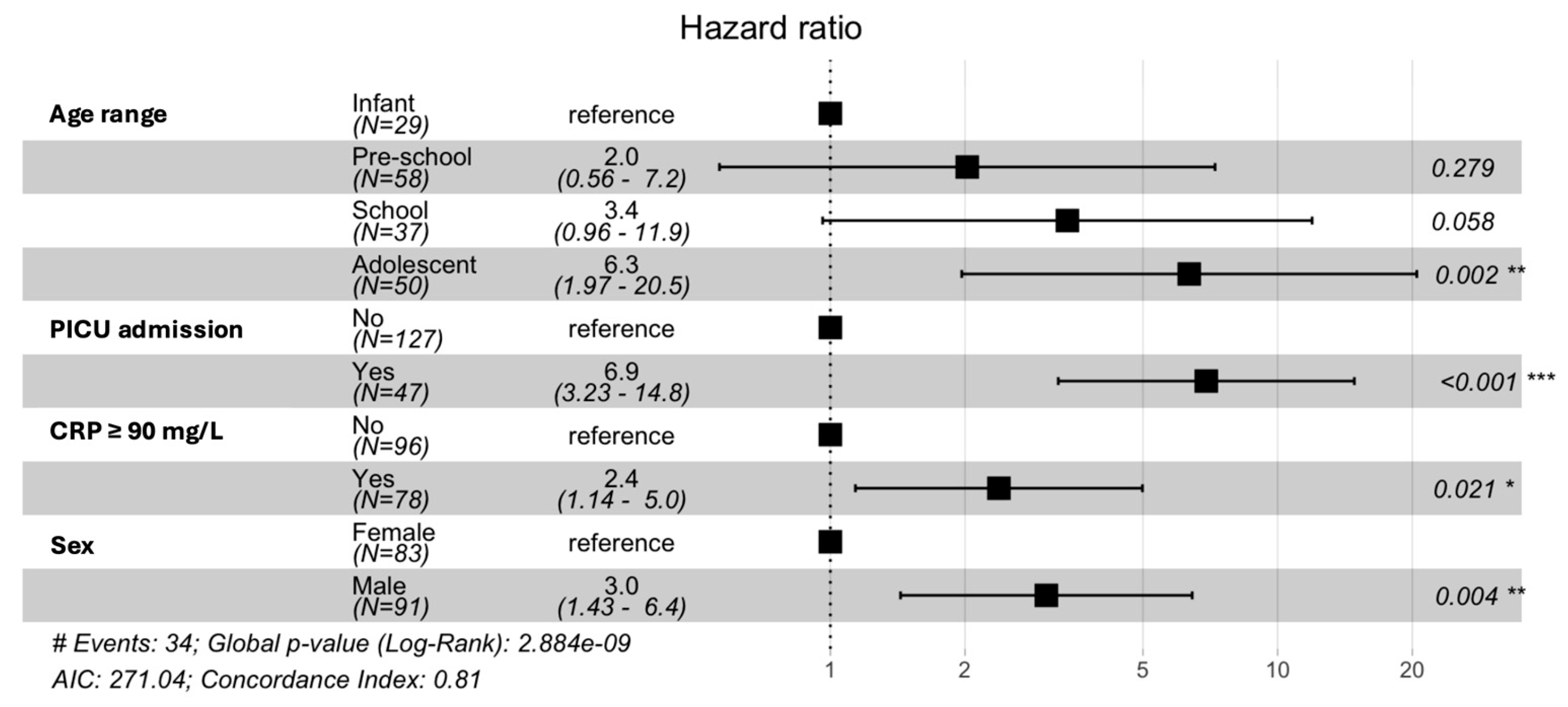
3.2. Risk Factors
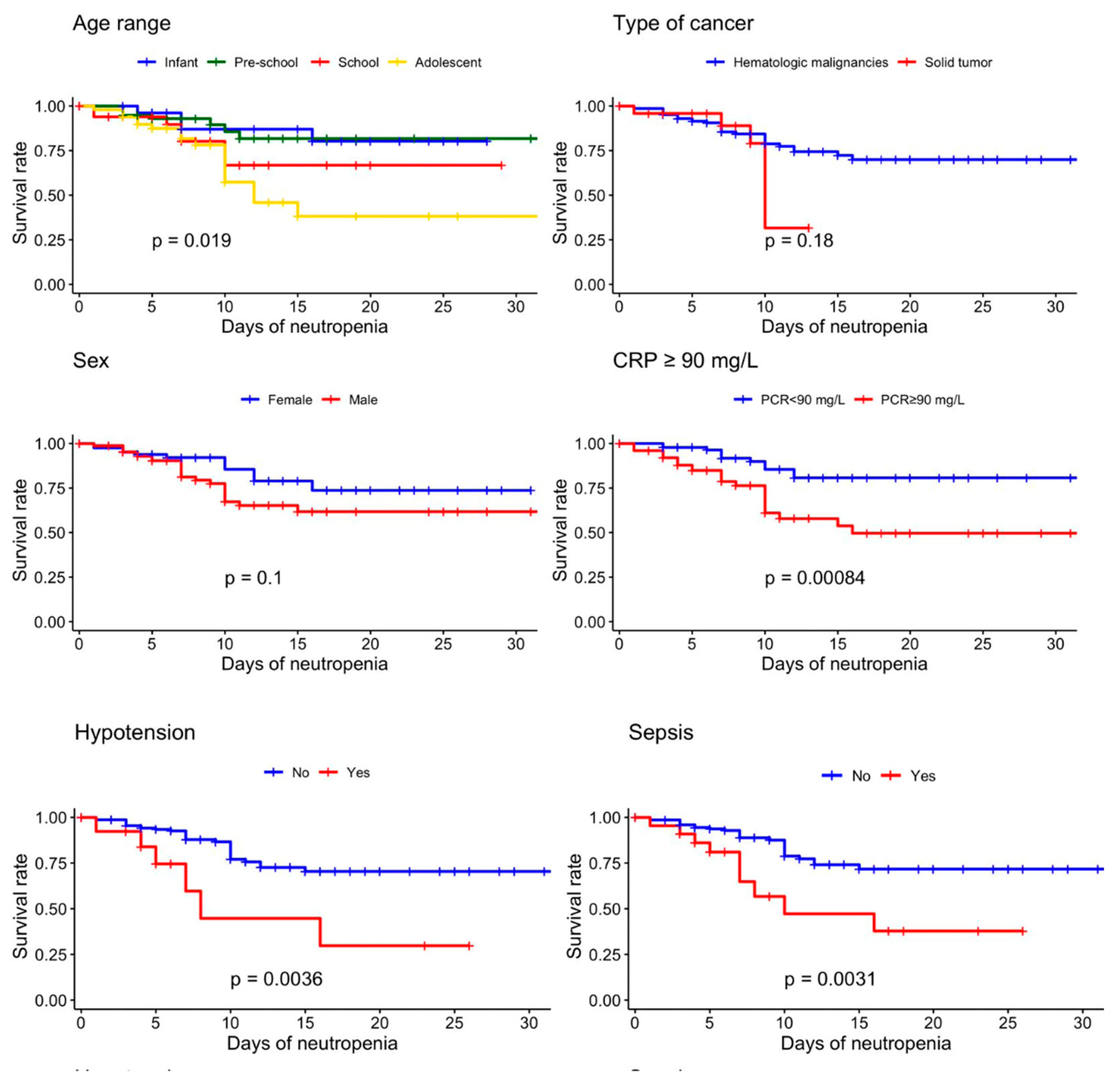
3.3. Event-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IFD | Invasive fungal disease |
| CRP | C-reactive protein |
| RF | Risk factor |
| HRFN | high-risk febrile neutropenia |
| PICU | Pediatric Intensive Care Unit |
| AML | Acute myeloid leukemia |
| HCT | Hematopoietic cell transplant |
| PINDA | National Child Program of Antineoplastic Drugs |
| IQR | Interquartile range |
| CI | confidence intervals |
| EFS | Event-free survival |
| HR | Hazard ratio |
| ROC | Receiver operating characteristic |
| CVC | Central venous catheter |
| GM | Galactomannan |
| CT | Computed tomography |
| BAL | Bronchoalveolar lavage |
| FB | Fungus ball |
| UC | Urine culture |
| BC | Blood culture |
Appendix A
Appendix A.1
| Variables Median (IRQ), N (%) | Mold infections(N=18) | Yeast infections(N=16) | P-Value |
| Age (Years) | 11.8 (9.0-13.7) | 7.4 (2.2-11.5) | 0.0198 |
| Age Range | 0.0929 | ||
| Infant | 0 | 4 (25.0) | |
| Pre-school | 3 (16.7) | 4 (25.0) | |
| Elementary School | 4 (22.2) | 2 (18.8) | |
| Adolescent | 11 (61.1)) | 5 (31.2) | |
| Male sex | 12 (66.7) | 11 (68.8) | 1 |
| Hematologic Malignancies | 17 (94.4) | 11(68.7) | 0.0782 |
| Solid tumors | 1 (5.6) | 5(31.3) | |
| CRP ≥ 90 mg/L | 11 (61.1) | 12 (75.0) | 0.4768 |
| Hypotension | 4 (22.2) | 1(12.5) | 0.6602 |
| Sepsis | 6 (33.3) | 6 (37.5) | 1 |
| PICU admission | 12 (66.7) | 10 (62.5) | 1 |
References
- Lehrnbecher T, Groll AH, Cesaro S, et al (2023) Invasive fungal diseases impact on outcome of childhood ALL – an analysis of the international trial AIEOP-BFM ALL 2009. Leukemia 37:72–78. [CrossRef]
- Lehrnbecher T, Schoning S, Poyer F, et al (2019) Incidence and Outcome of Invasive Fungal Diseases in Children With Hematological Malignancies and/or Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Prospective Multicenter Study. Front Microbiol 10:681. [CrossRef]
- Fisher BT, Robinson PD, Lehrnbecher T, et al (2018) Risk Factors for Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review. J Pediatric Infect Dis Soc 7:191–198. [CrossRef]
- Santolaya ME, Alvarez AM, Acuña M, et al (2018) Efficacy of pre-emptive versus empirical antifungal therapy in children with cancer and high-risk febrile neutropenia: A randomized clinical trial. Journal of Antimicrobial Chemotherapy 73:2860–2866. [CrossRef]
- Ruijters VJ, Oosterom N, Wolfs TFW, et al (2019) Frequency and Determinants of Invasive Fungal Infections in Children With Solid and Hematologic Malignancies in a Nonallogeneic Stem Cell Transplantation Setting: A Narrative Review. J Pediatr Hematol Oncol 41:345–354. [CrossRef]
- Kobayashi R, Hori D, Sano H, et al (2018) Risk Factors for Invasive Fungal Infection in Children and Adolescents With Hematologic and Malignant Diseases: A 10-year Analysis in a Single Institute in Japan. Pediatr Infect Dis J 37:1282–1285. [CrossRef]
- King J, Pana ZD, Lehrnbecher T, et al (2017) Recognition and Clinical Presentation of Invasive Fungal Disease in Neonates and Children. J Pediatric Infect Dis Soc 6: S12–S21. [CrossRef]
- Van Rhijn N, White PL (2025) Antifungal treatment strategies and their impact on resistance development in clinical settings. Journal of Antimicrobial Chemotherapy. [CrossRef]
- Gutiérrez V, Contardo V, De la Maza V, et al (2022) Enfermedad Fúngica Invasora :Experiencia en centros PINDA 2016-2020. Chile. Rev. chil. infectol.. 2023. Vol. 40(4):360-369. [CrossRef]
- Barraza M, Valenzuela R, Villarroel M, et al (2024) Epidemiological changes of invasive fungal disease in children with cancer: Prospective study of the National Child Program of Antineoplastic Drugs network, Chile. Mycoses 67:e13780. [CrossRef]
- Yeoh DK, Blyth CC, Clark JE, et al (2024) Invasive fungal disease and antifungal prophylaxis in children with acute leukaemia: a multicentre retrospective Australian cohort study. Lancet Reg Health West Pac 52:. [CrossRef]
- Jenks JD, Cornely OA, Chen SCA, et al (2020) Breakthrough invasive fungal infections: Who is at risk? Mycoses 63:1021–1032. [CrossRef]
- Boutin CA, Durocher F, Beauchemin S, et al (2024) Breakthrough Invasive Fungal Infections in Patients With High-Risk Hematological Disorders Receiving Voriconazole and Posaconazole Prophylaxis: A Systematic Review. Clinical Infectious Diseases 79:151–160. [CrossRef]
- Kanda Y, Kimura SI, Iino M, et al (2020) D-Index-Guided Early Antifungal Therapy Versus Empiric Antifungal Therapy for Persistent Febrile Neutropenia: A Randomized Controlled Noninferiority Trial. J Clin Oncol 38:815–822. [CrossRef]
- Wang L, Wang Y, Hu J, et al (2019) Clinical risk score for invasive fungal diseases in patients with hematological malignancies undergoing chemotherapy: China Assessment of Antifungal Therapy in Hematological Diseases (CAESAR) study. Front Med 13:365–377. [CrossRef]
- Alali M, Giurcanu M, Elmuti L, Kumar M (2021) Pediatric Invasive Fungal Risk Score in Cancer and Hematopoietic Stem Cell Transplantation Patients With Febrile Neutropenia. J Pediatr Hematol Oncol. [CrossRef]
- Portugal RD, Garnica M, Nucci M (2009) Index to predict invasive mold infection in high-risk neutropenic patients based on the area over the neutrophil curve. Journal of Clinical Oncology 27:3849–3854. [CrossRef]
- Peter Donnelly J, Chen SC, Kauffman CA, et al (2020) Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71:1367–1376. [CrossRef]
- Monsereenusorn C, Sricharoen T, Rujkijyanont P, et al (2021) Clinical Characteristics and Predictive Factors of Invasive Fungal Disease in Pediatric Oncology Patients with Febrile Neutropenia in a Country with Limited Resources. Pediatric Health Med Ther 12:335–345. [CrossRef]
- Moraitaki E, Kyriakidis I, Pelagiadis I, et al (2024) Epidemiology of Invasive Fungal Diseases: A 10-Year Experience in a Tertiary Pediatric Hematology–Oncology Department in Greece. Journal of Fungi 10:. [CrossRef]
- Gal Etzioni TR, Fainshtain N, Nitzan-Luques A, et al (2024) Invasive Fungal Infections in Children with Acute Leukemia: Epidemiology, Risk Factors, and Outcome. Microorganisms 12:145. [CrossRef]
- Hajri H Al, Al-Salmi W, Hinai K Al, et al (2023) Invasive fungal infections in children with leukemia in a tertiary hospital in Oman: An eight-year review. Curr Med Mycol 9(3), 16–22. [CrossRef]
- Roumani AM, Benmouffok N (2022) Risk Factors for Invasive Fungal Disease in Pediatric Oncology. International Journal of Clinical Research 3:167–172. [CrossRef]
- Sezgin Evim M, Tüfekçi Ö, Baytan B, et al (2022) Invasive Fungal Infections in Children with Leukemia: Clinical Features and Prognosis. Turkish Journal of Hematology 39:94–102. [CrossRef]
- Kobayashi R, Kaneda M, Sato T, et al (2008) The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: a 10-year analysis at a single institution at Japan. J Pediatr Hematol Oncol 30:886–890. [CrossRef]
- Lin GL, Chang HH, Lu CY, et al (2018) Clinical characteristics and outcome of invasive fungal infections in pediatric acute myeloid leukemia patients in a medical center in Taiwan. J Microbiol Immunol Infect 51:251–259. [CrossRef]
- Fang W, Wu J, Cheng M, et al (2023) Diagnosis of invasive fungal infections: challenges and recent developments. J Biomed Sci 30 (1), 42. [CrossRef]
- Cesaro S, Tridello G, Castagnola E, et al (2017) Retrospective study on the incidence and outcome of proven and probable invasive fungal infections in high-risk pediatric onco-hematological patients. Eur J Haematol 99:240–248. [CrossRef]
| Variable (Median; IQR) (n; %) | IFD (n=34) |
No IFD (n= 140) |
Total (n= 174) |
p-value |
| Demographic | ||||
| Age; years | 9.4 (5-13) | 6 (3.1-10.2) | 7.0 (3-13) | 0.0200* |
| Male sex | 23 (67.7) | 68 (48.6) | 91 (52.3) | 0.0550 |
| Age range | 0.0540** | |||
| Infant | 4 (11.8) | 25 (17.9) | 29 (16.7) | Ref |
| Pre-school | 7 (20.6) | 51 (36.4) | 58 (33.3) | 0.8196 |
| School | 7 (20.6) | 30 (21.4) | 37 (21.3) | 0.5805 |
| Adolescent | 16 (47.0) | 34 (24.3) | 50 (28.7) | 0.0810 |
| Clinical | ||||
| Type of cancer | 0.5868 | |||
| Hematologic malignancies | 28 (82.4) | 121 (86.4) | 149 (85.6) | |
| Solid tumors | 6 (17.6) | 19 (13.6) | 25 (14.4) | |
| Use of CVC | 32 (94.1) | 126 (91.3) | 158 (91.9) | 0.7390 |
| Previous IFD | 2 (5.9) | 5 (3.6) | 7 (4.0) | 0.7390 |
| Bacterial co-infection | 29 (85.3) | 99 (70.7) | 128 (73.6) | 0.1270 |
| CRP ≥ 90 mg/L | 23 (67.6) | 55 (39.3) | 78 (44.8) | 0.0052* |
| Hypotension | 6 (17.6) | 8 (5.7) | 21 (12.1) | 0.0331* |
| Sepsis | 9 (26.5) | 14 (10) | 23 (13.2) | 0.0205* |
| PICU admission | 22(64.7) | 25 (17.9) | 47(27.0) | 0.0001* |
|
Age (years) |
Gender |
Type of cancer/ Phase of treatment |
Source |
Clinical and microbiological findings |
Outcome |
| Proven IFD | |||||
| 13 | M | Solid Tumor* | Renal | FB, UC (+) C. albicans | Alive |
| 4 | M | Lymphoma* | Renal | FB, UC (+) C. albicans | Alive |
| 0.8 | F | AML/Consolidation | Candidemia | BC (+) C. parapsilosis | Dead |
| 6 | M | ALL relapse/Induction | Spleen | Splenic lesions, UC (+) C. glabrata | Alive |
| 14 | M | AML/Consolidation | Pneumonia | Thin hyphae in lung biopsy. Aspergillus sp. | Alive |
| 11 | F | ALL/Consolidation | Renal | Renal biopsy, abscess, C. lusitaniae | Alive |
| 3 | M | Solid Tumor* | Pneumonia | Serum GM (3.8), pulmonary CT, PCR Aspergillus sp. | Alive |
| 3 | F | ALL/Consolidation | Candidemia | BC (+) C. parapsilosis | Alive |
| 1 | M | AML/Consolidation | Catheter Infection | CVC (+) C. albicans | Alive |
| 8 | M | ALL/Consolidation | Candidemia | BC, UC (+) C. tropicalis | Alive |
| 1 | M | AML/Consolidation | Catheter Infection | CVC (+) C. parapsilosis | Alive |
| 9 | F | Solid Tumor* | Candidemia | BC (+) C. parapsilosis | Alive |
| 8 | M | AML/Consolidation | Hepatosplenic | Hepatosplenic images, UC (+) C. albicans | Alive |
| 12 | M | ALL/Induction | Pneumonia | Pulmonary CT, Biopsy Aspergillus fumigatus | Alive |
| 13 | F | Solid Tumor* | Esophagitis | Esophageal biopsy, C. dublinensis | Alive |
| 13 | M | ALL/Induction | Hepatosplenic | Hepatosplenic images, UC (+) C. albicans | Alive |
| 13 | F | ALL/Induction | Fungal Sinusitis | Nasal biopsy, serum GM (0.7) Scedosporium sp. | Dead |
| 14 | M | ALL relapse/Consolidation | Pneumonia | Pulmonary CT, nasal cavity scab, Aspergillus sp. | Alive |
| 2 | M | Solid Tumor* | Candidemia | BC (+) C. tropicalis | Alive |
| 14 | M | ALL/Maintenance | Fungemia | BC (+) Rhodotorula mucilaginosa | Alive |
| 0.9 | M | Solid Tumor* | Candidemia | BC (+) C. tropicalis | Alive |
| Probable IFD | |||||
| 8 | M | AML/Consolidation | Fungal Sinusitis | Thin septate hyphae in biopsy | Alive |
| 13 | M | AML/Consolidation | Fungal Sinusitis | Sparse septate hyphae with 90-degree bifurcation in biopsy | Alive |
| 18 | F | ALL relapse/Maintenance | Pneumonia | BAL GM (2.1), pulmonary CT | Alive |
| 9 | M | AML/Consolidation | Pneumonia | Serum GM (0.6), pulmonary CT | Alive |
| 4 | M | AML/Consolidation | Pneumonia | Serum GM (0.7), pulmonary CT | Alive |
| 17 | M | AML/Induction | Pneumonia | Serum GM (1.5), pulmonary CT | Alive |
| 9 | M | ALL/Induction | Pneumonia | Serum GM (0.8), pulmonary CT | Alive |
| 9 | F | ALL/Induction | Pneumonia | Serum GM (0.6), pulmonary CT | Alive |
| 14 | F | AML/Consolidation | Pneumonia | Serum (1.9) and BAL (4.9) GM, pulmonary CT | Alive |
| 6 | M | ALL/Consolidation | Pneumonia | BAL GM (4.1), pulmonary CT | Alive |
| 11 | M | AML/Consolidation | Pneumonia | Serum GM (1.1), pulmonary CT | Alive |
| 12 | F | ALL/Consolidation | Pneumonia | Serum (2.8) and BAL (6.5) GM (+), pulmonary CT | Alive |
| 11 | F | ALL/Induction | Pneumonia | Serum GM (0.8), pulmonary CT | Dead |
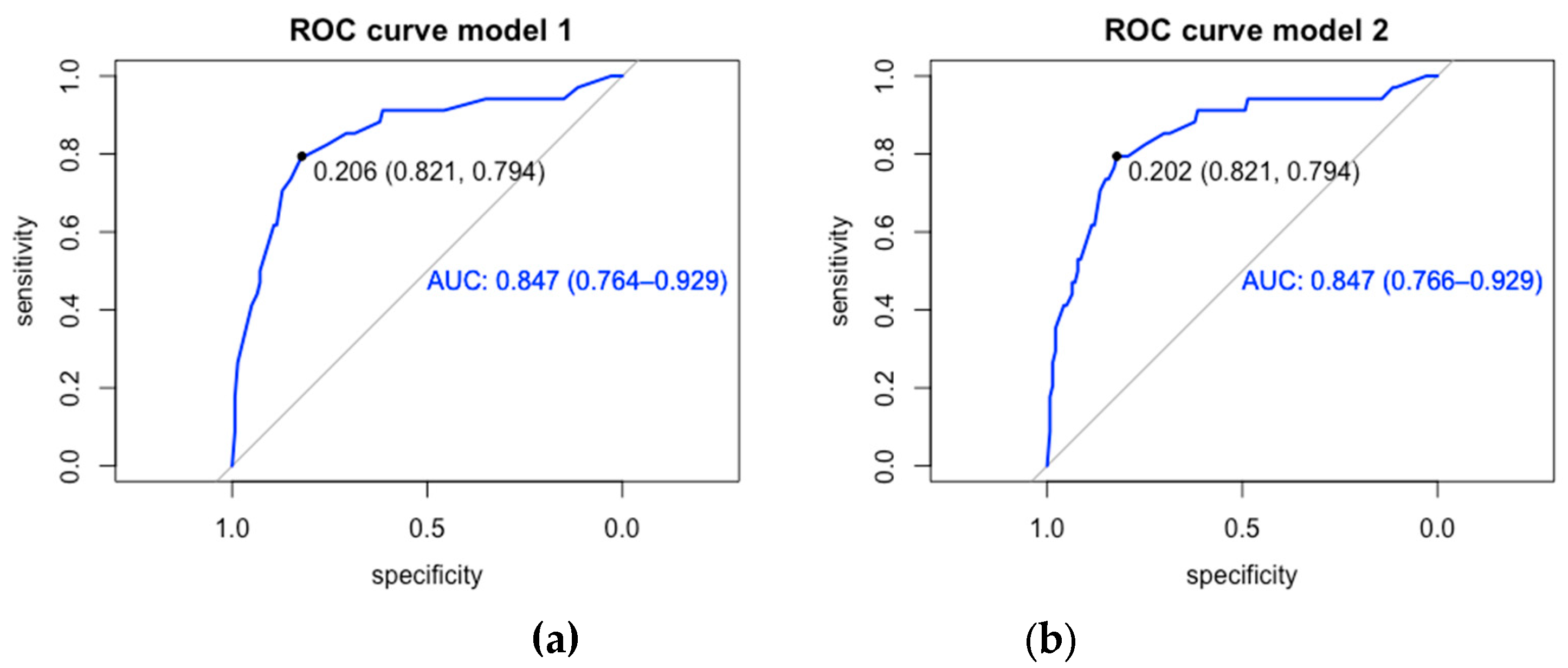
| Variable | Input model | Model 1 | Model 2 | |||
| CrudeOR | 95% CI | AdjustedOR | 95% CI | AdjustedOR | 95% CI | |
| Male sex | 2.21 | 1.02 - 5.04 | 4.04 | 1.58 - 11.38 | 4.04 | 1.58 – 11.36 |
| Age range (ref Infant) | ||||||
| Adolescent | 2.94 | 0.94 - 11.23 | 4.65 | 1.20 - 21.87 | 4.74 | 1.21 - 22.61 |
| CRP ≥ 90 mg/L | 3.23 | 1.49 - 7.39 | 3.13 | 1.26 - 8.20 | 3.05 | 1.20 – 8.15 |
| Hypotension | 3.54 | 1.09 - 10.98 | ||||
| Sepsis | 3.24 | 1.23 - 8.25 | ||||
| PICU admission | 8.43 | 3.76 -19.81 | 10.73 | 4.26 - 29.47 | 10.65 | 4.35 - 29.28 |
| Type of cancer (ref hematologic malignancy) | 1.36 | 0.46 - 3.57 | 1.18 | 0.32 - 3.97 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





