Submitted:
27 August 2025
Posted:
28 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Setting, Patients and Study Design
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Characteristics and Outcomes of Patients’ Cohort
3.2. Characteristics and Outcomes of Patients with Probable and Proven IFIs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Potential Conflicts of Interest
References
- Wasylyshyn, A.I.; Linder, K.A.; Kauffman, C.A.; Richards, B.J.; Maurer, S.M.; Sheffield, V.M.; Benitez Colon, L.; Miceli, M.H. Invasive Fungal Disease in Patients with Newly Diagnosed Acute Myeloid Leukemia. J Fungi (Basel) 2021, 7, 761. [Google Scholar] [CrossRef]
- Alkan, A.; Buyukasik, Y.; Uzun, O.; Demir, A.U.; Coplu, L. Invasive fungal infections in patients with acute leukemia: A retrospective cohort study at a tertiary-care hospital. Medicine (Baltimore) 2024, 103, 39959. [Google Scholar] [CrossRef]
- Girmenia, C.; Raiola, A.M.; Piciocchi, A.; Algarotti, A.; Stanzani, M.; Cudillo, L.; Pecoraro, C.; Guidi, S.; Iori, A.P.; Montante, B.; et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014, 20, 872–80. [Google Scholar] [CrossRef] [PubMed]
- Menzin, J.; Meyers, J.L.; Friedman, M.; Korn, J.R.; Perfect, J.R.; Langston, A.A.; Danna, R.P.; Papadopoulos, G. The economic costs to United States hospitals of invasive fungal infections in transplant patients. Am J Infect Control 2011, 39, 15–20. [Google Scholar] [CrossRef]
- Busca, A.; Passera, R.; Maffini, E.; Festuccia, M.; Brunello, L.; Dellacasa, C.M.; Aydin, S.; Frairia, C.; Manetta, S.; et al. Hematopoietic cell transplantation comorbidity index and risk of developing invasive fungal infections after allografting. Bone Marrow Transplant. 2018, 53, 1304–1310. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010, 50, 1091–100. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009, 48, 265–73. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.S.; Masouridi-Levrat, S.; Chalandon, Y.; Mamez, A.C.; Giannotti, F.; Riat, A.; Fischer, A.; Poncet, A.; Glampedakis, E.; Van Delden, C.; et al. Invasive Mold Infections in Allogeneic Hematopoietic Cell Transplant Recipients in 2020: Have We Made Enough Progress? Open Forum Infect Dis. 2021, 9, ofab596. [Google Scholar] [CrossRef]
- Robenshtok, E.; Gafter-Gvili, A.; Goldberg, E.; Weinberger, M.; Yeshurun, M.; Leibovici, L.; Paul, M. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007, 25, 5471–89. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, Z.; Yu, B.; Wang, B.; Wu, C.; Wu, J.; Lai, J.; Gao, X.; Chen, J. Network meta-analysis of triazole, polyene, and echinocandin antifungal agents in invasive fungal infection prophylaxis in patients with hematological malignancies. BMC Cancer. 2021, 21, 404. [Google Scholar] [CrossRef]
- Young, J.H.; Andes, D.R.; Ardura, M.I.; Arrieta, A.; Bow, E.J.; Chandrasekar, P.H.; Chen, S.C.A.; Hammond, S.P.; Husain, S.; Koo, S.; et al. Modeling Invasive Aspergillosis Risk for the Application of Prophylaxis Strategies. Open Forum Infect Dis. 2024, 11, ofae082. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018, 24, 1–38. [Google Scholar] [CrossRef]
- Dadwal, S.S.; Hohl, T.M.; Fisher, C.E.; Boeckh, M.; Papanicolaou, G.; Carpenter, P.A.; Fisher, B.T.; Slavin, M.A.; Kontoyiannis, D.P. American Society of Transplantation and Cellular Therapy Series, 2: Management and Prevention of Aspergillosis in Hematopoietic Cell Transplantation Recipients. Transplant Cell Ther. 2021, 27, 201–211. [Google Scholar] [CrossRef]
- Stemler, J.; Mellinghoff, S.C.; Khodamoradi, Y.; Sprute, R.; Classen, A.Y.; Zapke, S.E.; Hoenigl, M.; Krause, R.; Schmidt-Hieber, M.; Heinz, W.J.; et al. Primary prophylaxis of invasive fungal diseases in patients with haematological malignancies: 2022 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). J Antimicrob Chemother. 2023, 78, 1813–1826. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R. 3rd.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Maschmeyer, G.; Lamoth, F.; Blennow, O.; Xhaard, A.; Spadea, M.; Busca, A.; Cordonnier, C.; Maertens, J. Primary antifungal prophylaxis in hematological malignancies. Updated clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL). Leukemia. 2025, 39, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.A.; Durocher, F.; Beauchemin, S.; Ziegler, D.; Abou Chakra, C.N.; Dufresne, S.F. Breakthrough Invasive Fungal Infections in Patients With High-Risk Hematological Disorders Receiving Voriconazole and Posaconazole Prophylaxis: A Systematic Review. Clin Infect Dis. 2024, 79, 151–160. [Google Scholar] [CrossRef]
- Ishida, K.; Haraguchi, M.; Kimura, M.; Araoka, H.; Natori, A.; Reynolds, J.M.; Raja, M.; Natori, Y. Incidence of Breakthrough Fungal Infections in Patients With Isavuconazole Prophylaxis: A Systematic Review and Meta-analysis. Open Forum Infect Dis. 2025, 12, ofaf163. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin Infect Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef]
- Girmenia, C.; Busca, A.; Candoni, A.; Cesaro, S.; Luppi, M.; Nosari, A.M.; Pagano, L.; Rossi, G.; Venditti, A.; Aversa, F. Breakthrough invasive fungal diseases in acute myeloid leukemia patients receiving mould active triazole primary prophylaxis after intensive chemotherapy: An Italian consensus agreement on definitions and management. Med Mycol. 2019, 57, S127–S137. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016, 387, 760–9. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R., 3rd; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef] [PubMed]
- EMA (2015). Cresemba (isavuconazole). European Medicines Agency. EPAR summary for the public. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cresemba.
- FDA (2015). FDA approves Cresemba (isavuconazonium sulfate) for invasive aspergillosis and invasive mucormycosis. FDA (2024). Drugs@FDA: FDA-Approved Drugs. Application Number: 207500. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=207500.
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019, 19, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, H.; Athans, V.; Brizendine, K.D. Real-world use-Isavuconazole at a large academic medical center. Mycoses 2019, 62, 534–541. [Google Scholar] [CrossRef]
- Gow-Lee, V.; Abu Saleh, O.M.; Harris, C.E.; Gile, J.J.; Akhiyat, N.; Chesdachai, S. Outcomes of Invasive Fungal Infections Treated with Isavuconazole: A Retrospective Review. Pathogens 2024, 13, 886. [Google Scholar] [CrossRef]
- Gunathilaka, S.S.; Keragala, R.K.; Gunathilaka, K.M.; Wickramage, S.; Bandara, S.R.; Senevirathne, I.S.; Jayaweera, A.S. Use of isavuconazole in mucormycosis: a systematic review. BMC Infect Dis. 2025, 25, 25. [Google Scholar] [CrossRef] [PubMed]
- Dagher, H.; Hachem, R.; Chaftari, A.M.; Jiang, Y.; Ali, S.; Deeba, R.; Shah, S.; Raad, I. Real-World Use of Isavuconazole as Primary Therapy for Invasive Fungal Infections in High-Risk Patients with Hematologic Malignancy or Stem Cell Transplant. J Fungi (Basel) 2022, 8, 74. [Google Scholar] [CrossRef]
- Weng, J.; Du, X.; Fang, B.; Li, Y.; Huang, L.; Ju, Y. Efficacy and safety of isavuconazole versus voriconazole for the treatment of invasive fungal infections: a meta-analysis with trial sequential analysis. BMC Infect Dis. 2025, 25, 230. [Google Scholar] [CrossRef]
- Ellsworth, M.; Ostrosky-Zeichner, L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. J Fungi (Basel) 2020, 6, 324. [Google Scholar] [CrossRef]
- Lewis, J.S., 2nd; Wiederhold, N.P.; Hakki, M.; Thompson, G.R., 3rd. New Perspectives on Antimicrobial Agents: Isavuconazole. Antimicrob Agents Chemother. 2022, 66, 0017722. [Google Scholar] [CrossRef]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob Agents Chemother. 2018, 62, 00585–18. [Google Scholar] [CrossRef]
- Risum, M.; Vestergaard, M.B.; Weinreich, U.M.; Helleberg, M.; Vissing, N.H.; Jørgensen, R. Therapeutic Drug Monitoring of Isavuconazole: Serum Concentration Variability and Success Rates for Reaching Target in Comparison with Voriconazole. Antibiotics (Basel) 2021, 10, 487. [Google Scholar] [CrossRef]
- Lewis, R.; Niazi-Ali, S.; McIvor, A.; Kanj, S.S.; Maertens, J.; Bassetti, M.; Levine, D.; Groll, A.H.; Denning, D.W. Triazole antifungal drug interactions-practical considerations for excellent prescribing. J Antimicrob Chemother. 2024, 79, 1203–1217. [Google Scholar] [CrossRef]
- DiPippo, A.J.; Rausch, C.R.; Kontoyiannis, D.P. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses 2019, 62, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R., 3rd. Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–8. [Google Scholar] [PubMed]
- Pagano, L.; Busca, A.; Candoni, A.; Cattaneo, C.; Cesaro, S.; Fanci, R.; Nadali, G.; Potenza, L.; Russo, D.; Tumbarello, M.; et al. Risk stratification for invasive fungal infections in patients with hematological malignancies: SEIFEM recommendations. Blood Rev. 2017, 31, 17–29. [Google Scholar] [CrossRef]
- Batista, M.V.; Ussetti, M.P.; Jiang, Y.; Neofytos, D.; Cortez, A.C.; Feriani, D.; Schmidt-Filho, J.; França-Silva, I.L.A.; Raad, I.; Hachem, R. Comparing the Real-World Use of Isavuconazole to Other Anti-Fungal Therapy for Invasive Fungal Infections in Patients with and without Underlying Disparities: A Multi-Center Retrospective Study. J Fungi (Basel). 2023, 9, 166. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Monzó-Gallo, P.; Aguilar-Guisado, M.; Ramos, J.C.; Laporte-Amargós, J.; Machado, M.; Martin-Davila, P.; Franch-Sarto, M.; Sánchez-Romero, I.; Badiola, J.; et al. Breakthrough invasive fungal infection among patients with haematologic malignancies: A national, prospective, and multicentre study. J Infect. 2023, 87, 46–53. [Google Scholar] [CrossRef]
- Eigl, S.; Prattes, J.; Reinwald, M.; Thornton, C.R.; Reischies, F.; Sess, B.; Neumeister, P.; Zollner-Schwetz, I.; Raggam, R.B.; Flick, H.; Buchheidt, D.; et al. Influence of mould-active antifungal treatment on the performance of the Aspergillus-specific bronchoalveolar lavage fluid lateral-flow device test. Int J Antimicrob Agents 2015, 46, 401–5. [Google Scholar] [CrossRef]
- Eigl, S.; Hoenigl, M.; Spiess, B.; Heldt, S.; Prattes, J.; Neumeister, P.; Wolfler, A.; Rabensteiner, J.; Prueller, F.; Krause, R.; et al. Galactomannan testing and Aspergillus PCR in same-day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med Mycol. 2017, 55, 528–534. [Google Scholar] [CrossRef]
- Jenks, J.D.; Gangneux, J.P.; Schwartz, I.S.; Alastruey-Izquierdo, A.; Lagrou, K.; Thompson Iii, G.R.; Lass-Flörl, C.; Hoenigl, M. European Confederation of Medical Mycology (ECMM) Council Investigators. Diagnosis of Breakthrough Fungal Infections in the Clinical Mycology Laboratory: An ECMM Consensus Statement. J Fungi (Basel) 2020, 6, 216. [Google Scholar] [CrossRef]
- Ruhnke, M.; Cornely, O.A.; Schmidt-Hieber, M.; Alakel, N.; Boell, B.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Heinz, W.J.; Hentrich, M.; et al. Treatment of invasive fungal diseases in cancer patients-Revised 2019 Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Mycoses 2020, 63, 653–682. [Google Scholar] [CrossRef]
- Maertens, J.A.; Rahav, G.; Lee, D.G.; Ponce-de-León, A.; Ramírez Sánchez, I.C.; Klimko, N.; Sonet, A.; Haider, S.; Diego Vélez, J.; Raad, I.; et al. study investigators. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet 2021, 397, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.; Meis, J.F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin Infect Dis. 2016, 62, 362–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, LA.; Marini, B.L.; Benitez, L.; Nagel, J.L.; Miceli, M.; Berglund, C.; Perissinotti, A.J. Risk factors for subtherapeutic levels of posaconazole tablet. J Antimicrob Chemother. 2017, 72, 2902–2905. [Google Scholar] [CrossRef]
- Kovanda, L.L.; Marty, F.M.; Maertens, J.; Desai, A.V.; Lademacher, C.; Engelhardt, M.; Lu, Q.; Hope, W.W. ; Impact of Mucositis on Absorption and Systemic Drug Exposure of Isavuconazole. Antimicrob Agents Chemother. 2017, 61, e00101–17. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.; Su, Y.; Lee, Y.J.; Seo, S.; Shaffer, B.; Tamari, R.; Gyurkocza, B.; Barker, J.; Bogler, Y.; Giralt, S.; et al. A Single-Center, Open-Label Trial of Isavuconazole Prophylaxis against Invasive Fungal Infection in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2020, 26, 1195–1202. [Google Scholar] [CrossRef]
- Greene, R.E.; Schlamm, H.T.; Oestmann, J.W.; Stark, P.; Durand, C.; Lortholary, O.; Wingard, J.R.; Herbrecht, R.; Ribaud, P.; Patterson, T.F.; et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007, 44, 373–9. [Google Scholar] [CrossRef]
- Aguado, J.M.; Vázquez, L.; Fernández-Ruiz, M.; Villaescusa, T.; Ruiz-Camps, I.; Barba, P.; Silva, J.T.; Batlle, M.; Solano, C.; Gallardo, D. PCRAGA Study Group; Spanish Stem Cell Transplantation Group; Study Group of Medical Mycology of the Spanish Society of Clinical Microbiology and Infectious Diseases; Spanish Network for Research in Infectious Diseases. Serum galactomannan versus a combination of galactomannan and polymerase chain reaction-based Aspergillus DNA detection for early therapy of invasive aspergillosis in high-risk hematological patients: a randomized controlled trial. Clin Infect Dis. 2015, 60, 405–14. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective antifungal therapy (PATH) alliance(®): focus on mucormycosis. Mycoses 2014, 57, 240–6. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-Response Relationships for Isavuconazole in Patients with Invasive Aspergillosis and Other Filamentous Fungi. Antimicrob Agents Chemother. 2017, 61, e01034–17. [Google Scholar] [CrossRef] [PubMed]
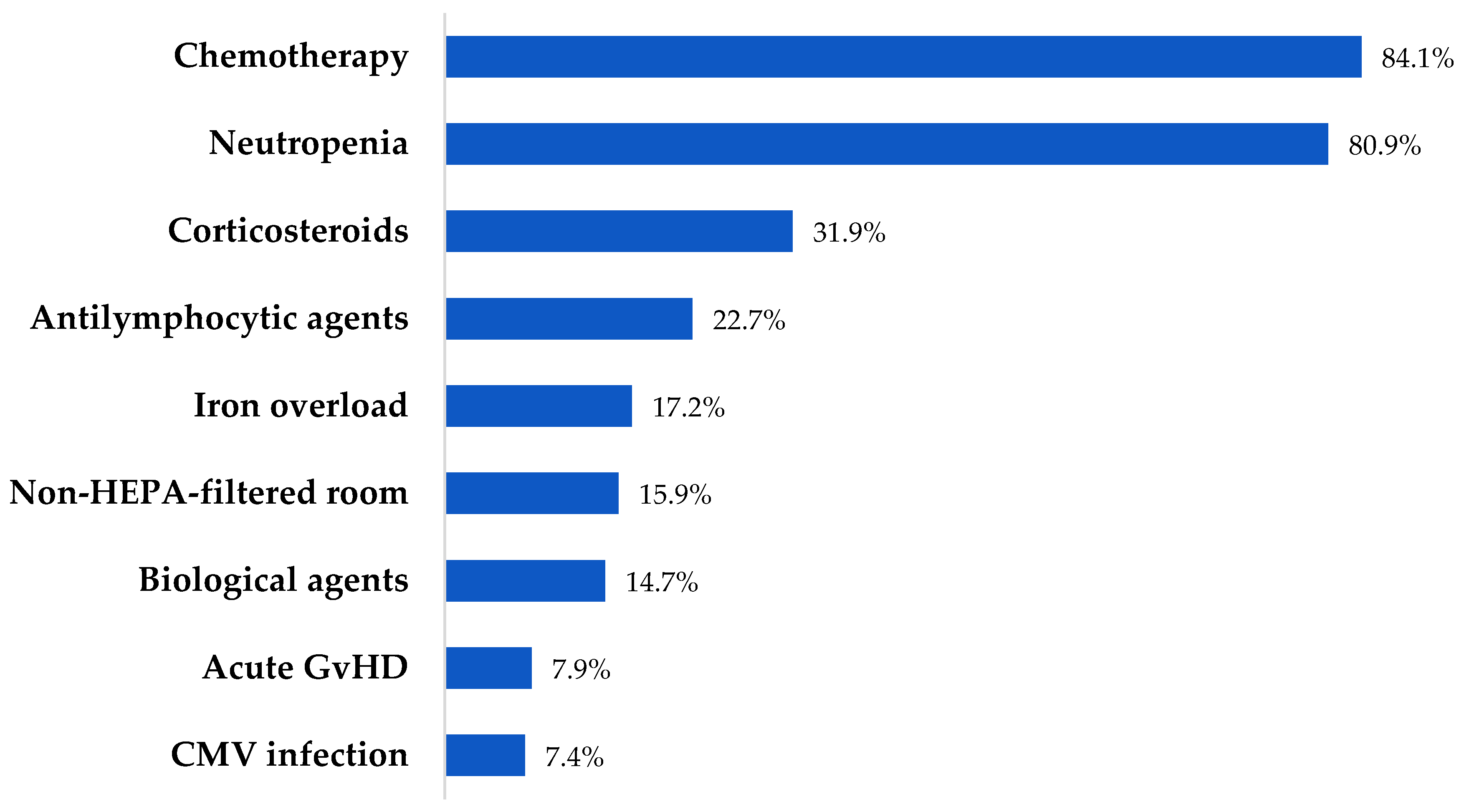
| Variables | Patients (n=163) N (%) |
|---|---|
| Age (years) median (IQR) | 50 (38-63) |
| Sex, male | 95 (58.3) |
| Charlson comorbidity index score ≥ 3 | 73 (44.8) |
| Underlying disease | |
| Acute myelogenous leukemia | 76 (46.6) |
| Acute lymphoblastic leukemia | 20 (12.3) |
| Non-Hodgkin lymphoma | 16 (9.8) |
| Myelodysplastic syndrome | 8 (4.9) |
| Hodgkin lymphoma | 11 (6.7) |
| Aplastic anemia | 9 (5.5) |
| Chronic myelogenous leukemia | 6 (3.7) |
| Chronic lymphoblastic leukemia | 4 (2.4) |
| Multiple myeloma | 7 (4.3) |
| Others | 6 (3.7) |
| Disease status | |
| Complete remission | 38 (23.3) |
| Partial remission | 8 (4.9) |
| Relapsed | 33 (20.2) |
| Refractory | 18 (11) |
| Recently diagnosed | 66 (40.5) |
| HCT | 49 (30.1) |
| Allogeneic | 30 (18.4) |
| HLA matching and donor type | |
| Haploidentical | 15 (9.2) |
| Matched unrelated donor | 5 (3.1) |
| Matched related donor | 10 (6.1) |
| T-cell depletion | 9 (5.5) |
| Acute GvHD | 13 (7.9) |
| Grade I | 2 (1.2) |
| Grade II | 4 (2.4) |
| Grade III | 7 (4.3) |
| Grade IV | 0 (0) |
| Chronic GvHD | 7 (4.3) |
| Variables | Patients (n=163) N (%) |
|---|---|
| IFI classification | |
| Proven | 22 (13.5) |
| Probable | 44 (26.9) |
| Possible | 97 (59.5) |
| IFI location | |
| Lungs | 147 (90.2) |
| Paranasal sinuses | 23 (14.1) |
| Liver | 5 (3.1) |
| Skin and soft tissue | 5 (3.1) |
| Central nervous system | 3 (1.8) |
| Disseminated | 3 (1.8) |
| Lung CT scan | |
| Nodules | 89 (54.6) |
| Ground glass appearance | 59 (36.2) |
| Halo sign | 37 (22.7) |
| Tree in bud | 22 (13.5) |
| Alveolar infiltrate | 14 (8.6) |
| Cavity | 3 (1.8) |
| Air crescent sign | 1 (0.6) |
| Reverse halo sign | 1 (0.6) |
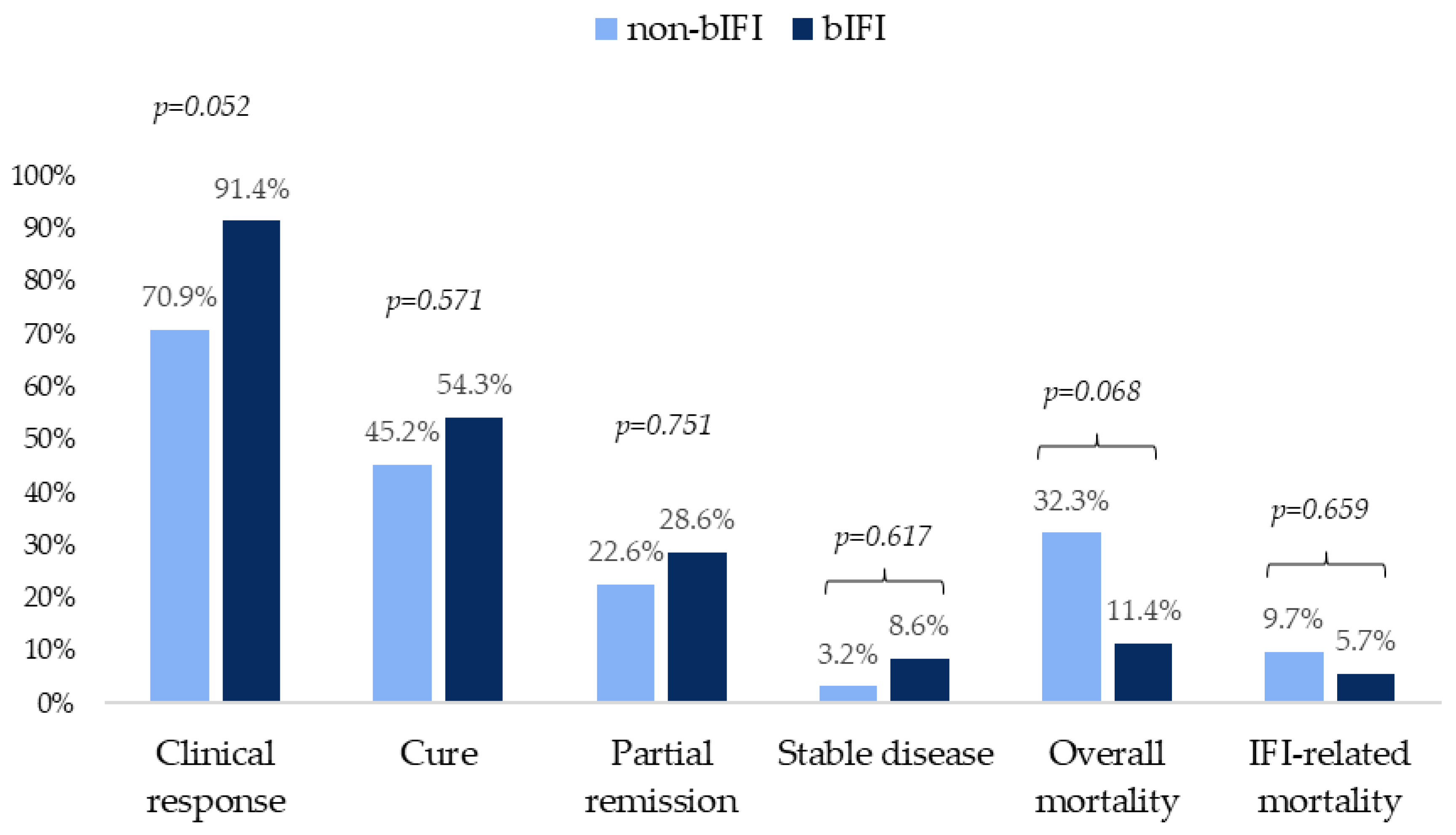
| Variable | Total (n = 66) |
non-bIFI (n = 31) |
bIFI (n = 35) |
p-value |
|---|---|---|---|---|
| Age, median (IQR) | 47 (39–61) | 54 (43–67) | 44 (36–52) | 0.014 |
| Male sex – n (%) | 40 (60.6) | 16 (51.6) | 24 (68.6) | 0.159 |
| Charlson comorbidity index ≥ 3 – n (%) | 29 (43.9) | 17 (54.8) | 12 (34.3) | 0.093 |
| Underlying disease – n (%) | ||||
| Acute myelogenous leukemia | 23 (34.8) | 11 (35.5) | 12 (34.3) | 0.918 |
| Acute lymphoblastic leukemia | 7 (10.6) | 1 (3.2) | 6 (17.1) | 0.41 |
| Myelodysplastic syndrome | 7 (10.6) | 2 (6.4) | 5 (14.3) | 0.433 |
| Non-Hodgkin lymphoma | 4 (6.1) | 4 (12.9) | 0 (0) | 0.043 |
| Hodgkin lymphoma | 10 (15.1) | 4 (12.9) | 6 (17.1) | 0.738 |
| Multiple myeloma | 7 (10.6) | 5 (16.1) | 2 (5.7) | 0.239 |
| Disease status – n (%) | ||||
| Complete remission | 16 (24.2) | 5 (16.1) | 11 (31.4) | 0.165 |
| Partial remission | 4 (6.1) | 1 (3.2) | 3 (8.6) | 0.616 |
| Relapsed | 17 (25.8) | 9 (29) | 8 (22.9) | 0.567 |
| Refractory | 9 (13.6) | 7 (22.6) | 2 (5.7) | 0.071 |
| Recently diagnosed | 20 (30.3) | 9 (29) | 11 (31.4) | 0.832 |
| HCT – n (%) | 27 (40.9) | 9 (29) | 18 (51.4) | 0.064 |
| Allogeneic | 13 (19.7) | 4 (12.9) | 9 (25.7) | 0.228 |
| Corticosteroid use – n (%) | 24 (364) | 11 (35.5) | 13 (37.1) | 0.888 |
| Biological agents – n (%) | 15 (22.7) | 7 (22.6) | 8 (22.9) | 0.978 |
| Antilymphocyte drugs – n (%) | 12 (18.2) | 3 (9.7) | 9 (25.7) | 0.117 |
| Neutropenia – n (%) | 48 (72.7) | 21 (67.7) | 27 (77.1) | 0.392 |
| Antifungal prophylaxis – n (%) | 35 (53) | 0 (0) | 35 (100) | – |
| Fluconazole | 13 (19.7) | 0 (0) | 13 (37.1) | – |
| Posaconazole | 10 (15.1) | 0 (0) | 10 (28.6) | – |
| Voriconazole | 2 (3) | 0 (0) | 2 (5.7) | – |
| L-AmB | 5 (7.6) | 0 (0) | 5 (14.3) | – |
| LC-AmB | 2 (3) | 0 (0) | 2 (5.7) | – |
| Caspofungin | 3 (4.5) | 0 (0) | 3 (8.6) | – |
| Variable | Total (n = 66) |
non-bIFI (n = 31) |
bIFI (n = 35) |
p-value |
|---|---|---|---|---|
| Type of IFI – n (%) | ||||
| Aspergillosis | 43 (65.1) | 22 (70.9) | 21 (60) | 0.351 |
| Mucormycosis | 8 (12.1) | 2 (6.4) | 6 (17.1) | 0.265 |
| Fusariosis | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
| Other hyalo or phaeohyphomycosis | 5 (7.6) | 2 (6.4) | 3 (8.6) | 1 |
| Histoplasmosis | 1 (1.5) | 1 (2.9) | 0 (0) | 0.470 |
| Cryptococcosis | 1 (1.5) | 1 (2.9) | 0 (0) | 0.470 |
| Unidentified hyphae | 8 (12.1) | 3 (9.7) | 5 (14.3) | 0.713 |
| Microscopic detection – n (%) | 16 (24.2) | 9 (29) | 7 (20) | 0.245 |
| Septate branched hyphae | 10 (15.1) | 7 (22.6) | 3 (8.6) | 0.170 |
| Coenocytic hyphae | 5 (7.6) | 1 (3.2) | 4 (11.4) | 0.360 |
| Cryptococcus yeast | 1 (1.5) | 1 (3.2) | 0 (0) | 0.470 |
| Culture isolates – n (%) | ||||
| Alternaria sp. | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
| Aspergillus sp. | 3 (4.5) | 1 (3.2) | 2 (5.7) | 1 |
| Aspergillus flavus complex | 5 (7.6) | 2 (6.4) | 3 (8.6) | 1 |
| Aspergillus fumigatus complex | 5 (7.6) | 4 (12.9) | 1 (2.9) | 0.178 |
| Aspergillus niger | 3 (4.5) | 2 (6.4) | 1 (2.9) | 0.596 |
| Cryptococcus neoformans var. neoformans | 1 (1.5) | 1 (2.9) | 0 (0) | 0.470 |
| Cunninghamella sp. | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
| Curvularia sp. | 2 (3) | 2 (6.4) | 0 (0) | 0.216 |
| Fusarium sp. | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
| Penicillium sp. | 2 (3) | 0 (0) | 2 (5.7) | 1 |
| Rhizopus sp. | 2 (3) | 0 (0) | 2 (5.7) | 1 |
| Rhizopus microsporum | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
| Rhizopus oryzae | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
| Rhizopus arrhizus | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
| Aspergillus GM – n (%) | ||||
| Positive in serum | 12 (18.2) | 5 (16.1) | 7 (20) | 0.684 |
| Positive GM in BAL | 17 (25.8) | 9 (29) | 8 (22.9) | 0.567 |
| Positive serum + BAL GM | 6 (9.1) | 3 (9.7) | 3 (8.6) | 1 |
| Histoplasma urinary antigen – n (%) | 1 (1.5) | 1 (2.9) | 0 (0) | 0.470 |
| Histopathology – n (%) | ||||
| Hyphal invasion of blood vessels | 7 (10.6) | 3 (9.7) | 3 (8.6) | 1 |
| Septate branched hyphae | 5 (7.6) | 3 (9.7) | 2 (5.7) | 0.659 |
| Coenocytic hyphae | 6 (9.1) | 2 (6.4) | 4 (11.4) | 0.676 |
| Yeast | 1 (1.5) | 1 (3.2) | 0 (0) | 0.470 |
| Variable | Total (n = 66) |
non-bIFI (n = 31) |
bIFI (n = 35) |
p-value |
|---|---|---|---|---|
| ISA treatment modality-n (%) | ||||
| Preemptive therapy | 39 (59.1) | 17 (54.8) | 22 (62.9) | 0.508 |
| Targeted therapy | 29 (43.9) | 14 (45.2) | 15 (42.8) | 0.851 |
| Treatment with ISA – n (%) | ||||
| As first-line therapy | 31 (46.9) | 16 (51.6) | 15 (42.9) | 0.477 |
| As continuation therapy | 35 (53%) | 15 (48.4) | 20 (57.1) | 0.477 |
| Other first-line therapy – n (%) | ||||
| L-AmB | 23 (34.8) | 8 (25.8) | 15 (42.9) | 0.147 |
| LC-AmB | 1 (1.5) | 1 (3.2) | 0 (0) | 0.470 |
| Voriconazole | 11 (16.7) | 8 (25.8) | 3 (8.6) | 0.097 |
| Posaconazole | 1 (1.5) | 1 (3.2) | 0 (0) | 0.470 |
| Fluconazole | 1 (1.5) | 1 (3.2) | 0 (0) | 0.470 |
| Caspofungin | 1 (1.5) | 0 (0) | 1 (2.9) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





