Introduction
Immune checkpoint inhibitors (ICPIs) have significantly improved clinical outcomes in patients with non-small cell lung cancer (NSCLC) and small-cell lung cancer [
1] They inhibit immunologic checkpoints, thereby restoring T-cell activation and antitumor immunity in patients with cancer [
2]. Pembrolizumab and nivolumab are monoclonal antibodies that block the interaction between programmed cell death 1 and its ligands, PD-L1 and PD-L2 [
3]. Whereas atezolizumab, sugemalimab, and durvalumab are monoclonal antibodies that bind programmed death ligand 1, blocking its engagement with PD-1 . ipilimumab is a monoclonal antibody that binds cytotoxic T-lymphocyte antigen-4, blocking its interaction with CD80 and CD86 [
1]. Despite these significant improvement in clinical outcomes with the advent of these AGENTS, immune-related adverse events (irAEs) such as pneumonitis continue to pose serious clinical challenges. Pneumonitis, while rare, can lead to significant morbidity and mortality, particularly in patients with pre-existing pulmonary comorbidities or those receiving combination therapies [
1]. The aggregate of current evidence suggests varying pneumonitis risks across different ICPIs, yet direct comparisons remain limited. Previous pairwise meta-analyses and pharmacovigilance reports have indicated potential differences in toxicity profiles between PD-1 and PD-L1 inhibitors [
2]. However, these analyses often lack the scope and statistical rigor of network-based approaches especially when those approaches are based not only on the broad adverse effects burden of ICPIs but rather on specific morbidities such as pneumonitis.
Immune checkpoint inhibitor–associated pneumonitis (ICI-pneumonitis) represents one of the most clinically significant immune-related adverse events of immunotherapy. Although relatively uncommon, occurring in approximately 2–5% of treated patients, it carries disproportionate morbidity and mortality. Severe (grade ≥3) cases occur in about 0.8%, frequently necessitating immunotherapy discontinuation, hospitalization, and high-dose corticosteroid therapy. Fatal outcomes are reported in up to 30% of severe cases. The burden extends beyond patient outcomes, as ICI-pneumonitis often mimics infection, radiation injury, or tumor progression, creating diagnostic uncertainty and delaying cancer therapy. Additionally, real-world data show higher incidence rates compared with clinical trials, likely reflecting broader patient comorbidities and combined ICI regimens. Given the expanding use of ICIs across malignancies, awareness, early detection, and standardized management are crucial to mitigate this growing clinical and healthcare burden.
This network meta-analysis aims to compare pneumonitis risk across multiple ICPIs using both direct and indirect evidence. It addresses limitations in prior reviews by including a broader set of studies and applying sensitivity analyses to assess robustness across study designs, treatment contexts, and patient subgroups.
Methods
Search Strategy
A comprehensive literature search was undertaken to identify randomized controlled trials evaluating pneumonitis risk associated with immune checkpoint inhibitors (ICPIs) in patients with lung cancer. The search was conducted across multiple electronic databases, including PubMed, Embase, Scopus, MEDLINE, and the Cochrane Database of Systematic Reviews, from database inception to June 2025. In addition, gray literature sources such as Google Scholar and trial registries (ClinicalTrials.gov and WHO ICTRP) were reviewed to ensure completeness and minimize publication bias. The search combined Medical Subject Headings (MeSH) and free-text terms relating to the interventions and outcome of interest. The final search syntax was structured as follows: (“immune checkpoint inhibitors” OR “PD-1” OR “PD-L1” OR “CTLA-4”) AND (“pneumonitis” OR “interstitial lung disease” OR “lung toxicity”) AND (“randomized controlled trial”) AND (“lung cancer” OR “SCLC” OR “NSCLC”). The reference lists of all included articles and relevant review papers were manually screened to identify additional eligible studies.
Eligibility Criteria
Eligible studies were randomized controlled trials that enrolled adult patients (≥18 years) with histologically confirmed non–small-cell lung cancer (NSCLC) or small-cell lung cancer (SCLC) who received treatment with at least one immune checkpoint inhibitor targeting PD-1, PD-L1, or CTLA-4 pathways, either as monotherapy or in combination with other agents. Studies were required to report extractable data on the incidence or number of pneumonitis cases of any grade, as defined by Common Terminology Criteria for Adverse Events (CTCAE). Trials that compared ICPIs with placebo, standard chemotherapy, or other ICPIs were included. Exclusion criteria were preclinical or non-oncologic studies, trials enrolling pediatric populations, studies involving non-randomized or single-arm designs, and those that did not provide sufficient data to calculate pneumonitis event rates or effect estimates.
Study Selection and Data Extraction
All retrieved citations were imported into Rayyan QCRI software for reference management and duplicate removal. Two reviewers (RE and MID) independently screened titles and abstracts to identify potentially eligible studies. The full texts of all potentially relevant articles were then obtained and reviewed in detail. Disagreements between reviewers regarding inclusion or exclusion were resolved by consensus, and if unresolved, arbitration was undertaken by a third reviewer (AA).
A standardized data extraction form was developed and piloted on ten randomly selected studies to ensure uniformity and reliability. The extracted information included details on study design, publication year, country, phase, sample size, histologic subtype, intervention type, comparator arm, ICPI dosage and regimen, follow-up duration, and the number of pneumonitis cases (any grade and grade ≥3, where available). When essential data were missing or unclear, corresponding authors were contacted to obtain clarification.
Risk of Bias Assessment
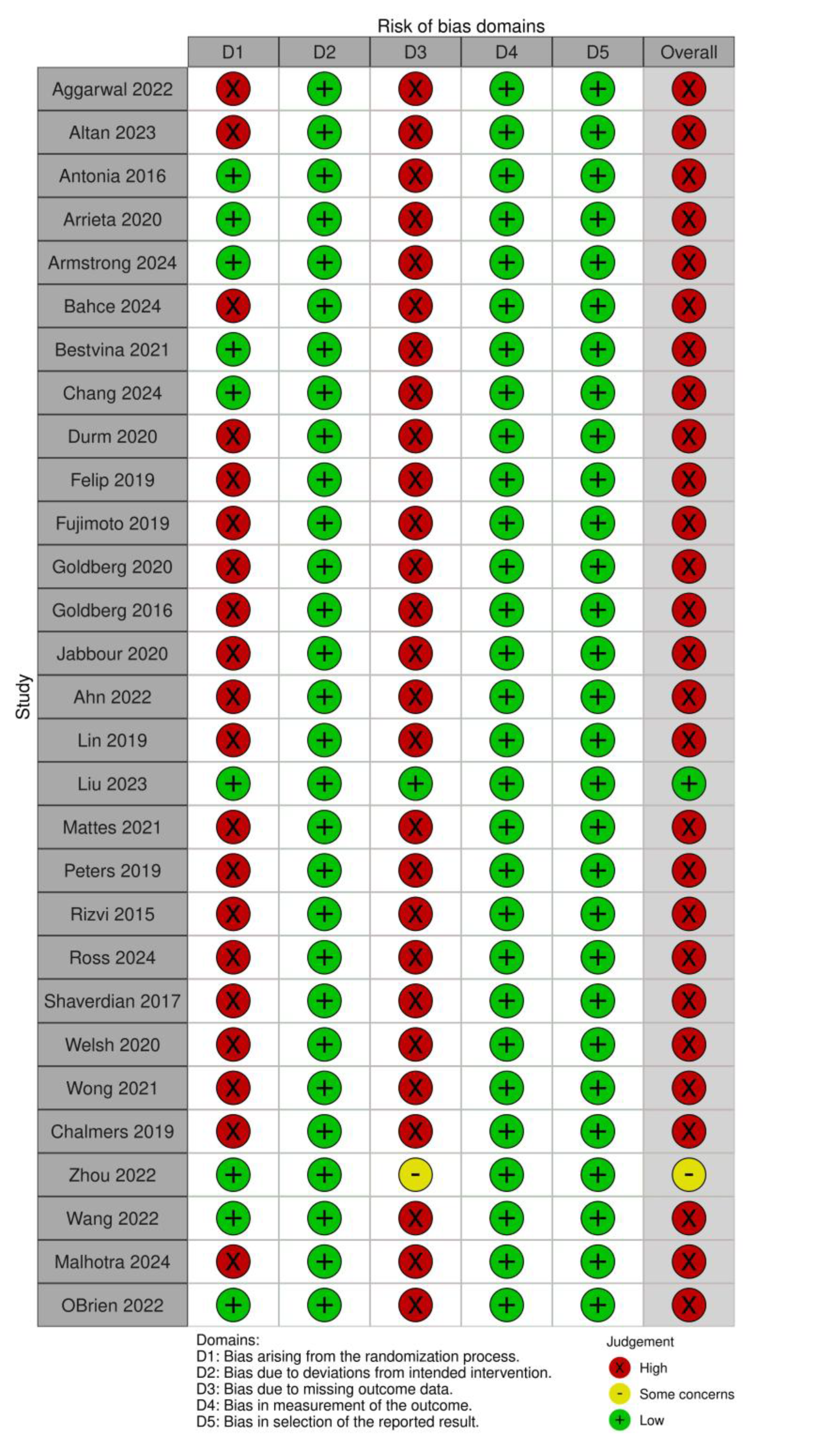
The methodological quality of included studies was evaluated using the Cochrane Risk of Bias 2.0 tool, and the results were visualized using the ROBVIS web application. This tool assesses the internal validity of randomized trials across six core domains: the randomization process, deviations from intended interventions, completeness of outcome data, accuracy of outcome measurement, selective reporting, and other potential sources of bias. Each domain was rated as low, some concerns, or high risk of bias. Two reviewers (AA and MID) performed independent assessments, and discrepancies were resolved through discussion. Studies classified as having a high risk of bias were subjected to sensitivity analyses to determine their influence on pooled estimates.
Network Geometry and Treatment Nodes
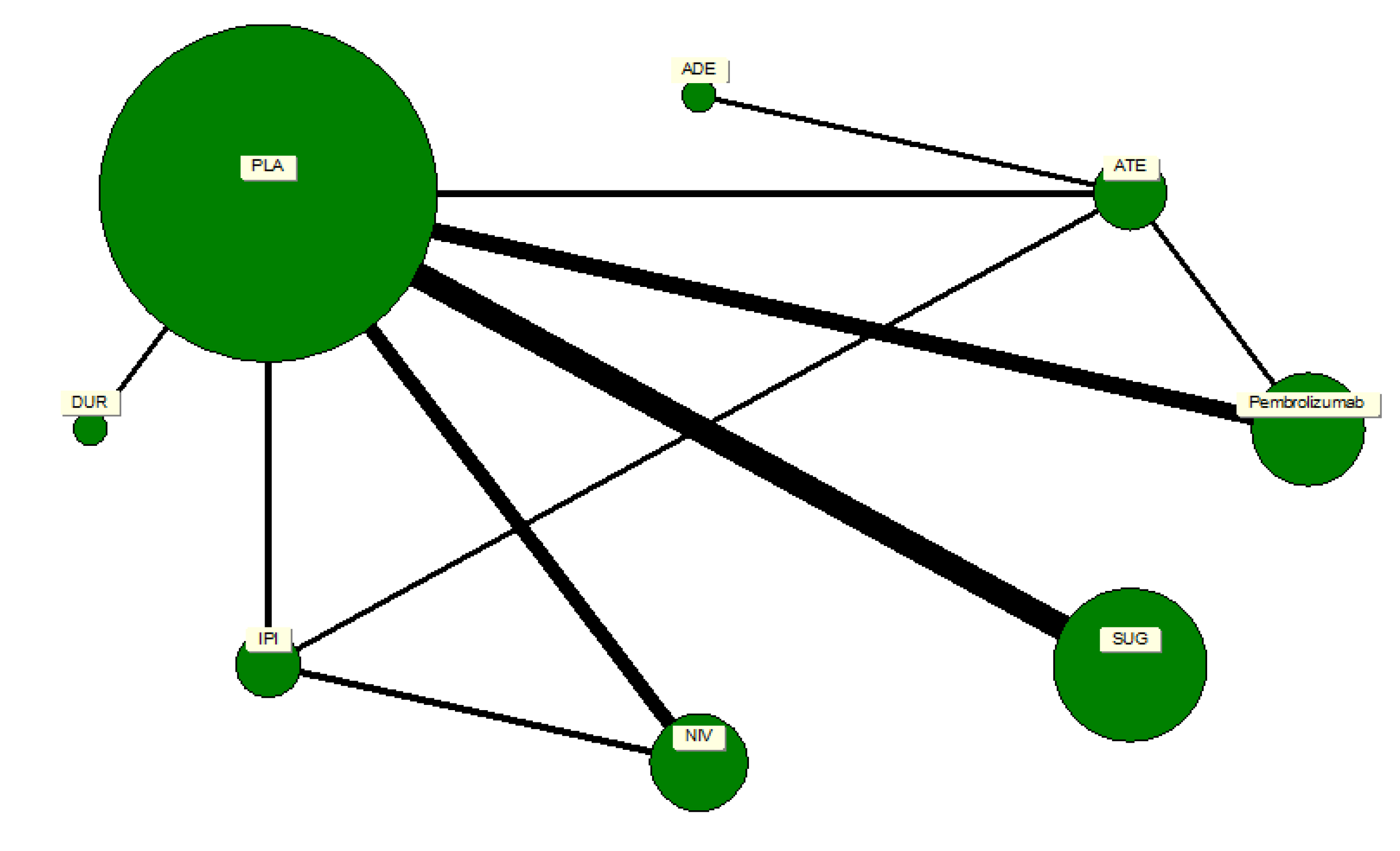
A network plot was constructed to illustrate the geometry of treatment comparisons among the included trials. Each node represented a distinct intervention, and edges denoted direct head-to-head comparisons between treatments. The node size was weighted according to the number of participants who received that particular treatment, while the thickness of each connecting line reflected the number of trials contributing to that comparison. Placebo served as the reference node, forming the largest and most interconnected point within the network. The network comprised seven active ICPIs: pembrolizumab, nivolumab, durvalumab, atezolizumab, avelumab, sugemalimab, and ipilimumab. The geometry revealed a well-connected, star-shaped structure centered on placebo, ensuring the feasibility of indirect comparisons across agents. There were no disconnected components or closed loops that would threaten the transitivity assumption required for network meta-analysis.
Statistical Analysis
All analyses adhered to PRISMA guidelines for network meta-analyses. The primary outcome was the odds ratio (OR) for any-grade pneumonitis associated with ICPI therapy. Initially, pairwise meta-analyses were performed for comparisons between each ICPI and placebo using a random-effects DerSimonian–Laird model to account for between-study heterogeneity. Heterogeneity was quantified using the I², Q, and H statistics, with an I² value exceeding 50% indicating substantial heterogeneity.
Subsequently, a frequentist random-effects network meta-analysis (NMA) was undertaken to estimate pooled odds ratios and 95% confidence intervals using placebo as the common comparator. The relative ranking of each intervention was determined using P-scores, which estimate the probability that a given treatment is among the most effective or least harmful options. The assumption of transitivity was verified by ensuring comparability of clinical and methodological characteristics across trials.
Consistency between direct and indirect evidence was examined using the design-by-treatment interaction model and node-splitting approaches, with p-values >0.05 indicating satisfactory global and local consistency, respectively. Sensitivity analyses were conducted to evaluate the robustness of results, excluding studies with high risk of bias and those evaluating combination regimens, thereby isolating the effects of monotherapy. Additional subgroup analyses were stratified by cancer type (NSCLC versus SCLC), ICPI class (PD-1 versus PD-L1 versus CTLA-4 inhibitors), and trial phase. Publication bias was assessed through visual inspection of funnel plot symmetry and confirmed using Egger’s regression test. All analyses were carried out in MetaXL (version 5.3, EpiGear International, Queensland, Australia), R (netmeta package), and Stata version 17 (metan and network commands)
Results
Study Characteristics
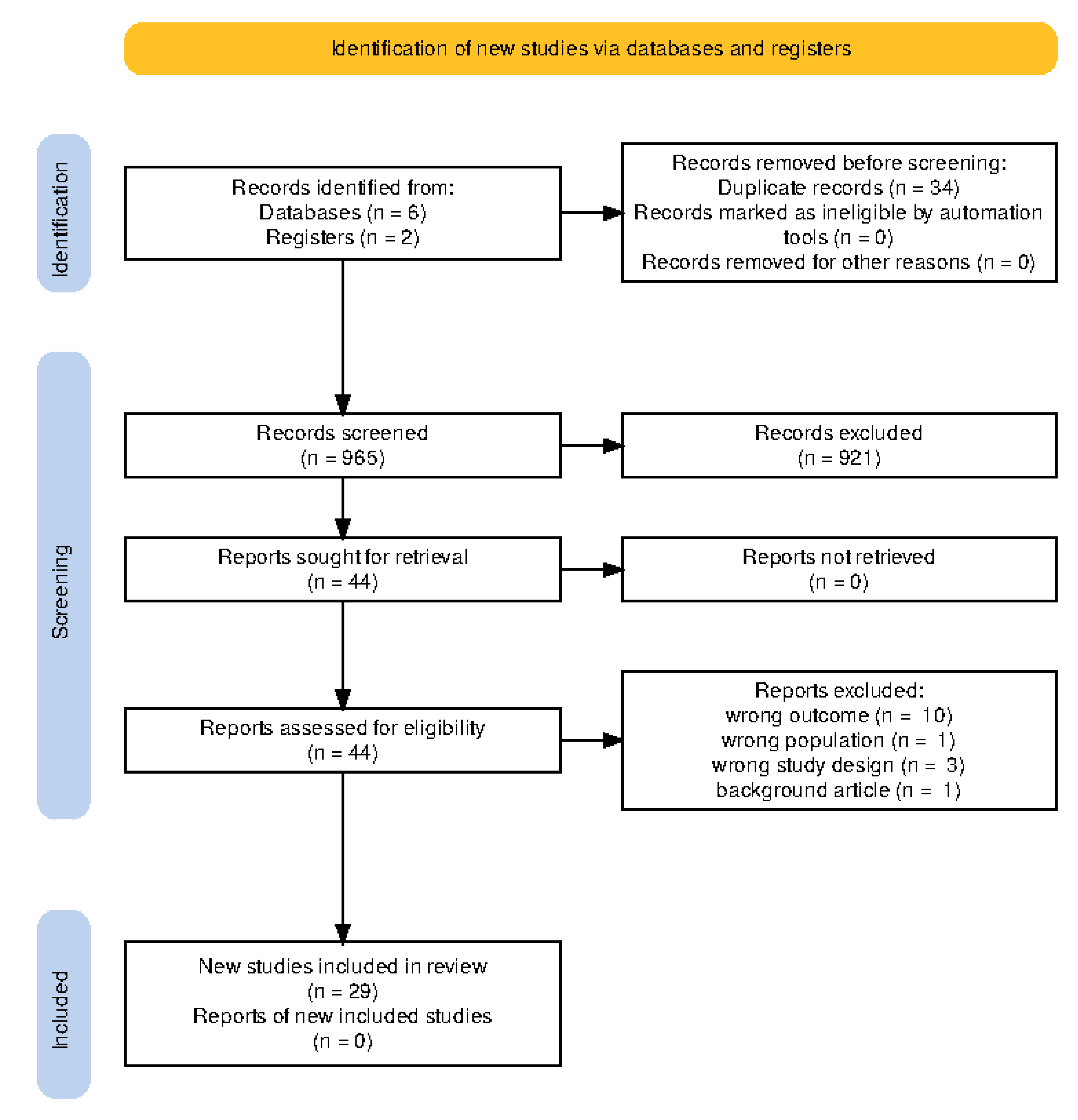
Table 1 shows the full the socio-demographic characteristics of the included studies. Electronic search of relevant databases returned n = 965 titles and abstracts from which n = 29 studies were eligible for inclusion into systemic review and network meta-analysis.
Figure 1. A total of 29 studies [
2,
3,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32] were included in this network meta-analysis, encompassing patients primarily with non-small cell lung cancer (NSCLC) 82.76%, and a minority with small-cell lung cancer (SCLC) 17.2%. The majority of the studies were conducted in early-phase settings: Phase I (27.6%)[
3,
11,
18,
20,
21,
31] , Phase II (62.1%) [
6,
7,
8,
9,
10,
14,
15,
16,
17,
19,
23,
26,
27,
28,
29,
32], Phase III (10.3%)[
12,
22,
23].
The sample sizes across trials varied widely, ranging from as few as 13 participants [
26] to over 10,000 [
12,
25], reflecting both exploratory and confirmatory trial designs. The median age of participants across studies ranged from 50 to 72 years, with most cohorts being predominantly male. Smoking history, although clinically relevant in pneumonitis risk, was inconsistently reported across the included studies.
Prevalence estimates stratified by type of ICPI
Regarding the types of immune checkpoint inhibitors (ICPIs) explored, pembrolizumab was the most frequently studied (40.7%), followed by nivolumab (22.2%), atezolizumab (14.8%), ipilimumab (11.1%), durvalumab (7.4%), and sugemalimab (3.7%) as shown in
Table 1. Combination regimens, involving concurrent chemotherapy, radiotherapy, or dual ICPI therapy, reflecting real-world treatment strategies and clinical trial complexity. The Common Terminology Criteria for Adverse Events (CTCAE) was used for adverse event grading in nearly all studies, although a few used modified criteria or did not specify the grading approach.
Small Study Effect and Publication Bias
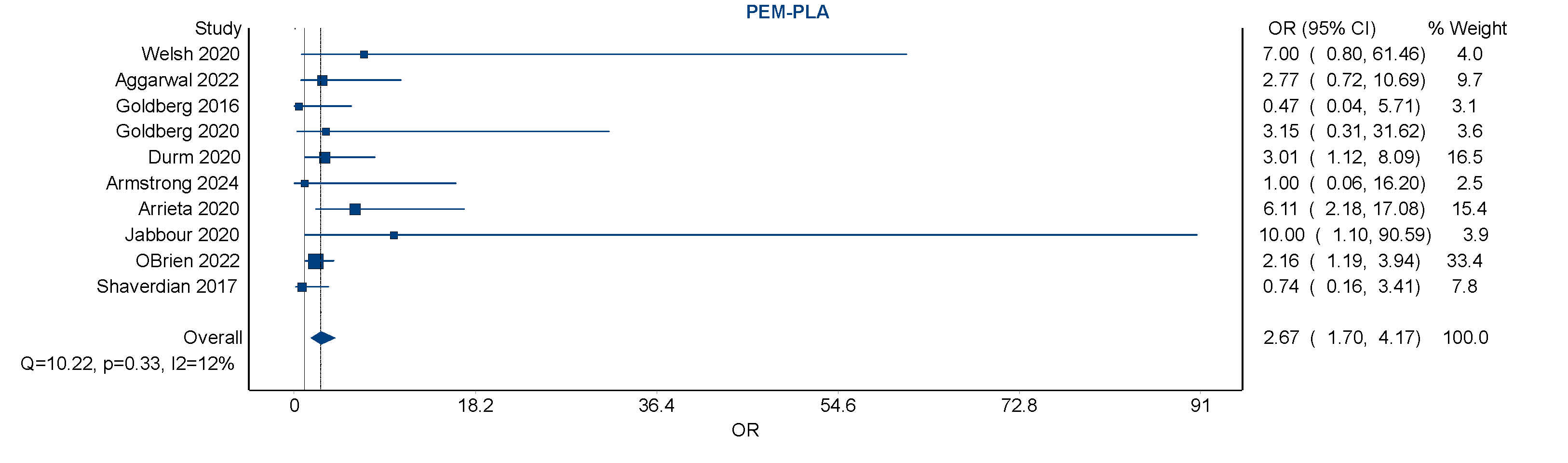
Visual inspection of the forest plot showed that although individual study effect estimates varied, they consistently favored an increased risk with pembrolizumab over placebo. A notable contributor to the weight of the analysis was the large RCT (O’Brien 2022) [
25], which alone accounted for over 33% of the pooled estimate, demonstrating the influence of large trials in driving overall findings. Smaller studies contributed less weight but did not significantly deviate from the overall trend.
Sensitivity Analyses
Sensitivity analyses were performed to assess the robustness of the primary findings. Exclusion of studies with high risk of bias had minimal impact on the pooled estimates, with the odds ratio for pembrolizumab vs. placebo decreasing slightly but remaining statistically significant, suggesting that the observed association was not driven by study quality alone. Additional sensitivity analyses were conducted to account for the impact of combination therapies. When studies employing concurrent chemo-radiotherapy or dual ICPI regimens were excluded, the pooled odds ratio for pembrolizumab decreased modestly from 2.67 to approximately 2.1. This attenuation suggests that combination regimens may contribute modestly to pneumonitis risk, but that pembrolizumab independently confers an elevated risk.
Subgroup analyses by cancer type (NSCLC vs. SCLC) and by trial phase (Phase III vs. earlier) revealed broadly similar patterns of risk, though statistical power was limited due to small numbers in some strata. Overall, the direction and magnitude of effect remained consistent across sensitivity analyses, lending confidence to the main findings.
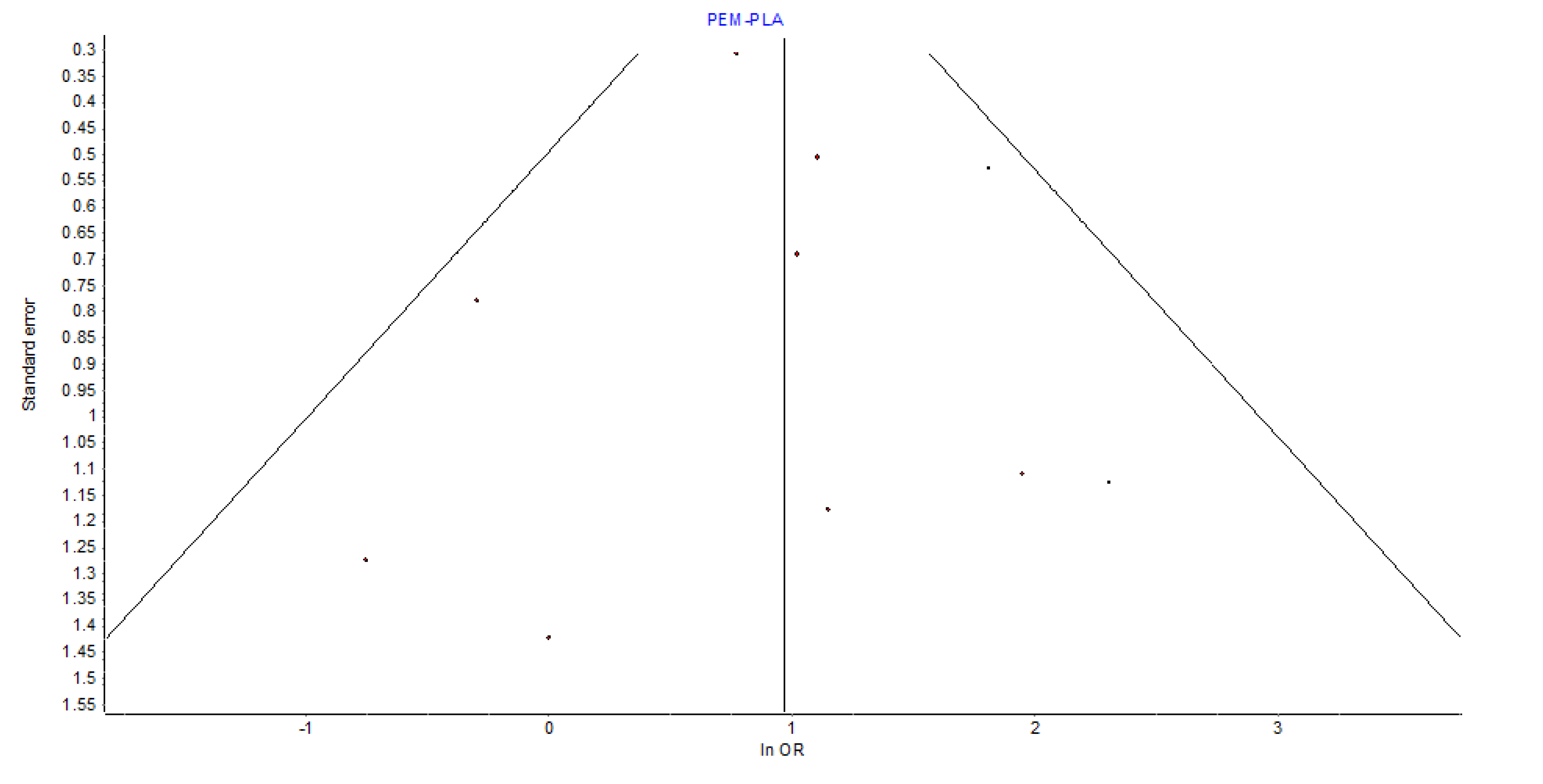
Heterogeneity and Publication Bias
The assessment of heterogeneity in the pairwise meta-analysis revealed low between-study variance, supported by an
I² of 12%,
Q = 10.22 (p = 0.33), and
H = 1.06. These values indicate that variation in effect sizes was likely due to chance rather than true heterogeneity. Visual inspection of the funnel plot for pembrolizumab
vs. placebo showed general symmetry, providing no strong evidence of small-study effects or publication bias.
Figure 4
Pneumonitis Outcomes and Adverse Event Reporting
Across studies, the incidence of grade ≥3 pneumonitis ranged from 0% to as high as 7% in the pembrolizumab arms, confirming that pneumonitis, though infrequent, can be clinically significant and occasionally life-threatening. All but four studies[
14,
21,
28,
30] used CTCAE criteria for grading adverse events, promoting consistency in reporting across the dataset. Notably, combination regimens tended to report higher rates of pneumonitis than monotherapy studies, underscoring the importance of evaluating treatment context. These findings are relevant for clinical decision-making in tailoring treatment based on patient risk profiles and expected toxicity burden.
Risk of Bias Assessment
Assessment of study quality revealed that 81.5% of studies were considered at high risk of bias, primarily due to open-label design, small sample sizes, or limited pneumonitis reporting. Only one large-scale RCT (Liu et al., 2023 [
12]) was deemed to have low risk of bias, while two other studies were rated as having some concerns.
Figure 5
Discussion
In this network meta-analysis of RCTs we found increased pneumonitis risk associated with pembrolizumab compared to other ICPTs. This network meta-analysis provides a nuanced evaluation of pneumonitis risk across different immune checkpoint inhibitors (ICPIs) in lung cancer treatment, highlighting pembrolizumab and sugemalimab as having the highest relative risks. These findings build upon prior meta-analyses while offering new insights enabled by network-level comparisons and robust sensitivity testing. The increased pneumonitis risk associated with pembrolizumab (odds ratio [OR] = 2.67, 95% confidence interval [CI]: 1.70–4.17) aligns with several previous meta-analyses and pharmacovigilance studies. For example, a systematic review by Wang et al. found that PD-1 inhibitors, particularly pembrolizumab, were associated with a higher incidence of immune-related pneumonitis compared to PD-L1 inhibitors, potentially due to broader immune activation by PD-1 blockade. Our findings extend this knowledge by confirming the elevated risk through both direct and indirect comparisons across a larger and more heterogeneous patient population.
In contrast, the point estimate for nivolumab (odds ratio [OR] = 2.69) was also elevated but accompanied by a wide confidence interval (95% confidence interval [CI]: 0.64–11.35), reflecting imprecise estimation due to fewer direct comparisons. A similar trend was noted in the work of Ramalingham et al.[
33], who observed that nivolumab carried a non-trivial pneumonitis risk, albeit slightly lower than pembrolizumab. Sugemalimab, a newer PD-L1 inhibitor, demonstrated a high odds ratio (odds ratio [OR] = 2.45, 95% confidence interval [CI]: 1.52–3.95), which may appear contradictory to the general perception that PD-L1 inhibitors carry a lower pneumonitis risk. However, its elevated risk in our analysis might be attributed to limited real-world safety data, smaller sample sizes, and the inclusion of high-risk populations in clinical trials. Additionally, sugemalimab trials have been conducted predominantly in Asian populations, and existing literature suggests that pneumonitis rates may vary by ethnicity, with higher susceptibility reported among East Asian patients [
33].
Atezolizumab and ipilimumab showed lower and less consistent associations with pneumonitis. Atezolizumab’s relatively favorable toxicity profile has been supported by prior pharmacovigilance reports and meta-analyses [
17]. Ipilimumab, an anti-CTLA-4 antibody, has a distinct mechanism of action that may result in different immune-related adverse event patterns, with colitis and dermatitis often more prominent than pneumonitis [
26,
33].
Socio-demographic factors, though not consistently reported across studies, are important to consider in interpreting pneumonitis risk. Age is a recognized risk factor; older patients may have decreased pulmonary reserve. Males were overrepresented, and smoking status was inconsistently documented, both of which could influence observed toxicity rates.
These findings underscore the need for personalized risk-benefit assessments in ICPI therapy selection and for standardized adverse event reporting in future trials.
Strengths and Limitations
Strengths of this study include the comprehensive scope of the network meta-analysis, which allowed both direct and indirect comparisons of multiple ICPIs. previous network metanalytical attempts have focused on general immune mediated adverse effect of ICPIs potentially diluting the requisite rigor required in the reporting in the exact burden of this adverse effect. The novelty of our approach is the laser focus on pneumonitis therefore providing exact comparative point estimate of its risk in these cohort of patients. The inclusion of monotherapy and combination therapy regimens reflects real-world practice. Robust sensitivity analyses, low heterogeneity, and symmetrical funnel plots further support the credibility of our findings.
Despite afore-mentioned strength analysis of these data schemes is fraught with lots of limitations. This includes the predominance of early-phase studies and variable pneumonitis definitions. Sparse data for some agents led to wide confidence intervals, and socio-demographic data were often missing or inconsistently reported, limiting detailed subgroup analysis. Nevertheless, the stability of our point estimates across both direct and indirect comparisons suggests that these limitations are unlikely to significantly impact on the burden of pneumonitis vis-à-vis ICPI exposure
Conclusion
Our analysis demonstrates that pneumonitis risk varies among ICPIs, with pembrolizumab and sugemalimab showing the highest odds. Although the absolute incidence is low, the potential severity of pneumonitis warrants vigilant monitoring. These results should guide clinicians in risk stratification, treatment planning, and support the development of standardized reporting criteria and further comparative research.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
M.I.D. involved review concept development, literature search, screening, risk of bias assessment, data analyses, result interpretation, writing of initial draft and final manuscript, and guarantor of the review. R.A.E. involved literature search, study registration on Prospero, screening, data curation, independent reviewer, risks of bias reviewer, result interpretation, writing of initial and final manuscript drafts. I.Y.A. involved data screening, data curation, independent reviewer, risks of bias reviewer, writing of initial and final manuscript drafts. A.A. was involved in data screening validation, data curation, independent reviewer, risks of bias reviewer, writing of initial and final manuscript drafts.
Funding
The article processing change of this manuscript on acceptance will be sought from Qatar National Library.
Data Availability Statement
Materials used for data analysis are available for the corresponding author on reasonable request.
Conflicts of Interest
None of the authors have any conflict of interest to declare with regards to this manuscript.
References
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol Off J Am Soc Clin Oncol. 2021 Dec;39(36):4073–126.
- Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2020 Feb;15(2):248–57.
- Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol. 2020 Jun;6(6):848–55.
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021 Jan;12(1):55–61.
- Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013 Nov;67(11):974–8.
- Durm GA, Jabbour SK, Althouse SK, Liu Z, Sadiq AA, Zon RT, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non–small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer [Internet]. 2020;126(19):4353–61. Available from: https://pubmed.ncbi.nlm.nih. 3269.
- Felip E, Ardizzoni A, Ciuleanu T, Cobo M, Laktionov K, Szilasi M, et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer [Internet]. 2020;127:160–72. Available from: https://pubmed.ncbi.nlm.nih. 3202.
- Fujimoto D, Yomota M, Sekine A, Morita M, Morimoto T, Hosomi Y, et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: A multicenter, open-label single-arm phase II trial. Lung Cancer [Internet]. 2019;134:274–8. Available from: https://pubmed.ncbi.nlm.nih. 3118.
- Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol [Internet]. 2020;21(5):655–63. Available from: https://pubmed.ncbi.nlm.nih. 3225.
- Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol [Internet]. 2016;17(7):976–83. Available from: https://pubmed.ncbi.nlm.nih. 2726.
- Ahn MJ, Cho BC, Ou X, Walding A, Dymond AW, Ren S, et al. Osimertinib Plus Durvalumab in Patients With EGFR-Mutated, Advanced NSCLC: A Phase 1b, Open-Label, Multicenter Trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2022 May;17(5):718–23.
- Liu Q, Zhang C, Huang Y, Huang R, Huang SM, Larkins E, et al. Evaluating Pneumonitis Incidence in Patients with Non–small Cell Lung Cancer Treated with Immunotherapy and/or Chemotherapy Using Real-world and Clinical Trial Data. Cancer Res Commun [Internet]. 2023;3(2):258–66. Available from: https://pubmed.ncbi.nlm.nih. 3686.
- Mattes MD, Eubank TD, Almubarak M, Wen S, Marano GD, Jacobson GM, et al. A Prospective Trial Evaluating the Safety and Systemic Response From the Concurrent Use of Radiation Therapy with Checkpoint Inhibitor Immunotherapy in Metastatic Non–Small Cell Lung Cancer. Clin Lung Cancer [Internet]. 2021;22(4):268–73. Available from: https://pubmed.ncbi.nlm.nih. 3360.
- Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—The ETOP NICOLAS trial. Lung Cancer [Internet]. 2019;133:83–7. Available from: https://pubmed.ncbi.nlm.nih. 3120.
- Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol [Internet]. 2015;16(3):257–65. Available from: https://pubmed.ncbi.nlm.nih. 2570.
- Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother cancer. 2022 Apr;10(4).
- Ross HJ, Kozono D, Wang XF, Urbanic JJ, Williams TM, Nelson GD, et al. Atezolizumab before and after Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase II Nonrandomized Controlled Trial. JAMA Oncol [Internet]. 2024;10(9):1212–9. Available from: https://pubmed.ncbi.nlm.nih. 3905.
- Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol [Internet]. 2017;18(7):895–903. Available from: https://pubmed.ncbi.nlm.nih. 2855.
- Welsh JW, Heymach J V, Guo C, Menon H, Klein K, Cushman TR, et al. Phase 1/2 Trial of Pembrolizumab and Concurrent Chemoradiation Therapy for Limited-Stage SCLC. J Thorac Oncol [Internet]. 2020;15(12):1919–27. Available from: https://pubmed.ncbi.nlm.nih. 3291.
- Wong DJ, Bauer TM, Gordon MS, Bene-Tchaleu F, Zhu J, Zhang X, et al. Safety and Clinical Activity of Atezolizumab Plus Ipilimumab in Locally Advanced or Metastatic Non–Small Cell Lung Cancer: Results From a Phase 1b Trial. Clin Lung Cancer [Internet]. 2022;23(3):273–81. Available from: https://pubmed.ncbi.nlm.nih. 3445.
- Chalmers AW, Patel S, Boucher K, Cannon L, Esplin M, Luckart J, et al. Phase I Trial of Targeted EGFR or ALK Therapy with Ipilimumab in Metastatic NSCLC with Long-Term Follow-Up. Target Oncol [Internet]. 2019;14(4):417–21. Available from: https://pubmed.ncbi.nlm.nih. 3134.
- Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3. Lancet Oncol [Internet]. 2022;23(2):209–19. Available from: https://pubmed.ncbi.nlm.nih. 3503.
- Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol [Internet]. 2022;23(6):739–47. Available from: https://pubmed.ncbi.nlm.nih. 3557.
- Malhotra J, Chiappori A, Fujioka N, Hanna NH, Feldman LE, Patel M, et al. Phase I/II trial of plinabulin in combination with nivolumab and ipilimumab in patients with recurrent small cell lung cancer (SCLC): Big ten cancer research consortium (BTCRC-LUN17-127) study. Lung Cancer [Internet]. 2024;195. Available from: https://pubmed.ncbi.nlm.nih. 3917.
- O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol [Internet]. 2022;23(10):1274–86. Available from: https://pubmed.ncbi.nlm.nih. 3610.
- Altan M, Wang Y, Song J, Welsh J, Tang C, Guha-Thakurta N, et al. Nivolumab and ipilimumab with concurrent stereotactic radiosurgery for intracranial metastases from non-small cell lung cancer: analysis of the safety cohort for non-randomized, open-label, phase I/II trial. J Immunother Cancer [Internet]. 2023;11(7). Available from: https://pubmed.ncbi.nlm.nih. 3740.
- Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol [Internet]. 2016;17(7):883–95. Available from: https://pubmed.ncbi.nlm.nih. 2726.
- Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol [Internet]. 2020;6(6):856–64. Available from: https://pubmed.ncbi.nlm.nih. 3227.
- Armstrong AJ, Geva R, Chung HC, Lemech C, Miller WH, Hansen AR, et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: a phase 2 randomized trial. Invest New Drugs [Internet]. 2024;42(1):145–59. Available from: https://pubmed.ncbi.nlm.nih. 3832.
- Bahce I, Dickhoff C, Schneiders FL, Veltman J, Heineman DJ, Hashemi SMS, et al. Single-arm trial of neoadjuvant ipilimumab plus nivolumab with chemoradiotherapy in patients with resectable and borderline resectable lung cancer: The INCREASE study. J Immunother Cancer [Internet]. 2024;12(9). Available from: https://pubmed.ncbi.nlm.nih. 3934.
- Bestvina CM, Pointer KB, Karrison T, Al-Hallaq H, Hoffman PC, Jelinek MJ, et al. A Phase 1 Trial of Concurrent or Sequential Ipilimumab, Nivolumab, and Stereotactic Body Radiotherapy in Patients With Stage IV NSCLC Study. J Thorac Oncol [Internet]. 2022;17(1):130–40. Available from: https://pubmed.ncbi.nlm.nih. 3450.
- Chang JY, Lin SH, Dong W, Liao Z, Gandhi SJ, Gay CM, et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet [Internet]. 2023;402(10405):871–81. Available from: https://pubmed.ncbi.nlm.nih. 3747.
- Ramalingam SS, Ciuleanu TE, Pluzanski A, Lee JS, Schenker M, Bernabe Caro R, et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1. J Clin Oncol [Internet]. 2025 Oct 22;38(15_suppl):9500. Available from. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).