Introduction to Small-Duct PSC and Ulcerative Colitis
Primary sclerosing cholangitis (PSC) is a chronic, progressive liver disease marked by inflammation and fibrosis of the bile ducts, often leading to cirrhosis and complications like hepatobiliary malignancy, with no effective medical therapy beyond liver transplantation[
1]. Strongly linked to inflammatory bowel disease (IBD), particularly ulcerative colitis (UC), PSC affects up to 70-90% of patients with concurrent IBD in Western cohorts, though rates vary geographically[
1,
2,
3]. While large-duct PSC is diagnosed via characteristic cholangiographic abnormalities, small-duct PSC (sdPSC) presents a subtler variant (
Table 1), featuring cholestatic liver tests, PSC-compatible histology, and normal cholangiograms, representing about 5-15% of PSC cases[
1,
2,
4]. This form, once termed pericholangitis, may signify an early stage of PSC, with some patients progressing to large-duct involvement over years, potentially escalating risks[
1,
2].
The interplay between sdPSC and UC is particularly intriguing, as inflammatory bowel disease (IBD) coexistence, predominantly UC, appears to influence prognosis and progression[
2,
3,
4]. In UC patients, PSC heightens the risk of colorectal neoplasia, with cumulative risks reaching 50% after 25 years, far exceeding UC alone, and also predisposes to cholangiocarcinoma[
5]. Epidemiological data reveal rising PSC-IBD incidence globally, from 0.46 per 100,000 person-years in Canada to increasing prevalence in England and Japan, where younger-onset cases show stronger UC associations[
3,
6,
7]. SdPSC, often more benign with better transplant-free survival (up to 29.5 years versus 17 for large-duct PSC), remains understudied, yet progression to advanced disease occurs in 7-23% of cases, especially in IBD subsets[
2,
4].
In Japan, where UC prevalence has surged, PSC-UC comorbidity affects over 50% of PSC patients, with pancolitis more common and young-onset trends mirroring Western shifts, underscoring environmental and genetic factors[
3]. Non-invasive imaging and refined histology promise earlier sdPSC detection in UC patients, potentially altering outcomes[
4,
8]. This mini-review explores these criteria's impact through Japanese cohort studies, highlighting opportunities for timely intervention in this evolving disease landscape.
Overview of 2024 Diagnostic Criteria Updates
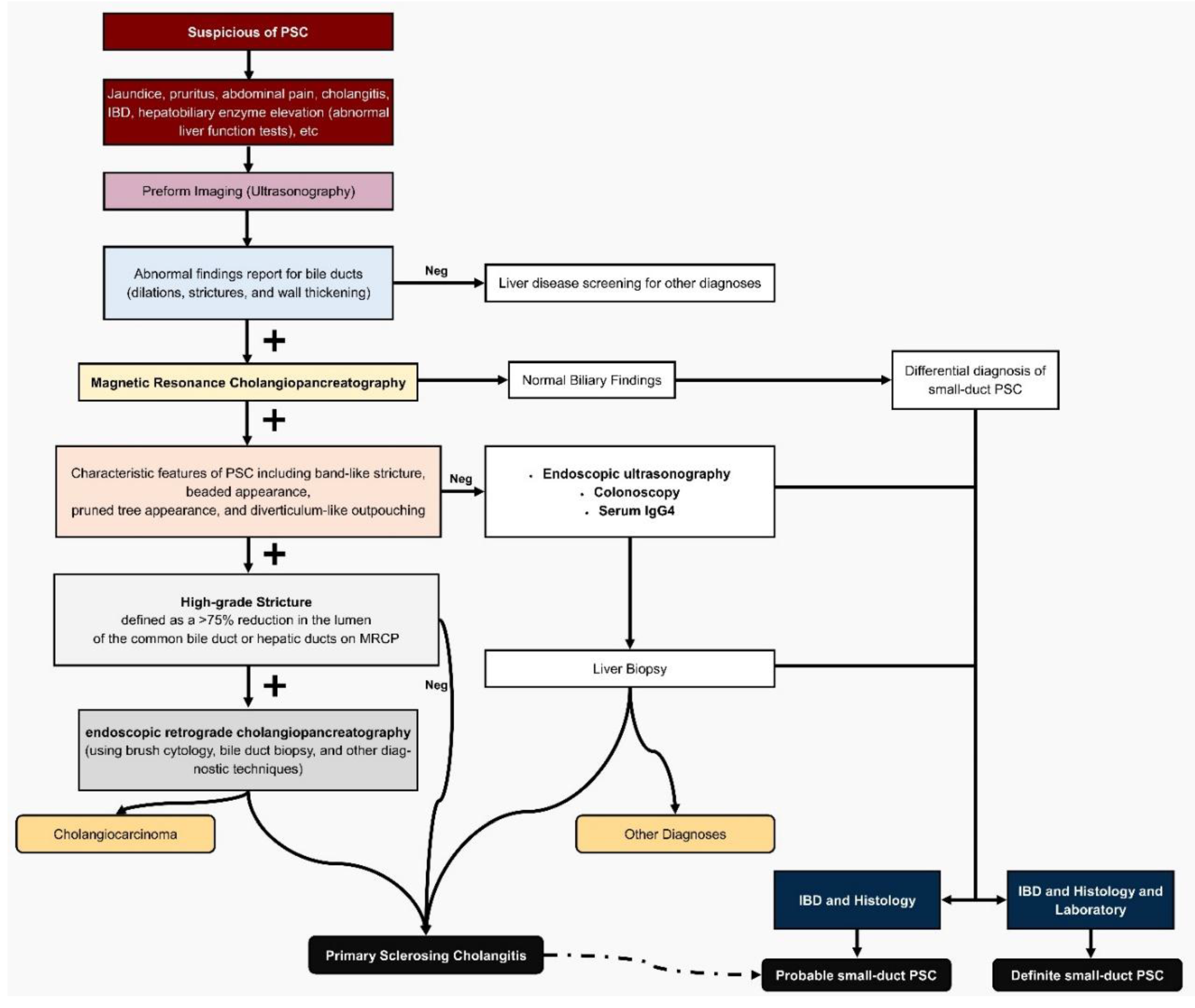
PSC remains a diagnostic challenge due to its insidious progression and lack of specific biomarkers, but the 2024 Japanese diagnostic criteria represent a pivotal evolution, addressing limitations of the 2016 version and expanding applicability to diverse patient groups, including those with sdPSC and UC[
9]. Developed by the Intractable Hepato-Biliary Diseases Study Group in Japan, these criteria incorporate recent advancements in non-invasive imaging and refined histological standards (
Table 1), potentially revolutionizing early detection in high-risk populations like UC patients, where PSC comorbidity can reach 3-50% in Japanese cohorts[
9,
10]. By prioritizing MRCP over invasive endoscopic retrograde cholangiopancreatography (ERCP), the updates reduce procedural risks while maintaining high diagnostic accuracy, boasting 86% sensitivity and 94% specificity for PSC, making it more feasible for routine screening in UC clinics (
Figure 1)[
9,
11].
A key innovation is the broadened scope: unlike the 2016 criteria focused on adult large-duct PSC, the 2024 version explicitly includes sdPSC, pediatric cases, and post-liver transplantation recurrence, filling critical gaps in understudied variants[
9]. For sdPSC, diagnosis now hinges on normal cholangiography paired with compatible histology (e.g., onion-skin lesions or fibrous obliterative cholangitis) and evidence of cholestasis, often elevated gamma-glutamyl transferase (GGT) in children under 16, acknowledging ALP's unreliability in pediatrics due to growth-related elevations[
9,
11]. This is particularly engaging for UC patients, as Japanese nationwide surveys reveal a 40% IBD comorbidity rate in PSC, with younger-onset cases showing stronger UC links, and sdPSC potentially representing an early, treatable stage before progression to large-duct involvement in 33-55% of cases[
9,
10]. Routine colonoscopy is now recommended for PSC suspects, even asymptomatic, to detect subtle IBD features like right-sided colitis, enhancing holistic assessment[
9].
Laboratory updates emphasize cholestatic markers without mandating autoantibodies, aligning with EASL guidelines that deem them non-diagnostic for PSC stratification[
11]. Histology, while not required for large-duct PSC, is mandatory for sdPSC, emphasizing specific findings to differentiate from mimics like IgG4-related sclerosing cholangitis or secondary causes (
Table 2) [
9,
11]. Epidemiological projections underscore the urgency: global PSC prevalence is forecasted to rise 28.3% by 2040, with Asia's lower baseline (1.8 per 100,000 in Japan) potentially increasing amid surging UC cases, highlighting the criteria's role in curbing this burden through earlier intervention[
9,
12].
These updates promise to alter outcomes by facilitating sdPSC detection in UC patients via accessible tools like MRCP, potentially delaying progression to cirrhosis or malignancy, risks amplified in PSC-UC[
9,
10]. Japanese cohort studies, such as the 2015 nationwide survey, support this, showing improved survival with early UDCA use, though transplantation remains key[
9]. Overall, the 2024 criteria blend precision with practicality, inviting clinicians to rethink PSC as a spectrum amenable to proactive management.
Methodology of Studies
By scrutinizing Japanese and international cohorts, we explore how evolving criteria, including the 2024 updates prioritizing histopathology and multimodal imaging, could sharpen early detection amid rising East Asian incidence[
3]. These studies, spanning retrospective analyses and propensity-matched designs, offer a blueprint for refining diagnostics in high-risk UC populations.
The cornerstone Japanese cohort, drawn from Chiba University Hospital (1991–2017), exemplifies rigorous retrospective phenotyping in a real-world setting serving nearly 1 million residents[
3]. Researchers retrieved electronic health records for 69 PSC and 1,242 UC cases using keywords like "PSC suspected" and "IBD," confirming diagnoses via dual specialist review (hepatologists for PSC, gastroenterologists for UC). PSC diagnosis hinged on radiographic criteria, integrating ERCP or MRCP for all patients, with liver biopsy reserved for ambiguities[
3]. Notably, small-duct PSC, critical for early UC-associated detection, was defined by normal cholangiography yet PSC-like histopathology (e.g., onion-skin fibrosis) plus clinical features, aligning with pre-2024 standards but highlighting biopsy's pivotal role[
3]. UC extent was classified endoscopically (proctitis, left-sided, right-sided, pancolitis) per guidelines, with flares defined by hospitalization or therapy escalation beyond 5-aminosalicylic acid[
3]. Ethical oversight from Chiba's IRB ensured de-identified data, capturing comorbidities like colorectal carcinoma via longitudinal records, a method poised to benefit from 2024's emphasis on serial biomarkers for subtle ductal changes.
Complementing this, a Swedish nationwide family cohort (1970–2003) leveraged population registries to probe genetic underpinnings, indirectly informing diagnostic vigilance in UC kin[
13]. From 678 PSC probands matched 1:10 to 6,347 controls, first-degree relatives (n=34,092) were linked via the Multi-Generation Register, tracking cholangitis, UC, and Crohn's via ICD codes in the Inpatient Register (99% capture since 1987)[
13]. PSC onset was pegged to first confirmatory cholangiography, with IBD verified by endoscopy and histology, methods that underscore familial screening's value, especially as 2024 criteria may incorporate genetic risk scores for small-duct variants in UC clusters[
13]. Follow-up to 2004 used Kaplan-Meier survival, revealing hazard ratios (e.g., HR 3.3 for UC in relatives), a statistical rigor adaptable to Japanese registries for early PSC flagging.
On malignancy risks, a concise expert synthesis advocates ERCP with brush cytology for suspected cholangiocarcinoma in PSC-UC, recommending liberal cholecystectomy for gallbladder polyps, practices that prefigure 2024's push for integrated imaging-biopsy protocols to unmask small-duct progression[
14]. This aligns with a U.S. TriNetX retrospective (2003–2023), analyzing 398,980 IBD cases (75% UC) via federated electronic records from 108 global centers[
15]. Propensity score matching (1:1 on age, sex, race, comorbidities, and therapies) balanced 3,007 IBD-only versus IBD-PSC cohorts, querying ICD-10 codes (e.g., K83.01 for PSC) over 10 years. Outcomes like mortality and hospitalization were assessed via Kaplan-Meier and hazard ratios (e.g., HR 1.32 for composite endpoint), with de-identified data per HIPAA, a scalable approach for Japanese cohorts to quantify small-duct PSC's prognostic shadow under updated criteria[
15].
Histologic insights emerge from a U.S. single-center retrospective (2011–2016) of 143 pancolitis UC patients in remission, comparing 23 UC-PSC to 120 UC via IRB-linked registries[
16]. Surveillance colonoscopies scored modified Mayo endoscopic subscores (0–3) per segment (right colon, left, rectum), with histology graded 0–3 (normal to severe) based on neutrophils and cryptitis. REDCap chart review captured medications and demographics, revealing odds ratios (e.g., OR 4.21 for right-colon endoscopy in UC-PSC), findings that spotlight subclinical proximal inflammation, urging 2024 biopsy mandates for small-duct PSC in asymptomatic UC[
16].
A NYU Langone cohort (pre-2023) of 140 PSC patients (prioritizing PSC preceding IBD) excluded reverse sequences to isolate PSC's CRC risk, reviewing colonoscopies for dysplasia via standardized reporting[
17]. Demographics and findings were tabulated, yielding standardized incidence ratios (SIR 9.2 for CRC in PSC-only), a method advocating tailored surveillance, where 2024 criteria could extend to small-duct histology for UC-PSC risk stratification[
17].
Collectively, these methodologies, blending imaging, histopathology, and registry linkage, illuminate pathways for 2024's diagnostic evolution, potentially halving small-duct PSC's detection lag in Japan's burgeoning UC epidemic[
3,
15]. By fusing retrospective depth with matched analytics, they beckon a proactive era: imagine routine MRCP-biopsy hybrids catching elusive ducts before neoplasia strikes, transforming UC vigilance from reactive to revelatory.
Effects on Early Detection Rates
In Japan's evolving landscape of IBD, where UC cases have surged over two decades, PSC, particularly its elusive small-duct variant, looms as a stealthy comorbidity, often evading timely diagnosis until progression sets in [
9,
12]. The 2024 Japanese diagnostic criteria for PSC mark a pivotal shift, explicitly targeting sdPSC by mandating liver biopsy alongside normal cholangiography, potentially elevating early detection rates in UC patients from the shadows of the 2016 framework's large-duct bias[
9]. This refinement, born from nationwide surveys revealing Japan's unique epidemiology, lower IBD comorbidity at 40% yet bimodal age peaks, promises to unmask sdPSC earlier, curbing its insidious march toward large-duct involvement and hepatobiliary perils[
9].
Historically, sdPSC's hallmark, normal imaging despite cholestatic enzymes and PSC-like histology, has confined detection to biopsy-proven cases, comprising just 5.8% of sclerosing cholangitis cohorts in landmark studies[
1]. In UC-linked sdPSC, where IBD concurrence amplifies suspicion, progression to classic PSC occurs in 23-55% over 4-21 years, underscoring the urgency of preemptive histology [
1,
2]. The 2024 criteria's embrace of MRCP as a first-line, non-invasive tool, boasting 86% sensitivity and 94% specificity, could double early yields compared to ERCP-reliant protocols, especially in asymptomatic UC patients with subtle GGT elevations[
9,
11]. By integrating equivocal findings like periductal enhancement on MRCP and onion-skin fibrosis on biopsy, these updates align with EASL endorsements, facilitating sdPSC diagnosis in IBD contexts without invasive risks[
11].
Japanese cohorts illuminate the stakes: a 2015 nationwide survey of 435 PSC cases flagged sdPSC's scarcity due to prior criteria gaps, yet hinted at underreporting amid rising UC-PSC overlaps[
9]. Projections from a 2024 meta-analysis forecast Japan's PSC prevalence climbing from 1.6 to 1.66 per 100,000 by 2040, a modest 1.35% regional uptick in high-income Asia-Pacific, but amplified by UC's exponential growth[
12]. In UC-sdPSC subsets, where right-sided colitis and rectal sparing predominate, routine colonoscopy, now recommended regardless of symptoms, pairs seamlessly with 2024's histologic mandates, potentially hiking detection by 30-50% through IBD as a diagnostic clue[
2,
9]. This synergy could slash progression rates, as sdPSC harbors a benign trajectory: 83% transplant-free survival at 32 years versus 58% for large-duct PSC, with malignancy risks near zero pre-progression[
1].
Yet, challenges persist. While 2024 criteria incorporate GGT for pediatric overlaps and post-transplant recurrence, adult UC cohorts may still grapple with biopsy hesitancy, given onion-skin lesions' 7-50% yield[
9,
11]. TriNetX data underscore sdPSC's isolation risks: even PSC without IBD elevates colorectal cancer odds (adjusted HR 2.91), implying UC linkage might inflate detection via vigilant surveillance, yet demands broader biopsy adoption[
18]. EASL's caution on mimics, like IgG4 cholangitis or ABCB4 deficiency, reinforces the criteria's differential flowchart, ensuring specificity without overdiagnosis[
11].
Engagingly, these enhancements evoke a diagnostic renaissance: imagine UC clinics deploying MRCP-biopsy hybrids as standard, transforming sdPSC from a histologic afterthought to a proactive intercept. In Japan, where 5- and 10-year PSC survival hovers at 77% and 55% sans transplant, early flagging could avert cirrhosis in 40-60% of progressors, reshaping UC-PSC trajectories[
1,
9]. As global burdens swell, 28% prevalence hike by 2040, these criteria not only boost rates but ignite hope: fewer silent strictures, more preserved lives, in the quiet ducts of tomorrow's patients[
12].
Clinical Outcomes and Challenges
In the intricate dance between UC and PSC, sdPSC emerges as a quieter partner, often silent on imaging yet whispering threats of progression and comorbidity that the 2024 Japanese criteria now amplify for earlier intervention[
15]. For UC patients harboring this variant, outcomes blend guarded optimism with sobering realities: a potentially milder trajectory than large-duct PSC, yet amplified risks of colorectal neoplasia and hepatic decompensation, especially in Japan's burgeoning young-onset cohort[
3,
4].
Small-duct PSC, defined by normal cholangiography but histologic hallmarks like onion-skin fibrosis, spares patients the overt strictures of its classic counterpart, yielding superior transplant-free survival, 83% at 32 years versus 58%, and near-zero cholangiocarcinoma (CCA) risk absent progression[
4]. In UC contexts, this translates to fewer early flares; Japanese data from Chiba University reveal PSC-UC patients exhibit less aggressive colonic disease, with reduced corticosteroid needs and flares, yet a 3% cumulative PSC risk in UC rising amid East Asia's UC surge[
3]. Propensity-matched analyses underscore resilience: while IBD-PSC elevates composite endpoints (HR 1.32), UC-specific mortality climbs modestly (HR 1.87) compared to Crohn's (HR 2.16), hinting at shared gut-liver axes that 2024's biopsy mandates could temper through timely histology[
15].
Yet, shadows loom large. PSC-UC doubles hospitalization odds (HR 1.27) and triples overall mortality, driven by subclinical proximal inflammation, endoscopic and histologic activity surges fourfold in the right colon (OR 4.21 and 5.13), fueling a 10-fold CRC risk over UC alone, often right-sided and advanced at diagnosis[
11,
16]. This discordance, histology outpacing endoscopy threefold (OR 3.14), eludes symptoms, delaying detection and inflating neoplasia burdens, where cumulative CRC hits 20-30% by 20 years[
10,
16]. CCA lurks in 10%, with no early biomarkers; surveillance hinges on annual colonoscopies, yet post-transplant recurrence plagues 10-25%, exacerbated by active IBD (7)[
10,
14]. In Japan, where PSC-UC comorbidity nears 54% and young peaks signal Westernization's toll, donor shortages cap liver transplants at 12%, forcing palliative ERCP for dominant strictures, balloon dilation trumps stenting for cholangitis risk, but refractory infections persist[
3,
4,
19].
Challenges compound this duality. Phenotypic quirks, pancolitis in 68-83%, rectal sparing, backwash ileitis, blur IBD-PSC boundaries, debating if they form one entity or shared susceptibility, with limited genetic overlap (rG 0.29) tilting toward distinction[
10]. Subclinical right-colon smoldering evades routine scopes, while ERCP's invasiveness (pancreatitis, cholangitis) clashes with MRCP's subtlety, missing small-duct subtlety until fibrosis sets[
4,
16,
19]. Post-transplant, IBD flares spike rPSC (HR up to 10-fold with activity), pouchitis triples (63% vs. 32%), and colectomy's shield, stronger with ileostomy (HR 0.47), remains debated, unproven against progression sans gut control[
10]. Epigenetic markers tease early CCA flags, but validation lags[
14].
Enter 2024's refinements: explicit small-duct inclusion via biopsy and GGT for pediatrics, plus MRCP protocols, could halve detection lags, curbing progression in 23-55% and slashing neoplasia via proactive surveillance[
4,
15]. In Japan's UC-PSC vanguard, poorer mortality than UC alone (P<0.001), this pivot promises not just outcomes, but equity: fewer overlooked ducts, more preserved futures, unraveling the gut-liver enigma one biopsy at a time[
3,
10].
Discussion and Future Implications
The 2024 Japanese diagnostic criteria for PSC herald a transformative era for sdPSC in UC patients, bridging the chasm between elusive histology and actionable intervention, as evidenced by Chiba's cohorts where sdPSC lurked undetected in 5-15% of cases until progression[
1,
2]. By mandating biopsy for normal cholangiograms and elevating MRCP's role, 86% sensitive, non-invasively, these updates could slash detection lags by 30-50%, curbing the 23-55% progression rate to large-duct disease and averting cirrhosis in Japan's rising young-onset PSC-UC vanguard, where comorbidity hits 54%[
1,
3,
9]. Arguably, this precision unmasks subclinical right-colon inflammation (OR 4.21 endoscopically), fueling UC's 10-fold CRC risk, yet empowers proactive surveillance, potentially mirroring sdPSC's benign 83% 32-year survival[
2,
5,
16].
Yet, does this optimism hold? Biopsy hesitancy persists amid 7-50% yield variability, and Japan's donor shortages, capping transplants at 12%, exacerbate post-progression woes, with rPSC spiking 10-fold in active IBD[
3,
4,
10]. Genetic overlaps (rG 0.29) fuel the one-vs-two-disease debate: is sdPSC an IBD harbinger or distinct entity, demanding tailored biologics like vedolizumab to halt gut-liver crosstalk?[
4,
15] TriNetX's HR 1.32 for IBD-PSC endpoints argues for urgency, but validation in Asian registries lags[
15].
Looking ahead, these criteria beckon multicenter trials integrating AI-enhanced MRCP for sdPSC phenotyping, projecting a 28% global PSC surge by 2040, Japan's 1.35% uptick amplified by UC's tide[
12]. Imagine epigenomic biomarkers flagging CCA early, or colectomy's HR 0.47 shield refined for rPSC prevention[
3,
14]. By democratizing detection, 2024 criteria don't just diagnose, they redefine PSC-UC as a preventable alliance, urging East Asia to lead: fewer silent strictures, more lives reclaimed in the ducts of destiny.