4. Discussion
Pulmonary hypertension (PH) in chronic obstructive pulmonary disease (COPD) is independently associated with reduced survival, higher rates of exacerbations, and impaired quality of life [
6]. Even in the setting of maximized inhaled therapy, many patients continue to experience progressive dyspnea and exercise intolerance, driven by pulmonary vascular remodeling and increased right ventricular (RV) afterload, which may ultimately lead to chronic RV dysfunction [
7].
The management of COPD-associated PH (COPD-PH) remains an area of considerable uncertainty, as no therapies are currently approved specifically for this indication. Current guidelines emphasize optimization of underlying COPD therapy, initiation of long term oxygenation in selected patients and reserve the use of pulmonary vasodilators for carefully selected cases within specialized centers [
3].
Patients with Group 3 PH, especially those with severe obstruction and gas exchange impairment, have been historically excluded from most pulmonary arterial hypertension (PAH) trials. As a result, clinicians are left with little guidance and few therapeutic options. The evidence of specific pulmonary vasodilator therapy in COPD associated PH is conflicting [
4]. Our observation suggests that ensifentrine may fill a critical gap in care for patients with dual bronchial and vascular pathology.
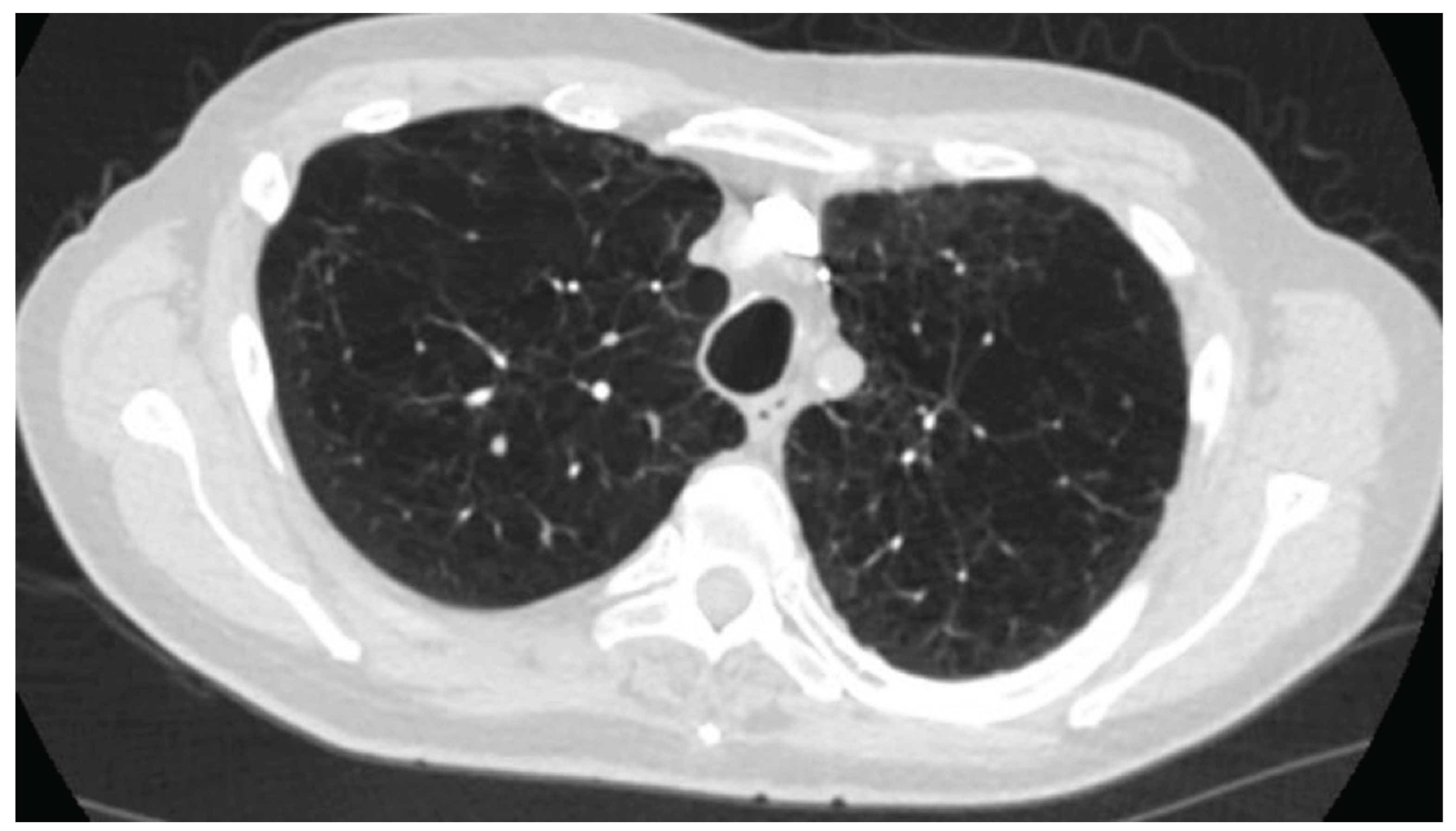
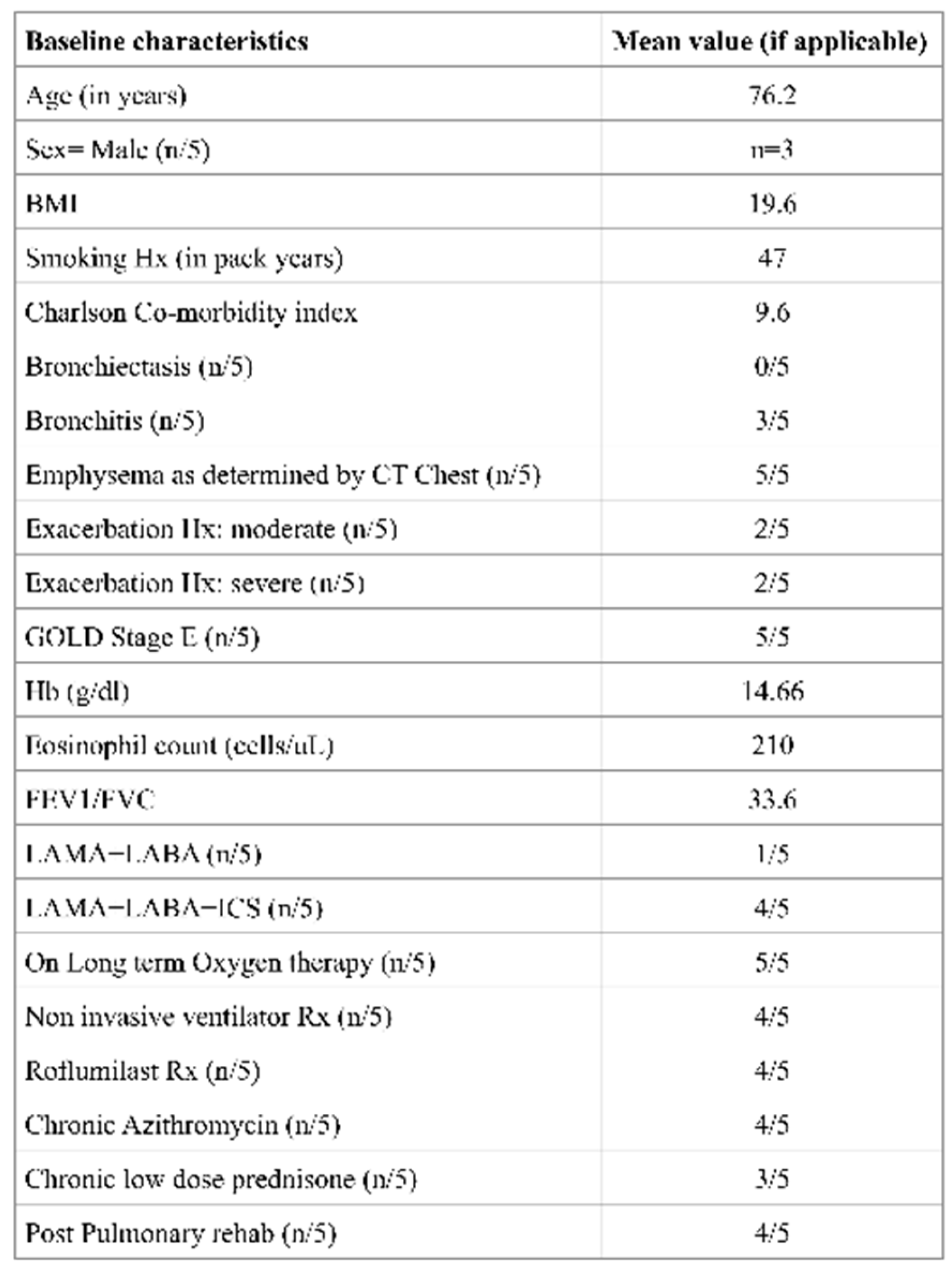
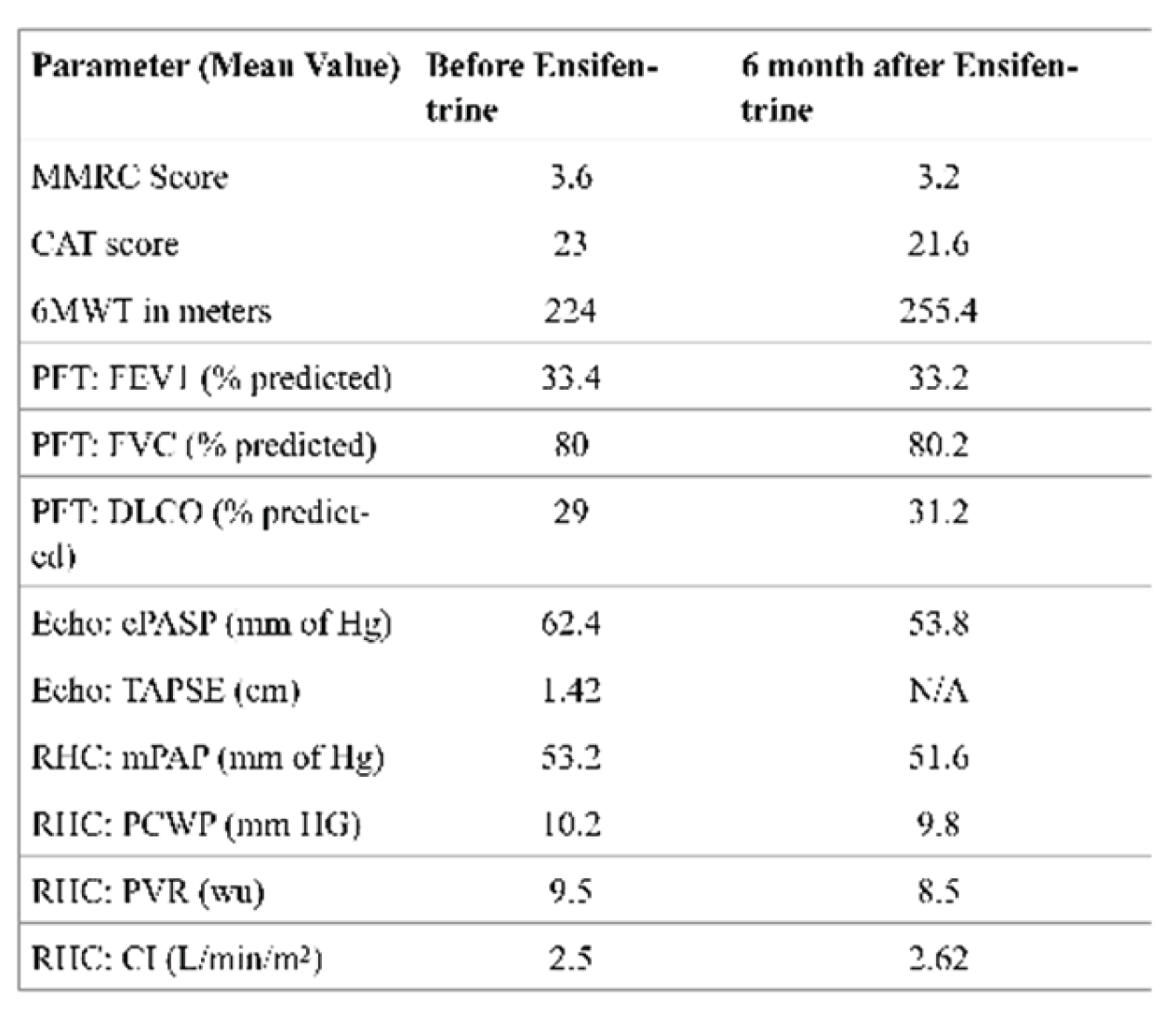
In this retrospective cohort, all five patients had very severe COPD (mean FEV₁ 33.4% predicted). Four of the five were receiving domiciliary noninvasive ventilation, and all had severe pre-capillary PH with a mean pulmonary vascular resistance (PVR) of 9.5 Wood units (WU). Echocardiography and hemodynamic data revealed evidence of RV dysfunction, with a mean tricuspid annular plane systolic excursion (TAPSE) of 1.42 cm and a cardiac index of 2.5 L/min/m², in the absence of significant coronary artery disease or HFpEF. These findings highlight the adverse remodeling of the RV that can occur in longstanding COPD-PH, resembling a form of “COPD-associated RV cardiomyopathy”. Functional impairment was profound, as demonstrated by a mean six-minute walk distance (6MWD) of only 224 m, despite optimized medical therapy and a severe emphysematous phenotype.
Traditional pulmonary vasodilators, such as endothelin receptor antagonists and phosphodiesterase-5 inhibitors (PDE5i such as sildenafil), are generally avoided in COPD due to concerns regarding ventilation-perfusion (V/Q) mismatch and hypoxemia, though they may be considered on a case-by-case basis in expert centers or within clinical trials [
4]. Previous studies of sildenafil in COPD-PH have yielded conflicting results, with consistent improvements in pulmonary hemodynamics but mixed effects on exercise capacity and oxygenation. A central concern with systemic vasodilators is their potential to exacerbate V/Q mismatch through nonselective vasodilation of poorly ventilated lung regions [
4]. By contrast, ensifentrine’s inhaled delivery may mitigate this risk by localizing its effects to better-ventilated areas of the lung.
Preclinical and Phase II studies support the mechanistic rationale for PDE3 and PDE-4 inhibition, showing dose dependent relaxation of airway constriction and anti-inflammatory effect [
8]. PDE-3 regulated cAMP and cGMPin airway smooth muscle, which mediates bronchial tone [
5]. It is also known that PDE-III inhibition leads to increased myocardial contractility by preventing cAMP breakdown and pulmonary vascular dilatation by preventing cGMP breakdown (e.g milrinone) [
9]. In our cohort, the addition of nebulized ensifentrine to standard therapy was associated with subjective improvements in dyspnea, as reflected in modified Medical Research Council (mMRC) and COPD Assessment Test (CAT) scores, although cough and sputum production remained unchanged. Importantly, all patients had a predominant severe emphysema phenotype with dyspnea as the leading symptom; none had coexistent bronchiectasis, and three had chronic bronchitic features.
Hemodynamic changes were only modest with a mean reduction in PVR by 1 WU and an increase in cardiac index of 0.1 L/min/m². These shifts may reflect a possible noise due to an interventional bias or a true therapeutic effect or, yet the consistency of improvement across multiple domains including 6MWD and diffusing capacity for carbon monoxide (DLCO) suggests biological plausibility. On average, 6MWD improved by 31 m. While modest, this gain is clinically meaningful in such a debilitated cohort, many of whom may already be functioning near their physiologic limits. Furthermore, the absence of oxygen desaturation or worsening gas exchange during the six-minute walk test reassures against exacerbation of V/Q mismatch, a common limitation of systemic vasodilator therapy.
The dual mechanism of ensifentrine provides a compelling rationale for its application in COPD-PH, atleast theoretically. PDE3 inhibition increases intracellular cyclic adenosine monophosphate (cAMP), producing smooth muscle relaxation in both the bronchial airways and pulmonary vasculature, leading to bronchodilation and mild pulmonary vasodilation without systemic hypotension. PDE4 inhibition exerts anti-inflammatory effects, potentially attenuating cytokine-mediated vascular remodeling and pulmonary arterial stiffness-key drivers of elevated PVR in Group 3 PH [
10]. Importantly, Ensifentrine’s inhaled formulation delivers the drug directly to the lungs, maximizing local efficacy while minimizing systemic side effects, including hypotension.
Our observations suggest that ensifentrine may improve pulmonary hemodynamics, functional outcomes, and right heart performance in patients with severe COPD-PH, although the extent to which these benefits derive from direct vascular effects versus improvements in airflow and inflammation remains uncertain. It is possible that enhanced bronchodilation and anti-inflammatory action with ensifentrine could have mitigated regional hypoxic pulmonary vasoconstriction resulting in improvement in pulmonary vascular resistance. Notably, this is the first report to our knowledge evaluating ensifentrine in patients with COPD-PH confirmed by right heart catheterization.
Data from the ENHANCE trials demonstrated improvements in lung function, symptom burden, and quality of life with ensifentrine in patients with moderate-to-severe COPD over 24 weeks [
5]. Our patients did not exhibit any significant improvement in FEV1. Out patients had very severe obstructive physiology and severe emphysema as a predominant phenotype compared to the trial population. Furthermore, our study was observational with very few number of subjects who likely had no reserve or salvageable expiratory airflow.
Combination therapy with ensifentrine and roflumilast also appeared to be well tolerated, without the adverse effects typically associated with PDE4 inhibition, such as weight loss, depression, or hepatotoxicity. One patient not included in the analysis experienced vivid dreams and nightmares requiring discontinuation of therapy within one week, underscoring the need for individualized monitoring. It is currently unknown whether the combination of Roflumilast and Ensifentrine could be considered and further evidence is needed for such an approach. If pursued as a part of salvage therapy for end-stage COPD, each medication should be introduced sequentially rather than simultaneously, with close monitoring for neuropsychiatric or gastrointestinal side effects as part of a shared decision-making process.
Following 6 months of ensifentrine therapy, two patients in this cohort did not tolerate Sildenafil therapy due to side effects, and three reported no clear subjective improvement. However, the absence of objective evaluation in these individuals, combined with the severity of their disease, may have limited the ability to detect meaningful clinical benefit.
Overall, while the study is constrained by its small sample size, retrospective design, and lack of a comparator group, the findings offer surprising insight that ensifentrine may result in favorable pulmonary hemodynamics. However our’s is a small observational case series with no control group. Due to a small number of patients, propensity score matching nor statistics could be implemented for a definitive conclusion. Furthermore, the findings could represent rather a noise than a signal, as many patients were excluded for various reasons and co-morbidities during the final analysis. Larger, prospective randomized trials are warranted to validate these results and determine whether ensifentrine can be integrated earlier (e.g PVR > 3 WU) into treatment algorithms for this challenging and under-treated population.
Strengths and Limitations
This study has several limitations that warrant consideration. The most notable is the very small sample size of only five patients, which limits statistical inference and substantially reduces the generalizability of our findings. The retrospective and non-randomized design, conducted within a single specialized center, introduces the potential for selection bias and further constrains external validity. In addition, the absence of an untreated or placebo comparator prevents definitive attribution of observed improvements to ensifentrine. The open-label nature of treatment may also have introduced observer or reporting bias, particularly for subjective outcomes such as dyspnea scores and patient-reported symptom assessments. Finally, while right heart catheterization remains the gold standard for hemodynamic evaluation, measurements in patients with severe obstructive lung disease may be affected by intrathoracic pressure variability, potentially influencing the precision of reported values.
Despite these limitations, this analysis also has several strengths. All patients had pre-capillary pulmonary hypertension confirmed by right heart catheterization, ensuring diagnostic accuracy. The cohort was well characterized clinically and phenotypically, with detailed functional, imaging, and hemodynamic assessments. Patients were also maintained on maximized guideline-directed COPD therapy, allowing the effects of ensifentrine to be assessed in the context of optimized standard care. Finally, the systematic collection of safety outcomes, including tolerability of combined ensifentrine and roflumilast therapy, adds practical insights for real-world clinical use.
Taken together, the study is hypothesis generating for a population in whom therapeutic options remain extremely limited and underscores the need for larger, prospective, multi-center randomized controlled trials to validate these findings.
Future Directions
The findings from this retrospective analysis highlight several important directions for future research. Foremost, larger multi-center randomized controlled trials are needed to rigorously evaluate the role of ensifentrine in patients with COPD-associated pulmonary hypertension, ideally employing co-primary endpoints that include both hemodynamic response and functional capacity. Beyond clinical outcomes, mechanistic studies using advanced imaging techniques—such as cardiac MRI, perfusion scans, or gas exchange assessments—would provide valuable insights into the drug’s potential impact on pulmonary vascular remodeling and right ventricular function. Additionally, future investigations should focus on identifying patient subgroups most likely to benefit from PDE3/4 inhibition. For example, analyses stratified by emphysema-dominant versus bronchitic phenotypes, as well as by prior responsiveness to phosphodiesterase inhibitors, may reveal clinically meaningful patterns of treatment response. Incorporating biomarker-driven approaches to stratification may further refine patient selection and optimize therapeutic benefit. Together, these avenues of research will be critical for establishing the role of ensifentrine in the management of this complex and under-treated population