Submitted:
30 December 2024
Posted:
03 January 2025
You are already at the latest version
Abstract
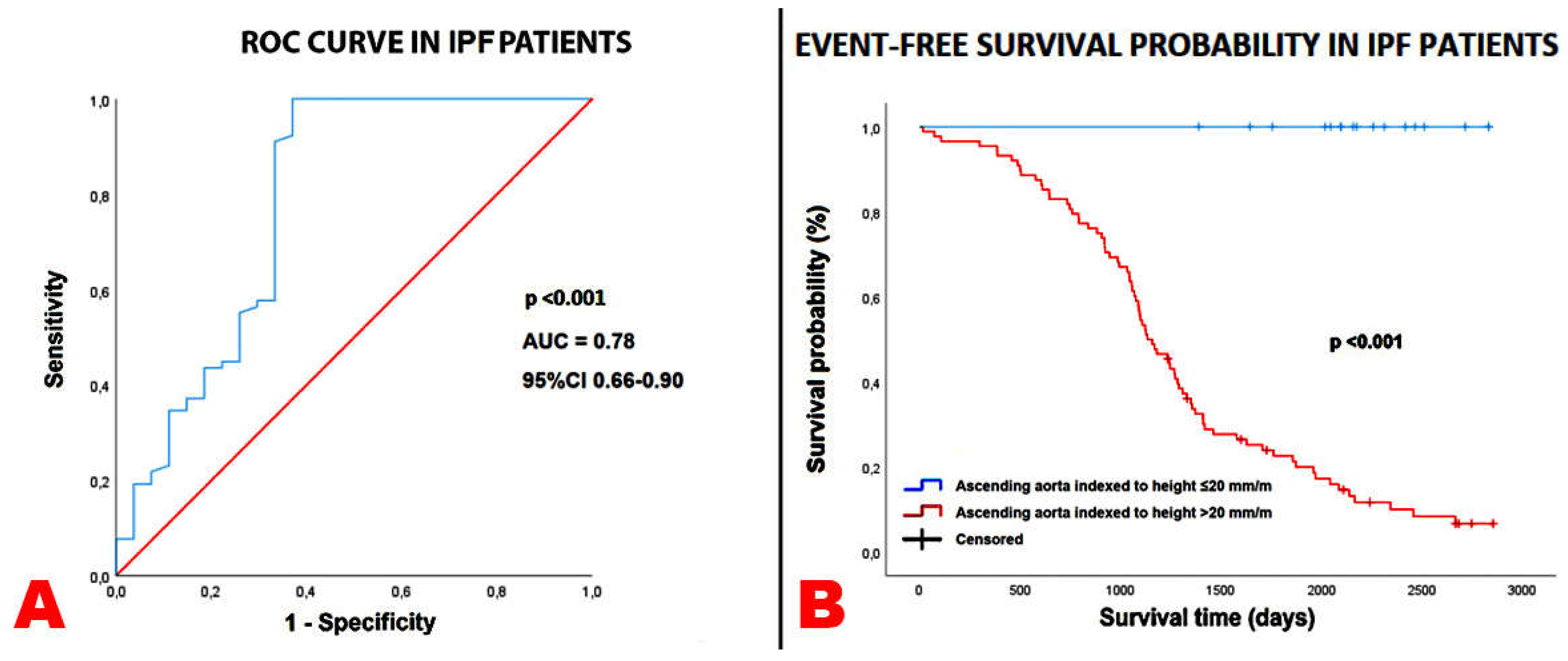
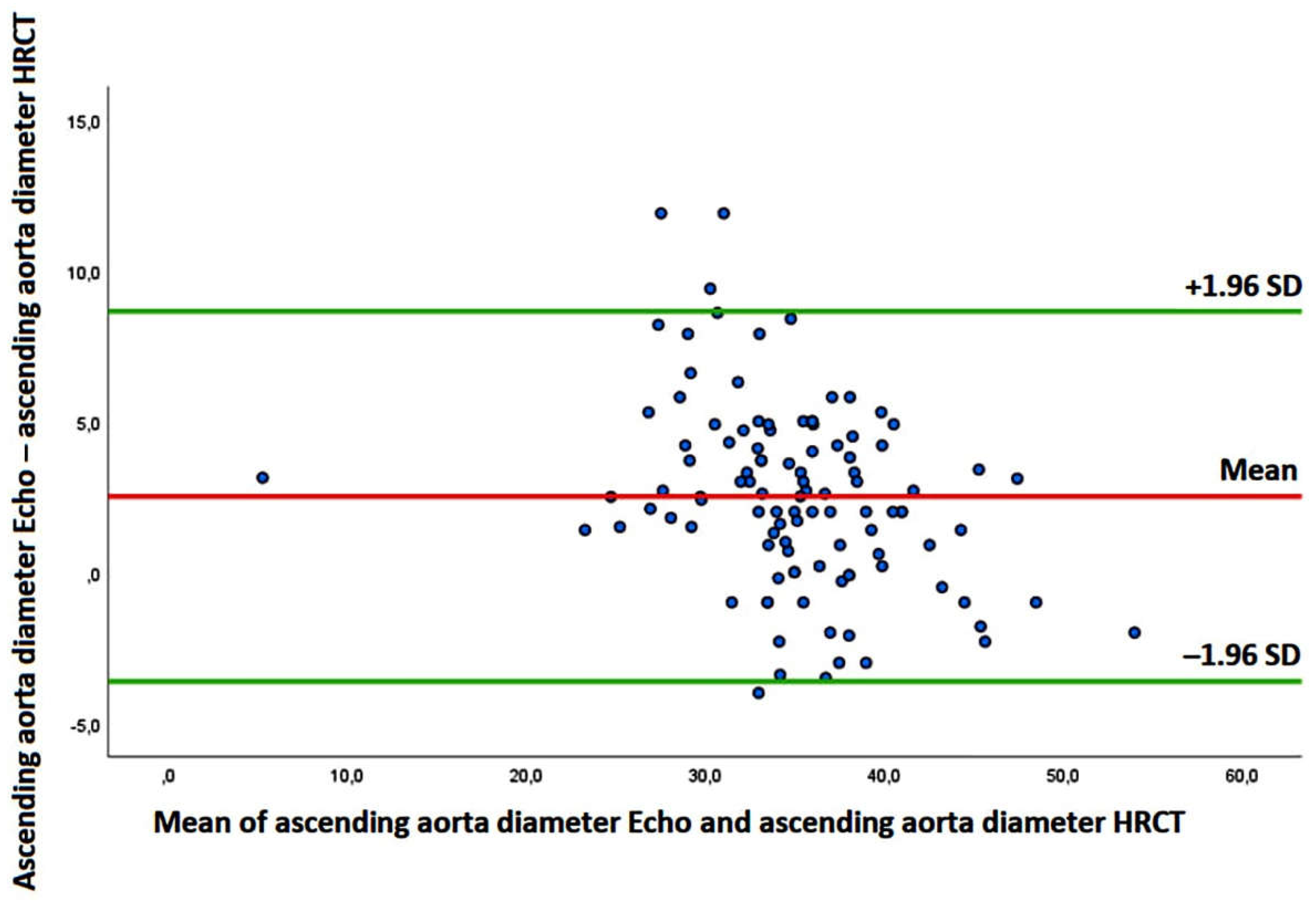
Background: No previous study was specifically focused on the ascending aorta (AA) diameter assessment in patients with idiopathic pulmonary fibrosis (IPF). The aim of this study was to investigate the prognostic role of the AA diameter in patients with mild-to-moderate IPF and to identify the main determinants of the AA dilatation. Methods: All IPF patients without severe pulmonary hypertension who underwent a multi-instrumental evaluation, comprehensive of high-resolution computed tomography (HRCT) and transthoracic echocardiography (TTE), between September 2017 and No-vember 2023, were retrospectively analyzed. The primary endpoint was the composite of “all-cause mortality or re-hospitalization for all causes”, over a medium-term follow-up. The secondary endpoint was to evaluate the independent predictors of AA dilatation. Additionally, Bland-Altman analysis was used to assess the accuracy and precision of echocardiography-derived AA diameters compared with non-ECG gated HRCT meaurements. Results: A total of 105 IPF patients and 102 age-, sex- and cardiovascular risk factors-matched controls without IPF were retrospectively evaluated. Over a follow-up of 3.9±1.9 yrs, 31 patients died and 47 were re-hospitalized. AA/height (HR 1.15, 95% CI 1.06-1.25, p < 0.001) was independently associated with the primary endpoint, whereas unindexed AA (HR 1.01, 95% CI 0.96-1.06, p = 0.83) and AA/BSA (HR 1.00, 95% CI 0.89-1.11, p = 0.39) were not. An AA/height >20 mm/m showed 100% sensitivity and 63% specificity (AUC = 0.78) for predicting the primary endpoint. C-reactive protein (OR 1.87; 95% CI 1.21-2.89, p = 0.005) and left ventricular mass index (OR 1.13, 95% CI 1.04-1.24, p = 0.006) were independently associated with an AA/height >20 mm/m in the whole study group. The Bland-Altman analysis revealed a bias of +2.51 mm (with the 95% limits of agreement ranging –3.62 to 8.65 mm) for AA estimation, suggesting a general overestimation of the AA diameter by TTE in comparison to HRCT. Conclusions: The AA dilatation is predictive of poor outcome in IPF patients without advanced lung disease, over a mid-term follow-up. The AA/height assessment may improve the prognostic risk stratification of IPF patients.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. High-Resolution Computed Tomography
2.3. Conventional Transthoracic Echocardiography
2.4. Endpoint Definition
2.5. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Instrumental Findings
3.3. Survival Analysis
3.4. Measurement Variability
4. Discussion
4.1. Main Findings of the Study
4.2. Prognostic Role of Ascending Aorta Dilatation
4.3. Pathophysiological Mechanisms of Ascending Aorta Dilatation in IPF Patients
4.4. Implications for Clinical Practice
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Nathan, S.D.; Shlobin, O.A.; Weir, N.; Ahmad, S.; Kaldjob, J.M.; Battle, E.; Sheridan, M.J.; du Bois, R.M. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011, 140, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Harari, S.; Caminati, A.; Zanobetti, A.; Schwartz, J.D.; Bertazzi, P.A.; Cesana, G.; Madotto, F. The association between air pollution and the incidence of idiopathic pulmonary fibrosis in Northern Italy. Eur. Respir J. 2018, 51, 1700397. [Google Scholar] [CrossRef] [PubMed]
- Luppi, F.; Kalluri, M.; Faverio, P.; Kreuter, M.; Ferrara, G. Idiopathic pulmonary fibrosis beyond the lung: Understanding disease mechanisms to improve diagnosis and management. Respir. Res. 2021, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Tonelli, R.; Murray, M.; Samarelli, A.V.; Spagnolo, P. Environmental Causes of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2023, 24, 16481. [Google Scholar] [CrossRef]
- Raghu, G.; Amatto, V.C.; Behr, J.; Stowasser, S. Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur. Respir. J. 2015, 46, 1113–1130. [Google Scholar] [CrossRef]
- Kärkkäinen, M.; Kettunen, H.P.; Nurmi, H.; Selander, T.; Purokivi, M.; Kaarteenaho, R. Effect of smoking and comorbidities on survival in idiopathic pulmonary fibrosis. Respir. Res. 2017, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Caminati, A.; Lonati, C.; Cassandro, R.; Elia, D.; Pelosi, G.; Torre, O.; Zompatori, M.; Uslenghi, E.; Harari, S. Comorbidities in idiopathic pulmonary fibrosis: An underestimated issue. Eur. Respir. Rev. 2019, 28, 190044. [Google Scholar] [CrossRef]
- Nathan, S.D.; Basavaraj, A.; Reichner, C.; Shlobin, O.A.; Ahmad, S.; Kiernan, J.; Burton, N.; Barnett, S.D. Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir. Med. 2010, 104, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Kizer, J.R.; Zisman, D.A.; Blumenthal, N.P.; Kotloff, R.M.; Kimmel, S.E.; Strieter, R.M.; Arcasoy, S.M.; Ferrari, V.A.; Hansen-Flaschen, J. Association between pulmonary fibrosis and coronary artery disease. Arch. Intern. Med. 2004, 164, 551–556. [Google Scholar] [CrossRef]
- Gardin, J.M.; Arnold, A.M.; Polak, J.; Jackson, S.; Smith, V.; Gottdiener, J. Usefulness of aortic root dimension in persons > or = 65 years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the Cardiovascular Health Study). Am. J. Cardiol. 2006, 97, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Chien, K.L.; Hsu, H.C.; Su, T.C.; Chen, M.F.; Lee, Y.T. Aortic root dimension as an independent predictor for all-cause death in adults . Echocardiography 2010, 27, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Facchetti, R.; Bombelli, M.; Re, A.; Cairoa, M.; Sala, C.; Tadic, M.; Grassi, G.; Mancia, G. Aortic root diameter and risk of cardiovascular events in a general population: Data from the PAMELA study. J. Hypertens. 2014, 32, 1879–1887. [Google Scholar] [CrossRef]
- Kamimura, D.; Suzuki, T.; Musani, S.K.; Hall, M.E.; Samdarshi, T.E.; Correa, A.; Fox, E.R. Increased Proximal Aortic Diameter is Associated With Risk of Cardiovascular Events and All-Cause Mortality in Blacks The Jackson Heart Study. J. Am. Heart Assoc. 2017, 6, e005005. [Google Scholar] [CrossRef] [PubMed]
- Canciello, G.; Mancusi, C.; Losi, M.A.; Izzo, R.; Trimarco, B.; de Simone, G.; De Luca, N. Aortic Root Dilatation Is Associated With Incident Cardiovascular Events in a Population of Treated Hypertensive Patients: The Campania Salute Network. Am. J. Hypertens. 2018, 31, 1317–1323. [Google Scholar] [CrossRef]
- Leone, D.; Airale, L.; Bernardi, S.; Mingrone, G.; Astarita, A.; Cesareo, M.; Sabia, L.; Avenatti, E.; Tosello, F.; Bruno, G.; et al. Prognostic role of the ascending aorta dilatation in patients with arterial hypertension. J. Hypertens. 2021, 39, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Caminati, A.; Lipsi, R.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Harari, S. Early left atrial dysfunction in idiopathic pulmonary fibrosis patients without chronic right heart failure. Int. J. Cardiovasc. Imaging. 2020, 36, 1711–1723. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Caminati, A.; Lipsi, R.; Lombardo, M.; Harari, S. Association between C-reactive protein and carotid plaque in mild-to-moderate idiopathic pulmonary fibrosis. Intern. Emerg. Med. 2021, 16, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Caminati, A.; Nicolosi, G.L.; Lombardo, M.; Harari, S. Incremental prognostic value of arterial elastance in mild-to-moderate idiopathic pulmonary fibrosis. Int. J. Cardiovasc. Imaging. 2022, 38, 1473–1485. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Caminati, A.; Re, M.; Elia, D.; Trevisan, R.; Granato, A.; Zompatori, M.; Lombardo, M.; Harari, S. Prognostic role of CHA2DS2-VASc score for mortality risk assessment in non-advanced idiopathic pulmonary fibrosis: A preliminary observation. Intern. Emerg. Med. 2023, 18, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Ohnesorge, B.; Flohr, T.; Fischbach, R.; Kopp, A.F.; Knez, A.; Schröder, S.; Schöpf, U.J.; Crispin, A.; Klotz, E.; Reiser, M.F.; et al. Reproducibility of coronary calcium quantification in repeat examinations with retrospectively ECG-gated multisection spiral CT. Eur. Radiol. 2002, 12, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging. 2019, 12, e009047. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O'Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Evangelista, A.; Sitges, M.; Jondeau, G.; Nijveldt, R.; Pepi, M.; Cuellar, H.; Pontone, G.; Bossone, E.; Groenink, M.; Dweck, M.R.; et al. Multimodality imaging in thoracic aortic diseases: A clinical consensus statement from the European Association of Cardiovascular Imaging and the European Society of Cardiology working group on aorta and peripheral vascular diseases. Eur. Heart J. Cardiovasc. Imaging. 2023, 24, e65–e85. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef]
- Zafar, M.A.; Li, Y.; Rizzo, J.A.; Charilaou, P.; Saeyeldin, A.; Velasquez, C.A.; Mansour, A.M.; Bin Mahmood, S.U.; Ma, W.G.; Brownstein, A.J.; et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J. Thorac. Cardiovasc. Surg. 2018, 155, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Caminati, A.; Grasso, E.; Colleoni, M.; Nicolosi, G.L.; Lombardo, M.; Harari, S. TAPSE/SPAP ratio stratifies mortality risk in mild-to-moderate idiopathic pulmonary fibrosis. Int. J. Tuberc. Lung Dis. 2024, 28, 183–188. [Google Scholar] [CrossRef]
- Dalleywater, W.; Powell, H.A.; Hubbard, R.B.; Navaratnam, V. Risk factors for cardiovascular disease in people with idiopathic pulmonary fibrosis: A population-based study. Chest. 2015, 147, 150–156. [Google Scholar] [CrossRef]
- Okamoto, M.; Shipley, M.J.; Wilkinson, I.B.; McEniery, C.M.; Valencia-Hernández, C.A.; Singh-Manoux, A.; Kivimaki, M.; Brunner, E.J. Does Poorer Pulmonary Function Accelerate Arterial Stiffening?: A Cohort Study With Repeated Measurements of Carotid-Femoral Pulse Wave Velocity. Hypertension. 2019, 74, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Driussi, C.; Bettio, M.; Ferrara, F.; D'Andrea, A.; Bossone, E. Aortic root dimensions and stiffness in healthy subjects. Am. J. Cardiol. 2013, 112, 1224–1229. [Google Scholar] [CrossRef]
- Milan, A.; Tosello, F.; Caserta, M.; Naso, D.; Puglisi, E.; Magnino, C.; Comoglio, C.; Rabbia, F.; Mulatero, P.; Veglio, F. Aortic size index enlargement is associated with central hemodynamics in essential hypertension. Hypertens. Res. 2011, 34, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, W.; Li, Q.; Hong, H. The positive correlation between brachial-ankle pulse wave velocity and aortic diameter in Chinese patients with diabetes. J. Clin. Hypertens. (Greenwich). 2022, 24, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, A.; Zhou, J.; Zhang, C.; Chen, M. Genetic association of circulating C-reactive protein levels with idiopathic pulmonary fibrosis: A two-sample Mendelian randomization study. Respir. Res. 2023, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Syed, M.B.J.; Fletcher, A.J.; Dweck, M.R.; Forsythe, R.; Newby, D.E. Imaging aortic wall inflammation. Trends Cardiovasc. Med. 2019, 29, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Totaro, S.; Rabbia, F.; Milan, A.; Urbina, E.M.; Veglio, F. Aortic root dilatation in the children and young adults: Prevalence, determinants, and association with target organ damage. J. Am. Soc. Hypertens. 2016, 10, 782–789. [Google Scholar] [CrossRef]
- Milan, A.; Tosello, F.; Naso, D.; Avenatti, E.; Leone, D.; Magnino, C.; Veglio, F. Ascending aortic dilatation, arterial stiffness and cardiac organ damage in essential hypertension. J. Hypertens. 2013, 31, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, C.R.; Pham, M.H.C.; Sigvardsen, P.E.; Kühl, J.T.; Sørgaard, M.; Taudorf, M.; Fuchs, A.; Nordestgaard, B.G.; Køber, L.V.; Kofoed, K.F. Aortic enlargement and coronary artery calcification in a general population cohort. Eur. Heart J. Cardiovasc. Imaging. 2022, 23, 855–862. [Google Scholar] [CrossRef]
- Kurata, A.; Kawakami, T.; Sato, J.; Sakamoto, A.; Muramatsu, T.; Nakabayashi, K. Aortic aneurysms in systemic lupus erythematosus: A meta-analysis of 35 cases in the literature and two different pathogeneses. Cardiovasc. Pathol. 2011, 20, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Takagi, A.; Miyata, T.; Takayama, Y. Aortic aneurysms in patients with autoimmune disorders treated with corticosteroids. Eur. J. Vasc. Endovasc. Surg. 1995, 10, 366–369. [Google Scholar] [CrossRef]
- Chen, S.W.; Kuo, C.F.; Huang, Y.T.; Lin, W.T.; Chien-Chia Wu, V.; Chou, A.H.; Lin, P.J.; Chang, S.H.; Chu, P.H. Association of Family History With Incidence and Outcomes of Aortic Dissection. J. Am. Coll. Cardiol. 2020, 76, 1181–1192. [Google Scholar] [CrossRef]
- Chen, S.W.; Chan, Y.H.; Lin, C.P.; Wu, V.C.; Cheng, Y.T.; Chen, D.Y.; Chang, S.H.; Hung, K.C.; Chu, P.H.; Chou, A.H. Association of Long-term Use of Antihypertensive Medications With Late Outcomes Among Patients With Aortic Dissection. JAMA Netw. Open. 2021, 4, e210469. [Google Scholar] [CrossRef] [PubMed]
- Pramana, K.A.A.P.; Pintaningrum, Y.; Rahmat, B. The effects of statin therapy on aneurysm size, growth rate, and matrix metalloproteinases-9 levels in patients with aortic aneurysm: A systematic review and meta-analysis. Egypt. Heart J. 2023, 75, 88. [Google Scholar] [CrossRef]
- van Cleemput, J.; Sonaglioni, A.; Wuyts, W.A.; Bengus, M.; Stauffer, J.L.; Harari, S. Idiopathic Pulmonary Fibrosis for Cardiologists: Differential Diagnosis, Cardiovascular Comorbidities, and Patient Management. Adv. Ther. 2019, 36, 298–317. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, M.; Paini, A.; Bertacchini, F.; Stassaldi, D.; Aggiusti, C.; Agabiti Rosei, C.; Bassetti, D.; Agabiti-Rosei, E.; Muiesan, M.L. Changes in left ventricular geometry during antihypertensive treatment. Pharmacol. Res. 2018, 134, 193–199. [Google Scholar] [CrossRef]

| CLINICAL VARIABLES | IPF patients (n = 105) |
Controls (n = 102) |
p-Value |
|---|---|---|---|
| Demographics and anthropometrics | |||
| Age (yrs) | 76.3 ± 6.8 | 76.4 ± 11.4 | 0.94 |
| Male sex (%) | 82 (78.1) | 72 (70.6) | 0.22 |
| Heigth (cm) | 166.0 ± 7.7 | 164.5 ± 9.3 | 0.21 |
| Weight (Kg) | 73.7 ± 13.3 | 70.7 ± 13.6 | 0.11 |
| BSA (m2) | 1.86 ± 0.18 | 1.85 ± 0.21 | 0.71 |
| BMI (Kg/m2) | 26.6 ± 3.5 | 26.3 ± 4.0 | 0.56 |
| Yrs from IPF diagnosis | 3.7 ± 1.9 | / | |
| Cardiovascular risk factors | |||
| Smoking history (%) | 86 (81.9) | 74 (72.5) | 0.11 |
| Hypertension (%) | 55 (52.4) | 60 (58.8) | 0.35 |
| Type 2 diabetes mellitus (%) | 34 (32.4) | 30 (29.4) | 0.64 |
| Dyslipidemia (%) | 52 (49.5) | 40 (39.2) | 0.13 |
| Atherosclerotic disease burden | |||
| ≥50% carotid artery stenosis (%) | 34 (32.4) | 19 (18.6) | 0.02 |
| Coronary artery calcification on HRCT (%) | 41 (39.0) | 25 (24.5) | 0.02 |
| Lower extremity peripheral artery disease (%) | 12 (11.4) | 4 (3.9) | 0.04 |
| Polidistrectual vasculopathy (%) | 24 (22.8) | 8 (7.8) | 0.003 |
| History of cardiovascular and/or cerebrovascular events | |||
| History of CAD (previous PCI/CABG) (%) | 23 (21.9) | 17 (16.7) | 0.34 |
| Previous stroke/TIA (%) | 7 (6.7) | 12 (11.8) | 0.20 |
| Non-cardiovascular comorbidities | |||
| Cancers (%) | 19 (18.1) | 13 (12.7) | 0.29 |
| COPD (%) | 19 (18.1) | 12 (11.8) | 0.20 |
| OSAS (%) | 9 (8.6) | 6 (5.9) | 0.45 |
| GERD (%) | 24 (22.8) | 15 (14.7) | 0.13 |
| Hypothyroidism (%) | 11 (10.5) | 6 (5.9) | 0.23 |
| Mixed anxiety–depressive disorder (%) | 9 (8.6) | 7 (6.9) | 0.64 |
| Blood tests | |||
| Serum hemoglobin (g/dl) | 14.0 ± 1.7 | 13.7 ± 1.9 | 0.23 |
| eGFR (ml/min/m2) | 80.7 ± 17.0 | 78.0 ± 17.9 | 0.27 |
| Serum glucose (mg/dl) | 115.5 ± 20.6 | 110.4 ± 33.4 | 0.19 |
| Serum NT-proBNP (pg/ml) | 404.2 ± 1247.2 | 631.1 ± 1385.0 | 0.22 |
| Serum CRP (mg/dl) | 1.7 ± 2.7 | 0.9 ± 2.1 | 0.02 |
| Serum LDL cholesterol (mg/dl) | 115.1 ± 33.0 | 107.6 ± 36.4 | 0.12 |
| Cardioprotective treatment | |||
| Antiplatelets (%) | 45 (42.8) | 50 (49.0) | 0.37 |
| Anticoagulants (%) | 12 (11.4) | 10 (9.8) | 0.70 |
| ACEi-ARBs (%) | 39 (37.1) | 48 (47.0) | 0.15 |
| Calcium channel blockers (%) | 21 (20.0) | 30 (29.4) | 0.12 |
| Beta blockers (%) | 25 (23.8) | 42 (41.2) | 0.007 |
| Diuretics (%) | 28 (26.7) | 24 (23.5) | 0.60 |
| Statins (%) | 22 (20.9) | 35 (34.3) | 0.03 |
| Antidiabetic drugs (%) | 28 (26.7) | 25 (24.5) | 0.72 |
| Proton pump inhibitors (%) | 22 (20.9) | 13 (12.7) | 0.11 |
| Respiratory treatment | |||
| Oxygen therapy (%) | 55 (52.4) | / | |
| Oral corticosteroids (%) | 38 (36.2) | / | |
| Inhalation therapy (%) | 11 (10.5) | / | |
| Pirfenidone (%) | 43 (40.9) | / | |
| Nintedanib (%) | 55 (52.4) | / | |
| INSTRUMENTAL PARAMETERS | IPF patients (n = 105) |
Controls (n = 102) |
p-Value |
|---|---|---|---|
| Radiological findings | |||
| Definite UIP (%) | 63 (60.0) | / | / |
| Probable UIP (%) | 26 (24.8) | / | / |
| Indeterminate pattern (%) | 16 (15.2) | / | / |
| CAC score (HU) | 698.9 ± 879.8 | / | / |
| Spirometry parameters | |||
| FVC (l) | 2.6 ± 0.6 | / | / |
| FVC (%) | 77.6 ± 16.7 | / | / |
| FEV1 (l) | 2.1 ± 0.5 | / | / |
| FEV1 (%) | 82.4 ± 16.1 | / | / |
| FEV1/FVC ratio | 0.8 ± 0.1 | / | / |
| TLC (l) | 4.8 ± 1.1 | / | / |
| TLC (%) | 76.4 ± 16.7 | / | / |
| DLCO (ml/min/mmHg) | 11.4 ± 4.0 | / | / |
| DLCO (%) | 47.9 ± 16.0 | / | / |
| Restrictive pattern (%) | 70 (66.7) | / | / |
| ΔSaO2 (%) | 6.6 ± 4.2 | / | / |
| 6MWT (m) | 399.3 ± 110.6 | / | / |
| ECG variables | |||
| Heart rate (bpm) | 74.9 ± 15.0 | 74.1 ± 12.2 | 0.67 |
| AF (%) | 12 (11.4) | 10 (9.8) | 0.70 |
| Intraventricular delay (%) | 21 (20.0) | 25 (24.5) | 0.43 |
| EchoDoppler parameters | |||
| LVEDD (mm) | 46.5 ± 5.7 | 47.0 ± 6.2 | 0.55 |
| RWT | 0.43 ± 0.06 | 0.43 ± 0.07 | >0.99 |
| LVMi (g/m2) | 97.5 ± 23.3 | 102.6 ± 29.2 | 0.16 |
| Normal LV geometric pattern (%) | 36 (34.3) | 30 (29.4) | 0.45 |
| LV concentric remodeling (%) | 48 (45.7) | 40 (39.2) | 0.34 |
| LV concentric hypertrophy (%) | 10 (9.5) | 16 (15.7) | 0.18 |
| LV eccentric hypertrophy (%) | 11 (10.5) | 16 (15.7) | 0.26 |
| LVEDVi (ml/m2) | 40.3 ± 11.8 | 38.7 ± 13.5 | 0.36 |
| LVESVi (ml/m2) | 15.8 ± 8.2 | 14.7 ± 10.3 | 0.39 |
| LVEF (%) | 61.9 ± 9.1 | 63.8 ± 9.4 | 0.14 |
| E/A ratio | 0.78 ± 0.18 | 0.75 ± 0.44 | 0.52 |
| E/average e’ ratio | 14.0 ± 4.5 | 11.9 ± 4.9 | 0.001 |
| LAVi (ml/m2) | 33.6 ± 10.9 | 34.1 ± 13.7 | 0.77 |
| More than mild MR (%) | 12 (11.4) | 11 (10.8) | 0.88 |
| More than mild AR (%) | 11 (10.5) | 8 (7.8) | 0.51 |
| More than mild TR (%) | 24 (22.8) | 7 (6.9) | 0.001 |
| RVIT (mm) | 33.1 ± 6.6 | 28.1 ± 4.3 | <0.001 |
| RV/LV basal diameter ratio | 0.77 ± 0.23 | 0.70 ± 0.19 | 0.02 |
| TAPSE (mm) | 22.0 ± 4.7 | 22.9 ± 3.7 | 0.13 |
| TRV (m/sec) | 3.3 ± 2.7 | 2.6 ± 0.3 | 0.009 |
| IVC (mm) | 19.7 ± 4.8 | 17.9 ± 3.8 | 0.003 |
| sPAP (mmHg) | 42.0 ± 13.3 | 27.7 ± 6.3 | <0.001 |
| TAPSE/sPAP (mm/mmHg) | 0.57 ± 0.24 | 0.86 ± 0.22 | <0.001 |
| Unindexed aortic root (mm) | 36.4 ± 3.8 | 34.9 ± 4.1 | 0.007 |
| Aortic root indexed to BSA (mm/m2) | 19.6 ± 2.2 | 19.3 ± 2.4 | 0.35 |
| Aortic root indexed to height (mm/m) | 22.0 ± 2.3 | 21.2 ± 2.1 | 0.009 |
| Unindexed ascending aorta (mm) | 36.6 ± 4.9 | 35.0 ± 3.9 | 0.01 |
| Ascending aorta indexed to BSA (mm/m2) | 19.8 ± 3.0 | 19.4 ± 2.7 | 0.31 |
| Ascending aorta indexed to height (mm/m) | 22.1 ± 2.9 | 21.3 ± 2.4 | 0.03 |
| UNIVARIATE COX REGRESSION ANALYSIS |
MULTIVARIATE COX REGRESSION ANALYSIS | |||||
|---|---|---|---|---|---|---|
| VARIABLES | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age (yrs) | 1.02 | 0.98-1.05 | 0.32 | |||
| Male sex | 1.33 | 0.77-2.31 | 0.31 | |||
| Smoking | 1.15 | 0.65-2.02 | 0.64 | |||
| CRP (mg/dl) | 1.12 | 1.05-1.20 | <0.001 | 1.09 | 1.01-1.18 | 0.03 |
| FVC (%) | 0.98 | 0.96-0.99 | 0.002 | 0.98 | 0.97-0.99 | 0.02 |
| LVEF (%) | 0.97 | 0.94-0.99 | 0.03 | 0.98 | 0.95-1.00 | 0.11 |
| TAPSE/sPAP ratio (mm/mmHg) | 0.10 | 0.03-0.34 | <0.001 | 0.23 | 0.07-0.76 | 0.02 |
| Unindexed ascending aorta diameter | 1.01 | 0.96-1.06 | 0.83 | |||
| Ascending aorta diameter indexed to BSA (mm/m2) | 1.00 | 0.89-1.11 | 0.39 | |||
| Ascending aorta diameter indexed to height (mm/m) | 1.18 | 1.09-1.27 | <0.001 | 1.15 | 1.06-1.25 | <0.001 |
| CAC score (HU) | 1.00 | 0.97-1.03 | 0.98 | |||
| Beta blocker treatment | 0.79 | 0.50-1.26 | 0.32 | |||
| UNIVARIATE LOGISTIC. REGRESSION ANALYSIS |
MULTIVARIATE LOGISTIC REGRESSION ANALYSIS |
|||||
|---|---|---|---|---|---|---|
| VARIABLES | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Age (yrs) | 1.03 | 0.95-1.11 | 0.43 | |||
| Male sex | 1.62 | 0.51-5.19 | 0.42 | |||
| BSA (m2) | 1.66 | 0.10-30.5 | 0.73 | |||
| Hypertension | 1.71 | 0.59-4.91 | 0.32 | |||
| Smoking | 2.20 | 0.67-7.23 | 0.19 | |||
| CRP (mg/dl) x 0.1 U increase | 2.00 | 1.30-3.06 | 0.001 | 1.87 | 1.21-2.89 | 0.005 |
| FVC (%) | 0.96 | 0.93-0.99 | 0.03 | 0.98 | 0.93-1.03 | 0.36 |
| LVMi (g/m2) | 1.08 | 1.04-1.13 | <0.001 | 1.13 | 1.04-1.24 | 0.006 |
| CAC score (HU) | 1.02 | 0.95-1.09 | 0.52 | |||
| Oral corticosteroids | 1.77 | 0.53-5.89 | 0.35 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





