Introduction
Metastatic prostate cancer (mPC) is an aggressive disease with a median overall survival ranging approximately between 18 and 36 months [
1]. Metastatic castrate resistant prostate (mCRPC) cancer is the most aggressive form, where patients develop resistance to androgen deprivation therapy [
2]. The heterogeneity in outcomes for mCRPC is suggested to be due to the underlying molecular genetic features. The most essential genomic mutations are alterations in DNA damage repair genes, including those involved in DNA homologous recombination repair (HRR), found in up to 30% of mPC. The most common HRR alterations in mCRPC are found in BRCA2, seen in approximately 3-15% of patients [
3]. BRCA2-mutated prostate cancers have a more aggressive course with a poor response to chemotherapy and hormonal agents; however, this mutation predicts response to poly ADP-ribose polymerase (PARP) inhibitors [
4,
5]. While BRCA2-mutated prostate cancer is recognized for its aggressive clinical course, paraneoplastic syndromes and thrombotic microangiopathy (TMA) have not been well described in this subset. Here we report a rare case of BRCA2-mutated metastatic prostate cancer complicated by the sequential development of SIADH and TMA, highlighting an unusual paraneoplastic scenario and a fatal course.
Case Presentation
A 72-year-old man with hypertension presented to the hospital with altered sensorium. He was drowsy with an otherwise unremarkable physical examination, including the absence of any focal neurological deficits. His blood pressure was 130/78 mmHg, heart rate 80/min, temperature 37.5 °C, and SPO2 97% on room air. The results of laboratory investigations are shown in
Table 1.
Computed tomography (CT) of the head did not show any abnormality. CT of the abdomen and pelvis with contrast revealed an enlarged prostate, retroperitoneal lymphadenopathy, and multiple osteoblastic bone lesions suggestive of metastatic prostate cancer. The prostate-specific antigen (PSA) was markedly elevated at 521 ng/mL. Urology was consulted, and a prostate biopsy was done, which revealed acinar adenocarcinoma with a Gleason score of 9. The cancer was high-risk, with extra prostatic extension and perineural invasion. Bone scan confirmed widespread metastatic lesions in the axial skeleton. Given his hypotonic hyponatremia in the setting of apparent euvolemia, an inappropriately concentrated urine, normal urine sodium, and absence of other factors that may cause hyponatremia, a diagnosis of SIADH was made.
The patient was treated with fluid restriction, furosemide, and a 3% NaCl solution, resulting in a gradual increase in serum sodium to 135 mEq/L. Triplet therapy consisting of docetaxel, androgen deprivation therapy (bicalutamide followed by Leuprolide), and darolutamide was started, and the patient was discharged in stable condition for outpatient follow-up. He went into remission after six cycles of docetaxel with normalization of PSA to 2.95 ng/mL, and he was continued on leuprolide and darolutamide.
After six months of chemotherapy, the patient presented with generalized weakness and hematuria. The results of laboratory investigations on this admission are shown in
Table 2.
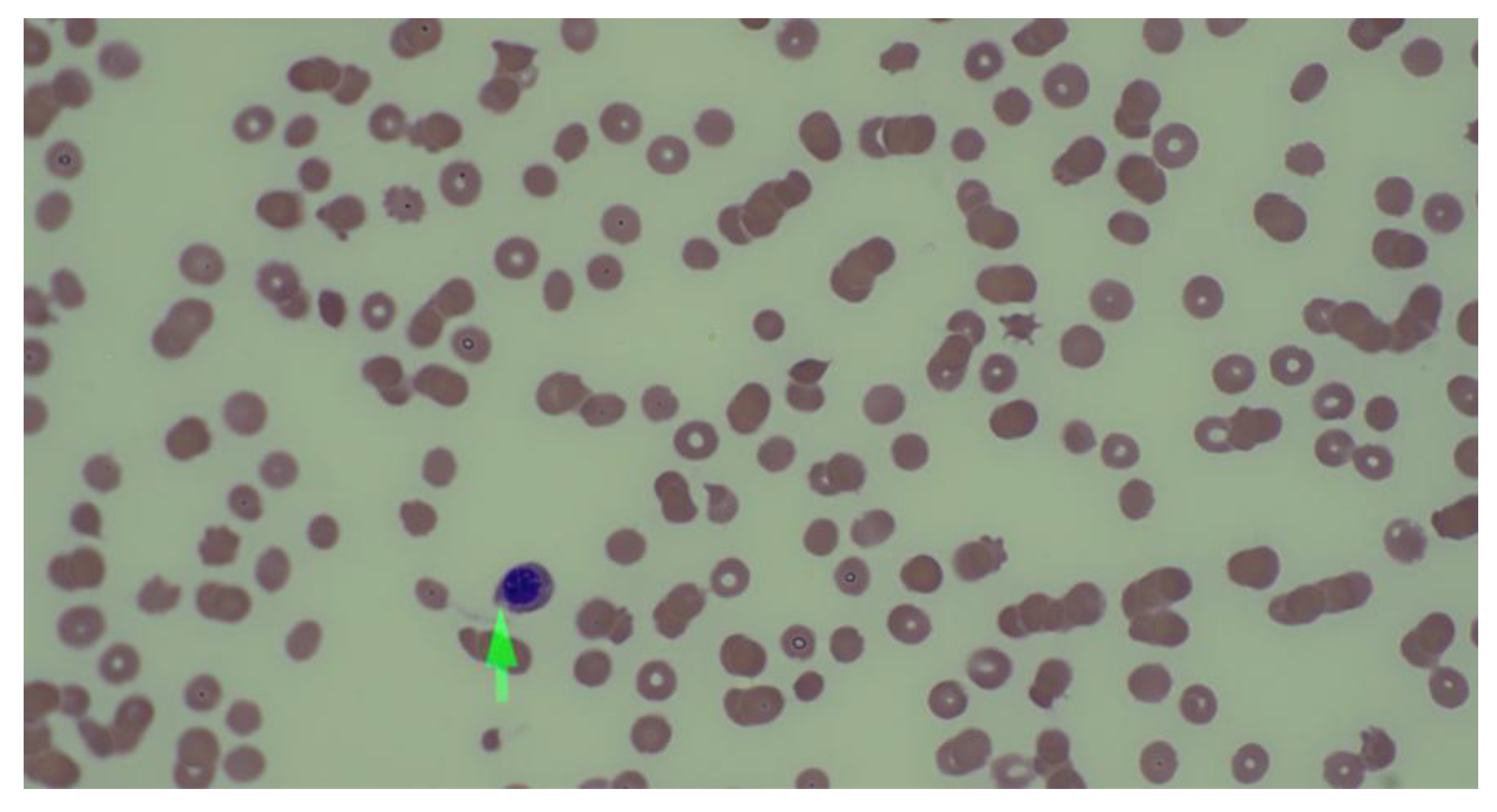
The PSA of 1,332 ng/ml was strongly suggestive of disease progression. The peripheral smear revealed schistocytes, nucleated RBCs, and immature granulocytes, suggesting TMA (
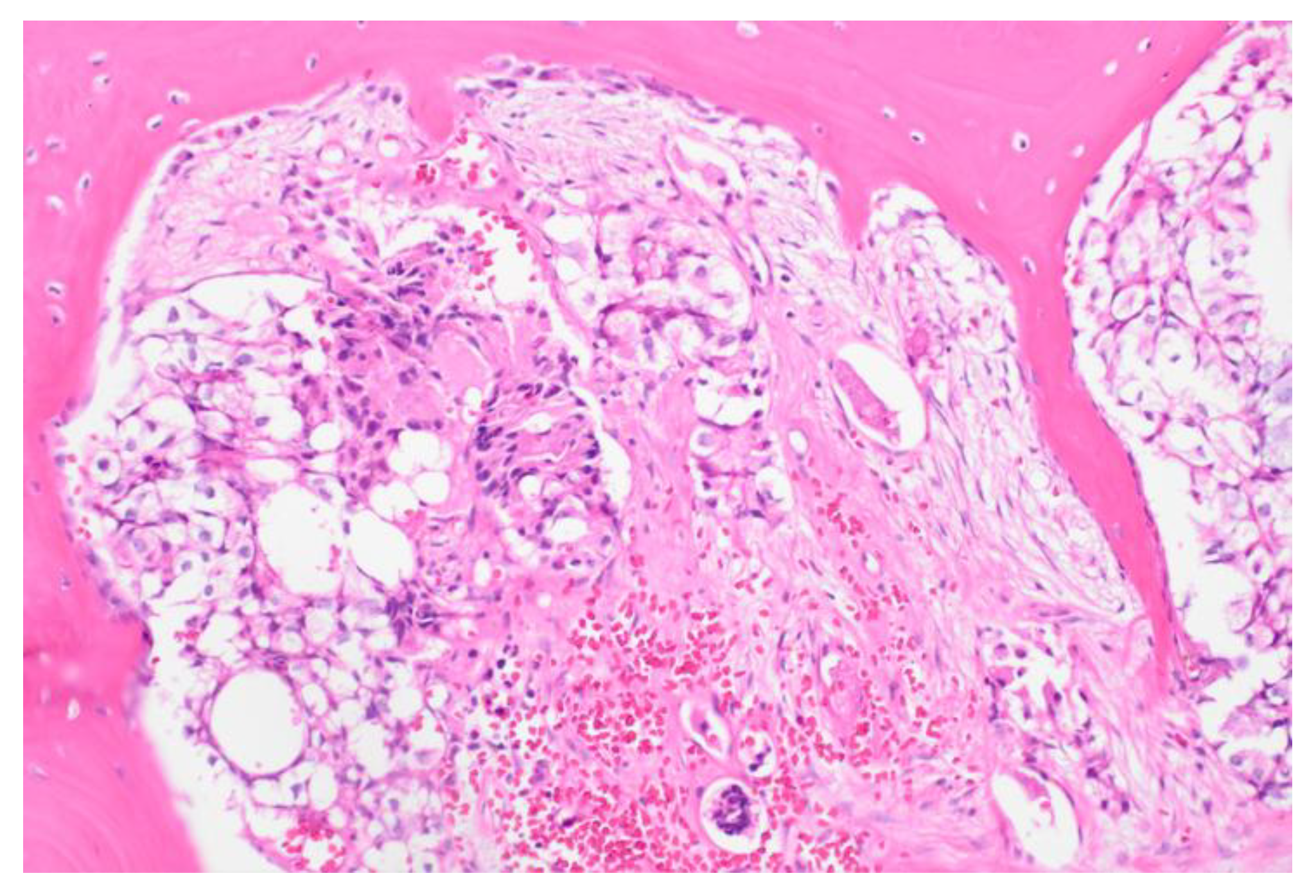
Figure 1). A bone marrow biopsy confirmed metastatic prostate adenocarcinoma and revealed a BRCA2 mutation (p.(trp1692MetfsTer3) (
Figure 2). The patient’s PLASMIC score was 4, and ADAMTS13 activity was normal (81%), ruling out thrombotic thrombocytopenic purpura. The patient was treated with five cycles of docetaxel/carboplatin along with blood product support. Hyponatremia was managed with fluid restriction. The patient showed marked clinical and laboratory improvement with normalization of hematological abnormalities, liver enzymes, renal functions, and hyponatremia. The patient was discharged on a combination of Olaparib, abiraterone, and leuprolide. Two months later, the patient relapsed and died.
Discussion
Paraneoplastic syndromes are rare in prostate cancer. SIADH in prostate cancer has been reported as case reports, with some reports showing an association with poorly differentiated or small cell histology resulting from ectopic secretion of ADH (vasopressin) from tumor cells. SIADH may be the presenting manifestation of cancer or develop during disease progression. Water restriction is the standard initial treatment, though the primary treatment lies in the control of the underlying cancer [
6,
7,
8]. Abe et al. reported SIADH and humoral hypercalcemia of malignancy in a patient with prostate cancer and pulmonary metastases [
8]. The patient was treated with water restriction, hypertonic saline, tolvaptan, and radiation to the prostate due to local symptoms. The serum sodium improved from 114 mEq/L at presentation to 135 mEq/L at discharge [
8]. In our patient, SIADH was the initial presentation of cancer and developed at the time of progression. Management with water restriction and treatment of the underlying cancer led to significant improvement in the patient’s clinical condition and electrolyte balance.
TMA refers to disorders characterized by microangiopathic hemolytic anemia, thrombocytopenia, and organ dysfunction due to thrombus formation affecting small or larger vessels. The most recognized forms of TMA are thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). Cancer-associated TMA has been reported to range from 6 to 15% [
9]. The exact mechanism by which solid tumors induce TMA is complex and multifactorial. The clinical spectrum may vary widely from asymptomatic abnormal laboratory tests to acute, severe, potentially life-threatening forms due to massive microvascular occlusion. Patients with TMA often present with fatigue, pallor, jaundice, easy bruising, bleeding complications, and acute kidney injury. The diagnosis typically involves a combination of anemia, thrombocytopenia, a peripheral blood smear showing schistocytes, elevated lactate dehydrogenase, non-conjugated bilirubin, and reticulocyte count, and low haptoglobin suggestive of hemolysis. A normal ADAMTS-13 activity rules out TTP. Cancer-associated TMA is mainly associated with adenocarcinoma histology and with advanced disease. The treatment involves treating the underlying cancer, as plasmapheresis, steroids, or other immunosuppressive agents used in TTP have no beneficial role. Complement inhibitors such as eculizumab have shown promise in treating HUS and chemotherapy-induced TMA [
9]. Cancer-associated TMA is a rare condition associated with poor prognosis [
10]. TMA associated with prostate cancer is rare and exists as case reports [
11,
12]. Newton et al. described a patient with prostate cancer who was diagnosed with TMA and found to have recurrent prostate cancer with mediastinal lymphadenopathy. The patient underwent two sessions of hemodialysis, blood and platelet transfusions, and a dose of eculizumab. He was started on hormonal treatment for prostate cancer. The patient improved and was discharged in stable condition [
11].
BRCA2-mutated prostate cancer is strongly associated with aggressive disease across the continuum. The patients present more often with high-grade tumors, nodal and distant metastases, and have inferior survival compared to BRCA2-negative tumors. In advanced disease, BRCA2 mutations predict earlier castration resistance and poor response to standard hormone therapy and chemotherapy, with real-world data confirming shorter survival. Pathologically, BRCA2 alterations correlate with intraductal/cribriform histology and genomic features such as BRCA2/RB1 co-loss, which drive aggressive variant clinical behavior [
3,
4]. Therapeutically, BRCA2 predicts sensitivity to PARP inhibitors and platinum, explaining our patient’s transient response to platinum-based chemotherapy and Olaparib. Nonetheless, responses are often short-lived in this aggressive subtype [
5].
Though our patient improved with supportive care and chemotherapy, hormone therapy, and Olaparib for prostate cancer, the remission was short-lasting, and he succumbed to the disease after 2 months.
Our case adds novelty with the occurrence of SIADH at presentation and cancer-associated TMA at progression. Both have been rarely described in prostate cancer, typically with advanced disease, but not explicitly linked to BRCA2. We hypothesize that tumor burden and marrow infiltration in BRCA2-driven disease created a pro-inflammatory, pro-thrombotic milieu predisposing to these syndromes.
Conclusions
To our knowledge, this is the first reported case of BRCA2-mutated metastatic prostate cancer complicated by SIADH and TMA. BRCA2 status is a key driver of aggressive biology in metastatic prostate cancer, and the unique combination of SIADH and TMA observed here underscores the need for clinicians to consider atypical paraneoplastic and microangiopathic phenomena in advanced disease and the importance of early molecular testing, timely BRCA-directed therapy, and trial-based strategies.
References
- Gillessen S, Omlin A, Attard G et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015 Aug;26(8):1589-604. [CrossRef]
- Shapoo N, Shapoo N, Boma N, et al. Resistance in Prostate Cancer: Mechanisms, Challenges and Therapeutic Innovations. J Clin Trials. 2024, S31:001. [CrossRef]
- Shore N, Oliver L, Shui I, et al. Systematic Literature Review of the Epidemiology of Advanced Prostate Cancer and Associated Homologous Recombination Repair Gene Alterations. J Urol. 2021 Apr;205(4):977-986. [CrossRef]
- Cui M, Gao XS, Gu X et al. BRCA2 mutations should be screened early and routinely as markers of poor prognosis: evidence from 8,988 patients with prostate cancer. Oncotarget. 2017 Jun 20;8(25):40222-40232. [CrossRef]
- Matsubara N, de Bono J, Olmos D et al. Olaparib Efficacy in Patients with Metastatic Castration-resistant Prostate Cancer and BRCA1, BRCA2, or ATM Alterations Identified by Testing Circulating Tumor DNA. Clin Cancer Res. 2023 Jan 4;29(1):92-99. [CrossRef]
- Bogdanos J, Karamanolakis D, Milathianakis C, et al. Syndrome of inappropriate antidiuretic hormone secretion in a patient with hormone refractory prostate cancer. Anticancer Res. 2003 Mar-Apr;23(2C):1755-6.
- Yamazaki T, Suzuki H, Tobe T, et al. Prostate adenocarcinoma producing syndrome of inappropriate secretion of antidiuretic hormone. Int J Urol. 2001 Sep;8(9):513-6. [CrossRef]
- Abe J, Muranaka T, Kunishima Y. Dual paraneoplastic syndrome with prostate cancer: A case of syndrome of inappropriate secretion of antidiuretic hormone (SIADH) and humoral hypercalcemia of malignancy (HHM). Urol Case Rep. 2025 Aug 6;62:103148. [CrossRef]
- Shapoo N, Boma N, Chaudhari S, et al. Solid Tumors, Liquid Challenges: The Impact of Coagulation Disorders. Hematol Rep. 2025 Feb 5;17(1):8. [CrossRef]
- Valério P, Barreto JP, Ferreira H, et al. Thrombotic microangiopathy in oncology - a review. Transl Oncol. 2021 Jul;14(7):101081. [CrossRef]
- Newton J, Floyd L, Ponnusamy A, et al. Thrombotic microangiopathy secondary to recurrent prostate cancer. Clin Nephrol Case Stud. 2021 Sep 10;9:105-109. [CrossRef]
- Biers SM, Sullivan ME, Roberts IS, et al. Thrombotic microangiopathy in advanced prostatic carcinoma. Urology. 2004 Feb;63(2):380-2. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).