1. Introduction
PD is a surgical procedure primarily indicated for premalignant or malignant tumors originating from the pancreas or periampullary structures—including the common bile duct, ampulla of Vater, or duodenum—and less commonly for chronic pancreatitis or traumatic injuries [
1]. In high-volume centers, perioperative mortality has decreased to 4–5%, while morbidity associated with the procedure remains high, reaching up to 60% [
2].
The most feared complication of the Whipple procedure is POPF. Despite significant advances in surgical techniques, the incidence of POPF remains high—typically ranging from 5% to 30%, and in some cases, reaching as high as 50%. One of the major factors implicated in the significant variation in the reported incidence of POPF is the lack of standardized definitions prior to the ISGPF definitions in 2005 and their update in 2016 [
3,
4,
5,
6].
To address the high incidence of POPF, the standard Whipple procedure has undergone over 70 variations, particularly concerning the type of anastomosis. However, no consensus has been reached regarding the superiority of any specific technique, leaving the choice to the surgeon's discretion—favoring the approach with which they are most comfortable and that is associated with the lowest complication rates [
7].
Conventional PD and PPPD are both recognized as acceptable surgical options for resection of periampullary lesions. PPPD may reduce the incidence of dumping syndrome and bile reflux gastritis; however, it is associated with a higher risk of DGE compared to conventional PD [
8,
9].
Reconstruction after PD involves three anastomoses: of the stomach or duodenum, the pancreatic remnant, and the common bile duct.
The most debated anastomosis—having the greatest impact on postoperative outcomes—is the reconstruction of the pancreatic stump, which can be performed using either PG or PJ [
5]. Alternative methods of pancreatic duct management, including duct ligation, occlusion with hemostatic or cyanoacrylate sealants, or even full pancreatectomy, have been suggested as ways to lower the incidence of postoperative pancreatic fistula in cases of pancreaticoduodenectomy [
10,
11]. Also, the use of internal or external stenting of PJ to protect the anastomosis and reduce the risk of POPF remains a subject of ongoing debate, with no clear consensus established [
12,
13].
Various technical modifications have been developed for pancreatic anastomosis following PD, including: end-to-side duct-to-mucosa configurations, constructed in either single-layer or double-layer fashion; end-to-side invagination techniques (also known as "dunking" methods) for PJ; telescoping-style end-to-end reconstructions; the binding method described by Peng; PPPD with gastric partition, followed by either duct-to-mucosa or invagination reconstruction; purse-string sutured anastomoses; use of internal or external stents; creation of a Roux-en-Y limb dedicated to isolated PJ; precolic versus retrocolic anastomosis relative to the transverse colon; techniques employing transpancreatic U-sutures with buttress material to reinforce the PJ or PG; and adjunctive strategies such as mesh reinforcement or omental wrapping to enhance anastomotic integrity[
13,
14,
15,
16].
Several RCTs have demonstrated either comparable outcomes between the two techniques or a lower incidence of postoperative fistulas—particularly POPF—following PG [
17,
18,
19,
20,
21,
22,
23,
24,
25]. However, one of the most highly regarded trials reported a higher rate of grade A/B PPH associated with PG [
23].
To reduce the risk of hemorrhage, two key aspects are essential: identifying arterial anomalies or stenosis and facilitating a safe and effective dissection. For this purpose, two main pancreatic head dissection strategies have been developed—the posterior (artery-first) and anterior (uncinate-first) approaches—along with several technical variants and alternative approaches, including inferior supracolic, inferior infracolic (mesenteric), combined anterior-posterior (hanging maneuver), right/medial uncinate, superior, and left posterior approaches, as well as combinations of multiple artery-first techniques [
26].
The present study is a retrospective cohort analysis conducted at a single high-volume pancreatic surgery center. The primary aim is to determine which method—PG or PJ—is preferable in terms of postoperative outcomes. Specifically, we compare the two techniques with respect to complication rates, length of hospitalization, and in-hospital mortality, in order to provide real-world evidence that may guide clinical decision-making. The findings are intended to contribute to the ongoing debate surrounding the most effective and safest approach, particularly in high-risk patients and complex operative settings.
2. Materials and Methods
2.1. Study Design
The design of our study consisted of a monocentric retrospective cohort study, undertaken at the Surgical Department of the Regional Institute of Gastroenterology and Hepatology “Prof. Dr. Octavian Fodor”, Cluj-Napoca, Romania, between 01.01.2019 and 31.05.2025. We enrolled 604 patients who presented in our service with resectable periampullary tumors, either benign or malignant. As for the surgical treatment with curative intent, standard PD was performed in 569 patients, and PPPD was performed in the remaining 35 patients. In all patients, a standard lymphadenectomy was performed during pancreaticoduodenectomy, encompassing the peripancreatic, hepatoduodenal ligament, pancreaticoduodenal, pyloric, superior mesenteric, and celiac/hepatic artery lymph node stations, in accordance with ISGPS and NCCN guidelines. The aim of our study was to establish which type of pancreatic anastomosis confers a better postoperative outcome. For that reason, we constructed two groups of patients based on pancreatic anastomosis, with 415 patients having PG and 189 patients with PJ. We must mention that in our centre, the standard technique for PG is the binding PG developed by Peng—a combination of invagination and purse-string sutures—while for PJ, we perform a duct-to-mucosa PJ, stented or non-stented.
The choice between the two anastomotic techniques is based on several factors, including the surgeon’s experience and preference, the consistency of the pancreatic parenchyma, and the diameter of the main pancreatic duct (Wirsung). As a general rule in our center, PG is preferred for a soft pancreatic remnant with an undilated duct, whereas PJ is typically reserved for cases involving a firm pancreatic stump, often associated with features of chronic pancreatitis and a dilated Wirsung duct (>3 mm). All cases of periampullary tumors are evaluated preoperatively by a multidisciplinary team comprising gastroenterologists, anesthesiologists, radiologists, oncologists, and surgeons, in order to determine the indication for surgery and the most appropriate operative strategy. Our institution is a high-volume pancreatic surgery center, routinely performing complex pancreaticoduodenectomies, including those requiring venous resections involving the portal vein, superior mesenteric vein, porto-mesenteric confluence, and splenic vein. In accordance with institutional standards, patients deemed suitable for radical resection were managed under the supervision of the surgical team, which maintained primary responsibility for preoperative assessment and the overall therapeutic strategy.
Comparisons between the two groups were conducted with respect to demographics, intraoperative characteristics, and postoperative outcomes.
Our work is reported by respecting the STROBE guidelines, with the STROBE checklist attached to
supplementary materials.
2.3. Data Collection
Patient characteristics, surgical treatment details, tumor histology, and postoperative outcomes were retrieved from our institution’s electronic database. PD was performed either as a classic Whipple procedure or as a PPPD, with portal or superior mesenteric vein reconstruction undertaken in cases of venous tumor involvement. Pancreatic reconstruction was achieved either by duct-to-mucosa pancreaticojejunostomy or by invaginated pancreaticogastrostomy. Intraoperative variables assessed comprised estimated blood loss (mL) and operative duration (minutes). Postoperative complications were categorized as follows: infectious complications—wound infection, intra-abdominal abscess, and sepsis; fistulas—POPF, POBF, gastrojejunostomy leakage, and lymphatic leakage; other specific complications—PPH, DGE, celiac axis ischemia, and mesenteric infarction; and general complications—pulmonary complications, cardiovascular events, and Clostridium difficile infection. Bile culture results, when present, were classified as negative or positive. Relaparotomy, length of postoperative hospital stay, and 90-day mortality were analyzed across groups as key outcomes with significant prognostic impact.
2.4. Outcomes and Definitions
The overall complication rate was defined as the proportion of patients who developed at least one postoperative adverse event of any type or severity during the follow-up period. Infectious complications were evaluated and included: sepsis, defined as a systemic response to infection manifesting with altered temperature, abnormal leukocyte count, and evidence of organ dysfunction; intra-abdominal abscess, characterized by a localized infected fluid collection confirmed either radiologically or during reoperation; and SSI, identified by erythema, purulent discharge, or a positive wound culture necessitating therapeutic intervention [
27,
28]. SSI remains one of the most common and burdensome complications following major abdominal procedures, particularly in HPB surgery, where reported rates may reach up to 30% and significantly impact postoperative recovery, hospital stay, and overall outcomes [
29]. PPH was defined in accordance with the criteria of the ISGPS as any episode of bleeding after pancreatic resection, independent of its timing, anatomical site, or severity [
30]. Chyle leak was defined as the detection of lymphatic fluid in postoperative abdominal drainage, characterized by a milky or opalescent aspect and confirmed by a triglyceride level ≥110 mg/dL (1.2 mmol/L) [
31]. In line with ISGLS criteria, a POBF was defined as bilious drainage fluid with a bilirubin concentration at least threefold higher than the corresponding serum level [
32] According to the ISGPS definition, a POPF is diagnosed when fluid collected from surgical drains on or after the third postoperative day demonstrates an amylase concentration exceeding three times the upper limit of normal serum values, in association with a clinically relevant impact such as infection, requirement for therapeutic intervention, or persistence of drainage. Per ISGPS criteria, DGE is characterized by impaired gastric motility after surgery, manifested by failure to resume a solid oral diet within the expected postoperative period or intolerance to oral intake requiring continued nasogastric decompression [
33]. PPAP was defined as an acute inflammatory condition of the pancreatic remnant occurring within the first three postoperative days after partial pancreatic resection. The diagnosis required sustained POH, with serum amylase exceeding the institutional upper limit of normal for at least 48 hours, in association with clinically relevant features and radiologic alterations consistent with pancreatitis [
34].
2.5. Statistical Analysis
Data was analyzed using R Commander 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as mean ± SD, while qualitative data were presented as frequencies, reported as absolute numbers and percentages, according to the nature of the variable. For qualitative data Chi-square test or Fisher exact test were used, to evaluate the difference in frequencies between the groups. For quantitative data, comparisons between groups were conducted in two forms –parametric (Student-T test) or non-parametric tests (Mann Whitney U) for independent groups. Normality of quantitative data analyzed was assessed by Shapiro-Wilk test for small sample size (n < 50) or Kolmogorov-Smirnov for large sample size (n > 50). Normality was assessed also by Anderson Darling test for better sensitivity. In the case or normally distributed data, equality of variances was tested by Levene’s or Barlett’s tests. For equal variances, Fischer’s ANOVA was used, while for unequal variances, Welch’s ANOVA was chosen. Postoperative survival was analyzed using Kaplan–Meier survival curves, with the log-rank test employed to compare 90-day mortality between groups. Cox proportional-hazards regression was performed to estimate HR and 95% CI for the influence of anastomotic technique on early postoperative survival. All p values <0.05 were considered statistically significant.
3. Results
Between January 1, 2019, and May 31, 2025, a total of 604 patients underwent PD at our institution, a high-volume tertiary referral center for hepatopancreatobiliary surgery. All procedures were performed with curative intent for malignant or benign periampullary disease, and patients with palliative resections or incomplete records were excluded. Reconstruction of the pancreatic remnant was carried out according to institutional practice and intraoperative findings. In 415 patients (68.7%), continuity was restored by PG, performed as a binding technique according to the Peng modification, while in 189 patients (31.3%) reconstruction was achieved by PJ, using a duct-to-mucosa approach with or without internal stenting. The allocation of reconstruction type was guided primarily by gland texture and duct size, with PG preferred in patients with a soft pancreatic remnant and small duct, and PJ in those with a firm gland and dilated duct. This distribution reflects both surgeon preference and established consensus on tailoring reconstruction to intraoperative pancreatic characteristics.
3.1. Baseline Characteristics
A total of 604 patients underwent PD during the study period, with pancreatic reconstruction performed by PG in 415 cases (68.7%) and by PJ in 189 cases (31.3%). The mean age of the entire cohort was 62.3 ± 10.4 years, with no significant difference between the PG and PJ groups (63.6 ± 9.93 vs. 62.9 ± 9.84 years; p = 0.442). Sex distribution was also comparable, with males representing 58.6% of the cohort (p = 0.565).
Histopathological analysis revealed pancreatic adenocarcinoma as the predominant diagnosis (52.3%), followed by ampullary carcinoma (20.5%) and distal cholangiocarcinoma (10.6%). The distribution of tumor types did not differ significantly between the groups (p = 0.358). Regarding the type of surgical procedure, classic pancreaticoduodenectomy was performed in 94.2% of cases, while pylorus-preserving pancreaticoduodenectomy was more frequent in the PJ group compared to PG (9.5% vs. 4.1%; p = 0.008).
Vascular invasion was identified in 7.0% of patients, with similar distribution between groups (p = 0.992). Among those requiring vascular reconstruction, tangential suture was the most common technique, used in 90.2% of cases. PBD was performed in 59.6% of patients, most frequently by endoscopic methods (50.3%), with no significant differences between groups (p = 0.701). Positive bile cultures were more frequently observed in the PJ group compared with PG (57.1% vs. 49.4%; p = 0.001).
Mean operative time was 282.2 ± 68.3 minutes and mean intraoperative blood loss was 318.0 ± 259.0 mL, with no significant differences between groups (p = 0.155 and p = 0.841, respectively). Overall, most baseline variables were comparable between groups, except for a higher proportion of pylorus-preserving procedures and positive bile cultures in the PJ subgroup.
Table 1 summarizes the analysis of these clinical variables, which was undertaken to assess baseline comparability and clinical homogeneity between the subgroups.
3.2. Postoperative Outcomes
The distribution of postoperative complications, together with key prognostic indicators relevant to short-term outcomes (relaparotomy, postoperative hospital stay and 90-day mortality), are summarized and graphically illustrated in
Table 2.
The overall complication rate was 44.9% in the entire cohort, with no significant difference between PG (43.9%) and PJ (47.1%; p = 0.481). Rates of infectious complications, including wound infection, intra-abdominal abscess, sepsis, and Clostridium difficile colitis, were comparable between the two groups. Similarly, the incidence of pulmonary and cardiovascular complications, postoperative acute pancreatitis, lymphatic leakage, mesenteric infarction, and celiac axis ischemia did not differ significantly.
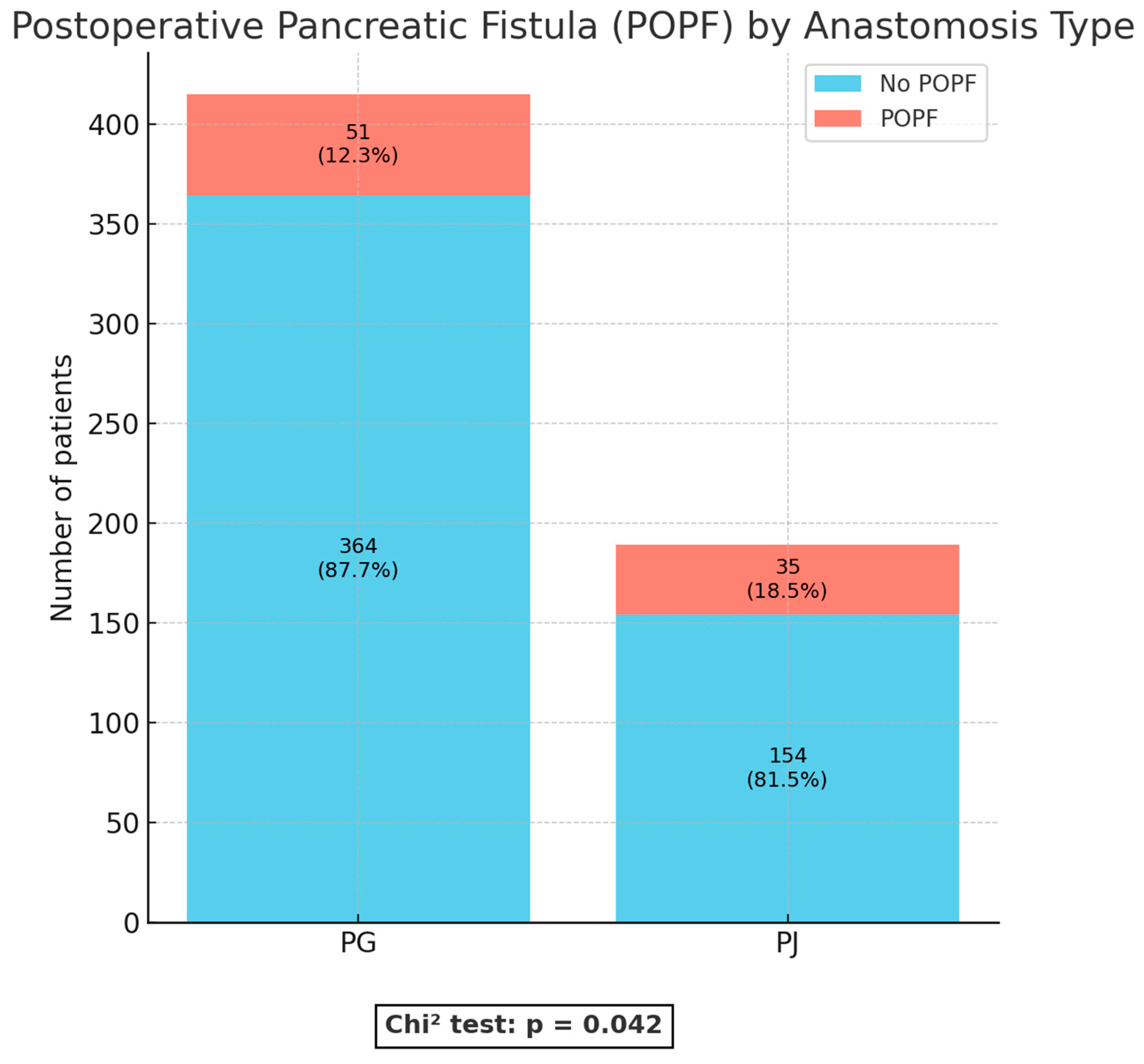
With regard to anastomosis-related outcomes, important differences were observed. POPF, defined as either a biochemical leak (Grade A) or a clinically relevant fistula (Grade B/C), occurred more frequently in the PJ group compared with the PG group (18.5% vs. 12.3%; p = 0.042) (
Figure 1).
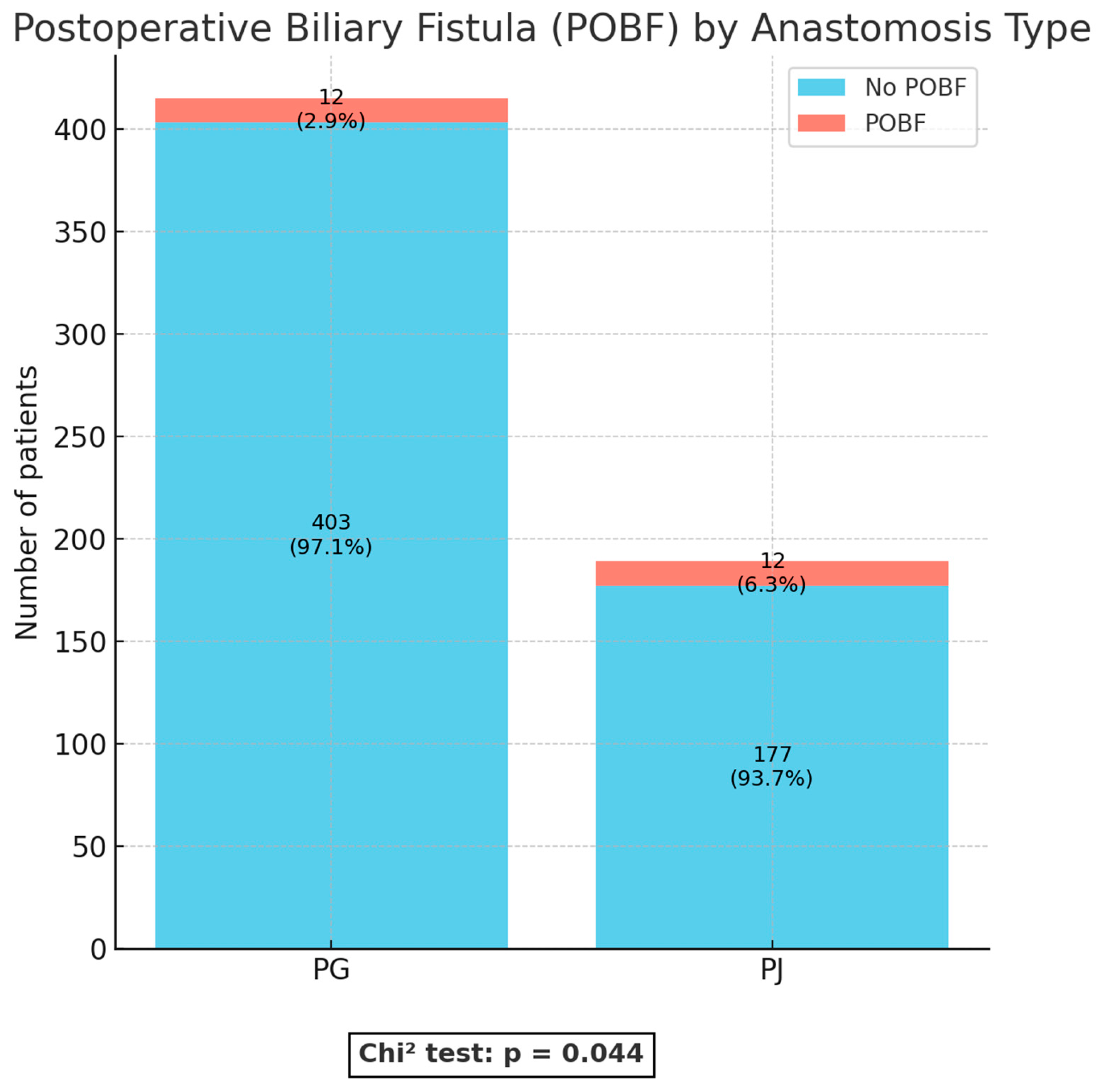
POBF, encompassing Grade A (biochemical leak) and Grade B/C (clinically relevant fistula) was also more common after PJ than PG (6.3% vs. 2.9%; p = 0.044) (
Figure 2).
In contrast, no significant differences were noted in other specific post-pancreatectomy complications such as delayed gastric emptying (7.4% vs. 7.2%; p = 0.938) or postpancreatectomy hemorrhage (12.7% vs. 15.7%; p = 0.341). Gastrojejunostomy leakage was rare and similar in both groups (2.1% vs. 0.7%; p = 0.138).
Regarding prognostic outcomes, relaparotomy rates (6.3% vs. 8.0%; p = 0.487), mean postoperative hospital stay (16.0 vs. 14.6 days; p = 0.135), and 90-day mortality (10.1% vs. 8.9%; p = 0.768) were comparable between PJ and PG groups.
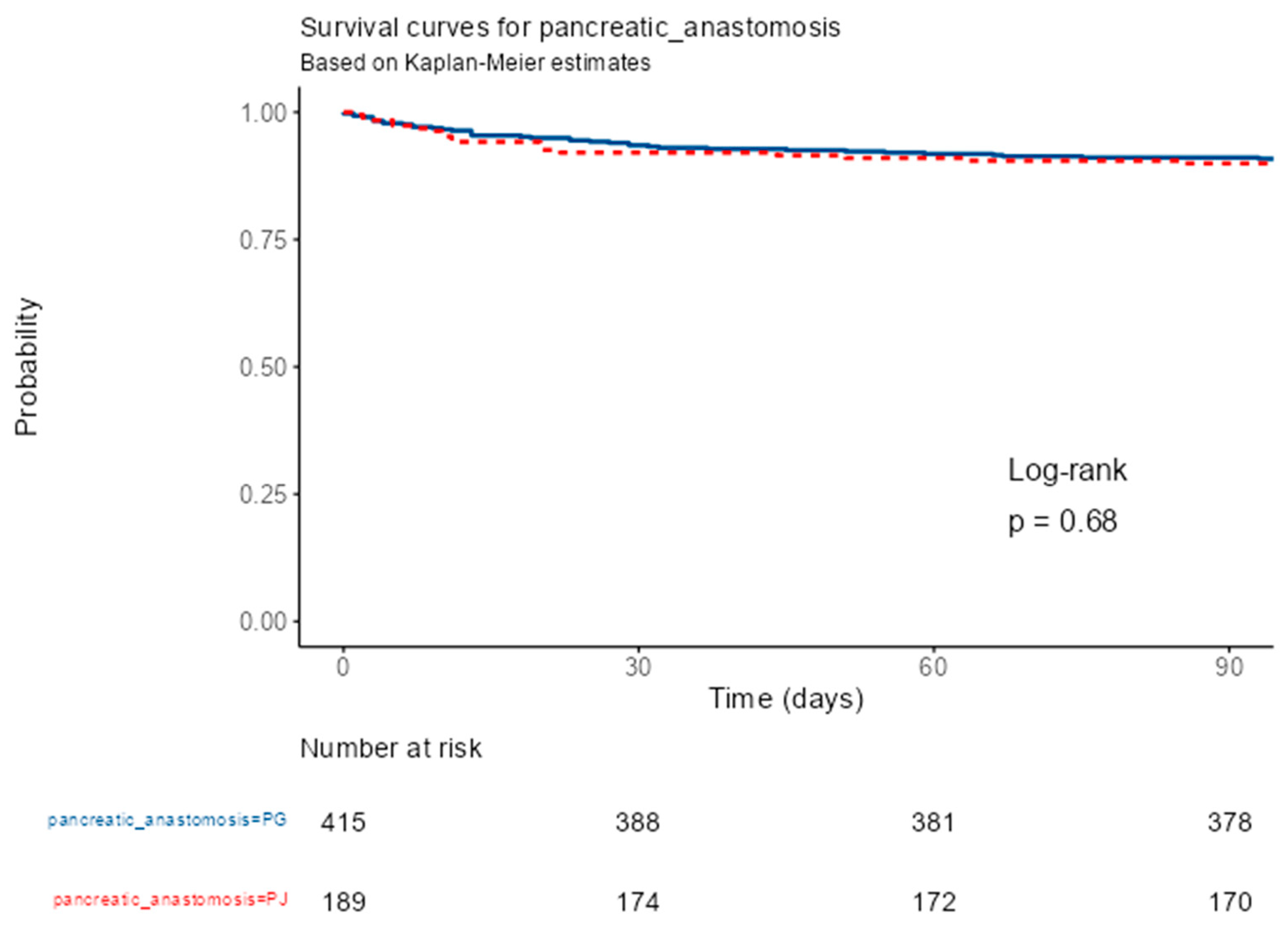
Kaplan–Meier survival analysis showed no significant difference in 90-day postoperative survival between patients reconstructed by PG and those by PJ after PD. Both survival curves remained nearly superimposed throughout the observation period, with survival probabilities consistently above 95%. At 30, 60, and 90 days, the numbers of patients at risk were 388, 381, and 378 in the PG group, and 174, 172, and 170 in the PJ group, respectively. The log-rank test indicated no statistically significant difference between groups (p = 0.68). These results demonstrate comparable early postoperative survival for both types of pancreatic anastomosis.
In total, 69 deaths occurred within the analyzed follow-up, 46 (11.1%) in the PG group and 23 (12.2%) in the PJ group. Cox proportional-hazards regression confirmed the absence of a statistically significant association between the type of pancreatic anastomosis and early postoperative survival. Compared with PG, PJ reconstruction was associated with a HR of 1.11 (95% CI 0.67–1.83, p = 0.682). Model concordance was 0.511 (SE 0.028), with an R² of 0.000 and a likelihood-ratio test p = 0.684, indicating no predictive contribution of the anastomotic technique to survival outcomes. These findings indicate that, despite differences in perioperative complication profiles, the choice of pancreatic anastomosis did not significantly influence short-term survival (
Figure 3).
4. Discussion
This retrospective cohort study compared the outcomes of PG and PJ following PD for periampullary tumors in a large series of 604 patients. In line with the literature, our analysis confirmed that POPF remains the most relevant and feared complication after PD, with a reported incidence between 5–30% in previous studies and persisting despite advances in surgical technique [
3,
4,
5,
17,
35].
The retrospective cohort study of Mastalier et al. demonstrated that although overall outcomes between PG and PJ were comparable, PG was associated with significantly lower rates of postoperative pancreatic fistula in high-risk patients, particularly those with soft pancreatic texture, nondilated ducts, hypoalbuminemia, weight loss, or high intraoperative blood loss. Based on these predictors, the authors proposed a simplified risk score to guide individualized selection of the anastomotic technique, highlighting PG as the preferred option in patients at elevated risk of fistula [
35].
Our institution’s practice pattern—favoring PG in cases with soft pancreatic remnants and small ducts, and PJ in those with firm glands and dilated ducts—reflects international consensus that reconstruction technique must be tailored to gland texture and duct size. Nevertheless, the current analysis, performed in a high-volume pancreatic surgery center, demonstrated significant differences in outcomes between PG and PJ despite these selection principles.
Specifically, the incidence of POPF was significantly lower in the PG group (12.3%) compared with PJ (18.5%, p = 0.042). A similar trend was observed for postoperative biliary fistula (POBF: 2.9% vs. 6.3%, p = 0.044). These findings support several RCTs and meta-analyses that suggested PG may reduce anastomotic leakage rates compared with PJ [
18,
21,
25,
36,
37]. From a physiological standpoint, PG offers a well-vascularized, thick gastric wall for anastomosis and exposes pancreatic secretions to the acidic gastric environment, potentially reducing premature enzymatic activation and local tissue injury.
In a recent retrospective cohort from our institution, Moldovan et al. reported that endoscopic PBD was associated with higher rates of postoperative pancreatic fistula and intra-abdominal abscess compared with surgical or no drainage, while 90-day mortality remained similar across groups [
38]. Moreover, in our previous systematic review and meta-analysis on PBD in periampullary tumors, ERBD was associated with higher rates of postoperative intra-abdominal abscess and delayed gastric emptying compared with external drainage methods (PTBD/ENBD), emphasizing the importance of an appropriate selection of the drainage technique according to clinical context [
39]. These findings highlight how perioperative management strategies can influence postoperative morbidity without affecting short-term survival. In the present study, however, no differences were observed between the PG and PJ groups regarding the type or impact of preoperative biliary drainage, indicating that outcomes related to the anastomotic technique were not influenced by prior biliary drainage.
Importantly, other postoperative outcomes—including delayed gastric emptying, postpancreatectomy hemorrhage, intra-abdominal abscess, relaparotomy, hospital stay, and 90-day mortality—were not significantly different between the groups. This suggests that, aside from fistula formation, both reconstruction methods are largely comparable in terms of global morbidity and mortality.
When placed in the context of existing evidence, our results—showing a higher rate of POPF after PJ compared with PG—contrast with the large randomized trials by Bassi et al. and Keck et al., both of which demonstrated comparable fistula rates between the two reconstruction techniques [
17,
23]. These findings have been further supported by high-quality meta-analyses. Cheng et al. conducted a comprehensive systematic review and meta-analysis including randomized controlled trials and observational studies, and demonstrated that PG and PJ are largely comparable in terms of overall postoperative outcomes, with no clear superiority of either technique regarding clinically relevant postoperative pancreatic fistula, morbidity, or mortality [
40]. Similarly, Lyu et al. (2018), in an updated meta-analysis restricted to randomized controlled trials and applying the standardized ISGPS 2016 definitions, reported no significant difference between PG and PJ with respect to the incidence of clinically relevant POPF, postoperative hemorrhage, or delayed gastric emptying [
5].
In a prospective single institution matched historical control study, Wang et al. (2016) reported that the Blumgart PJ technique was associated with superior outcomes compared to PG. Specifically, the incidence and severity of CR-POPF, as well as surgical mortality, were significantly lower in the Blumgart PJ group, regardless of pancreatic texture, duct size, or underlying pathology. Based on these results, the authors advocated Blumgart PJ as a rapid, straightforward, and safe option for pancreatic reconstruction following pancreaticoduodenectomy [
41].
In our series, PPH rates did not differ significantly between the two groups compared, possibly due to surgical standardization and meticulous hemostatic technique in our center. However, our results differ from the RECOPANC trial, which reported higher rates of postpancreatectomy hemorrhage with PG [
23].
Bassi et al. (2005) reported that PG was associated with significantly fewer POBFs, postoperative collections, DGE, and a lower frequency of multiple surgical complications. In contrast, our study also demonstrated a lower rate of POBF after PG, but found comparable rates of DGE and intra-abdominal abscess between the two techniques [
17].
In the present cohort, early postoperative survival did not differ significantly between PG and PJ following PD. Both reconstruction methods achieved a 90-day survival exceeding 95%, and the median survival was undefined in each group due to the absence of a 50% event rate during follow-up. These findings suggest that the type of pancreatic anastomosis has no measurable impact on short-term mortality, which is consistent with previously published randomized and observational data. The RECOPANC trial and subsequent meta-analyses likewise demonstrated comparable early survival outcomes between PG and PJ, emphasizing that the choice of anastomotic technique primarily influences postoperative morbidity—particularly the incidence and severity of clinically relevant pancreatic fistula—rather than mortality [
23]. The present results therefore reinforce the notion that both PG and PJ are safe reconstructive options in experienced hands, and that the selection of technique should be guided by surgeon expertise, intraoperative findings, and institutional preference rather than concerns regarding early postoperative survival.
The present study also contributes real-world evidence by examining a consecutive series of patients managed in a high-volume Eastern European pancreatic surgery center, where binding PG (Peng technique) and duct-to-mucosa PJ are standard. By applying standardized ISGPS/ISGLS definitions for POPF, DGE, PPH, and POBF, we ensured comparability with international literature.
This study has limitations inherent to its retrospective design, including potential selection bias, unmeasured confounders, and surgeon preference influencing reconstruction choice. Nevertheless, the large sample size, homogeneity of operative standards, and detailed complication reporting strengthen the validity of the findings.
Taken together, our results support the use of PG as the preferred anastomotic technique in patients at higher risk of POPF, particularly those with soft pancreas and small ducts. While PJ remains a viable option, especially in firm, dilated glands, its association with higher fistula rates in our cohort underscores the need for careful patient selection and possibly adjunctive protective measures. Future prospective, randomized studies are warranted to confirm whether PG should be recommended as the first-line reconstruction in pancreaticoduodenectomy.
5. Conclusions
This large single-center retrospective cohort study compared PG and PJ following PD for periampullary tumors. Our findings demonstrated that PG was associated with significantly lower rates of POPF and POBF compared with PJ, while other postoperative outcomes—including DGE, PPH, intra-abdominal abscess, relaparotomy, hospital stay, and 90-day mortality—were similar between the two groups. These results suggest that PG may represent the preferred anastomotic technique in patients with high-risk gland characteristics, particularly those with a soft pancreatic remnant and a nondilated duct, whereas PJ remains an acceptable option in cases with a firm gland and dilated duct. Taken together, our data support an individualized, risk-adapted approach to pancreatic reconstruction after PD and highlight the need for future prospective multicenter randomized trials to confirm whether PG should be adopted as the first-line reconstructive method.
6. Limitations
This study has several limitations that should be acknowledged.
First, its retrospective, single-center design introduces the potential for selection bias, residual confounding, and dependence on the accuracy of institutional records. The choice of anastomotic technique was not randomized but based on intraoperative gland texture, duct size, and surgeon preference, which may have influenced postoperative outcomes.
Second, although the overall cohort was large, subgroup analyses (such as patients with pylorus-preserving procedures or those requiring vascular resections) may have been underpowered to detect small differences.
Third, long-term outcomes, including exocrine and endocrine pancreatic function, quality of life, and survival, were not evaluated, limiting the conclusions to short-term perioperative results.
Fourth, the generalizability of our findings may be restricted, as all procedures were performed in a high-volume pancreatic surgery center with standardized techniques (binding PG and duct-to-mucosa PJ), which may not reflect practice in lower-volume institutions.
Finally, heterogeneity in perioperative management and unmeasured factors such as nutritional status or subtle variations in surgical technique could have influenced complication rates.
Moreover, the study represents a level III–IV grade of evidence, given its retrospective observational design, and therefore cannot establish causal relationships. External validation through prospective multicenter RCTs is essential to confirm these findings and to define the optimal reconstructive approach after PD across diverse practice settings.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, SAM, NAH, EIM, AS and FZ; methodology, AD, SAM, TM and LF; software, RB and VIN; validation, RB, NAH, TM and FG; formal analysis, SAM, VIN and LF; investigation, SAM, AD, SM and MSM; resources, SAM, FG and LF; data curation, AD, MSM, FG and VIN; writing—original draft preparation SAM, SM, MSM and TM; writing—review and editing SAM, NAH, EIM, SM; visualization, MSM, RB, TM and FZ; supervision, NAH, EIM, AS and FZ; project administration, SAM, NAH, EIM, AS and FZ; funding acquisition, SAM, MSM and EIM. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Iuliu Hațieganu” University of Medicine and Pharmacy Cluj-Napoca, Romania, Doctoral Research Program, through the project PCD 778/2/13.01.2025, 3rd year of implementation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of “Octavian Fodor” Regional Institute of Gastroenterology and Hepatology (8331/14.07.2025) and of “Iuliu Hațieganu” University of Medicine and Pharmacy (AVZ 288/06.10.2025) from Cluj-Napoca, Romania.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data were obtained from the databases. The author has sorted out all the data and attached them to the attachment at the end of the article.
Acknowledgments
We acknowledge the support of the 3rd Department of Surgery, University of Medicine and Pharmacy ‘Iuliu Hațieganu’, Cluj-Napoca, and the Regional Institute of Gastroenterology and Hepatology ‘Prof. O. Fodor’, Cluj-Napoca, Romania.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
CI
CR-POPF
DGE |
Confidence interval
Clinically relevant postoperative pancreatic fistula
Delayed gastric emptying |
ERBD
ENBD |
Endoscopic retrograde biliary drainage
Endoscopic nasobiliary drainage |
GIST
HPB
HR |
Gastrointestinal stromal tumor
Hepatopancreatobiliary
Hazard ratio |
IVC
ISGPF |
Inferior vena cava
International Study Group on Pancreatic Fistula |
| ISGLS |
International Study Group of Liver Surgery |
| ISGPS |
International Study Group of Pancreatic Surgery |
| NCCN |
National Comprehensive Cancer Network |
| NET |
Neuroendocrine tumor |
| PBD |
Preoperative biliary drainage |
| PD |
Pancreaticoduodenectomy |
| PG |
Pancreaticogastrostomy |
| PJ |
Pancreaticojejunostomy |
POBF
POH |
Postoperative biliary fistula
Postoperative hyperamylasemia |
POPF
PPAP |
Postoperative pancreatic fistula
Postpancreatectomy acute pancreatitis |
| PPH |
Post-pancreatectomy hemorrhage |
| PPPD |
Pylorus-preserving pancreaticoduodenectomy |
| PTBD |
Percutaneous transhepatic biliary drainage |
| PV |
Portal vein |
| PV-SMV |
Portal vein-superior mesenteric vein confluence |
| RCT |
Randomized controlled trial |
SD
SE |
Standard deviation
Standard error |
| SMV |
Superior mesenteric vein |
| SSI |
Surgical site infection |
| STROBE |
STrengthening the Reporting of OBservational studies in Epidemiology |
| SV |
Splenic vein |
| T-T |
Termino-terminal |
References
- Cameron, J.L.; Riall, T.S.; Coleman, J.; Belcher, K.A. One Thousand Consecutive Pancreaticoduodenectomies. Ann Surg 2006, 244, 10–15. [CrossRef]
- Giuliani, T.; Marchegiani, G.; Di Gioia, A.; Amadori, B.; Perri, G.; Salvia, R.; Bassi, C. Patterns of Mortality after Pancreatoduodenectomy: A Root Cause, Day-to-Day Analysis. Surgery 2022, 172, 329–335. [CrossRef]
- Shahzad, N. Pancreaticojejunostomy versus Pancreaticogastrostomy after Pancreaticoduodenectomy to Prevent Post-Operative Pancreatic Fistula, a Dissonance between Evidence and Practice. Archives of Clinical Gastroenterology 2018, 008–011. [CrossRef]
- Yap, P.Y.; Hwang, J.S.; Bong, J.J. A Modified Technique of Pancreaticogastrostomy with Short Internal Stent: A Single Surgeon’s Experience. Asian J Surg 2018, 41, 250–256. [CrossRef]
- Lyu, Y.; Li, T.; Cheng, Y.; Wang, B.; Chen, L.; Zhao, S. Pancreaticojejunostomy Versus Pancreaticogastrostomy After Pancreaticoduodenectomy: An Up-to-Date Meta-Analysis of RCTs Applying the ISGPS (2016) Criteria. Surg Laparosc Endosc Percutan Tech 2018, 28, 139–146. [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 Update of the International Study Group (ISGPS) Definition and Grading of Postoperative Pancreatic Fistula: 11 Years After. Surgery 2017, 161, 584–591. [CrossRef]
- El Nakeeb, A.; Hamdy, E.; Sultan, A.M.; Salah, T.; Askr, W.; Ezzat, H.; Said, M.; Zeied, M.A.; Abdallah, T. Isolated Roux Loop Pancreaticojejunostomy versus Pancreaticogastrostomy after Pancreaticoduodenectomy: A Prospective Randomized Study. HPB 2014, 16, 713–722. [CrossRef]
- Hüttner, F.J.; Fitzmaurice, C.; Schwarzer, G.; Seiler, C.M.; Antes, G.; Büchler, M.W.; Diener, M.K. Pylorus-Preserving Pancreaticoduodenectomy (Pp Whipple) versus Pancreaticoduodenectomy (Classic Whipple) for Surgical Treatment of Periampullary and Pancreatic Carcinoma. Cochrane Database of Systematic Reviews 2016, 2016. [CrossRef]
- Diener, M.K.; Knaebel, H.-P.; Heukaufer, C.; Antes, G.; B??chler, M.W.; Seiler, C.M. A Systematic Review and Meta-Analysis of Pylorus-Preserving Versus Classical Pancreaticoduodenectomy for Surgical Treatment of Periampullary and Pancreatic Carcinoma. Ann Surg 2007, 245, 187–200. [CrossRef]
- F Feo, C. Wirsung Duct Occlusion Versus Pancreaticojejunostomy after Pancreaticoduodenectomy. International Journal of Surgery Research and Practice 2017, 4. [CrossRef]
- Marczell, A.P.; Stierer, M. Partial Pancreaticoduodenectomy (Whipple Procedure) for Pancreatic Malignancy: Occlusion of a Non-Anastomosed Pancreatic Stump with Fibrin Sealant. HPB Surg 1992, 5, 251–259; discussion 259-60. [CrossRef]
- Singh, K.; Kaman, L.; Tandup, C.; Raypattanaik, N.; Dahiya, D.; Behera, A. Internal Stenting across the Pancreaticojejunostomy and Main Pancreatic Duct after Pancreaticoduodenectomy. Polish Journal of Surgery 2021, 93, 40–47. [CrossRef]
- Zhou, Y.; Zhou, Q.; Li, Z.; Lin, Q.; Gong, Y.; Chen, R. The Impact of Internal or External Transanastomotic Pancreatic Duct Stents Following Pancreaticojejunostomy. Which One Is Better? A Meta-Analysis. Journal of Gastrointestinal Surgery 2012, 16, 2322–2335. [CrossRef]
- Bhoriwal, S.K.; Kumar, S.; Deo, S.; Sharma, J.; Mishra, A.; Kumar, N.; Saikia, J.; Dhall, K. Clinical Outcomes and Technical Description of Unstented End to Side Pancreaticogastrostomy by Small Posterior Gastrotomy. Ann Hepatobiliary Pancreat Surg 2021, 25, 251–258. [CrossRef]
- Peng, S.Y.; Wang, J.W.; Li, J.T.; Mou, Y.P.; Liu, Y.B.; Cai, X.J. Binding Pancreaticojejunostomy – a Safe and Reliable Anastomosis Procedure. HPB 2004, 6, 154–160. [CrossRef]
- Ohwada, S.; Ogawa, T.; Kawate, S.; Tanahashi, Y.; Iwazaki, S.; Tomizawa, N.; Yamada, T.; Ohya, T.; Morishita, Y. Results of Duct-To-Mucosa Pancreaticojejunostomy for Pancreaticoduodenectomy Billroth I Type Reconstruction in 100 Consecutive Patients. J Am Coll Surg 2001, 193, 29–35. [CrossRef]
- Bassi, C.; Falconi, M.; Molinari, E.; Salvia, R.; Butturini, G.; Sartori, N.; Mantovani, W.; Pederzoli, P. Reconstruction by Pancreaticojejunostomy Versus Pancreaticogastrostomy Following Pancreatectomy. Ann Surg 2005, 242, 767–773. [CrossRef]
- Topal, B.; Fieuws, S.; Aerts, R.; Weerts, J.; Feryn, T.; Roeyen, G.; Bertrand, C.; Hubert, C.; Janssens, M.; Closset, J. Pancreaticojejunostomy versus Pancreaticogastrostomy Reconstruction after Pancreaticoduodenectomy for Pancreatic or Periampullary Tumours: A Multicentre Randomised Trial. Lancet Oncol 2013, 14, 655–662. [CrossRef]
- Takano, S.; Ito, Y.; Watanabe, Y.; Yokoyama, T.; Kubota, N.; Iwai, S. Pancreaticojejunostomy versus Pancreaticogastrostomy in Reconstruction Following Pancreaticoduodenectomy. Journal of British Surgery 2000, 87, 423–427. [CrossRef]
- Wellner, U.F.; Sick, O.; Olschewski, M.; Adam, U.; Hopt, U.T.; Keck, T. Randomized Controlled Single-Center Trial Comparing Pancreatogastrostomy Versus Pancreaticojejunostomy After Partial Pancreatoduodenectomy. Journal of Gastrointestinal Surgery 2012, 16, 1686–1695. [CrossRef]
- Duffas, J.-P.; Suc, B.; Msika, S.; Fourtanier, G.; Muscari, F.; Hay, J.M.; Fingerhut, A.; Millat, B.; Radovanowic, A.; Fagniez, P.-L. A Controlled Randomized Multicenter Trial of Pancreatogastrostomy or Pancreatojejunostomy after Pancreatoduodenectomy. The American Journal of Surgery 2005, 189, 720–729. [CrossRef]
- Rault, A.; SaCunha, A.; Klopfenstein, D.; Larroudé, D.; Dobo Epoy, F.N.; Collet, D.; Masson, B. Pancreaticojejunal Anastomosis Is Preferable to Pancreaticogastrostomy after Pancreaticoduodenectomy for Longterm Outcomes of Pancreatic Exocrine Function. J Am Coll Surg 2005, 201, 239–244. [CrossRef]
- Keck, T.; Wellner, U.F.; Bahra, M.; Klein, F.; Sick, O.; Niedergethmann, M.; Wilhelm, T.J.; Farkas, S.A.; Börner, T.; Bruns, C.; et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767). Ann Surg 2016, 263, 440–449. [CrossRef]
- Fernández-Cruz, L.; Cosa, R.; Blanco, L.; López-Boado, M.A.; Astudillo, E. Pancreatogastrostomy With Gastric Partition After Pylorus-Preserving Pancreatoduodenectomy Versus Conventional Pancreatojejunostomy. Ann Surg 2008, 248, 930–938. [CrossRef]
- Figueras, J.; Sabater, L.; Planellas, P.; Muñoz-Forner, E.; Lopez-Ben, S.; Falgueras, L.; Sala-Palau, C.; Albiol, M.; Ortega-Serrano, J.; Castro-Gutierrez, E. Randomized Clinical Trial of Pancreaticogastrostomy versus Pancreaticojejunostomy on the Rate and Severity of Pancreatic Fistula after Pancreaticoduodenectomy. British Journal of Surgery 2013, 100, 1597–1605. [CrossRef]
- Sanjay, P.; Takaori, K.; Govil, S.; Shrikhande, S. V; Windsor, J.A. ‘Artery-First’ Approaches to Pancreatoduodenectomy. British Journal of Surgery 2012, 99, 1027–1035. [CrossRef]
- Bassetti, M.; Eckmann, C.; Giacobbe, D.R.; Sartelli, M.; Montravers, P. Post-Operative Abdominal Infections: Epidemiology, Operational Definitions, and Outcomes. Intensive Care Med 2020, 46, 163–172. [CrossRef]
- Moreno, R.; Rhodes, A.; Piquilloud, L.; Hernandez, G.; Takala, J.; Gershengorn, H.B.; Tavares, M.; Coopersmith, C.M.; Myatra, S.N.; Singer, M.; et al. The Sequential Organ Failure Assessment (SOFA) Score: Has the Time Come for an Update? Crit Care 2023, 27, 15. [CrossRef]
- Feier, C.V.I.; Gaborean, V.; Faur, I.F.; Vonica, R.C.; Faur, A.M.; Rus, V.I.; Dragan, B.S.; Muntean, C. A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications. J Clin Med 2025, 14, 5191. [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy Hemorrhage (PPH)–An International Study Group of Pancreatic Surgery (ISGPS) Definition. Surgery 2007, 142, 20–25. [CrossRef]
- Besselink, M.G.; van Rijssen, L.B.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Asbun, H.J.; Bockhorn, M.; Strobel, O.; Büchler, M.W.; et al. Definition and Classification of Chyle Leak after Pancreatic Operation: A Consensus Statement by the International Study Group on Pancreatic Surgery. Surgery 2017, 161, 365–372. [CrossRef]
- Koch, M.; Garden, O.J.; Padbury, R.; Rahbari, N.N.; Adam, R.; Capussotti, L.; Fan, S.T.; Yokoyama, Y.; Crawford, M.; Makuuchi, M.; et al. Bile Leakage after Hepatobiliary and Pancreatic Surgery: A Definition and Grading of Severity by the International Study Group of Liver Surgery. Surgery 2011, 149, 680–688. [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed Gastric Emptying (DGE) after Pancreatic Surgery: A Suggested Definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [CrossRef]
- Marchegiani, G.; Barreto, S.G.; Bannone, E.; Sarr, M.; Vollmer, C.M.; Connor, S.; Falconi, M.; Besselink, M.G.; Salvia, R.; Wolfgang, C.L.; et al. Postpancreatectomy Acute Pancreatitis (PPAP). Ann Surg 2022, 275, 663–672. [CrossRef]
- Mastalier, B.; Cauni, V.; Tihon, C.; Septimiu Petrutescu, M.; Ghita, B.; Popescu, V.; Andras, D.; Radu, I.M.; Vlasceanu, V.G.; Floroiu, M.F.; et al. Pancreaticogastrostomy versus Pancreaticojejunostomy and the Proposal of a New Postoperative Pancreatic Fistula Risk Score. J Clin Med 2023, 12, 6193. [CrossRef]
- Hallet, J.; Zih, F.S.W.; Deobald, R.G.; Scheer, A.S.; Law, C.H.L.; Coburn, N.G.; Karanicolas, P.J. The Impact of Pancreaticojejunostomy versus Pancreaticogastrostomy Reconstruction on Pancreatic Fistula after Pancreaticoduodenectomy: Meta-analysis of Randomized Controlled Trials. HPB 2015, 17, 113–122. [CrossRef]
- Wellner, U.F.; Sick, O.; Olschewski, M.; Adam, U.; Hopt, U.T.; Keck, T. Randomized Controlled Single-Center Trial Comparing Pancreatogastrostomy Versus Pancreaticojejunostomy After Partial Pancreatoduodenectomy. Journal of Gastrointestinal Surgery 2012, 16, 1686–1695. [CrossRef]
- Moldovan, S.A.; Moiș, E.I.; Graur, F.; Nechita, V.I.; Furcea, L.; Zaharie, F.; Bodea, R.; Mirel, S.; Moldovan, M. Ș.; Mocan, T.; et al. Biliary Drainage for the Preoperative Management of Periampullary Neoplasms: A Retrospective Cohort Study. Medicina (B Aires) 2025, 61, 1565. [CrossRef]
- Moldovan, S.A.; Moiș, E.I.; Graur, F.; Puia, I.C.; Vlad, I.; Nechita, V.I.; Furcea, L.; Zaharie, F.; Popa, C.; Leucuța, D.C.; et al. A Systematic Review and Meta-Analysis of Preoperative Biliary Drainage Methods in Periampullary Tumors. J Clin Med 2025, 14, 7097. [CrossRef]
- Cheng, Y.; Lai, M.; Wang, X.; Tu, B.; Cheng, N.; Gong, J. Pancreaticogastrostomy versus Pancreaticojejunostomy Reconstruction for the Prevention of Pancreatic Fistula Following Pancreaticoduodenectomy. In Cochrane Database of Systematic Reviews; Cheng, Y., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2016.
- Wang, S.-E.; Chen, S.-C.; Shyr, B.-U.; Shyr, Y.-M. Comparison of Modified Blumgart Pancreaticojejunostomy and Pancreaticogastrostomy after Pancreaticoduodenectomy. HPB 2016, 18, 229–235. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).