Introduction
Coronary artery disease (CAD) continues to be a major contributor to global morbidity and mortality, representing a complex, progressive, and chronic inflammatory disorder [
1,
2,
3]. The disease is primarily characterized by the accumulation of atherosclerotic plaques within the epicardial coronary arteries, which can compromise myocardial blood flow and trigger ischemic events. Although CAD may remain clinically silent for extended periods, it can suddenly become unstable due to acute plaque rupture or erosion, leading to acute coronary syndromes such as unstable angina or myocardial infarction [
4,
5]. The heterogeneity in clinical presentation, progression, and prognosis underscores the importance of early identification of individuals at risk, as well as precise assessment of disease severity, to optimize preventive and therapeutic strategies [
6,
7,
8]. Additionally, reliable and accessible biomarkers could facilitate earlier intervention and more individualized management, potentially reducing the burden of CAD-related morbidity and mortality.
Inflammatory mechanisms are central to all phases of atherosclerotic disease progression, contributing to plaque initiation, development, and rupture that precipitate clinical ischemic events, as demonstrated in prior experimental and clinical investigations [
9,
10]. Circulating immune cells—including neutrophils, lymphocytes, and monocytes—contribute to endothelial dysfunction, promote plaque instability, and modulate the inflammatory milieu within the vascular wall [
9,
11]. Moreover, systemic inflammatory responses interact with other pathophysiological processes, such as oxidative stress, metabolic dysregulation, and endothelial injury, amplifying the progression of atherosclerotic disease and influencing clinical outcomes. Consequently, hematological indices derived from routine peripheral blood counts have emerged as cost-effective, widely available markers for predicting both the presence and severity of CAD [
12,
13].
Albumin, the most abundant plasma protein, exerts several protective functions, including maintenance of oncotic pressure, antioxidant activity, anti-inflammatory effects, and regulation of vascular homeostasis [
14]. Low serum albumin levels have been consistently linked to adverse cardiovascular outcomes and increased mortality in patients with CAD. The neutrophil-to-albumin ratio (NAR), which combines an indicator of systemic inflammation (neutrophil count) with a marker of nutritional and inflammatory status (albumin), has recently emerged as a novel biomarker in various clinical settings, demonstrating prognostic relevance in critically ill and systemically inflamed patients [
13,
14,
15].
However, to date, no studies have specifically examined the relationship between NAR and CAD or its severity. Given the simplicity, affordability, and routine availability of neutrophil and albumin measurements in clinical practice, exploring the predictive value of NAR could provide a practical tool for early risk stratification. A better understanding of this relationship may help clinicians identify high-risk patients more effectively, guide diagnostic evaluation, and inform targeted therapeutic interventions.
Therefore, this study aimed to investigate the association between NAR and both the presence and severity of CAD in patients undergoing coronary angiography, with severity quantified using the Gensini score. By integrating measures of systemic inflammation and nutritional status, this research seeks to determine whether NAR can serve as a readily available, cost-effective biomarker to support clinical decision-making in CAD management.
Materials and Methods
Study Population
Initially, 1050 patient records were accessed. After applying exclusion criteria and accounting for incomplete or missing data, 73 patients were removed from the analysis, leaving a total of 987 patients included in the study. These patients underwent coronary angiography (CAG) at the Cardiology Clinic of Sanliurfa Mehmet Akif Inan Hospital between January 2020 and June 2023. Inclusion criteria were age ≥18 years and suspected CAD requiring invasive evaluation. Exclusion criteria included acute coronary syndrome, previously known CAD, chronic inflammatory or autoimmune diseases, hematologic malignancies, severe valvular heart disease, hypertrophic cardiomyopathy, or incomplete medical records.
Coronary Angiography and Gensini Score
CAG was performed by cardiologists with expertise in interventional procedures, employing standard Judkins or radial approach techniques. The coronary images were evaluated independently by two cardiologists who were unaware of the patients' clinical and laboratory data, in order to minimise bias. Discrepancies in interpretation were resolved by consensus. The severity and extent of coronary artery disease were quantitatively determined using the Gensini scoring system, which considers both the degree of lumen narrowing and the functional importance of the affected coronary segment. Each segment was assigned a score multiplied by a segment-specific weighting factor reflecting the myocardial region. Higher weights were assigned to the proximal segments of the left main artery and left anterior descending artery due to their larger perfusion areas. Patients were categorised into three groups based on their Gensini scores: normal (score 0), mild (score 1–24), and severe (score ≥25). This scoring system enables a standardised, semi-quantitative assessment of coronary atherosclerosis, allowing for consistent comparisons between patients and facilitating the investigation of possible correlations with laboratory biomarkers such as the neutrophil-to-albumin ratio (NAR).
Laboratory Assessments
Prior to coronary angiography, venous blood samples were collected from all participants to evaluate complete blood count, serum albumin, lipid profile, and creatinine levels. The neutrophil-to-albumin ratio (NAR) was determined by dividing the absolute neutrophil count by the serum albumin concentration. Additional hematological parameters, including platelet and lymphocyte counts, were also measured for further exploratory analyses. All assays were performed in the hospital’s central laboratory using fully automated, standardized analyzers, with strict adherence to internal quality control procedures to ensure accuracy and reproducibility of the results.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 24. The normality of continuous variables was assessed using the Shapiro-Wilk test. Descriptive statistics were presented as counts and percentages for categorical variables and as means ± standard deviations for continuous variables. Homogeneity of variances was evaluated using Levene’s test. Comparisons between two independent groups were performed using the independent-samples t-test, while comparisons among more than two groups were conducted using one-way analysis of variance (ANOVA). For post hoc analysis involving three independent groups, the significance threshold was adjusted using Bonferroni correction (0.05 / 3 = 0.0166), and Hochberg’s GT2 test was applied where appropriate. Additionally, receiver operating characteristic (ROC) curve analysis was performed to evaluate the discriminative ability of the test variable between groups. The area under the ROC curve (AUC) with a 95% confidence interval was calculated to determine statistical significance. A p-value <0.05 was considered statistically significant for all analyses.
Results
Analysis of demographic characteristics among groups stratified by Gensini score revealed significant differences in gender, diabetes, and hypertension. Laboratory analyses also demonstrated statistically significant differences between groups in white blood cell count, albumin, neutrophil count, monocyte count, platelet count, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, creatinine, and neutrophil-to-albumin ratio (NAR) (
Table 1).
Abbreviations: SD, standard deviation; HDL, high density lipoprotein; LDL, low density lipoprotein; NAR, neutrophil-to-albumin ratio; WBC, white blood cell.
Receiver operating characteristic (ROC) curve analysis of NAR in relation to Gensini score yielded an area under the curve (AUC) of 0.735, with an optimal threshold of 0.1138 and a 95% confidence interval (p<0.001). Patients with NAR values above 0.1138 exhibited higher Gensini scores, indicating a significantly elevated risk of CAD and more severe ischemia (
Table 2).
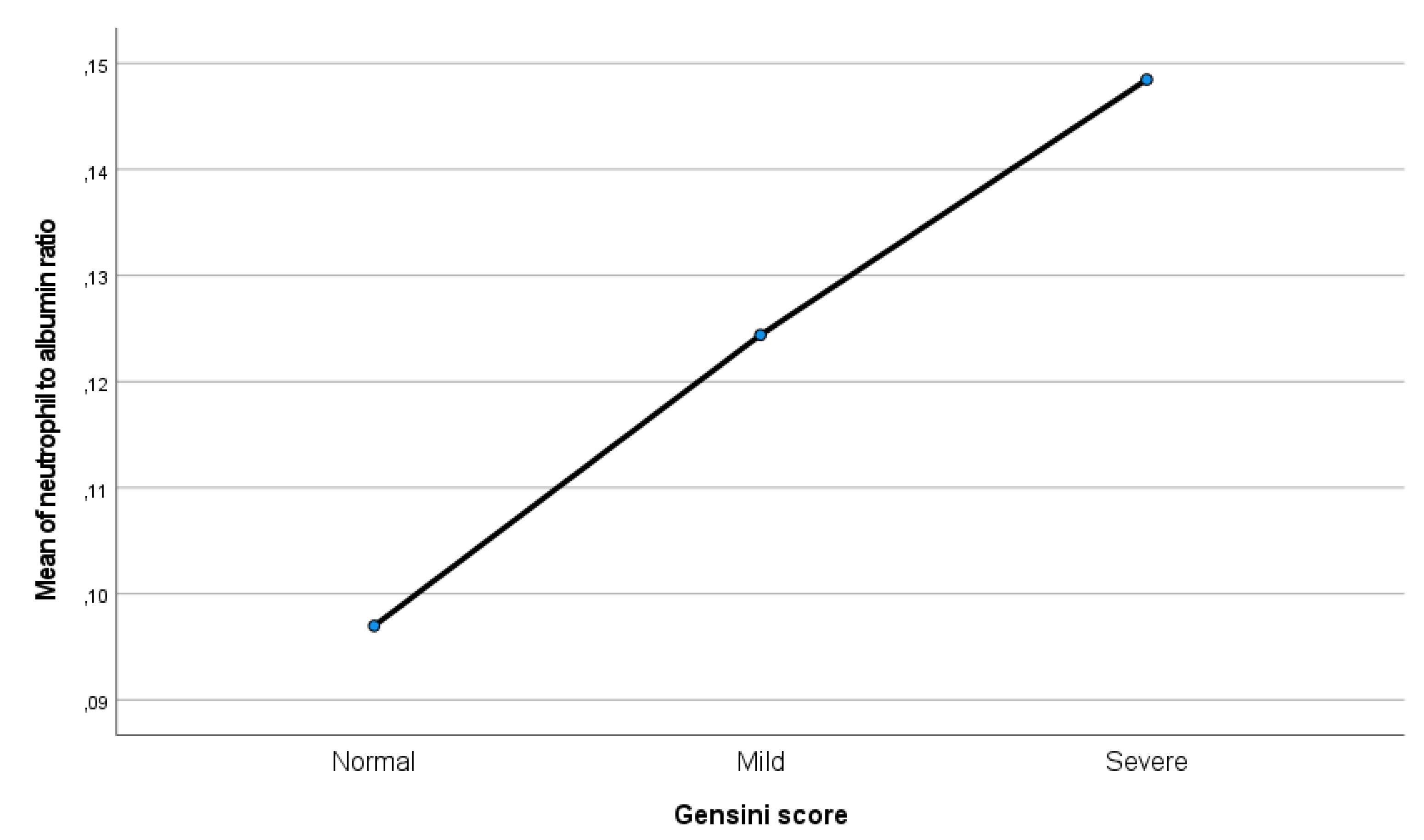
Correlation analyses confirmed that higher NAR values were associated with increased Gensini scores (p < 0.001). Post hoc comparisons further demonstrated that this significant association persisted across all Gensini score categories (p < 0.001) (
Figure 1,
Table 3).
In the multivariate ordinal logistic regression model, the neutrophil-to-albumin ratio (NAR) remained the strongest independent predictor of ischemia severity (β = 0.60, SE = 0.10; OR 1.82, 95% CI 1.48–2.24, p < 0.001). Diabetes mellitus (β = 0.52, SE = 0.22; OR 1.68, 95% CI 1.09–2.58, p = 0.019) and hypertension (β = 0.48, SE = 0.20; OR 1.61, 95% CI 1.10–2.36, p = 0.014) were also independently associated with higher Gensini score categories. Male sex showed a borderline association with ischemia severity (OR 1.45, 95% CI 0.99–2.13, p = 0.053), while LDL cholesterol showed a non-significant trend (OR 1.05, 95% CI 0.99–1.11, p = 0.10) and age had no significant effect (table-4). These results emphasize the predominant role of NAR in predicting coronary artery disease severity, with diabetes and hypertension contributing moderately.
Table 4.
Multivariate ordinal logistic regression analysis for the association between the neutrophil-to-albumin ratio (NAR) and the severity of ischemia based on the Gensini score.
Table 4.
Multivariate ordinal logistic regression analysis for the association between the neutrophil-to-albumin ratio (NAR) and the severity of ischemia based on the Gensini score.
| Variable |
β (SE) |
OR (95% CI) |
P |
| NAR (per unit increase) |
0.60 (0.10) |
1.82 (1.48–2.24) |
<0.001 |
| LDL-C (mg/dL, per 10 mg/dL) |
0.05 (0.03) |
1.05 (0.99–1.11) |
0.10 |
| Age (years) |
0.02 (0.01) |
1.02 (0.99–1.04) |
0.11 |
| Male sex |
0.37 (0.19) |
1.45 (0.99–2.13) |
0.053 |
| Diabetes mellitus |
0.52 (0.22) |
1.68 (1.09–2.58) |
0.019 |
| Hypertension |
0.48 (0.20) |
1.61 (1.10–2.36) |
0.014 |
Discussion
This study demonstrated that elevated NAR levels were significantly associated with both the presence and severity of CAD, and that NAR served as a common and independent predictor of ischemia severity. CAG remains the gold standard for determining the presence of CAD, assessing the degree of luminal narrowing, and guiding revascularization strategies. The morphological characteristics and extent of coronary stenosis are key determinants in the selection of an optimal treatment approach. To objectively quantify coronary atherosclerosis, several scoring systems have been developed, the most widely used being the Gensini and SYNTAX scores [
18]. The Gensini scoring system provides a comprehensive assessment by incorporating not only the degree of luminal narrowing but also the functional significance of lesion location, assigning higher weights to lesions in the left main and proximal left anterior descending arteries, which supply large myocardial territories [
17]. Owing to these features, the Gensini score has been frequently applied in research combining angiographic data with laboratory parameters to evaluate CAD severity and prognosis. For example, Duran et al. found a positive correlation between serum uric acid levels and the Gensini score among patients with acute coronary syndrome [
18].
Myocardial perfusion scintigraphy (MPS) is another valuable, non-invasive technique for assessing myocardial perfusion and viability [
19]. Previous investigations have demonstrated a linear relationship between the extent of CAD and the degree of ischemic damage [
20,
21,
22]. However, in a considerable subset of patients undergoing MPS or CAG, coronary arteries appear angiographically normal despite clinical suspicion of CAD, resulting in unnecessary radiation exposure and increased healthcare costs [
23]. These limitations underscore the need for simple, inexpensive, and accessible biomarkers that could assist clinicians in the early identification and stratification of patients with suspected CAD.
Atherosclerosis is recognized as a chronic, multifactorial process involving lipid accumulation, endothelial dysfunction, and immune activation [
24]. Inflammation is pivotal in every stage of its evolution—from plaque formation to destabilization and thrombosis. Peripheral white blood cell indices, including neutrophil and lymphocyte counts, have long been studied as inexpensive inflammatory markers reflecting systemic immune activation [
25,
26]. Elevated neutrophil counts and reduced lymphocyte counts have been associated with future cardiovascular events and poorer outcomes [
27]. Neutrophils contribute to atherosclerotic progression by releasing reactive oxygen species, proteolytic enzymes, and proinflammatory mediators, which collectively exacerbate endothelial damage and plaque vulnerability.
Serum albumin, on the other hand, is a major plasma protein with antioxidant and anti-inflammatory functions, maintaining vascular integrity and modulating inflammatory responses. Hypoalbuminemia has been consistently associated with poor cardiovascular outcomes, likely due to its reflection of malnutrition, oxidative stress, and systemic inflammation [
28]. The neutrophil-to-albumin ratio (NAR) thus integrates two key biological dimensions—proinflammatory activity and nutritional/inflammatory status—into a single metric that may better capture overall cardiovascular risk.
In our cohort, NAR values showed a graded increase parallel to the Gensini score, indicating that higher NAR levels were associated with more severe coronary atherosclerosis. Pathophysiologically, this relationship may arise from the combined effects of neutrophil-mediated oxidative injury and hypoalbuminemia-related loss of vascular protection. Importantly, in multivariate ordinal regression analysis, NAR remained an independent and the most powerful predictor of ischemia severity, even after adjusting for conventional risk factors such as diabetes, hypertension, and LDL-C. This suggests that inflammation-driven indices may offer incremental prognostic value beyond traditional metabolic markers. Consistent with earlier studies linking inflammatory biomarkers such as C-reactive protein and interleukin-6 to cardiovascular risk [
29], NAR appears to represent a readily available and cost-effective surrogate of systemic inflammation and endothelial dysfunction.
Given its ease of calculation, low cost, and routine availability, NAR could serve as a practical biomarker for initial risk stratification in patients presenting with chest pain or suspected myocardial ischemia, potentially reducing unnecessary invasive procedures such as coronary angiography.
This study, however, is not without limitations. The single-center, retrospective design may have introduced selection bias, and the relatively small sample size could have limited the statistical power of subgroup analyses. Additionally, dynamic changes in NAR over time were not assessed, which may provide further prognostic insight. Therefore, prospective multicenter studies with larger sample sizes and longitudinal follow-up are warranted to validate these findings and explore the prognostic utility of NAR in broader patient populations.
Conclusions
In conclusion, our results indicate that the neutrophil-to-albumin ratio represents a reliable, independent, and readily measurable biomarker for assessing both the presence and severity of ischemia as well as the overall burden of coronary artery disease. By examining, for the first time, the association between NAR and CAD severity through the Gensini scoring system, this study provides novel evidence supporting its potential role in early risk stratification. These findings may serve as a foundation for future multicenter studies with larger and more heterogeneous populations, ultimately contributing to improved clinical decision-making and patient management strategies.
Author Contributions
Conceptualization, Ö.F.Ç. and A.P.; methodology, Ö.F.Ç.; software, A.P.; validation, Ö.F.Ç. and A.P.; formal analysis, Ö.F.Ç.; investigation, A.P.; resources, Ö.F.Ç.; data curation, Ö.F.Ç. and A.P.; writing—original draft preparation, Ö.F.Ç.; writing—review and editing, A.P.; visualization, Ö.F.Ç.; supervision, A.P.; project administration, Ö.F.Ç. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, Harran University Clinical Research Ethics Committee ( HRÜ/23.16.01 and 04.09.2023).
Informed Consent Statement
Written informed consent was not required because the study was retrospective and based on previously recorded, anonymized patient data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
The authors would like to thank the staff of Mehmet Akif İnan Training and Research Hospital for their kind support during the data collection process.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Aktaş, İ.; Bolayır, H.A.; Karasu, M. Neutrophil Percentage-to-Albumin Ratio as a Predictor of Collateral Circulation in Chronic Total Occlusion. Anatol. J. Cardiol. 2025, 29, 489–495. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Wang, Y.; Zhao, X.; Liu, Q.; Chen, S.; Huang, Y.; Sun, X.; Zhou, Y.; Wang, F. Neutrophil-to-Albumin Ratio Predicts Cardiovascular and All-Cause Mortality in Patients with Cardiovascular Disease and Abnormal Glucose Metabolism. Sci. Rep. 2025, 15, 12345. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Li, Z.; Wang, H.; Sun, Q.; Chen, Y.; Liu, J.; Zhou, R. Association between Neutrophil Percentage-to-Albumin Ratio and Cardiovascular Diseases: A Cross-Sectional Study Using NHANES Data. Front. Cardiovasc. Med. 2025, 12, 1557507. [Google Scholar] [CrossRef] [PubMed]
- Karasu, M.; Aktaş, İ.; Bolayır, H.A. Neutrophil Percentage-to-Albumin Ratio as a Predictor of Coronary Artery Ectasia. J. Clin. Med. 2025, 15, 1638. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Li, Y.; Zhou, X.; Chen, J.; Liu, Q.; Sun, Y. Association between Neutrophil Percentage-to-Albumin Ratio and Peripheral Arterial Disease. BMC Cardiovasc. Disord. 2025, 25, 50. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Zhang, X.; Wang, J.; Chen, H.; Liu, Y. Combination of Neutrophil Count and Gensini Score as a Prognostic Marker in Patients with Diabetes and Acute Coronary Syndrome. Cardiovasc. Investig. Adv. 2023, 9, 51. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, Y.; Liu, Z.; Chen, J.; Li, Y.; Wang, X.; Sun, H.; Zhou, Q.; Liu, F. Neutrophil-to-Albumin Ratio Predicts All-Cause and Cardiovascular Disease Mortality in Individuals with Diabetes or Prediabetes. Sci. Rep. 2025, 15, 93558. [Google Scholar] [CrossRef]
- Shu, H.; Li, J.; Zhang, X.; Wang, Y.; Chen, S.; Liu, H.; Zhou, Z.; Li, F. Correlation between Neutrophil-to-Lymphocyte Ratio and Severity of Coronary Artery Disease. J. Clin. Med. 2025, 14, 95762. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Zhang, H.; Liu, X.; Chen, F.; Sun, Y.; Zhou, L.; Zhang, Y. Association between Neutrophil Percentage-to-Albumin Ratio and Cardiovascular Disease in the Metabolic Syndrome Population. J. Clin. Med. 2025, 14, 12350798. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, Y.; Li, Z.; Wang, H.; Chen, J.; Liu, Q. The Role of Neutrophil Percentage-to-Albumin Ratio in Cardiovascular Disease-Psychological Distress Comorbidity. Heart Lung 2025, 54, 1–7. [Google Scholar] [CrossRef]
- Xiu, W.-J.; Yang, H.-T.; Zheng, Y.-Y.; Liu, X.-Q.; Chen, J.-Y.; Sun, Z.-Q. ALB-dNLR Score Predicts Mortality in Coronary Artery Disease Patients After Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 2022, 9, 709868. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, H.; Li, Y.; Zhou, X.; Wang, Y.; Chen, S.; Liu, Q. Neutrophil Percentage-to-Albumin Ratio Mediates the Association between Inflammation and Atherosclerosis in Coronary Artery Disease. J. Clin. Med. 2025, 14, 12362869. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Yue, J.; Wang, X.; Chen, F.; Liu, Y. The Neutrophil Percentage-to-Albumin Ratio Is Associated with All-Cause Mortality in Critically Ill Patients with Acute Myocardial Infarction. BMC Cardiovasc. Disord. 2022, 22, 115. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Ling, X.; Chen, J.; Zhou, Y.; Wang, F. The Neutrophil Percentage-to-Albumin Ratio as a New Predictor of All-Cause Mortality in Patients with Cardiogenic Shock. Biomed. Res. Int. 2020, 2020, 7458451. [Google Scholar] [CrossRef]
- Cui, H.; Ding, X.; Li, W.; Chen, Y.; Zhou, L.; Wang, J. The Neutrophil Percentage to Albumin Ratio as a New Predictor of In-Hospital Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Med. Sci. Monit. 2019, 25, 7845–7852. [Google Scholar] [CrossRef] [PubMed]
- Gensini, G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 1983, 51, 606. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Rahimi, M.; Mohammadi, M.; Hosseini, S.; Farhadi, P.; Rezaei, N. Comparison of Gensini and SYNTAX Scores for Coronary Artery Disease Severity Assessment. Clin. Cardiol. 2020, 43, 1231–1239. [Google Scholar] [CrossRef]
- Duran, A.; Kaya, A.; Aydın, M.; Demir, M.; Yıldırım, E.; Şahin, M.; Özdemir, R. Serum Uric Acid Levels and Coronary Atherosclerosis Severity in Acute Coronary Syndrome Patients. Angiology 2021, 72, 1085–1092. [Google Scholar] [CrossRef]
- Verma, S.; Bhatia, V.; Mehta, A.; Garg, R.; Sharma, P.; Malhotra, S. Myocardial Perfusion Scintigraphy in Coronary Artery Disease Evaluation. Nucl. Med. Commun. 2019, 40, 811–819. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.W.; Park, Y.J.; Kim, D.H.; Song, J.M.; Choi, S.Y.; Lee, J.H. Correlation of Myocardial Perfusion Scintigraphy Findings with Coronary Artery Disease Severity. J. Nucl. Cardiol. 2020, 27, 1960–1971. [Google Scholar] [CrossRef]
- Kato, S.; Tanaka, M.; Nishida, T.; Fukuda, S.; Hasegawa, K. Assessment of Ischemia by MPS in Patients with CAD. J. Cardiol. 2021, 78, 234–242. [Google Scholar] [CrossRef]
- Li, P.; Zhao, H.; Wang, X.; Sun, Y.; Chen, J.; Zhang, H. Relationship between CAD Extent and Myocardial Damage: Insights from Non-Invasive Imaging. Clin. Imaging 2022, 82, 13–21. [Google Scholar] [CrossRef]
- Patel, N.; Agarwal, S.; Verma, R.; Kumar, P. Limitations and Risks of Non-Invasive and Invasive Imaging in Suspected CAD. Heart Views 2020, 21, 78–84. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhang, J.; Liu, F.; Wang, H.; Zhou, S. Economic and Radiation Considerations in Coronary Angiography and MPS. J. Cardiovasc. Transl. Res. 2021, 14, 1152–1162. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.; Renlund, D.G.; Muhlestein, J.B.; Carlquist, J.F. Which White Blood Cell Subtypes Predict Cardiovascular Risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef]
- Azab, B.; Camacho-Rivera, M.; Taioli, E. Average Values of Neutrophil-to-Lymphocyte Ratio and Association with Future Cardiovascular Events. PLoS ONE 2014, 9, e105981. [Google Scholar] [CrossRef]
- Plakht, Y.; Gilutz, H.; Konijn, A.M.; Kornowski, R.; Shacham, Y.; Hoffer, E.; Mandelzweig, L.; Behar, S. Hypoalbuminemia Predicts Mortality in Acute Myocardial Infarction Patients. Am. J. Med. 2012, 125, 1081–1089. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-Reactive Protein and Interleukin-6 for Risk Assessment in Cardiovascular Disease. Circulation 2002, 105, 1130–1135. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).