Submitted:
14 August 2025
Posted:
14 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Study Design and Population
- Age ≥18 years at presentation
- According to current ESC/AHA guidelines, the clinical presentation is consistent with acute coronary syndrome (STEMI, NSTEMI, or unstable angina).
- Untreated state (absence of prior use of lipid-lowering medicines, antiplatelet drugs other than aspirin for primary prevention, or other cardiovascular therapies)
- First acute coronary syndrome presentation (no previous myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting)
- Complete admission lipid profile obtained within 24 hours of symptom onset
- Adequate angiographic images suitable for quantitative coronary analysis
- Previous history of coronary artery disease, including prior myocardial infarction, PCI, or coronary artery bypass grafting
- Use of any lipid-lowering medications, including statins, ezetimibe, PCSK9 inhibitors, or fibrates
- Severe renal dysfunction (estimated glomerular filtration rate <30 mL/min/1.73 m² or requiring dialysis)
- Severe hepatic dysfunction
- Active infection or inflammatory conditions that could affect lipid metabolism
- Malignancy with ongoing treatment or recent chemotherapy (within 6 months)
- Pregnancy or lactation
- Inability to undergo coronary angiography due to contrast allergy or other contraindications
- Incomplete clinical data or lipid profile measurements
- Poor angiographic image quality preventing accurate quantitative analysis
2.2. Diagnostic Biomarker Assessment
- Total cholesterol: measured using the cholesterol esterase/cholesterol oxidase method
- Triglycerides: measured using the glycerol phosphate oxidase method
- HDL cholesterol: measured using a direct homogeneous assay with selective solubilization
- TG/HDL-C ratio: triglycerides (mg/dL) divided by HDL cholesterol (mg/dL)
- TC/HDL-C ratio: total cholesterol (mg/dL) divided by HDL cholesterol (mg/dL)
- LDL-C/HDL-C ratio: LDL cholesterol (mg/dL) divided by HDL cholesterol (mg/dL)
2.3. Statistical Analysis
- ANOVA with linear trend testing across lesion groups
- Pearson correlation for biomarker-plaque burden relationships
- Multivariable linear regression adjusting for age, sex, diabetes, hypertension, smoking
- Sensitivity/specificity analysis for TG/HDL thresholds (Youden index)
- Assumptions (linearity, normality, and homoscedasticity) were verified. p<0.05 is significant. SPSS v23.0. The sample size provided >80% power to detect r≥0.2 (α=0.05).
- Missing Data Management: The primary method for addressing missing data was complete case analysis, due to the retrospective design of the study and the high completeness of essential variables. Sensitivity analyses employing multiple imputations were intended if missing data were over 5% for any critical variable.
3. Results
3.1. Baseline Characteristics
3.2. Diagnostic Performance of Lipid Biomarkers
3.3. Diagnostic Accuracy of TG/HDL-C Ratio
- >3.0: Sensitivity 82.1%, Specificity 65.3%, PPV 71.4%, NPV 77.8%
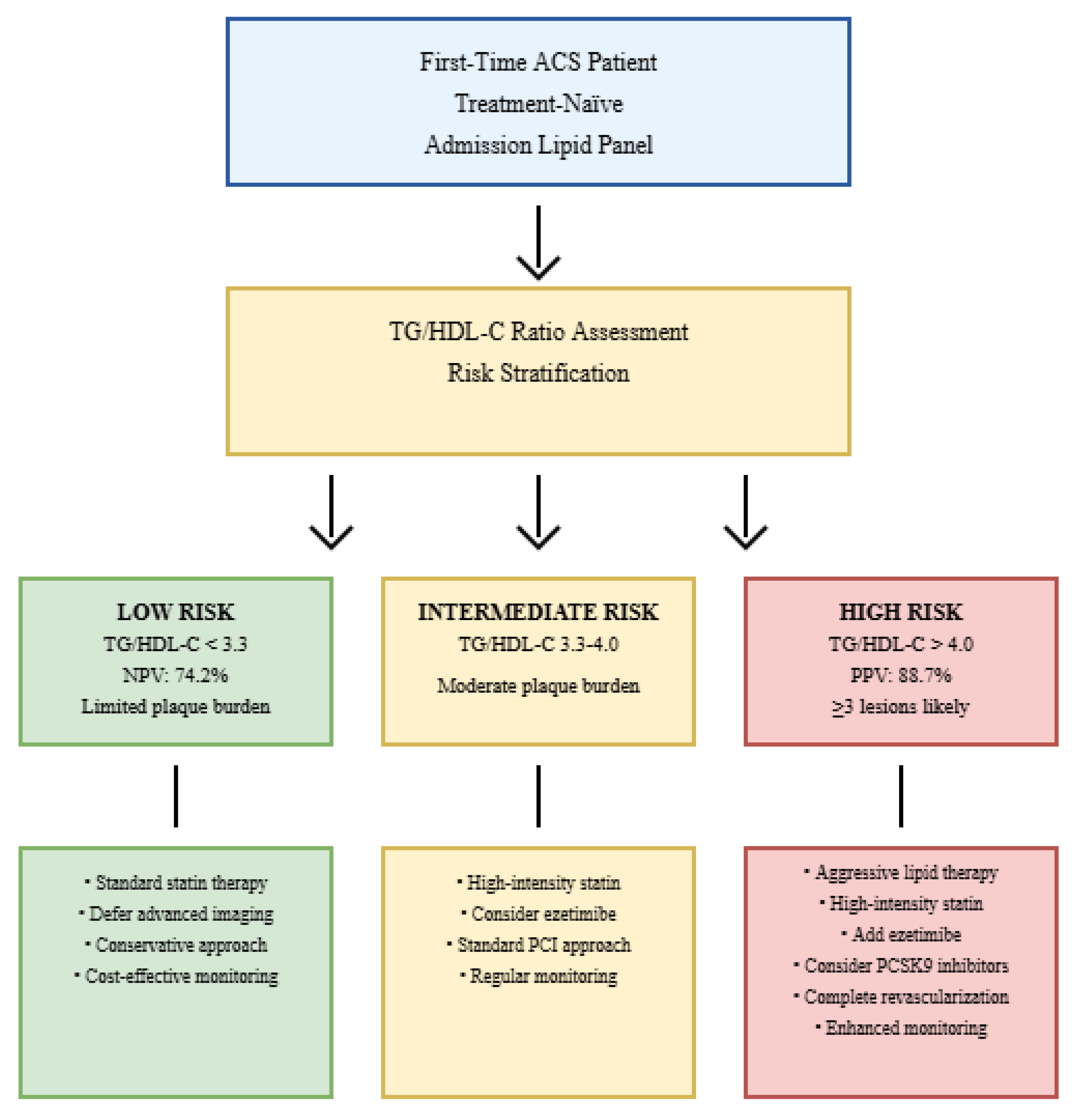
- >3.3: Sensitivity 76.5%, Specificity 76.8%, PPV 78.9%, NPV 74.2%
- >4.0: Sensitivity 68.4%, Specificity 89.5%, PPV 88.7%, NPV 70.1%
- >4.5: Sensitivity 52.1%, Specificity 93.7%, PPV 91.3%, NPV 61.2%
3.4. Multivariable Analysis
3.4.1. Independent Predictor Analysis
3.4.2. Subgroup Analysis Results
3.5. Cost-Effectiveness and Implementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| AUC | Area Under the Curve |
| CAD | Coronary Artery Disease |
| CHD | Coronary Heart Disease |
| HDL-C | High-Density Lipoprotein Cholesterol |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| MetS | Metabolic Syndrome |
| NPV | Negative Predictive Value |
| NSTEMI | Non-ST-Elevation Myocardial Infarction |
| OCT | Optical Coherence Tomography |
| PAD | Peripheral Artery Disease |
| PCI | Percutaneous Coronary Intervention |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| PPV | Positive Predictive Value |
| QCA | Quantitative Coronary Analysis |
| sdLDL | Small Dense Low-Density Lipoprotein |
| STEMI | ST-Segment Elevation Myocardial Infarction |
| TC | Total Cholesterol |
| TG | Triglycerides |
References
- Otrante A, Bounafaa A, Berrougui H, Essamadi AK, Nguyen M, Fülöp T, Khalil A. Small Dense LDL Level and LDL/HDL Distribution in Acute Coronary Syndrome Patients. Biomedicines. 2023 Apr 18;11(4):1198. [CrossRef] [PubMed] [PubMed Central]
- Guijarro C, Cosin-Sales J. LDL cholesterol and atherosclerosis: The evidence. Clin Investig Arter Publ Off Soc Esp Arterioscler. 2021; 33(Suppl. S1):25-32.
- Welty, FK. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr Cardiol Rep. 2013;15:400.
- Bulnes JF, González L, Velásquez L, Orellana MP, Venturelli PM, et al. Role of inflammation and evidence for the use of colchicine in patients with acute coronary syndrome. Front Cardiovasc Med. 2024 Jun 27;11:1356023. [CrossRef] [PubMed] [PubMed Central]
- Writing C, Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Covington AM, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr, et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80:1366-1418.
- Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J Am Coll Cardiol. 2020;75:2122-2135.
- Kosmas CE, Rodriguez Polanco S, Bousvarou MD, Papakonstantinou EJ, Peña Genao E, Guzman E, Kostara CE. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics (Basel). 2023 Mar 1;13(5):929. [CrossRef] [PubMed] [PubMed Central]
- Bergmark BA, Mathenge N, Merlini PA, Lawrence-Wright MB, Giugliano RP. Acute coronary syndromes. Lancet. 2022 Apr 2;399(10332):1347-1358. [CrossRef] [PubMed] [PubMed Central]
- Tani, S. The Ratio of Triglyceride to High-density Lipoprotein Cholesterol as an Indicator of Risk Stratification for Atherosclerotic Cardiovascular Disease in a Clinical Setting. Intern Med. 2020;59:2639-2640.
- Onea HL, Spinu M, Homorodean C, Ober MC, Olinic M, Lazar FL, Achim A, Tataru DA, Olinic DM. Superficial Calcified Plates Associated to Plaque Erosions in Acute Coronary Syndromes. Life (Basel). 2023 Aug 11;13(8):1732. [CrossRef] [PubMed] [PubMed Central]
- Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodules in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013 Nov 5;62(19):1748-58. [CrossRef] [PubMed] [PubMed Central]
- Sugiyama T, Yamamoto E, Fracassi F, Lee H, Yonetsu T, Kakuta T, Soeda T, Saito Y, Yan BP, Kurihara O, Takano M, Niccoli G, Crea F, Higuma T, Kimura S, Minami Y, Ako J, Adriaenssens T, Boeder NF, Nef HM, Fujimoto JG, Fuster V, Finn AV, Falk E, Jang IK. Calcified Plaques in Patients With Acute Coronary Syndromes. JACC Cardiovasc Interv. 2019 Mar 25;12(6):531-540. [CrossRef] [PubMed]
- Alfonso F, Gonzalo N, Nuñez-Gil I, Bañuelos C. Coronary thrombosis from large, nonprotruding, superficial calcified coronary plaques. J Am Coll Cardiol. 2013;62:2254.
- Pogran E, Burger AL, Zweiker D, Kaufmann CC, Muthspiel M, Rega-Kaun G, Wenkstetten-Holub A, Wojta J, Drexel H, Huber K. Lipid-Lowering Therapy after Acute Coronary Syndrome. J Clin Med. 2024 Apr 1;13(7):2043. [CrossRef] [PubMed] [PubMed Central]
- Chen H, Chen X. PCSK9 inhibitors for acute coronary syndrome: the era of early implementation. Front Cardiovasc Med. 2023 May 2;10:1138787. [CrossRef] [PubMed] [PubMed Central]
- Kim N, Cho JM, Yang IH. Recurrent Acute Coronary Syndrome in Young Man with Familial Hypercholesterolemia: Efficacy of Evolocumab Add-On Treatment Demonstrated via Serial Coronary Angiography. Biomedicines. 2024 May 17;12(5):1113. [CrossRef] [PubMed] [PubMed Central]
- Lagace, TA. PCSK9 and LDLR Degradation. Curr Opin Lipidol. 2014;25:387-393.
- Cohen M, Visveswaran G. Defining and managing patients with non-ST-elevation myocardial infarction: Sorting through type 1 vs other types. Clin Cardiol. 2020 Mar;43(3):242-250. [CrossRef] [PubMed] [PubMed Central]
- Lee ZV, Lam H. Aggressive lipid-lowering therapy after percutaneous coronary intervention - for whom and how?: Aggressive lipid-lowering therapy after PCI. AsiaIntervention. 2022 Mar;8(1):24-31. [CrossRef] [PubMed] [PubMed Central]
- Wu G, Yu G, Zheng M, Peng W, Li L. Recent Advances for Dynamic-Based Therapy of Atherosclerosis. Int J Nanomedicine. 2023 Jul 13;18:3851-3878. [CrossRef] [PubMed] [PubMed Central]
- Chen X, Rong C, Qi P, Bai W, Yao W, Zhang Y, Dang Y. LDL-C and Total Stent Length are Independent Predictors of Periprocedural Myocardial Injury and Infarction for Unstable Angina Patients Undergoing Elective Percutaneous Coronary Intervention. Int J Gen Med. 2021 Apr 16;14:1357-1365. [CrossRef] [PubMed] [PubMed Central]
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, et al; ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-3826. doi: 10.1093/eurheartj/ehad191. Erratum in: Eur Heart J. 2024 Apr 1;45(13):1145. [CrossRef] [PubMed]
- Zhou S, Qiu M, Wang K, Li J, Li Y, Han Y. Triglyceride to high density lipoprotein cholesterol ratio and major adverse cardiovascular events in ACS patients undergoing PCI. Sci Rep. 2024 Dec 30;14(1):31752. [CrossRef] [PubMed] [PubMed Central]
- Koide Y, Miyoshi T, Nishihara T, Nakashima M, Ichikawa K, Miki T, et al. The Association of Triglyceride to High-Density Lipoprotein Cholesterol Ratio with High-Risk Coronary Plaque Characteristics Determined by CT Angiography and Its Risk of Coronary Heart Disease. J Cardiovasc Dev Dis. 2022 Sep 28;9(10):329. [CrossRef] [PubMed] [PubMed Central]
- Kosmas CE, Silverio D, Tsomidou C, Salcedo MD, Montan PD, Guzman E. The Impact of Insulin Resistance and Chronic Kidney Disease on Inflammation and Cardiovascular Disease. Clin Med Insights Endocrinol Diabetes. 2018;11:1179551418792257.
- Atamas SP, Chapoval SP, Keegan AD. Cytokines in chronic respiratory diseases. F1000 Biol Rep. 2013;5:3.
- Li Y, Deng S, Liu B, Yan Y, Du J, et al. The effects of lipid-lowering therapy on coronary plaque regression: a systematic review and meta-analysis. Sci Rep. 2021 Apr 12;11(1):7999. [CrossRef] [PubMed] [PubMed Central]
- Lin X, Huang J, Lin H, Chen P. Apelin-to-total cholesterol ratio predicts long-term major adverse cardiovascular events in ST-elevation myocardial infarction patients after primary percutaneous coronary intervention: a retrospective cohort analysis. BMC Cardiovasc Disord. 2025 Jul 4;25(1):478. [CrossRef] [PubMed] [PubMed Central]
- Dimitriadis K, Pyrpyris N, Iliakis P, Beneki E, Adamopoulou E, Papanikolaou A, Konstantinidis D, Fragkoulis C, Kollias A, Aznaouridis K, Tsioufis K. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Patients Following Acute Coronary Syndromes: From Lipid Lowering and Plaque Stabilization to Improved Outcomes. J Clin Med. 2024 Aug 25;13(17):5040. [CrossRef] [PubMed] [PubMed Central]
- Wu TT, Gao Y, Zheng YY, Ma YT, Xie X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018;17:197.
- Liu T, Liu J, Wu Z, Lv Y, Li W. Predictive value of the atherogenic index of plasma for chronic total occlusion before coronary angiography. Clin Cardiol. 2021;44:518-525.
- Bhardwaj S, Bhattacharjee J, Bhatnagar MK, Tyag S. Atherogenic index of plasma, Castelli risk index and atherogenic coefficient- new parameters in assessing cardiovascular risk. Int J Pharm Biol Sci. 2013;3:354-364.
- 33Mortensen MB, Dzaye O, Bøtker HE, Jensen JM, Maeng M, et al. Low-Density Lipoprotein Cholesterol Is Predominantly Associated With Atherosclerotic Cardiovascular Disease Events in Patients With Evidence of Coronary Atherosclerosis: The Western Denmark Heart Registry. Circulation. 2023 Apr 4;147(14):1053-1063. [CrossRef] [PubMed]
- Yu M, Yang Y, Dong SL, Zhao C, Yang F, et al. Effect of Colchicine on Coronary Plaque Stability in Acute Coronary Syndrome as Assessed by Optical Coherence Tomography: The COLOCT Randomized Clinical Trial. Circulation. 2024 Sep 24;150(13):981-993. [CrossRef] [PubMed]
- Priest VL, Scuffham PA, Hachamovitch R, Marwick TH. Cost-effectiveness of coronary computed tomography and cardiac stress imaging in the emergency department: a decision analytic model comparing diagnostic strategies for chest pain in patients at low risk of acute coronary syndromes. JACC Cardiovasc Imaging. 2011;4:549-56.
- Baron SJ, Korjian S, Gibson CM, Reynolds MR. Cost-Effectiveness of the CADScor System in Low-Risk Patients Presenting to the Emergency Department with Chest Pain. Pharmacoecon Open. 2025 Jun 23. Epub ahead of print. [CrossRef] [PubMed]
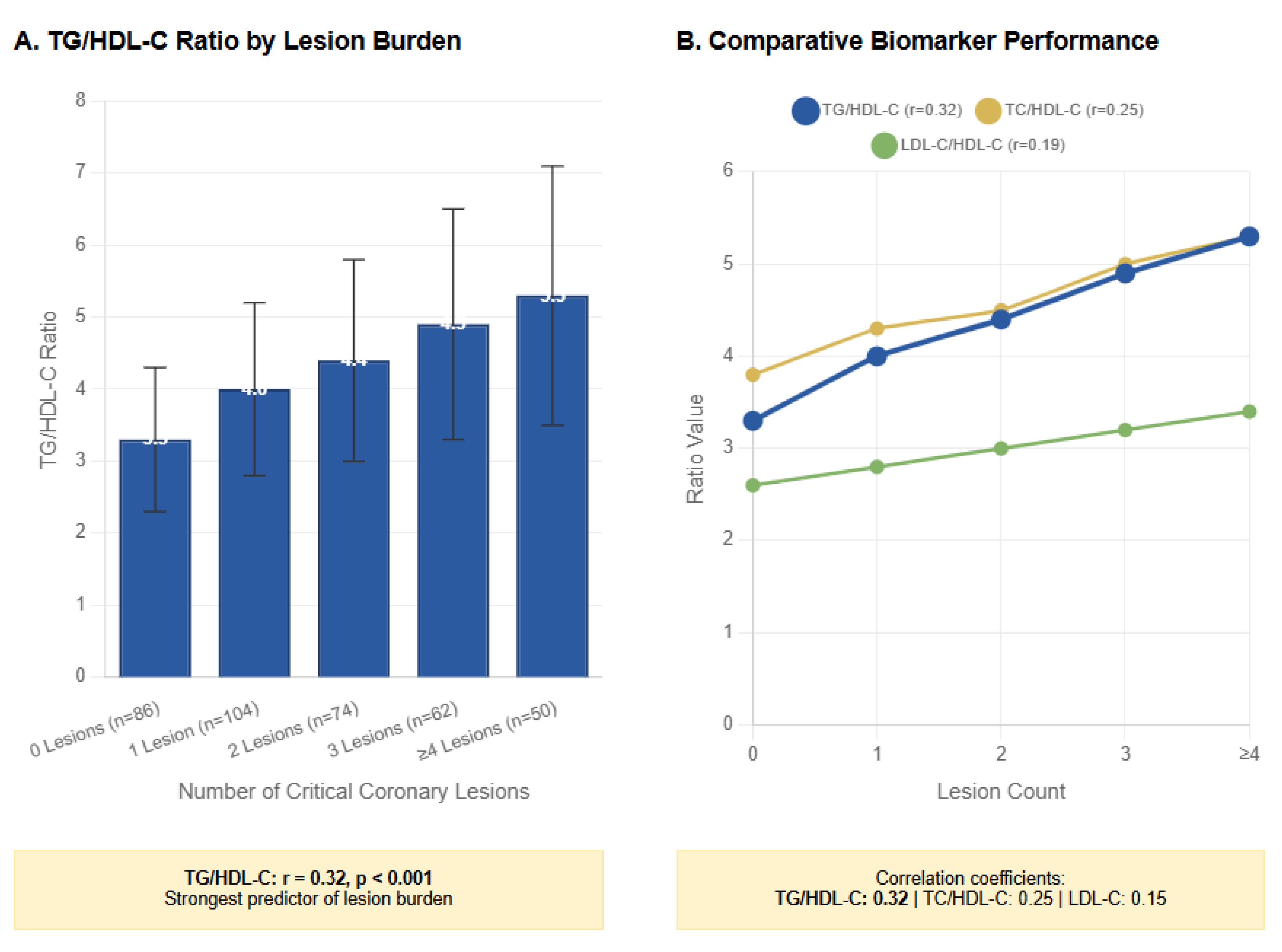
| Variable | 0 Lesions (n=86) | 1 Lesion (n=104) | 2 Lesions (n=74) | 3 Lesions (n=62) | ≥4 Lesions (n=50) | p-value* |
|---|---|---|---|---|---|---|
| Diagnostic Cohort | ||||||
| Age (years) | 62.1 ± 9.8 | 64.3 ± 10.2 | 65.8 ± 9.7 | 66.2 ± 10.5 | 67.5 ± 9.9 | 0.12 |
| Male sex, n (%) | 60 (69.8) | 75 (72.1) | 50 (67.6) | 40 (64.5) | 30 (60.0) | 0.45 |
| Cardiovascular Risk | ||||||
| Hypertension, n (%) | 50 (58.1) | 60 (57.7) | 45 (60.8) | 35 (56.5) | 25 (50.0) | 0.67 |
| Diabetes, n (%) | 30 (34.9) | 40 (38.5) | 25 (33.8) | 20 (32.3) | 15 (30.0) | 0.89 |
| Current smoking, n (%) | 40 (46.5) | 50 (48.1) | 35 (47.3) | 30 (48.4) | 20 (40.0) | 0.75 |
| ACS Presentation | ||||||
| STEMI, n (%) | 54 (62.8) | 62 (59.6) | 48 (64.9) | 41 (66.1) | 35 (70.0) | 0.75 |
| NSTEMI, n (%) | 30 (34.9) | 40 (38.5) | 25 (33.8) | 20 (32.3) | 15 (30.0) | 0.85 |
| Unstable angina, n (%) | 2 (2.3) | 2 (1.9) | 1 (1.4) | 1 (1.6) | 0 (0.0) | 0.80 |
| Lipid Biomarker (mg/dL) | 0 Lesions (n=86) | 1 Lesion (n=104) | 2 Lesions (n=74) | 3 Lesions (n=62) | ≥4 Lesions (n=50) | p-value* | Correlation (r) |
|---|---|---|---|---|---|---|---|
| LDL-C | 110.0 ± 25.0 | 115.0 ± 30.0 | 120.0 ± 35.0 | 125.0 ± 40.0 | 130.0 ± 45.0 | 0.03 | 0.15 |
| HDL-C | 42.0 ± 8.0 | 41.0 ± 7.0 | 40.0 ± 9.0 | 39.0 ± 8.0 | 38.0 ± 7.0 | 0.25 | -0.12 |
| Triglycerides | 140.0 ± 50.0 | 160.0 ± 60.0 | 175.0 ± 65.0 | 190.0 ± 70.0 | 200.0 ± 80.0 | 0.20 | 0.18 |
| Lipid Ratios | |||||||
| LDL-C/HDL-C | 2.6 ± 0.7 | 2.8 ± 0.8 | 3.0 ± 0.9 | 3.2 ± 1.0 | 3.4 ± 1.1 | 0.04 | 0.19 |
| TC/HDL-C | 3.8 ± 0.9 | 4.3 ± 1.0 | 4.5 ± 1.1 | 5.0 ± 1.2 | 5.3 ± 1.3 | 0.02 | 0.25 |
| TG/HDL-C | 3.3 ± 1.0 | 4.0 ± 1.2 | 4.4 ± 1.4 | 4.9 ± 1.6 | 5.3 ± 1.8 | 0.01 | 0.32 |
| TG/HDL-C Threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC (95% CI) | Optimal Use Case |
|---|---|---|---|---|---|---|
| >3.0 | 82.1 | 65.3 | 71.4 | 77.8 | 0.68 (0.61–0.75) | Rule out low burden |
| >3.3 | 76.5 | 76.8 | 78.9 | 74.2 | 0.71 (0.64–0.78) | Low-risk identification |
| >3.7 | 70.3 | 81.2 | 82.1 | 69.0 | 0.73 (0.66–0.80) | Intermediate-risk screening |
| >4.0 | 68.4 | 89.5 | 88.7 | 70.1 | 0.72 (0.65–0.79) | High-risk identification |
| >4.5 | 52.1 | 93.7 | 91.3 | 61.2 | 0.70 (0.63–0.77) | Confirmatory testing |
| Predictor | β-coefficient | Standard Error | p-value | Diagnostic Odds Ratio (95% CI) | Contribution to Model |
|---|---|---|---|---|---|
| TG/HDL-C | 0.18 | 0.05 | 0.02 | 1.20 (1.07–1.34) | 42% |
| LDL-C | 0.14 | 0.07 | 0.04 | 1.15 (1.01–1.31) | 33% |
| Age | 0.09 | 0.06 | 0.11 | 1.09 (0.98–1.22) | 12% |
| Male sex | 0.11 | 0.13 | 0.09 | 1.12 (0.87–1.44) | 8% |
| Diabetes | 0.13 | 0.14 | 0.09 | 1.14 (0.87–1.49) | 5% |
| Hypertension | 0.08 | 0.12 | 0.15 | 1.08 (0.86–1.36) | 2% |
| Current smoking | 0.10 | 0.11 | 0.08 | 1.11 (0.89–1.38) | 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





