# These authors contributed equally to this work and should be considered co-first authors.
1. Introduction
The increasing frequency of bacterial resistance, coupled with the global antibiotic crisis, highlights the urgent need for innovation in antimicrobial research.[
1], with small-molecule antimicrobial peptide mimics emerging as a promising research field.. These mimics not only have the broad-spectrum activity and multi-target mechanism of natural antimicrobial peptides, but also show better performance in terms of stability, cost and bioavailability, laying a solid foundation for the development of new antimicrobial drugs.[
2].
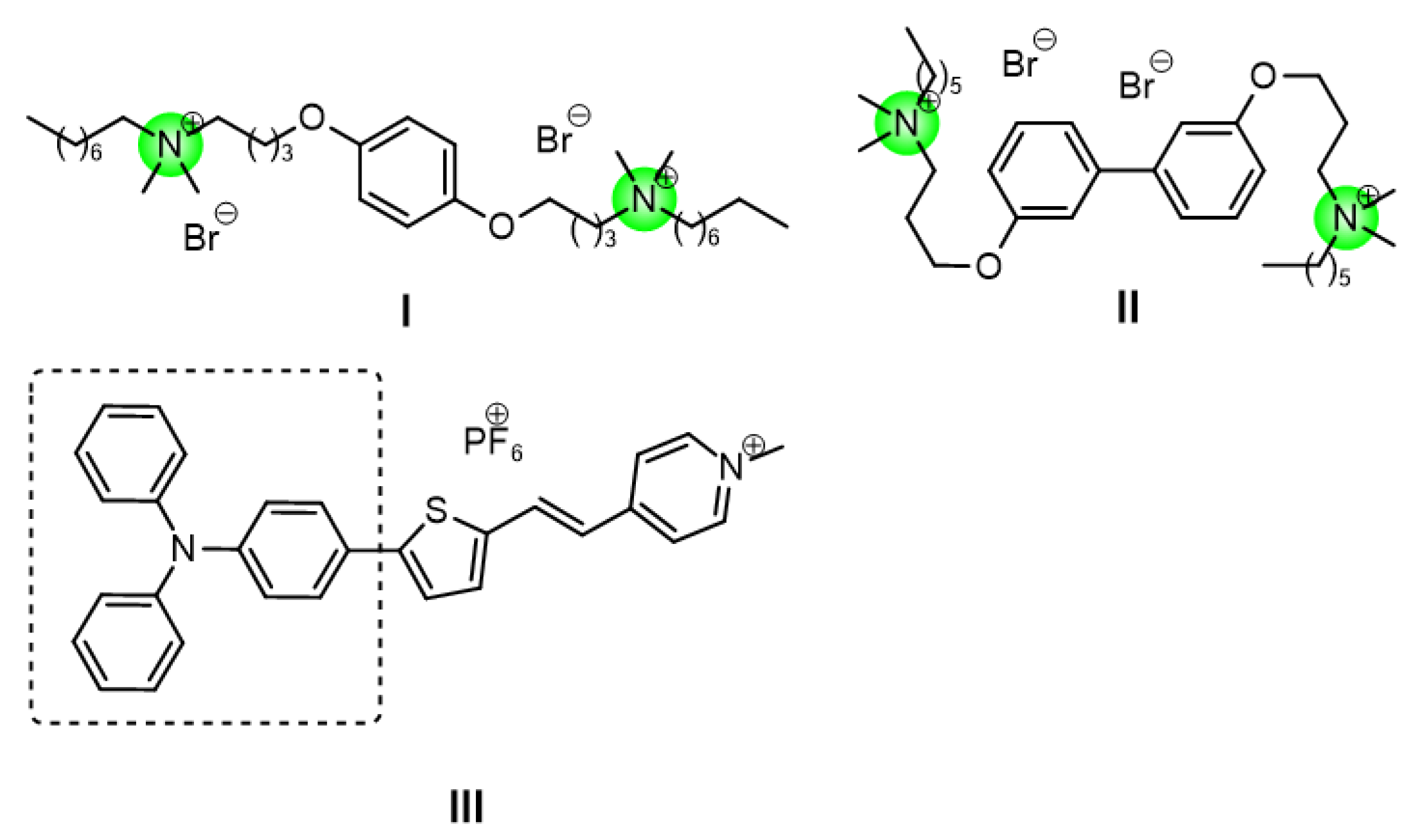
Small-molecule antimicrobial peptide mimics of quaternary ammonium salts are a class of compounds centered on positively charged quaternary ammonium cationic head groups. They form amphiphilic linear structures by combining with hydrophobic long-chain alkyl or aromatic groups. These mimics target bacterial cell membranes through their amphiphilic structures and achieve bactericidal effects by physically disrupting membrane integrity. This unique membrane-lytic mechanism is less likely to induce bacterial drug resistance, thus providing a promising strategy to address the problem of bacterial resistanc. Our research group has also been continuously exploring new small molecule antimicrobial peptide mimics to identify potent mimetics with good biocompatibility (
Figure 1,
I,
II)[
3,
4].
Triphenylamine (TPA) is a tertiary amine with a central nitrogen atom bonded to three benzene rings[
5]. The central nitrogen atom is coplanar with the three adjacent carbon atoms, and the three benzene rings adopt a propeller-like arrangement. The triphenylamine (TPA) moiety exhibits high hydrophobicity, and a significant hyper-conjugative electronic effect; its propeller-like twisted configuration and strong electron-donating ability make it highly favorable for constructing photoactive fluorescent materials[
6,
7,
8]. In recent years, a series of D−π−A (donor−π−acceptor) structures based on triphenylamine derivatives have been reported. Amongst these, pyridine-substituted triphenylamine derivatives have been the most extensively studied and are widely used in the fields of bacterial detection, imaging, and elimination[
9,
10,
11,
12,
13]. Therefore, triphenylamine is regarded as an ideal building block for constructing multifunctional antimicrobial agents. Tang et al. used pyridinium cation as the electron acceptor and triphenylamine as the electron donor to synthesize triphenylamine derivatives (with a D-π-A structure) (
Figure 1, III).[
9].
In this work, triphenylamine quaternary ammonium salt derivatives (TPQ) were designed and synthesized, which provides a new idea for the design of antibacterial agents with excellent biocompatibility.
2. Results and Discussion
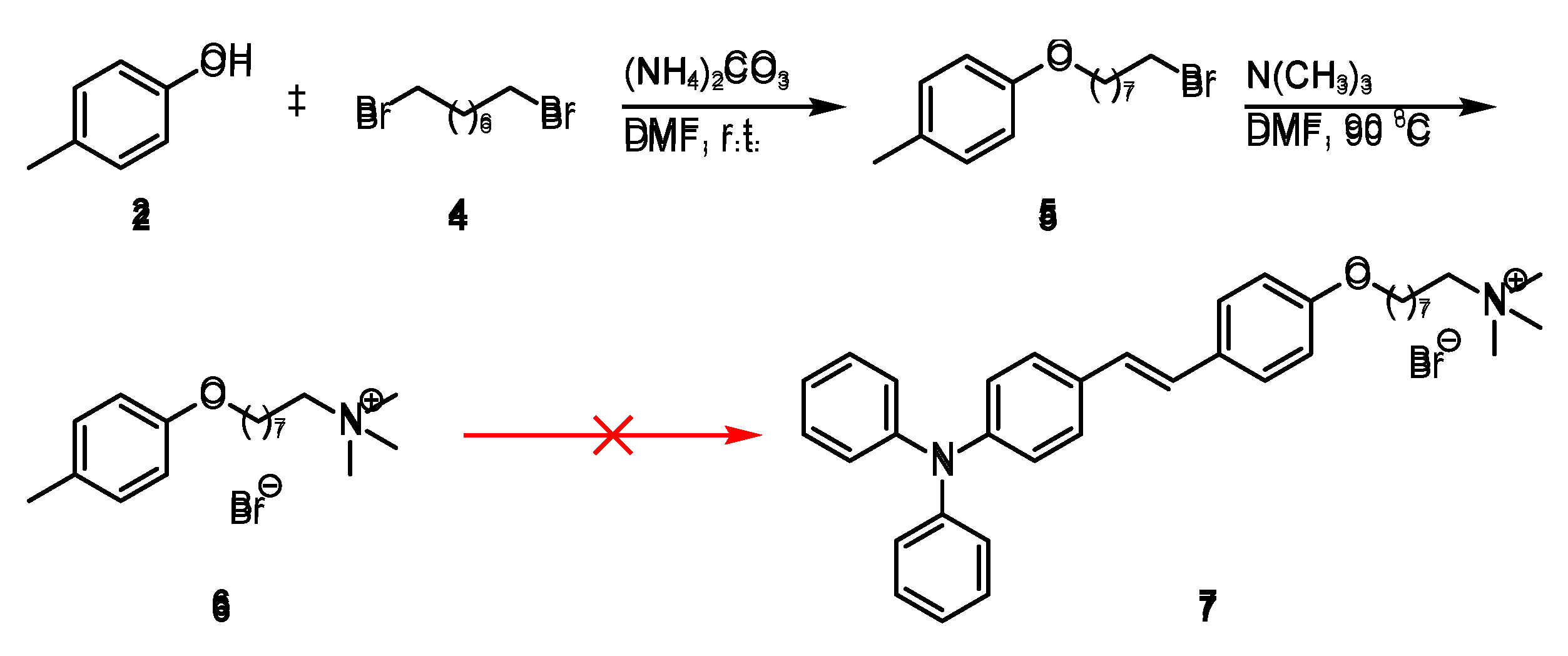
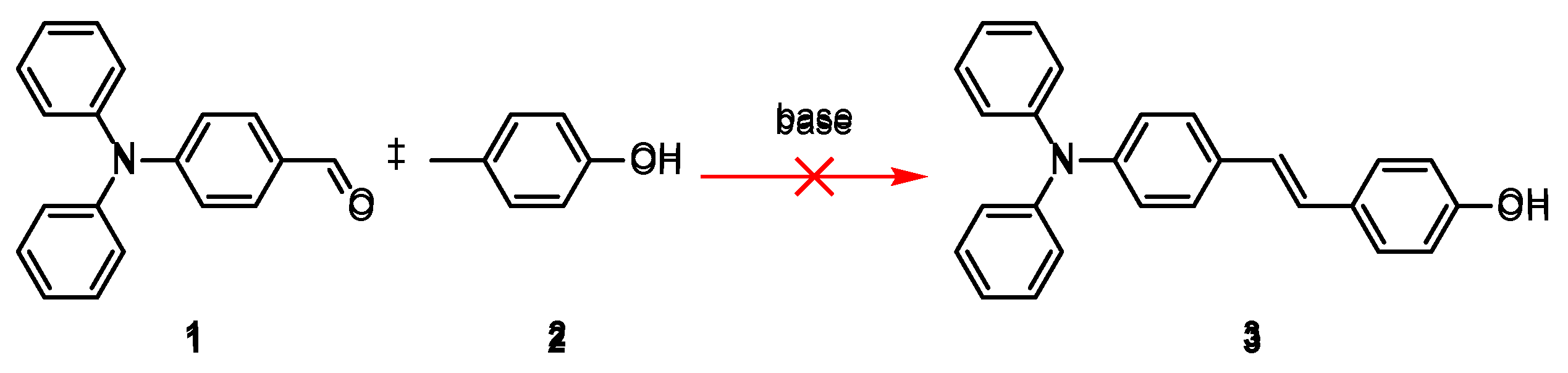
Following the previously reported synthetic route for pyridinium cations [
9], piperidine was used as the base, but the expected product was not obtained (
Scheme 1 and
Scheme 3). Therefore, it is speculated that this result is due to the insufficient electron-withdrawing ability of p-cresol, which leads to the low reactivity of the benzene ring.
Therefore,
p-cresol was planned to be quaternized (to become a cation) to increase its reactivity (
Scheme 2). First, ammonium carbonate was used as a base and
N,
N-dimethylformamide (DMF) was used as a solvent, and the reaction was carried out at room temperature overnight. The phenolic hydroxyl group of
p-cresol acts as a nucleophile and attacks the carbon atom connected to the bromine atom in the molecule to afford compound
5. In the next step, nucleophilic substitution occurs when trimethylamine attacks the terminal bromine atom of the aliphatic chain in compound
5, causing the bromine atom to act as the leaving group. Using DMF as solvent, the reaction was carried out at 90 °C for 12 h to generate a quaternary ammonium salt compound
6. Finally, attempts to react compound
6 with compound 1 were unsuccessful, despite the presence of various catalysts such as piperidine,
p-toluenesulfonic acid, and sodium ethoxide.
Scheme 2.
Attempts on the synthetic route of (E)-2-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium (7).
Scheme 2.
Attempts on the synthetic route of (E)-2-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium (7).
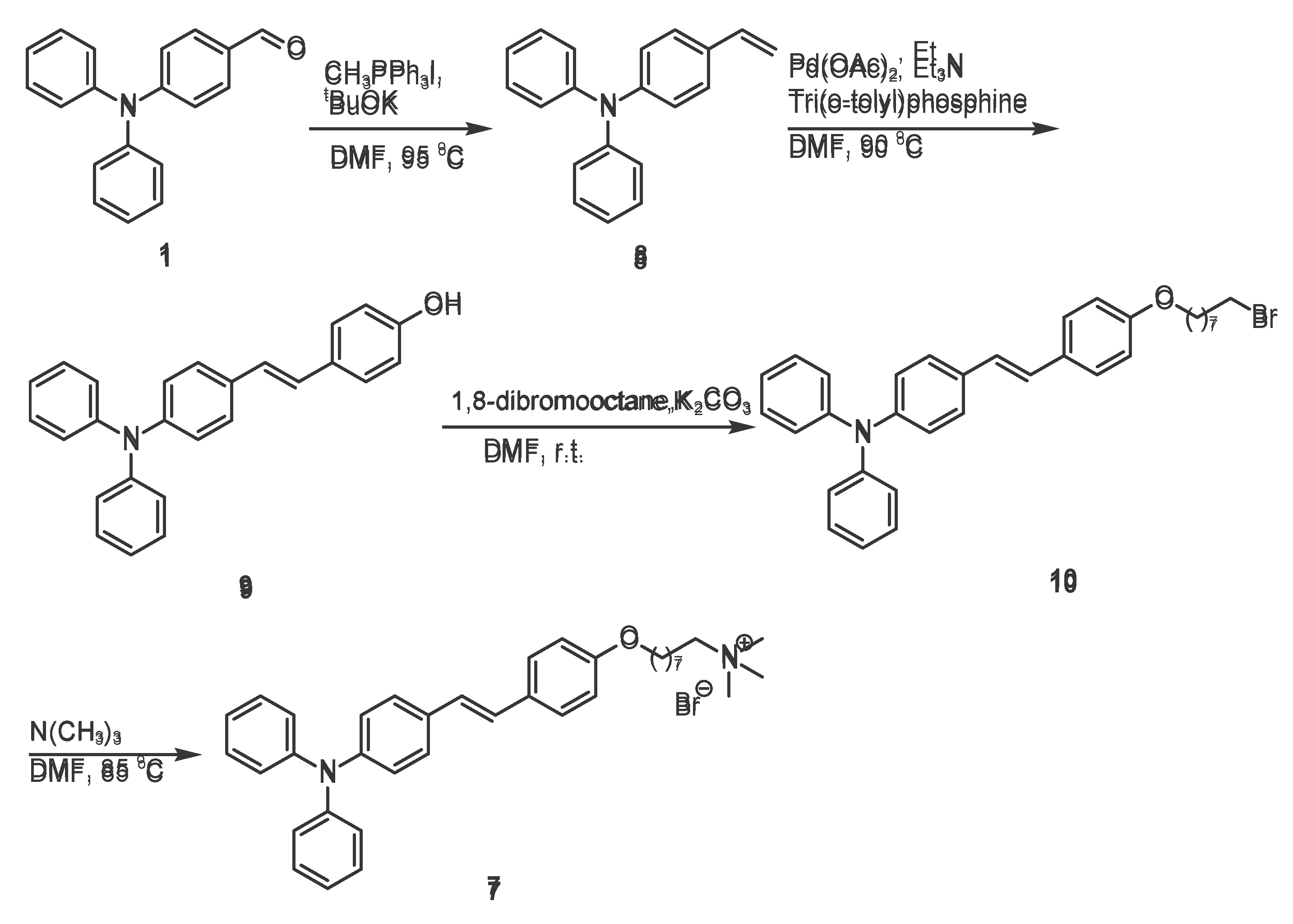
Scheme 3.
Synthetic Route of (E)-2-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium (7).
Scheme 3.
Synthetic Route of (E)-2-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium (7).
To guide the design of the next experiments, a comprehensive review of the literature was conducted. [
14]. With 4-(diphenylamino) benzaldehyde (
Scheme 3, 1) as the starting material, a Wittig reaction was carried out in N,N-dimethylformamide (DMF) using methyltriphenylphosphine iodide and potassium tert-butoxide as the Wittig reagent and base, respectively. The reaction gave 4-(diphenylamino)styrene (compound 8), which provided the key carbon-carbon double bond for the subsequent Heck coupling reaction.. A Heck reaction was then carried out with p-bromophenol in DMF at 90 °C for 10 h, using palladium acetate as the catalyst, tris(o-tolyl)phosphine as the ligand, and triethylamine as the base. In this reaction, the carbon-carbon double bond of 4-(diphenylamino)styrene
8 coupled with the brominated benzene ring of
p-bromophenol, a hydroxyl group (-OH) was introduced simultaneously to generate an intermediate
9 containing both a double bond and a hydroxyl group. This intermediate provides a hydroxyl site for subsequent substitution reactions (
Scheme 3).
Subsequently, the hydroxyl group in the intermediate
9 (Scheme 3) molecule acts as a nucleophile, attacking the brominated carbon atom of 1,8-dibromooctane molecule and displacing the bromide atom. This nucleophilic substitution reaction forms an ether bond and simultaneously introduces a brominated alkyl chain, thereby generating an intermediate
10 that serves as a reaction site for the subsequent quaternization reaction.
The final triphenylamine quaternary ammonium derivative
7 was obtained by reacting the intermediate
10 (
Scheme 3) with trimethylamine in DMF at 85 °C overnight. In this quaternization reaction, the bromine atom of the intermediate
10 is replaced by the nitrogen atom of trimethylamine to generate an alkyl-linked quaternary ammonium group and introduce an eight-carbon hydrophobic chain to give the final compound
7.
This synthetic route employs a stepwise construction strategy, in which functional groups and structural motifs are installed sequentially. Starting from a simple aldehyde, a carbon-carbon double bond, a hydroxyl functional group, a brominated alkyl chain, and a quaternary ammonium unit are sequentially introduced; each transformation provides the desired structural control for subsequent reactions.
3. Materials and Methods
3.1. Chemicals and Instrumentations
Unless otherwise specified, all reagents used were of analytical grade. The key reagents included: 4-(diphenylamino)benzaldehyde, methyltriphenylphosphonium iodide, potassium tert-butoxide, N,N-dimethylformamide, p-bromophenol, palladium acetate, tris(o-tolyl)phosphine, triethylamine, 1,8-dibromooctane, and potassium carbonate. The main instruments were as follows: Thermostatic heating magnetic stirrer (Model DF-101S), manufactured by Shanghai Yukang Science and Education Instrument Equipment Co, Ltd , Rotary evaporator (Model N-1100V), obtained from EYELA (Tokyo Rika Kikai Co., Ltd., Japan); Chemical diaphragm pump (Model MZ 2C NT), product of Vacuubrand GmbH, Germany; Vacuum drying oven (Model DZF-6020), supplied by Gongyi Jinghua Instrument Co., Ltd.; Circulating water vacuum pump (Model SHZ-D(Ⅲ)), produced by Zhengzhou Yuxiang Instrument Equipment Co., Ltd.; Electronic balance (Model ME203E), brand of Mettler Toledo, Switzerland; Triple-purpose UV analyzer (Model ZF-7), manufactured by Shanghai Kanghua Biochemical Instrument Co., Ltd.; Ultrasonic cleaner (Model KQ5200), obtained from Kunshan Ultrasonic Instrument Co., Ltd.; Automatic image melting point apparatus (Model SGW®-650), produced by Shanghai Yidian Physical Optics Instrument Co., Ltd.; High-resolution quadrupole-time-of-flight mass spectrometer (Model Q-TofMicro), product of Waters-Micromass Co., Ltd., USA; Superconducting nuclear magnetic resonance spectrometer (Model DPX-400), manufactured by Bruker Corporation, Germany.
3.2. N,N-Diphenyl-4-Vinylaniline (8)
To a solution of 4-(diphenylamino)benzaldehyde (3.00 g, 10.98 mmol, 1.00 equiv) and methyltriphenylphosphonium iodide (5.04 g, 12.48 mmol, 1.14 equiv) in DMF (30 mL), a DMF solution (45 mL) of potassium tert-butoxide (1.46 g, 12.99 mmol, 1.18 equiv) was added dropwise, stirred at room temperature for 30 minutes, and then heated to 95 °C for 13 h. After completion of the reaction, the mixture was extracted with water and dichloromethane (DCM). The organic layer was washed successively with water (80 mL) and 1 M HCl. The combined organic layer was dried over anhydrous Na2SO4, filtered, concentrated and then purified the crude product by column chromatography (Dichloromethane (DCM): Petroleum ether = 1:7, v: v) to obtain intermediate 8 (2.73 g ) as a white solid, with a yield of 91%.1H NMR (400 MHz, Chloroform-d) δ 7.22 – 7.10 (m, 6H), 7.04 – 6.96 (m, 4H), 6.96 – 6.87 (m, 4H), 6.56 (dd, J = 17.6, 10.9 Hz, 1H), 5.54 (dd, J = 17.6, 1.0 Hz, 1H), 5.05 (dd, J = 10.8, 1.0 Hz, 1H); 13C NMR (101 MHz, Chloroform-d) δ 147.74, 147.61, 136.34, 132.00, 129.38, 127.19, 124.50, 123.75, 123.05, 112.28.
3.3.(. E)-4-(4-(Diphenylamino)Styryl)Phenol (9)
In a three-necked round-bottomed flask (50 mL) , intermediate 8 (2 g, 7.37 mmol, 1.00 eq.), 4-bromophenol (1.28 g, 7.37 mmol, 1.00 eq.), palladium acetate (0.17 g, 0.74 mmol, 0.1 eq.) and o-trimethylphenylphosphine (0.45 g, 1.47 mmol, 0.2 eq.) were dissolved in a mixed solution consisting of TEA/DMF (4:1, v/v, 15 mL ) and triethylamine (2.05 mL, 14.74 mmol, 2.00 eq.). Under nitrogen protection, the reaction mixture was heated to 90 °C and stirred for 6 hours. After completion of the reaction, the mixture cooled to room temperature, and then extracted with water (30 mL) and DCM (30 mL) The combined organic phases were washed with water (30 mL) three times, then washed with saturated sodium chloride solution (10 mL) 3 times, dried over anhydrous anhydrous Na2SO4, filtered, and concentrated by vacuum rotary evaporation. The crude product was purified by silica gel column chromatography (eluent: dichloromethane: petroleum ether = 2:1, v: v) to afford intermediate 9 (0.99 g) as a yellow solid with a yield of 50%. 1H NMR (400 MHz, Chloroform-d) δ 7.36 (dd, J = 10.2, 7.7 Hz, 4H), 7.29 – 7.19 (m, 4H), 7.16 – 6.97 (m, 8H), 6.92 (d, J = 2.9 Hz, 2H), 6.85 – 6.76 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 163.32, 155.91, 147.75, 147.02, 132.20, 130.18, 129.40, 127.81, 127.19, 126.99, 125.91, 124.46, 123.99, 123.01, 115.87, 37.02, 31.96.
3.4.(. E)-4-(4-(2-Bromooctyl)Oxy)Styryl)-N,N-Diphenylaniline (10)
In a round-bottomed flask (50 mL), intermediate 9 (2 g, 5.5 mmol, 1.00 eq.), 1,8-dibromooctane (0.72 mL, 7.15 mmol, 1.30 eq.), and potassium carbonate (1.52 g, 11.01 mmol, 2 eq.) were dissolved in N,N-dimethylformamide (DMF, 15 mL). The reaction mixture was stirred at room temperature for 24 h. After completion of the reaction, the mixture cooled to room temperature, and then extracted with water (20 mL) and DCM (20 mL). The combined organic phase was washed with water (3 × 20 mL) and saturated NaCl solution (3 × 10 mL), then dried over anhydrous Na₂SO₄.. After filtration, the mixture was concentrated by rotary evaporation under reduced pressure. The crude product was purified by silica gel column chromatography (dichloromethane: petroleum ether = 1: 5, v/v), yielding intermediate 10 (0.88 g) as a pale yellow solid with a yield of 44%. 1H NMR (400 MHz, Chloroform-d) δ 7.38 (dd, J = 21.5, 8.7 Hz, 4H), 7.26 (s, 2H), 7.22 (s, 2H), 7.13 – 7.07 (m, 4H), 7.05 – 6.98 (m, 4H), 6.96 – 6.84 (m, 4H), 3.96 (s, 2H), 3.41 (d, J = 6.7 Hz, 2H), 1.89 – 1.65 (m, 4H), 1.49 – 1.32 (m, 8H). 13C NMR (101 MHz, Chloroform-d) δ 158.74, 147.72, 147.03, 132.13, 130.35, 129.36, 127.59, 127.16, 126.85, 126.08, 124.45, 123.95, 122.99, 114.79, 68.07, 34.12, 32.88, 29.33, 29.30, 28.80, 28.20, 26.06.
3.5.(. E)-2-(4-(4-(Diphenylamino)Styryl)Phenoxy)-N,N,N-Trimethyloctan-1-Aminium (7)
In a round-bottomed flask (25 mL), compound 10 (0.2 g, 0.41 mmol, 1.00 eq.) and trimethylamine (0.78 mL, 2.06 mmol, 5 eq.) were dissolved in N,N-dimethylformamide (DMF, 8 mL). The reaction mixture was heated to 85 °C and stirred for 12 hours. After the reaction was completed and cooled to room temperature, the solvent was removed by rotary evaporation. The product was precipitated with petroleum ether (100 mL), followed by filtration, yielding the final product 7( 0.1 g) as a yellow solid with a yield of 54% and a melting range of 279.6 – 281.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.53 – 7.43 (m, 4H), 7.30 (t, J = 7.7 Hz, 4H), 7.09 – 6.88 (m, 12H), 3.97 (t, J = 6.5 Hz, 2H), 3.34 – 3.28 (m, 2H), 3.08 (s, 9H), 1.76 – 1.61 (m, 4H), 1.35 (t, J = 6.6 Hz, 8H).13C NMR (101 MHz, DMSO-d6) δ 158.73, 147.56, 146.80, 132.41, 130.33, 130.10, 128.09, 127.81, 127.18, 126.05, 124.49, 123.82, 123.66, 115.19, 67.96, 65.75, 52.63, 51.31, 29.16, 29.06, 28.97, 26.23, 25.95, 22.55. HRMS (ESI) Calculated for C37H45N2O+: 533.3526, found: 533.3531.