Introduction
Peritoneal metastasis (PM) is a common pattern of distant metastasis in adenocarcinoma of the stomach (GC) or esophagogastric junction (AEG). Despite the fact that preoperative staging involving both endoscopy and computed tomography (CT) is a standard requirement, typically the diagnostic accuracy, whether used separately or in combination, remains relatively low in reliably detecting PM [
1]. According to the European Society for Medical Oncology clinical practice guideline, laparoscopy with or without peritoneal washing is recommended in stage IB-II GC in order to exclude occult PM, especially in tumor stage T3/T4 disease [
2]. The Japanese Gastric Cancer Treatment Guidelines also include staging laparoscopy to determine PM [
3]. The Korean Practice Guidelines for Gastric Cancer stated that peritoneal washing cytology could be helpful in the staging of advanced disease [
4]. Previous studies reported comparable low survival prognosis and high recurrence in occult PM with cytology-positive peritoneal lavage, such as in patients with clear and obvious macroscopic peritoneal dissemination. The 5-year survival rate in those studies ranged from 2-5.8% [
5,
6,
7]. However, recent advancement in systemic therapies and surgical techniques, have contributed to the evolution of approaches. The current strategy involves the administration of neoadjuvant chemotherapy prior to performing curative surgical resection, aiming to improve patient outcomes [
8,
9,
10]. Notably, achieving negative cytology following preoperative systemic treatment has been strongly correlated with improvement in OS and reduction in peritoneal recurrence rate [
11,
12]. Consistent with this approach, cytological evaluation is required before undergoing radical gastrectomy.
To detect free peritoneal cancer cells (FPCC) in these patients, peritoneal lavage for cytology will benefit both diagnosis and prognosis. These FPCC typically resulted from spontaneous shedding of malignant cells from primary tumor. However, this can also be triggered by surgical trauma during tumor manipulation, intraoperative perforation, or lymphadenectomy. These cells can be implanted on the peritoneal surface with the aid of adhesive molecules. Following attachment, they can infiltrate the sub-peritoneal layer and continue to proliferate and spread. Various methods have proven effective for detecting FPCC. Newer techniques have been established to increase the sensitivity and specificity of diagnosis [
13,
14,
15]. Still, the most available and highly specific technique remains the gold standard conventional cytological evaluation by Papanicolaou staining [
16]. Determination of the factors that most highly suggest cytology-positive confer the most clinical benefit. Earlier studies reported poor differentiation, advanced T-stage, and lymph node involvement as factors that predict positivity of FPCC [
17,
18,
19]. A clear and confident understanding of the associated factors will help to identify patients who would benefit from preoperative systemic therapy before surgery. Accordingly, the aim of this study was to identify the independent predictors of FPCC, and to investigate survival outcomes relative to peritoneal cytology status among patients who underwent intended curative gastrectomy for GC or AEG.
Materials and Methods
This study was conducted at the Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. All patients aged 18 years or older diagnosed with GC or EGJ who underwent intended curative radical gastrectomy with lymphadenectomy and preoperative peritoneal cytology during January 2005 to December 2020 were reviewed. Demographic and clinicopathologic characteristics were collected and recorded. Lymph node ratio (LNR) is reported as a ratio of metastatic lymph nodes to total harvested lymph nodes. Depth of tumor invasion (T-stage) and lymph node metastasis (N-stage) were classified according to the American Joint Committee on Cancer 7th edition TNM staging system. Patients who received neoadjuvant therapy, pathologically confirmed metastatic disease prior to surgery, or active second primary cancer were excluded.
After general anesthesia, peritoneal washing for cytology was performed in all patients with high risk of occult PM including tumor with poorly differentiation, clinical T3-4 and/or presence of regional lymph node metastasis from preoperative imaging. Peritoneal cytology was obtained from peritoneal lavage using 200 ml of 0.9% sodium chloride solution administered into the peritoneal cavity at the upper abdominal cavity region and cul-de-sac before beginning the resection. The solution was collected by aspiration after 5 minutes and sent to the Department of Pathology at our center for processing and examination. Cytologic evaluation was performed using Giemsa and Papanicolaou staining. The results were categorized as negative for malignancy, positive for malignancy, or presence of atypical cells. Those with atypical cells exhibited the cytological features suggestive of malignancy but do not meet the definitive criteria for cancerous cells. They may display the nuclear enlargement, irregular nuclear contours, hyperchromasia, increased nuclear-to-cytoplasmic ratio, and mild to moderate pleomorphism. However, the unequivocal characteristics of malignant cells, such as prominent nucleoli, marked pleomorphism, or clear evidence of invasive growth were absence. After peritoneal washing was collected, the extent of surgery and lymphadenectomy were determined by adequate margin for resection and preoperative staging according to the Japanese Gastric Cancer Treatment Guideline [
3]. After surgery, standard regimen of adjuvant chemotherapy was given as indicated by pathology result, also the postoperative surveillance was scheduled in accordance with the Japanese Gastric Cancer Treatment Guideline.
The primary outcome was to identify the factors independently associated with positive peritoneal cytology in those without gross PM. Survival analyses among the three cytology groups were also analyzed. Overall survival (OS) was defined as the time from the date of surgery to the date of death from any cause. Disease-free survival (DFS) was defined as the time from the date of surgery to the date of recurrence, including locoregional, peritoneal and distant metastasis.
All statistical analyses were performed using SPSS Statistics for Windows version 21 (SPSS, Inc., Chicago, IL, USA). Categorical data are described as n(%). Data are described as mean ± standard deviation for continuous data with normal distribution, and median (interquartile range, IQR) for non-normally distributed continuous data. Categorical data were compared using chi-square test or Fisher’s exact test. Comparisons of continuous data with normal distribution were made using Student’s t-test for unpaired data, and using Mann-Whitney U test for non-normally distributed data. Cox univariate and multivariate logistic regression was used to identify independent predictors of positive cytology. Survival rates were determined using Kaplan-Meier survival analysis. Log-rank test was used to distinguish differences among three groups. A p-value less than 0.05 was considered statistically significant.
Results
A total of 392 patients diagnosed with GC or AEG who underwent intended curative surgery with cytological examination were included. Of those, 43 cases of pathologically confirmed metastatic disease were excluded. The medical records of the remaining 349 patients were retrospectively reviewed and analyzed. The mean age of patients was 62.1±13.8 years. 68 (19.5%) patients were diagnosed with AEG (Siewert type II and III), and remaining were diagnosed with GC. 82 (23.5%) patients underwent simultaneous diagnostic laparoscopy during the surgical resection procedure. D2 lymphadenectomy was performed in 288 (82.5%) patients. Microscopic negative surgical margin was completed in 257 (73.6%) patients. For preoperative clinical staging in the cytology-positive group, there were 2 (6.3%) patients in clinical stage I, 5 (15.7%) patients in clinical stage II, and 25 (78.2%) patients in clinical stage III. Patients with FPCC had a significantly higher clinical T-stage (cT1 6.3% vs. 11.4%, cT2 6.3% vs. 15.8%, cT3 21.9% vs. 34.2%, and cT4 65.7% vs. 38.0%, respectively;
p=0.001) and a significantly higher clinical N-stage (cN0 9.4% vs. 29.2%, cN1 15.6% vs. 11.9%, cN2 9.4% vs. 18.9%, and cN3 65.6% vs. 39.5%, respectively;
p=0.004) compared to the cytology-negative group. Clinicopathological characteristics are shown in
Table 1. Adjuvant treatments were recommended according to the postoperative recovery and pathological result at the time. For the negative, positive, and atypical cell groups, chemotherapy was given in 75 (42.1%) patients, 20 (62.5%) patients, and 50 (45.9%) patients. Concurrent chemoradiation was given in 28 (15.7%) patients, 2 (6.3%) patients, and 27 (24.8%) patients, respectively.
Cox univariate regression analysis revealed poor differentiation (odds ratio [OR]: 2.67, 95% confidence interval [CI]: 1.04-6.82;
p=0.041), pT4 tumor (OR: 3.82, 95%CI: 1.24-11.82;
p=0.020), and pN3 stage (OR: 2.70, 95%CI: 1.12-6.47;
p=0.026), and metastatic LNR >0.40 (OR: 8.22, 95%CI: 2.24-30.17;
p=0.002) to be significantly associated with the presence of FPCC. Borrmann type, tumor size, angiolymphatic invasion, and perineural invasion were not found to be significantly correlated with positive cytology. Similar to the univariate analysis, Cox multivariate regression analysis demonstrated poor differentiation (adjusted OR [aOR]: 2.63, 95%CI: 1.04-6.82;
p=0.015), pT4 tumor (aOR: 4.62, 95%CI: 1.28-14.34;
p=0.018), pN3 stage (aOR: 4.13, 95%CI: 1.14-15.03;
p=0.031), and metastatic LNR >0.4 (aOR: 6.49, 95%CI: 1.44-29.14;
p=0.015) to be independently associated with the presence of FPCC, all as shown in
Table 2.
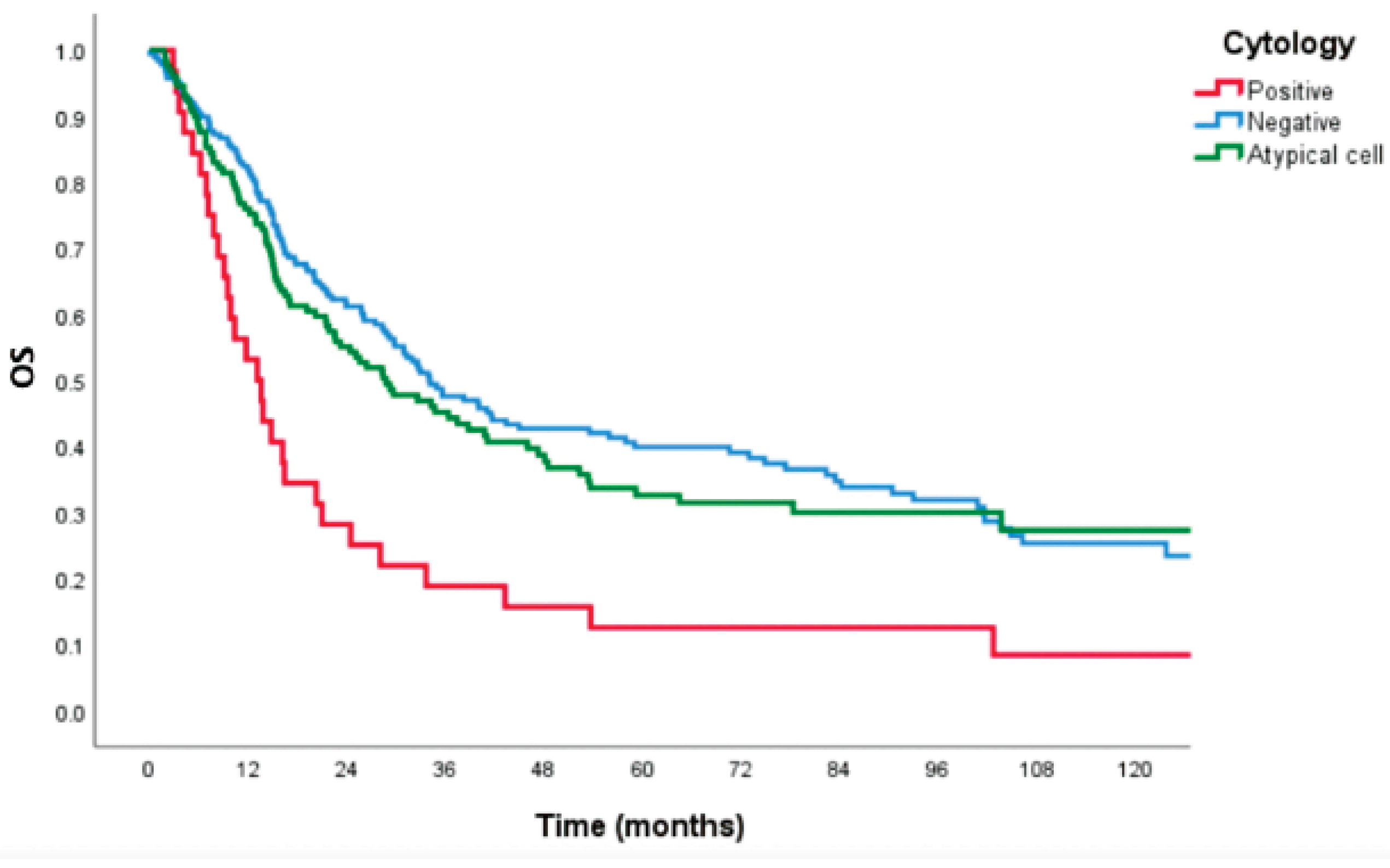
The median survival time of patients with negative, positive, and atypical cells from cytology was 34.1 months (IQR 26.1-42.0), 13.1 months (IQR 8.3-17.9), and 28.7 months (IQR 18.2-39.3), respectively (
p<0.001). 5-year OS was 27.2%, 8.3%, and 25.3%, respectively (
p<0.001), as shown in
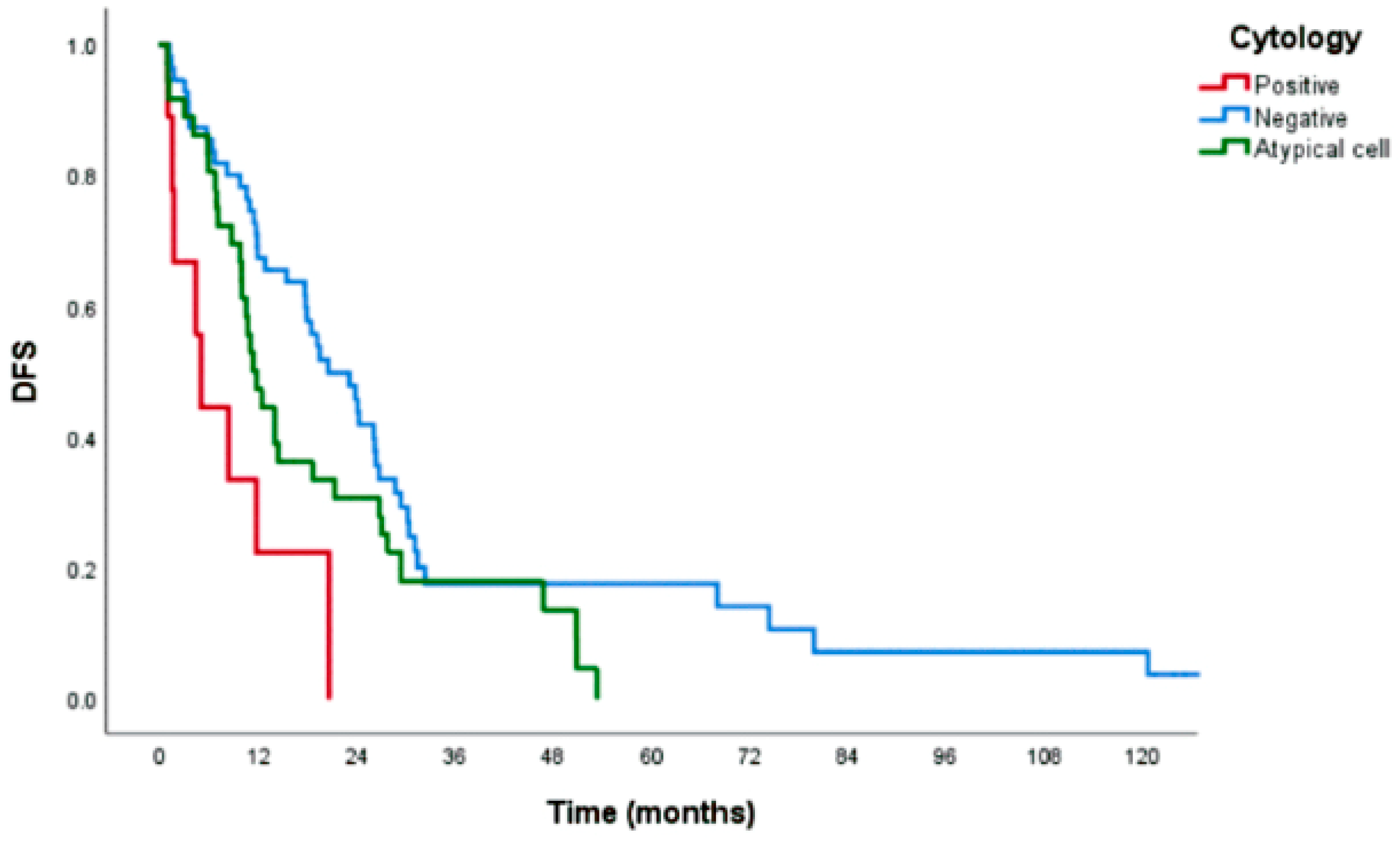
Figure 1. 3-year DFS was 17.8%, 0.0%, and 17.4%, respectively (
p<0.001), as shown in
Figure 2. The median time to recurrence was 20.5, 4.9, and 11.3 months (
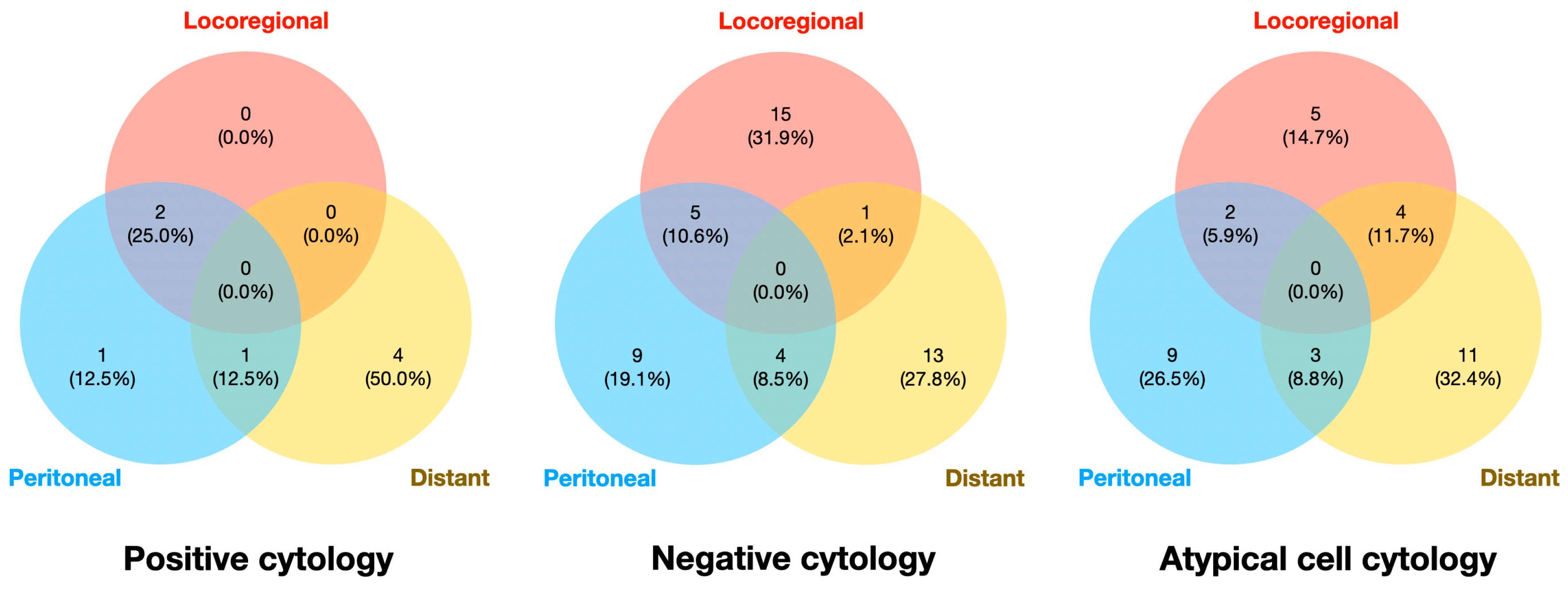
p<0.001). Pattern of recurrence was classified into locoregional recurrence, PM, and distant metastasis. In the negative cytology group, locoregional recurrence was the most common site of recurrence (31.9%) followed by distant metastasis (27.8%). However, distant metastasis was the most common pattern of recurrence in the positive cytology (50.0%) and atypical cell cytology (32.4%) groups. Details are shown in
Figure 3.
Discussion
Identifying PM can be particularly challenging, especially in case without obvious peritoneal nodules detectable on preoperative imaging. The difficulty in diagnosing microscopic peritoneal dissemination underscores the need for additional diagnostic tools to improve detection accuracy. Several studies reported the necessity of peritoneal lavage cytology prior to curative surgery since preoperative cytology worsens the oncologic outcome [
20,
21]. Patients with positive cytology results often have survival outcomes similar to those with overt PM. Even in the absence of visible peritoneal nodules, the presence of FPCC can indicate the micrometastasis, which significantly increases the likelihood of peritoneal recurrence after surgery. A highly sensitive and accurate method of detecting preoperative FPCC would alert clinicians to the need for neoadjuvant treatment prior to curative surgery to improve outcomes. During surgery, intraperitoneal chemotherapy is also an optional adjunct in cytology-positive cases. The integration of these advanced diagnostic and therapeutic strategies may help improve long-term survival rates in patients with GC.
The incidence of FPCC in GC cases was reported to range from 8.3% to 27% [
22,
23], which is comparable to the 9.2% in our study. The low incidence can be explained by the exclusion of confirmed PM cases before surgery. Recurrent disease had been reported up to 57% in those with positive cytology [
24]. The independent predictors of FPCC positivity in our study were poorly differentiated, pathological T4, pathological N3, and metastatic LNR >0.40. These findings are largely consistent with previously published results [
17,
18,
19]. However, it should be noted that these findings are based on data obtained after both surgery and complete pathological examination. For clinical application, these factors need to be addressed at the time of diagnosis. Accurate staging evaluation and awareness of the likelihood of the presence of gastric serosal tumor invasion and multiple lymph node metastasis would influence the need for pre-resection cytologic evaluation. Based on the findings of this study, diagnostic laparoscopy with cytologic examination should be applied in patients with independent predictors of FPCC.
The presence of FPCC was found to be significantly associated with a poorer prognosis in our study. Moreover, we compared the survival outcome and disease recurrence from the atypical cell group to other groups. Although the atypical cells from cytology may arise due to
reactive changes, inflammation, or
early-stage of tumor cell exfoliation, our results found some similarities and differences compared to other two groups. Interestingly, the median survival time of the positive group was less than half of that of the negative group. Conversely, comparable survival time was observed between the atypical cell group and negative group. This could be explained by the etiology of non-cancerous cause of atypical cells. Regarding the patterns of metastasis, the positive and atypical cell groups had mostly distant metastasis, whereas the negative group had mostly locoregional recurrence. This may be attributed to the possibility of early tumor cell exfoliation, where the detached cells share similar characteristics with malignant cancer cells. Further study is needed to further explain the natural course and prediction of recurrence to determine whether PM is a consequence of positive cytology or not. Therefore, adjuvant treatment by intraperitoneal chemotherapy can be given to control these FPCC intraoperatively [
25,
26].
This study has some mentionable limitations. First, consistent with our study’s retrospective design, some preoperative clinical staging and postoperative long-term follow-up data were missing in some cases. Furthermore, since our study analyzed data spanning a 15-year period, variations in treatment approaches and advancements in surgical techniques may have impacted differences in survival outcomes, even though the prescribed treatments adhered to the guidelines in place during that time. Future prospective multicenter study will yield more complete and current data regarding the influence of FPCC in patients with GC or AEG.
Conclusions
Poorly differentiated histology, tumor stage T4, node stage N3, and metastatic LNR > 0.40 are independent predictors of FPCC in patients with GC or AEG. The presence of FPCC is significantly associated with poor survival and disease recurrence outcomes.
Author Contributions
All authors-Conceptualization; TP, AS, CN, AM, JS-Data curation; TP, AS, JS-Formal analysis; TP, AS, CN-Investigation; TP, AS, CN-Methodology; TP, AS, CN-Project administration; TP, AM, JS-Resources; TP, AS, AM-Software; TP, AS, CN, AM-Supervision; TP, AS, CN, JS, AM-Validation; TP, AS, CN, JS, AM-Visualization; All authors-Writing - original draft; All authors-Writing - Review and editing.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Siriraj Institutional Review Board (COA no. Si 359/2019) on 14 February 2021.
Informed Consent Statement
This study was conducted in accordance with ethical and data protection regulations. Due to the retrospective nature of this study, written informed consent to participate was not obtained from study patients.
Data Availability Statement
The datasets of this study are available upon reasonable request.
Acknowledgments
The authors gratefully acknowledge Miss Wathanaphirom Mangmee and Miss Chorlada Keatrungarun for their assistance with data collection, and Dr. Saowalak Hunnangkul for her assistance with statistical analysis.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Tustumi F, Bernardo WM, Dias AR, Ramos MF, Cecconello I, Zilberstein B, et al. Detection value of free cancer cells in peritoneal washing in gastric cancer: a systematic review and meta-analysis. Clinics (Sao Paulo). 2016;71(12):733-45. [CrossRef]
- Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-v49. [CrossRef]
- Japanese Gastric Cancer A. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26(1):1-25.
- Kim IH, Kang SJ, Choi W, Seo AN, Eom BW, Kang B, et al. Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline). J Gastric Cancer. 2025;25(1):5-114. [CrossRef]
- Koganti SB, Boddepalli S, Nambada M, Thumma VM, Nagari B, Sastry RA. Positive Peritoneal Lavage Cytology -Implications for Staging and Management of Gastric Cancer. Indian J Surg Oncol. 2016;7(4):430-5. [CrossRef]
- Pecqueux M, Fritzmann J, Adamu M, Thorlund K, Kahlert C, Reissfelder C, et al. Free intraperitoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget. 2015;6(34):35564-78. [CrossRef]
- Allen CJ, Newhook TE, Vreeland TJ, Das P, Minsky BD, Blum M, et al. Yield of peritoneal cytology in staging patients with gastric and gastroesophageal cancer. J Surg Oncol. 2019;120(8):1350-7. [CrossRef]
- Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19(2):329-38. [CrossRef]
- Yoshida K, Yasufuku I, Terashima M, Young Rha S, Moon Bae J, Li G, et al. International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann Gastroenterol Surg. 2022;6(2):227-40. [CrossRef]
- Valletti M, Eshmuminov D, Gnecco N, Gutschow CA, Schneider PM, Lehmann K. Gastric cancer with positive peritoneal cytology: survival benefit after induction chemotherapy and conversion to negative peritoneal cytology. World J Surg Oncol. 2021;19(1):245. [CrossRef]
- Jamel S, Markar SR, Malietzis G, Acharya A, Athanasiou T, Hanna GB. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer. 2018;21(1):10-8.
- Bausys A, Ümarik T, Dobrzhanskyi O, Luksta M, Kondratskyi Y, Reinsoo A, et al. Neoadjuvant Chemotherapy Followed by Gastrectomy for Cytology-Positive Gastric Cancer without Any Other Non-Curative Factors in a Western Setting: An International Eastern European Cohort Study. Cancers. 2023;15(24):5794. [CrossRef]
- Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S27-37. [CrossRef]
- Gęca K, Skórzewska M, Rawicz-Pruszyński K, Mlak R, Sędłak K, Pelc Z, et al. Prognostic value of molecular cytology by one-step nucleic acid amplification (OSNA) assay of peritoneal washings in advanced gastric cancer patients. Scientific Reports. 2022;12(1):12477. [CrossRef]
- Hayes N, Wayman J, Wadehra V, Scott DJ, Raimes SA, Griffin SM. Peritoneal cytology in the surgical evaluation of gastric carcinoma. Br J Cancer. 1999;79(3-4):520-4. [CrossRef]
- Lisiecki R, Kruszwicka M, Spychała A, Murawa D. Prognostic significance, diagnosis and treatment in patients with gastric cancer and positive peritoneal washings. A review of the literature. Rep Pract Oncol Radiother. 2017;22(6):434-40. [CrossRef]
- Sakata T, Takahata T, Kimura T, Yasuhara I, Kojima T, Akazai Y, et al. A single-institution retrospective analysis of gastric carcinoma with positive peritoneal lavage cytology and without serosal invasion: A case series. Ann Med Surg (Lond). 2019;39:10-5. [CrossRef]
- Kano Y, Kosugi S, Ishikawa T, Otani T, Muneoka Y, Sato Y, et al. Prognostic significance of peritoneal lavage cytology at three cavities in patients with gastric cancer. Surgery. 2015;158(6):1581-9. [CrossRef]
- La Torre M, Ferri M, Giovagnoli MR, Sforza N, Cosenza G, Giarnieri E, et al. Peritoneal wash cytology in gastric carcinoma. Prognostic significance and therapeutic consequences. Eur J Surg Oncol. 2010;36(10):982-6. [CrossRef]
- Higaki E, Yanagi S, Gotohda N, Kinoshita T, Kuwata T, Nagino M, et al. Intraoperative peritoneal lavage cytology offers prognostic significance for gastric cancer patients with curative resection. Cancer Sci. 2017;108(5):978-86. [CrossRef]
- Kobayashi H, Honda M, Kawamura H, Takiguchi K, Muto A, Yamazaki S, et al. Clinical impact of gastrectomy for gastric cancer patients with positive lavage cytology without gross peritoneal dissemination. J Surg Oncol. 2022;125(4):615-20. [CrossRef]
- Jiang CG, Xu Y, Wang ZN, Sun Z, Liu FN, Yu M, et al. Clinicopathological analysis and prognostic significance of peritoneal cytology in Chinese patients with advanced gastric cancer. ANZ J Surg. 2011;81(9):608-13. [CrossRef]
- Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17(12):3173-80. [CrossRef]
- Parakonthun T, Parichardsombat N, Salomon H, Paredes R, Phalanusittheph C, Taweerutchana V, et al. Significance of Microscopic Residual Tumor in Adenocarcinoma of Stomach and Esophagogastric Junction after Gastrectomy with D2 Lymphadenectomy. Siriraj Med J. 2018;70(2):95-102.
- Graversen M, Rouvelas I, Ainsworth AP, Bjarnesen AP, Detlefsen S, Ellebaek SB, et al. Feasibility and Safety of Laparoscopic D2 Gastrectomy in Combination with Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Patients with Gastric Cancer at High Risk of Recurrence-The PIPAC-OPC4 Study. Ann Surg Oncol. 2023;30(7):4433-41.
- Imano M, Imamoto H, Itoh T, Satou T, Peng YF, Yasuda A, et al. Impact of intraperitoneal chemotherapy after gastrectomy with positive cytological findings in peritoneal washings. Eur Surg Res. 2011;47(4):254-9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).