1. Introduction
Bovine spongiform encephalopathy (BSE) is classified among transmissible spongiform encephalopathies (TSEs); a group of progressively degenerative neurological disorders caused by prion (proteinaceous infectious particle) which are associated in humans with a variant form of Creutzfeldt-Jakob disease (Banerjee et al 2023). Prions are infectious agents composed of misfolded protein without DNA or and characterized by incubation periods ranging from months to years depending on the host (Banerjee et al 2023). Since BSE is transmitted through animal feed, its prevention relies primarily on banning proteins from animal tissues in ruminant feed.
Commission Regulation (EU) No 142/2011 establishes detailed guidelines for processing and using animal by-products in feed, including blood and milk products. Due to concerns over TSEs, the use of blood products in ruminant feed is generally prohibited. The regulation also covers the inclusion of milk and milk products in feed. While milk and milk products are allowed in feed, their inclusion in animal feed is subject to regulations that ensure their safety and suitability for specific animals. Commission Implementing Regulation (EU) 2023/1446 recently renewed the approval of hydrolyzed proteins in feed under specific conditions depending on the source material and the intended application.
Regulatory enforcement against prohibited proteins in animal feed hinges on detecting animal by- proteins prohibited in feed. Methods for detecting animal by-products in feed materials are largely based on microscopy and DNA analysis (Method of reference and SOPs - EURL-AP). While microscopy identifies animal particles, it struggles with species-level identification (Golinelli et al., 2016). DNA-based methods like Polymerase Chain Reaction (PCR) offer high sensitivity for species identification but can be confounded by damaged DNA from feed processing. Both microscopy analysis and PCR methods are complementary and together ensure feed safety and regulatory compliance (Method of reference and SOPs - EURL-AP).
The use of animal plasma in the EU feed is regulated in order to safeguard public and animal health. Commission Implementing Regulation (EU) No 483/2014 specifically addresses the importation of spray-dried blood and blood plasma of porcine origin for feed production. It outlines specific health requirements covering processing techniques, microbiological standards, and transport protocols to mitigate the risk of transmitting diseases such as porcine epidemic diarrhea virus (PEDv). Regulation (EU) No 142/2011 establishes broader framework for the handling of blood products, including plasma intended for feed requiring health certification to ensure that derived products that complies with rigorous microbiological safety criteria.
Spray-dried animal plasma is used in wet and dry dog foods formulations due to its valuable properties including water retention, gel strength, and fat emulsion capacities (Polo). In aquaculture, SDAP has demonstrated benefits in improving the health status and enhancing disease resistance (Chuchird et al. 2021). Although advantages of SDAP on animal health and performance are well documented have been documented, concerns may arise regarding its safety, considering its origin from abattoir-collected animal blood, especially in the context of emerging or re-emerging diseases within animal populations (Kazimierska and Biel, 2023). These concerns highlight the importance of rigorous regulations to ensure product safety. Bovine spongiform encephalopathy (BSE) was the most thoroughly documented animal disease in the United Kingdom. Epidemiological studies linked the outbreak to contaminated feed ingredients, primarily meat meal and meat-and-bone meal (MBM), that carried transmissible spongiform encephalopathy (TSE) agents. These agents spread further by the recycling of infected cattle carcasses through the rendering process. In response, the European Commission imposed a ban on UK ruminant exports in 1996. As a result, many countries have since prohibited the use of MBM in ruminant feed. Analysis and control of these products remain essential to prevent future BSE outbreaks.
In this review, we discuss limits of current methods of analysis to detect some blood products, milk and hydrolyzed proteins in feed.
2. Methods for analysis of animal by-products in feed
Methods for detecting animal material in feed are based on analyses of either bones, muscles and other fragments, proteins or DNA. The details and limitations of these methods are described in the following sections.
2.1. PCR (Polymerase Chain Reaction):
Even after the heating processes used in feed production PCR is a highly sensitive method for detecting animal and to identify the species of origin. However, the efficiency of DNA recovery and the possibility of detecting DNA from permitted ingredients (like milk powder) rather than banned materials are challenging. The rendering process used in feed preparation relies on heat treatment, which inherently denatures proteins and degrades DNA which is inherent limitation for this method. Heat treatment induces protein denaturation by disrupting hydrogen bonds and other non-covalent interactions, resulting in the unfolding of the protein and changes to its secondary and tertiary structures (Li et al., 2021). Under elevated temperatures and pressures, covalent bonds between amino acids may also break, fragmenting the protein into shorter polypeptide chains (Li et al., 2021). Consequently, any analytical method used to assess heat-treated proteins must consider these structural modifications to ensure accurate results.
2.2. Feed microscopy
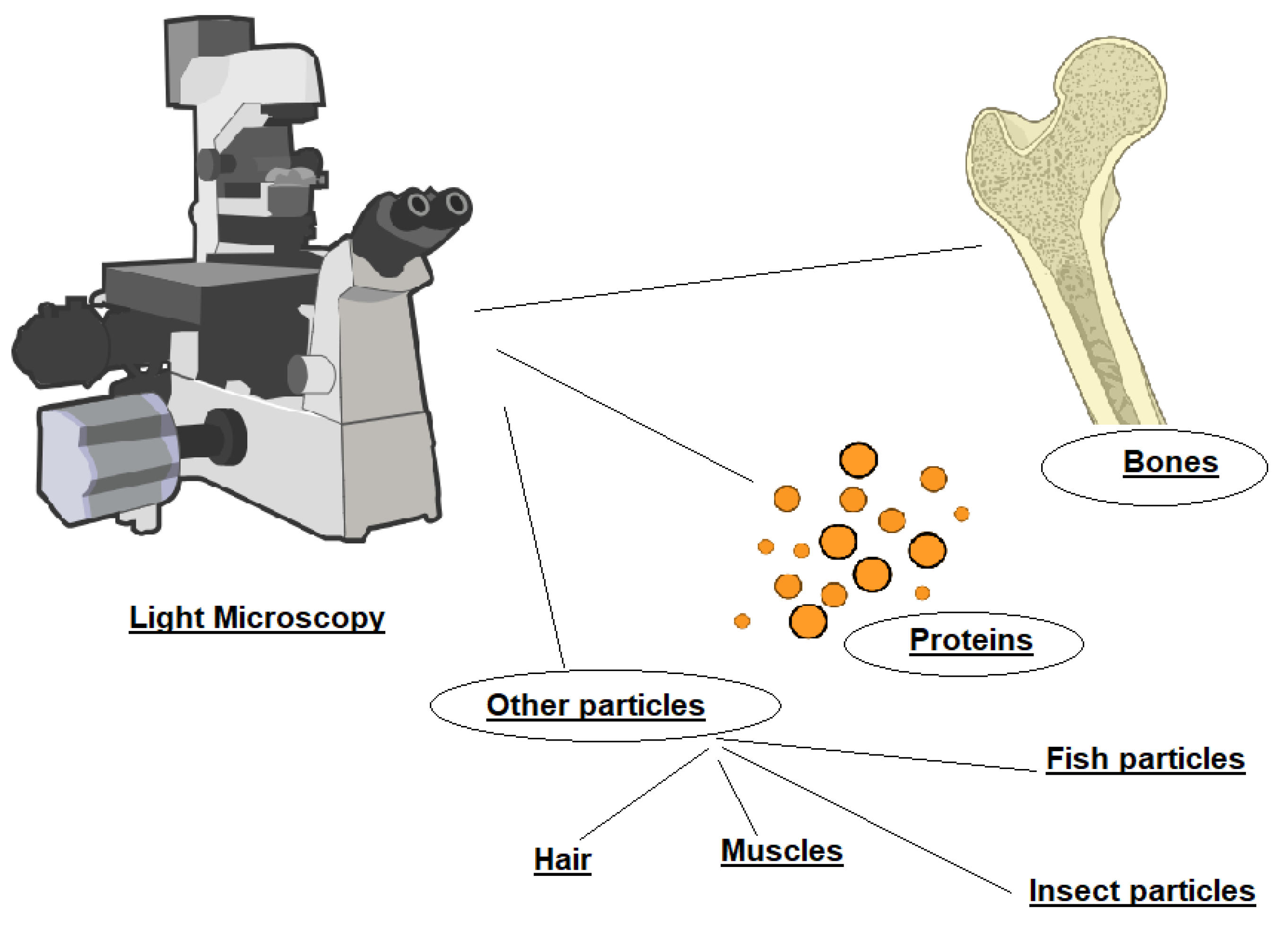
Feed microscopy is the official cost-effective method widely used to detect animal-derived particles and proteins in feeds. The technique is based on the examination of fragments to assess morphological and histological characteristics on the basis of microscopically identifiable characteristics (
Figure. 1) such as bones, cartilage, muscle fibres, hair, blood products, milk globules, lactose crystals, feathers, egg shells, or bristles, cuticular fragments, insect tracheal structures in the case of terrestrial invertebrate and fish bones, otoliths and scales in the case of fish flour ((EC) 152/2009). According to the updated and consolidated version of Commission Regulation (EC) 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed, no more than six slides of the sediment, and depending on the operator’s choice, either on the flotate or on the raw material should be examined.
According to the updated and consolidated version of Commission Regulation (EC) 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed; no more than six slides of the sediment, and depending on the operator’s choice, either on the flotate or on the raw material should be examined. Even though the microscopy method is qualitative, the limit of decision is set to 5 particles from animal origin in case of one determination or 10 in case of two determinations. The method is effective at distinguishing terrestrial animals from fish but has limitations in accurately differentiating between mammalian and avian bone structures and can be dependent on the analyst’s experience.
Bone fragments, in particular, vary in color and shape. A critical diagnostic feature is the presence of lacunae, small cavities within the bone matrix, whose morphology differs among animal classes. By analyzing lacunar shape, it is possible to infer the species origin of the tissue, distinguishing between mammalian, avian, and fish (Domenis et al. 2009; van Raamsdonk et al. 2012). However, microscopy alone cannot be used for precise animal species identification. Despite this limitation, it remains the official tool in feed analysis due to its low cost and minimal sample preparation requirements.
3. Blood Products identification
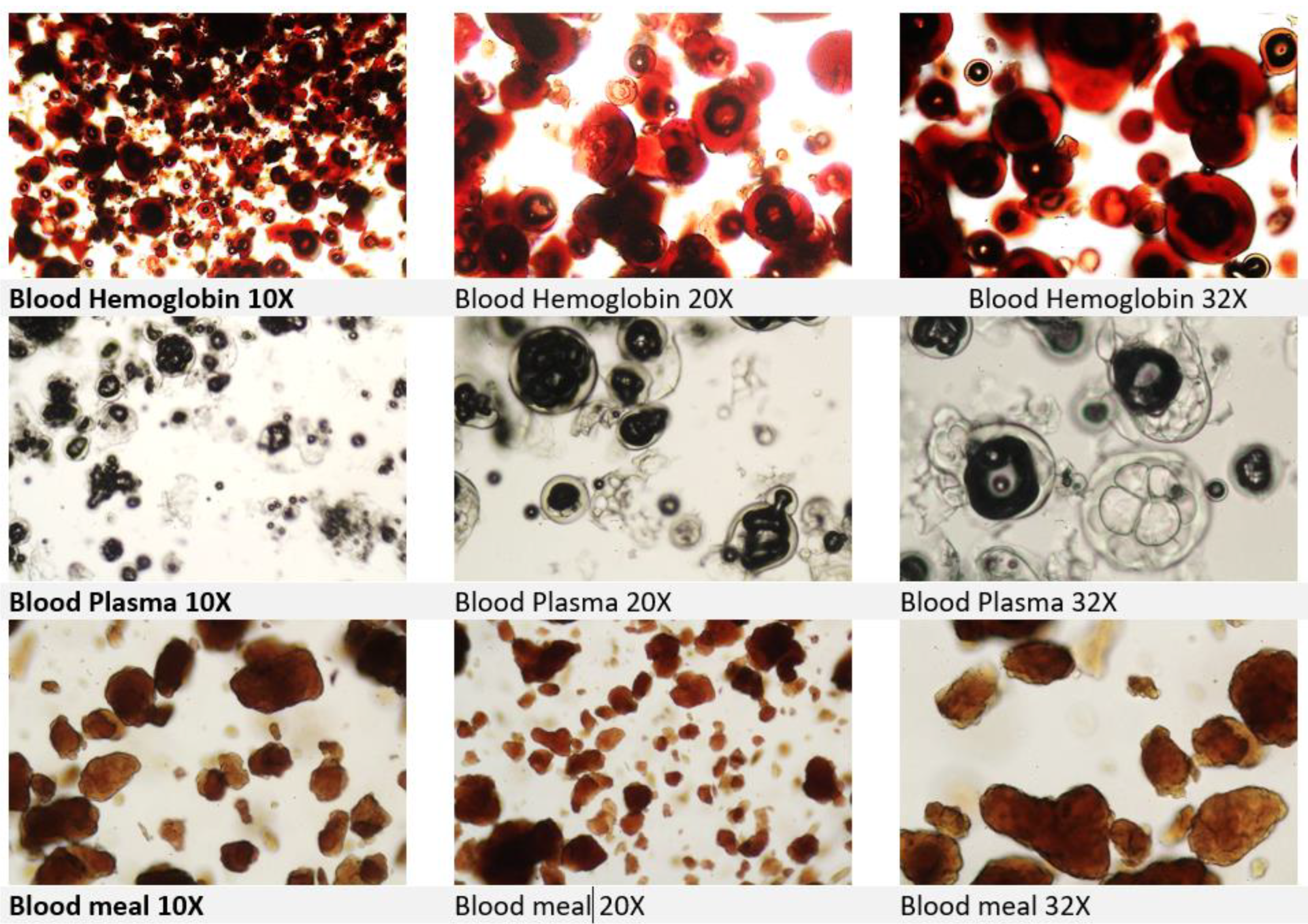
Analytical methods are crucial to detect and differentiate between authorized and unauthorized blood products in feed to ensure compliance with regulations. Blood products in feed includes dried plasma, whole blood, or hymoglobin (
Figure. 2). Blood meal is considered PAP’s.
More than half of young calves raised in the United States are fed all-milk protein-based milk replacers as part of their liquid diet which are considered the most digestible for calves (Drackley, 2008; Henrichs et al., 2021). However, due to the rising demand for milk proteins in human nutrition, alternative ingredient solutions are being explored for use in calf nutrition such as spray-dried plasma protein, known for its favourable amino acid profile, high digestibility, and comparable growth performance to all-milk protein sources. Additionally, its use has been associated with reduced morbidity and mortality in milk-fed calves (Drackley, 2008; Henrichs et al., 2021; Quigley & Wolfe, 2003). An additional alternative protein source evaluated is enzymatically hydrolyzed wheat protein (Henrichs et al 2021). Including, at conventional rates, Spray-dried plasma and wheat protein in milk replacers to partially replace whey protein has been shown to perform similarly to the use of milk replacers formulated exclusively with whey, improving growth and intestinal function in young calves when fed with milk replacers.
The exemption of Bovine spray-dried plasma protein from ruminant product ban associated with Bovine Spongiform Encephalopathy (BSE) is based on its processing methods, based on its separation from high-risk tissues and viral inactivation through spray-drying and post-drying heat treatments (Blázquez et al., 2020).
While the use of spray-dried bovine plasma protein that meets regulatory standards and derived from animal sources compliant with human food safety standards is allowed in the United States in animal feed, including pet food, calf milk replacers, and swine diets, provided it (Kazimierska and Biel, 2023); it is still not allowed in the European Union or Japan, among other countries.
van Raamsdonk et al. (2011) investigated blood plasma in milk formula after an alert issued in Spain about the presence of plasma in milk formula “KUVU” for calves, the presence of blood plasma in milk formula was investigated by RIKILT, a Dutch research institute, conducted further analysis on the suspect sample. The investigation found blood plasma in a sample of calf milk formula in 2010, detected by tetramethylbenzidine (TMB) staining and confirmed by PCR tests showing porcine DNA. The Dutch research institute RIKILT performed a Polymerase Chain Reaction (PCR) test and discovered a low level of porcine (pig) DNA. The presence of porcine DNA was the likely reason for the positive TMB stain. As TMB is a sensitive screening method based on the peroxidase activity of hemoglobin in blood products for detecting blood and blood products, it can produce false positives in the presence of other substances. Microscopic staining techniques such as Alizarin Red for bone fragments and TMB for blood plasma are commonly used to support the identification of animal particles in feed. However, both techniques have shown issues with specificity and sensitivity (van Raamsdonk, Scholtens, et al. 2011; van Raamsdonk et al. 2017).
4. Milk and Milk Products
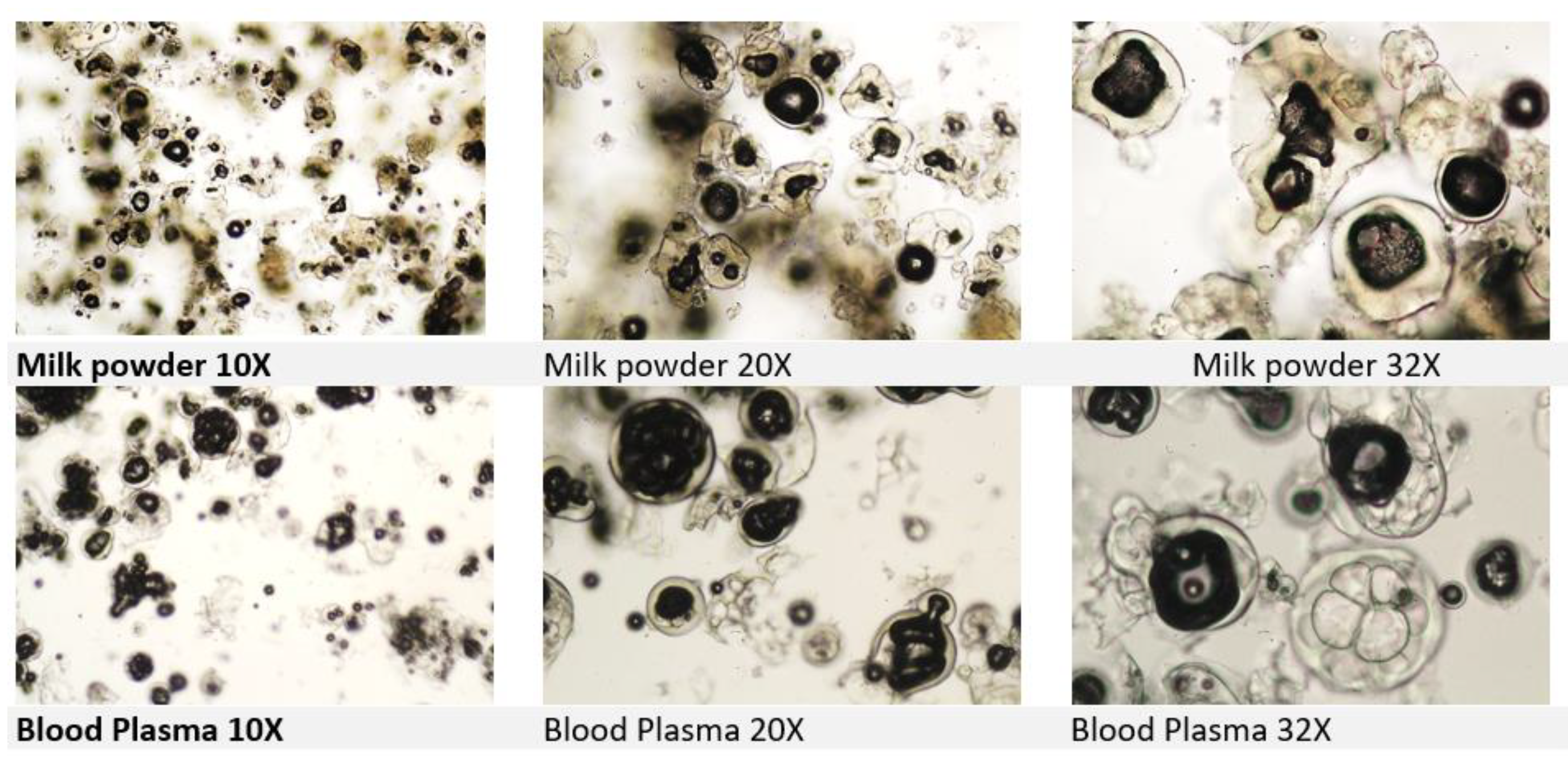
Milk and milk-based products include milk, milk-derived products, colostrum, and colostrum products. Milk globules observed under light microscope might look quite similar to spray dried plasma.
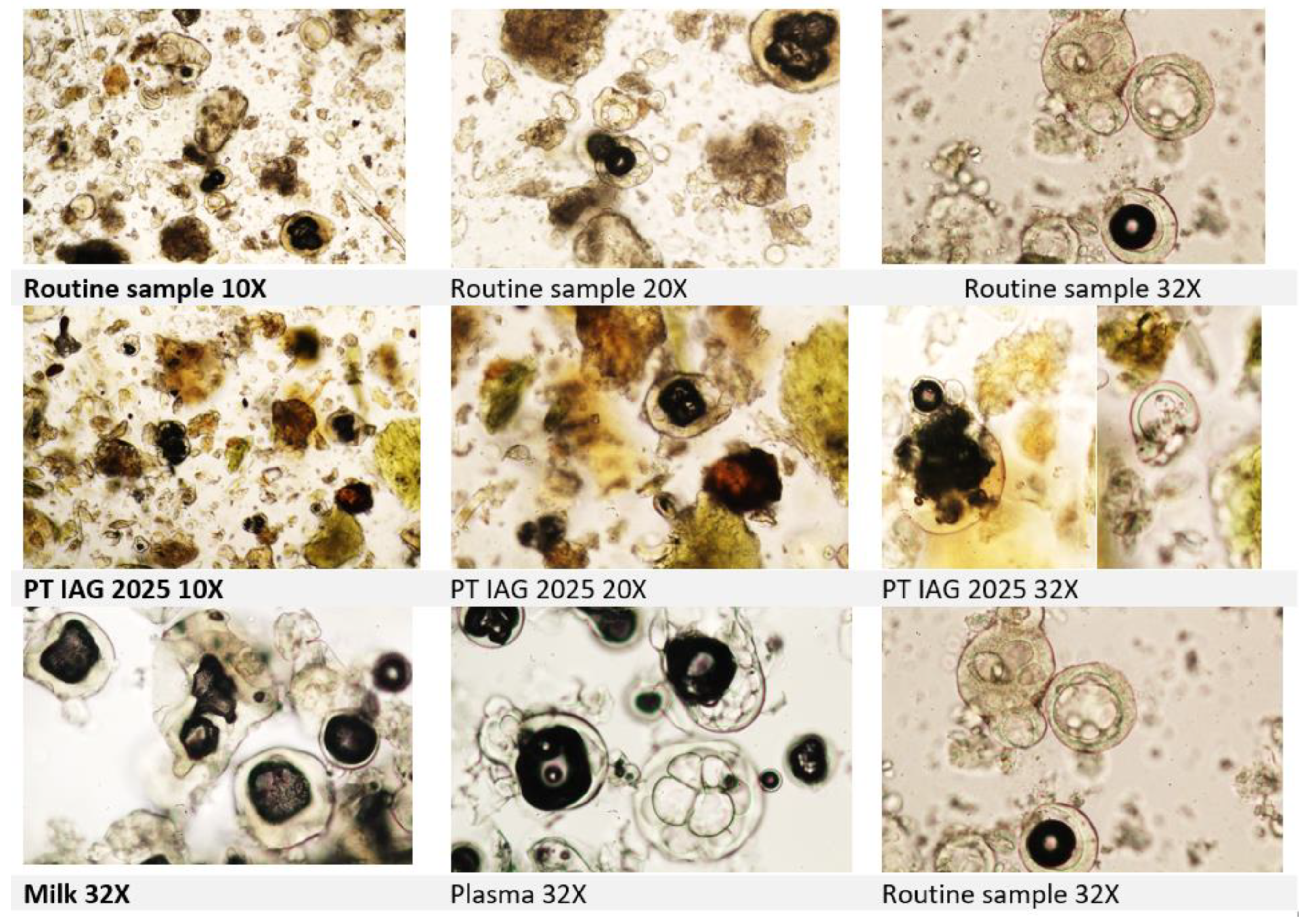
The confirmation should include searching for lactose crystals and sample investigation with polarized light microscopy as discussed in the latest proficiency test of the International Association for Feedingstuff Analysis - Section Feedingstuff Microscopy. The Annual Meeting IAG 2025 held in Fribourg and Posieux indeed focused on the challenge of detecting the plasma and milk powder, even at a 2% inclusion rate in feed. During the meeting, a practical workshop on milk and blood products focused on milk and blood products identification with significant input on operators’ identification skills. Plasma globules and milk powder globules might look quite similar under microscope (
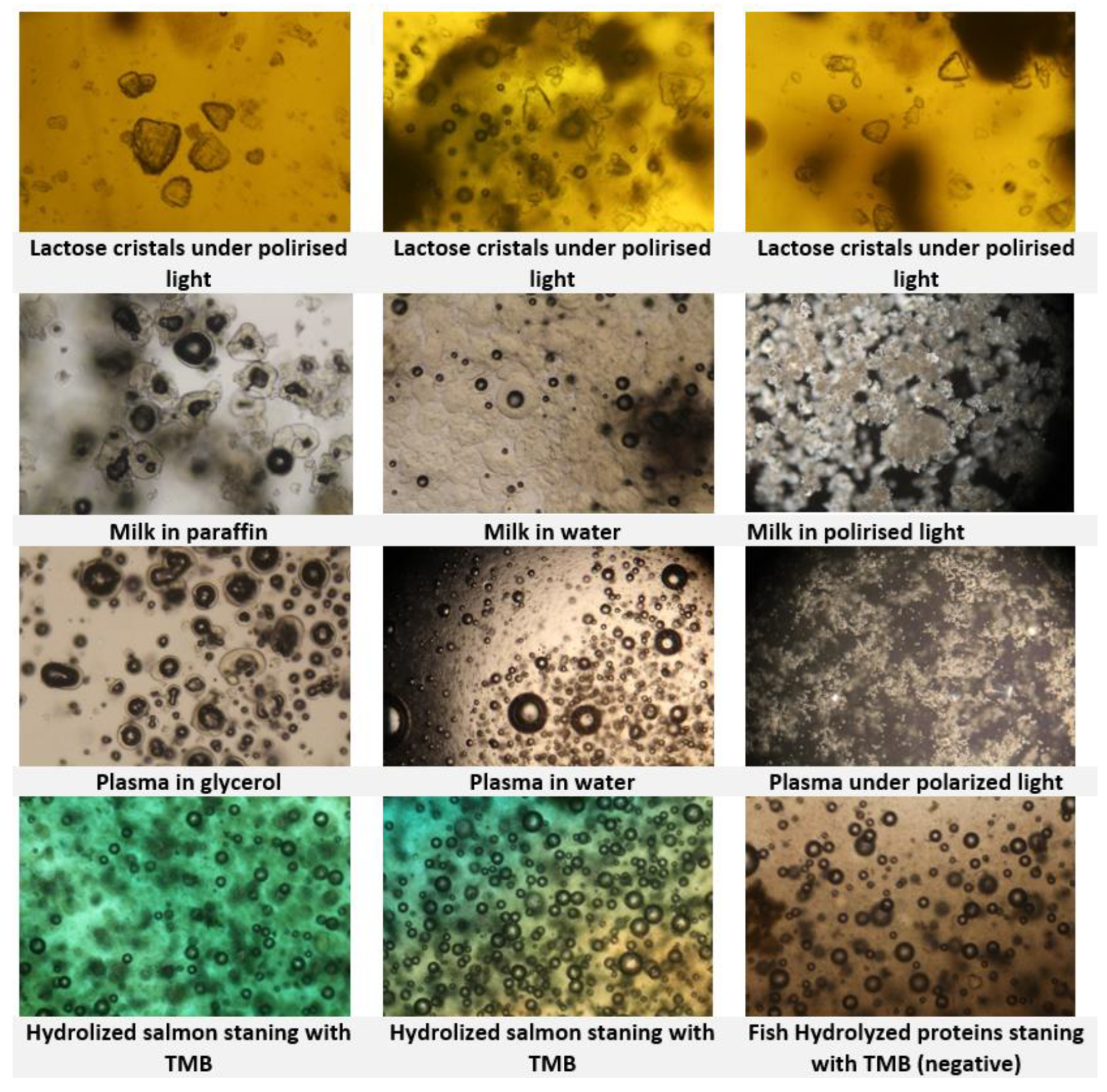
Figure. 3). To improve identification, an additional staining step with TMB + H2O2 is essential. Furthermore, the use of polarised light microscopy for lactose crystals may enhance analytical accuracy in future (
Figure. 4).
5. Hydrolized proteins
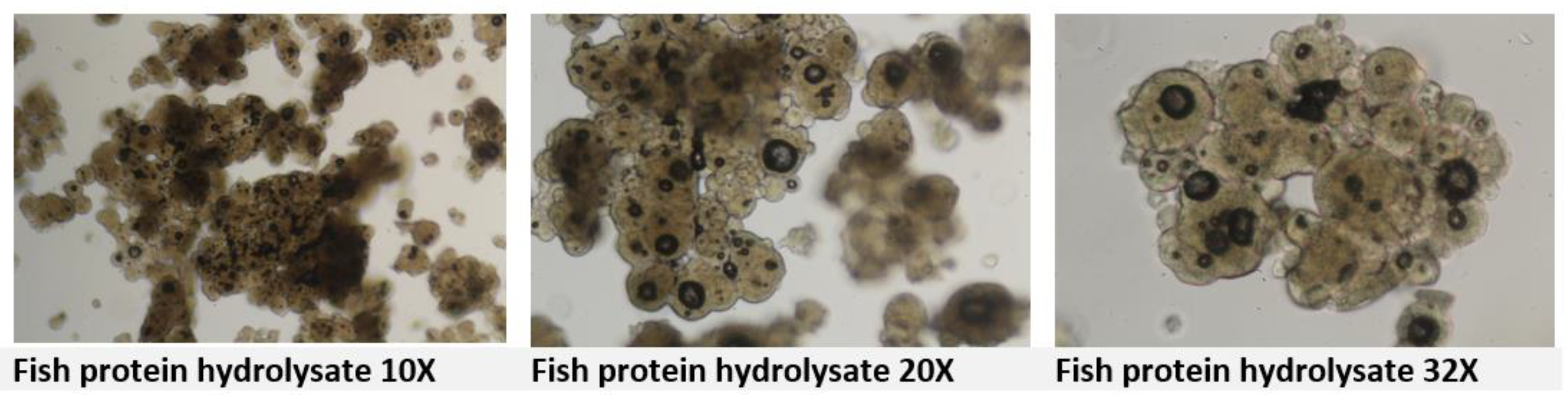
Hydrolyzed proteins in feed are derived from proteins that have been broken down into smaller peptides and amino acids, often through enzymatic hydrolysis. As for the milk and plasma globules, proteins are also quite challenging under light microscope with morphological similarities it is difficult to classify such globules origin from either animal or vegetal sources (
Figure. 5).
Once an operator identifies globular structures during sample examination, microscopic analysis becomes limited for tracing their origin.
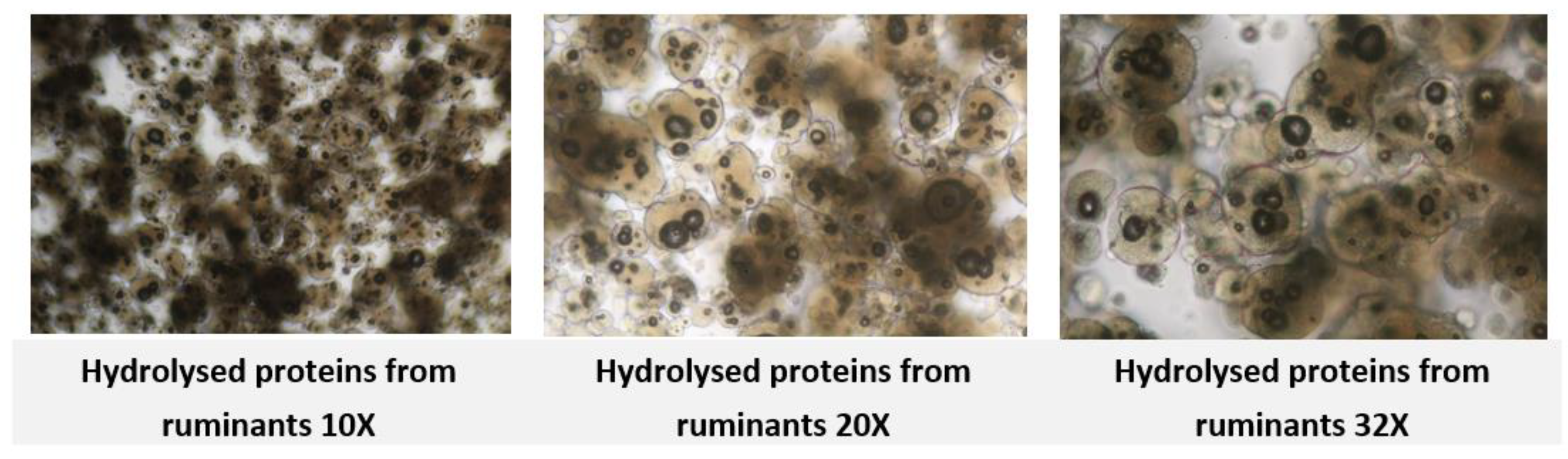
The European regulations and associated Standard Operating Procedures (SOPs) do not provide explicit guidance on how to proceed in such cases It remains unclear whether PCR testing should be systematically applied whenever these particles are detected, or if alternative practical methods exist to distinguish them from globules of hydrolyzed ruminant proteins (
Figure. 6 and
Figure. 7). This poses challenges for laboratories aiming to ensure compliance while maintaining analytical precision.
6. Actual challenges in microscopy
Microscopic identification of animal proteins in feed remains a persistent challenge due to the complexity of sample composition and the limitations of current techniques (
Figure. 8). A particularly critical issue is the detection of plasma fraud in milk replacers, especially when other components are present that interfere with tetramethylbenzidine (TMB) staining. These interferences can compromise the reliability of results ensuring accurate identification.
Milk replacers are commonly used for replacement dairy calves. Milk replacer adulteration, involving the addition or the substitution of expensive milk proteins like whey or casein with bovine or porcine plasma. Milk replacer fraud is a form of economically motivated adulteration as plasma proteins seems to be nutritionally valuable and generally less expensive than milk proteins (Quigley and Wolfe, 2003). Accurate testing and detection methods are crucial to prevent and mitigate this type of fraud common and serious problem in many countries.
Qualitative methods to detect adulterants in milk products consist of simple, rapid, and often color-based chemical reactions (Azad and Ahmed, 2016). Staining techniques like tetramethylbenzidine (TMB) staining can be employed to detect blood plasma under light microscope as referenced in EU Commission Regulation (EC) 152/2009 and corresponding Standard Operating Procedures (SOPs) established by the European Union Reference Laboratory for Animal Proteins (EURL-AP).
Tetramethylbenzidine (TMB) is a chemical compound widely used in colorimetric assays to detect the presence of hemoglobin, particularly in plasma. It acts as a substrate for peroxidase-like enzymes, and its oxidation by hydrogen peroxide in the presence of hemoglobin results in a color change, typically to blue or green, making it a useful presumptive test for blood presence. TMB is a highly sensitive reagent, capable of detecting even very diluted blood samples. However, its sensitivity also presents a limitation: it can yield false positives when other substances with peroxidase-like activity are present, potentially compromising the specificity of the test.
TMB staining method serves only as a preliminary screening test and must be followed by a confirmatory method to verify the presence of animal proteins. According to the standard protocol, a slight green coloration should be observed in a suspect sample indicates the presence of blood plasma and blood products. In this review, TMB staining was used to compare various products, including skimmed milk powder, fish flour, blood meal, and blood plasma (
Figure. 9). Strong coloring responses were observed with blood meal and blood plasma, whereas skimmed milk showed no coloration within the specified reaction time. The coloration shown in additives is probably due to the presence of copper sulfate as an ingredient which makes difficult the discrimination between milk globules and plasma globules in the sample. The hydrolyzed fish shows a strong coloration confirmed with a positive PCR result for terrestrial vertebrates. The TMB coloration was not always accurate as for example in the case of starfish and crabs. Those samples should not contain any blood products.
Benzidine, since its discovery in 1904 (Jules, 1975), was recognized as a sensitive and specific test for peroxidase. However, its use has been discouraged due to concerns about associated health risks.
In 1964, Culliford and Nickolls (1964) published in a review about the benzidine test where they reported that the benzidine test could yield false-positive results caused contamination factors such as chemical oxidants, catalysts, and vegetable peroxidases. Following the ban on benzidine as a staining agent, identifying safer and equally reliable alternatives became a priority.
Holland et al. (1974) described the synthesis of 3,3’,5,5’-tetramethylbenzidine (TMB) and suggested its initial possible use for blood detection. They also investigated carcinogenic properties of TMB. All tumors found in rats exposed to TMB were either benign or age-related with no direct association to TMB exposure. These findings established TMB as a promising substitute to carcinogen benzidine for presumptive blood testing in forensic science. According to Holland et al. (1974), the sensitivity of both benzidine and TMB reagents was found to be unaffected by storage conditions, including protection from light and temperature with an initial sensitivity of 1 ppm blood. However, within 24 hours, this sensitivity decreases tenfold to 10 ppm, a level that remains stable through the fifth day (Holland et al., 1974). Holland et al. (1974) found that by the eighth day, sensitivity declines by another factor of ten, reaching 100 ppm. Authors assumed that the loss of sensitivity occurred gradually during this period regardless the reagent protection the from light and temperature during storage.
As expected, due to structural similarities between benzidine and 3,3’,5,5’-tetramethylbenzidine (TMB), experimental comparisons have shown no significant differences in their sensitivities and specificities for blood detection (Holland et al., 1974). Nevertheless, TMB rises concerns about yielding false positives with on certain papers, experience suggests that the reagent can be reliable in hand of experienced operators. TMB carcinogenic tested negative for mutagenicity in the Ames test and did not induce tumor formation in a single-arm study involving 24 rats (Holland et al., 1974). Based on this evidence, TMB has been adopted as a safer alternative to carcinogenic compounds such as benzidine (Holland et al., 1974).
3,3′,5,5′-Tetramethylbenzidine (TMB) can yield false-positive reactions when certain components in feed exhibit peroxidase-like activity, complicating the interpretation of results and making decision-making more challenging. Garner et al. (1976) found that TMB can yield false-positive results reactions after a prolonged time to certain types of paper such as typing paper, recycled paper, filter paper, and white construction paper. Certain papers and fruits contain substances with peroxidase-like activity that can catalyze the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB). This reaction mimics the colorimetric response used to detect blood leading in false-positive results and compromising the specificity of the technique (Garner et al., 1976).
For example, the dihydrogen phosphate ion (H2PO4-) reacts with TMB (3,3’,5,5’-tetramethylbenzidine) in the presence of a suitable oxidizing agent such as hydrogen peroxide (H2O2), and a peroxidase enzyme, such as horseradish peroxidase (HRP), to produce a blue-colored product. TMB reacts also with benzoyl peroxide (BPO) in wheat flour causing a color change that can be used as naked eye detection method to detect benzoyl peroxide, a common food additive used as a bleaching agent in wheat flour, actually banned in the European Union due to concerns about potential health effects and nutrient degradation (Hu et al., 2013).
TMB reacts also with Dextrose (glucose) in the presence of glucose oxidase (GOx) and hydrogen peroxide (H2O2) to produce a blue-colored product. This reaction is the basis for several colorimetric assays used for the detection and quantification of glucose. The intensity of the blue color is directly proportional to the amount of glucose present in the sample (Li et al., 2019).
Copper sulfate (CuSO4) is a common ingredient in premixes and additives for feed. It can also generate reaction with TMB in presence of oxidizing agent, like hydrogen producing blue-color (Jing et al 2013). Previous studies have shown that Ag+ can also induce a blue color by oxidizing TMB, a color reaction that also occurs when Cu2+ ions exists in aqueous solution (Zhang et al 2022).
TMB is widely used compared to other staining reagent (Zhang et al 2022). However, its application in feed analysis is complicated due to its potential reactions with components that might lead false-positive. In presence of certain materials such as specific types of paper, Copper sulfate (CuSO4) commonly used in feed additives and premixes, dextrose wheat flour and other components that exhibit peroxidase-like activity. These interferences can catalyze TMB oxidation and mimic the colorimetric response typically associated with blood plasma making result interpretation particularly difficult in mixed feed samples. In presence of such materials, distinguishing between milk powder and spray-dried plasma for example using only TMB staining is unreliable, underscoring the need for complementary analytical techniques to ensure accurate identification
7. Conclusion
The safety of animal feed remains a fundamental priority within EU legislation. Traceability of feed materials including blood products, milk, and hydrolyzed proteins along with analytical precision are key element of effective risk management. Current methods can’t specifically distinguish between prohibited material and authorized one, particularly in case of rendering processes involving heat treatment that denatures and degrades proteins and DNA compromising molecular and / or morphological integrity. These limitations underscore the urgent need for improving analytical methods for animal by-products detection and identification with greater specificity. In this review, the dairy products adulteration with plasma, often motivated by economic gain, was discussed and challenging of the microscopic identification particularly in presence of components that interfere with TMB (3,3′,5,5′-Tetramethylbenzidine) coloration assays, was highlighted. Additionally, microscopic differentiation between proteins globules remains limited, as most hydrolyzed animal protein globules appear similar under the microscope. Addressing these challenges requires research, regulatory updates, and advanced technologies to ensure the integrity and safety of feed across the supply chain.
8. Methodology
Slides observation and material preparation were carried out in the Feed and Food Microscopy Laboratory at AGROLAB Alimentalia (Italy). The Tetramethylbenzidine–Hydrogen Peroxide reagent used for staining was prepared according to the specifications outlined in Section 2.1.2.1.4.4 of Annex VI to Regulation (EC) No 152/2009 and the EURL-AP Standard Operating Procedure (SOP) for staining reagents. The 3,3’,5,5’-Tetramethylbenzidine (TMB) was sourced from Sigma-Aldrich. Microscopic analysis was performed using the Zeiss Axiovert 25 Inverted Phase Contrast Microscope. Observations were conducted at magnifications of 10X, 20X, and 32X, each paired with a 10X ocular lens. A 0.4 N.A. condenser equipped with a stage and phase slider was employed during examination. Photographic documentation of the slides included an additional 2.5X magnification.
Acknowledgments
The author expresses sincere gratitude to Dr. Giulio Lora, Site Manager at AGROLAB Alimentalia S.r.l., Italy, for granting access to microscopy tools and internal reference materials essential to this work. Special thanks are extended to Dr.ssa Arianna Salvador, Lab Leader of Microbiology and Molecular Biology, for permitting the use of the food and feed microscopy laboratory for image capture and slide observation. Appreciation is also due to Dr. Enrico Goldin, Head of CRM Food, and his team for kindly providing samples and reference material analyzed in this study.
References
- Balan, P.; Staincliffe, M.; Moughan, P.J. Effects of spray-dried animal plasma on the growth performance of weaned piglets—A review. J Anim Physiol Anim Nutr. 2021, 105, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Adhikary, K.; Sarkar, R.; Chakraborty, S.; Sasmita, J. 2023. Chapter 53—Prion diseases: A rare group of neurodegenerative disorders Antimicrobial, Host Defense, and Therapeutic Strategies, 651-666. [CrossRef]
- Blázquez, E.; Rodríguez, C.; Ródenas, J.; Segalés, J.; Pujols, J.; Polo, J. Biosafety Steps in the Manufacturing Process of Spray-Dried Plasma: A Review with Emphasis on the Use of Ultraviolet Irradiation as a Redundant Biosafety Procedure. Porc. Health Manag. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Chuchird, N.; Rairat, T.; Keetanon, A.; Phansawat, P.; Chou, C.-C.; Campbell, J. Effects of Spray-Dried Animal Plasma on Growth Performance, Survival, Feed Utilization, Immune Responses, and Resistance to Vibrio Parahaemolyticus Infection of Pacific White Shrimp (Litopenaeus vannamei). PLoS ONE 2021, 16, e0257792. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed (Text with EEA relevance).
- Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein (OJ L 138, 25.5.2017, p. 92).
- Commission Regulation (EU) 2021/1372 of 17 August 2021 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards the prohibition to feed non-ruminant farmed animals, other than fur animals, with protein derived from animals (OJ L 295, 18.8.2021, p. 1).
- Csurka, T.; Szücs, F.; Csehi, B.; Friedrich, L.F.; Pásztor-Huszár, K. Substitution of milk allergen ingredient by blood plasma powder in custard with different sweeteners. Progress in Agricultural Engineering Sciences 2021, 17 (Suppl. 1), 77–85. [Google Scholar] [CrossRef]
- Drackley, J.K. Calf nutrition from birth to breeding. Vet. Clin. North Am. Food Anim. Pract. 2008, 24, 55–86. [Google Scholar] [CrossRef]
- Domenis, L.; Squadrone, S.; Marchis, D.; Abete, M.C. Osteocyte lacunae features in different chicken bones Biotechnol. Agron. Soc. Environ. 2009, 13, 29–32. [Google Scholar]
- Garner, D.D.; Cano, K.M.; Peimer, R.S.; Yeshion, T.E. An Evaluation of Tetramethylbenzidine as a Presumptive Test for Blood. J. Forensic Sci. 1976, 21, 816–821. [Google Scholar] [CrossRef]
- Golinelli, L.P.; Silva, J.T.; Carvalho, A.C.; Paschoalin, V.M.F. Detection of Animal Products in Ruminant Feeds by Microscopy and Real-Time PCR. Journal of Veterinary Science & Technology 2016, 7, 1000316. [Google Scholar] [CrossRef]
- Henrichs, B.S.; Brost, K.N.; Hayes, C.A.; Campbell, J.M.; Drackley, J.K. Effects of spray-dried bovine plasma protein in milk replacers fed at a high plane of nutrition on performance, intestinal permeability, and morbidity of Holstein calves. Journal of Dairy Science. 2021, 104, 7856–7870. [Google Scholar] [CrossRef]
- Holland, V.R.; Saunders, B.C.; Rose, F.L.; Walpole, A.L. A Safer Substitute for Benzidine in the Detection of Blood. Tetrahedron 1974, 30, 3299–3302. [Google Scholar] [CrossRef]
-
https://www.eurl.craw.eu/legal-sources-and-sops/method-of-reference-and-sops/.
- Hu, J.; Dong, Y.-L.; Zhang, H.-J.; Chen, X.-J.; Chen, X.-G.; Zhang, H.-G.; Chen, H.-L. Naked eye detection of benzoyl peroxide in wheat flour using 3,3′,5,5′-tetramethylbenzidine as a chromogenic agent. RSC Adv. 2013, 48, 26307–26312. [Google Scholar] [CrossRef]
- Ionescu, A.-D.; Cîrîc, A.I.; Begea, M. A Review of Milk Frauds and Adulterations from a Technological Perspective. Appl. Sci. 2023, 13, 9821. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, H.; Zou, J.; Zhu, G.; Huang, Y. CuCeTA nanoflowers as an efficient peroxidase candidate for direct colorimetric detection of glyphosate. J Mater Chem B. 2023, 11, 9630–9638. [Google Scholar] [CrossRef] [PubMed]
- Kazimierska, K.; Biel, W. Chemical Composition and Functional Properties of Spray-Dried Animal Plasma and Its Contributions to Livestock and Pet Health: A Review. Animals 2023, 13, 2484. [Google Scholar] [CrossRef]
- Li, X.; Gao, L.; Chen, Z. Highly sensitive colorimetric detection of glucose through glucose oxidase and Cu2+-catalyzed 3,3’,5,5’-tetramethylbenzidine oxidation. Spectrochim Acta A Mol Biomol Spectrosc. 2019, 213, 37–41. [Google Scholar] [CrossRef]
- Li, H.; Zhao, T.; Li, H.; Yu, J. Effect of Heat Treatment on the Property, Structure, and Aggregation of Skim Milk Proteins. Frontiers in Nutrition. Sec. Food Chemistry 2021, 8. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Sun, X. Highly sensitive and selective colorimetric detection of Ag(I) ion using 3,3′,5,5′,-tetramethylbenzidine (TMB) as an indicator. Sensors and Actuators B: Chemical 2012, 165, 44–47. [Google Scholar] [CrossRef]
- Polo, J.; Rodríguez, C.; Ródenas, J.; Morera, S.; Saborido, N. The Use of Spray-Dried Animal Plasma in Comparison with Other Binders in Canned Pet Food Recipes. Anim. Feed Sci. Technol. 2009, 154, 241–247. [Google Scholar] [CrossRef]
- Quigley, J.D.; Kost, C.J.; Wolfe, T.A. Effects of Spray-Dried Animal Plasma in Milk Replacers or Additives Containing Serum and Oligosaccharides on Growth and Health of Calves. Journal of Dairy Science. 2002, 85, 413–421. [Google Scholar] [CrossRef]
- Quigley, J.D.; Wolfe, T.M. Effects of Spray-Dried Animal Plasma in Calf Milk Replacer on Health and Growth of Dairy Calves. Journal of Dairy Science 2003, 86, 586–592. [Google Scholar] [CrossRef]
- Raamsdonk, Leo van & Scholtens, I.M.J. & Ossenkoppele, J.s & Egmond, Harry & Groot, M.J.. (2011). Investigation into blood plasma in milk formula. Landscape Online.
- Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies (OJ L 147, 31.5.2001, p. 1).
- Sezer, B.; Durna, S.; Bilge, G.; Berkkan, A.; Yetisemiyen, A.; Boyaci, H.I. Identification of milk fraud using laser-induced breakdown spectroscopy (LIBS). International Dairy Journal 2018, 81, 1–7. [Google Scholar] [CrossRef]
- Standefer, J.C.; Vanderjagt, D. Use of tetramethylbenzidine in plasma hemoglobin assay. Clin Chem. 1977, 23, 749–751. [Google Scholar] [CrossRef] [PubMed]
- van Raamsdonk, L.W.D.; Scholtens-Toma, I.M.J.; Ossenkoppele, J.S.; van Egmond, H.J.; Groot, M.J. (2011). Investigation into blood plasma in milk formula. (Report / RIKILT; No. 2011.003). RIKILT. https://edepot.wur.nl/184869.
- van Raamsdonk, L.W.D.; Pinotti, L.; Veys, P.; Campagnoli, A.; Paltanin, C.; Crespo, C.B.; Jørgensen, J.S. Chapter 6 Markers for microscopic detection. In “Detection, identification and quantification of processed animal proteins in feedingstuffs” Editors J.S Jorgensen, V. Baeten; Presses universitaires de Namur pp 59-69 ISBN (Print)9782875510297.
- Vasconcellos, R.S.; Henríquez, L.B.F.; Lourenço, P.D.S. Spray-Dried Animal Plasma as a Multifaceted Ingredient in Pet Food. Animals 2023, 13, 1773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wood, D.R.; Blome, R.M.; Ribeiro, L.C.; Keunen, A.J.; Keunen, B.W.; Crenshaw, J.D.; Campbell, J.M.; Renaud, D.L. Effect of porcine plasma on growth and health of Holstein calves. JDS Commun. 2021, 2, 340–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Zhao, W.; Hu, C.; Guo, D.; Liu, Y. Colorimetric copper (Ⅱ) ions detection in aqueous solution based on the system of 3′3′5′5′-tetramethylbenzidine and AgNPs in the presence of Na2S2O3. Journal of Science: Advanced Materials and Devices. 2022, 7, 100420. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Chai, T.-Q.; Peng, L.-J.; Zhang, W.-Y.; Tian, T.; Zhang, H.; Yang, F.-Q. Bisubstrate multi-colorimetric assay based on the peroxidase-like activity of Cu2+-triethylamine complex for copper ion detection. Dyes and Pigments 2023, 210, 111028. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).