Submitted:
14 October 2025
Posted:
16 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
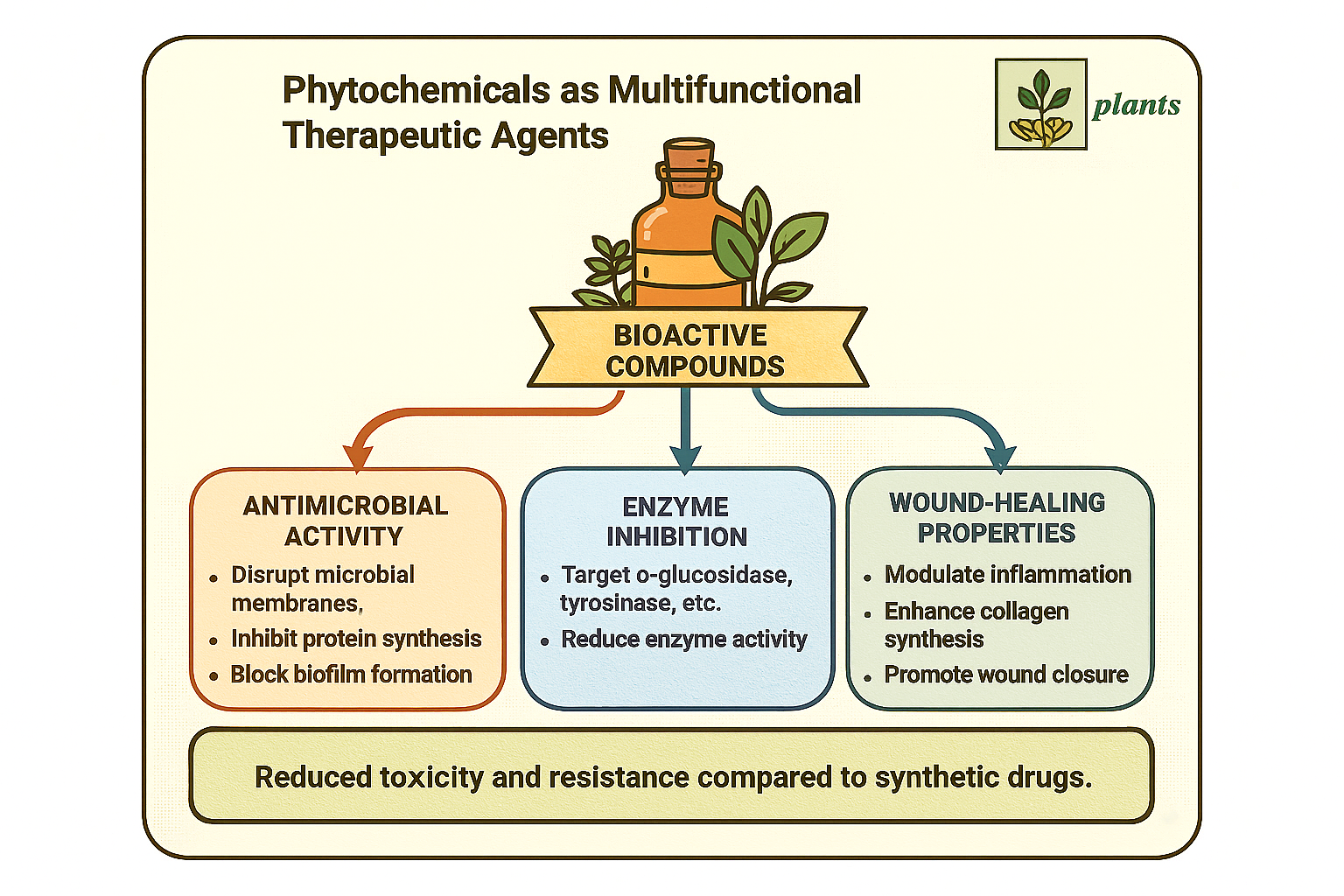
2.1. Antimicrobial Properties of Phytochemicals
2.2. Enzyme Inhibitory Activity of Plant-Derived Compounds
2.3. Wound-Healing Effects of Phytochemicals
2.4. Multifunctional Synergy: Antimicrobial, Enzyme Inhibition, and Wound Healing
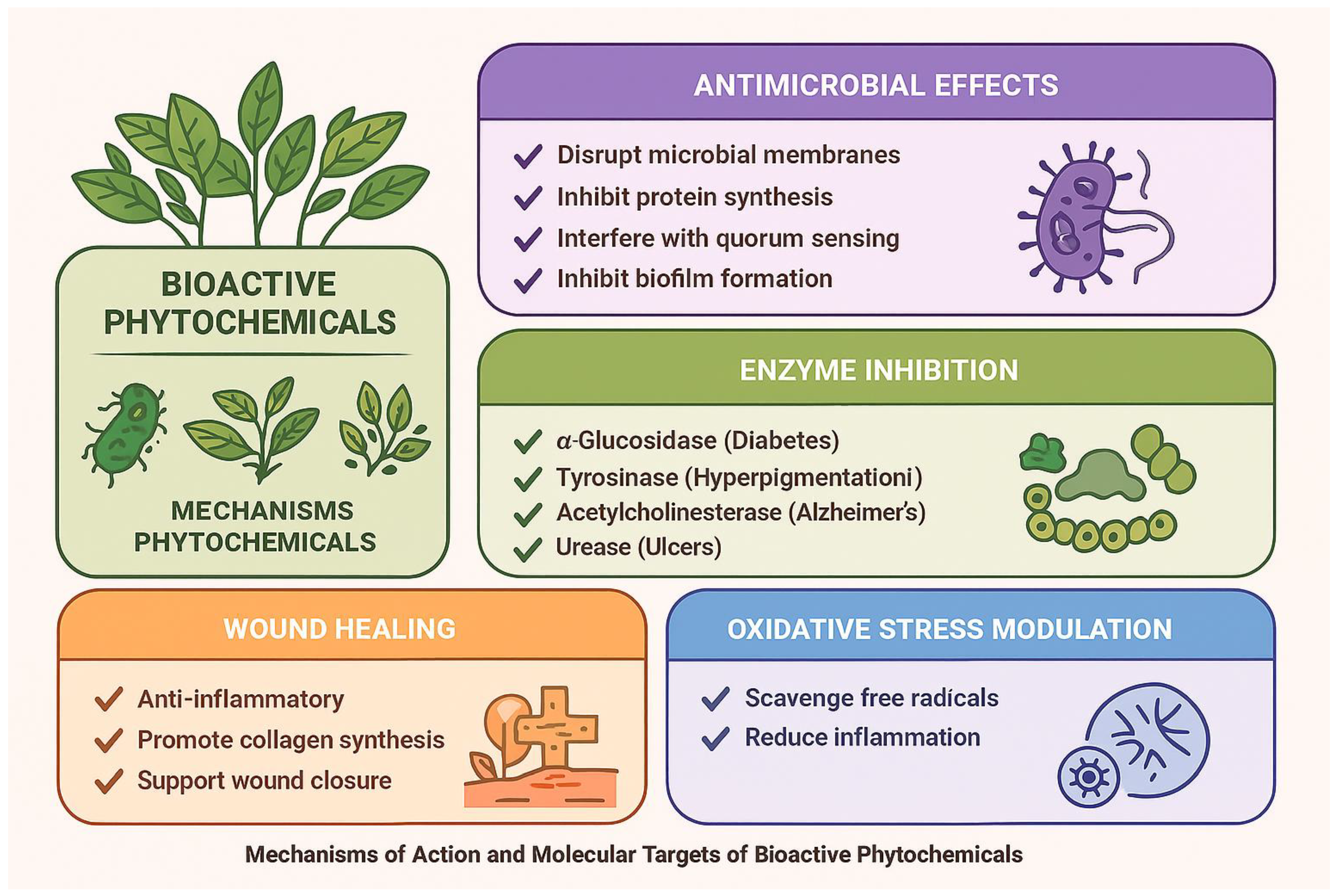
3. Mechanisms of Action and Molecular Targets of Bioactive Phytochemicals
3.1. Antimicrobial Mechanisms of Phytochemicals
3.2. Mechanisms of Enzyme Inhibition
3.3. Phytochemical Mechanisms in Wound Healing
3.4. Multi-Target and Synergistic Interactions
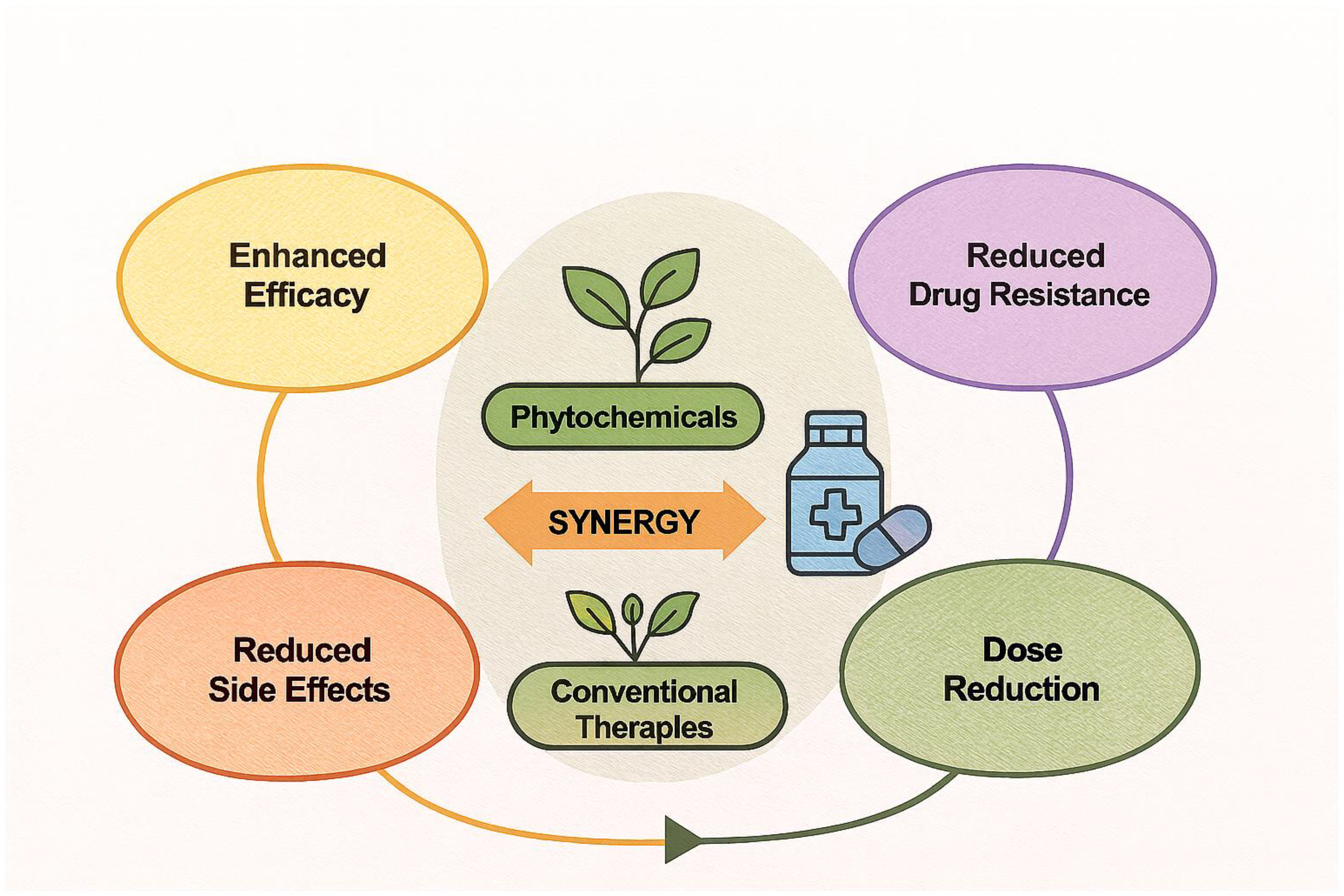
4. Synergistic Interactions Between Phytochemicals and Conventional Therapeutics
4.1. Rationale for Synergy
4.2. Synergistic Enhancement of Antibacterial Agents
4.3. Synergy with Antifungal and Antiviral Agents
4.4. Potentiation of Enzyme Inhibitors and Anti-inflammatory Drugs
4.5. Mechanisms Underlying Synergistic Effects
- Biofilm disruption: Many plant compounds interfere with biofilm matrix formation, exposing pathogens to drug action [50].
- Enzyme inhibition: Co-inhibition of target enzymes by phytochemicals and drugs amplifies metabolic disruption [56].
- Pharmacokinetic modulation: Some phytochemicals act as bioenhancers (e.g., piperine), increasing the bioavailability of poorly absorbed drugs [51].
4.6. Clinical Potential and Challenges
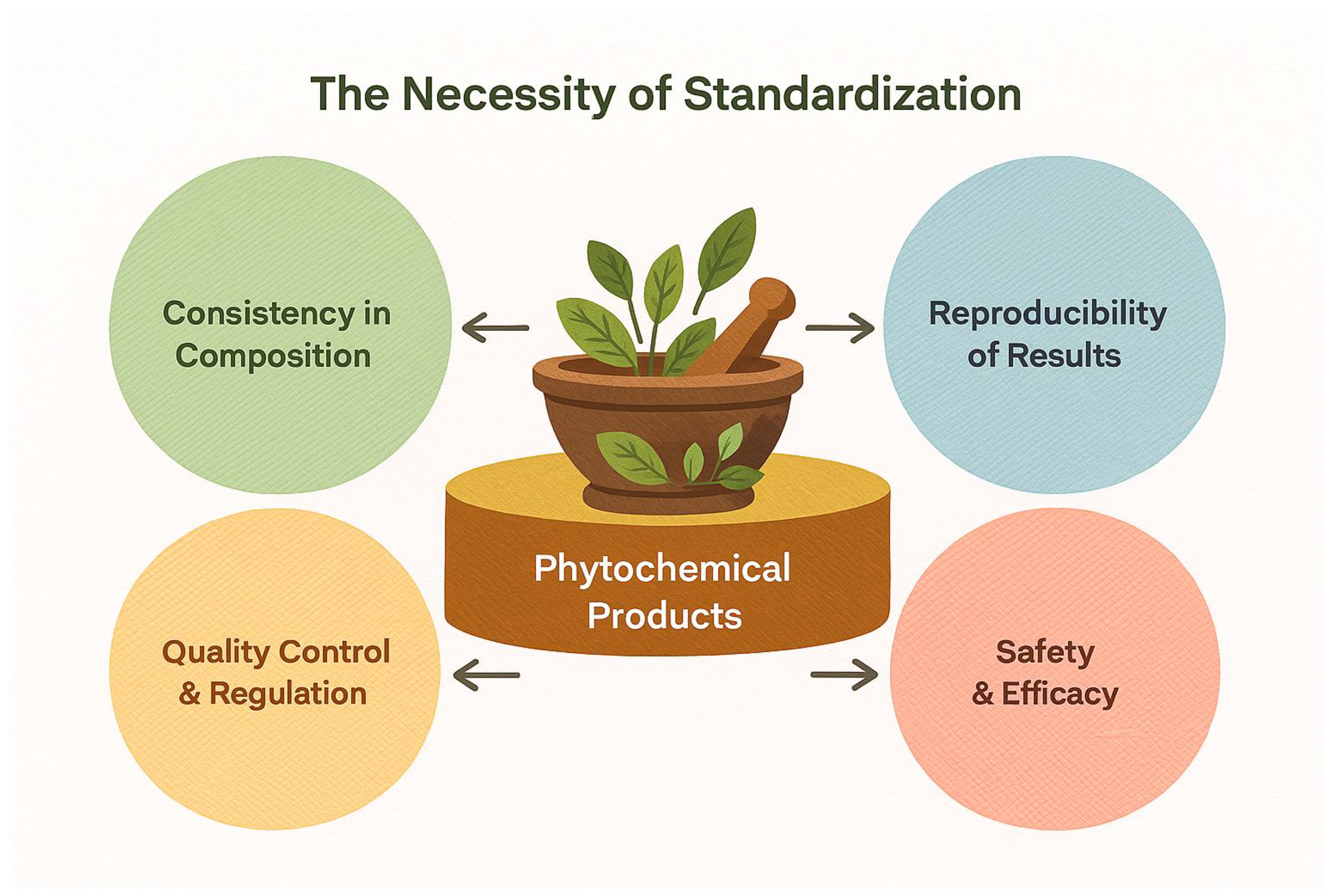
5. Standardization, Safety, and Toxicity Assessments of Bioactive Plant Extracts
5.1. The Necessity of Standardization
5.2. Safety Assessment and Dose Determination
5.3. Toxicological Challenges in Polyherbal Formulations
5.4. Regulatory Frameworks and Quality Control
5.5. Ethical Considerations and Future Directions
6. Integration of Ethnopharmacological Knowledge into Modern Therapeutics
Funding
Acknowledgments
Conflicts of Interest
References
- Karageçili, H.; Gülçin, İ. The Lamiaceae Family Plants: Ethnobotanical Properties, Ethnopharmacological Uses, Phytochemical Studies and Their Utilization in Public or Current Clinical Practices—A Review. Rec. Nat. Prod. 2025, 19, 466–487. [Google Scholar] [CrossRef]
- Kaushal, K.; Kumar, M.; Thakur, A.; Kaur, T. An Overview of the Phytochemical and Therapeutic Potential of the White Button Mushroom (Agaricus bisporus (J.E. Lange) Imbach). J. Phytonanotechnol. Pharm. Sci. 2025, 5, 34–40. [Google Scholar] [CrossRef]
- Abbigeri, M.B.; Thokchom, B.; Singh, S.R.; Bhavi, S.M. Antioxidant and Antidiabetic Potential of the Green Synthesized Silver Nanoparticles Using Martynia annua Root Extract. Nano TransMed 2025, 6, 9–17. [Google Scholar]
- Mali, S.N.; Saied, E.M.; dos Santos, C.B.R.; Cruz, J.N. Medicinal Chemistry for Neglected Tropical Diseases Using In Vitro, In Vivo and In Silico Approaches. Front. Chem. 2025, 13, 1689034. [Google Scholar] [CrossRef]
- Topcu, K.S.B.; Genç, N.; Celik, A.; Sağ, V.; Yıldırım, B.; Karaismailoğlu, M.C.; Kisa, D. Liquid Chromatography–Tandem Mass Spectrometry Analysis of Three Endemic Plants in Brassicaceae Family: An Integrative Analysis with In Vitro and In Silico Study to Assess Biological Potential. Chem. Biodivers. 2025, 22, e202500715. [Google Scholar] [CrossRef]
- Bouaïfa, Z.; Boudjelal, A.; Bouaziz-Terrachet, S. Arisarum vulgare: Bridging Tradition and Science through Phytochemical Characterization and Exploring Therapeutic Potential via In Vitro, In Vivo, and In Silico Biological Activities. ChemistrySelect 2024, 9, e202402754. [Google Scholar] [CrossRef]
- Sahariah, G.; Dutta, K.N.; Gam, S.; Talukdar, S.; Boro, D.; Deka, K.; Siangshai, S.; Bora, N.S. A Review of the Ethnopharmacology, Phytochemistry and Pharmacological Activities of Mikania micrantha Kunth.: An Asian Invasive Weed. Int. J. Plant Res. 2025. [Google Scholar] [CrossRef]
- Deore, D.A.; Ahire, S.; Mahajan, S.K. Herbal Medicine Meets Nanotechnology: A Transformative Approach. Asian J. Pharm. Res. 2025. [Google Scholar] [CrossRef]
- Alshahrani, M.Y.; Alshahrani, K.M.; Tasleem, M.; Akeel, A.; Almeleebia, T.M.; Ahmad, I.; Asiri, M.; Alshahrani, N.A.; Alabdallah, N.M.; Saeed, M. Computational Screening of Natural Compounds for Identification of Potential Anti-Cancer Agents Targeting MCM7 Protein. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B. Ethnopharmacology and Drug Discovery. J. Ethnopharmacol. 2005, 100, 50–52. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Sabry, A.; Abolibda, T.Z.; Guo, Z.; Nahar, L.; Sarker, S.D.; Saeed, A.; Karav, S. Unraveling the Role of Globularia Species in Modern Medicine Based on Evidence from Phytochemistry, Traditional Uses and Biological Activities. Phytomedicine 2025, 156466. [Google Scholar] [CrossRef] [PubMed]
- Jaafer, M.F.; Mahood, W.S.; Alyam, M.S.S. Adiantum capillus-veneris L.: A Comprehensive Review of Its Phytochemical Composition, Pharmacological Activities, and Therapeutic Potential. Baghdad J. Biochem. Appl. Biol. Sci. 2025, 6, 85–99. [Google Scholar] [CrossRef]
- Khan, M.A.; El-Kersh, D.M.; Islam, M.S. Mikania micrantha Kunth: An Ethnopharmacological Treasure Trove of Therapeutic Potential. Chem. Biodivers. 2023, 20(8), e202300392. [Google Scholar] [CrossRef]
- Kashkooe, A.; Sardari, F.A.; Mehrabadi, M.M. M. Curr. Drug Discov. Technol. 2021, 18(4), 522–534. [Google Scholar] [CrossRef]
- Darko, D.; Kwekutsu, E.; Idoko, B.; Idoko, I.P. Synergistic Effects of Phytochemicals in Combating Chronic Diseases with Insights into Molecular Mechanisms and Nutraceutical Development. Int. J. Innov. Sci. Res. Technol. 2025, 10, 1865–1883. [Google Scholar] [CrossRef]
- Kumari, S.; Swer, T. Acacia nilotica Linn: A Comprehensive Review of Its Nutritional Profile, Pharmacological Activities, and Food Applications. Phytochem. Rev. 2025. [Google Scholar] [CrossRef]
- Farahin, P.N.; Nadia, N.; Susanti, D.; Hasniza, N.; Abd Halim, K.B.; Haron, N. Molecular Docking of Polyphenol Compounds from Anacardium occidentale with Alpha-Glucosidase and Dipeptidyl-Peptidase-4 Enzymes. Malays. J. Fundam. Appl. Sci. 2021, 17, 202–216. [Google Scholar] [CrossRef]
- Vadaga, A.; Gudla, S.S. Phytochemical Composition and Pharmacological Benefits of Majuphal. Pharmacol. Res.—Nat. Prod. 2025, 8, 100342. [Google Scholar] [CrossRef]
- Kumari, S.; Swer, T. Acacia nilotica Linn: A Comprehensive Review of Its Nutritional Profile, Pharmacological Activities, and Food Applications. Phytochem. Rev. 2025. [CrossRef]
- Ghalloo, B.A.; Khan, K.-u.-R.; Ahmad, S.; Aati, H.Y.; Al-Qahtani, J.H.; Ali, B.; Mukhtar, I.; Hussain, M.; Shahzad, M.N.; Ahmed, I. Phytochemical Profiling, In Vitro Biological Activities, and In Silico Molecular Docking Studies of Dracaena reflexa. Molecules 2022, 27, 913. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Campos-García, J.; Contreras-Garduño, J.; Lugo-Ortiz, C. Use of Medicinal Plants in the Process of Wound Healing. Pharmaceutics 2024, 17, 303. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Alhussaini, M.S.; Asad, M.; Joseph, B. Effect of Moringa oleifera Leaf Extract on Excision Wound Infections in Rats: Antioxidant, Antimicrobial, and Gene Expression Analysis. Molecules 2022, 27, 4481. [Google Scholar] [CrossRef] [PubMed]
- Alsarayreh, A.Z.; Oran, S.A.; Shakhanbeh, J.M. In Vitro and In Vivo Wound Healing Activities of Globularia arabica Leaf Methanolic Extract in Diabetic Rats. J. Cosmet. Dermatol. 2022, 21(10), 4888–4900. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; Ismail, A.F.H.; Sarian, M.N. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mithi, F.M.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef]
- Omojate, G.C.; Enwa, F.O.; Jewo, A.O.; Eze, C.O. Mechanisms of Antimicrobial Actions of Phytochemicals against Enteric Pathogens—A Review. Res. J. Pharm. Biol. Chem. Sci. 2014, 2, 77–85. [Google Scholar]
- Elsaman, T.; Mohamed, M.A.; Mohamed, M.S.; Eltayib, E.M.; Abdalla, A.E. Microbial-Based Natural Products as Potential Inhibitors Targeting DNA Gyrase B of Mycobacterium tuberculosis: An In Silico Study. Front. Chem. 2025, 13, 1524607. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; Ismail, A.F.H.; Sarian, M.N. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.-K. Quorum Sensing Inhibitors as Antipathogens: Biotechnological Applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Wichayapreechar, P.; Prasansuklab, A.; Charoongchit, P.; Charoenjittichai, R. The Potential of Tecoma stans (Linn.) Flower Extract as a Natural Antioxidant and Anti-Aging Agent for Skin Care Products. Cosmetics 2024, 11, 214. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, R.; Liu, F.; Ge, L.; Zhang, X.; et al. On Mechanism behind UV-A Light Enhanced Antibacterial Activity of Gallic Acid and Propyl Gallate against Escherichia coli O157:H7. Sci. Rep. 2017, 7, 3027. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Ao, J.; Hou, Y.; Xi, M.; Cai, Y.; Li, M.; Luo, A. ; Luo, A. Structure-activity relationships and the underlying mechanism of α-amylase inhibition by hyperoside and quercetin: multi-spectroscopy and molecular docking analyses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121797. [Google Scholar] [CrossRef]
- Man, Z.; Feng, Y.; Xiao, J.; Yang, H.; Wu, X. Structural Changes and Molecular Mechanism Study on the Inhibitory Activity of Epigallocatechin against α-Glucosidase and α-Amylase. Front. Nutr. 2022, 9, 948027. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Anwar, F.; Naz, F.; Mehmood, T.; Saari, N. Anti-Helicobacter pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants. Molecules 2013, 18, 2135–2149. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Muñoz, E.; García-Serna, J.; García-Lobo, J.M.; et al. J. Enzyme Inhib. Med. Chem. 2019, 34(1), 279–309. [CrossRef]
- Mushtaq, Z.; Sadeer, N.B.; Hussain, M.; Mahwish, *!!! REPLACE !!!*; Alsagaby, S.A.; Imran, M.; Mumtaz, T.; Umar, M.; Tauseef, A.; Al Abdulmonem, W.; Al Jbawi, E.; Mahomoodally, M.F. Therapeutical Properties of Apigenin: A Review on the Experimental Evidence and Basic Mechanisms. Toxicol. Mech. Methods 2023, 33, 632–650. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Crețu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Alhussaini, M.S.; Asad, M.; Joseph, B. Moringa oleifera Leaf Extract Promotes Healing of Infected Wounds in Diabetic Rats: Evidence of Antimicrobial, Antioxidant and Proliferative Properties. Pharmaceuticals 2022, 15, 528. [Google Scholar] [CrossRef]
- Shady, N.H.; Mostafa, N.M.; Fayez, S.; Abdel-Rahman, I.M.; Maher, S.A.; Zayed, A.; Saber, E.A.; Khowdiary, M.M.; Elrehany, M.A.; Alzubaidi, M.A.; Altemani, F.H.; Shawky, A.M.; Abdelmohsen, U.R. Mechanistic Wound Healing and Antioxidant Potential of Moringa oleifera Seeds Extract Supported by Metabolic Profiling, In Silico Network Design, Molecular Docking, and In Vivo Studies. Antioxidants 2022, 11, 1743. [Google Scholar] [CrossRef]
- Genah, S.; Ciccone, V.; Filippelli, A.; et al. Erucin, a Natural Isothiocyanate, Exerts Pro-Angiogenic Properties in Cultured Endothelial Cells and Reverts Angiogenic Impairment Induced by High Glucose. Phytother. Res. 2024, 38(6), 2641–2655. [Google Scholar] [CrossRef]
- Yao, R.-Q.; Zhang, L.; Wang, W.; Li, L. Cornel Iridoid Glycoside Promotes Neurogenesis and Angiogenesis and Improves Neurological Function after Focal Cerebral Ischemia in Rats. Brain Res. Bull. 2009, 79, 69–76. [Google Scholar] [CrossRef]
- Friščić, M.; Petlevski, R.; Kosalec, I.; Madunić, J.; Matulić, M.; Bucar, F.; Hazler Pilepić, K.; Maleš, Ž. Globularia alypum L. and Related Species: LC–MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential. Pharmaceuticals 2022, 15, 506. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Tegos, G.P.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug Pump Inhibitors Uncover Remarkable Activity of Plant Antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef]
- Abrar, A.; Zafar, A.; Fatima, M.; Muntaqua, D.; Naz, I.; Fatima, H.; Ul Haq, I. Mechanistic Insight into the Synergistic Antimicrobial Potential of Fagonia indica Burm.f. Extracts with Cefixime. Saudi Pharm. J. 2024, 32, 101893. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, W.; Cao, Q.; Li, Y.; Bao, G.; Lin, T.; Bao, J.; Chang, C.; Yang, C.; Yin, Y.; Xu, J.; Ren, Z.; Jin, Y.; Lu, F. Global Downregulation of Penicillin Resistance and Biofilm Formation by MRSA Is Associated with the Interaction between Kaempferol Rhamnosides and Quercetin. Microbiol. Spectr. 2022, 10(6), e02782-22. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Cheng, B.; Gao, L.; Qin, C.; Zhang, L.; Zhang, X.; Wang, J.; Wan, Y. Discovery of Quercetin and Its Analogs as Potent OXA-48 Beta-Lactamase Inhibitors. Front. Pharmacol. 2022, 13, 926104. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Chandrasekar, M.J.N. Plant-Based Natural Products as Inhibitors for Efflux Pumps to Reverse Multidrug Resistance in Staphylococcus aureus: A Mini Review. Mini-Rev. Med. Chem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, W.; Cai, L.; Yang, T. Potentiation and Mechanism of Berberine as an Antibiotic Adjuvant Against Multidrug-Resistant Bacteria. Infect. Drug Resist. 2023, 16, 3545–3562. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Cho, Y.S.; Schiller, N.L.; Oh, K.H. Antibacterial Effects of Green Tea Polyphenols on Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. Curr. Microbiol. 2008, 57(6), 542–546. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal Activity of Thymol and Carvacrol by Disrupting Ergosterol Biosynthesis and Membrane Integrity Against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S. Mechanistic Perspectives on Herpes Simplex Virus Inhibition by Phenolic Acids and Tannins: Interference with the Herpesvirus Life Cycle. Int. J. Mol. Sci. 2025, 26(13), 5932. [Google Scholar] [CrossRef]
- Liao, Y.; Mai, X.; Wu, X.; Hu, X.; Luo, X.; Zhang, G. Exploring the Inhibition of Quercetin on Acetylcholinesterase by Multispectroscopic and In Silico Approaches and Evaluation of Its Neuroprotective Effects on PC12 Cells. Molecules 2022, 27, 7971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, X.; Sun, W.; Xing, Y.; Xiu, Z. Dietary Flavonoids and Acarbose Synergistically Inhibit α-Glucosidase and Lower Postprandial Blood Glucose. J. Agric. Food Chem. 2017, 65(49), 10877–10884. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Modulation of the Immune System by Boswellic Acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Lin, C.-W.; Cheng, N.-C.; Cazzell, S.M.; Chen, H.-H.; Huang, K.-F.; Tung, K.-Y.; Huang, H.-L.; Lin, P.-Y.; Perng, C.-K.; Shi, B.; Liu, C.; Ma, Y.; Cao, Y.; Li, Y.; Xue, Y.; Yan, L.; Li, Q.; Ning, G.; Chang, S.-C. Effect of a Novel Macrophage-Regulating Drug on Wound Healing in Patients with Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4(9), e2122607. [Google Scholar] [CrossRef]
- Tao, L.; Willighagen, E.L.; Allard, P.-M.; Wishart, D.S.; Choudhary, K.; van Santen, J.A.; Schulze, T.; Fan, T.; Chen, Y.; Wolfender, J.-L.; Zhang, L. Artificial Intelligence for Natural Product Drug Discovery. Nat. Rev. Drug Discov. 2024, 23, 135–153. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing Herbal Medicine: Enhancing Product Quality and Safety through Robust Quality Control Practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Obasohan, P.; Makia, R.S.; Abiola, T.O.; Umelo, E.C.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Essaghah, A.E.A.; Ahmed, D.S.; Umar, H. The Use of Quality Control Parameters in the Evaluation of Herbal Drugs: A Review. Discover Medicine 2024, 1, 168. [Google Scholar] [CrossRef]
- Anukanon, S.; Saeng-ngoen, K.; Ngamnon, Y.; Rapan, N.; Seelarat, W.; Takolpuckdee, P.; Pakvilai, N.; Chatree, Y. Comparative Analysis of Curcuminoid Content, Antioxidant Capacity, and Target-Specific Molecular Docking of Turmeric Extracts Sourced from Thailand. Food Chem. (Oxf.) 2025, 11, 100291. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Banerjee, S.; Das Gupta, B.; Kar, A. Evidence-Based Validation of Herbal Medicine: Translational Approach. In Evidence-Based Validation of Herbal Medicine; Academic Press: Cambridge, MA, USA, 2022; pp. 1–41. [Google Scholar] [CrossRef]
- Lee, K.-M.; Jeon, J.-Y.; Lee, B.-J.; Lee, H.; Cho, H.-K. Application of Metabolomics to Quality Control of Natural Product Derived Medicines. Biomol. Ther. (Seoul) 2017, 25(6), 559–568. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Chattopadhyay, R.R. Possible Biochemical Mode of Anti-Inflammatory Action of Azadirachta indica A. Juss. in Rats. Indian J. Exp. Biol. 1998, 36, 418–420. [Google Scholar]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn.—A Reservoir Plant for Therapeutic Applications: An Overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef]
- Flores-Peña, R.; Monroy-Ramirez, H.C.; Caloca-Camarena, F.; Arceo-Orozco, S.; Salto-Sevilla, J.A.; Galicia-Moreno, M.; Armendariz-Borunda, J. Naringin and Naringenin in Liver Health: A Review of Molecular and Epigenetic Mechanisms and Emerging Therapeutic Strategies. Antioxidants 2025, 14, 979. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory Effects of Quercetin and Its Main Methyl, Sulfate, and Glucuronic Acid Conjugates on Cytochrome P450 Enzymes, and on OATP, BCRP and MRP2 Transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Boudreau, M.D.; Beland, F.A. An Evaluation of the Biological and Toxicological Properties of Aloe Barbadensis (Miller), Aloe Vera. J. Environ. Sci. Health C 2006, 24, 103–154. [Google Scholar] [CrossRef] [PubMed]
- Balekundri, A.; Mannur, V. Quality Control of the Traditional Herbs and Herbal Products: A Review. Future J. Pharm. Sci. 2020, 6, 67. [Google Scholar] [CrossRef]
- Klein-Junior, L.C.; de Souza, M.R.; Viaene, J.; Bresolin, T.M.B.; de Gasper, A.L.; Henriques, A.T.; Vander Heyden, Y. Quality Control of Herbal Medicines: From Traditional Techniques to State-of-the-art Approaches. Planta Med. 2021, 87, 964–988. [Google Scholar] [CrossRef]
- Vandebroek, I.; Balick, M.J. Globalization and Loss of Plant Knowledge: Challenging the Paradigm. PLoS ONE 2012, 7, e37643. [Google Scholar] [CrossRef]
- Zingales, V.; Esposito, M.R.; Torriero, N.; Taroncher, M.; Cimetta, E.; Ruiz, M.J. The Growing Importance of Three-Dimensional Models and Microphysiological Systems in the Assessment of Mycotoxin Toxicity. Toxins 2023, 15, 422. [Google Scholar] [CrossRef] [PubMed]
- 79. Mukherjee, P.K.; Wahile, A. Integrated Approaches Towards Drug Development from Ayurveda and Other Indian Systems of Medicines. J. Ethnopharmacol. 2006, 103, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. The Discovery of Artemisinin (Qinghaosu) and Gifts from Chinese Medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ. Health Perspect. 2001, 109 (Suppl 1), 69–75. [Google Scholar] [CrossRef]
- Heinrich, M.; Gibbons, S. Ethnopharmacology in drug discovery: an analysis of its role and potential contribution. J. Pharm. Pharmacol. 2001, 53, 425–432. [Google Scholar] [CrossRef]
- Heinrich, M.; Gibbons, S. Ethnopharmacology in Drug Discovery: An Analysis of Its Role and Potential Contribution. J. Pharm. Pharmacol. 2001, 53, 425–432. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Fong, H.H.S. Integration of Herbal Medicine into Modern Medical Practices: Issues and Prospects. Integr. Cancer Ther. 2002, 1, 287–293. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A.; Ndjoko, K.; Wolfender, J.-L. The Potential of African Plants as a Source of Drugs. Curr. Org. Chem. 2000, 4, 973–1010. [Google Scholar] [CrossRef]
- Heinrich, M.; Edwards, S.; Moerman, D.E.; Leonti, M. Ethnopharmacological Field Studies: A Critical Assessment of Their Conceptual Basis and Methods. J. Ethnopharmacol. 2009, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Booker, A.; Johnston, D.; Heinrich, M. Value Chains of Herbal Medicines—Research Needs and Key Challenges in the Context of Ethnopharmacology. J. Ethnopharmacol. 2012, 140, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Chen, S.; Liang, Y.; Wang, X.; Tian, R.; Upton, R. Chromatographic Fingerprint Analysis—A Rational Approach for Quality Assessment of Traditional Chinese Herbal Medicines. J. Chromatogr. A 2006, 1112, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.H.; Laxman, L.K.P. A Review of the International Framework for Access and Benefit Sharing of Genetic Resources with Special Reference to the Nagoya Protocol. Asia Pac. J. Environ. Law 2013, 16, 105–139. [Google Scholar]
- Sarma, S.K.; Kumar, A.A.; Vishnuvardhan, S.; Yamini, C.; Santhalahari, C.; Lahari, C.; Chaitany, G.; Ejitha, M. Antiurolithiatic Activity on Aerva lanata. J. Adv. Zool. 2024, 45, 48–57. [Google Scholar]
- Singh, M.P.; Bharghava, S.; Bhaduaria, R.S.; Sharma, C.S. Wound Healing Potential of Alcoholic Extract of Mimosa pudica Linn. Leaves. Pharmacologyonline 2010, 2, 32–38. [Google Scholar]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, F.; Wang, Y.; Li, C.; Zhang, X.; Li, H.; Diao, L.; Gu, J.; Wang, W.; Li, D.; He, F. BATMAN-TCM: A Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B. Where Lies the Future of Ayurveda-Inspired Drug Discovery? Expert Opin. Drug Discov. 2023, 18, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Einstein, G.P.; Tulp, O.L.; Sainvil, F.; Branly, R. Introduction to Traditional Medicine and Their Role in Prevention and Treatment of Emerging and Re-Emerging Diseases. Biomolecules 2022, 12, 1442. [Google Scholar] [CrossRef]
- Khare, S.; Andersson, S.; Mahadik, S.; Diwan, V. Why Do We Need an Integrative Approach to Solve the Health Problems in the Modern Society? Cent. India J. Med. Res. 2024, 3. [Google Scholar] [CrossRef]
| Plant Species | Key Phytochemicals | Antimicrobial Activity | Enzyme Inhibitory Activity | Wound Healing Activity |
|---|---|---|---|---|
| Mikania micrantha | Essential oils, Flavonoids | Strong (bacteria/fungi) | Cholinesterase | Anti-inflammatory, antioxidant [13] |
| Adiantum capillus-veneris | Triterpenoids, Phenolic acids | Antibacterial, Antifungal | Cholinesterase | Collagen stabilization [14] |
| Majuphal (Quercus infectoria) | Gallotannins, Polyphenols | Broad-spectrum | α-glucosidase, Tyrosinase | Anti-inflammatory [18] |
| Acacia nilotica | Flavonoids, Alkaloids | Antibacterial | Urease, AChE | Tissue repair promotion [19] |
| Moringa oleifera | Isothiocyanates, Flavonoids | Antibacterial | MMPs (indirect) | VEGF, TGF-β modulation [22] |
| Globularia species | Iridoids, Phenolic glycosides | Moderate | Multiple enzymes | Keratinocyte proliferation [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





