Submitted:
14 October 2025
Posted:
15 October 2025
You are already at the latest version
Abstract
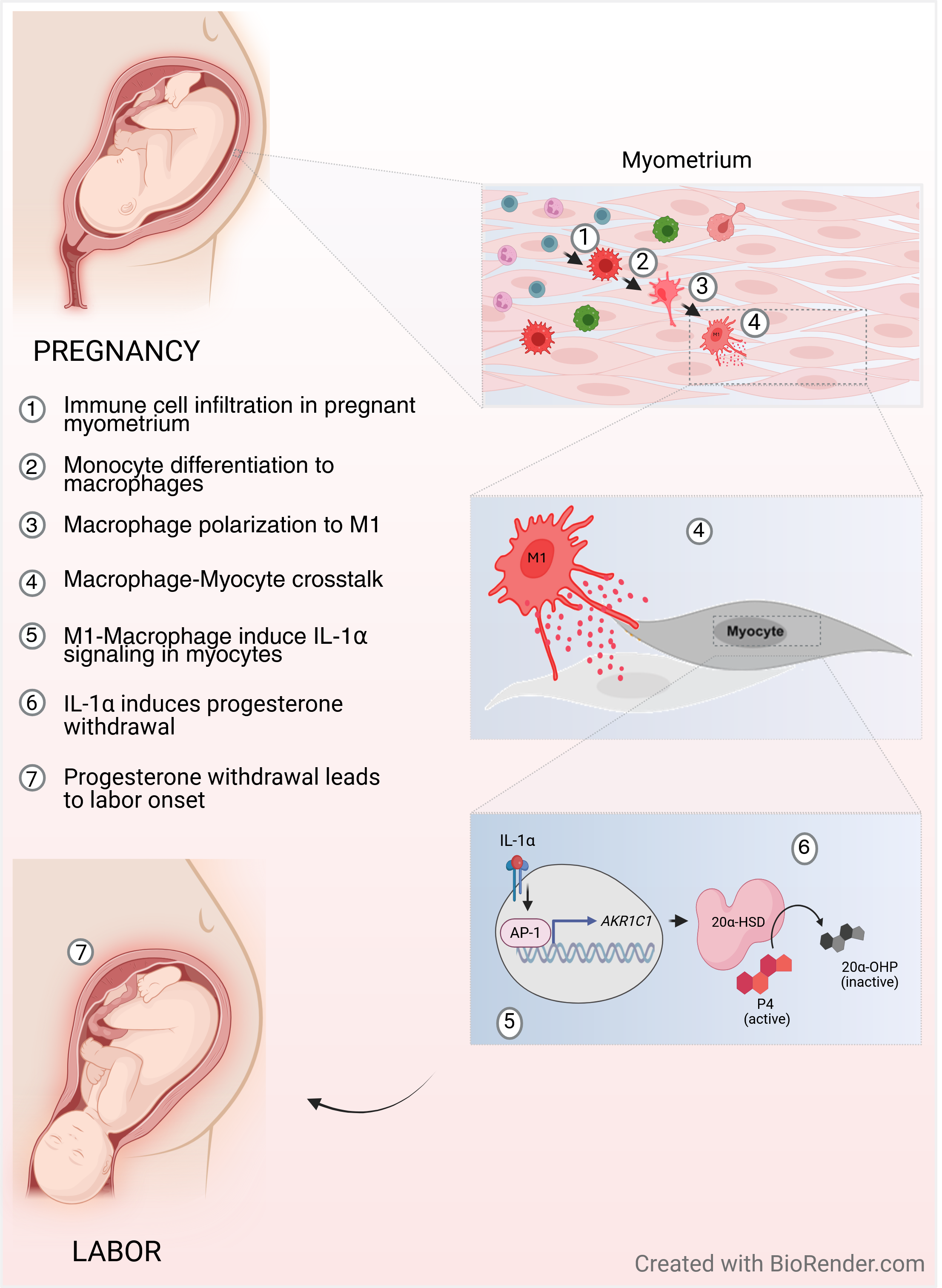
Keywords:
Introduction
Materials and Methods
1. Ethics Approval
2. Immunohistochemistry of Human Myometrium
3. Primary Myometrial Cell Lines
3. Myometrial Cell Treatment Regimen
4. Peripheral Blood Derived Monocytes
5. In-Vitro Differentiation and Polarization of Macrophages
6. Immunocytochemistry (ICC)
7. Image Analysis
8. Protein Extraction
9. Immunoblotting
10. Real-Time PCR
11. Collagen Gel Contraction Assay
12. Statistical Analysis
Results
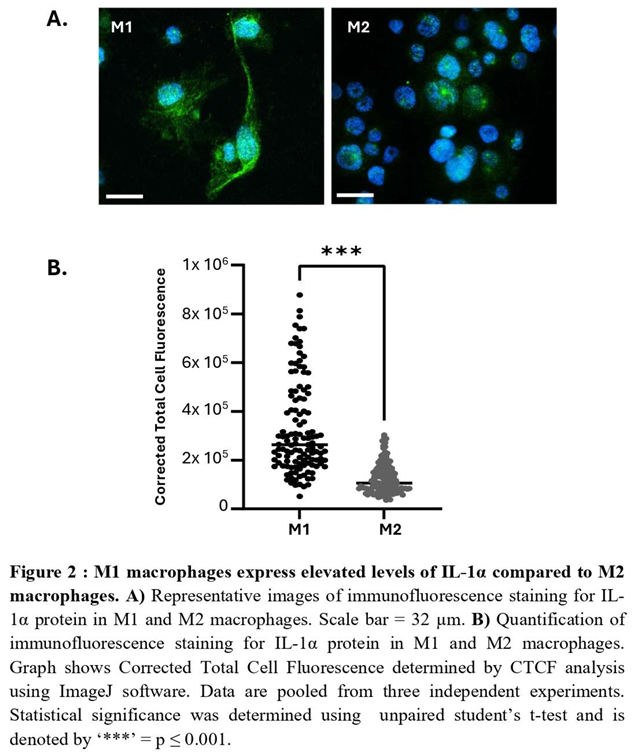
1. M1 Macrophages Express Elevated Levels of IL-1α Compared to M2 Macrophages

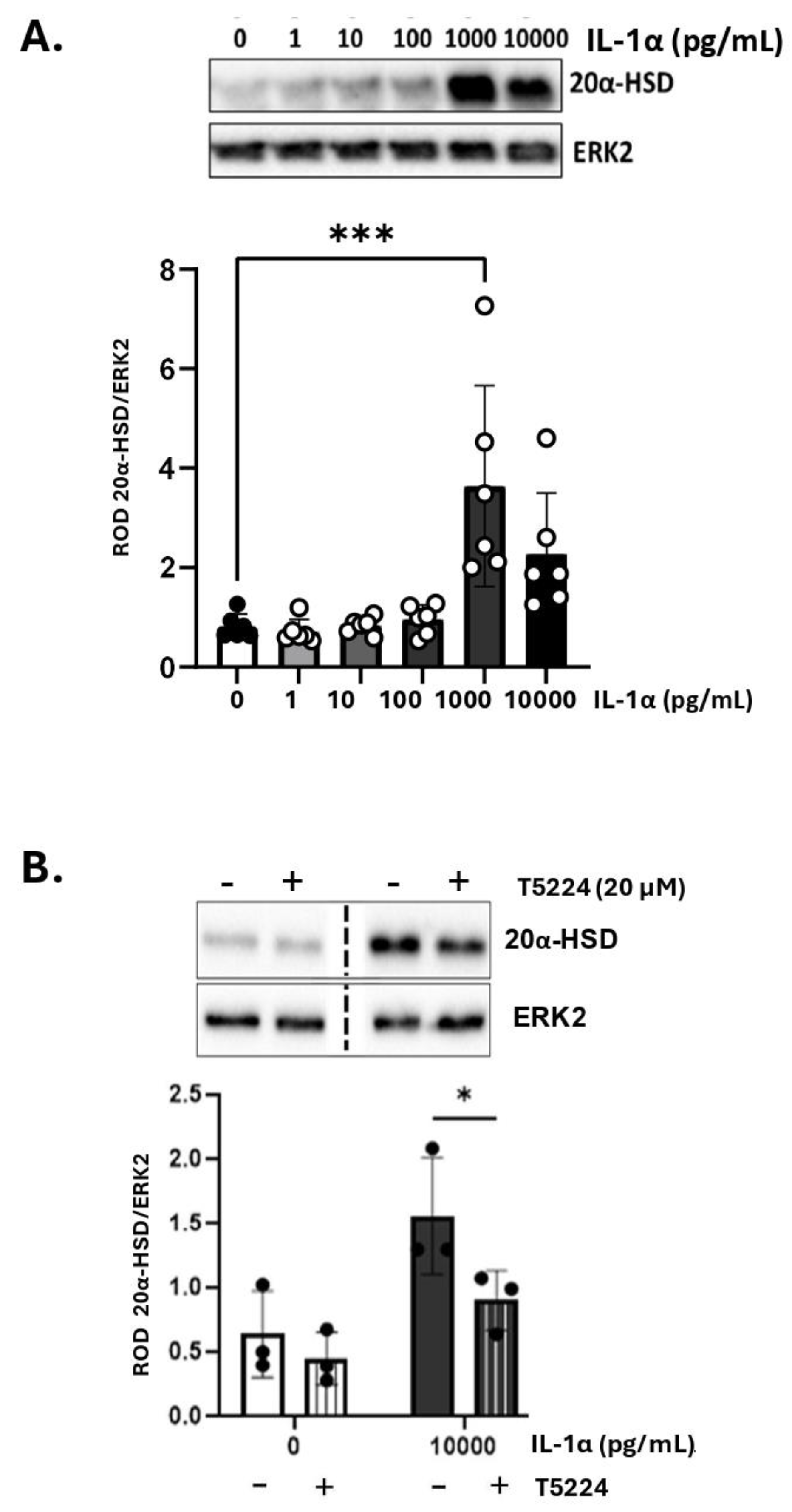
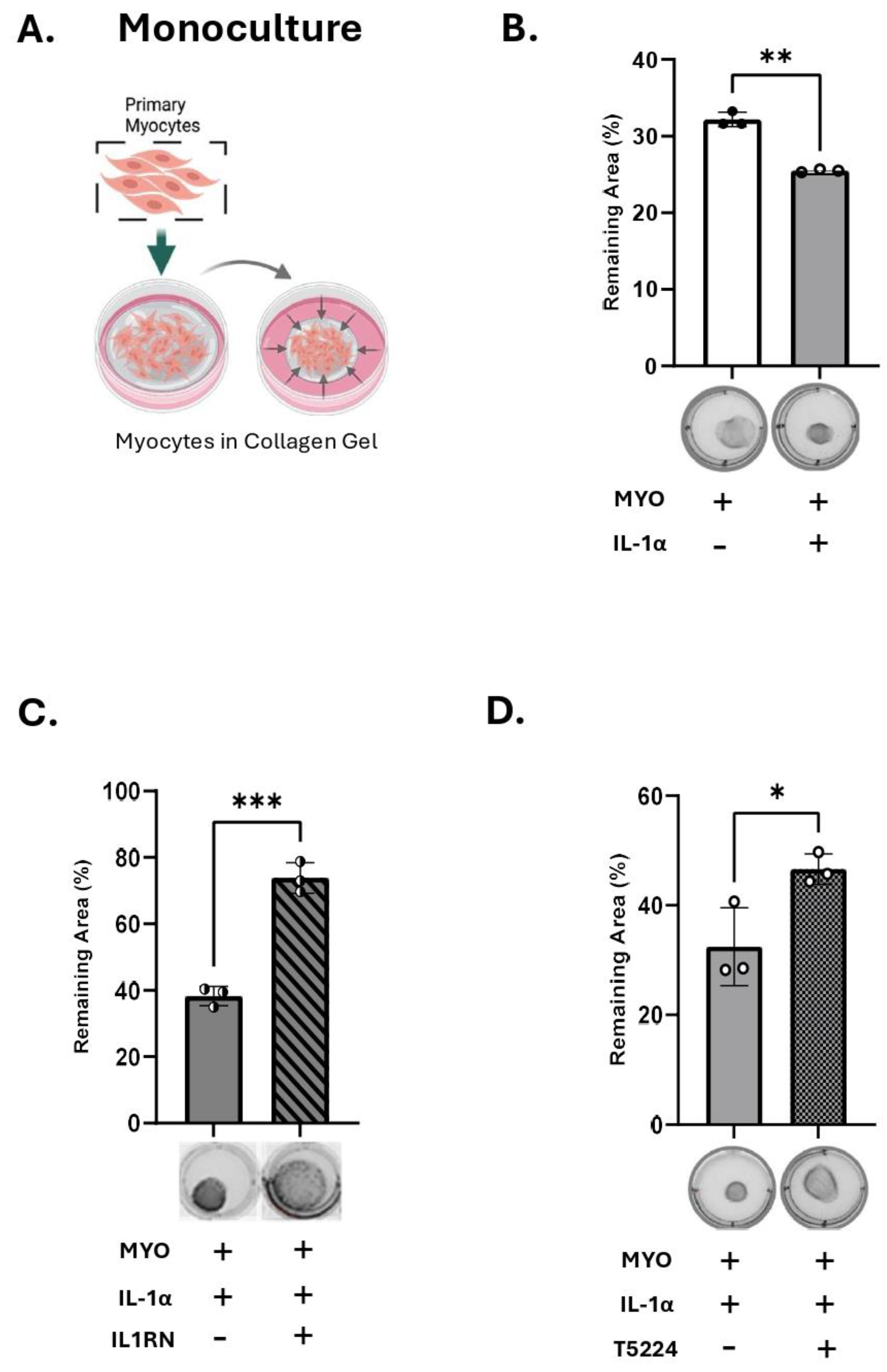
2. IL-1α Induces 20a-HSD Levels and Contractility in the Myocytes and this Effect Is Mediated by AP-1 Transcription Factors
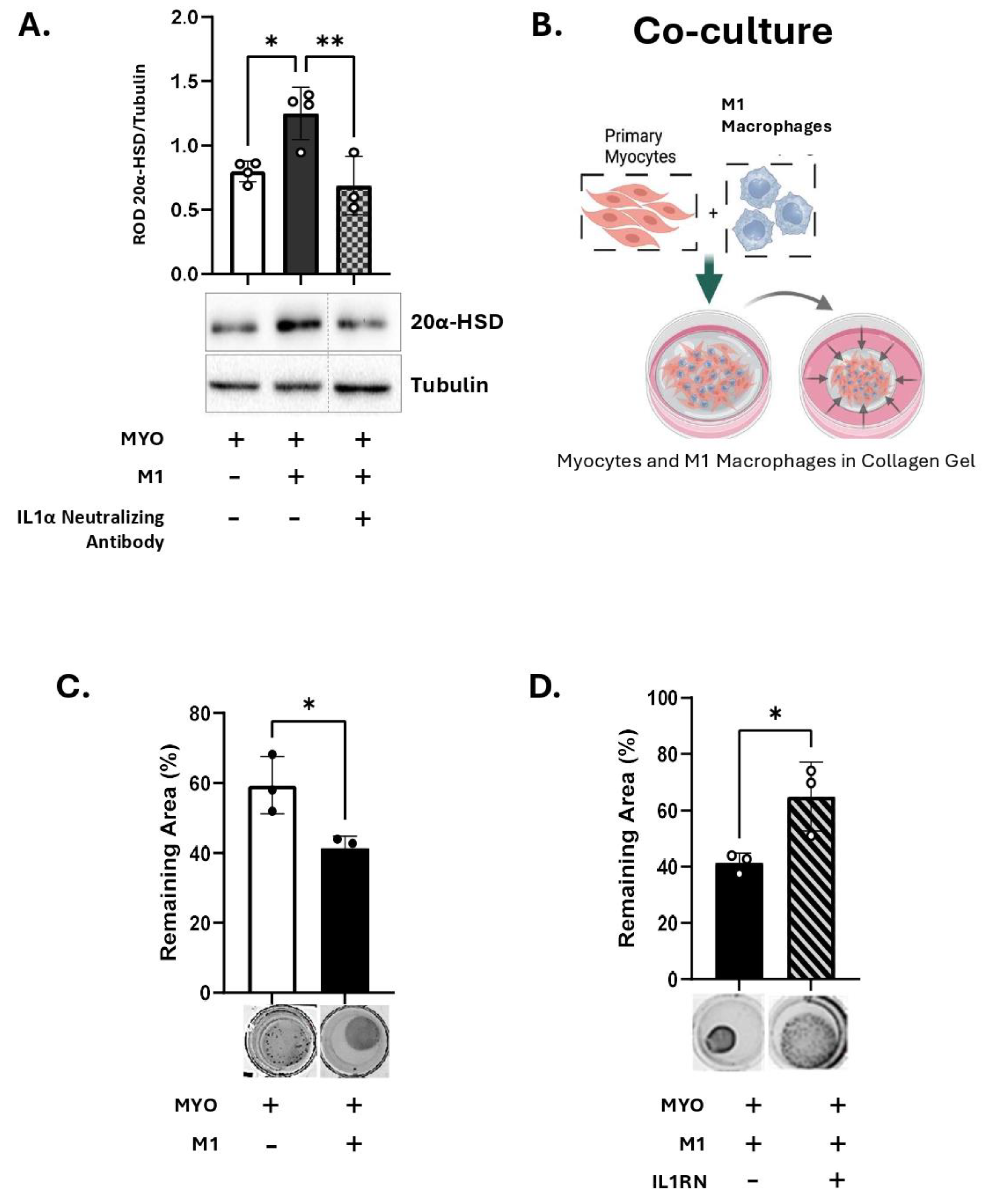
3. M1-Macrophages Induce 20α-HSD Protein Expression in Human MYO, While Inhibition of IL-1α Blocks This Effect
4. M1-Macrophage Produced IL-1α Induces Myocyte Contractility
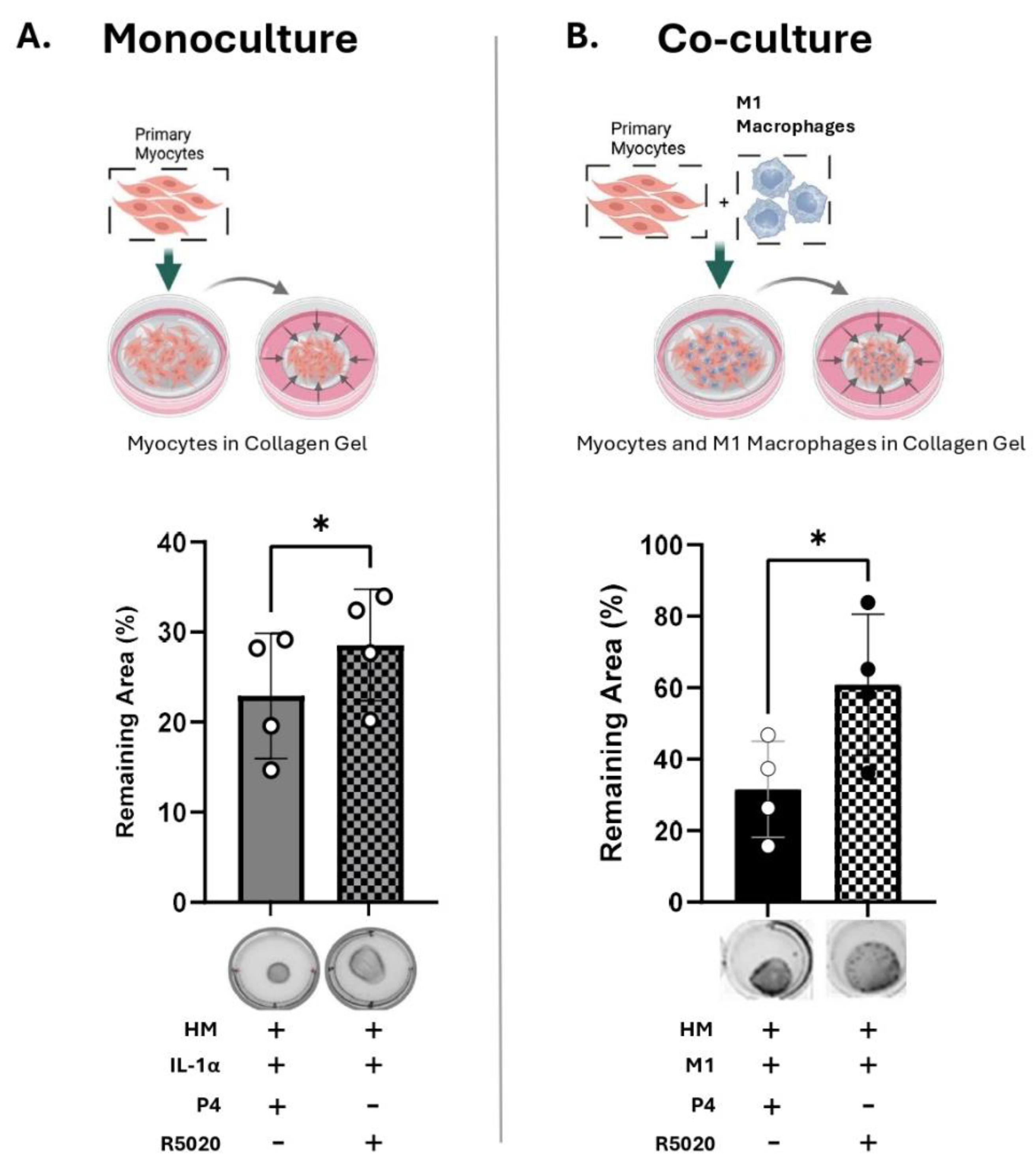
5. R5020 Blocks the Effect of IL-1α and M1-Macrophages on Myocyte Contractility
Discussion
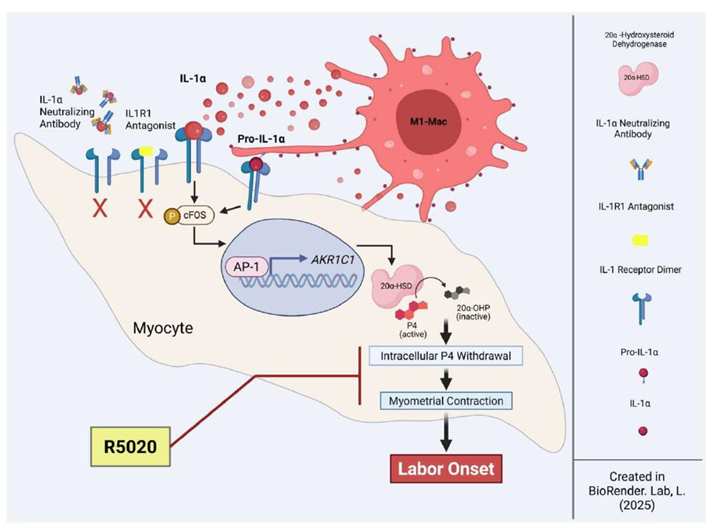
Study limitations
Conclusions
Supplementary Materials
Author’s Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth Analg 2015, 120, 1337–1351. [Google Scholar] [CrossRef]
- Ward, R.M.; Beachy, J.C. Neonatal complications following preterm birth. BJOG 2003, 110 Suppl 20, 8–16. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Kim, C.J.; et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014, 71, 330–358. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod 2012, 86, 39. [Google Scholar] [CrossRef]
- Short, R.V. Blood progesterone levels in relation to parturition. J Reprod Fertil 1960, 1, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Csapo, A. Progesterone “block”. American Journal of Anatomy 1956, 98, 273–291. [Google Scholar] [CrossRef]
- Csapo, A.I.; Pohanka, O.; Kaihola, H.L. Progesterone deficiency and premature labour. Br Med J 1974, 1, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Schummers, L.; Darling, E.K.; Dunn, S.; McGrail, K.; Gayowsky, A.; Law, M.R.; Laba, T.L.; Kaczorowski, J.; Norman, W.V. Abortion Safety and Use with Normally Prescribed Mifepristone in Canada. N Engl J Med 2022, 386, 57–67. [Google Scholar] [CrossRef]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun 2016, 7, 11565. [Google Scholar] [CrossRef]
- Nadeem, L.; Balendran, R.; Dorogin, A.; Mesiano, S.; Shynlova, O.; Lye, S.J. Pro-inflammatory signals induce 20alpha-HSD expression in myometrial cells: A key mechanism for local progesterone withdrawal. J Cell Mol Med 2021, 25, 6773–6785. [Google Scholar] [CrossRef]
- Shynlova, O.; Nadeem, L.; Dorogin, A.; Mesiano, S.; Lye, S.J. The selective progesterone receptor modulator-promegestone-delays term parturition and prevents systemic inflammation-mediated preterm birth in mice. Am J Obstet Gynecol 2022, 226, 249 e241–249 e221. [Google Scholar] [CrossRef]
- Nadeem, A.; Nadeem, L.; Lye, S.J.; Shynlova, O. Promegestone Prevents Lipopolysaccharide-Induced Cervical Remodeling in Pregnant Mice. Cells 2025, 14, 242. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Nguyen, T.; Lye, S.J. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med 2013, 17, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Tsui, P.; Dorogin, A.; Lye, S.J. Monocyte Chemoattractant Protein-1 (CCL-2) Integrates Mechanical and Endocrine Signals That Mediate Term and Preterm Labor1. The Journal of Immunology 2008, 181, 1470–1479. [Google Scholar] [CrossRef]
- Mizoguchi, M.; Ishida, Y.; Nosaka, M.; Kimura, A.; Kuninaka, Y.; Yahata, T.; Nanjo, S.; Toujima, S.; Minami, S.; Ino, K.; et al. Prevention of lipopolysaccharide-induced preterm labor by the lack of CX3CL1-CX3CR1 interaction in mice. PLoS One 2018, 13, e0207085. [Google Scholar] [CrossRef]
- Shan, Y.; Shen, S.; Long, J.; Tang, Z.; Wu, C.; Ni, X. Term and Preterm Birth Initiation Is Associated with the Macrophages Shifting to M1 Polarization in Gestational Tissues in Mice. Biology (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J Leukoc Biol 2003, 73, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Brough, D.; Le Feuvre, R.A.; Wheeler, R.D.; Solovyova, N.; Hilfiker, S.; Rothwell, N.J.; Verkhratsky, A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol 2003, 170, 3029–3036. [Google Scholar] [CrossRef]
- Romero, R.; Mazor, M.; Brandt, F.; Sepulveda, W.; Avila, C.; Cotton, D.B.; Dinarello, C.A. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992, 27, 117–123. [Google Scholar] [CrossRef]
- Romero, R.; Parvizi, S.T.; Oyarzun, E.; Mazor, M.; Wu, Y.K.; Avila, C.; Athanassiadis, A.P.; Mitchell, M.D. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990, 35, 235–238. [Google Scholar]
- Heng, Y.J.; Liong, S.; Permezel, M.; Rice, G.E.; Di Quinzio, M.K.; Georgiou, H.M. The interplay of the interleukin 1 system in pregnancy and labor. Reprod Sci 2014, 21, 122–130. [Google Scholar] [CrossRef]
- Hirsch, E.; Filipovich, Y.; Mahendroo, M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol 2006, 194, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H. The entry of fetal and amniotic fluid components into the uterine vessel circulation leads to sterile inflammatory processes during parturition. Front Immunol 2012, 3, 321. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Grivel, J.C.; Tarca, A.L.; Chaemsaithong, P.; Xu, Z.; Fitzgerald, W.; Hassan, S.S.; Chaiworapongsa, T.; Margolis, L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 2015, 213, 836 e831–836 e818. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Mazor, M.; Tartakovsky, B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991, 165, 969–971. [Google Scholar] [CrossRef]
- Motomura, K.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Xu, Y.; Galaz, J.; Slutsky, R.; Levenson, D.; Gomez-Lopez, N. The alarmin interleukin-1alpha causes preterm birth through the NLRP3 inflammasome. Mol Hum Reprod 2020, 26, 712–726. [Google Scholar] [CrossRef]
- Srikhajon, K.; Shynlova, O.; Preechapornprasert, A.; Chanrachakul, B.; Lye, S. A new role for monocytes in modulating myometrial inflammation during human labor. Biol Reprod 2014, 91, 10. [Google Scholar] [CrossRef]
- Chimal-Ramirez, G.K.; Espinoza-Sanchez, N.A.; Chavez-Sanchez, L.; Arriaga-Pizano, L.; Fuentes-Panana, E.M. Monocyte Differentiation towards Protumor Activity Does Not Correlate with M1 or M2 Phenotypes. J Immunol Res 2016, 2016, 6031486. [Google Scholar] [CrossRef]
- Shiratori, H.; Feinweber, C.; Luckhardt, S.; Linke, B.; Resch, E.; Geisslinger, G.; Weigert, A.; Parnham, M.J. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol 2017, 88, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Dallot, E.; Pouchelet, M.; Gouhier, N.; Cabrol, D.; Ferré, F.o.; Breuiller-Fouché, M. Contraction of Cultured Human Uterine Smooth Muscle Cells after Stimulation with Endothelin-1. Biology of Reproduction 2003, 68, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Romero, R.; Miller, D.; Kadam, L.; Mial, T.N.; Plazyo, O.; Garcia-Flores, V.; Hassan, S.S.; Xu, Z.; Tarca, A.L.; et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol 2016, 196, 2476–2491. [Google Scholar] [CrossRef]
- Fleenor, D.L.; Pang, I.-H.; Clark, A.F. Involvement of AP-1 in Interleukin-1α–Stimulated MMP-3 Expression in Human Trabecular Meshwork Cells. Investigative Ophthalmology & Visual Science 2003, 44, 3494–3501. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu Rev Immunol 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Mallers, T.M.; Larsen, B.; Kwak-Kim, J.; Chaouat, G.; Gilman-Sachs, A.; Beaman, K.D. V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 2012, 143, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Lee, Y.H.; Shynlova, O.; Lye, S.J. Stretch-induced human myometrial cytokines enhance immune cell recruitment via endothelial activation. Cell Mol Immunol 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Tsao, F.Y.; Wu, M.Y.; Chang, Y.L.; Wu, C.T.; Ho, H.N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J Formos Med Assoc 2018, 117, 204–211. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Gilman-Sachs, A.; Chaouat, G.; Beaman, K.D. Placental ATPase expression is a link between multiple causes of spontaneous abortion in mice. Biol Reprod 2011, 85, 626–634. [Google Scholar] [CrossRef]
- Diamond, A.K.; Sweet, L.M.; Oppenheimer, K.H.; Bradley, D.F.; Phillippe, M. Modulation of monocyte chemotactic protein-1 expression during lipopolysaccharide-induced preterm delivery in the pregnant mouse. Reprod Sci 2007, 14, 548–559. [Google Scholar] [CrossRef]
- Garcia-Flores, V.; Liu, Z.; Romero, R.; Pique-Regi, R.; Xu, Y.; Miller, D.; Levenson, D.; Galaz, J.; Winters, A.D.; Farias-Jofre, M.; et al. Homeostatic Macrophages Prevent Preterm Birth and Improve Neonatal Outcomes by Mitigating In Utero Sterile Inflammation in Mice. J Immunol 2024, 213, 1620–1634. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, M.; Cui, D.; Yang, H. The pathologic changes of human placental macrophages in women with hyperglycemia in pregnancy. Placenta 2022, 130, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sisino, G.; Bouckenooghe, T.; Aurientis, S.; Fontaine, P.; Storme, L.; Vambergue, A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochim Biophys Acta 2013, 1832, 1959–1968. [Google Scholar] [CrossRef]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Boriboonhirunsarn, D.; Tanpong, S. Rate of Spontaneous Preterm Delivery Between Pregnant Women With and Without Gestational Diabetes. Cureus 2023, 15, e34565. [Google Scholar] [CrossRef] [PubMed]
- Köck, K.; Köck, F.; Klein, K.; Bancher-Todesca, D.; Helmer, H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern Fetal Neonatal Med 2010, 23, 1004–1008. [Google Scholar] [CrossRef]
- Dudley, D.J.; Collmer, D.; Mitchell, M.D.; Trautman, M.S. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig 1996, 3, 328–335. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) pathway. Sci Signal 2010, 3, cm1. [Google Scholar] [CrossRef]
- Niu, J.; Li, Z.; Peng, B.; Chiao, P.J. Identification of an autoregulatory feedback pathway involving interleukin-1alpha in induction of constitutive NF-kappaB activation in pancreatic cancer cells. J Biol Chem 2004, 279, 16452–16462. [Google Scholar] [CrossRef]
- McCarthy, D.A.; Ranganathan, A.; Subbaram, S.; Flaherty, N.L.; Patel, N.; Trebak, M.; Hempel, N.; Melendez, J.A. Redox-control of the alarmin, Interleukin-1α. Redox Biology 2013, 1, 218–225. [Google Scholar] [CrossRef]
- Rider, P.; Kaplanov, I.; Romzova, M.; Bernardis, L.; Braiman, A.; Voronov, E.; Apte, R.N. The transcription of the alarmin cytokine interleukin-1 alpha is controlled by hypoxia inducible factors 1 and 2 alpha in hypoxic cells. Front Immunol 2012, 3, 290. [Google Scholar] [CrossRef] [PubMed]
- Freigang, S.; Ampenberger, F.; Weiss, A.; Kanneganti, T.-D.; Iwakura, Y.; Hersberger, M.; Kopf, M. Fatty acid–induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nature Immunology 2013, 14, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Hayashi, H.; Miyazawa, K.; Kojima, S.; Akahoshi, T.; Onozaki, K. 17β-Estradiol Induces IL-1α Gene Expression in Rheumatoid Fibroblast-Like Synovial Cells through Estrogen Receptor α (ERα) and Augmentation of Transcriptional Activity of Sp1 by Dissociating Histone Deacetylase 2 from ERα1. The Journal of Immunology 2007, 178, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Bakouche, O.; Brown, D.C.; Lachman, L.B. Subcellular localization of human monocyte interleukin 1: evidence for an inactive precursor molecule and a possible mechanism for IL 1 release. J Immunol 1987, 138, 4249–4255. [Google Scholar] [CrossRef]
- Menon, R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol 2014, 5, 567. [Google Scholar] [CrossRef]
- Schmiedecke, S.S.; Estrada, S.M.; Burd, I.; Napolitano, P.G.; Ieronimakis, N.M. 12: Evidence for the crucial role of estrogen signaling with preterm labor and perinatal neuroinflammation. American Journal of Obstetrics & Gynecology 2019, 220, S10–S11. [Google Scholar] [CrossRef]
- Challis, J.R.G. Mechanism of parturition and preterm labor. Obstet Gynecol Surv 2000, 55, 650–660. [Google Scholar] [CrossRef]
- Lamacchia, C.; Rodriguez, E.; Palmer, G.; Gabay, C. Endogenous IL-1α is a chromatin-associated protein in mouse macrophages. Cytokine 2013, 63, 135–144. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Beller, D.I.; Mizel, S.B.; Unanue, E.R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A 1985, 82, 1204–1208. [Google Scholar] [CrossRef]
- Chan, J.N.E.; Humphry, M.; Kitt, L.; Krzyzanska, D.; Filbey, K.J.; Bennett, M.R.; Clarke, M.C.H. Cell surface IL-1alpha trafficking is specifically inhibited by interferon-gamma, and associates with the membrane via IL-1R2 and GPI anchors. Eur J Immunol 2020, 50, 1663–1675. [Google Scholar] [CrossRef]
- Brody, D.T.; Durum, S.K. Membrane IL-1: IL-1 alpha precursor binds to the plasma membrane via a lectin-like interaction. J Immunol 1989, 143, 1183–1187. [Google Scholar] [CrossRef]
- Lopez, T.E.; Zhang, H.; Bouysse, E.; Neiers, F.; Ye, X.Y.; Garrido, C.; Wendremaire, M.; Lirussi, F. A pivotal role for the IL-1beta and the inflammasome in preterm labor. Sci Rep 2024, 14, 4234. [Google Scholar] [CrossRef]
- Peters, G.A.; Yi, L.; Skomorovska-Prokvolit, Y.; Patel, B.; Amini, P.; Tan, H.; Mesiano, S. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology 2017, 158, 158–169. [Google Scholar] [CrossRef]
- Doring, B.; Shynlova, O.; Tsui, P.; Eckardt, D.; Janssen-Bienhold, U.; Hofmann, F.; Feil, S.; Feil, R.; Lye, S.J.; Willecke, K. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J Cell Sci 2006, 119, 1715–1722. [Google Scholar] [CrossRef]
- Mesiano, S. Progesterone withdrawal and parturition. J Steroid Biochem Mol Biol 2022, 224, 106177. [Google Scholar] [CrossRef]
- Raynaud, J.P.; Ojasoo, T. [Promegestone, a new progestin]. J Gynecol Obstet Biol Reprod (Paris) 1983, 12, 697–710. [Google Scholar]
- Kuhl, H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005, 8 Suppl 1, 3–63. [Google Scholar] [CrossRef]
- Kirby, M.A.; Heuerman, A.C.; Custer, M.; Dobyns, A.E.; Strilaeff, R.; Stutz, K.N.; Cooperrider, J.; Elsissy, J.G.; Yellon, S.M. Progesterone Receptor-Mediated Actions Regulate Remodeling of the Cervix in Preparation for Preterm Parturition. Reprod Sci 2016, 23, 1473–1483. [Google Scholar] [CrossRef]
- Kuon, R.J.; Garfield, R.E. Actions of progestins for the inhibition of cervical ripening and uterine contractions to prevent preterm birth. Facts Views Vis Obgyn 2012, 4, 110–119. [Google Scholar]
- Winneker, R.C.; Bitran, D.; Zhang, Z. The preclinical biology of a new potent and selective progestin: trimegestone. Steroids 2003, 68, 915–920. [Google Scholar] [CrossRef]
- Anonymous. Trimegestone. Drugs in R & D 1999, 1, 228–229. [Google Scholar] [CrossRef]
- Kuon, R.J.; Shi, S.Q.; Maul, H.; Sohn, C.; Balducci, J.; Maner, W.L.; Garfield, R.E. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am J Obstet Gynecol 2010, 202, 455.e451–459. [Google Scholar] [CrossRef]
- Shi, S.Q.; Maner, W.L.; Mackay, L.B.; Garfield, R.E. Identification of term and preterm labor in rats using artificial neural networks on uterine electromyography signals. Am J Obstet Gynecol 2008, 198, 235.e231–234. [Google Scholar] [CrossRef]
- Yellon, S.M.; Dobyns, A.E.; Beck, H.L.; Kurtzman, J.T.; Garfield, R.E.; Kirby, M.A. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS One 2013, 8, e81340. [Google Scholar] [CrossRef]
- Neilson, J.P. Mifepristone for induction of labour. Cochrane Database Syst Rev 2000, Cd002865. [Google Scholar] [CrossRef]
- Thong, K.J.; Baird, D.T. Induction of abortion with mifepristone and misoprostol in early pregnancy. Br J Obstet Gynaecol 1992, 99, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Tribe, R.M.; Moriarty, P.; Dalrymple, A.; Hassoni, A.A.; Poston, L. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol Reprod 2003, 68, 1842–1849. [Google Scholar] [CrossRef]
- Papatheodorou, D.C.; Karagiannidis, L.K.; Paltoglou, G.; Margeli, A.; Kaparos, G.; Valsamakis, G.; Chrousos, G.P.; Creatsas, G.; Mastorakos, G. Pulsatile interleukin-6 leads CRH secretion and is associated with myometrial contractility during the active phase of term human labor. J Clin Endocrinol Metab 2013, 98, 4105–4112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




