Submitted:
14 October 2025
Posted:
15 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
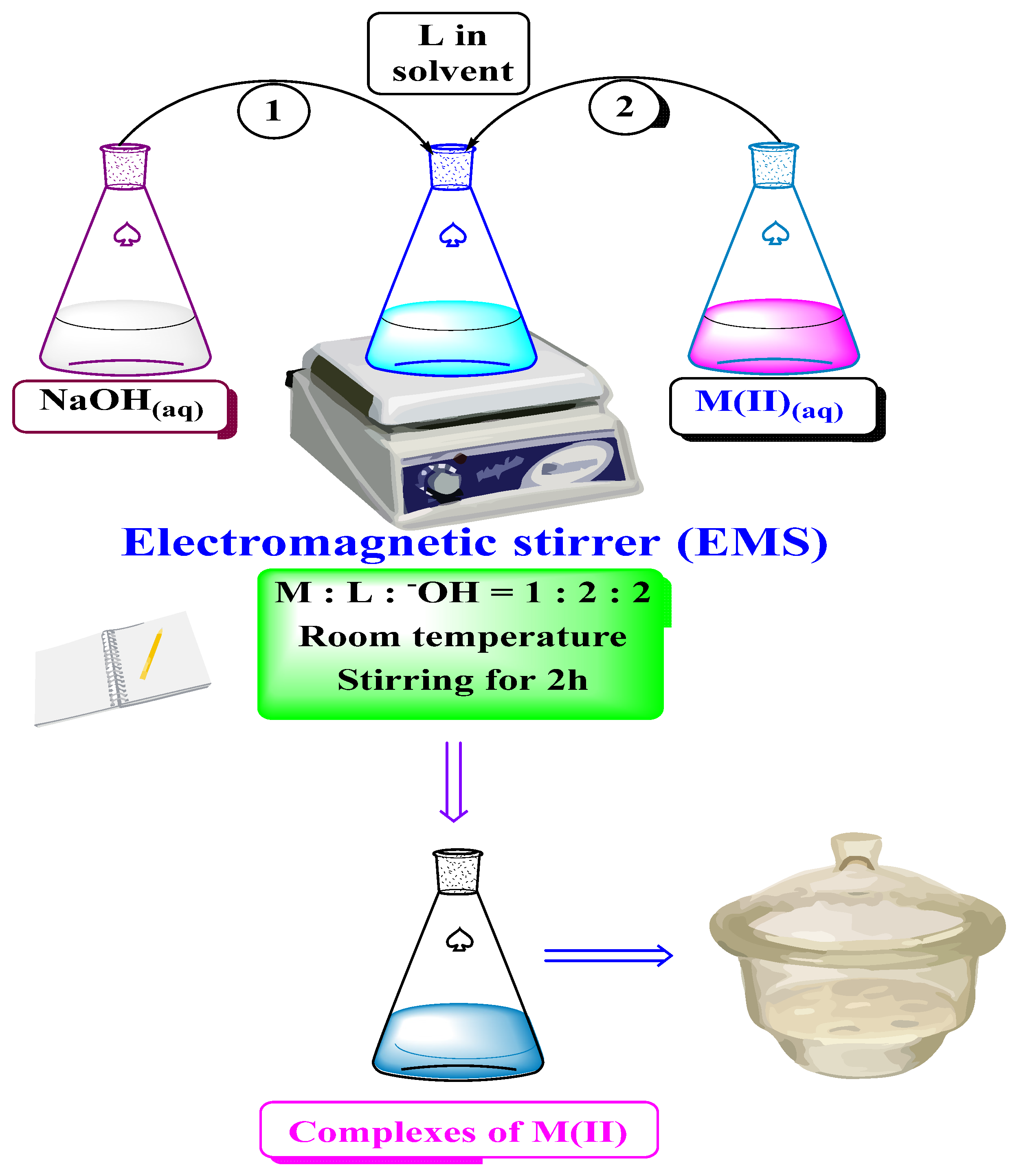
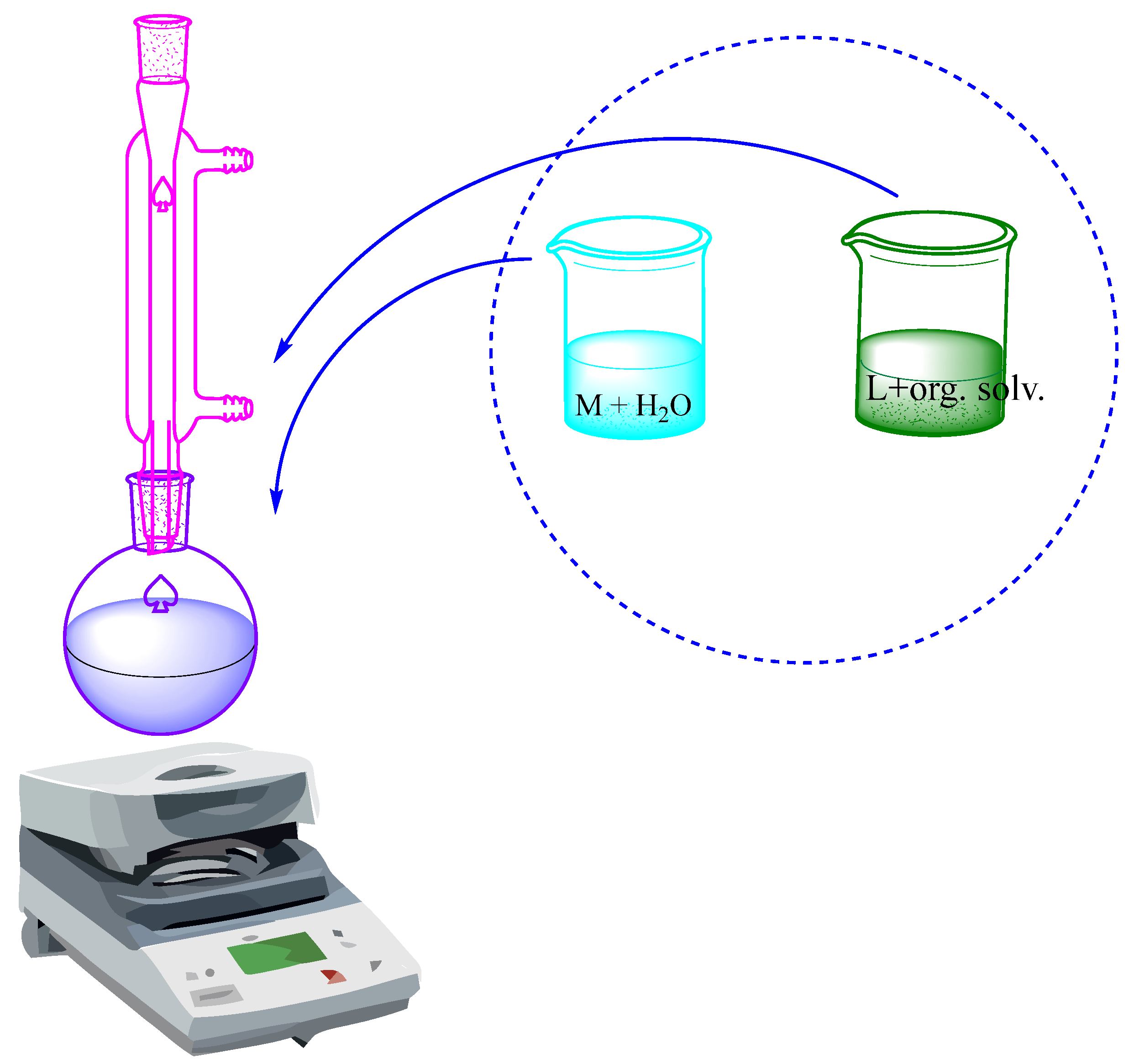
1.1. The Synthesis of Metal Complexes
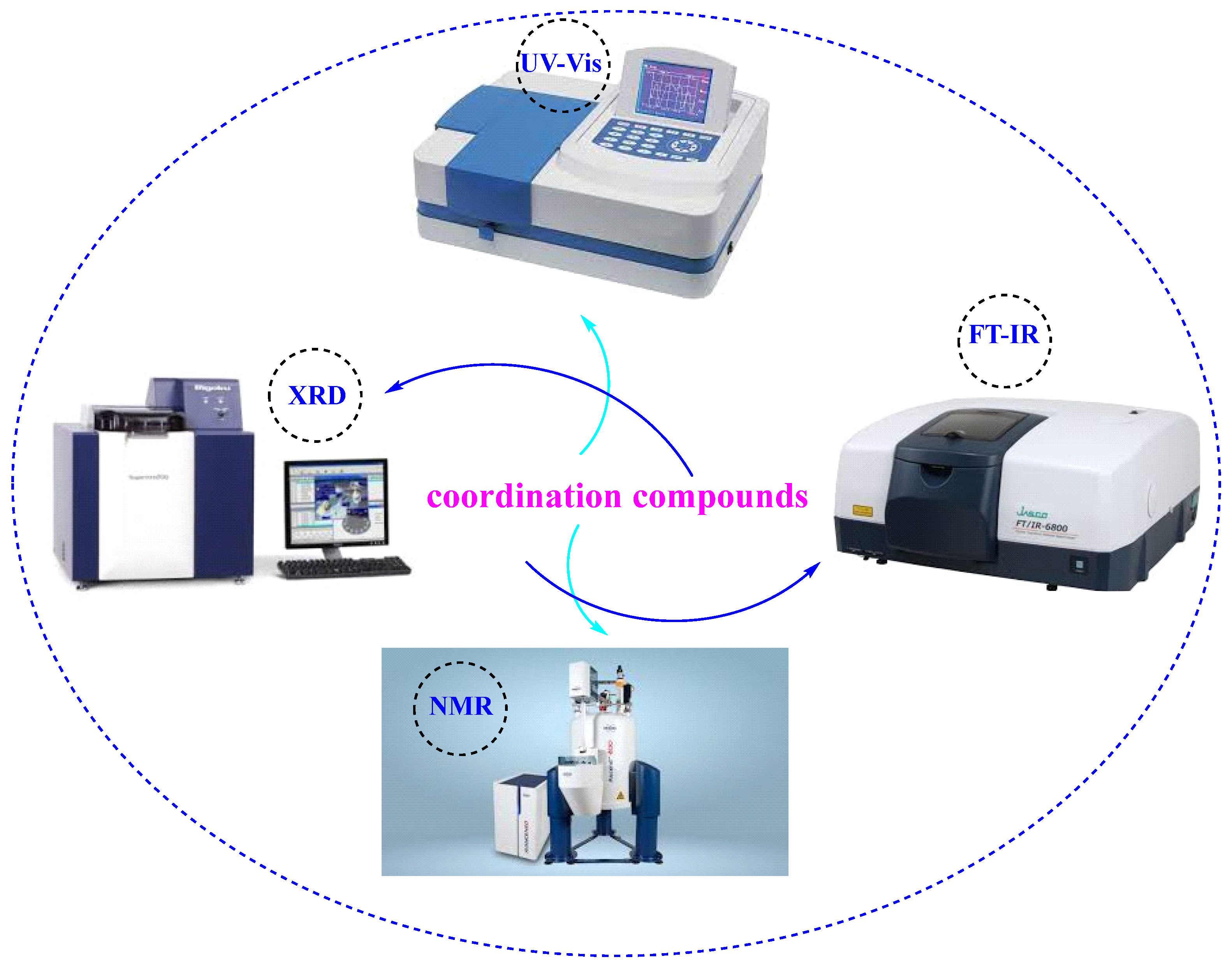
1.2. Analytical Approaches, Including Spectroscopic, Crystallographic, and Electrochemical Techniques for Characterization of Coordination Compounds
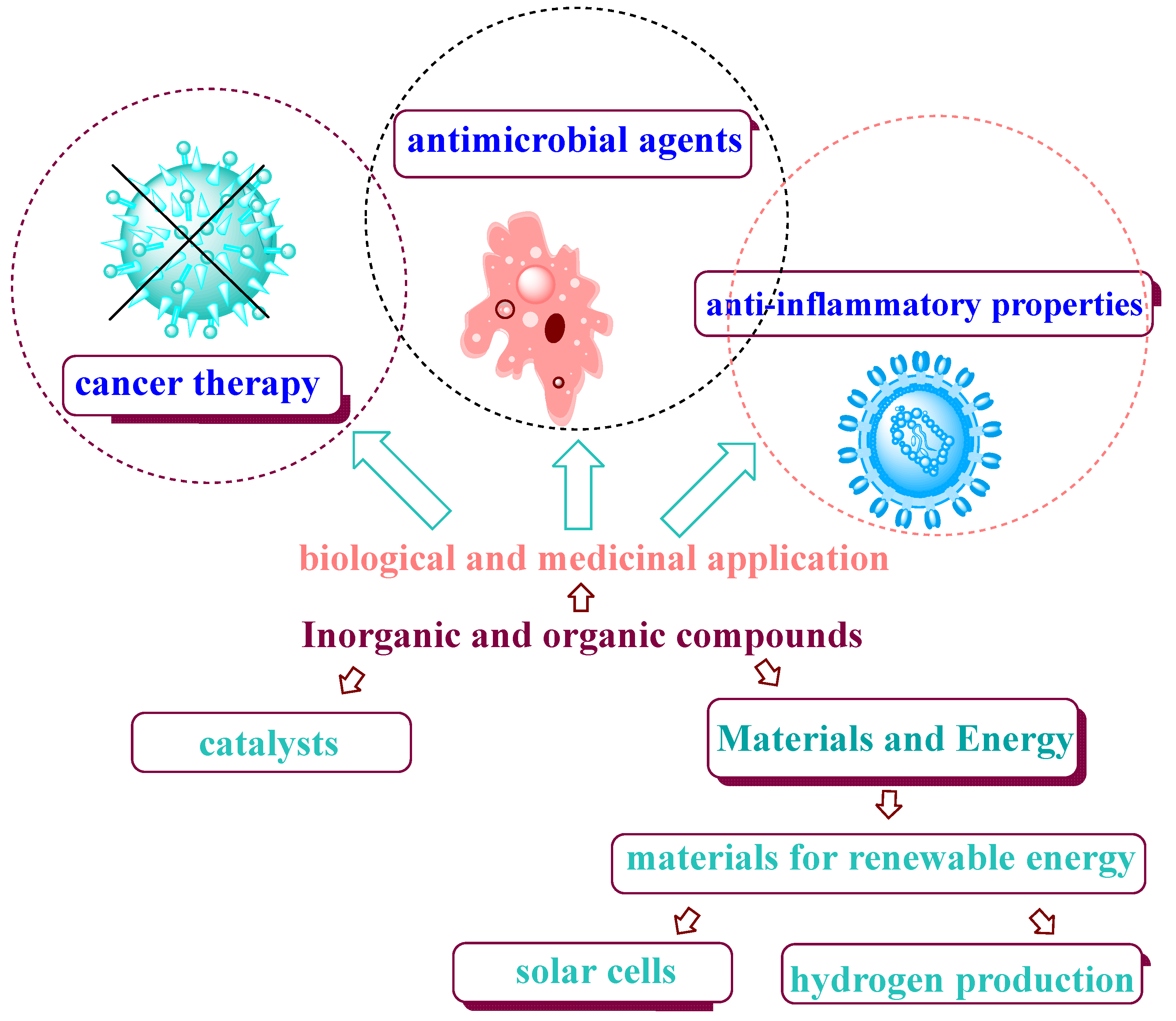
1.3. Biological and Medicinal Application of Inorganic Compounds
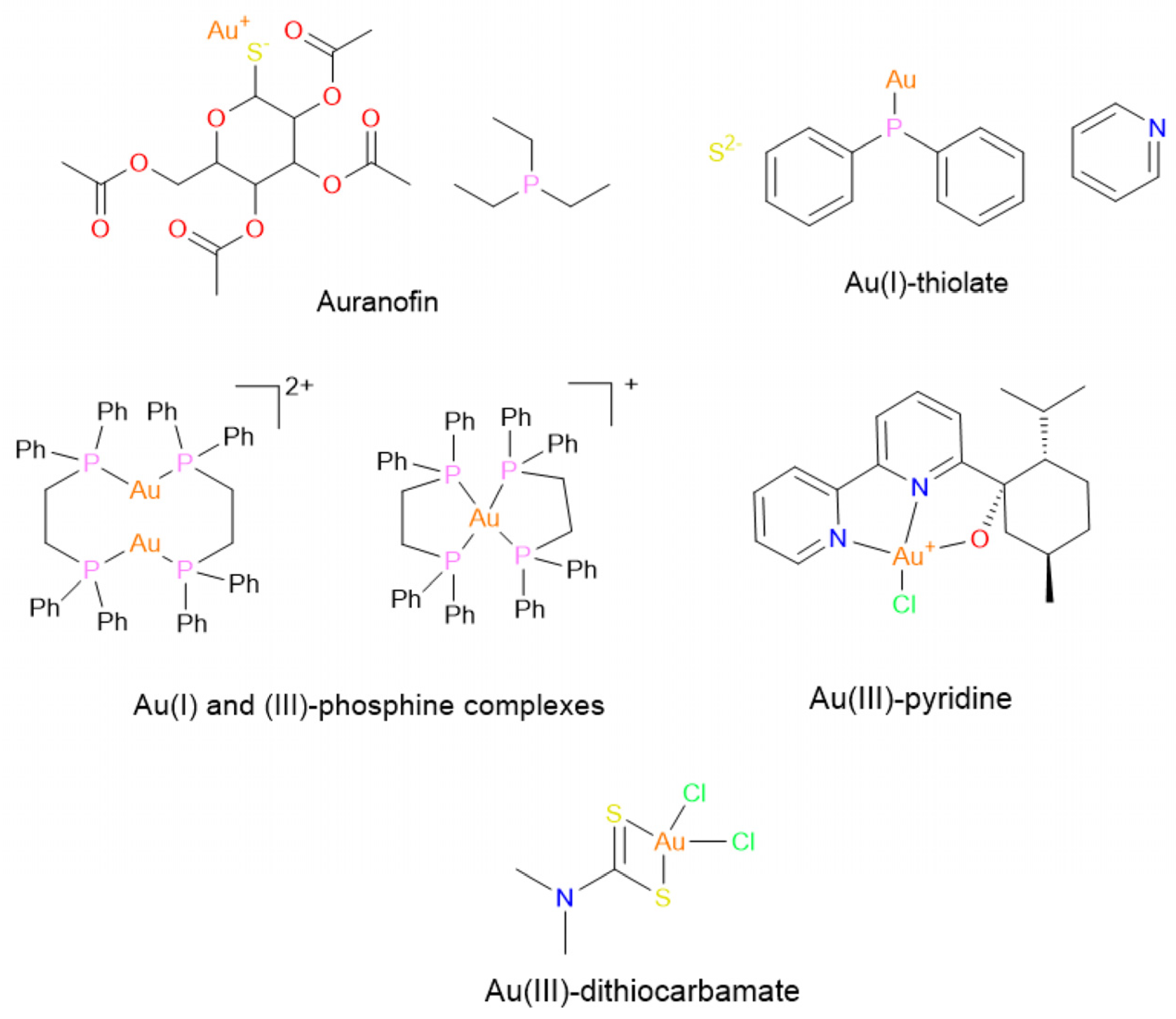
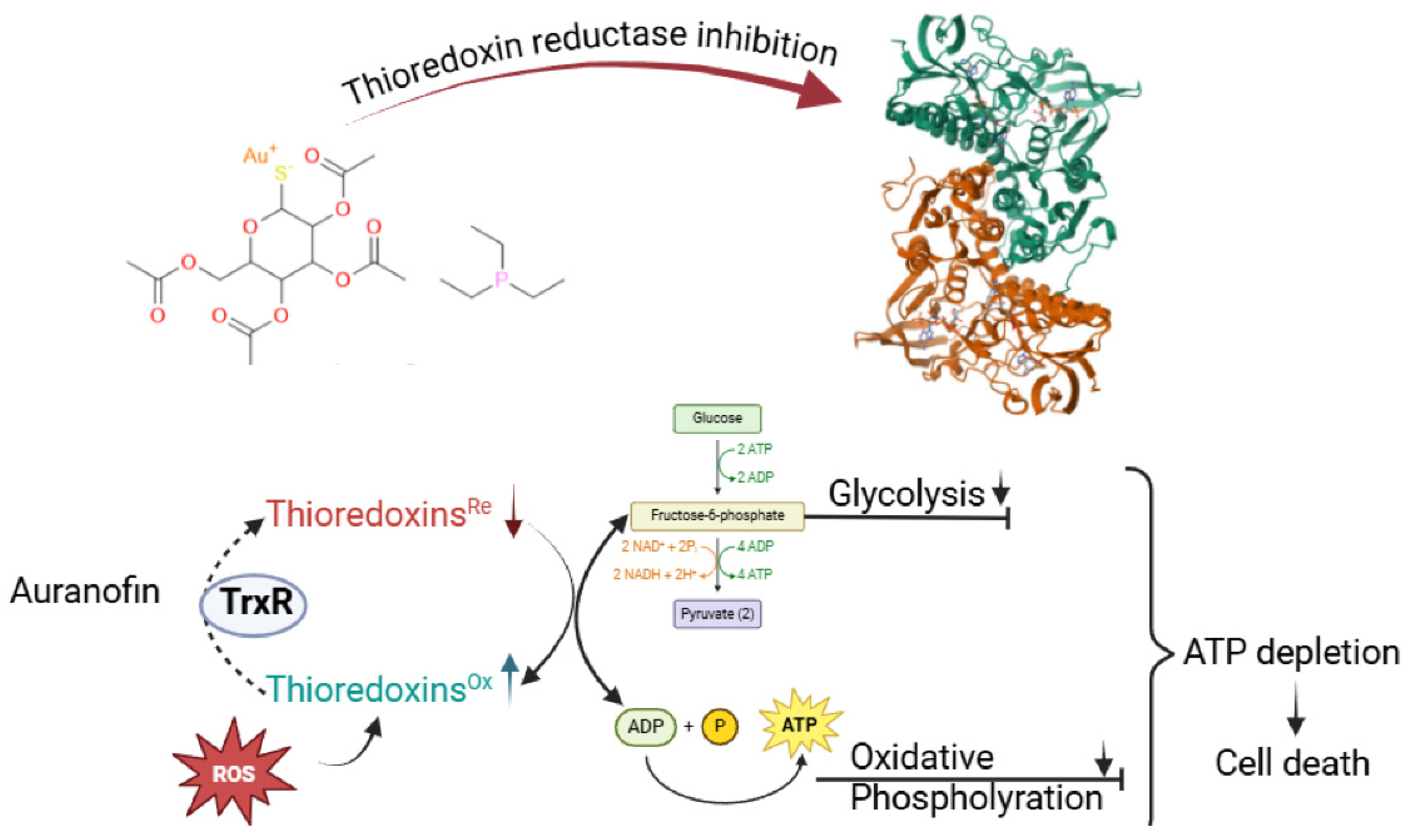
Gold(I/III)-Based Anti-Cancer Compounds
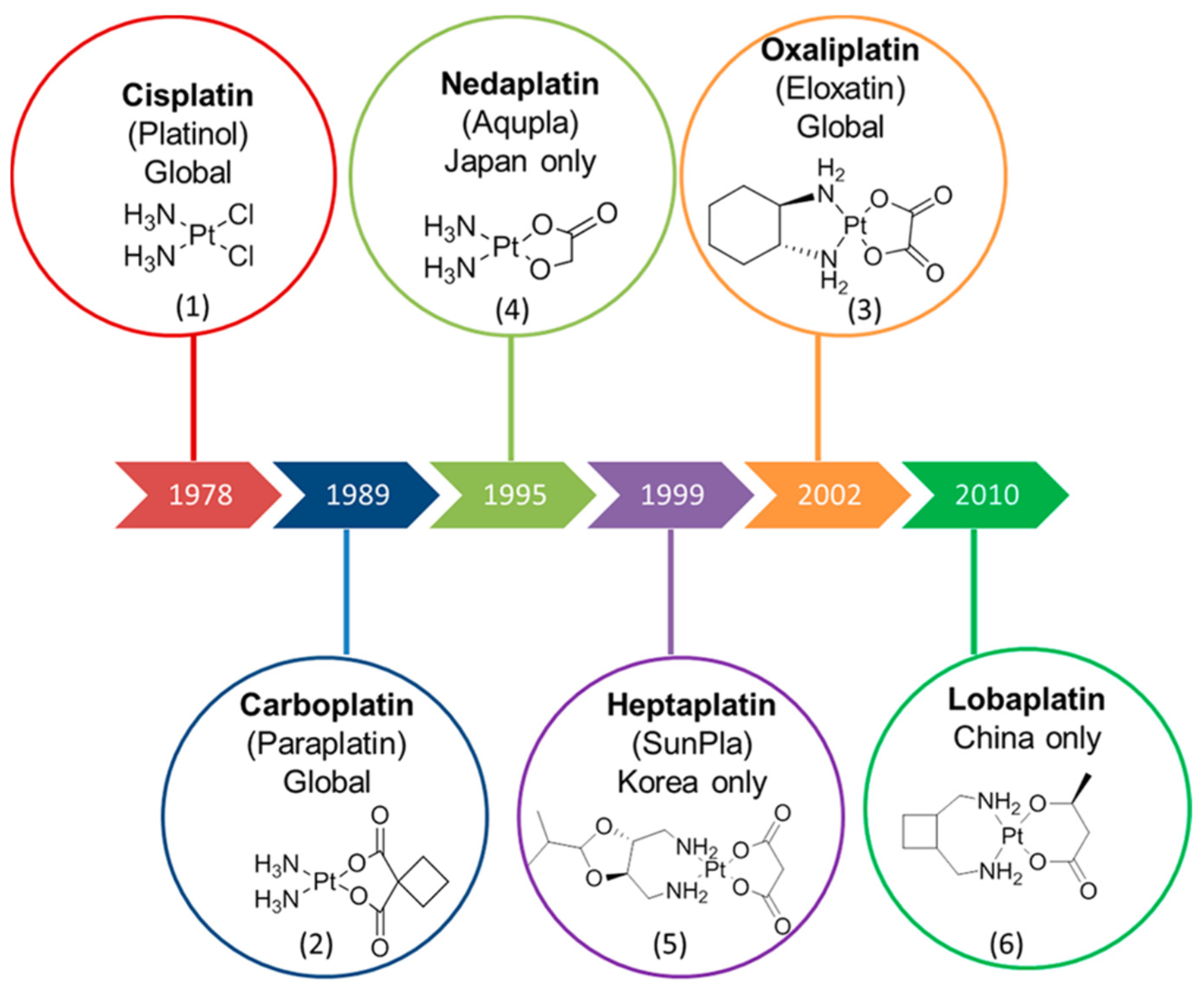
Platinum-Based Anti-Cancer Compounds
Platinum(II)-Based Anti-Cancer Drugs
Platinum(IV)-Based Anti-Cancer Drugs
| Structure of Platinum(IV) Complexes | In Vitro Activity | In Vivo Activity | References |
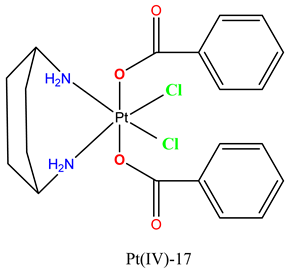 |
No data available. | In a murine LLC model, administration of the complex (5 mg/kg) reduced tumor mass by 72.5%. | Barbanente et al. 2022 [102] |
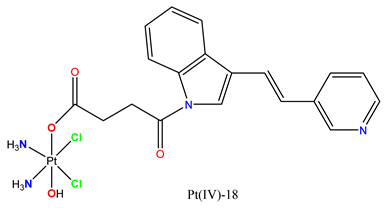 |
Caused cell death in HEPG2 cells. | In mice, HepG2 tumor xenograft growth was inhibited, with activation of T cells enhancing antitumor immunity. | Hua et al. 2019 [103] |
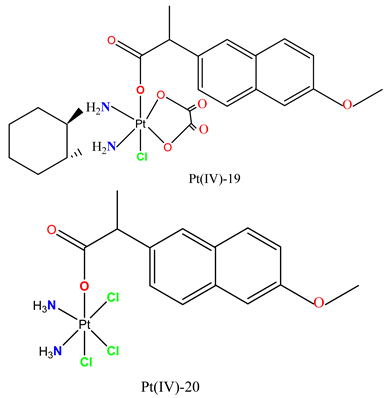 |
Reduced growth of cancer cells such as A549, A549R, SKOV-3, and CT-26. | In BALB/c mice, CT26 tumor growth was inhibited to a level comparable with oxaliplatin and cisplatin. | Chen et al. 2020 [104] |
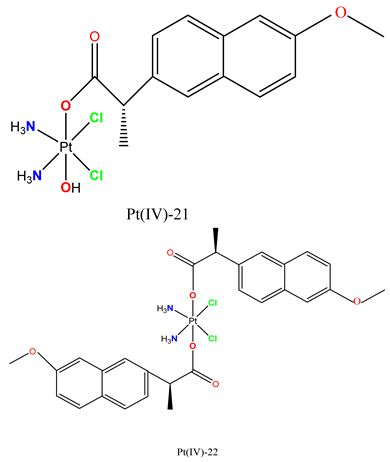 |
In human cancer cell lines (MCF-7, MDA-MB-435, MDA-MB-231), proliferation was inhibited and cytotoxicity induced, while MCF-7 cell migration was delayed in a wound healing assay. | In female Balb/C mice, MDA-MB-231 tumor growth was inhibited. | Jin et al. 2020 [106] |
Ruthenium-Based Anti-Cancer Compounds
Organic Compounds with Anti-Cancer Properties
Inorganic and Organic Compounds with Antimicrobial Properties
Gold(I/III)-Based Compounds with Antimicrobial Properties
Platinum (II/IV)-Based Compounds with Antimicrobial Activities
Ruthenium(II/III)-Based Compounds with Antimicrobial Activities
Inorganic and Organic Compounds with Anti-Inflammatory Properties
2. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| 1H NMR | proton nuclear magnetic resonance |
| 13C NMR | carbon-13 nuclear magnetic resonance |
| ESI-HRMS | electrospray ionization high-resolution mass spectrometry |
| ESI-MS | electrospray ionization mass spectrometry |
| FT-IR | fourier transform infrared |
| ICP-MS | inductively coupled plasma mass spectrometry |
| TrxR | thioredoxin reductase |
| DMSO | dimethyl sulfoxide |
| AuNPs | gold nano particles |
| NP | nano particle |
| ATP | adenosine triphosphate |
| PEG | polyethylene glycol |
| PET | positron emission tomography |
| MM | multiple myeloma |
| HCC | hepatocellular carcinoma |
| ROS | reactive oxygen species |
| FDA | Food and Drug Administration |
| 4T1 | mouse mammary carcinoma cell line |
| Bcl-2 | B-cell lymphoma 2 |
| Apaf-1 | Apoptotic Protease-Activating Factor 1 |
| MDA-MB-231 | A human breast adenocarcinoma cell line established from a patient with metastatic mammary adenocarcinoma |
| MCF-7 | Another human breast cancer cell line used in cancer research |
| A549 | A human lung carcinoma cell line |
| PC3 | A human prostate cancer cell line |
| BXPC-3 | A human pancreatic cancer cell line |
| PBMCn | Peripheral Blood Mononuclear Cells |
| SAR | Structure–activity relationship |
| CT-DNA | calf thymus-Deoxyribonucleic acid |
| MMP | mitochondrial membrane potential |
| CDK1 | cyclin-dependent kinase 1 |
| Cdc25A | cell division cycle 25 A |
| B16-F10 | a specific murine melanoma cell line derived from the B16 tumor line |
| A2780 | ovarian cancer cell line |
| ASNS | asparagine synthetase |
| HepG2 | A human liver carcinoma cell line |
| HeLa | A common human cervical cancer cell line |
| SKOV3 | A human ovarian cancer cell line |
| BEL-7404 | A human hepatocellular carcinoma cell line |
| NCI-H460 | A human large cell lung carcinoma cell line |
| U251 | A human glioblastoma cell line |
| SMMC-7721 | A human hepatocellular carcinoma cell line |
| LLC | Lewis Lung Carcinoma |
| C57BL | inbred laboratory mouse strain |
| TDO | tryptophan-2,3-dioxygenase |
| AHR | aryl hydrocarbon receptor |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| COX-2 | cyclooxygenase-2 |
| COXs | cyclooxygenases |
| IL | interleukin |
| OA | octanoate |
| HL-7702 | non-malignant human liver cells |
| CT26 | murine (mouse) cell line representing a highly immunogenic colorectal carcinoma |
| SGC-7901 | human gastric cancer cell line |
| dppz | dipyridophenazine |
| HCT116 | human colorectal cell line |
| HSA | human serum albumin |
| AIE | aggregation-induced emission |
| PARP | poly (ADP-ribose) polymerase |
| MIC | minimum inhibitory concentration |
| TPPMS | triphenylphosphine monosulfonate |
| MMCs | minimum microbiocidal concentration |
| MRSA | Methicillin-Resistant Staphylococcus Aureus |
| Ca2+-Mg2+-ATPase | Calcium Magnesium adenosine triphosphatase |
| PGE₂ | prostaglandin E₂ |
| iNOS | nitric oxide synthase |
| TNF-α | Tumor Necrosis Factor-alpha |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
References
- Xu, M.; Wu, R.; Wang, S.; Xu. H.; Zhong S. Preparation and Room Temperature Phosphorescent Properties of Cd(II) Homo- and Zn(II), Cd(II) Heterometallic Coordination Polymers Microstructures. J Inorg Organomet Polym 2024, 34, 4072–4081. [CrossRef]
- Tsoneva, S.; Milusheva, M.; Burdzhiev, N.; Marinova, P.; Varbanova, E.; Tumbarski, Y.; Mihaylova, R.; Cherneva, E.; Nikolova, S. Antimicrobial Activity of Ethyl (2-(Methylcarbamoyl)phenyl) carbamate and Its Mixed Ligand Ni(II) and Co(II) Complexes. Inorganics, 2025, 13, 267. [CrossRef]
- Marinova, P.E.; Tsoneva, S.H.; Nikolova, S.A.; Ivanov, I.I. Novel complexes of n-substituted-4,5-dimethoxy-phenylethyl-2-arylketoamides with metal ions. Bulg. Chem. Commun. 2019, 51, 8–11.
- Enikeeva K.R., Kasimov A.I., Litvinov I.A., Lyubina A.P., Voloshina A.D., Musina E.I., Karasik A.A. Synthesis of Nickel(II) Complexes Based on Dialkylphosphorylpyridines and Their Cytotoxic Activity. Žurnal neorganičeskoj himii. 2023; 68(9), 1137-1145. https://journals.rcsi.science/0044-457X/article/view/136457/114391. [CrossRef]
- Petja Marinova, Stoyanka Nikolova, Slava Tsoneva, Synthesis of N-(1-(2-acetyl-4,5-dimethoxyphenyl)propan-2-yl)benzamide and its copper(II) complex, Russ. J Gen. Chem., 2023, 93(1), 161-165. [CrossRef]
- Marinova, P.; Hristov,M.; Tsoneva, S.; Burdzhiev,N.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Synthesis, Characterization, and Antibacterial Studies of New Cu(II) and Pd(II) Complexes with 6-Methyl-2-Thiouracil and 6-Propyl-2-Thiouracil. Appl. Sci. 2023, 13, 13150. [CrossRef]
- Kulikova V.A., Shubina E.S., Filippov O.A., Yakhvarov D.G., Sakhapov I.F., Kagilev A.A., Gafurov Z.N., Gutsul E.I., Kirkina V.A., Belkova N.V. Basicity and Hydride-Donating Ability of Palladium(II) Hydride Complex with Diarylamido-bis-phosphine Pincer Ligand, Žurnal neorganičeskoj himii. 2023, 68(9), 1226-1234. [CrossRef]
- Agnieszka Czylkowska, Bartłomiej Rogalewicz, Anita Raducka, Natalia Błaszczyk, Tomasz Maniecki, Kinga Wieczorek and Paweł Mierczy ’ nski. Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands. Materials 2020, 13, 3217; [CrossRef]
- Golobiˇc, A.; Dojer, B.; Jagodiˇc, M.; Siher, A.; Pegan, A.; Kristl, M. Synthesis and Characterization of New Copper(II) Coordination Compounds with Methylammonium Cations. Inorganics 2024, 12, 261. [CrossRef]
- Cheng, S.-Y.; Zhang, Q.; Tang, Q.; Neary, M.C.; Zheng, S. Diverse Cobalt(II) and Iron(II/III) Coordination Complexes/Polymers Based on 4′-Pyridyl: 2,2′;6′,2′′-Terpyridine: Synthesis, Structures, Catalytic and Anticancer Activities. Chemistry 2024, 6, 1099–1110. [CrossRef]
- Yan V. Demyanov, Taisiya S. Sukhikh, Irina Yu. Bagryanskaya, Alexander S. Novikov, Marianna I. Rakhmanova, Alexander V. Artem’ev. Homo- and heterometallic complexes designed on group 11 metals and tris(6-methyl-2-pyridyl)phosphine: Synthesis, metallophilic interactions and room-temperature phosphorescence. Polyhedron, 2024, 252, 116901. [CrossRef]
- Atefe Haghi Khaje Ghiaci, Robabeh Alizadeh, Hakimeh Ahmadi, Nasim Qaedi Nejat, Parastoo Bayrami Aghabagher, Vahid Amani, Two new lead(II) discrete complex and coordination polymer & ultrasound-assisted synthesis of their nanostructures: synthesis, characterization, crystal structure determination, thermal & X-ray powder diffraction studies. Journal of Molecular Structure, 2023, 1289, 135874. [CrossRef]
- N.L. Sheeba, S. Meenakshi Sundar, Critical evaluation of silver nanoparticles synthesized at room temperature/microwave irradiation: A green approach, Next Nanotechnology, 2024, 6, 100083, . [CrossRef]
- Beauty Kumari, Khursheed Ahmad, Copper Coordination Dynamics: Synthesis and Structural Insights Utilizing DFT, Hirshfeld, and Antimicrobial Analysis, Inorg. Chem. Commun., 2024, 160, 111992. [CrossRef]
- Siddiqui SA, Prado-Roller A, Shiozawa H. Room temperature synthesis of a luminescent crystalline Cu-BTC coordination polymer and metal-organic framework. Mater Adv. 2021 3(1), 224-231. [CrossRef]
- Castiñeiras A, Fernández-Hermida N, García-Santos I, Gómez-Rodríguez L, Frontera A, Niclós-Gutiérrez J. Synthesis, Structural Characterisation, and Electrochemical Properties of Copper(II) Complexes with Functionalized Thiosemicarbazones Derived from 5-Acetylbarbituric Acid. Molecules. 2024, 29(10), 2245. [CrossRef]
- Mónica Benito, Ghodrat Mahmoudi, Elies Molins, Ennio Zangrando, Masoumeh Servati Gargari, Rosa M. Gomila, Antonio Frontera and Damir A. Safin. Versatile copper(II) discrete and polymeric coordination compounds with (pyridine-2-yl)methylenenicotinohydrazide and azelaic acid. Cryst Eng Comm, 2025, 27, 164-175. [CrossRef]
- Wei, Y-S., Fan, Z., Luo, C. Horike. S. Desolvation of metal complexes to construct metal–organic framework glasses. Nat. Synth 2024, 3, 214–223. [CrossRef]
- Zhen-Zhong Mei, Hong-Yu Wang, Chao Ren, Ying Yang and Jin-Zhong Gu. Hydrothermal synthesis, structures, and catalytic performance of five coordination compounds driven by 5-aminoisophthalic acid. RSC Adv., 2024, 14, 28160-28167. [CrossRef]
- Gabano, E.; Ravera, M. Microwave-Assisted Synthesis: Can Transition Metal Complexes Take Advantage of This “Green” Method? Molecules 2022, 27, 4249. [CrossRef]
- Er, A., Ay, Ç., Yılmaz, N. Bozkuş, S. İ.; Acar, N.; Karaca B.; Atakol. O. Two novel energetic Ag(I) complexes: synthesis, crystal structure, and thermal investigations. J Therm Anal Calorim 2025, 150, 10935–10949. [CrossRef]
- Baimuratova, R.K., Zhinzhilo, V.A., Uflyand, I.E. et al. Low-Temperature Synthesis of Metal–Organic Coordination Polymers Based on Oxo-centered Iron Complexes: Magnetic and Adsorption Properties. Russ. J. Phys. Chem. 2023, 97, 735–748. [CrossRef]
- Trofimova O.Y., Pashanova K.I., Ershova I.V., Arseniev M.V., Yakushev I.A., Dorovatovsky P.V., Aisin R.R., Piskunov A.V. Copper(II) Catecholate Complexes with Polypyridyl Ligands, Žurnal neorganičeskoj himii. 2023, 68(9), 1154-1164. https://journals.rcsi.science/0044-457X/article/view/136462/114396. [CrossRef]
- Ivakhnenko E.P., Vitkovskaya Y.G., Lysenko K.A., Kislitsyn S.E., Starikov A.G., Knyazev P.A., Tereshchenko A.A., Minkin V.I. Zinc(II) Chelate Complexes with Redox-Active o-Indophenols: Synthesis and Structure, Žurnal neorganičeskoj himii. 2023. 68(9), 1181-1191. [CrossRef]
- Petrov P.A., Nikolaevskii S.A., Yambulatov D.S., Starikova A.A., Sukhikh T.S., Kiskin M.A., Sokolov M.N., Eremenko I.L. Heteroleptic Anionic Cobalt(II) Pivalate Complex with a Bridging Trimethylsiloxy Ligand: Synthesis, Structure, and Formation Mechanism. Žurnal neorganičeskoj himii. 2023. 68(9), 1255-1264. [CrossRef]
- Kal’tenberg A.A., Bashilova A.D., Somov N.V., Malysheva Y.V., Grishin I.D. Ruthenium Complexes Based on C2B9-nido-Carborane and Tridentate Phosphorus- and Nitrogen-Containing Ligands. Žurnal neorganičeskoj himii. 2023. 68(9), 1277-1286. [CrossRef]
- Lavrenova, L.G.; Sukhikh, T.S.; Glinskaya, L.A.; Trubina, S.V.; Zvereva, V.V.; Lavrov, A.N.; Klyushova, L.S.; Artem’ev, A.V. Synthesis, Structure, and Magnetic and Biological Properties of Copper(II) Complexes with 1,3,4-Thiadiazole Derivatives. Int. J. Mol. Sci. 2023, 24, 13024. [CrossRef]
- Giorgio Cagossi, Polo P. Mazzeo, Aessia Bacchi, Polo Pelagatti, Comparison between mechanochemical and solution synthesis of Zn and Cu complexes containing pyridine and p-halogen substituted benzoates. RSC Mechanochemistry 2025, 2(3), 475-481. [CrossRef]
- Vakhid A. Mamedov, Vera L. Mamedova, Victor V. Syakaev, Tmur A. Kushatov, Ditry E. Korshin Il’dar Kh Rizvanov, Aidar T. Gubaidullin. Synthesis and crystal structure of the new copper(II) coordination polymer with N1-(2-сarboxyphenyl)-N2-(4-ethylcarboxyphenyl)oxalamide ligand. Tetrahedron 2024, 150, 133751. [CrossRef]
- Shyam Sundar Mothuku, Uwe Monkowius, Marko Hapke. Triptycepenes: Synthesis, Metal Complexes, and Their Reactivity in Catalytic Reactions. Organometallics 2025, 44, 11, 1200–1209. [CrossRef]
- Majumdar, D., Philip, J.E., Roy, S. B. Gassoumi, Ghalla, H. Synthesis, characterization, crystal engineering, DFT, and biological evaluation of a novel Cu(II)-perchlorate Schiff base complex. BMC Chemistry 2025, 19, 227. [CrossRef]
- Dmitry I. Nazarov, Jorge Labella, Alexey V. Kuzmin, Maxim A. Faraonov, Evgenii N. Ivanov, Salavat S. Khasanov, Tomas Torres, Mikhail K. Islyaikin and Dmitri V. Konarev. Expanded Porphyrin Exhibiting Off-Centered Out-of-Plane Coordination to Dysprosium. CCS Chem. 2024, 6, 1731–1738. [CrossRef]
- Zhong, R. M., Xu, Q. B., Wang, G. Y., Zheng, L. L., Wu, J. Z., & Ou, Y. C. Synthesis, crystal structures, and electrochemical properties of metal complexes based on 1,10-phenanthroline-5,6-dione. Journal of Coordination Chemistry, 2023, 76(2), 271–278. [CrossRef]
- Roger D. Sommer. How to grow crystals for X-ray crystallography. 2024, 80(8), 337-342. [CrossRef]
- Blake, A. J. Crystal Growth, Evaluation and Handling. 2024. https://www.nottingham.ac.uk/~pczajb2/growcrys.htm. Accessed: March 5, 2024.
- Boyle, P. D. Crystal Growing Guides. 2023. https://xray.chem.uwo.ca/Guides.html. Accessed: December 14, 2023.
- Carroll, R. C. & Coles, S. J. Developing the application of the crystalline sponge method. Acta Cryst. A 2023, 79, C1303.
- Tamás Pivarcsik, Jakob Kljun, Sergio Clemente Rodriguez, David Cortéz Alcaraz, Uroš Rapuš, Márta Nové, Egon F Várkonyi, József Nyári, Anita Bogdanov, Gabriella Spengler, Iztok Turel, Éva A. Enyedy. Structural and Solution Speciation Studies on fac-Tricarbonylrhenium(I) Complexes of 2,2′-Bipyridine Analogues. ACS Omega 2024, 9, 44, 44601–44615. [CrossRef]
- Tyler, A. R., Ragbirsingh, R., McMonagle, C. J., Waddell, P. G., Heaps, S. E., Steed, J. W., Thaw, P., Hall, M. J. & Probert, M. R. Encapsulated Nanodroplet Crystallization of Organic-Soluble Small Molecules. Chem, 2020, 6, 1755–1765. https://www.cell.com/action/showPdf?pii=S2451-9294%2820%2930177-7.
- Virovets, A. V., Peresypkina, E. & Scheer, M. Structural Chemistry of Giant Metal Based Supramolecules. Chem. Rev. 2021, 121, 14485–14554. [CrossRef]
- Shrestha, P. UV-Vis Spectroscopy: Principle, Parts, Uses, Limitations. 2023. https://microbenotes.com/uv-vis-spectroscopy/.
- Khalid, K.; Ishak, R.; Chowdhury, Z.Z. Chapter 15—UV–Vis spectroscopy in non-destructive testing. In Non-Destructive Material Characterization Methods; Elsevier: Amsterdam, The Netherlands, 2024; pp. 391–416. [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 7th ed.; 3rd printing; W. H. Freeman: New York, NY, USA, 2007.
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [CrossRef]
- Namioka, T. Diffraction Gratings. In Vacuum Ultraviolet Spectroscopy; Experimental Methods in Physical Sciences; Elsevier: Amsterdam, The Netherlands, 2000; Volume 1, pp. 347–377. [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009.
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009.
- El-Azazy, M.; Al-Saad, K.; El-Shafie, A.S. (Eds.) Infrared Spectroscopy: Perspectives and Applications; Books on Demand: 2023. ISBN 1803562811/9781803562810. London, UNITED KINGDOM.
- James, M. Thompson, Infrared Spectroscopy, 1st ed.; Jenny Stanford Publishing, New York, NY, USA, 2018. [CrossRef]
- Barbara, H. Stuart, Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. ISBN 9780470854273. [CrossRef]
- Idim, V.D. The transition metal-based coordination complex: Synthesis, spectroscopic characterization and the review of the applications. International Journal of Chemical and Biological Sciences 2023, 5(2), 49-52. [CrossRef]
- Graci, M. R., Awwadi, F. F., Dickie, D. A., Landee, C. P., & Turnbull, M. M. Synthesis, coordination modes, structures, and magnetic properties of halogen-substituted 2-hydroxypyridine copper(II) chloride coordination compounds. Journal of Coordination Chemistry, 2024, 77(1–2), 79–100. [CrossRef]
- An, Y.; Sedinkin, S.L.; Venditti, V. Solution NMR methods for structural and thermodynamic investigation of nanoparticle adsorption equilibria. Nanoscale Adv. 2022, 4, 2583–2607. [CrossRef]
- Mohan, M.; Andersen, A.B.A.; Mareš, J.; Jensen, N.D.; Nielsen, U.G.; Vaara, J. Unravelling the effect of paramagnetic Ni2+ on the 13C NMR shift tensor for carbonate in Mg2−xNix Al layered double hydroxides by quantum-chemical computations. Phys. Chem. Chem. Phys. 2023, 25, 24081–24096. [CrossRef]
- Zhang, Y.; Fei, H.T.; Liu, G.T.; Wang, W.; Sun, Y.J.; Wu, C.J. Exploring paramagnetic NMR and EPR for studying the bonding in actinide complexes. Dalton Trans. 2023. [CrossRef]
- Göthner, E.; Lehmann, K.; Grote, D.; Spek, A.L.; Hill, J.P.; Ikeda, M. NMR studies on manganese(II) complexes for enhanced relaxivity in MRI applications. Inorg. Chem. 2023. [CrossRef]
- Shin, H.K.; Lee, S.J.; Kim, T.J.; Lee, K.G.; Kang, D.H.; Son, J.T. Analyzing 31P NMR shifts in molybdenum phosphide nanoclusters. J. Mol. Struct. 2023, 1289, 135896. [CrossRef]
- Adams, D.; Hines, C.J.; Rodriguez, M.L. Utilizing high-field NMR for detection of low-spin iron(III) centers in bioinorganic complexes. J. Inorg. Biochem. 2023. [CrossRef]
- Bai, T.; Lee, A.Y.; Chen, J.; Ma, Z. Coordination dynamics in copper(I) complexes: A study through variable-temperature NMR. Inorg. Chem. Commun. 2023. [CrossRef]
- Brett, G.L.; Armstrong, R.D.; Thomas, S.P.; McQueen, C.J. Exploring transition metal complex environments via 1H and 31P NMR spectroscopy. Chem. A Eur. J. 2023. [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi. J.; Luciani, N.; Carn, F.; Gazeau, F. Gold-based therapy: From past to present. Proc. Natl. Acad. Sci. 2020, 117, 22639–22648, . [CrossRef]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent development of gold(I) and gold(III) complexes as therapeutic agents for cancer diseases. Chem. Soc. Rev. 2022, 51, 5518–5556, . [CrossRef]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold nanoparticles as a potent radiosensitizer: a transdisciplinary approach from physics to patient, Cancers 2020, 12, 2021, . [CrossRef]
- Ansari, E; Kumar, R.; Ratnam, A. Gold–NHC complexes: from synthetic aspects to anti-cancer activity. Dalton Trans. 2025, 54, 7553-7601, . [CrossRef]
- Stefanache, A.; Miftode, A.M.; Constantin, M.; Bogdan Goroftei, R.E.; Olaru, I.; Gutu, C.; Vornicu, A.; Lungu, I.I. Noble Metal Complexes in Cancer Therapy: Unlocking Redox Potential for Next-Gen Treatments. Inorganics, 2025, 13(2), 64. [CrossRef]
- Conceição, N. R.; Mahmoud, A. G.; Dietl, M. C.; Caligiuri, I.; Rizzolio, F.; Carabineiro, S. A. C.; Rudolph, M.; Fátima M. C. Guedes da Silva; Pombeiro, A. J. L.; Stephen, A.; Hashmi, K.; Scattolin, T. Exploring the catalytic and anticancer activity of gold(I) complexes bearing 1,3,5-triaza-7-phosphaadamantane (PTA) and related ligands. New J. Chem., 2025, 49, 7216-7226. [CrossRef]
- Reddy, T. S.; Privér, S. H.; Ojha, R.; Mirzadeh, N.; Velma, G. R.; Jakku, R.; Hosseinnejad, T.; Luwor, R.; Ramakrishna, S.; Wlodkowic, D.; Plebanski, M.; Bhargava, S. K. Gold(I) complexes of the type [AuL{κC-2-C6H4P(S)Ph2}] [L = PTA, PPh3, PPh2(C6H4-3-SO3Na) and PPh2(2-py)]: Synthesis, characterisation, crystal structures, and In Vitro and In Vivo anticancer properties. Eur J Med Chem, 2025, 281, 117007 http://dx.doi.org/10.1016/j.ejmech.2024.117007.
- Mirabelli, C.K.; Johnson, R.K.; Sung, C.M.; Faucette, L.; Muirhead, K.; Crooke, S.T. Evaluation of the in vivo antitumor activity and in vitro cytotoxic properties of auranofin, a coordinated gold compound, in murine tumor models, Cancer Res 1985, 45, 32–39.
- Du, Y.; Xia, L.; Jo, A.; Davis, R.M.; Bissel, P.; Ehrich, M.F.; Kingston, D.G.I. Synthesis and evaluation of doxorubicin-loaded gold nanoparticles for tumor-targeted drug delivery, Bioconjug. Chem. 2018, 29, 420–430, . [CrossRef]
- Astolfi, P.; Pisani, M.; Giorgini, E.; Rossi, B.; Damin, A.; Vita, F.; Francescangeli, O.; Luciani, L.; Galassi, R. Synchrotron characterization of hexagonal and cubic lipidic phases loaded with azolate/phosphane gold(I) compounds: a new approach to the uploading of gold(I)-based drugs, Nanomaterials 2020, 10, 1851, . [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A. K.; Pathak, S. A review on metal complexes and its anti-cancer activities: Recent updates from in vivo studies. Biomedicine & Pharmacotherapy 2024, 171, 116211. [CrossRef]
- Walther, W.; Althagafi, D.; Curran, D.; O’Beirne, C.; Carthy, C. Mc; Ott, I.; Basu, U.; Büttner, B.; Sterner-Kock, A.; Müller-Bunz, H.; S’anchez-Sanz, G.; Zhu, X.; Tacke, M. In-vitro and in-vivo investigations into the carbene-gold anticancer drug candidates NHC*-Au-SCSNMe2 and NHC*-Au-S-GLUC against advanced prostate cancer PC3, Anticancer. Drugs 2020, 31, 672–683, . [CrossRef]
- Guarra, F.; Terenzi, A.; Pirker, C.; Passannante, R.; Baier, D.; Zangrando, E.; G’omez-Vallejo, V.; Biver, T.; Gabbiani, C.; Berger, W.; Llop, J.; Salassa, L. 124 I Radiolabeling of a Au III -NHC Complex for In Vivo Biodistribution Studies, Angew. Chem. Int. Ed. 2020, 59, 17130–17136, . [CrossRef]
- Sze, J.H.; Raninga, P.V.; Nakamura, K.; Casey, M.; Khanna, K.K.; Berners-Price, S.J.; Trapani, G. Di; Tonissen, K.F. Anticancer activity of a Gold(I) phosphine thioredoxin reductase inhibitor in multiple myeloma, Redox Biol. 2020, 28, 101310, . [CrossRef]
- Sankarganesh, M.; Raja, J.D.; Revathi, N.; Solomon, R.V.; Kumar, R.S. Gold(III) complex from pyrimidine and morpholine analogue Schiff base ligand: Synthesis, characterization, DFT, TDDFT, catalytic, anticancer, molecular modeling with DNA and BSA and DNA binding studies, J. Mol. Liq. 2019, 294, 111655, . [CrossRef]
- Mirzadeh, N.; Telukutla, S.R.; Luwor, R.; Priv’er, S.; Velma, G.R.; Jakku, R.K.; Andrew S. N.; Plebanski, M.; Christian, H.; Bhargava, S. Dinuclear orthometallated gold(I)-gold(III) anticancer complexes with potent in vivo activity through an ROS-dependent mechanism, Metallomics 2021, 13 . [CrossRef]
- Arojojoye, A.S.; Kim, J.H.; Olelewe, C.; Parkin, S.; Awuah, S.G. Chiral gold(III) complexes: speciation, in vitro, and in vivo anticancer profile, Chem. Commun. 2022, 58, 10237–10240, . [CrossRef]
- Pooran Kumar, P.N. Navya, Amrin Begum, Ruchika Ojha, Magdalena Plebanski, Suresh K. Bhargava. Unveiling the anticancer potential of gold-sulfur complexes: Mechanisms, delivery strategies, and clinical progression. Coord. Chem. Rev. 2025, 541, 216809 . [CrossRef]
- Checconi, P.; Mariconda, A.; Catalano, A.; Ceramella, J.; Pellegrino, M.; Aquaro, S.; Sinicropi, M.S.; Longo, P. Searching for New Gold(I)-Based Complexes as Anticancer and/or Antiviral Agents. Molecules 2025, 30, 1726. [CrossRef]
- Marinova, P.; Tsoneva, S.; Frenkeva, M.; Blazheva, D.; Slavchev A.; Penchev, P. New Cu(II), Pd(II) and Au(III) complexes with 2-thiouracil: Synthesis, Characteration and Antibacterial Studies, Russ. J. Gen. Chem. 2022, 92(8), 1578-1584. [CrossRef]
- Marinova, P.; Stoitsov, D.; Burdzhiev, N.; Tsoneva, S.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Investigation of the Complexation Activity of 2,4-Dithiouracil with Au(III) and Cu(II) and Biological Activity of the Newly Formed Complexes. Appl. Sci., 2024, 14, 6601. [CrossRef]
- Marinova, P.; Burdzhiev, N.; Blazheva, D.; Slavchev, A. Synthesis and Antibacterial Studies of a New Au(III) Complex with 6-Methyl-2-Thioxo-2,3-Dihydropyrimidin-4(1H)-One. Molbank 2024, M1827. [CrossRef]
- Marinova, P.E.; Tamahkyarova, K.D. Synthesis and Biological Activities of Some Metal Complexes of 2-Thiouracil and Its Derivatives: A Review. Compounds 2024, 4, 186–213. [CrossRef]
- Komeda S.; Casini, A. Next-generation anticancer metallodrugs. Curr. Top. Med. Chem., 2012, 12(3), 219–235. [CrossRef]
- Todorov, L.; Kostova, I. Recent Trends in the Development of Novel Metal-Based Antineoplastic Drugs, Molecules, 2023, 28(4), 1959. [CrossRef]
- Wang Y.; Yuan H.; Fang R.; Lu J.; Duo J.; Li G.; Wang W.J. A new gold(I) phosphine complex induces apoptosis in prostate cancer cells by increasing reactive oxygen species. Mol Cell Biochem. 2025, 480(4), 2265-2276. [CrossRef]
- Kauffman, G.B.; Pentimalli, R.; Doldi, S.; Hall, M.D. Michele Peyrone (1813-1883), discoverer of cisplatin, Platin. Met. Rev. 2010, 54, 250–256, . [CrossRef]
- Rosenberg, B. Van Camp, L.; Krigas, T. Inhibition of cell division in escherichia coli by electrolysis products from a platinum electrode, Nature 1965, 205, 698–699, . [CrossRef]
- Shen, D.-W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes, Pharmacol. Rev. 2012, 64, 706–721, . [CrossRef]
- Johnstone, T.C.; Park, G.Y.; Lippard, S.J. Understanding and improving platinum anticancer drugs–phenanthriplatin, Anticancer Res 2014, 34, 471–476. http://www.ncbi.nlm.nih.gov/pubmed/24403503.
- Kenny, R. G.; Marmion C. J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem Rev., 2019, 119(2), 1058–1137. [CrossRef]
- Bazsefidpar, P.; Eftekhar, E.; Jahromi, M.Z.; Nikpoor, A.R.; Moghadam, M.E.; Zolghadri, S. In-vitro cytotoxicity and in-vivo antitumor activity of two platinum complexes with 1,3-dimethyl pentyl glycine ligand against breast cancer, J. Inorg. Biochem. 2023, 241 112144, . [CrossRef]
- Dimitrijević Stojanovi’c, M.N.; Franich, A.A.; Juriˇsevi’c, M.M.; Gajovi’c, N.M.; Arsenijevi’c, N. N.; Jovanovi’c, I.P.; Stojanovi’c, B.S.; Mitrovi’c, S.L.; Kljun, J.; Rajkovi’c, S.; ˇZivkovi’c, M.D. Platinum(II) complexes with malonic acids: synthesis, characterization, in vitro and in vivo antitumor activity and interactions with biomolecules, J. Inorg. Biochem. 2022, 231, 111773, . [CrossRef]
- Qin, L.-Q.; Wei, Z.-Z.; Yang, L.; Qin, Q.-P.; Zeng, J.-J.; Tan, M.-X.; Liang, H. Strong in vitro and in vivo cytotoxic effects of two platinum(II) complexes with cryptolepine derivatives, Med. Chem. Res. 2021, 30, 1419–1426, https://doi. org/10.1007/s00044-021-02739-0.
- Maciel, L.L.F.; Silva, M.B.; Moreira, R.O.; Cardoso, A.P.; Fernandes, C.; Horn, A.; Almeida, J. C. de Aquino; Kanashiro, M.M. In vitro and in vivo relevant antineoplastic activity of platinum(II) complexes toward triple-negative MDA-MB-231 breast cancer cell line, Pharmaceutics 2022, 14, 2013, . [CrossRef]
- Ruiz, M.C.; Resasco, A.; Di Virgilio, A.L.; Ayala, M.; Cavaco, I.; Cabrera, S.; Aleman, J.; Le’on, I. E. In vitro and in vivo anticancer effects of two quinoline–platinum(II) complexes on human osteosarcoma models, Cancer Chemother. Pharmacol. 2019, 83, 681–692, . [CrossRef]
- Mo, X.; Chen, K.; Chen, Z.; Chu, B.; Liu, D.; Liang, Y.; Xiong, J.; Yang, Y.; Cai, J.; Liang, F. Antitumor activities for two Pt(II) complexes of tropolone and 8-hydroxyquinoline derivative, Inorg. Chem. 2021, 60, 16128–16139, https://doi. org/10.1021/acs.inorgchem.1c01763.
- A.A. Franich, M.D. ˇZivkovi’c, T. Ili’c-Tomi’c, I.S. Đorđevi’c, J. Nikodinovi’c-Runi’c, A. Pavi’c, G.V. Janji’c, S. Rajkovi’c, New minor groove covering DNA binding mode of dinuclear Pt(II) complexes with various pyridine-linked bridging ligands and dual anticancer-antiangiogenic activities, J. Biol. Inorg. Chem. 25 2020, 395–409, . [CrossRef]
- S. Gadre, M. Manikandan, P. Duari, S. Chhatar, A. Sharma, S. Khatri, J. Kode, M. Barkume, N.K. Kasinathan, M. Nagare, M. Patkar, A. Ingle, M. Kumar, U. Kolthur-Seetharam, M. Patra, A rationally designed bimetallic platinum (II)- ferrocene antitumor agent induces non-apoptotic cell death and exerts in vivo efficacy, Chem. – A Eur. J. 2022, 28, . [CrossRef]
- D. Hu, C. Yang, C. Lok, F. Xing, P. Lee, Y.M.E. Fung, H. Jiang, C. Che, An Antitumor Bis(N-Heterocyclic Carbene)Platinum(II) Complex That Engages Asparagine Synthetase as an Anticancer Target, Angew. Chem. 2019, 131, 11030–11034, . [CrossRef]
- G.-B. Liang, Y.-C. Yu, J.-H. Wei, W.-B. Kuang, Z.-F. Chen, Y. Zhang, Design, synthesis and biological evaluation of naphthalenebenzimidizole platinum (II) complexes as potential antitumor agents, Eur. J. Med. Chem. 2020, 188, 112033, . [CrossRef]
- A. Barbanente, V. Gandin, C. Ceresa, C. Marzano, N. Ditaranto, J.D. Hoeschele, G. Natile, F. Arnesano, C. Pacifico, F.P. Intini, N. Margiotta, Improvement of kiteplatin efficacy by a benzoato Pt(IV) prodrug suitable for oral administration, Int. J. Mol. Sci. 2022, 23, 7081, . [CrossRef]
- S. Hua, F. Chen, G. Xu, S. Gou, Multifunctional platinum(IV) complexes as immunostimulatory agents to promote cancer immunochemotherapy by inhibiting tryptophan-2,3-dioxygenase, Eur. J. Med. Chem. 2019, 169, 29–41, . [CrossRef]
- Y. Chen, Q. Wang, Z. Li, Z. Liu, Y. Zhao, J. Zhang, M. Liu, Z. Wang, D. Li, J. Han, Naproxen platinum( <scp>iv</scp>) hybrids inhibiting cycloxygenases and matrix metalloproteinases and causing DNA damage: synthesis and biological evaluation as antitumor agents in vitro and in vivo, Dalt. Trans. 2020, 49, 5192–5204, . [CrossRef]
- B.W.J. Harper, E. Petruzzella, R. Sirota, F.F. Faccioli, J.R. Aldrich-Wright, V. Gandin, D. Gibson, Synthesis, characterization and in vitro and in vivo anticancer activity of Pt( <scp>iv</scp>) derivatives of [Pt(1S,2S-DACH)(5,6- dimethyl-1,10-phenanthroline)], Dalt. Trans. 2017, 46, 7005–7019, https://doi. org/10.1039/C7DT01054K.
- S. Jin, N. Muhammad, Y. Sun, Y. Tan, H. Yuan, D. Song, Z. Guo, X. Wang, Multispecific platinum(IV) complex deters breast cancer via interposing inflammation and immunosuppression as an inhibitor of COX-2 and PD-L1, Angew. Chem. Int. Ed. 2020, 59, 23313–23321, . [CrossRef]
- J. Yang, X. Sun, W. Mao, M. Sui, J. Tang, Y. Shen, Conjugate of Pt(IV)–histone deacetylase inhibitor as a prodrug for cancer chemotherapy, Mol. Pharm. 2012, 9, 2793–2800, . [CrossRef]
- M. Alessio, I. Zanellato, I. Bonarrigo, E. Gabano, M. Ravera, D. Osella, Antiproliferative activity of Pt(IV)-bis(carboxylato) conjugates on malignant pleural mesothelioma cells, J. Inorg. Biochem. 2013, 129, 52–57, https://doi. org/10.1016/j.jinorgbio.2013.09.003.
- V. Novohradsky, I. Zanellato, C. Marzano, J. Pracharova, J. Kasparkova, D. Gibson, V. Gandin, D. Osella, V. Brabec, Epigenetic and antitumor effects of platinum(IV)-octanoato conjugates, Sci. Rep. 2017, 7, 3751, . [CrossRef]
- G. Tamasi, A. Merlino, F. Scaletti, P. Heffeter, A.A. Legin, M.A. Jakupec, W. Berger, L. Messori, B.K. Keppler, R. Cini, Ru(CO) x}-Core complexes with benzimidazole ligands: synthesis, X-ray structure and evaluation of anticancer activity in vivo, Dalt. Trans. 2017, 46, 3025–3040, . [CrossRef]
- Z. Xu, J. Huang, D. Kong, Y. Yang, L. Guo, X. Jia, G. Zhong, Z. Liu, Potent half-sandwich Ru(II) N^N (aryl-BIAN) complexes: lysosome-mediated apoptosis, in vitro and in vivo anticancer activities, Eur. J. Med. Chem. 2020, 207, 112763, . [CrossRef]
- S. Swaminathan, J. Haribabu, N.K. Kalagatur, M. Nikhil, N. Balakrishnan, N.S. P. Bhuvanesh, K. Kadirvelu, P. Kolandaivel, R. Karvembu, Tunable anticancer activity of furoylthiourea-based RuII–arene complexes and their mechanism of action, Chem. – A Eur. J. 2021, 27, 7418–7433, . [CrossRef]
- C. Gossens, I. Tavernelli, U. Rothlisberger, DNA structural distortions induced by ruthenium arene anticancer compounds, J. Am. Chem. Soc. 2008, 130, 10921–10928, . [CrossRef]
- S.A. Elsayed, S. Harrypersad, H.A. Sahyon, M.A. El-Magd, C.J. Walsby, Ruthenium(II)/(III) DMSO-based complexes of 2-aminophenyl benzimidazole with in vitro and in vivo anticancer activity, Molecules 2020, 25, 4284, . [CrossRef]
- Y. Chen, W. Li, Y. Yang, R. Zhong, H. Hu, C. Huang, J. Chen, L. Liang, Y. Liu, Significant increase of anticancer efficacy in vitro and in vivo of liposome entrapped ruthenium(II) polypyridyl complexes, Eur. J. Med. Chem. 2023, 257, 115541, . [CrossRef]
- B. Kar, P. Paira, One pot three component synthesis of DNA targeting phototoxic Ru( <scp>ii</scp>)- p -cymene dipyrido [3,2- a:2,3′- c]phenazine analogues, Dalt. Trans. 2022, 51, 15686–15695, . [CrossRef]
- S. Nikoli’c, J. Arakelyan, V. Kushnarev, S. Mutasim Alfadul, D. Stankovi’c, Y. I. Kraynik, S. Grguri’c-ˇSipka, M.V. Babak, Coordination of Ru(II)-Arene Fragments to Dipyridophenazine Ligands Leads to the Modulation of Their In Vitro and In Vivo Anticancer Activity, Inorg. Chem. 2023, 62, 8188–8199, . [CrossRef]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry, Molecules 2019, 24, 1995. [CrossRef]
- Thota, S.; Rodrigues, D. A.; Crans D. C.; Barreiro, E. J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 14, 5805–5821. [CrossRef]
- Zarić, R. Ž.; Pirković, M. S.; Hamzagić, N. Ruthenium(II) Complexes as Potential Apoptosis Inducers in Cancer Therapy. Experimental and Applied Biomedical Research (EABR), 2024, 25(1), 71–79. [CrossRef]
- Shi, J.; Xie, L.; Gong, W.; Bai, H.; Wang, W.; Wang, A.; Cao, W.; Tong, H.; Wang, H. Insight into the anti-proliferation activity and photoinduced NO release of four nitrosylruthenium isomeric complexes and their HSA complex adducts. Metallomics, 2024, 16(2). [CrossRef]
- Khan, T. A.; Bhar, K.; Samanta, R.; Bhatt, S.; Singh, M.; Rani, R.; Kumar, V.; Sharma, A.K. A bis-quinoline ruthenium(II) arene complex with submicromolar cytotoxicity in castration-resistant prostate cancer cells. Chem. Commun., 2024, 60, 1579-1582. [CrossRef]
- Chen, L.; Yu, W.; Tang, H.; Zhang, S.; Wang, J.; Ouyang, Q.; Guo, M.; Zhu, X.; Huang, Z.; Chen, J. Cyclometalated ruthenium complexes overcome cisplatin resistance through PI3K/mTOR/Nrf2 signaling pathway. Metallomics, 2024, 16(1), mfae002. [CrossRef]
- Wei, L.; He, X.; Zhao, D.; Kandawa-Shultz, M.; Shao, G.; Wang, Y. Biotin-conjugated Ru(II) complexes with AIE characteristics as mitochondria-targeted photosensitizers for enhancing photodynamic therapy by disrupting cellular redox balance.Eur. J Med.Chem., 2024, 264, 115985. [CrossRef]
- Bhattacharya, S.; Adon, T.; Dsouza, K.; Kumar, H. Y. Exploring the Future of Metal-Based Anticancer Agents: A Comprehensive Review of Ruthenium-Based Complexes. Chemistry Select, 2025, 10(9). [CrossRef]
- Rahman, A.F.M.M.; Alam, M.S.; Kwon, Y. Editorial: Small organic molecules with anticancer activity. Front Chem. 2023, 11, 1254312. PMID: 37681208; PMCID: PMC10482230. [CrossRef]
- Liang X, Wu P, Yang Q, Xie Y, He C, Yin L, Yin Z, Yue G, Zou Y, Li L, Song X, Lv C, Zhang W, Jing B. An update of new small-molecule anticancer drugs approved from 2015 to 2020. Eur J Med Chem. 2021, 220,113473. [CrossRef]
- Zhang Y, Fu L, Farooq Awan MU, Wang Y. Editorial: Discovery of small molecule lead compounds: a driving force to unravel new anti-cancer targets and mechanisms, Volume II. Front Pharmacol. 2024, 15, 1519858. PMID: 39744135; PMCID: PMC11688810. [CrossRef]
- Muhammad Aown Hashmia, Aqsa Kanwala, Umme Habibah Siddiquab, Nasir Rasoola and Ayesha Malik. From molecule to medicine: introducing emerging strategies to synthesize potent anticancer purine scaffolds. RSC Adv., 2025, 15, 21604-21638. [CrossRef]
- Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; Supuran, C. T. Natural products in drug discovery: advances and opportunities, Nat Rev Drug Discov. 2021, 20, 200-216. [CrossRef]
- Newman, D. J.; Cragg, G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod. 2020. [CrossRef]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines 2024, 12, 201. [CrossRef]
- Liu, G.-H.; Chen, T.; Zhang, X.; Ma, X.-L.; Shi, H.-S. Small molecule inhibitors targeting the cancers. Med Comm. 2022, 3:e181. [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; Yang S. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021, 6:201. [CrossRef]
- Xue, X.; Lindstrom, A.; Qu, H.; Li, Y. Recent advances on small-molecule nanomedicines for cancer treatment. WIREs Nanomed Nanobiotechnol. 2019, e1607. [CrossRef]
- Feng, G., Wu, Y., Hu, Y. et al. Small molecule inhibitors targeting m6A regulators. J Hematol Oncol 2024, 17, 30. [CrossRef]
- Mendoza Lara DF, Hernández-Caballero ME, Terán JL, Ramírez JS, Carrasco-Carballo A. Anticancer Activities of Natural and Synthetic Steroids: A Review. ACS Omega. 2025, 10(8), 7493-7509. [CrossRef]
- Pradhan G, Juvale K. Structure activity relationship for anticancer activities of spirooxindole derivatives: A comprehensive review. Bioorg Chem. 2025, 154,107975. [CrossRef]
- Boga, C.; Micheletti, G. Design and Synthesis of Organic Molecules as Antineoplastic Agents. Molecules 2020, 25, 2808. [CrossRef]
- Amewu, R.K.; Sakyi, P.O.; Osei-Safo, D.; Addae-Mensah, I. Synthetic and Naturally Occurring Heterocyclic Anticancer Compounds with Multiple Biological Targets. Molecules 2021, 26, 7134. [CrossRef]
- Ohta, K.; Kaise, A.; Taguchi, F.; Aoto, S.; Ogawa, T.; Endo, Y. Design and Synthesis of Novel Breast Cancer Therapeutic Drug Candidates Based upon the Hydrophobic Feedback Approach of Antiestrogens. Molecules 2019, 24, 3966. [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hakobyan, E.K.; Sahakyan, H.; Arabyan, E.; Zakaryan, H.; Nersesyan, L.E.; Aharonyan, A.S.; Danielyan, I.S.; et al. Synthesis, Antitumor Activity, and Docking Analysis of New Pyrido [3′,2′:4,5]furo(thieno)[3,2-d]pyrimidin-8-amines. Molecules 2019, 24, 3952. [CrossRef]
- An, R.; Hou, Z.; Li, J.-T.; Yu, H.-N.; Mou, Y.-H.; Guo, C. Design, Synthesis and Biological Evaluation of Novel 4-Substituted Coumarin Derivatives as Antitumor Agents. Molecules 2018, 23, 2281. [CrossRef]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’yakonov, V.; Dzhemilev, U. Synthesis and Evaluation of Anticancer Activities of Novel C-28 Guanidine-Functionalized Triterpene Acid Derivatives. Molecules 2018, 23, 3000. [CrossRef]
- Kędzia, J.; Bartosik, T.; Drogosz, J.; Janecka, A.; Krajewska, U.; Janecki, T. Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones. Molecules 2019, 24, 1868. [CrossRef]
- Defant, A.; Mancini, I. Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Molecules. Molecules 2019, 24, 2224. [CrossRef]
- Smolobochkin, A.; Gazizov, A.; Sazykina, M.; Akylbekov, N.; Chugunova, E.; Sazykin, I.; Gildebrant, A.; Voronina, J.; Burilov, A.; Karchava, S.; et al. Synthesis of Novel 2-(Het)arylpyrrolidine Derivatives and Evaluation of Their Anticancer and Anti-Biofilm Activity. Molecules 2019, 24, 3086. [CrossRef]
- Calonghi, N.; Boga, C.; Telese, D.; Bordoni, S.; Sartor, G.; Torsello, C.; Micheletti, G. Synthesis of 9-Hydroxystearic Acid Derivatives and Their Antiproliferative Activity on HT 29 Cancer Cells. Molecules 2019, 24, 3714. [CrossRef]
- Constantinescu T, Lungu CN. Anticancer Activity of Natural and Synthetic Chalcones. Int J Mol Sci. 2021, 22(21):11306. PMID: 34768736; PMCID: PMC8582663. [CrossRef]
- Saidin S, Jumat MA, Mohd Amin NAA, Saleh Al-Hammadi AS. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater Sci Eng C Mater Biol Appl. 2021, 118, 111382. [CrossRef]
- Hess, J. Rational approaches towards inorganic and organometallic antibacterials. Biol Chem. 2021, 403(4), 363–375. [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: structures, antibiotic effects and future perspectives. Chem Commun (Camb). 2018, 54(10), 1153-1169. [CrossRef]
- Biegański P, Szczupak Ł, Arruebo M, Kowalski K. Brief survey on organometalated antibacterial drugs and metal-based materials with antibacterial activity. RSC Chem Biol. 2021, 2(2), 368-386. [CrossRef]
- Rofeal M, Abdelmalek F, Steinbüchel A. Naturally-Sourced Antibacterial Polymeric Nanomaterials with Special Reference to Modified Polymer Variants. Int J Mol Sci. 2022, 23(8), 4101. [CrossRef]
- Torres, N. S.,; Abercrombie, J. J.; Srinivasan, A.; Lopez-Ribot, J. L.; Ramasubramanian, A. K.; Leung, K. P. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother 2016. 60, 5663–5672. [CrossRef]
- Wiederhold, N. P.; Patterson, T. F.; Srinivasan, A.; Chaturvedi, A. K.; Fothergill, A. W.; Wormley, F. L.; et al. Repurposing auranofin as an antifungal: in-vitro activity against a variety of medically important fungi. Virulence 2017, 8, 138–142. [CrossRef]
- Cassetta, M. I.; Marzo, T.;, Fallani, S.; Novelli, A.; Messori, L. Drug repositioning: auranofin as a prospective antimicrobial agent for the treatment of severe Staphylococcal infections. Biometals 2014, 27, 787–791. [CrossRef]
- de Almeida, A.M.; de Oliveira, B.A.; de Castro, P.P. et al. Lipophilic gold(I) complexes with 1,3,4-oxadiazol-2-thione or 1,3-thiazolidine-2-thione moieties: synthesis and their cytotoxic and antimicrobial activities. Biometals 2017, 30, 841–857. [CrossRef]
- Epstein, T.D.; Wu, B.; Moulton, K.D.; Yan, M.; Dube, D.H. Sugar-Modified Analogs of Auranofin Are Potent Inhibitors of the Gastric Pathogen Helicobacter pylori. ACS Infectious Diseases 2019, 5 (10), 1682-1687. [CrossRef]
- Wu, B.; Yang, X.; Yan. M. Synthesis and Structure–Activity Relationship Study of Antimicrobial Auranofin against ESKAPE Pathogens. Journal of Medicinal Chemistry 2019, 62 (17), 7751-7768. [CrossRef]
- Frik, M.; Jiménez, J.; Gracia, I.; Falvello, L. R.; Abi-Habib, S.; Suriel, K. et al. Luminescent di- and polynuclear organometallic gold(I)-metal (Au2, {Au2Ag}n and {Au2Cu}n) compounds containing bidentate phosphanes as active antimicrobial agents. Chem. Eur. J. 2012, 18, 3659–3674. [CrossRef]
- Chen, X.; Sun, S.; Huang, S.; Yang, H.; Ye, Q.; Lv, L.; Liang, Y.; Shan, J.; Xu, J.; Liu, W.; Ma, T. Gold(I) selenium N-heterocyclic carbene complexes as potent antibacterial agents against multidrug-resistant gram-negative bacteria via inhibiting thioredoxin reductase. Redox Biology 2023, 60, 102621. [CrossRef]
- Ndugire, W.; Raviranga, N.G.H.; Lao, J.; Ramström, O.; Yan, M. Gold Nanoclusters as Nanoantibiotic Auranofin Analogues. Adv. Healthcare Mater. 2022, 11, 2101032. [CrossRef]
- Ratia, C.; Ballén, V.; Gabasa, Y.; Soengas, R.G.; Velasco-de Andrés, M.; Iglesias M.J.; Cheng, Q.; Lozano, F.; Arnér, E.S.J.; López-Ortiz, F.; Soto, S.M. Novel gold(III)-dithiocarbamate complex targeting bacterial thioredoxin reductase: antimicrobial activity, synergy, toxicity, and mechanistic insights. Front Microbiol. 2023, 14, 1198473. [CrossRef]
- Pintus, A.; Aragoni, M. C.; Cinellu, M. A.; Maiore, L.; Isaia, F.; Lippolis, V, et al. [Au(pyb-H)(mnt)]: a novel gold(III) 1,2-dithiolene cyclometalated complex with antimicrobial activity (pyb-H=C-deprotonated 2-benzylpyridine; mnt=1,2-dicyanoethene-1,2-dithiolate). J. Inorg. Biochem. 2017, 170, 188–194. [CrossRef]
- Chakraborty, P.; Oosterhuis, D.; Bonsignore, R.; Casini, A.; Olinga, P.; Scheffers, D. J. An organogold compound as potential antimicrobial agent against drug-resistant bacteria: initial mechanistic insights. Chem. Med. Chem. 2021, 16, 3060–3070. [CrossRef]
- Büssing, R.; Karge, B.; Lippmann, P.; Jones, P. G.; Broönstrup, M.; Ott, I. Gold(I) and gold(III) N-heterocyclic carbene complexes as antibacterial agents and inhibitors of bacterial thioredoxin reductase. Chem. Med. Chem. 2021, 16, 3402–3409. [CrossRef]
- Pereira, A. K.DS; Manzano, C.M.; Nakata, D.H.; Clavijo, J.C.T.; Pereira, D.H.; Lustri, W.R.; Cobri, P.P. Synthesis, crystal structures, DFT studies, antibacterial assays and interaction assessments with biomolecules of new platinum(ii) complexes with adamantane derivatives. New J. Chem., 2020, 44, 11546-11556. [CrossRef]
- Yu, W.-H.; Yiu, S.-C.; Lau, M.-T.; Ho, P.-Y.; Lam, P.-L.; Chui, C.-H.; Wong, W.-Y. Synthesis and Characterization of Neutral Cyclometalated Platinum(II) Complexes and their Antibacterial and Cytotoxic Evaluations. European Journal of Inorganic Chemistry 2023, 26 (3), 1434-1948. [CrossRef]
- Lunagariya, M.V.; Thakor, K.P.; Varma, R.R.; Waghela, B.N.; Pathak, C.; Patel, M.N. Synthesis, characterization and biological application of 5-quinoline 1,3,5-trisubstituted pyrazole based platinum(ii) complexes. Med. Chem. Commun. 2018, 9, 282-298. [CrossRef]
- Lacerda, M.L.d.; Rossi, D.A.; Lourenzatto, E.C.A.; Takeuchi, M.G.; Souza, W.A.; Silva, R.T.C.; Julio, L.G.; Guerra, W.; Melo, R.T.d. Antimicrobial Resistance Challenged with Platinum(II) and Palladium(II) Complexes Containing 1,10-Phenanthroline and 5-Amino-1,3,4-Thiadiazole-2(3H)-Thione in Campylobacter jejuni. Antibiotics 2022, 11, 1645. [CrossRef]
- Tümer, M.; Ekinci, D.; Tümer, F.; Bulut, A. Synthesis, characterization and properties of some divalent metal(II) complexes: Their electrochemical, catalytic, thermal and antimicrobial activity studies. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2007, 67(3–4), 916–929. [CrossRef]
- Radić, G.P.; Glođović, V.V.; Ratković, Z.R.; Novaković, S.B.; Garcia-Granda, S.; Roces, L.; Menéndez-Taboada, L.; Radojević, I.D.; Stefanović, O.D.; Čomić, L.R.; Trifunović, S.R. Synthesis, characterization and antimicrobial activity of novel platinum(IV) and palladium(II) complexes with meso-1,2-diphenyl-ethylenediamine-N,N′-di-3-propanoic acid – Crystal structure of H2-1,2-dpheddp·2HCl·H2O. Journal of Molecular Structure 2012, 1029, 180-186. [CrossRef]
- Nam-Cha, S.H.; Domínguez-Jurado, E.; Tinoco-Valencia, S.L.; Pérez-Tanoira, R.; Morata-Moreno, N.; Alfaro-Ruiza, R.; Lara-Sánchez, A.; Esteban, J.; Luján, R.; Alonso-Moreno, C.; Seguí, P.; Ocaña, A.; Gónzalez, Á.L.; Aguilera-Correa, J.J.; Pérez-Martínez, F.C.; Alarcón, M.M. () Synthesis, characterization, and antibacterial activities of a heteroscorpionate derivative platinum complex against methicillin-resistant Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2023, 13, 1100947. [CrossRef]
- Poljarević, J. M.; Krstić, M. P.; Grgurić-Šipka, S.; Sovilj, S. P.; Mišić, D. R.; Sabo, T. J. Platinum(IV) complexes with N-alkylphenothiazines: synthesis, characterization, and antibacterial activity. Journal of Coordination Chemistry 2013, 66(21), 3760–3769. [CrossRef]
- Frei, A.; Ramu, S.; Lowe, G. J.; Dinh, H.; Semenec, L.; Elliott, A. G.; Zuegg, J.; Deckers, A.; Jung, N.; Bräse, S.; Cain, A. K.; Blaskovich, M. A. T. Platinum Cyclooctadiene Complexes with Activity against Gram-positive Bacteria. Chem. Med. Chem. 2021, 16, 3165. [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.G.; Dowson, C.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [CrossRef]
- de Sousa, A.P.; Ellena, J.; Gondim, A.C.S.; Lopes, L.G.F.; Sousa, E.H.S.; de Vasconcelos, M.A.; Teixeira, E.H.; Ford, P.C.; Holanda, A.K.M. Antimicrobial activity of cis-[Ru(bpy)2(L)(L′)]n+ complexes, where L = 4-(4-chlorobenzoyl)pyridine or 4-(benzoyl)pyridine and L′ = Cl− or CO. Polyhedron 2018, 144, 88–94. [CrossRef]
- Liao, X.; Jiang, G.; Wang, J.; Duan, X.; Liao, Z.; Lin, X.; Shen, J.; Xiong, Y.; Jiang, G. Two ruthenium polypyridyl complexes functionalized with thiophen: Synthesis and antibacterial activity against Staphylococcus aureus. New J. Chem. 2020, 44, 17215–17221. [CrossRef]
- Bu, S.; Jiang, G.; Jiang, G.; Liu, J.; Lin, X.; Shen, J.; Xiong, Y.; Duan, X.; Wang, J.; Liao, X. Antibacterial activity of ruthenium polypyridyl complexes against Staphylococcus aureus and biofilms. J. Biol. Inorg. Chem. 2020, 25, 747–757. [CrossRef]
- Li, F.; Weber, D.K.; Morgan, J.L.; Collins, J.G.; Keene, F.R. An approach to therapeutic agents through selective targeting of destabilised nucleic acid duplex sequences. Dalt. Trans. 2012, 41, 6528–6535. [CrossRef]
- Sun, B.; Sundaraneedi, M.K.; Southam, H.M.; Poole, R.K.; Musgrave, I.F.; Keene, F.R.; Collins, J.G. Synthesis and biological properties of tetranuclear ruthenium complexes containing the bis [4(4′-methyl-2,2′-bipyridyl)]-1,7-heptane ligand. Dalt. Trans. 2019, 48, 14505–14515. [CrossRef]
- Southam, H.M.; Smith, T.W.; Lyon, R.L.; Liao, C.; Trevitt, C.R.; Middlemiss, L.A.; Cox, F.L.; Chapman, J.A.; El-Khamisy, S.F.; Hippler, M.; et al. A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biol. 2018, 18, 114–123. [CrossRef]
- Nobre, L.S.; Jeremias, H.; Romão, C.C.; Saraiva, L.M. Examining the antimicrobial activity and toxicity to animal cells of different types of CO-releasing molecules. Dalt. Trans. 2016, 45, 1455–1466. [CrossRef]
- Wang, L.; Liu, L.; Wang, X.; Tan, Y.; Duan, X.; Zhang, C.; Cheng, J.; Xiong, Y.; Jiang, G.; Wang, J.; Liao, X. Ruthenium(II) complexes targeting membrane as biofilm disruptors and resistance breakers in Staphylococcus aureus bacteria. European Journal of Medicinal Chemistry 2022, 238, 114485. [CrossRef]
- Al-Wahish, M.A.; Saadh, M.; Salama, A.H. Synthesis, characterization and antibacterial activity of ruthenium complex bearing 3,3’-dicarboxy-2,2’-bipyridine ligand. Pharmacia 2023, 70(2), 405-410. [CrossRef]
- Huang, H.-Y.; Wang, Q.; Zhang, C.-Y.; Chen, Z.-X.; Wang, J.-T.; Liao, X.-W.; Yu, R.-J.; Xiong, Y.-S. Synthesis and biological evaluation of ruthenium complexes containing phenylseleny against Gram-positive bacterial infection by damage membrane integrity and avoid drug-resistance. Journal of Inorganic Biochemistry 2023, 242, 112175. [CrossRef]
- Namiecińska, E.; Grazul, M.; Sadowska, B.; Więckowska-Szakiel, M.; Hikisz, P.; Pasternak, B,; Budzisz, E. Arene-Ruthenium(II) Complexes with Carbothiamidopyrazoles as a Potential Alternative for Antibiotic Resistance in Human. Molecules. 2022, 27(2), 468. [CrossRef]
- Zhou, R,; Gao, J.; Li, Y.; Raziq, K.; Afrasiyab, Chen, L.; Sun, D. An aromatic ruthenium complex with high antimicrobial efficiency by disrupting biofilms and recruiting M2 macrophages. Inorganica Chimica Acta 2025, 578, 122546. [CrossRef]
- Gorle, A.K.; Feterl, M.; Warner, J.M.; Wallace, L.; Keene, F.R.; Grant Collins, J. Tri- and tetra-nuclear polypyridyl ruthenium(ii) complexes as antimicrobial agents. Dalton Trans. 2014, 43, 16713-16725. [CrossRef]
- Desmard, M.; Davidge, K.S.; Bouvet, O.; Morin, D.; Roux, D.; Foresti, R.; Ricard, J.D.; Denamur, E.; Poole, R.K.; Montravers, P.; Morterlini, R.; Boczkowski, J. A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. The FASEB Journal 2009, 23, 1023-1031. [CrossRef]
- Tavares, A.F.; Parente, M.R.; Justino, M.C.; Oleastro, M.; Nobre, L.S. et al. () The Bactericidal Activity of Carbon Monoxide–Releasing Molecules against Helicobacter pylori. PLoS ONE 2013, 8(12), e83157. [CrossRef]
- Carvalho, S.M.; Marques, J.; Romão, C.C.; Saraiva, L.M. Metabolomics of Escherichia coli Treated with the Antimicrobial Carbon Monoxide-Releasing Molecule CORM-3 Reveals Tricarboxylic Acid Cycle as Major Target. Antimicrob. Agents Chemother. 2019, 63 . [CrossRef]
- Stinger, T.; Seldon, R.; Liu, N.; Warner, D.F.; Tam, C.; Cheng, L.W.; Land, K.M.; Smith, P.J.; Chibale, K.; Smith, G.S. Antimicrobial activity of organometallic isonicotinyl and pyrazinyl ferrocenyl-derived complexes. Dalton Trans. 2017, 46, 9875-9885. [CrossRef]
- Tao, R.; Lu, Y.; Xia, W.; Zhang, C.; Wang, C. Characterization and antibacterial activity of ruthenium-based shikimate cross-linked chitosan composites. International Journal of Biological Macromolecules 2022, 217, 890-901. [CrossRef]
- Vadivel, T.; Dhamodaran, M. Synthesis, characterization and antibacterial studies of ruthenium(III) complexes derived from chitosan schiff base. International Journal of Biological Macromolecules 2016, 90, 44-52. [CrossRef]
- Pancu DF, Scurtu A, Macasoi IG, Marti D, Mioc M, Soica C, Coricovac D, Horhat D, Poenaru M, Dehelean C. Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity-A Pharmaco-Toxicological Screening. Antibiotics (Basel). 2021,10(4), 401. [CrossRef]
- Shabalina, A.V.; Kozlov, V.A.; Popov, I.A.; Gudkov, S.V. A Review on Recently Developed Antibacterial Composites of Inorganic Nanoparticles and Non-Hydrogel Polymers for Biomedical Applications. Nanomaterials 2024, 14, 1753. [CrossRef]
- Butler MS, Vollmer W, Goodall ECA, Capon RJ, Henderson IR, Blaskovich MAT. A Review of Antibacterial Candidates with New Modes of Action. ACS Infect Dis. 2024, 10(10), 3440-3474. [CrossRef]
- Meneghetti F, Barlocco D. Novel Antibacterial Agents 2022. Pharmaceuticals (Basel). 2024, 17(3), 370. PMID: 38543156; PMCID: PMC10974457. [CrossRef]
- Liu G, Gui Y, Shi W, Yang H, Feng S, Liang S, Zhou C, Zhou Q, Li H, Li G, Si H, Ou C. Therapeutic efficacy of compound organic acids administration on methicillin-resistant Staphylococcus aureus-induced arthritis in broilers. Poult Sci. 2024, 103(12), 104219. [CrossRef]
- Nasr, A.M.; Abdel-Latif, H.M.R.; Abdel-Rahman, M.S.; Shalaby, M.A.; Abdel-Rahman, A.M. Pyrimidines as anti-inflammatory agents: Structure–activity relationship and mechanism of action. Eur. J. Med. Chem. 2020, 199, 112399. [CrossRef]
- Costa, P.A.; Vieira, H.L.A.; Silva, E.M.; Gallium compounds as potential anti-inflammatory agents: A systematic review. J. Inorg. Biochem. 2021, 218, 111371. [CrossRef]
- de Freitas, M.R.; Santos, R.C.; Organometallic NSAID complexes: Enhanced anti-inflammatory activity and reduced side effects. J. Med. Chem. 2019, 62, 10435–10450. [CrossRef]
- Ahmed, S.; Ali, R.; Flavonoid and chromone metal complexes as anti-inflammatory and antioxidant agents. Pharmacol. Res. 2021, 167, 105534. [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Natural organosulfur compounds: Mechanisms of anti-inflammatory and antioxidant action. Molecules 2015, 20, 12502–12529. [CrossRef]
- Semenok, D.; Medvedev, J.; Giassafaki, L.-P.; Lavdas, I.; Vizirianakis, I.S.; Eleftheriou, P.; Gavalas, A.; Petrou, A.; Geronikaki, A. 4,5-Diaryl 3(2H)Furanones: Anti-Inflammatory Activity and Influence on Cancer Growth. Molecules 2019, 24, 1751. [CrossRef]
- Dos Santos, G.; Brás, J.L.; Anti-inflammatory activity of inorganic nanoparticles: Case of IF-WS₂ fullerene-like nanoparticles. Int. J. Mol. Sci. 2020, 21, 9543. [CrossRef]
- Stoyanova, M.; Milusheva, M.; Gledacheva, V.; Todorova, M.; Kircheva, N.; Angelova, S.; Stefanova, I.; Pencheva, M.; Vasileva, B.; Hristova-Panusheva, K.; et al. Silver Nanoparticles with Mebeverine in IBS Treatment: DFT Analysis, Spasmolytic, and Anti-Inflammatory Effects. Pharmaceutics 2025, 17, 561. [CrossRef]
- Ivanov, I.; Manolov, S.; Bojilov, D.; Stremski, Y.; Marc, G.; Statkova-Abeghe, S.; Oniga, S.; Oniga, O.; Nedialkov, P. Synthesis of Novel Benzothiazole–Profen Hybrid Amides as Potential NSAID Candidates. Molecules 2025, 30, 107. [CrossRef]
- Dimitrova, D.; Manolov, S.; Ivanov, I.; Bojilov, D.; Dimova, N.; Marc, G.; Oniga, S.; Oniga, O. Trimetazidine–Profen Hybrid Molecules: Synthesis, Chemical Characterization, and Biological Evaluation of Their Racemates. Pharmaceuticals 2025, 18, 1251. [CrossRef]
- Ivanov, I.; Manolov, S.; Bojilov, D.; Marc, G.; Dimitrova, D.; Oniga, S.; Oniga, O.; Nedialkov, P.; Stoyanova, M. Novel Flurbiprofen Derivatives as Antioxidant and Anti-Inflammatory Agents: Synthesis, In Silico, and In Vitro Biological Evaluation. Molecules 2024, 29, 385. [CrossRef]
- Bojilov, D.; Manolov, S.; Ahmed, S.; Dagnon, S.; Ivanov, I.; Marc, G.; Oniga, S.; Oniga, O.; Nedialkov, P.; Mollova, S. HPLC Analysis and In Vitro and In Silico Evaluation of the Biological Activity of Polyphenolic Components Separated with Solvents of Various Polarities from Helichrysum italicum. Molecules 2023, 28, 6198. [CrossRef]
- Manolov, S.; Bojilov, D.; Ivanov, I.; Marc, G.; Bataklieva, N.; Oniga, S.; Oniga, O.; Nedialkov, P. Synthesis, Molecular Docking, Molecular Dynamics Studies, and In Vitro Biological Evaluation of New Biofunctional Ketoprofen Derivatives with Different N-Containing Heterocycles. Processes 2023, 11, 1837. [CrossRef]
- Manolov, S.; Ivanov, I.; Bojilov, D.; Nedialkov, P. Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives. Molecules 2023, 28, 151. [CrossRef]
- Panova, N.; Gerasimova, A.; Tumbarski, Y.; Ivanov, I.; Todorova, M.; Dincheva, I.; Gentscheva, G.; Gledacheva, V.; Slavchev, V.; Stefanova, I.; et al. Metabolic Profile, Antioxidant, Antimicrobial, Contractile, and Anti-Inflammatory Potential of Moringa oleifera Leaves (India). Life 2025, 15, 583. [CrossRef]
- Todorova, M.; Bakalska, R.; Feizi-Dehnayebi, M.; Ziarani, G.M.; Pencheva, M.; Stojnova, K.; Milusheva, M.; Nedialkov, P.; Cherneva, E.; Kolev, T.; et al. Synthesis, Anti-Inflammatory Activity, and Docking Simulation of a Novel Styryl Quinolinium Derivative. Appl. Sci. 2025, 15, 284. [CrossRef]
- Stoyanova, M.; Milusheva, M.; Gledacheva, V.; Stefanova, I.; Todorova, M.; Kircheva, N.; Angelova, S.; Pencheva, M.; Stojnova, K.; Tsoneva, S.; et al. Spasmolytic Activity and Anti-Inflammatory Effect of Novel Mebeverine Derivatives. Biomedicines 2024, 12, 2321. [CrossRef]
- Gerasimova, A.; Nikolova, K.; Petkova, N.; Ivanov, I.; Dincheva, I.; Tumbarski, Y.; Yanakieva, V.; Todorova, M.; Gentscheva, G.; Gavrilova, A.; et al. Metabolic Profile of Leaves and Pulp of Passiflora caerulea L. (Bulgaria) and Their Biological Activities. Plants 2024, 13, 1731. [CrossRef]
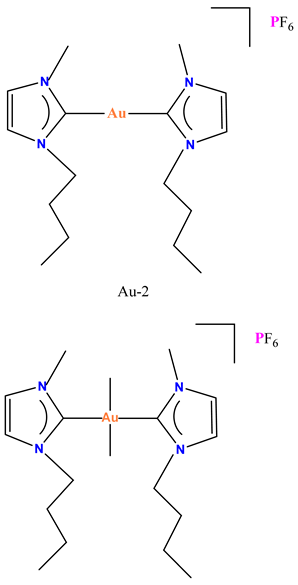
| Structure of Gold Complexes | In Vitro Activity | In Vivo Activity | References |
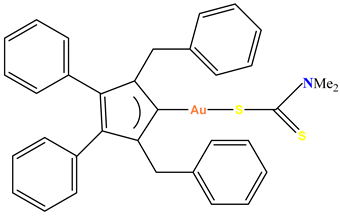 Au-1 |
demonstrated significant anti-tumor effect towards prostate cancer cell line PC3 |
Inhibited tumor progression in mice bearing PC3 xenografts.. | Walther et al. 2020 [72] |
 Au-3 |
Both compounds exhibited potent antiproliferative effects against multiple cancer cell lines, including A2780, A2780cis, HCT116-p53wt, and MCF-7. | Following labeling with radioactive iodine and administration to rats, the second complex rapidly distributed to major organs within 1–5 minutes. | Guarra et al. 2020 [73] |
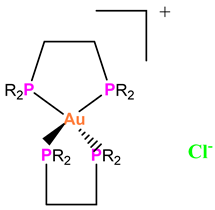 Au-4 |
Strongly blocked TrxR activity in myeloma cells, whether sensitive or resistant to bortezomib, which slowed their growth. | Reduced the growth of RPMI8226 myeloma tumors in NOD/SCID mice.. | Sze et al. 2020 [74] |
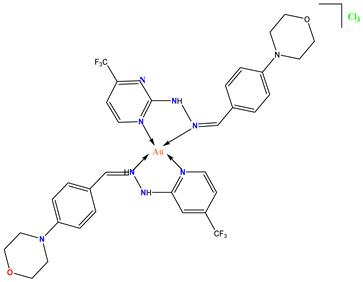 Au-5 |
The Au(III) compound attacked cancer cells but did not harm normal cells. | In vivo studies in tumor-bearing mice demonstrated the anticancer potential of the Au(III) derivative. | Sankaeganesh et al. 2019 [75] |
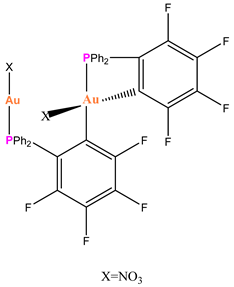 Au-6 |
Exhibited cytotoxicity and suppressed the proliferation of multiple cancer cell lines, including HeLa and DU145. | Reduced the growth of HeLa tumors in Balb/c nude mice. | Mirzadeh et al. 2021 [76] |
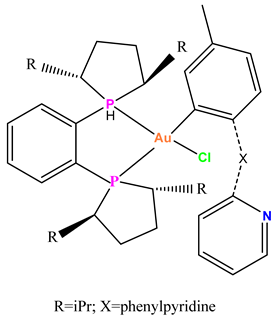 Au-7 |
Showed toxic activity against four aggressive cancer cell types, including H460, MDA-MB-231, MDA-MB-468 and BT-33 glioblastoma at micromolar levels. | At 10 mg/kg, tumor growth in 4T1-inoculated Balb/c mice was slightly lower than in untreated mice.. | Arojojoye et al. 2022 [77] |
| Structure of Platinum(II) Complexes | In Vitro Activity | In Vivo Activity | References |
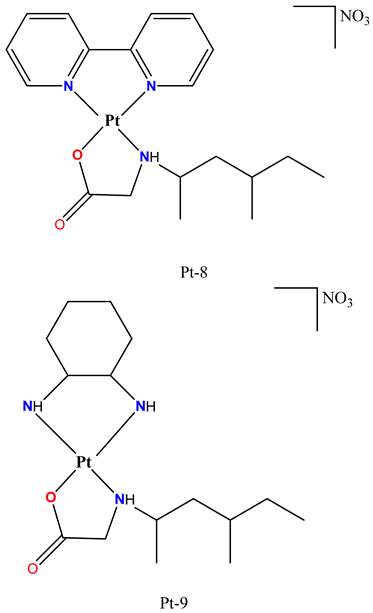 |
Exhibited stronger cytotoxicity against SKBR3 cells than oxaliplatin and carboplatin. | Showed anticancer activity in a 4T1 allotransplanted breast tumor model in Balb/c mice, significantly reducing tumor volume. | Bazsefidpar et al. 2023 [92] |
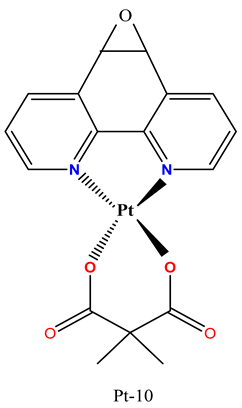 |
Exhibited significant cytotoxic effect against multiple cancer cell lines, including HCT116, 4T1 and CT26. | Pt-10 showed significant anticancer activity in an orthotopic 4T1 mouse tumor model without histopathological toxicity in the heart, lung, liver, or kidney. | Dimitrijević Stojanović et al. 2022 [93] |
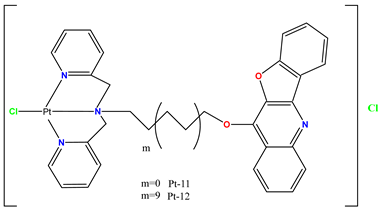 |
Induced programmed cell death in T-24 cells more efficiently than cisplatin. | Administration of the Pt(II) complex (2.0 mg/kg every 2 days) reduced T-24 xenograft growth in mice. | Qin et al. 2021 [94] |
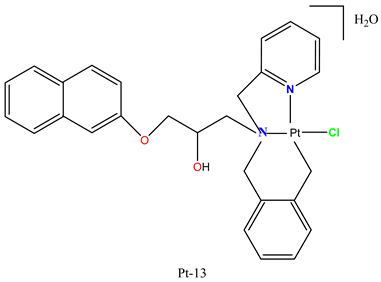 |
Pt-13 induced cytotoxicity in multiple tumor cell lines, including A549, PC3, MDA-MB-231, MCF-7, BXPC-3, and PBMC. | Strongly suppressed MDA-MB-231 tumor xenograft growth in BALB/c nude mice. | Maciel et al. 2022 [95] |
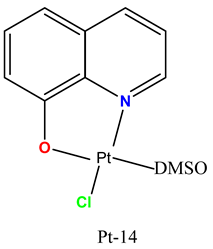 |
Quinoline–platinum complexes induced cytotoxicity in cisplatin-resistant human osteosarcoma MG-63 cells. | Inhibited growth of human osteosarcoma xenografts in mice. | Ruiz et al. 2019 [96] |
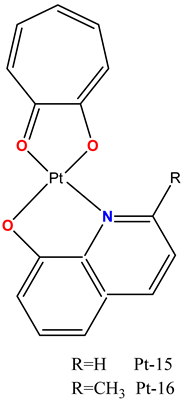 |
Induced cytotoxicity in HeLa, A549, T24, and NCI-H460 cells more efficiently than cisplatin. | In female Balb/c nude mice, tumor xenograft growth was inhibited with efficacy comparable to cisplatin. | Mo et al. 2021 [97] |
| Structure of Ruthenium Complexes | In Vitro Activity | In Vivo Activity | References |
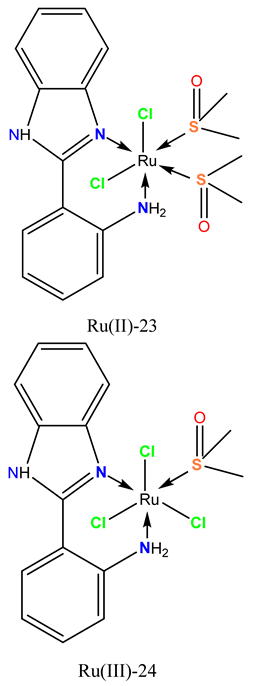 |
Exhibited cytotoxicity against multiple human tumor cell lines, including MCF-7 and Caco-2. | In the EAC mouse model, the treatment inhibited liver cancer cell proliferation by inducing apoptosis, increasing Bax and Caspase-3 levels, and decreasing Bcl-2 levels in the liver. | Elsayed et al. 2020 [114] |
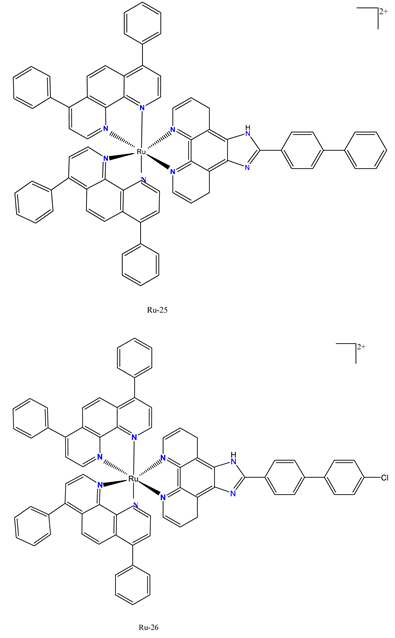 |
The Ru-25 and Ru-26- exhibited strong antiproliferative effect against SGC-7901cells. |
In nude mice, SGC-7901 tumor xenograft growth was inhibited by 53.5% and 72.9% at doses of 1.23 and 2.46 mg/kg, respectively. | Chen et al. 2023 [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





