1. Introduction
Cholangiocarcinoma is a rare but aggressive malignant tumor arising from the biliary epithelium, accounting for approximately 3% of all gastrointestinal cancers. It is classified according to its anatomical site of origin into intrahepatic, perihilar, or distal cholangiocarcinoma. The majority of patients (up to 70%) present at diagnosis with locally advanced or metastatic disease, making them ineligible for surgical resection. In such cases, the prognosis is poor: median survival with chemotherapy alone is less than 12 months, and it decreases to 7.5 months with best supportive care only. [
1]
Most patients with perihilar or distal cholangiocarcinoma present with obstructive jaundice. Therefore, biliary drainage plays a crucial role in patients with advanced disease, as it helps prevent episodes of cholangitis, reduces bilirubin levels to those compatible with chemotherapy administration, improves quality of life, and potentially increases survival. [
1]
The introduction of self-expandable metal stents (SEMS) has significantly improved the palliative management of jaundice by reducing occlusion rates and the need for reintervention compared with plastic stents. However, the problem of re-obstruction persists: despite the use of SEMS, many patients experience recurrent jaundice or cholangitis due to tumor ingrowth into the stent or biliary sludge formation. [
2] These complications can disrupt the continuity of chemotherapy and negatively affect morbidity and mortality.
This highlights the need for additional strategies to control intraductal tumor growth, prolong stent patency, and potentially improve survival. Among these, photodynamic therapy and intraluminal brachytherapy have been investigated in the past but have not entered routine clinical practice due to significant limitations. Photodynamic therapy is costly and associated with premature stent displacement and systemic phototoxicity. [
3] Intraluminal brachytherapy, which requires a surgical or percutaneous approach and careful handling of radioactive material, poses logistical challenges and is associated with serious adverse effects such as duodenal stenosis, gastrointestinal bleeding, and hemobilia. [
4]
In this context, endobiliary radiofrequency ablation (RFA) has emerged as a promising and relatively safe therapeutic option that can be applied concurrently with palliative biliary drainage.
2. Endobiliary Radiofrequency Ablation
2.1. RATIONALE AND MECHANISM OF ACTION OF RFA
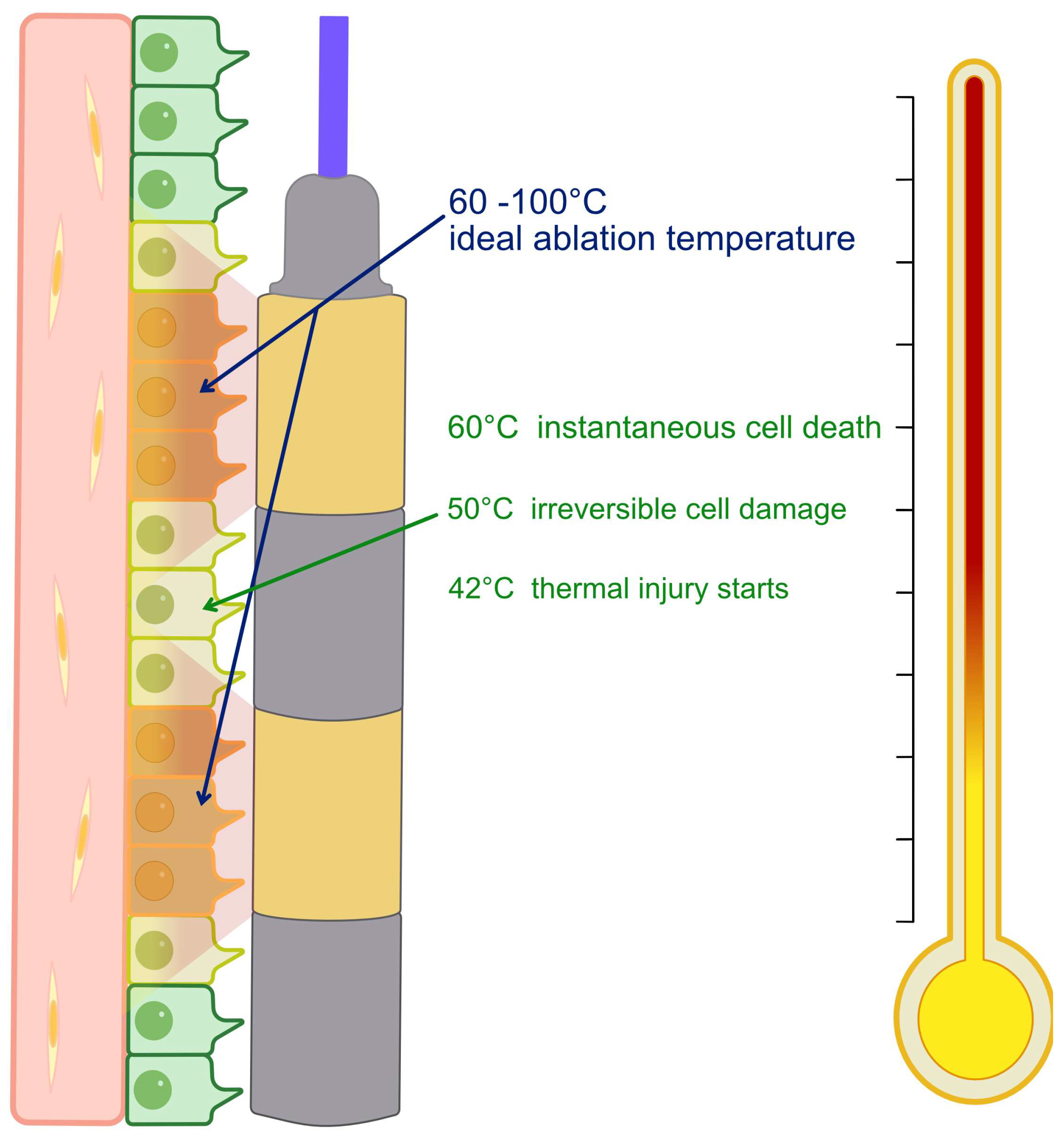
RFA consists of delivering high-frequency alternating current through an electrode positioned within the malignant biliary stricture. This method relies on the interaction between a high-frequency alternating current (approximately 400–500 kHz) and biological tissue. The energy delivered through the electrode causes ionic agitation and frictional heating, producing temperatures in excess of 60 °C that denature proteins and result in coagulative necrosis. [
5,
6,
7] The heating gradient is greatest near the electrode and declines with distance through thermal conduction. The central ablation zone is surrounded by a peripheral area that is exposed to sublethal temperatures (40–60 °C), in which cells may undergo apoptosis or recover (
Figure 1). However, excessive heat (>100 °C) can cause carbonisation and tissue impedance, which limits current flow and treatment efficacy. [
5,
6,
7,
8] Ablation depth depends on electrode geometry, tissue conductivity, temperature, and exposure time. Animal studies indicate that energy delivery for 30–120 s at 7–10 W achieves necrosis from the bile duct wall to adjacent tissue. Vascular cooling (“heat-sink” effect) may reduce efficacy near vessels >3 mm. [
9,
10,
11] Cell necrosis disrupts membrane integrity and releases intracellular antigens, heat-shock proteins, and nucleic acids, which can stimulate local and systemic immune activation. [
12,
13]
The goal of local thermal ablation is to reduce tumor burden and slow intraductal neoplastic growth, with two main expected benefits: prolonging stent patency (by reducing tumor ingrowth and the formation of hyperplastic granulation tissue) and potentially improving patient survival. Indeed, maintaining biliary duct patency for a longer time prevents recurrence of jaundice and cholangitis, allowing uninterrupted chemotherapy and improving the overall clinical condition.
Moreover, it has been hypothesized that tumor necrosis induced by RFA exerts a local immunomodulatory effect by releasing tumor antigens that can stimulate an antineoplastic immune response. This local immune response could further contribute to disease control and may partly explain the survival benefit observed in some studies. [
14]
2.2. CLINICAL EVIDENCE
Since its introduction following the first pilot study in 2011, [
15] RFA has been the subject of numerous investigations—initially mostly retrospective series, later followed by randomized controlled trials (RCTs) in recent years. The first reports indicated a clinical benefit from adding RFA to stenting in the management of malignant biliary obstruction: early studies conducted on small cohorts of patients showed prolonged stent patency and increased survival compared with stenting alone in patients with malignant biliary strictures. [
16,
17]
The first RCTs published on the efficacy of this technique in cholangiocarcinoma subsequently confirmed these positive findings, demonstrating that the combination of intraductal RFA and stent placement was associated with longer stent patency and improved survival compared with stenting alone. [
18,
19] A 2021 meta-analysis including 17 studies (of which only 3 were RCTs) substantially confirmed the benefits of the technique. [
20] However, as higher-quality evidence accumulated, the results became less consistent. Two recent European RCTs investigating RFA efficacy in malignant biliary strictures found no significant differences in either survival or stent patency, raising some doubts about the universal effectiveness of the technique. [
21,
22]
To clarify these findings, several groups conducted systematic reviews and meta-analyses of available RCTs. One meta-analysis including 6 RCTs and 439 patients with malignant biliary obstruction of mixed etiology showed that RFA combined with stenting was associated with a moderate survival benefit of 85 days. Subgroup analysis revealed that the survival improvement was limited to patients with distal malignant strictures, while the stent patency benefit was observed in both distal and perihilar strictures. [
23]
The systematic review published by our group in 2024 included 5 RCTs comprising a total of 370 patients with unresectable extrahepatic cholangiocarcinoma. [
24] Results indicated that, compared with stenting alone, RFA plus stent significantly improved stent patency (HR 0.64; 95% CI 0.45–0.90; p = 0.01). However, overall survival did not differ significantly between the two groups (HR 0.62; 95% CI 0.36–1.07; p = 0.09), except for a favorable trend in subgroup analysis: in trials using plastic stents, patients treated with RFA demonstrated better survival (HR 0.42; 95% CI 0.22–0.80; p = 0.009) than those with stenting alone. This may be explained by the possibility of performing repeated RFA sessions in studies using plastic stents, suggesting a potential cumulative therapeutic effect. Importantly, the rate of adverse events was not globally increased by RFA (odds ratio 1.21; p = 0.50), indicating that adding ablation does not entail a higher incidence of complications compared with stenting alone.
A more recent meta-analysis by Ramai et al. included 9 RCTs for a total of 750 patients with malignant biliary obstruction. [
25] This broader analysis, which also encompassed obstructions caused by other malignancies—such as pancreatic, ampullary, and gallbladder adenocarcinomas—found no significant difference in 3-month stent patency between RFA + stent and stent alone (RR 1.01; 95% CI 0.92–1.11; I²=4%), indicating comparable proportions of patent stents at 3 months. Interestingly, however, the 6-month survival rate was significantly higher in patients treated with RFA (RR 0.84; 95% CI 0.73–0.96; p = 0.01). Notably, the subgroup analysis by etiology revealed that patients with cholangiocarcinoma experienced the greatest survival benefit, with a mean difference of 4.9 months.
Regarding safety, Ramai et al. confirmed no overall significant differences in major complications between the two approaches, except for acute cholecystitis, which was more frequent in patients undergoing endobiliary RFA (~5% vs. <1% with stenting alone). This increased risk is likely related to ablation near the cystic duct orifice, possibly leading to ischemic/inflammatory damage to the gallbladder. [
26]
Finally, a meta-analysis published by Zhou et al. in 2025 specifically investigated patients with hilar cholangiocarcinoma, including 11 studies and 874 patients. The pooled results showed a significant survival benefit for those treated with RFA plus stenting compared with stenting alone (HR 0.74; 95% CI 0.61–0.89; p = 0.002) and improved stent patency (HR 0.77; 95% CI 0.61–0.97; p = 0.03). No significant differences were observed in overall or procedure-related adverse events (OR 1.48; p > 0.05), including cholangitis, pancreatitis, and liver abscess. Importantly, patients who also received chemotherapy demonstrated markedly better survival outcomes (HR 0.57; 95% CI 0.40–0.81), suggesting a potential synergistic effect between RFA and systemic therapy. These findings reinforce the role of RFA as a safe and effective adjunct in the palliative management of unresectable hilar cholangiocarcinoma, while emphasizing the importance of multimodal treatment strategies. [
27] The results of the published meta-analyses are summarized in
Table 1.
Overall, although the data are not yet conclusive, available evidence on the role of endobiliary RFA in cholangiocarcinoma indicates a potential benefit both in delaying stent re-obstruction and in improving overall survival. These effects may have meaningful clinical implications in the palliative setting, potentially reducing episodes of cholangitis and hospitalizations and allowing more complete administration of planned chemotherapy regimens.
Nevertheless, study results remain inconsistent. The reasons for these discrepancies may lie in differences in study design (inclusion criteria, stent type—plastic vs. SEMS, follow-up duration), patient characteristics (performance status, tumor location and extent—hilar vs. distal—and concomitant chemotherapy), and technical variables (ablation parameters, endoscopic vs. percutaneous approach, number of RFA sessions performed).
It is also important to note that RFA acts primarily as a complementary therapy: its positive impact may be most evident when integrated into a broader strategy that includes systemic treatment. Some data suggest that combining endobiliary RFA with chemotherapy improves survival compared with chemotherapy alone—at least in locally advanced disease—although such benefits are not confirmed in metastatic settings. [
29]
The recently published EASL guidelines on the management of extrahepatic cholangiocarcinoma confirm that RFA may be considered in combination with stenting to improve patency, although they issue a weak recommendation due to the heterogeneity of available evidence. [
1]
Optimal candidates for endobiliary RFA are patients with unresectable, locally advanced extrahepatic cholangiocarcinoma confined to the biliary tree (no distant metastases) who are eligible for systemic chemotherapy. In metastatic disease with limited extrahepatic spread, RFA may still prolong stent patency, but any survival impact is likely modest. Contraindications include poor performance status (ECOG > 2) or general unfitness for active treatment, and benign biliary strictures. Precautions are warranted when the cystic duct is patent and the target lesion is proximal in the common hepatic duct (risk of acute cholecystitis) and in very long or multifocal strictures, where achieving uniform ablation is challenging. Multidisciplinary assessment is recommended to balance expected benefits with procedural risk. [
1]
In summary, current evidence supports the use of endobiliary RFA as an additional palliative tool in the treatment of unresectable extrahepatic cholangiocarcinoma. However, further RCTs are needed to better define the magnitude of benefit—particularly in terms of survival—and to identify the subgroups of patients most likely to derive maximum advantage.
2.3. TECHNIQUE
Radiofrequency ablation can be performed either endoscopically or percutaneously. In the endoscopic approach, the radiofrequency application is performed during endoscopic retrograde cholangiopancreatography (ERCP). In patients with a native papilla, sphincterotomy is preferable. Prophylactic antibiotic therapy is also suggested. [
30]
A cholangiogram is required to properly visualize and measure both the length and diameter of the stricture before advancing the guidewire with the ablation catheter. Pneumatic dilation of the stricture may also be necessary before performing the ablation.
Currently, two probes are commercially available for endobiliary RFA: the Habib™ EndoHPB-RF (Boston Scientific, Marlborough, MA, USA) and the ELRA™ RF (Taewoong–STARmed, Koyang, South Korea). Both are bipolar catheters designed for intraductal use and are advanced over a guidewire into the bile duct under fluoroscopic guidance, ensuring that the electrode rings are in close contact with the target tissue.
The ELRA™ system, coupled with its dedicated VIVA Combo™ generator, integrates dual safety and control mechanisms: a temperature sensing system, which maintains a consistent ablation temperature by modulating energy delivery in real time to prevent carbonization, and an impedance monitoring system, which continuously assesses tissue contact and electrical resistance, automatically reducing or interrupting energy output when poor contact or tissue desiccation is detected. These features allow for precise, reproducible ablation zones and reduce the risk of excessive thermal spread.
In contrast, the Habib™ EndoHPB catheter does not include active temperature or impedance feedback and is typically powered by third-party generators (e.g., ERBE or RITA systems). Energy delivery is therefore controlled by preset power and time parameters, without real-time adjustment based on tissue response. While technically simpler and widely available, this configuration may result in less consistent energy distribution and potentially higher variability in ablation depth compared with temperature-controlled systems.
Energy and application time are variable (7–10 W, 60–120 seconds) and have yet to be definitively standardized. Excessive energy output or prolonged application should be avoided to limit thermal injury beyond the target area. The number of radiofrequency applications varies according to stricture length; when the stenosis exceeds 15 mm, using a probe with a longer electrode or performing multiple overlapping applications is recommended to achieve uniform ablation along the entire lesion. Treatment should proceed from the proximal to the distal end of the stricture, minimizing overlap to reduce the risk of complications. A one-minute interval between applications is recommended to prevent treated tissue from adhering to the probe.
After probe removal, a balloon catheter is used to clear necrotic debris, and a follow-up cholangiogram is performed to rule out complications. Finally, a biliary stent is placed to ensure adequate drainage. The use of plastic stents is indicated when periodic RFA sessions are planned, whereas self-expandable metal stents (SEMS) are preferred when a single RFA treatment is performed during biliary drainage.
To maximize procedural efficacy and minimize complications, the probe electrodes must be correctly positioned within the malignant stricture. In some cases, cholangiographic assessment of the stricture may be insufficient. In such cases, cholangioscopy can be performed to obtain a direct view of the lesion before positioning the electrodes under direct visualization. Cholangioscopy can also be repeated after the procedure to verify the absence of immediate complications such as bleeding or perforation. [
31]
Post-procedure, patients should be observed for signs of cholecystitis—particularly when ablation is performed near the cystic duct confluence—and, in cases with plastic stents, repeat endobiliary RFA may be considered every 3 ± 1 months or according to clinical judgment, while maintaining routine oncologic follow-up.
Table 2.
Technical features of the two commercially available endobiliary RFA systems.
Table 2.
Technical features of the two commercially available endobiliary RFA systems.
| Feature |
STARmed (Korea) |
Boston Scientific (USA) |
| Main brand |
ELRA™ Electrode |
Habib™ EndoHPB |
| Polarity |
Bipolar |
Bipolar |
| Electrode size |
11, 18, 22, 33 mm(11, 22 mm = 2-ring type; 18, 33 mm = 4-ring type) |
24 mm(2-ring type) |
| Catheter diameter |
7 Fr (2.31 mm) |
8 Fr (2.6 mm) |
| Radiofrequency generator |
STARmed VIVA Combo (dedicated generator) |
No dedicated generator (commonly ERBE or RITA) |
| Temperature control |
Available (automatic thermal feedback) |
Not available |
| Ablation power |
10 W for 33 mm 7 W for 18 mm |
10 W |
| Ablation duration |
120 s |
90 s |
| If electrode contacts lesion poorly |
Generator automatically stops and alarms poor contact; energy is delivered only with adequate tissue contact |
Generator continues to deliver energy regardless of contact quality |
| Device control and feedback |
Thermal- and impedance-controlled (automatic feedback via VIVA Combo™ generator) |
Power-controlled (no impedance or temperature feedback) |
3. Discussion
Despite its growing adoption, the clinical value of endobiliary RFA in unresectable extrahepatic cholangiocarcinoma remains uncertain. While early studies and meta-analyses suggested that RFA combined with stenting might prolong stent patency and potentially improve survival, [
16,
17,
18,
19,
20,
23,
24,
25] more recent randomized trials have yielded inconsistent results, with some showing no significant advantage compared with stenting alone. [
21,
22] These discrepancies may reflect heterogeneity in study design, patient selection, and procedural parameters such as energy delivery, duration of application, and stent type.
The type of malignancy could also influence outcomes, as the effect of RFA may differ among cholangiocarcinoma, pancreatic, and ampullary adenocarcinomas. Similarly, the choice of stent may play a role: plastic stents could allow repeated ablation sessions, helping to maintain ductal patency during chemotherapy, whereas self-expandable metal stents generally limit treatment to a single session. Consequently, RFA might be most beneficial in selected patients—those with good performance status, limited disease extent, and receiving systemic therapy—although this remains to be demonstrated.
Importantly, the interaction between RFA and chemotherapy has not been adequately investigated. The two modalities could act synergistically, but the lack of prospective studies designed to assess their combined effect prevents firm conclusions.
The immunologic impact of RFA also remains hypothetical. Experimental models suggest that necrosis may induce the release of tumor antigens and promote immune activation, but clinical confirmation is still lacking. Likewise, although RFA is generally well tolerated, safety concerns persist—particularly the potential for acute cholecystitis when ablating near the cystic duct—and the absence of standardized procedural settings complicates reproducibility.
Overall, RFA plus stenting appears to have comparable rates of major adverse events to stenting alone in randomized and pooled analyses, with cholangitis and pancreatitis occurring at similar frequencies; however, acute cholecystitis may be more frequent after ablation near the cystic duct orifice. Severe events (e.g., bleeding, perforation, liver abscess) are uncommon but reported. Recent meta-analyses and RCTs did not show a global increase in adverse events with RFA, although a higher risk of cholecystitis has been noted in some series. Careful electrode positioning and avoidance of prolonged/high-energy applications near the cystic duct are advisable. [
21,
22,
25,
26,
27]
5. Conclusions
Endobiliary RFA may offer an additional palliative option for patients with unresectable extrahepatic cholangiocarcinoma. By improving local tumor control and maintaining stent patency, RFA could help preserve chemotherapy continuity and quality of life. Although current evidence remains inconclusive, recent data and guideline recommendations support its use in selected patients with locally advanced disease eligible for systemic therapy. RFA should be regarded as a potential adjunct within a multimodal approach rather than a stand-alone treatment. Further randomized studies are needed to refine patient selection and standardize procedural protocols.
Author Contributions
Conceptualization, M.Mo. and D.B.; methodology, A.S.; software, M.Mo.; validation, A.B., L.M., M.Ma., G.T., M.V., E.P. and M.E.A.; resources, M.Mo. and D.B.; data curation, M.Mo. and D.B.; writing—original draft preparation, M.Mo., F.M, and D.B.; writing—review and editing, A.B., L.M., M.Ma., G.T., M.V., E.P., A.S. and M.E.A.; visualization, M.Mo. and D.B.; supervision, L.M.; project administration, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript: CI: Confidence interval; EASL: European Association for the Study of the Liver; ECOG: Eastern Cooperative Oncology Group (performance status); ELRA™: Endobiliary RFA electrode (STARmed/Taewoong); ERCP: Endoscopic retrograde cholangiopancreatography; Fr: French (catheter size); HR: Hazard ratio; I²: Inconsistency (heterogeneity) index; kHz: Kilohertz; MD: Mean difference; OR: Odds ratio; RFA: Radiofrequency ablation; RCT: Randomized controlled trial; RR: Risk ratio (relative risk); SEMS: Self-expandable metal stent(s); VIVA Combo: STARmed radiofrequency generator; WMD: Weighted mean difference.
References
- Marzioni, M.; Maroni, L.; Aabakken, L.; Carpino, G.; Koerkamp, B.G.; Heimbach, J.; Khan, S.; Lamarca, A.; Saborowski, A.; Vilgrain, V.; et al. EASL Clinical Practice Guidelines on the Management of Extrahepatic Cholangiocarcinoma. J. Hepatol. 2025, 83, 211–238. [CrossRef]
- Shah, T.; Desai, S.; Haque, M.; Dakik, H.; Fisher, D. Management of Occluded Metal Stents in Malignant Biliary Obstruction: Similar Outcomes with Second Metal Stents Compared to Plastic Stents. Dig. Dis. Sci. 2012, 57, 2765–2773. [CrossRef]
- Inoue, T.; Yoneda, M. Recent Updates on Local Ablative Therapy Combined with Chemotherapy for Extrahepatic Cholangiocarcinoma: Photodynamic Therapy and Radiofrequency Ablation. Curr. Oncol. 2023, 30, 2159–2168. [CrossRef]
- John, E.S.; Tarnasky, P.R.; Kedia, P. Ablative Therapies of the Biliary Tree. Transl. Gastroenterol. Hepatol. 2021, 6, 63. [CrossRef]
- Gazelle, G.S.; Goldberg, S.N.; Solbiati, L.; Livraghi, T. Tumor Ablation with Radio-Frequency Energy. Radiology 2000, 217, 633–646. [CrossRef]
- Goldberg, S.N. Radiofrequency Tumor Ablation: Principles and Techniques. Eur. J. Ultrasound Off. J. Eur. Fed. Soc. Ultrasound Med. Biol. 2001, 13, 129–147. [CrossRef]
- Goldberg, S.N.; Gazelle, G.S. Radiofrequency Tissue Ablation: Physical Principles and Techniques for Increasing Coagulation Necrosis. Hepatogastroenterology. 2001, 48, 359–367.
- van den Bijgaart, R.J.E.; Eikelenboom, D.C.; Hoogenboom, M.; Fütterer, J.J.; den Brok, M.H.; Adema, G.J. Thermal and Mechanical High-Intensity Focused Ultrasound: Perspectives on Tumor Ablation, Immune Effects and Combination Strategies. Cancer Immunol. Immunother. CII 2017, 66, 247–258. [CrossRef]
- Itoi, T.; Isayama, H.; Sofuni, A.; Itokawa, F.; Tamura, M.; Watanabe, Y.; Moriyasu, F.; Kahaleh, M.; Habib, N.; Nagao, T.; et al. Evaluation of Effects of a Novel Endoscopically Applied Radiofrequency Ablation Biliary Catheter Using an Ex-Vivo Pig Liver. J. Hepato-Biliary-Pancreat. Sci. 2012, 19, 543–547. [CrossRef]
- Zacharoulis, D.; Lazoura, O.; Sioka, E.; Potamianos, S.; Tzovaras, G.; Nicholls, J.; Koukoulis, G.; Habib, N. Habib EndoHPB: A Novel Endobiliary Radiofrequency Ablation Device. An Experimental Study. J. Investig. Surg. Off. J. Acad. Surg. Res. 2013, 26, 6–10. [CrossRef]
- Atar, M.; Kadayifci, A.; Daglilar, E.; Hagen, C.; Fernandez-Del Castillo, C.; Brugge, W.R. Ex Vivo Human Bile Duct Radiofrequency Ablation with a Bipolar Catheter. Surg. Endosc. 2018, 32, 2808–2813. [CrossRef]
- Dromi, S.A.; Walsh, M.P.; Herby, S.; Traughber, B.; Xie, J.; Sharma, K.V.; Sekhar, K.P.; Luk, A.; Liewehr, D.J.; Dreher, M.R.; et al. Radiofrequency Ablation Induces Antigen-Presenting Cell Infiltration and Amplification of Weak Tumor-Induced Immunity. Radiology 2009, 251, 58–66. [CrossRef]
- Jarosova, J.; Macinga, P.; Hujova, A.; Kral, J.; Urban, O.; Spicak, J.; Hucl, T. Endoscopic Radiofrequency Ablation for Malignant Biliary Obstruction. World J. Gastrointest. Oncol. 2021, 13, 1383–1396. [CrossRef]
- Jarosova, J.; Macinga, P.; Krupickova, L.; Fialova, M.; Hujova, A.; Mares, J.; Urban, O.; Hajer, J.; Spicak, J.; Striz, I.; et al. Impact of Endoluminal Radiofrequency Ablation on Immunity in Pancreatic Cancer and Cholangiocarcinoma. Biomedicines 2022, 10, 1331. [CrossRef]
- Steel, A.W.; Postgate, A.J.; Khorsandi, S.; Nicholls, J.; Jiao, L.; Vlavianos, P.; Habib, N.; Westaby, D. Endoscopically Applied Radiofrequency Ablation Appears to Be Safe in the Treatment of Malignant Biliary Obstruction. Gastrointest. Endosc. 2011, 73, 149–153. [CrossRef]
- Sharaiha, R.Z.; Natov, N.; Glockenberg, K.S.; Widmer, J.; Gaidhane, M.; Kahaleh, M. Comparison of Metal Stenting with Radiofrequency Ablation versus Stenting Alone for Treating Malignant Biliary Strictures: Is There an Added Benefit? Dig. Dis. Sci. 2014, 59, 3099–3102. [CrossRef]
- Kallis, Y.; Phillips, N.; Steel, A.; Kaltsidis, H.; Vlavianos, P.; Habib, N.; Westaby, D. Analysis of Endoscopic Radiofrequency Ablation of Biliary Malignant Strictures in Pancreatic Cancer Suggests Potential Survival Benefit. Dig. Dis. Sci. 2015, 60, 3449–3455. [CrossRef]
- Yang, J.; Wang, J.; Zhou, H.; Zhou, Y.; Wang, Y.; Jin, H.; Lou, Q.; Zhang, X. Efficacy and Safety of Endoscopic Radiofrequency Ablation for Unresectable Extrahepatic Cholangiocarcinoma: A Randomized Trial. Endoscopy 2018, 50, 751–760. [CrossRef]
- Gao, D.-J.; Yang, J.-F.; Ma, S.-R.; Wu, J.; Wang, T.-T.; Jin, H.-B.; Xia, M.-X.; Zhang, Y.-C.; Shen, H.-Z.; Ye, X.; et al. Endoscopic Radiofrequency Ablation plus Plastic Stent Placement versus Stent Placement Alone for Unresectable Extrahepatic Biliary Cancer: A Multicenter Randomized Controlled Trial. Gastrointest. Endosc. 2021, 94, 91-100.e2. [CrossRef]
- Tarar, Z.I.; Farooq, U.; Gandhi, M.; Ghous, G.; Saleem, S.; Kamal, F.; Imam, Z.; Jamil, L. Effect of Radiofrequency Ablation in Addition to Biliary Stent on Overall Survival and Stent Patency in Malignant Biliary Obstruction: An Updated Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 646–653. [CrossRef]
- Jarosova, J.; Zarivnijova, L.; Cibulkova, I.; Mares, J.; Macinga, P.; Hujova, A.; Falt, P.; Urban, O.; Hajer, J.; Spicak, J.; et al. Endoluminal Radiofrequency Ablation in Patients with Malignant Biliary Obstruction: A Randomised Trial. Gut 2023, 72, 2286–2293. [CrossRef]
- Albers, D.; Schmidt, A.; Schiemer, M.; Caca, K.; Wannhoff, A.; Sauer, P.; Wiesweg, M.; Schumacher, B.; Dechene, A. Impact of Endobiliary Radiofrequency Ablation on Biliary Drainage in Patients with Malignant Biliary Strictures Treated with Uncovered Self-Expandable Metal Stents: A Randomized Controlled Multicenter Trial. Gastrointest. Endosc. 2022, 96, 970–979. [CrossRef]
- de Oliveira Veras, M.; de Moura, D.T.H.; McCarty, T.R.; de Oliveira, G.H.P.; Gomes, R.S.A.; Landim, D.L.; Nunes, F.G.; Franzini, T.A.P.; Lera Dos Santos, M.E.; Bernardo, W.M.; et al. Intraductal Radiofrequency Ablation plus Biliary Stent versus Stent Alone for Malignant Biliary Obstruction: A Systematic Review and Meta-Analysis. Endosc. Int. Open 2024, 12, E23–E33. [CrossRef]
- Balducci, D.; Montori, M.; Martini, F.; Valvano, M.; De Blasio, F.; Argenziano, M.E.; Tarantino, G.; Benedetti, A.; Bendia, E.; Marzioni, M.; et al. The Impact of Radiofrequency Ablation on Survival Outcomes and Stent Patency in Patients with Unresectable Cholangiocarcinoma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers 2024, 16, 1372. [CrossRef]
- Ramai, D.; Maida, M.; Smith, E.R.; Wang, Y.; Spadaccini, M.; Previtera, M.; Chandan, S.; Huang, Y.; Tokmak, S.; Bhandari, P.; et al. Endoluminal Radiofrequency Ablation with Stenting versus Stenting Alone in Patients with Malignant Biliary Obstruction: A Meta-Analysis of Randomized Trials. Endoscopy 2025, 57, 272–281. [CrossRef]
- Inoue, T.; Yoneda, M. Updated Evidence on the Clinical Impact of Endoscopic Radiofrequency Ablation in the Treatment of Malignant Biliary Obstruction. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2022, 34, 345–358. [CrossRef]
- Zhou, H.; Khizar, H.; Wang, J.; Yang, J. Assessing the Impact of Radiofrequency Ablation on Hilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Int. J. Surg. 10.1097/JS9.0000000000003242. [CrossRef]
- de Jong, D.M.; Fritzsche, J.A.; Audhoe, A.S.; Yi, S.S.L.; Bruno, M.J.; Voermans, R.P.; van Driel, L.M.J.W. Comparison of Intraductal RFA Plus Stent versus Stent-Only Treatment for Unresectable Perihilar Cholangiocarcinoma-A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2079. [CrossRef]
- Gonzalez-Carmona, M.A.; Möhring, C.; Mahn, R.; Zhou, T.; Bartels, A.; Sadeghlar, F.; Bolch, M.; Vogt, A.; Kaczmarek, D.J.; Heling, D.J.; et al. Impact of Regular Additional Endobiliary Radiofrequency Ablation on Survival of Patients with Advanced Extrahepatic Cholangiocarcinoma under Systemic Chemotherapy. Sci. Rep. 2022, 12, 1011. [CrossRef]
- Lanza, D.; Casty, A.; Schlosser, S.H. Endobiliary Radiofrequency Ablation for Malignant Biliary Obstruction over 32-Month Follow-Up. Gastrointest. Tumors 2022, 9, 12–18. [CrossRef]
- Ogura, T.; Onda, S.; Sano, T.; Takagi, W.; Okuda, A.; Miyano, A.; Nishioka, N.; Imanishi, M.; Amano, M.; Masuda, D.; et al. Evaluation of the Safety of Endoscopic Radiofrequency Ablation for Malignant Biliary Stricture Using a Digital Peroral Cholangioscope (with Videos). Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 712–717. [CrossRef]
- ELRATM Endobiliary RFA. TaeWoong Med. USA.
- HabibTM EndoHPB Bipolar Radiofrequency Catheter Available online: https://www.bostonscientific.com/en-EU/products/rf-ablation/habib-endohpb-bipolar-radiofrequency-catheter.html (accessed on 10 October 2025).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).