Submitted:
10 October 2025
Posted:
15 October 2025
You are already at the latest version
Abstract
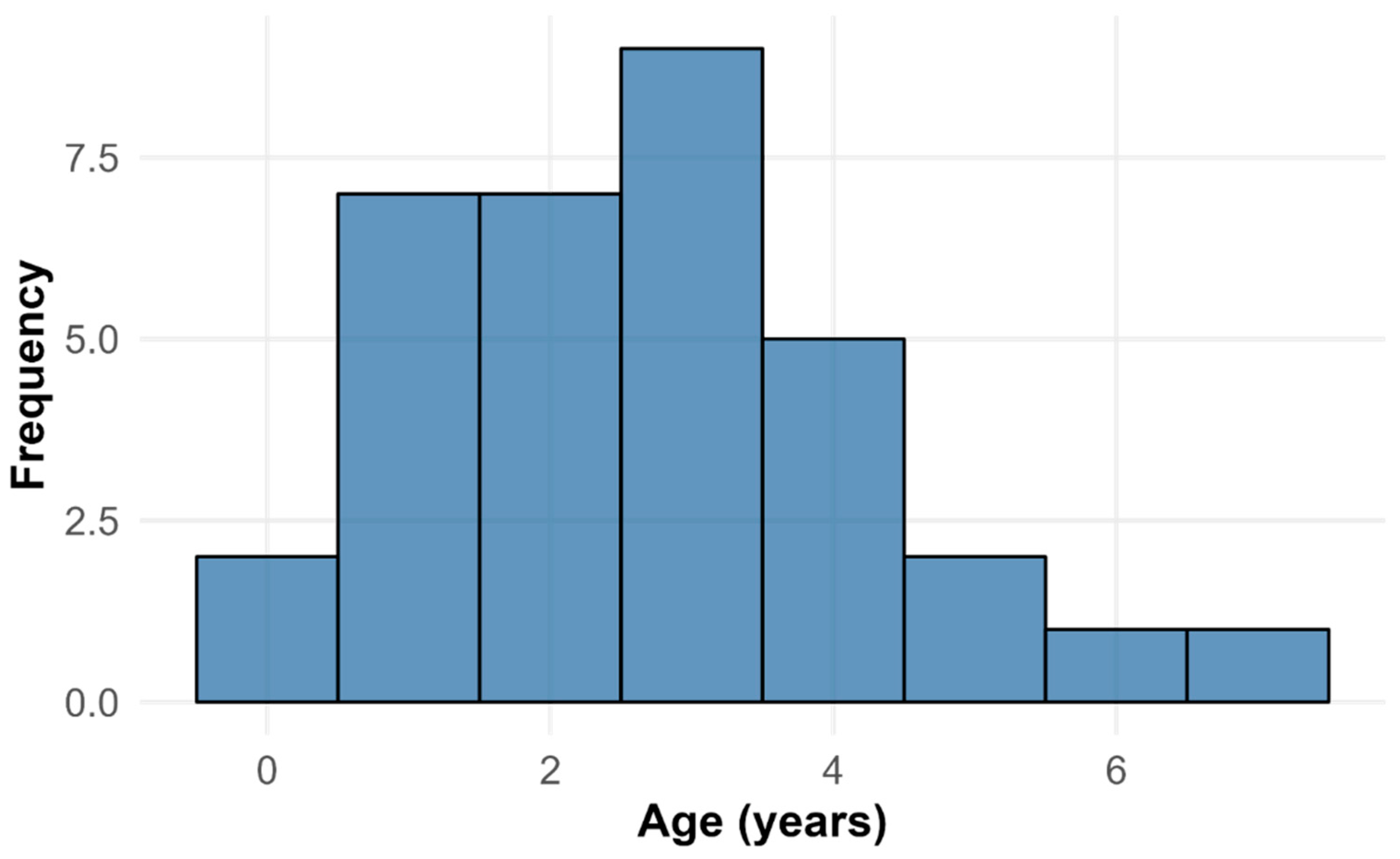
Understanding the drivers of population dynamics in long-lived, K-selected species such as bats is critical for conservation, particularly during vulnerable life-history stages like hibernation. We reviewed winter mortality records from more than 109 monitored hibernacula in Bulgaria. We found that significant die-offs (>30 individuals from at least three species) were recorded at only three sites, suggesting that such events are rare but potentially consequential for local populations. To reveal how habitat use shapes vulnerability during winter, we investigated hibernation preferences of cave-dwelling bats in Bulgaria. We further analysed the age structure of deceased Miniopterus schreibersii (Bonaparte, 1837) from Bulgaria’s largest hibernation colony following mortality events in winter 2022. Carcasses spanned a wide range of age classes, yet younger individuals predominated, consistent with the idea that early-life mortality represents a key demographic filter in bats. These findings emphasise the need for consistent mortality monitoring in bats, using standardised protocols that account for detection biases, scavenger removal, and site-specific variation. Such efforts are essential for clarifying the roles of environmental extremes, disease, and human disturbance in winter mortality.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Hibernacula survey
2.2. Bat sampling
2.3. Laboratory processing and age determination
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Shea, T.J.; Ellison, L.E.; Stanley, T.R. Survival estimation in bats: historical overview, critical appraisal and suggestions for new approaches. In Sampling Rare or Elusive Species: Concepts, Designs, and Techniques for Estimating Population Parameters; Thompson, W.L., Ed.; Island Press: Washington, DC, USA, 2004; pp. 297–336. [Google Scholar]
- Touzalin, F.; Petit, É.; Cam, E.; Stagier, C.; Teeling, E.C.; Puechmaille, S.J. Mark loss can strongly bias estimates of demographic rates in multi-state models: a case study with simulated and empirical datasets. Peer Community Journal 2023, 3, e348. [Google Scholar] [CrossRef]
- Blehert, D.S.; Hicks, A.C.; Behr, M.; Meteyer, C.U.; Berlowski-Zier, B.M.; Buckles, E.L.; et al. Bat white-nose syndrome: an emerging fungal pathogen? Science 2009, 323(5911), 227. [Google Scholar] [CrossRef]
- Fritze, M.; Puechmaille, S.J. Identifying unusual mortality events in bats: a baseline for bat hibernation monitoring and white-nose syndrome research. Mammal Review 2018, 48(3), 224–228. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Cryan, P.M.; Hayman, D.T.S.; Plowright, R.K.; Streicker, D.G. Multiple mortality events in bats: a global review. Mammal Review 2016, 46(3), 175–190. [Google Scholar] [CrossRef] [PubMed]
- Vindenes, Y.; Langangen, Ø. Individual heterogeneity in life histories and eco-evolutionary dynamics. Ecology Letters 2015, 18(5), 417–432. [Google Scholar] [CrossRef]
- Lord, R.D.; Muradali, F.; Lázaro, L. Age composition of vampire bats (Desmodus rotundus) in northern Argentina and southern Brazil. Journal of Mammalogy 1976, 57(3), 573–575. [Google Scholar] [CrossRef]
- Schowalter, D.B.; Harder, L.D.; Treichel, B.H. Age composition of some vespertilionid bats as determined by dental annuli. Canadian Journal of Zoology 1978, 56(2), 355–358. [Google Scholar] [CrossRef]
- Brunet-Rossinni, A.K.; Austad, S.N. Ageing studies on bats: a review. Biogerontology 2004, 5(4), 211–222. [Google Scholar] [CrossRef]
- Toshkova, N.; Kolev, M.; Deleva, S.; Simeonov, T.; Popov, V. Winter is (not) coming: Acoustic monitoring and temperature variation across important bat hibernacula. Biodiversity Data Journal 2025, 13, e141801. [Google Scholar] [CrossRef]
- Dietz, C.; von Helversen, O. Illustrated Identification Key to the Bats of Europe; Vol. 1; Dietz & von Helversen: Tübingen, Germany, 2004; pp. 1–74. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Batulevicius, D.; Paužienė, N.; Pauža, D.H. Dental incremental lines in some small species of the European vespertilionid bats. Acta Theriologica 2001, 46(1), 33–42. [Google Scholar] [CrossRef]
- Brunet-Rossini, A.K.; Wilkinson, G. Methods for estimating age in bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T.H., Parsons, S., Eds.; The Johns Hopkins University Press: Baltimore, USA, 2009; pp. 315–326. [Google Scholar]
- Brunet-Rossinni, A.K.; Austad, S.N. Ageing studies on bats: a review. Biogerontology 2004, 5(4), 211–222. [Google Scholar] [CrossRef]
- Ivanova, T. Important bat underground habitats (IBuH) in Bulgaria. Acta Zoologica Bulgarica 2005, 57(2), 197–206. [Google Scholar]
- Deleva, S.; Toshkova, N.; Kolev, M.; Tanalgo, K.C. Important underground roosts for bats in Bulgaria: current state and priorities for conservation. Biodiversity Data Journal 2023, 11, e98734. [Google Scholar] [CrossRef]
- Ivanova, T.; Petrov, B. A new stage in bat (Chiroptera) research in Bulgaria. Historia naturalis bulgarica 2001, 13, 88, [In Bulgarian]. [Google Scholar]
- Fleischer, T.; Gampe, J.; Scheuerlein, A.; Kerth, G. Rare catastrophic events drive population dynamics in a bat species with negligible senescence. Scientific Reports 2017, 7(1), 6298. [Google Scholar] [CrossRef]
- López-Roig, M.; Piera, E.; Serra-Cobo, J. Thinner bats to face hibernation as response to climate warming. Scientific Reports 2024, 14(1), 1024. [Google Scholar] [CrossRef]
- Bernard, R.F.; Willcox, E.V.; Jackson, R.T.; Brown, V.A.; McCracken, G.F. Feasting, not fasting: winter diets of cave hibernating bats in the United States. Frontiers in Zoology 2021, 18(1), 43. [Google Scholar] [CrossRef] [PubMed]
- Toshkova, N.; Dimitrova, K.; Langourov, M.; Zlatkov, B.; Bekchiev, R.; Ljubomirov, T.; et al. Snacking during hibernation? Winter bat diet and prey availabilities, a case study from Iskar Gorge, Bulgaria. Historia naturalis bulgarica 2023, 45, 125–142. [Google Scholar] [CrossRef]
- Constant, T.; Giroud, S.; Viblanc, V.A.; Tissier, M.L.; Bergeron, P.; Dobson, F.S.; et al. Integrating mortality risk and the adaptiveness of hibernation. Frontiers in Physiology 2020, 11, 706. [Google Scholar] [CrossRef]
- Theimer, T.C.; Dyer, A.C.; Keeley, B.W.; Gilbert, A.T.; Bergman, D.L. Ecological potential for rabies virus transmission via scavenging of dead bats by mesocarnivores. Journal of Wildlife Diseases 2017, 53(2), 382–385. [Google Scholar] [CrossRef]
- Vicente, J.; VerCauteren, K.C. The role of scavenging in disease dynamics. In Wildlife Research Monographs; Springer: Cham, Switzerland, 2019; pp. 161–182. [Google Scholar] [CrossRef]
- Mühldorfer, K.; Speck, S.; Wibbelt, G. Diseases in free-ranging bats from Germany. BMC Veterinary Research 2011, 7(1), 61. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, P. Wildlife Necropsy and Forensics; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Colombino, E.; Lelli, D.; Canziani, S.; Quaranta, G.; Guidetti, C.; Leopardi, S.; et al. Main causes of death of free-ranging bats in Turin province (north-western Italy): gross and histological findings and emergent virus surveillance. BMC Veterinary Research 2023, 19(1), 34. [Google Scholar] [CrossRef]
- Gol’din, P.; Godlevska, L.; Ghazali, M. Age-related changes in the teeth of two bat species: dental wear, pulp cavity and dentine growth layers. Acta Chiropterologica 2019, 20(2), 519–526. [Google Scholar] [CrossRef]
- Phillips, C.J.; Steinberg, B.M.; Kunz, T.H. Dentin, cementum, and age determination in bats: a critical evaluation. Journal of Mammalogy 1982, 63(2), 197–207. [Google Scholar] [CrossRef]
- Baagøe, H.J. Age determination in bats (Chiroptera). Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening 1977, 140, 53–92. [Google Scholar]
- Cool, S.M.; Bennet, M.; Romaniuk, K. Age estimation of pteropodid bats (Megachiroptera) from hard tissue parameters. Wildlife Research 1994, 21(3), 353–361. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Adams, D.M.; Haghani, A.; Lu, A.T.; Zoller, J.A.; Breeze, C.E.; et al. DNA methylation predicts age and provides insight into exceptional longevity of bats. Nature Communications 2021, 12(1), 1615. [Google Scholar] [CrossRef]
- Adams, D.M.; Rayner, J.G.; Hex, S.B.S.W.; Wilkinson, G.S. DNA methylation dynamics reflect sex and status differences in mortality rates in a polygynous bat. Molecular Ecology 2025, 34(9), 2331–2346. [Google Scholar] [CrossRef]
- Sendor, T.; Simon, M. Population dynamics of the pipistrelle bat: effects of sex, age and winter weather on seasonal survival. Journal of Animal Ecology 2003, 72(2), 308–320. [Google Scholar] [CrossRef]
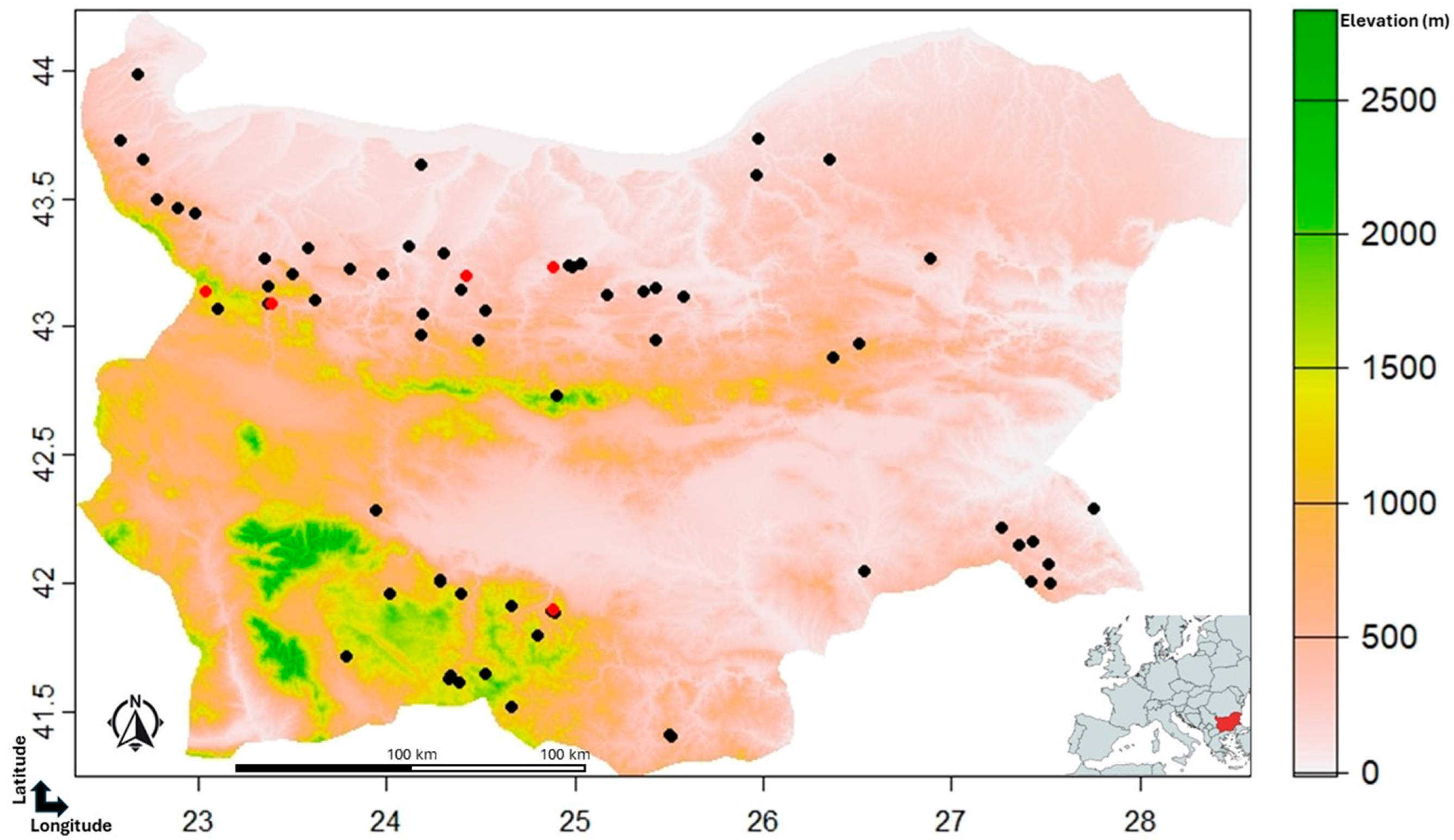
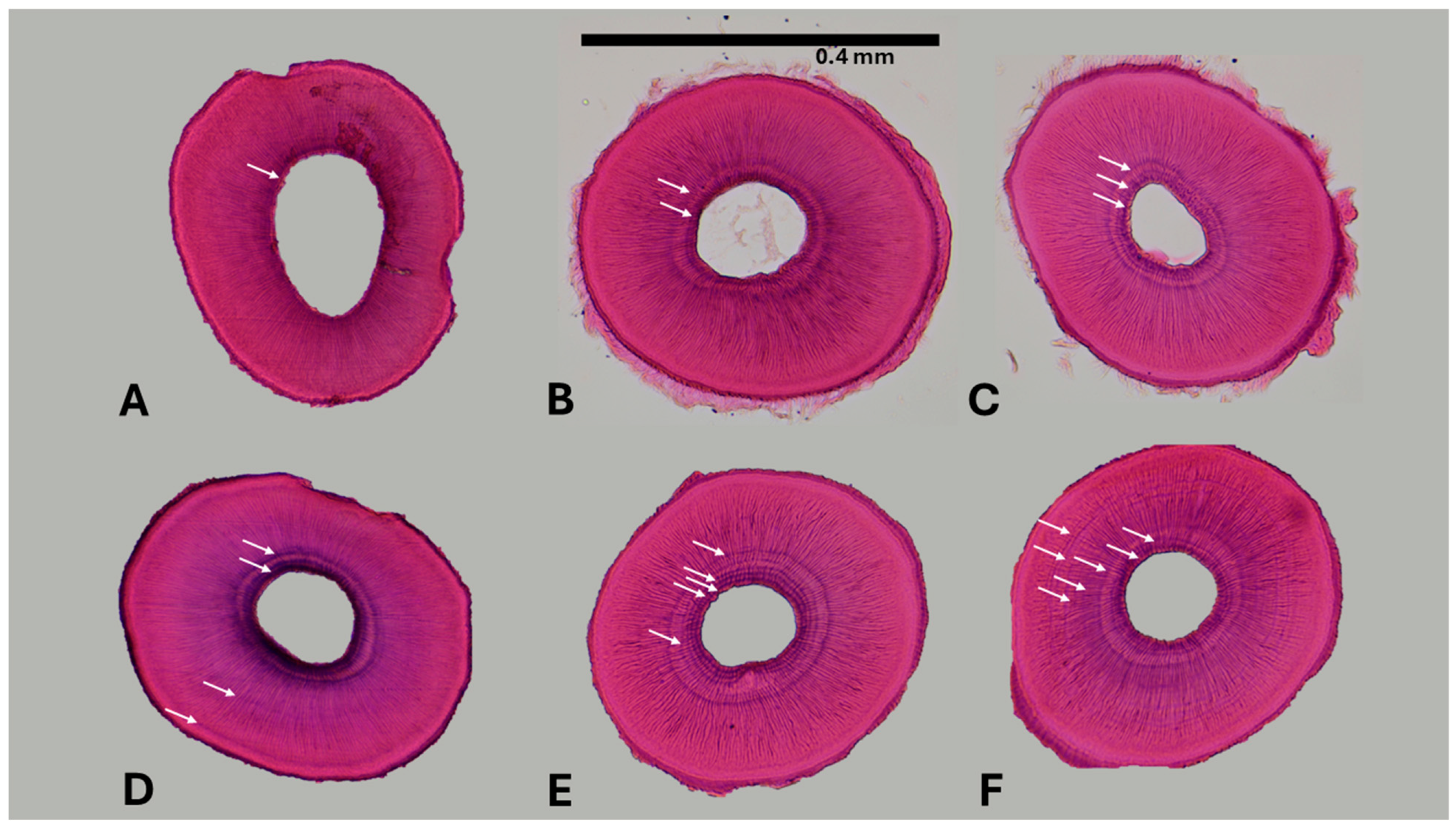
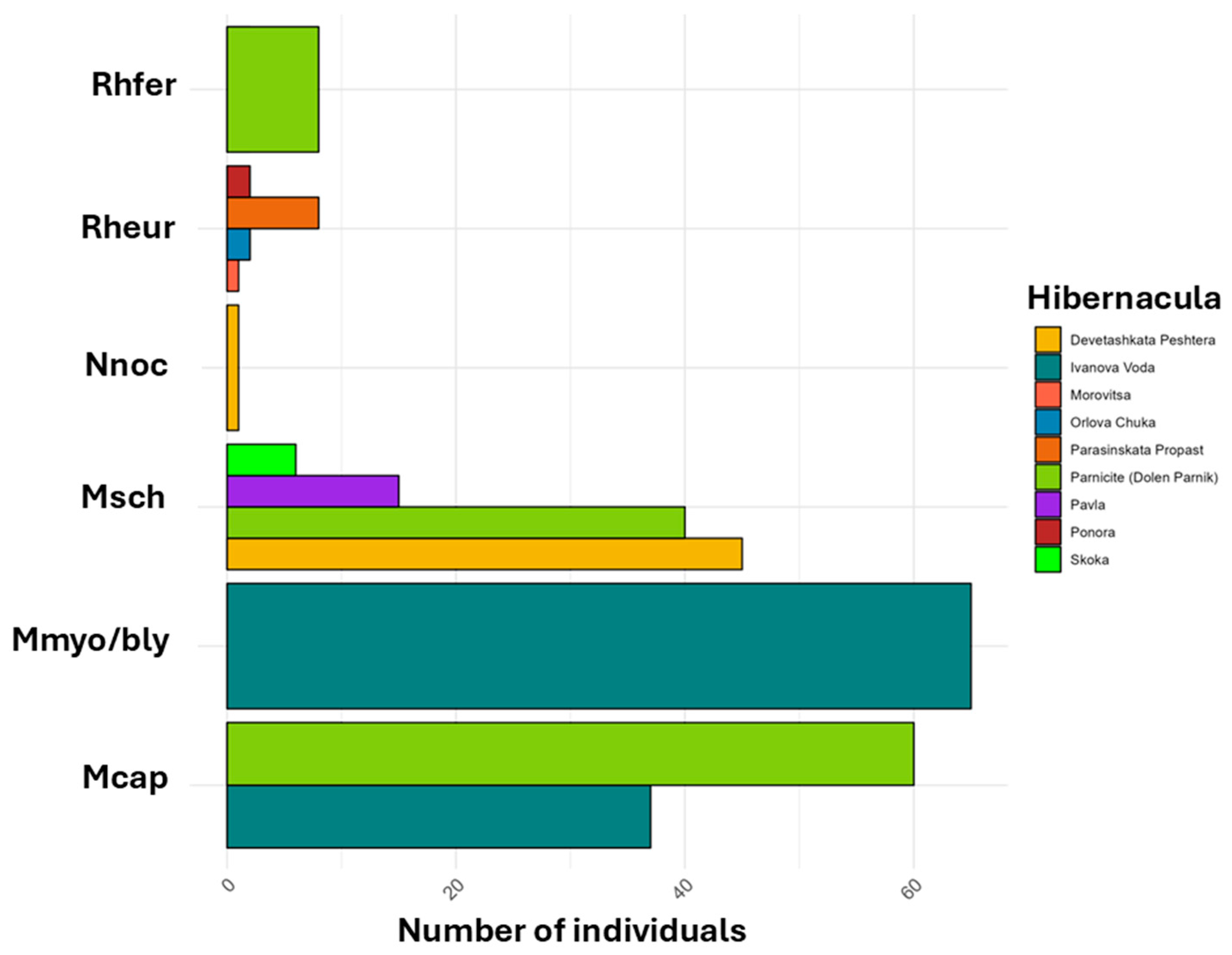
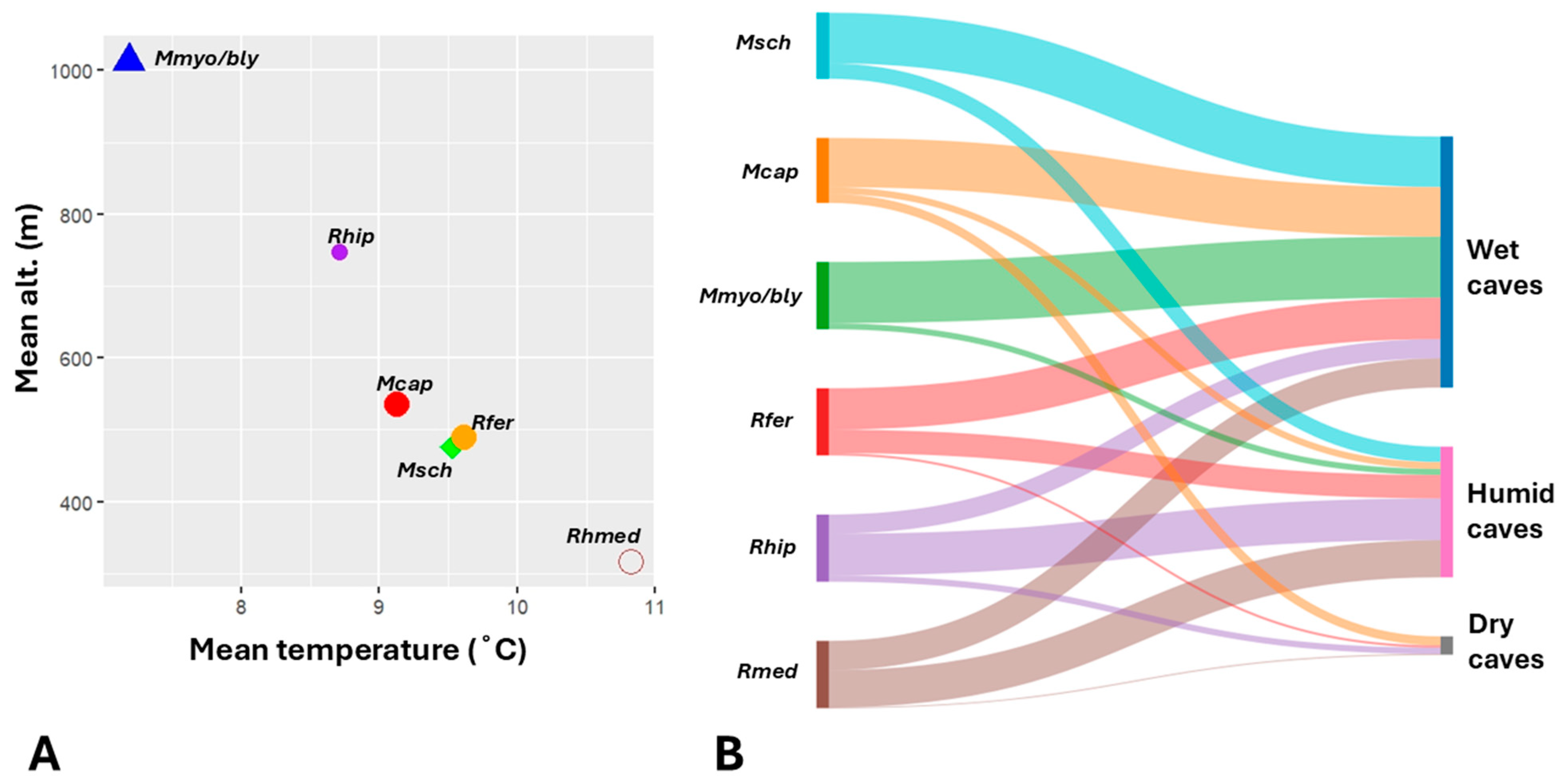
| Site | Total visits | Years covered | Notes (e.g. multiple visits per year) |
|---|---|---|---|
| Devetashka | 14 | 1997–2024 | Several years with >1 visit |
| Parnicite (Dolen parnik) | 14 | 1996–2023 | Several years with >1 visit |
| Ivanova Voda | 6 | 1997–2022 | Surveyed every 2–3 years |
| Orlova Chuka | 12 | 1991–2022 | Several years with >1 visit |
| Pavla (Ravnogorskata Peshtera) | 4 | 2008–2014 | Occasional surveys |
| Skoka | 2 | 2008–2016 | Occasional surveys |
| Ponora | 7 | 2012–2022 | Surveyed every 2–3 years |
| Site | Species | Year | n |
|---|---|---|---|
| Devetashka Cave | Miniopterus schreibersii | 2012 | 75 |
| Ivanova Voda | Myotis capaccinii | 2012 | 37 |
| Ivanova Voda | Myotis myotis/blythii | 2012 | 65 |
| Parnicite Cave | Miniopterus schreibersii | 2012 | 15 |
| Parnicite Cave | Miniopterus schreibersii | 2022 | 40 |
| Pavla | Miniopterus schreibersii | 2014 | 2 |
| Skoka Cave | Miniopterus schreibersii | 2012 | 6 |
| Parasinskata Propast | Rhinolophus euryale | 2012 | 8 |
| Ponora | Rhinolophus euryale | 2012 | 6 |
| Orlova Chuka | Rhinolophus euryale | 2021-2022 | 5 |
| Morovitsa | Rhinolophus euryale | 2023 | 1 |
| Parnicite Cave | Rhinolophus ferrumequinum | 2012 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





