Submitted:
09 October 2025
Posted:
09 October 2025
You are already at the latest version
Abstract
Keywords:
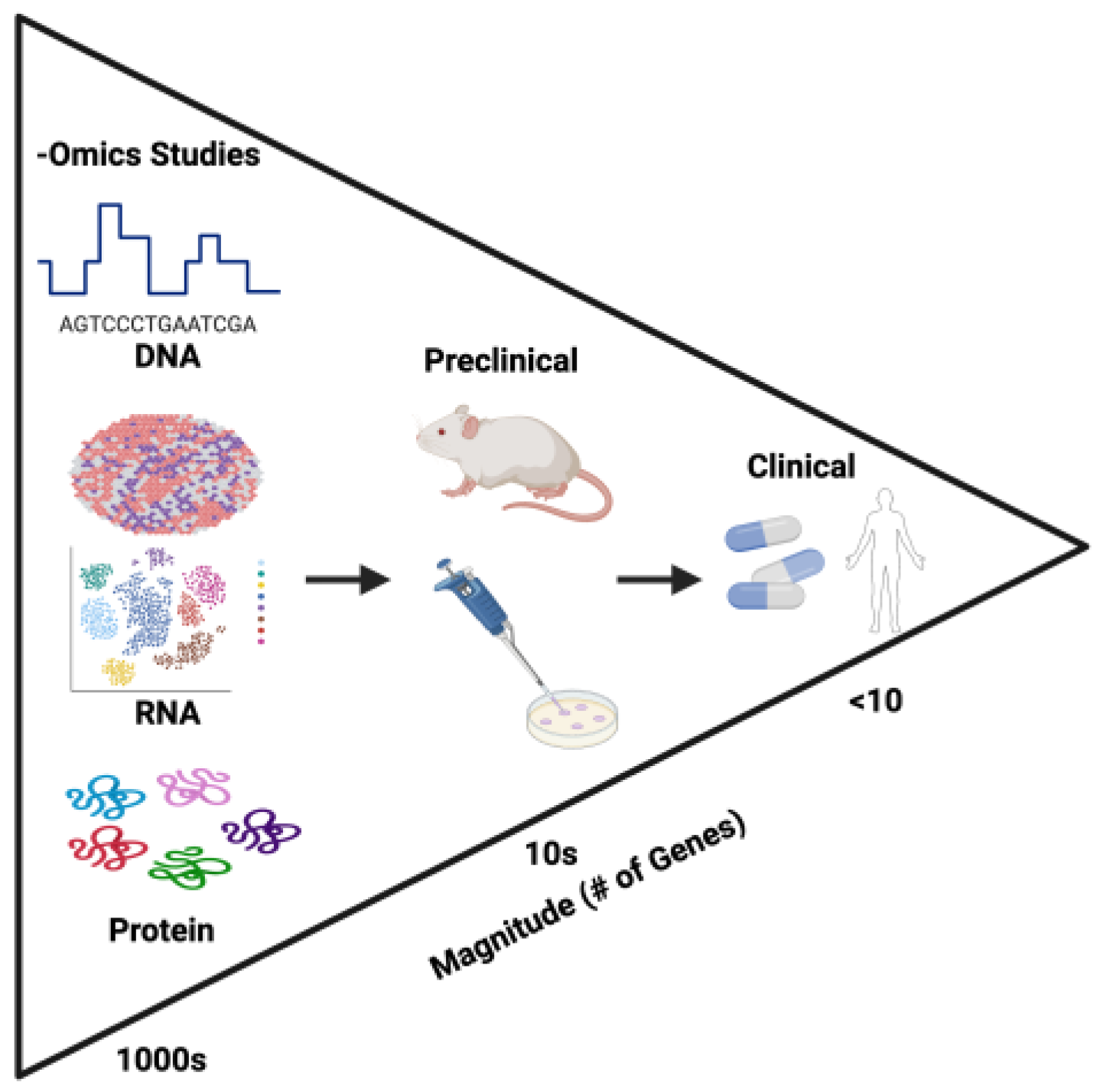
1. Introduction
2. Multiomic Approaches to Cancer Research
3. Multiomic Approaches in Triple Negative Breast Cancer
3.1. Triple Negative Breast Cancer Molecular Subtypes Based on Gene Set Enrichment Analysis
| Subtype | Gene Findings | Citation |
| BL1 | Upregulated DNA/RNA synthesis, cell division, and nuclear export | [35] |
| BL2 | Upregulated extracellular matrix, collagen, cell junction, and cell membrane components | [35] |
| M | Lowly express PD-L1, making immunotherapy less effective | [36] |
| LAR | PRC-2, enhances chemotherapy response Genetic dependency on CCND1 GPX4, can be inhibited to cause ferroptosis Activating mutation in PIK3CA |
[33,34,36,37] |
3.2. Differentially Expressed Gene Analysis of Triple Negative Breast Cancer
3.2.1. Immune-Related
3.2.2. Epithelial Cells
3.3. Additional Applications of -Omics in TNBC
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br J Radiol 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. The Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Nassar, S.F.; Raddassi, K.; Wu, T. Single-Cell Multiomics Analysis for Drug Discovery. Metabolites 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Papalexi, E.; Satija, R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol 2018, 18, 35–45. [Google Scholar] [CrossRef]
- Ziegenhain, C.; Vieth, B.; Parekh, S.; Reinius, B.; Guillaumet-Adkins, A.; Smets, M.; Leonhardt, H.; Heyn, H.; Hellmann, I.; Enard, W. Comparative Analysis of Single-Cell RNA Sequencing Methods. Mol Cell 2017, 65, 631–643.e634. [Google Scholar] [CrossRef]
- Zheng, B.; Fang, L. Spatially resolved transcriptomics provide a new method for cancer research. J Exp Clin Cancer Res 2022, 41, 179. [Google Scholar] [CrossRef]
- Xiao, X.; Juan, C.; Drennon, T.; Uytingco, C.R.; Vishlaghi, N.; Sokolowskei, D.; Xu, L.; Levi, B.; Sammarco, M.C.; Tower, R.J. Spatial transcriptomic interrogation of the murine bone marrow signaling landscape. Bone Research 2023, 11, 59. [Google Scholar] [CrossRef]
- Viode, A.; van Zalm, P.; Smolen, K.K.; Fatou, B.; Stevenson, D.; Jha, M.; Levy, O.; Steen, J.; Steen, H. A simple, time- and cost-effective, high-throughput depletion strategy for deep plasma proteomics. Sci Adv 2023, 9, eadf9717. [Google Scholar] [CrossRef]
- Nurchis, M.C.; Radio, F.C.; Salmasi, L.; Heidar Alizadeh, A.; Raspolini, G.M.; Altamura, G.; Tartaglia, M.; Dallapiccola, B.; Pizzo, E.; Gianino, M.M.; et al. Cost-Effectiveness of Whole-Genome vs Whole-Exome Sequencing Among Children With Suspected Genetic Disorders. JAMA Netw Open 2024, 7, e2353514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Peng, M.; Tang, L.; Ouyang, J.; Xiong, F.; Guo, C.; Tang, Y.; Zhou, Y.; Liao, Q.; et al. Single-cell RNA sequencing in cancer research. J Exp Clin Cancer Res 2021, 40, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Taramelli, R.; Pedrini, E.; Knijnenburg, T.; Huang, S. Extracting Intercellular Signaling Network of Cancer Tissues using Ligand-Receptor Expression Patterns from Whole-tumor and Single-cell Transcriptomes. Scientific Reports 2017, 7, 8815. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Tao, Y.; Chen, G.; Shi, T. Systematic expression analysis of ligand-receptor pairs reveals important cell-to-cell interactions inside glioma. Cell Communication and Signaling 2019, 17, 48. [Google Scholar] [CrossRef]
- Hosein, A.N.; Huang, H.; Wang, Z.; Parmar, K.; Du, W.; Huang, J.; Maitra, A.; Olson, E.; Verma, U.; Brekken, R.A. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Jankovic, V.; Golubov, J.; Poon, P.; Oswald, E.M.; Gurer, C.; Wei, J.; Ramos, I.; Wu, Q.; et al. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8(+) T cell dysfunction and maintain memory phenotype. Sci Immunol 2018, 3. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.-C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biology 2016, 17, 174. [Google Scholar] [CrossRef]
- Calbo, J.; van Montfort, E.; Proost, N.; van Drunen, E.; Beverloo, H.B.; Meuwissen, R.; Berns, A. A Functional Role for Tumor Cell Heterogeneity in a Mouse Model of Small Cell Lung Cancer. Cancer Cell 2011, 19, 244–256. [Google Scholar] [CrossRef]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bartonicek, N.; et al. A single-cell and spatially resolved atlas of human breast cancers. Nat Genet 2021, 53, 1334–1347. [Google Scholar] [CrossRef]
- Vickovic, S.; Eraslan, G.; Salmén, F.; Klughammer, J.; Stenbeck, L.; Schapiro, D.; Äijö, T.; Bonneau, R.; Bergenstråhle, L.; Navarro, J.F.; et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat Methods 2019, 16, 987–990. [Google Scholar] [CrossRef]
- Andersson, A.; Larsson, L.; Stenbeck, L.; Salmén, F.; Ehinger, A.; Wu, S.; Al-Eryani, G.; Roden, D.; Swarbrick, A.; Borg, Å. Spatial deconvolution of HER2-positive breast tumors reveals novel intercellular relationships. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yoosuf, N.; Navarro, J.F.; Salmén, F.; Ståhl, P.L.; Daub, C.O. Identification and transfer of spatial transcriptomics signatures for cancer diagnosis. Breast Cancer Res 2020, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sahoo, S.; Brien, R.; Jung, S.; Humphries, B.; Lee, W.; Cheng, Y.H.; Zhang, Z.; Luker, K.E.; Wicha, M.S.; et al. Single-cell RNA-sequencing of migratory breast cancer cells: Discovering genes associated with cancer metastasis. Analyst 2019, 144, 7296–7309. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. 2), S26–S35. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J Clin Oncol 2023, 41, 4192–4199. [Google Scholar] [CrossRef]
- Abramson, V.G.; Lehmann, B.D.; Ballinger, T.J.; Pietenpol, J.A. Subtyping of triple-negative breast cancer: Implications for therapy. Cancer 2015, 121, 8–16. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 2015, 24 (Suppl. 2), S36–S40. [Google Scholar] [CrossRef]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J Natl Compr Canc Netw 2020, 18, 479–489. [Google Scholar] [CrossRef]
- Steiner, M.; Tan, A.R. The evolving role of immune checkpoint inhibitors in the treatment of triple-negative breast cancer. Clin Adv Hematol Oncol 2021, 19, 305–315. [Google Scholar]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Masuda, H.; Baggerly, K.A.; Wang, Y.; Zhang, Y.; Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Valero, V.; Lehmann, B.D.; Pietenpol, J.A.; Hortobagyi, G.N.; et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 2013, 19, 5533–5540. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol 2014, 232, 142–150. [Google Scholar] [CrossRef]
- Zhang, W.; Li, E.; Wang, L.; Lehmann, B.D.; Chen, X.S. Transcriptome Meta-Analysis of Triple-Negative Breast Cancer Response to Neoadjuvant Chemotherapy. Cancers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Kalecky, K.; Modisette, R.; Pena, S.; Cho, Y.R.; Taube, J. Integrative analysis of breast cancer profiles in TCGA by TNBC subgrouping reveals novel microRNA-specific clusters, including miR-17-92a, distinguishing basal-like 1 and basal-like 2 TNBC subtypes. BMC Cancer 2020, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Colaprico, A.; Silva, T.C.; Chen, J.; An, H.; Ban, Y.; Huang, H.; Wang, L.; James, J.L.; Balko, J.M.; et al. Multi-omics analysis identifies therapeutic vulnerabilities in triple-negative breast cancer subtypes. Nat Commun 2021, 12, 6276. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, Y.; Ding, J.H.; Jin, X.; Ma, D.; Li, D.Q.; Shi, J.X.; Huang, W.; Wang, Y.P.; Jiang, Y.Z.; et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab 2023, 35, 84–100.e108. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A signature for cancer progression. Biomed Pharmacother 2021, 138, 111528. [Google Scholar] [CrossRef]
- Knoll, S.; Emmrich, S.; Pützer, B.M. The E2F1-miRNA cancer progression network. Adv Exp Med Biol 2013, 774, 135–147. [Google Scholar] [CrossRef]
- O’Donnell, K.A.; Wentzel, E.A.; Zeller, K.I.; Dang, C.V.; Mendell, J.T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005, 435, 839–843. [Google Scholar] [CrossRef]
- Chawra, H.S.; Agarwal, M.; Mishra, A.; Chandel, S.S.; Singh, R.P.; Dubey, G.; Kukreti, N.; Singh, M. MicroRNA-21’s role in PTEN suppression and PI3K/AKT activation: Implications for cancer biology. Pathol Res Pract 2024, 254, 155091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, G.; Zhang, Q.; Wu, Q.; Li, B.; Zhang, Z. MicroRNA-20b promotes cell growth of breast cancer cells partly via targeting phosphatase and tensin homologue (PTEN). Cell Biosci 2014, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, B.; Church, S.E.; Gorman, K.M.; North, K.; Richardson, E.T.; DiLullo, M.; Attaya, V.; Kasparian, J.; Mohammed-Abreu, A.; Kirkner, G.; et al. Integrative multi-omic profiling of triple-negative breast cancer for identifying suitable therapies. Clinical Cancer Research 2024. [Google Scholar] [CrossRef] [PubMed]
- Khozyainova, A.A.; Valyaeva, A.A.; Arbatsky, M.S.; Isaev, S.V.; Iamshchikov, P.S.; Volchkov, E.V.; Sabirov, M.S.; Zainullina, V.R.; Chechekhin, V.I.; Vorobev, R.S.; et al. Complex Analysis of Single-Cell RNA Sequencing Data. Biochemistry (Mosc) 2023, 88, 231–252. [Google Scholar] [CrossRef]
- Potter, S.S. Single-cell RNA sequencing for the study of development, physiology and disease. Nat Rev Nephrol 2018, 14, 479–492. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Kumar, A.R.; Devan, A.R.; Nair, B.; Vinod, B.S.; Nath, L.R. Harnessing the immune system against cancer: Current immunotherapy approaches and therapeutic targets. Mol Biol Rep 2021, 48, 8075–8095. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.; McArthur, H.; Kümmel, S.; Bergh, J.; Park, Y.H.; Hui, R.; et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: Exploratory analysis from KEYNOTE-522. Ann Oncol 2024, 35, 429–436. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Yan, L.; Wu, M.; Wang, T.; Yuan, H.; Zhang, X.; Zhang, H.; Li, T.; Pandey, V.; Han, X.; Lobie, P.E.; et al. Breast Cancer Stem Cells Secrete MIF to Mediate Tumor Metabolic Reprogramming That Drives Immune Evasion. Cancer Res 2024, 84, 1270–1285. [Google Scholar] [CrossRef]
- Balogh, K.N.; Templeton, D.J.; Cross, J.V. Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses. PLoS ONE 2018, 13, e0197702. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Bucala, R. The immunobiology of MIF: Function, genetics and prospects for precision medicine. Nat Rev Rheumatol 2019, 15, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, H.; Hong, B.; Xiao, Y.; Qian, Y. MIF as a potential diagnostic and prognostic biomarker for triple-negative breast cancer that correlates with the polarization of M2 macrophages. Faseb j 2024, 38, e23696. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.W.; Lin, J.; Hou, R.; Cai, Z.; Gong, Y.; He, P.A.; Yang, J. Single-cell RNA-seq reveals the metabolic status of immune cells response to immunotherapy in triple-negative breast cancer. Comput Biol Med 2024, 169, 107926. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, X.; Zhang, J.; Liu, D.; He, J.; Huang, C.; Liao, J.; Wang, Y.; Feng, Y.; Li, H.; et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat Cancer 2023, 4, 1273–1291. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Yang, Y.; Liu, F.; Jiang, Z.; Jiang, Z. TNFSF9 promotes metastasis of pancreatic cancer by regulating M2 polarization of macrophages through Src/FAK/p-Akt/IL-1β signaling. Int Immunopharmacol 2022, 102, 108429. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 2021, 39, 1578–1593.e1578. [Google Scholar] [CrossRef]
- Ma, X.; Wan, R.; Wen, Y.; Liu, T.; Song, Y.; Zhu, Y. Deubiquitinating enzyme OTUD4 regulates metastasis in triple-negative breast cancer by stabilizing Snail1. Exp Cell Res 2024, 434, 113864. [Google Scholar] [CrossRef]
- Zhu, Y.; Banerjee, A.; Xie, P.; Ivanov, A.A.; Uddin, A.; Jiao, Q.; Chi, J.J.; Zeng, L.; Lee, J.Y.; Xue, Y.; et al. Pharmacological suppression of the OTUD4/CD73 proteolytic axis revives antitumor immunity against immune-suppressive breast cancers. J Clin Invest 2024, 134. [Google Scholar] [CrossRef]
- Kim, M.; Yang, W.; Hong, D.; Won, H.S.; Yoon, S. A Retrospective View of the Triple-Negative Breast Cancer Microenvironment: Novel Markers, Interactions, and Mechanisms of Tumor-Associated Components Using Public Single-Cell RNA-Seq Datasets. Cancers 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed Pharmacother 2021, 133, 111068. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.M.; Dunworth, M.; Sharma, G.; Loth, M.; Tandurella, J.; Cimino-Mathews, A.; Gentz, M.; Bracht, S.; Haynes, M.; Fertig, E.J.; et al. Triple-negative breast cancer metastasis involves complex epithelial-mesenchymal transition dynamics and requires vimentin. Sci Transl Med 2022, 14, eabn7571. [Google Scholar] [CrossRef]
- Winter, M.; Meignan, S.; Völkel, P.; Angrand, P.O.; Chopin, V.; Bidan, N.; Toillon, R.A.; Adriaenssens, E.; Lagadec, C.; Le Bourhis, X. Vimentin Promotes the Aggressiveness of Triple Negative Breast Cancer Cells Surviving Chemotherapeutic Treatment. Cells 2021, 10. [Google Scholar] [CrossRef]
- Al Saleh, S.; Al Mulla, F.; Luqmani, Y.A. Estrogen receptor silencing induces epithelial to mesenchymal transition in human breast cancer cells. PLoS ONE 2011, 6, e20610. [Google Scholar] [CrossRef]
- Alnuaimi, A.R.; Nair, V.A.; Malhab, L.J.B.; Abu-Gharbieh, E.; Ranade, A.V.; Pintus, G.; Hamad, M.; Busch, H.; Kirfel, J.; Hamoudi, R.; et al. Emerging role of caldesmon in cancer: A potential biomarker for colorectal cancer and other cancers. World J Gastrointest Oncol 2022, 14, 1637–1653. [Google Scholar] [CrossRef]
- Sobue, K.; Sellers, J.R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem 1991, 266, 12115–12118. [Google Scholar] [CrossRef]
- Ma, W.Q.; Miao, M.C.; Ding, P.A.; Tan, B.B.; Liu, W.B.; Guo, S.; Er, L.M.; Zhang, Z.D.; Zhao, Q. CALD1 facilitates epithelial-mesenchymal transition progression in gastric cancer cells by modulating the PI3K-Akt pathway. World J Gastrointest Oncol 2024, 16, 1029–1045. [Google Scholar] [CrossRef]
- De Marchi, T.; Timmermans, A.M.; Smid, M.; Look, M.P.; Stingl, C.; Opdam, M.; Linn, S.C.; Sweep, F.C.; Span, P.N.; Kliffen, M.; et al. Annexin-A1 and caldesmon are associated with resistance to tamoxifen in estrogen receptor positive recurrent breast cancer. Oncotarget 2016, 7, 3098–3110. [Google Scholar] [CrossRef]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018, 173, 879–893.e813. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G.; Lehmann, B.D.; Pietenpol, J.A. New strategies for triple-negative breast cancer--deciphering the heterogeneity. Clin Cancer Res 2014, 20, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Gómez, P.; Greil, R.; Braga, S.; Climent, M.A.; Wardley, A.M.; Kaufman, B.; Stemmer, S.M.; Pêgo, A.; Chan, A.; et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 2013, 31, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Rugo, H.S.; Marcom, P.K.; Mayer, E.L.; Esteva, F.J.; Ma, C.X.; Liu, M.C.; Storniolo, A.M.; Rimawi, M.F.; Forero-Torres, A.; et al. TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 2012, 30, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Elias, A.D.; Rugo, H.S.; Cobleigh, M.A.; Wolff, A.C.; Eisenberg, P.D.; Lehman, M.; Adams, B.J.; Bello, C.L.; DePrimo, S.E.; et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2008, 26, 1810–1816. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Eidtmann, H.; Rezai, M.; Fasching, P.A.; Tesch, H.; Eggemann, H.; Schrader, I.; Kittel, K.; Hanusch, C.; Kreienberg, R.; et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med 2012, 366, 299–309. [Google Scholar] [CrossRef]
- Turner, N.; Lambros, M.B.; Horlings, H.M.; Pearson, A.; Sharpe, R.; Natrajan, R.; Geyer, F.C.; van Kouwenhove, M.; Kreike, B.; Mackay, A.; et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene 2010, 29, 2013–2023. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.; García-García, C.; Serra, V.; He, L.; Torres-Lockhart, K.; Prat, A.; Anton, P.; Cozar, P.; Guzmán, M.; Grueso, J.; et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov 2012, 2, 1036–1047. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Abramson, V.G.; Dees, E.C.; Shah, P.D.; Ballinger, T.J.; Isaacs, C.; Santa-Maria, C.A.; An, H.; Gonzalez-Ericsson, P.I.; Sanders, M.E.; et al. Atezolizumab in Combination With Carboplatin and Survival Outcomes in Patients With Metastatic Triple-Negative Breast Cancer: The TBCRC 043 Phase 2 Randomized Clinical Trial. JAMA Oncol 2024, 10, 193–201. [Google Scholar] [CrossRef]
- Marco Salas, S.; Kuemmerle, L.B.; Mattsson-Langseth, C.; Tismeyer, S.; Avenel, C.; Hu, T.; Rehman, H.; Grillo, M.; Czarnewski, P.; Helgadottir, S.; et al. Optimizing Xenium In Situ data utility by quality assessment and best-practice analysis workflows. Nat Methods 2025, 22, 813–823. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Wei, W.; Lu, Y.; Zou, B.; Zhang, L.; Shan, J.; Zhu, Y.; Wang, S.; Wu, H.; et al. A precise microdissection strategy enabled spatial heterogeneity analysis on the targeted region of formalin-fixed paraffin-embedded tissues. Talanta 2024, 278, 126501. [Google Scholar] [CrossRef]
- Pinkney, H.R.; Black, M.A.; Diermeier, S.D. Single-Cell RNA-Seq Reveals Heterogeneous lncRNA Expression in Xenografted Triple-Negative Breast Cancer Cells. Biology 2021, 10. [Google Scholar] [CrossRef]
| Technique | Description | Strength | Weakness |
| Single-cell transcriptomics (scRNA-seq) | Cellular level analysis of mRNA expression | High resolution. Identify cell populations in heterogeneous samples [6] | Cost [7]. Lacks spatial context. |
| Spatial transcriptomics | Analysis of mRNA from sample on slide | Trends with spatial context. Entire transcriptome probe sets available. Promising future as more advanced products are released | Cost [8]. Low resolution. Relatively low transcript capture in certain tissue types (e.g., mineralized tissue [9]) |
| Microarray gene expression analysis | Collection of mRNAs using array of known probes | Targeted studies | Can only identify expression of known probes. |
| Bulk RNA-seq | Analysis of mRNA from whole sample | More sensitive than microarray and doesn’t require probes. | Primarily for broad sample-wide trends |
| Proteomics | Analysis of pro-tein structure and function | Clinically relevant protein identification. Cost-effective methods [10] | Complexity of protein structure. Difficulty for studying post-translational modifications. |
| Genomics | Analysis of DNA sequencing | Whole-genome sequencing becoming more readily available. Identification of mutations. Exome sequencing can identify copy number variations. | Cost [11]. May not reflect transcribed/translated protein. Variants detected of may be of varying significance. |
| Subtype | Pathways | |
| Basal-like | Basal-like 1 (BL1) | Proliferative gene pathways (cell cycle, DNA replication), usually associated with high Ki-67 |
| Basal-like 2 (BL2) | Growth factor genes | |
| Immunomodulatory (IM)* | Immune cell signaling | |
| Mesenchymal | Mesenchymal-like (M) | Cell motility, cell differentiation, WNT, ALK, Extracellular matrix |
| Mesenchymal stem-like (MSL)** | Growth factor and epithelial-to-mesenchymal transition | |
| Luminal | Luminal androgen receptor (LAR) | Androgen/estrogen metabolism, Steroid biosynthesis, Porphyrin metabolism |
| Markers | Techniques | Function | Citations |
| MIF | RNA-seq, scRNA-seq, Spatial transcriptomics | Regulates glucocorticoid immunosuppression mediating cell survival | [52,53,54,55] |
| CXCL13 | scRNA-seq | Expressed in T cells to induce proinflammatory signaling in macrophages | [59] |
| CD73/OTUD4 | Proteomics and Spatial transcriptomics | CD73 stabilizes OTUD4, causing accumulation and immunosuppression | [60,61] |
| VIM | scRNA-seq | Intermediate filament protein found in mesenchymal cells. Drives epithelial to mesenchymal transition | [65,66,67,68] |
| CALD1 | scRNA-seq | Actin-binding protein involved in cell motility. Drives epithelial to mesenchymal transition. | [68,69,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





