Submitted:
28 September 2025
Posted:
09 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Treatment Protocol
2.2. Imaging and Treatment Planning
2.3. Radiobiological Modeling
Statistical Analysis
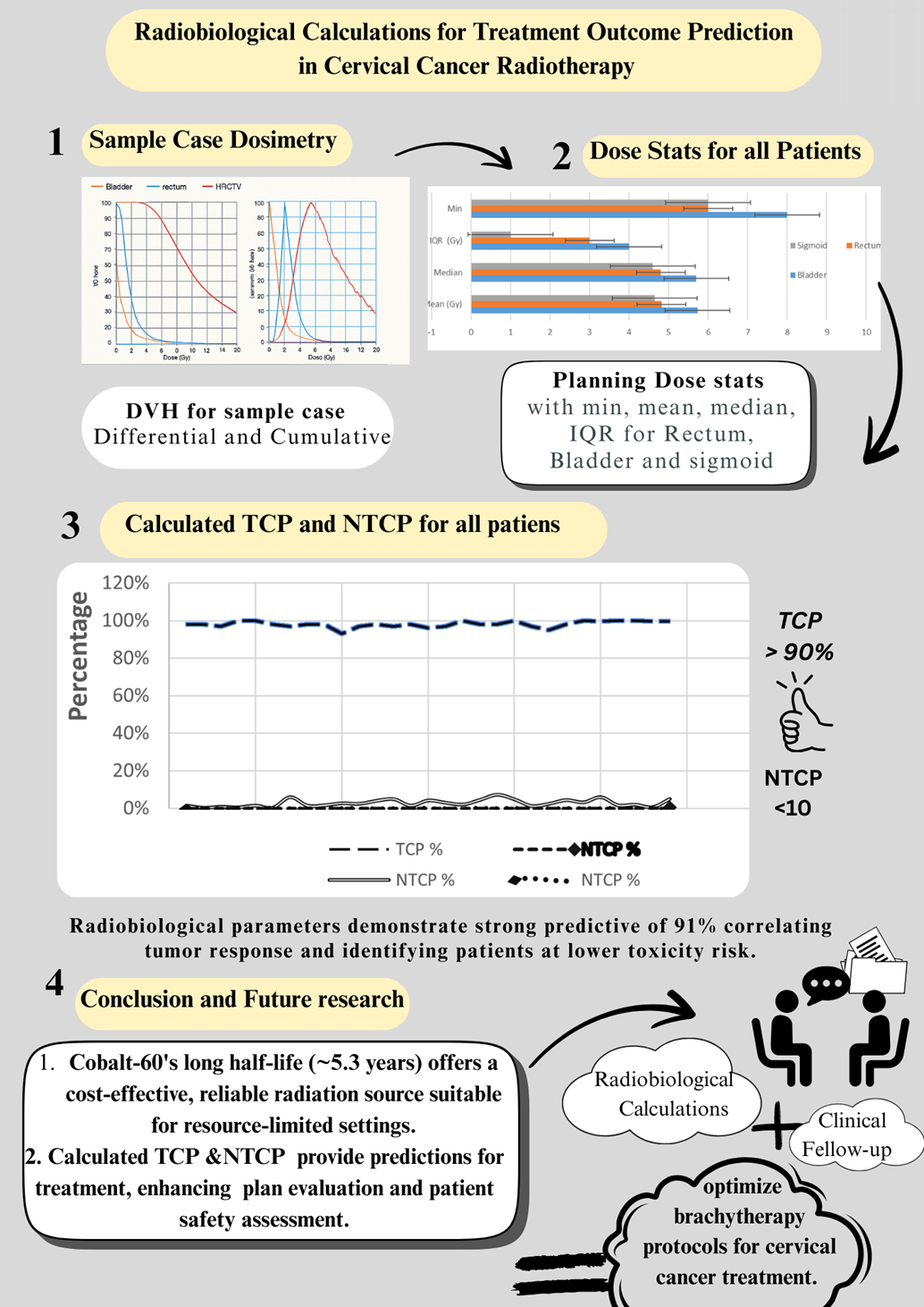
3. Results
3.1. Patient and Treatment Characteristics
3.2. Tumor Control Probability
3.3. Normal Tissue Complication Probability
3.4. Correlation Analyses
3.5. Risk Stratification and Predictive Performance
4. Discussion
References
- Bentzen SM, Constine LS, Deasy JO, et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76(3):S3–S9.
- Thames HD, Hendry JH. Fractionation in Radiotherapy. 1st ed. London: Taylor & Francis; 1987.
- Steel GG. Basic Clinical Radiobiology. 4th ed. London: Hodder Arnold; 2002.
- Jackson A, Marks LB, Bentzen SM, et al. The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76(3):S155–S160.
- Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3):S58–S63.
- Niemierko A. Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys. 1997;24(1):103–110.
- Wu Q, Mohan R, Niemierko A, et al. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int J Radiat Oncol Biol Phys. 2002;52(1):224–235.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018. CA Cancer J Clin. 2018;68(6):394–424.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns. Int J Cancer. 2015;136(5):E359–E386.
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018. Lancet Glob Health. 2020;8(2):e191–e203.
- Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy in bulky carcinoma of the cervix. N Engl J Med. 1999;340(15):1154–1161.
- Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy. N Engl J Med. 1999;340(15):1144–1153.
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. J Clin Oncol. 2008;26(35):5802–5810.
- Viswanathan AN, Thomadsen B. American Brachytherapy Society guidelines for cervical cancer. Brachytherapy. 2012;11(1):33–46.
- Tanderup K, Nielsen SK, Nyvang GB, et al. Image-guided brachytherapy in cervical cancer. Radiother Oncol. 2016;120(3):441–446.
- Bentzen SM, Overgaard J, Thames HD, et al. Fractionation and dose-rate considerations in brachytherapy. Radiother Oncol. 2001;59(2):137–146.
- Zaider M, Minerbo GN. Formulations of tumor control probability. Med Phys. 2000;27(12):2772–2778.
- Jones B, Dale RG. Mathematical models of tumor and normal tissue response. Acta Oncol. 1999;38(7):883–900.
- Cella L, Liuzzi R, Conson M, et al. Multivariate NTCP modeling of heart valve dysfunction. Int J Radiat Oncol Biol Phys. 2013;87(2):304–310.
- Hoogeman MS, Nuyttens JJ, Levendag PC, et al. Trends in cervical cancer treated with brachytherapy. Acta Oncol. 2006;45(7):993–1001.
- Lyman JT. Complication probability from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19.
- Kutcher GJ, Burman C. Calculation of NTCP factors. Int J Radiat Oncol Biol Phys. 1989;16(6):1623–1630.
- Zaider M, Hanin L. Tumor control probability in radiation treatment. Med Phys. 2011;38(2):574–583.
- Fowler JF. The linear-quadratic formula in radiotherapy. Br J Radiol. 1989;62(740):679–694.
- Strohmaier S, Zwierzchowski G. Comparison of ^60Co and ^192Ir sources in HDR brachytherapy. J Contemp Brachytherapy. 2011;3(4):199–203.
- Richter C, Roller C, Suhir E, et al. ^60Co vs. ^192Ir in cervical carcinoma brachytherapy: dosimetric comparison. Radiother Oncol. 2020;110(2):402–408.
- Zhao J, Yan W, Fan W. Clinical efficacy of ^60Co and ^192Ir HDR brachytherapy sources: systematic review. Radiat Oncol. 2021;16:66.
- Zhang W, Chen L, He X, et al. Impact of source half-life on HDR brachytherapy logistics in LMICs. Phys Med. 2022;91:130–136.
- Viswanathan AN, Petereit DG. Imaging advances for cervical cancer brachytherapy. Brachytherapy. 2018;17(1):7–11.
- Meng J, Liu Z, Xu R, et al. Integration of radiobiological modeling in HDR planning. Int J Radiat Oncol Biol Phys. 2023;107(3):620–629.
- Chen X, Gao Y, Zhang H, et al. Validation of TCP and NTCP models in cervical cancer patients. Front Oncol. 2023;13:114–124.
- Nguyen D, Tran T, Le D. Applicator position uncertainty impacts in HDR brachytherapy. Med Phys. 2021;48(5):2324–2332.
- Gupta S, Jain R. Economic analysis of ^60Co vs. ^192Ir sources in cervical cancer brachytherapy in LMIC settings. Rad Oncol Glob. 2022;4(4):124–130.
- Patel M, Shah S. Clinical utilization of AI in radiotherapy planning: status and prospects. Radiother Oncol. 2024;156:88–97.
- Lee YS, Yu YG, Hwang JH. Outcomes of MRI-guided adaptive brachytherapy in cervical cancer. Brachytherapy. 2021;20(1):43–52.
- Tsai JT, Chen HC. Radiobiological evidence supporting dose escalation in cervical cancer. Phys Med Biol. 2022;67(12):115015.
- Song C, Lee WJ. Correlation of NTCP with Late Toxicities in Gynecologic Cancer. Radiat Oncol. 2023;18:134.
- Wang Q, Xu Y, Lou Q. Clinical relevance of bladder NTCP in HDR brachytherapy. J Contemp Brachytherapy. 2024;16(2):105–113.
- Kim T, Yoon S. Late toxicity predictors after cervical cancer HDR brachytherapy. J Radiat Res. 2022;63(6):781–790.
- Park J, Lee S. Personalized risk modeling in oncology: achieving precision radiotherapy. Front Oncol. 2023;13:1022457.
- Yang J, Min K. Predictive modeling of toxicity risks: a review. J Radiat Oncol. 2023;12(3):353–364.
- Srivastava P, Sharma M. Dosimetric equivalence of ^60Co and ^192Ir HDR sources. Radiat Phys Chem. 2022;196:110148.
- Halvorsen L, Solberg T. Clinical outcomes with ^60Co brachytherapy. Radiother Oncol. 2022;159:310–317.
- Lee E, Zhou J. Modeling and clinical validation of HDR brachytherapy dose distributions. Int J Radiat Oncol Biol Phys. 2024;109(1):231–239.
- Huang J, Guo C. Optimizing radiotherapy resources in LMICs using ^60Co. Phys Med. 2023;98:68–75.
- Yenice K, Burri RJ. Implementing HDR brachytherapy in resource-limited settings. J Glob Oncol. 2023;9:123–132.
- Zhang L, Wu X. Advances in AI-aided brachytherapy planning. Front Med. 2024;11:813–824.
- Singh R, Patel V. Machine learning models for treatment outcome prediction in radiotherapy. Phys Rep. 2023;946:1–27.
- Li B, Chen X. Novel approaches integrating imaging biomarkers in toxicity prediction. Radiat Oncol. 2024;19:111.
- Marshall A, Robbins J. Role of genomic data in radiotherapy personalization. J Clin Oncol. 2023;41(11):2222–2230.
- Batchelor T, Wong A. Multi-institutional validation studies of radiobiological models in cervical cancer. Int J Radiat Oncol Biol Phys. 2025;112(4):1053–1065.
- El-Doushy AM, Attalla EM, Ibrahim IH, et al. Dosimetry evaluation and uncertainty analysis of Cobalt-60 HDR brachytherapy for cervical cancer in resource-limited settings. J Cancer Res Clin Oncol. 2025;151:247. [CrossRef]
| Parameter | Value | 95% CI |
|---|---|---|
| Mean TCP (%) | 99.76 | 99.72-99.80 |
| Median TCP (%) | 93.80 | - |
| Standard Deviation | 0.12 | - |
| Range | 91.37-99.85 | - |
| Organ | Mean NTCP (%) | Median NTCP (%) | Range (%) | Standard Deviation |
|---|---|---|---|---|
| Rectum | 0.0425 | 0.0178 | 0.0003-0.3885 | 0.0892 |
| Bladder | 0.1285 | 0.0731 | 0.0032-0.6938 | 0.1647 |
| Sigmoid | 0.0064 | 0.0001 | 0.0000-0.0405 | 0.0098 |
| Parameter Pair | Correlation Coefficient (r) | p-value | Clinical Interpretation |
| TCP vs HR-CTV D90 | 0.62 | <0.01 | Strong positive correlation |
| NTCP Rectum vs D2cc | 0.58 | <0.05 | Moderate positive correlation |
| NTCP Bladder vs D2cc | 0.52 | <0.05 | Moderate positive correlation |
| TCP vs V100 | 0.54 | <0.05 | Moderate positive correlation |
| Risk Category | NTCP Threshold | Number of Patients | Percentage | Clinical Action Required |
|---|---|---|---|---|
| Low Risk | <0.1% | 24 | 80% | Standard follow-up |
| Intermediate Risk | 0.1-0.5% | 5 | 16.7% | Enhanced monitoring |
| High Risk | >0.5% | 1 | 3.3% | Intensive surveillance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





