Submitted:
16 January 2025
Posted:
17 January 2025
You are already at the latest version
Abstract
Background/Objectives: To evaluate the efficacy and safety of unique magnetic resonance imaging (MRI)-guided interstitial brachytherapy (ISBT) for locally advanced cervical cancer that is unsuitable for intracavitary brachytherapy (ICBT) or intracavitary/interstitial brachytherapy (ICISBT). Methods: We analyzed the clinical outcomes, including toxicity, of 68 previously untreated patients with cervical cancer treated between 2014 and 2024. Results: The median high-risk clinical target volume (HR-CTV) was 53.20 cc (range, 16.34–147.03 cc) at ISBT. With a median follow-up time of 37.5 months (7-115 months), the three-year local control, progression-free survival, and overall survival rates were 89.8%, 52.4%, and 70.9%, respectively. Multivariate analyses showed significant associations of histology with local control, overall treatment times, HR-CTV volume with overall survival rate, over all treatment times, and HR-CTV volume and M category with progression-free survival. Toxicity grade 3 were occurred in 12 patients (17.6%) consist of 4 genitourinary (5.8%), 7 gastrointestinal (10.2%) toxicities. Conclusion: MRI-guided ISBT is an effective treatment strategy for obtaining a favorable local control rate for selected advanced diseases with an acceptable complication rate. Future research is warranted to elucidate who is a good candidate for MRI-guided ISBT.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient and Treatment
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| ISBT | Interstitial brachytherapy |
| ICBT | Intracavitary brachytherapy |
| ICISBT | Intracavitary/interstitial brachytherapy |
| HR-CTV | High-risk clinical target volume |
| PALN | Para-aortic lymph node |
| CS | Center-shielded |
| EBRT | External beam radiotherapy |
| EQD2 | The equivalent dose in 2 Gy fractions |
| OS | Overall survival |
| PFS | Progression-free survival |
| LC | Local control |
| OTT | Overall treatment time |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-249. [CrossRef]
- Green, J.A.; Kirwan, J.M.; Tierney, J.F.; Symonds, P.; Fresco, L.; Collingwood, M., et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781-6. [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R., et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428-433. [CrossRef]
- Haie-Meder, C.; Pötter, R.; Van Limbergen, E., et al. Gynaecological (GYN) GEC-ESTRO Working Group. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235-45. [CrossRef]
- Kirisits, C.; Lang, S.; Dimopoulos, J.; et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys 2006;65: 624–630. [CrossRef]
- Yamazaki, H.; Inoue, T.; Ikeda, H.; Tang, J.T.; Murayama, S.; Teshima, T.; Otani, M.; Kozuka, T. High-dose-rate remote afterloading intestinal radiotherapy employing the template technique for recurrent cancer in the pelvic area. Strahlenther Onkol. 1993;169:481-5.
- Beriwal, S.; Bhatnagar, A.; Heron, D.E.; et al. High-dose-rate inter stitial brachytherapy for gynecologic malignancies. Brachytherapy 2006;5:218–222. [CrossRef]
- Demanes, D.J.; Rodriguez, R.R.; Bendre, D.D.; et al. High dose rate transperineal interstitial brachytherapy for cervical cancer: High pelvic control and low complication rates. Int J Radiat Oncol Biol Phys 1999;45:105–112. [CrossRef]
- Kuipers, T.; Hoekstra, C.J.; van’t Riet, A.; et al. HDR brachytherapy applied to cervical carcinoma with moderate lateral expansion: Modified principles of treatment. Radiother Oncol 2001;58: 25–30. [CrossRef]
- Yoshida, K.; Yamazaki, H.; Takenaka, T.; Kotsuma, T.; Yoshida, M.; Furuya, S., et al. A dose-volume analysis of magnetic resonance imaging-aided high-dose-rate image-based interstitial brachytherapy for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2010;77:765-72. [CrossRef]
- Yoshida, K.; Yamazaki, H.; Kotsuma, T.; Takenaka, T.; Ueda, M.M.; Miyake, S.; et al. Simulation analysis of optimized brachytherapy for uterine cervical cancer: Can we select the best brachytherapy modality depending on tumor size? Brachytherapy. 2016;15:57-64. [CrossRef]
- Kokabu, T.; Masui, K.; Tarumi, Y.; Noguchi, N.; Aoyama, K.; Kataoka, H., et al. 3D-Image-Guided Multi-Catheter Interstitial Brachytherapy for Bulky and High-Risk Stage IIB-IVB Cervical Cancer. Cancers 2022 ;14:1257. [CrossRef]
- Takenaka, T.; Yamazaki, H.; Suzuki, G.; Masui, K.; Shimizu, D.; Kotsuma, T., et al. Initial tumor volume as an important predictor for indication of intra-cavitary brachytherapy, intra-cavitary/interstitial brachytherapy, and multi-catheter sole interstitial brachytherapy in cervical cancer patients treated with chemoradiotherapy. J Contemp Brachytherapy. 2023;15:191-197.. [CrossRef]
- Nag, S.; Erickson, B.; Thomadsen, B., et al. The American Brachy therapy Society recommendations for high-dose-rate brachy therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2000;48:201–211. [CrossRef]
- Pötter, R.; Haie-Meder, C.; Van Limbergen, E.; Barillot I, De Brabandere, M.; Dimopoulos, J., et al. GEC ESTRO Working Group. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67-77. [CrossRef]
- Viswanathan, A.N.; Beriwal, S.; De Los Santos, J.F.; Demanes, D.J.; Gaffney, D.; Hansen, J., et al. American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy. 2012;11:47-52. [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [CrossRef]
- Hande, V.; Chopra, S.; Kalra, B.; Abdel-Wahab, M.; Kannan, S.; Tanderup, K., et al. Point-A vs. volume-based brachytherapy for the treatment of cervix cancer: A meta-analysis. Radiother Oncol. 2022;170:70-78. [CrossRef]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C., et al. EMBRACE Collaborative Group. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021;22:538-547. [CrossRef]
- Schmid, M.P.; Lindegaard, J.C.; Mahantshetty, U., et al. Risk Factors for Local Failure Following Chemoradiation and Magnetic Resonance Image-Guided Brachytherapy in Locally Advanced Cervical Cancer: Results From the EMBRACE-I Study. J Clin Oncol. 2023;41:1933-1942. [CrossRef]
- Mazeron, R.; Castelnau-Marchand, P.; Dumas, I.; del Campo, E.R.; Kom, L.K.; Martinetti, F., et al. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother Oncol. 2015;114:257-63. [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P. et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lancet 2024;403:1341-1350. [CrossRef]
- Okazaki, S.; Murata, K.; Noda, S.E.; Kumazaki, Y.; Hirai, R.; Igari, M., et al. Dose-volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J Radiat Res. 2019 ;60:490-500. [CrossRef]
- Tamaki, T.; Noda, S.E.; Ohno, T.; Kumazaki, Y.; Kato, S.; Nakano, T. Dose-volume histogram analysis of composite EQD2 dose distributions using the central shielding technique in cervical cancer radiotherapy. Brachytherapy. 2016;15:598-606. [CrossRef]
- Lindegaard, J.C., Petric, P.; Schmid, M.P.; Nesvacil, N.; Haie-Meder, C.; Fokdal, L.U., et al. Prognostic Implications of Uterine Cervical Cancer Regression During Chemoradiation Evaluated by the T-Score in the Multicenter EMBRACE I Study. Int J Radiat Oncol Biol Phys. 2022;113:379-389. [CrossRef]
- Ohtaka, T.; Ando, K.; Oike, T.; Noda, S.E.; Kaminuma, T.; Murata, K., et al. The prognostic effect of tumor volume, reduction ratio, and cumulative doses on external beam radiotherapy with central-shielding method and image-guided adaptive brachytherapy for cervical cancer. Front Oncol 2024;14:1366777. [CrossRef]
- Schernberg, A.; Bockel, S.; Annede, P.; Fumagalli, I.; Escande, A.; Mignot, F., et al. Tumor Shrinkage During Chemoradiation in Locally Advanced Cervical Cancer Patients: Prognostic Significance, and Impact for Image-Guided Adaptive Brachytherapy. Int J Radiat Oncol Biol Phys 2018 ;102:362-372. [CrossRef]
- Cordoba, A.; Durand, B.; Escande, A.; Taieb, S.; Amor, M.B.H.; Le Deley, M.C., et al. Prognostic impact of tumor size reduction assessed by magnetic resonance imaging after radiochemotherapy in patients with locally advanced cervical cancer. Front Oncol. 2022 Dec 2;12:1046087. [CrossRef]
- Sun, C.; Wang, S.; Ye, W.; Wang, R.; Tan, M.; Zhang, H., et al. The Prognostic Value of Tumor Size, Volume and Tumor Volume Reduction Rate During Concurrent Chemoradiotherapy in Patients With Cervical Cancer. Front Oncol. 2022 Jul 14;12:934110. PMID: 35912169; PMCID: PMC9329537. [CrossRef]
- Angeles, M.A.; Baissas, P.; Leblanc, E.; Lusque, A.; Ferron, G.; Ducassou, A., et al. Magnetic resonance imaging after external beam radiotherapy and concurrent chemotherapy for locally advanced cervical cancer helps to identify patients at risk of recurrence. Int J Gynecol Cancer. 2019 Mar;29(3):480-486. Epub 2019 Feb 1. PMID: 30712019. [CrossRef]
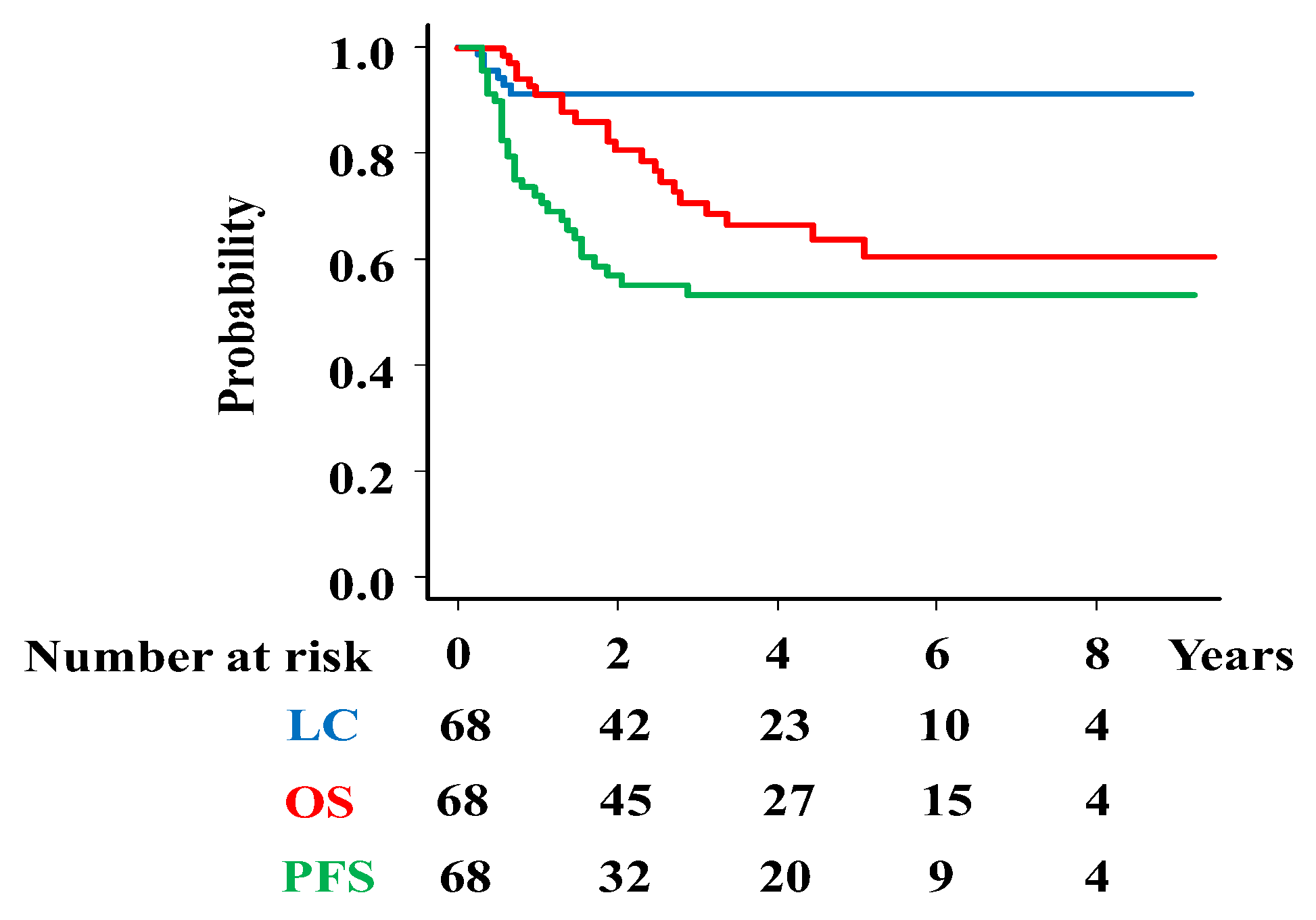

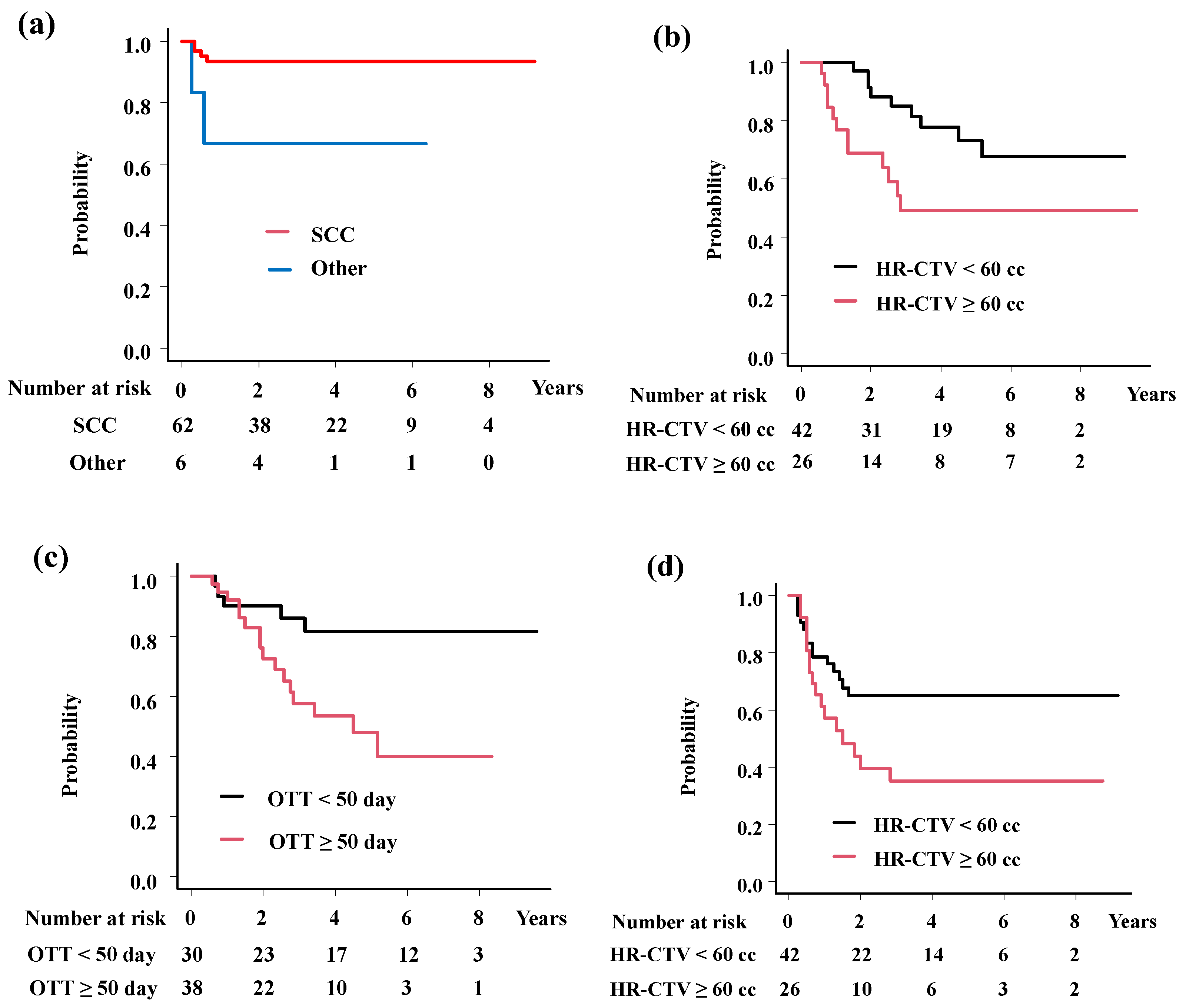
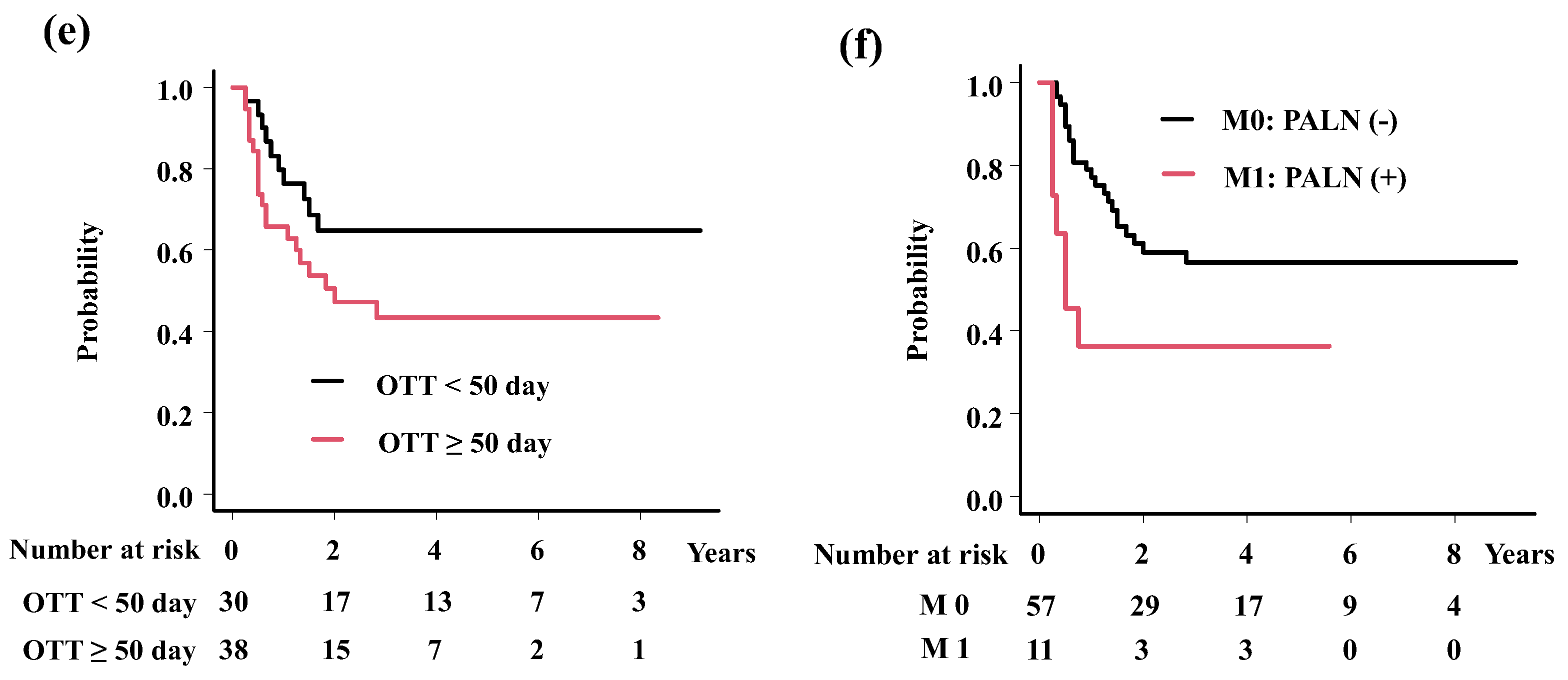

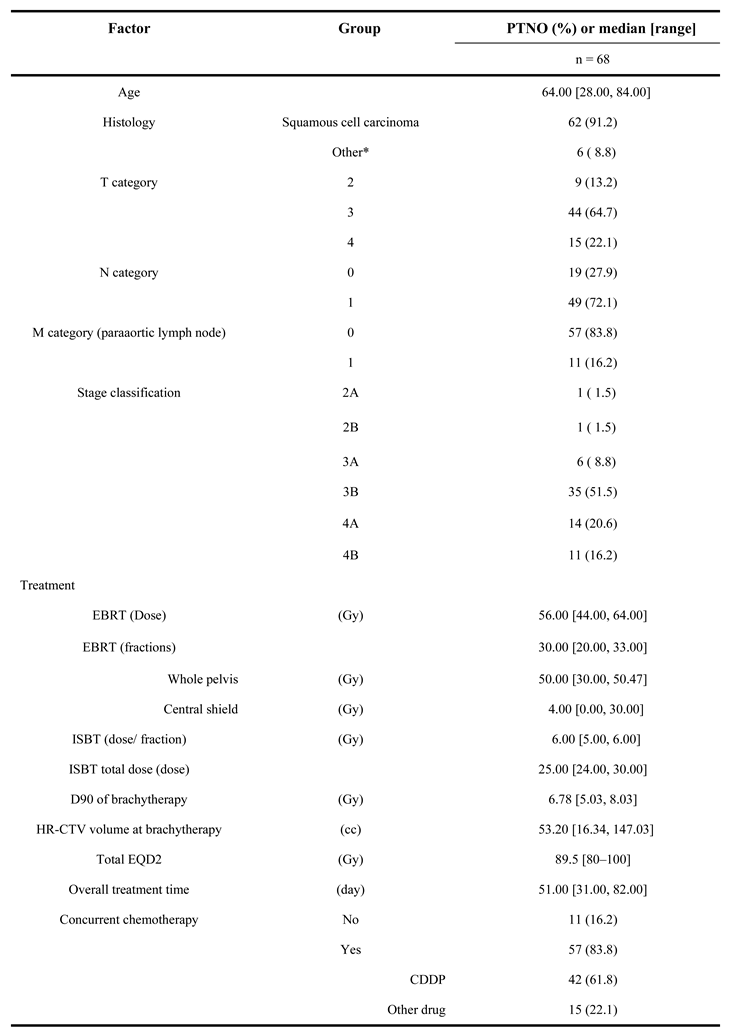
| Endpoint | Variable | Category | PTNO | 3-year (%) | Hazard Ratio (95%CI) | p-value |
|---|---|---|---|---|---|---|
| Local control | Histology | Squamous cell carcinoma | 62 | 93.5 | 0.16 (0.03-0.9) | 0.037 |
| Others | 6 | 66.7 | ||||
| Overall survival | HR-CTV volume | < 60 cc | 53 | 77.1 | 5.76 (2.17-15.27) | 0.00044 |
| ≥ 60 cc | 15 | 49.5 | ||||
| OTT | < 50 day | 30 | 85.9 | 6.69 (2.21-20.26) | 0.00078 | |
| ≥ 50 day | 38 | 57.5 | ||||
| Progressing free survival | HR-CTV volume | < 60 cc | 53 | 58 | 2.19 (1.05-4.57) | 0.036 |
| ≥ 60 cc | 15 | 37.5 | ||||
| OTT | < 50 day | 30 | 64.8 | 2.21 (1.02-4.77) | 0.045 | |
| ≥ 50 day | 38 | 43.3 | ||||
| M category | Paraaortic LN (+) | 11 | 36.4 | 2.47 (1.04-5.85) | 0.04 | |
| Paraaortic LN (-) | 57 | 56.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





