1. Introduction
The palliative treatment of very large tumors remains a major challenge in oncology. These lesions are often characterized by poor vascularization, hypoxic subregions, and numerous pre-treatments including previous radiation, limiting normal tissue tolerance of re-radiation.
Lattice Radiotherapy (LRT), a form of spatially fractionated radiation therapy (SFRT), has emerged as a promising strategy to address these challenges. By integrating high dose sub-volumes (“vertices”) within the gross tumor volume (GTV), LRT aims to achieve enhanced tumoricidal effects while maintaining tolerable dose distributions across critical structures. The underlying rationale is supported by radiobiological insights into immuno-genic modulation, bystander effects, and vascular disruption mechanisms triggered by steep intra-tumoral dose gradients.
Since first LRT reports ~15 years ago, there is increasing but still limited clinical knowledge reported in the literature. SFRT has been used for thousands of patients with favorable and encouraging outcomes, however, this modality remains insufficiently understood. Many questions on SFRT/LRT remain open, like ideal geometric arrangements, dosage, dose-volume arrangements, time interval for decision to repeat LRT – beside the lack of knowledge regarding underlying immuno-biological mechanisms of action. A comprehensive critical review on the recent status of clinical and preclinical studies and knowledge gaps has been recently published by Prezado et al, addressing clinical, physical as well as immuno-biological aspects and open questions on SFRT [
1].
Our formerly published clinical outcome data demonstrated the effectiveness and favorable safety of LRT in a heterogeneous cohort of 45 patients with 56 large (>/=7 cm) tumors treated in a palliative setting [
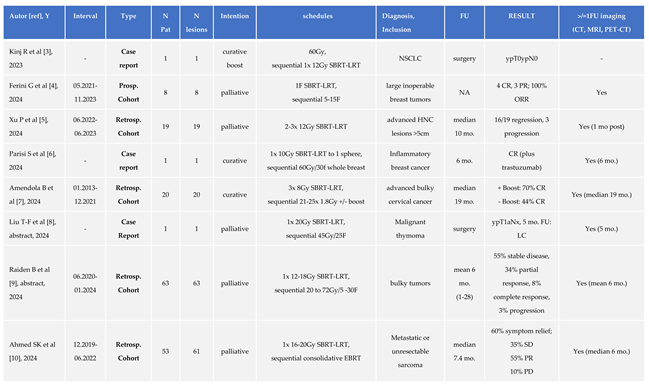
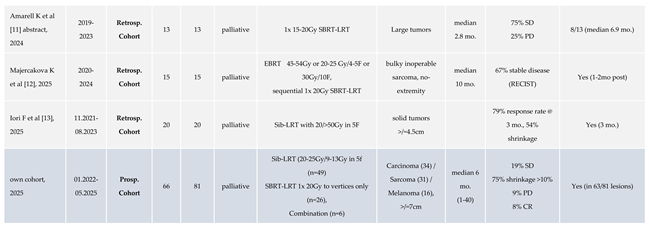
2]. LRT yielded encouraging rates of symptom relief and radiologic response, persisting for mean >6 months. In the meantime, the clinical application of LRT at our center has continued to expand, supported by increasing familiarity with the LRT concept and clinical outcome, and growing multidisciplinary interest. A summary of published clinical LRT results up to 2023 has been listed in Table 1 of our previous report [
1]. Additional clinical reports on LRT published in the literature since then are listed in Table 1 of this work [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13], mirroring an increasing interest in/spectrum of LRT indications, while orchestrated multi-center trial activities are still scant.
A recent publication informs about the formation of the Radiosurgery Society, GRID, LAT-TICE, Microbeam and FLASH (GLMF) Working Groups as a framework for these efforts, focused on advancing the understanding of the biology, technical/physical parameters, trial design, and clinical practice of these new radiation therapy modalities [
14].
Li et al analyzing the effectiveness and safety of LRT in large tumors >5 cm in a systematic review and meta-analysis based on single-arm clinical studies [
15]. Pooling 187 patients treated for 209 lesions out of 7 eligible publications, the authors found the 3-month complete response rate and partial response rate were 36.67% and 42.49%, respectively, while the three-month progressive disease rate was 7.10%. The tumor volume was reduced by 48.95%. The pooled 6-month overall survival rate was found 79.27%, with a median response time of 4.25 months. The pooled rates of mild and moderate-to-severe adverse events were 19.40% and 3.37%, respectively.
The following analysis represents an update of our single center expanded patient cohort with extended FU. The focus was on consistency of LRT effectiveness across histologies and different LRT regimens applied in patients with limited or no alternative therapeutic options.
2. Materials and Methods
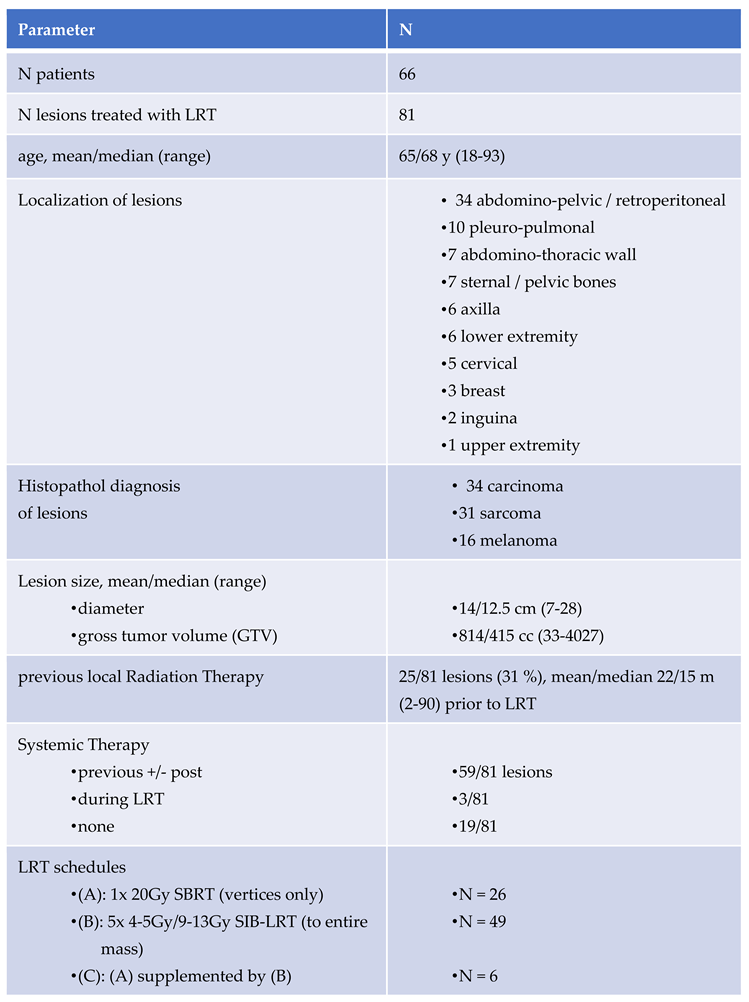
2.1. Patients (Table 2)
A total of 66 patients with 81 large lesions (≥7 cm diameter) were treated in palliative in-tent between January 2022 and May 2025. Histopathological diagnoses included carcinoma (n=34), sarcoma (n=31), and melanoma (n=16). Median gross tumor volume (GTV) was 415 cc (range: 33–4027 cc). In 63/81 (78%) lesions at least one follow-up (FU) imaging was avail-able for volumetric analysis.
Prior radiation was documented in 31% of all lesions.
Systemic therapy was administered in 73% of cases, progress of lesions under systemic therapy led to referral for LRT in most cases.
2.2. LRT
Our Lattice Radiation Treatment (LRT) protocol includes the following regimens (*):
A) single-fraction stereotactic LRT (SBRT-LRT, n=26) of 20 Gy to vertices only, as re-ported by Jiang et al and Dincer et al [
16,
17]
B) simultaneous integrated boost LRT (sib-LRT, n=49) applying 5x 4-5 Gy to the entire mass with sib of 9-13 Gy to lattice vertices, as described by Duriseti et al [
18]
C) combination: A), followed by B) - after typically 4-8 weeks: realized in only 6/81 lesions, aiming to improve treatment response to A)
Detailed information regarding LRT contouring/planning has been reported in our former publication [
2].
(*): When we started our LRT program, regimen A) was mainly used for very large and/or previously irradiated lesions. This single fraction SBRT-LRT regimen was then found to be similarly effective as regimen B), while most convenient for palliative patients. This observation led us to the standard application of the single fraction SBRT-LRT regimen A) as the regimen of first choice - if anatomically feasible, followed by regimen B) in cases of unsatisfactory response to regimen A).
2.3. Definition of Volumetric Response
FU-imaging was available from 63/81 lesions (78%). The following definitions of response were used (decision was against the RECIST score, which is only based on diameter of lesions):
- Progressive disease (PD): >10% increase of initial volume; please note: clinically obvious PD was also counted for lesions with no FU scans
- stable disease (SD): +/-10% of initial volume - taking the uncertainty given by edema reactions following LRT into account
- shrinkage (>10% reduction of initial tumor volume)
- complete remission (CR): no residual tumor in diagnostic scans
2.4. Follow up
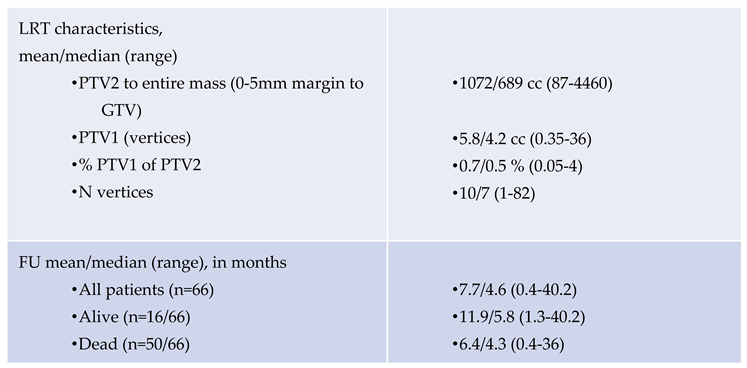
Clinical and radiological FU was conducted on an individualized basis, tailored to the individual needs of these palliative patients, i.e. FU imaging was not performed solely for analytical purposes, resulting in incomplete radiographic FU (63/81 lesions were examined with at least 1 magnetic resonance imaging or computed tomography scan, accounting for 78 % of cases). In consequence, time-related volumetric change analysis was not assessable in defined time intervals. Clinical FU was assessed at our department (physical or phone call visits) and based on chart notes from other involved disciplines.
2.5. PROMS (Patient Reported Outcome Measure)
PROMS were collected from all patients able to state their experienced symptom changes, using a Visual Analogue Scale (VAS, ranging from 0 = no symptoms, to 10 = unbearable symptoms),
Table 3.
3. Results
The mean/median overall survival (OS) of the cohort was 7.7/4.6 months (0.4-40.2); the respective OS of 16 alive patients (07.2025) was 11.9/5.8 (1.3-40.2), and 6.4/4.3 months for 50 died patients.
Table 4 shows detailed characteristics of treated lesions related to histology. Sarcomatous lesions were characterized by largest mean/median volumes.
All included lesions measured >/= 7cm in diameter.
3.1. Subjective Benefit/PROMS
82% symptomatic patients (45/55) reported fast relief of symptoms,
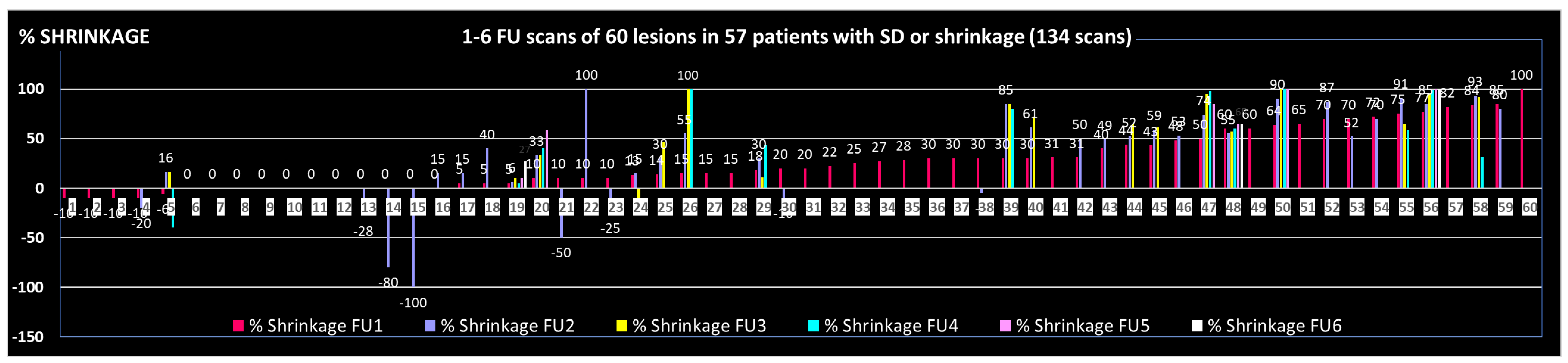
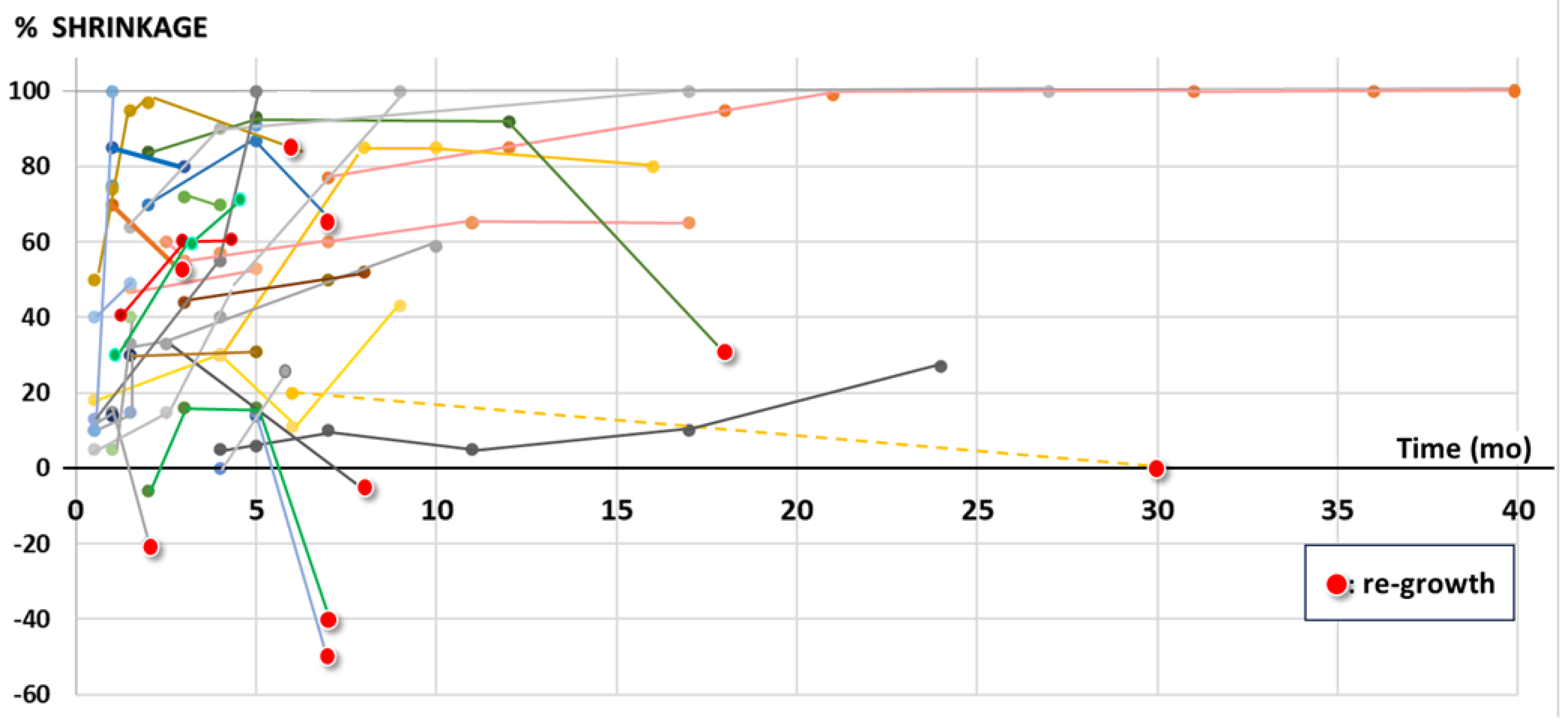
Table 3, with a life-long duration of this effect in most cases, which is supported by the objective duration of volumetric shrinkage as shown in
Figure 1: 9/37 initially shrunk lesions with > 1 FU scan showed re-growth; 4 of these 9 lesions remained smaller than initially, i.e. in only 5/37 (14%) a ‘clinically relevant’ failure during the observed FU time or lifetime was found.
The FU time, based on last available imaging of depicted 37 lesions with at least 2 FU scans was mean/median 9.2/5.0 months (1-40).
3.2. Radiologic/Volumetric Response, Table 5, Figure 2
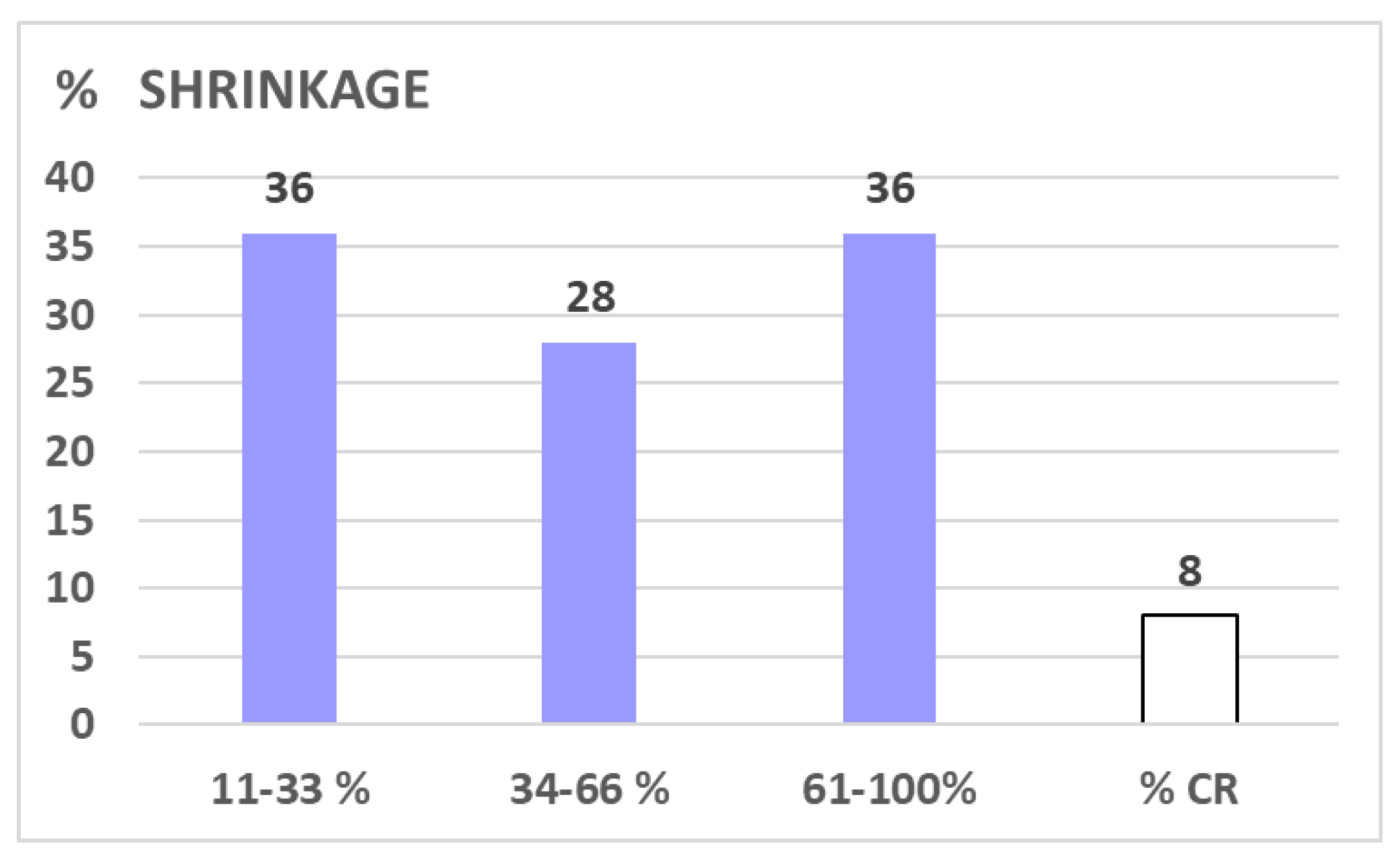
9% of all 81 lesions and 6% of all FU scanned lesions, respectively, failed to respond to LRT (progressive disease, PD: increase of >10% of pre-therapeutic volume). 19% of all lesions with at least one FU scan showed stable disease (SD, defined as +/-10% volume change compared to the pre-LRT volume); the remaining 75% of FU-scanned lesions showed ≥10% shrinkage as compared to the initial volume – mostly already in first FU (i.e. mean 2.8 months post LRT), with a mean/median maximum tumor volume reduction of 47%/63% after 5.5 months. Regarding the extent of shrinkage, about one third of cases reached 11-33%, 34-66%, and 67-100% shrinkage,
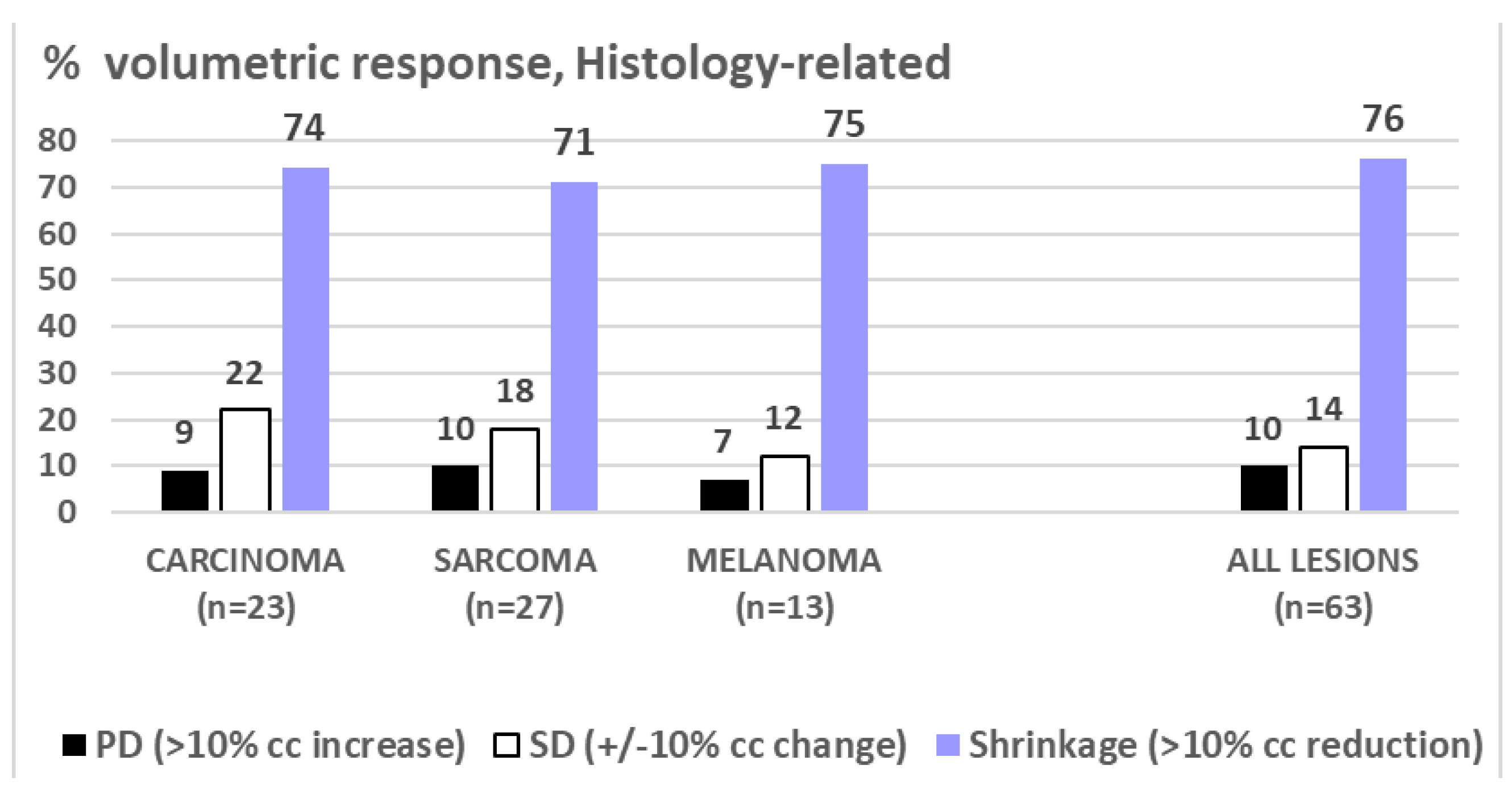
Figure 3. The duration of volumetric re-sponse was mean/median 9.2/5.0 months (1-40) at the time of this analysis. While the number of shrunk lesions/response rate was similar between all three assessed histologies (
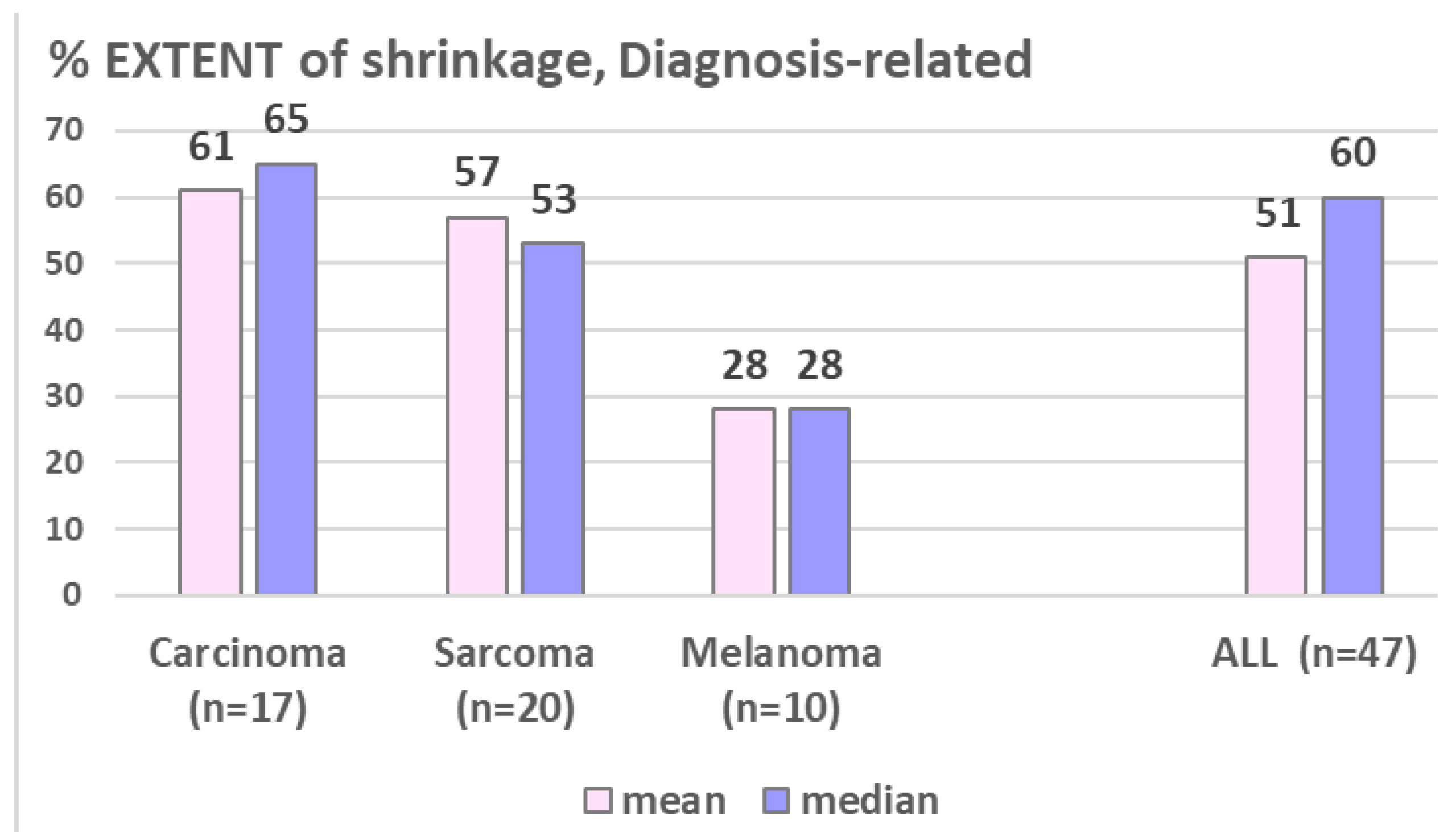
Figure 4), the EXTENT of shrinkage seemed lower in melanomatous compared to carcinomatous and sarcomatous lesions (
Figure 5) – however this observation is to take with caution considering the small and unbalanced sample sizes.
Table 5.
Volumetric response, related to histopathologic diagnosis.
Table 5.
Volumetric response, related to histopathologic diagnosis.
| PARAMETER |
CARCINOMA |
SARCOMA |
MELANOMA |
ALL analyzed LESIONS |
| FU imaging available |
23/34 (68 %) |
28/31 (90 %) |
12/16 (75 %) |
63/81 (78 %) |
| Volumetric response to LRT |
|
|
|
|
| •PD (>10% of initial cc)
|
3/34 (9 %) |
3/31 (10 %) |
1/16 (6 %) |
7/81 (9 %) |
|
•SD (+/-10% of inital cc)
|
5/23 (22 %) |
5/28 (18 %) |
2/12 (17 %) |
12/63 (19 %) |
|
•Shrinkage (<10% of initial cc)
|
17/23 (74 %) |
20/28 (71 %) |
10/12 (83 %) |
47/63 (75 %) |
Figure 2.
Development of the tumor volume in 60 lesions with SD or shrinkage, based on 1 to 6 FU scans/lesion.
Figure 2.
Development of the tumor volume in 60 lesions with SD or shrinkage, based on 1 to 6 FU scans/lesion.
Figure 3.
shows the EXTENT of shrinkage in % in 47 shrunk lesions: about one third of lesions each was found to shrink 1/3, 2/3 and ~3/3. In 8 % of our cohort, macroscopic radiologic complete remission was stated.
Figure 3.
shows the EXTENT of shrinkage in % in 47 shrunk lesions: about one third of lesions each was found to shrink 1/3, 2/3 and ~3/3. In 8 % of our cohort, macroscopic radiologic complete remission was stated.
Figure 4.
shows very similar PERCENTAGE of response (PD/SD/Shrinkage) to LRT among the assessed histopathological diagnoses.
Figure 4.
shows very similar PERCENTAGE of response (PD/SD/Shrinkage) to LRT among the assessed histopathological diagnoses.
Figure 5.
shows the diagnosis-related EXTENT of shrinkage, which seems lower in melanomatous lesion.
Figure 5.
shows the diagnosis-related EXTENT of shrinkage, which seems lower in melanomatous lesion.
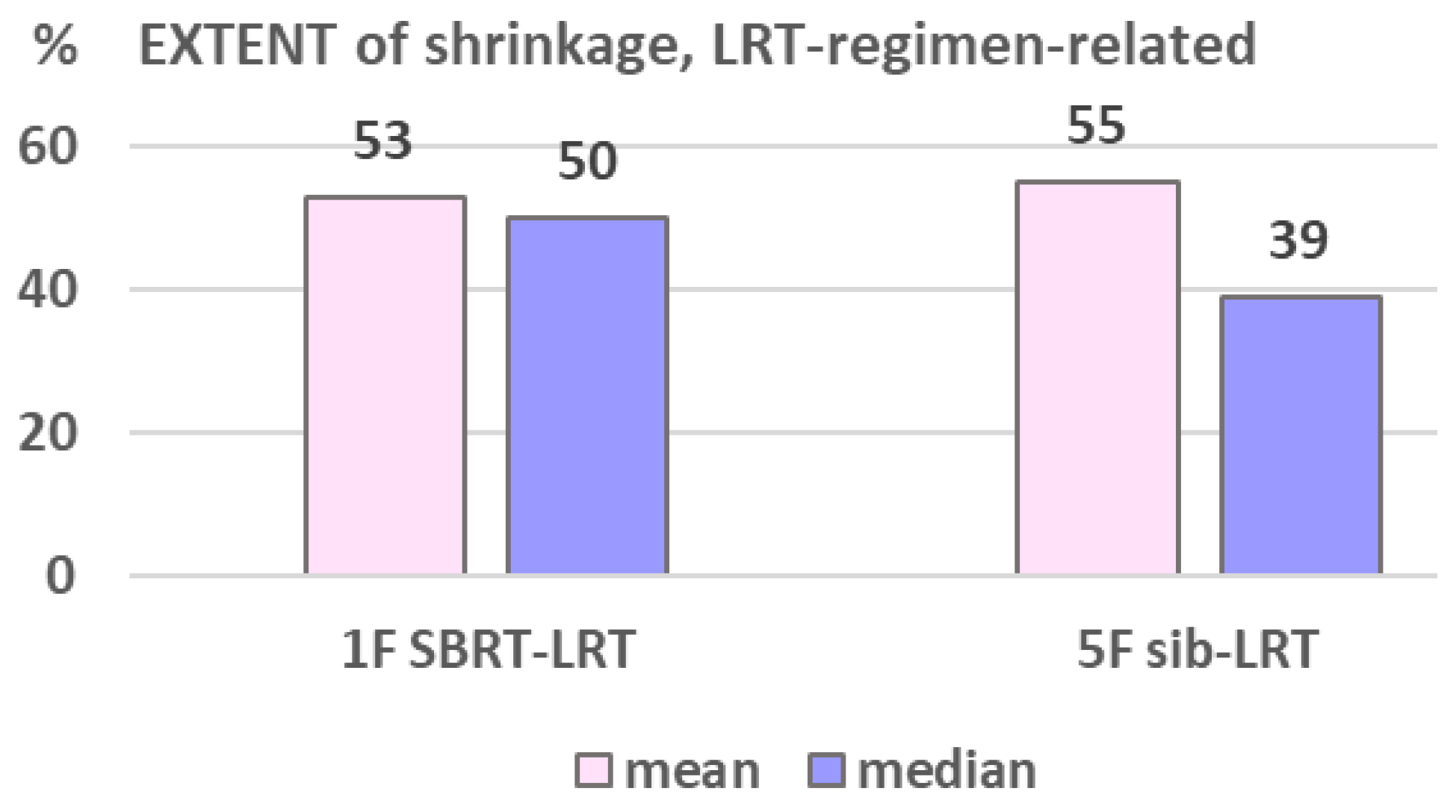
The volumetric response as related to the two used LRT regimens is shown in
Table 6: shrinkage and extent of shrinkage (
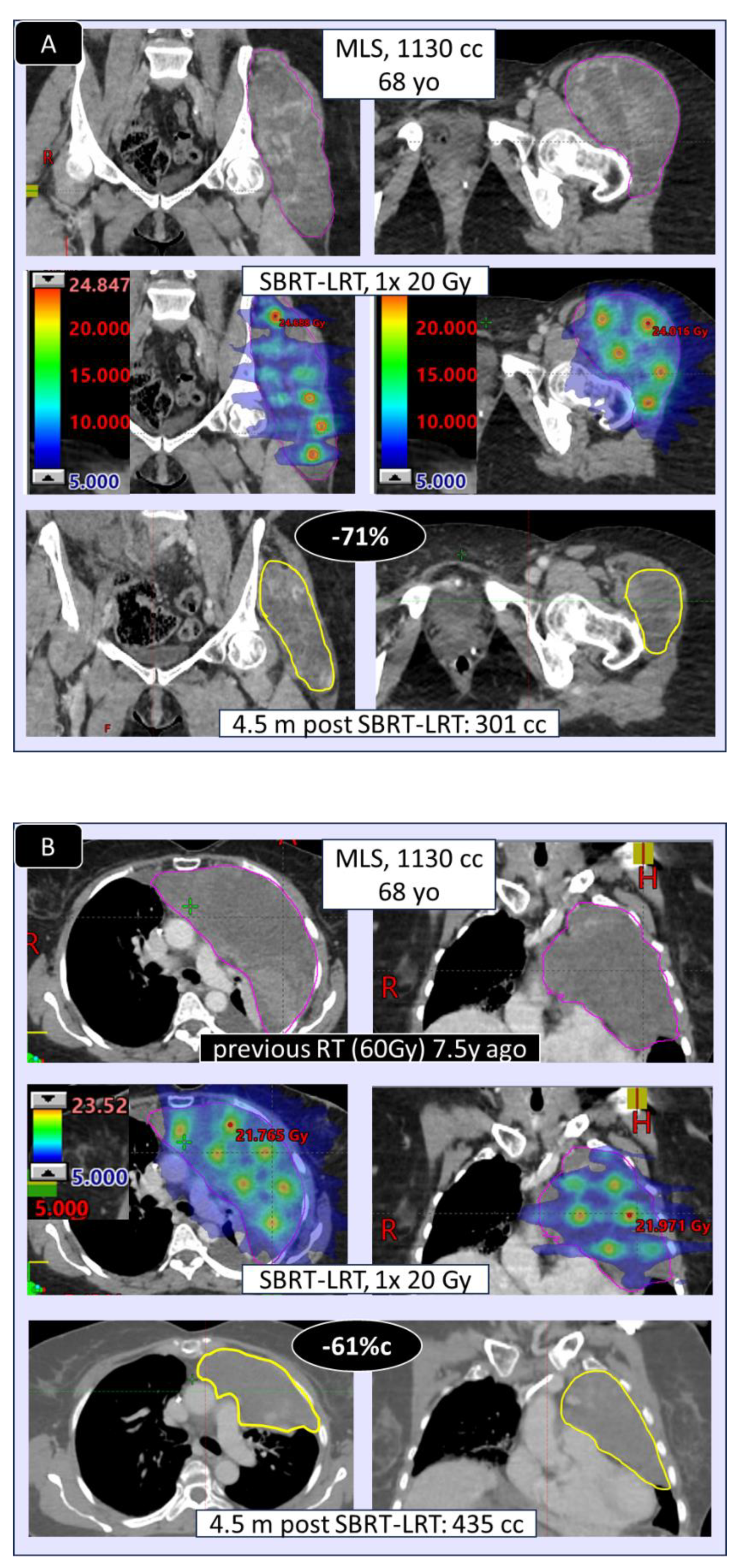
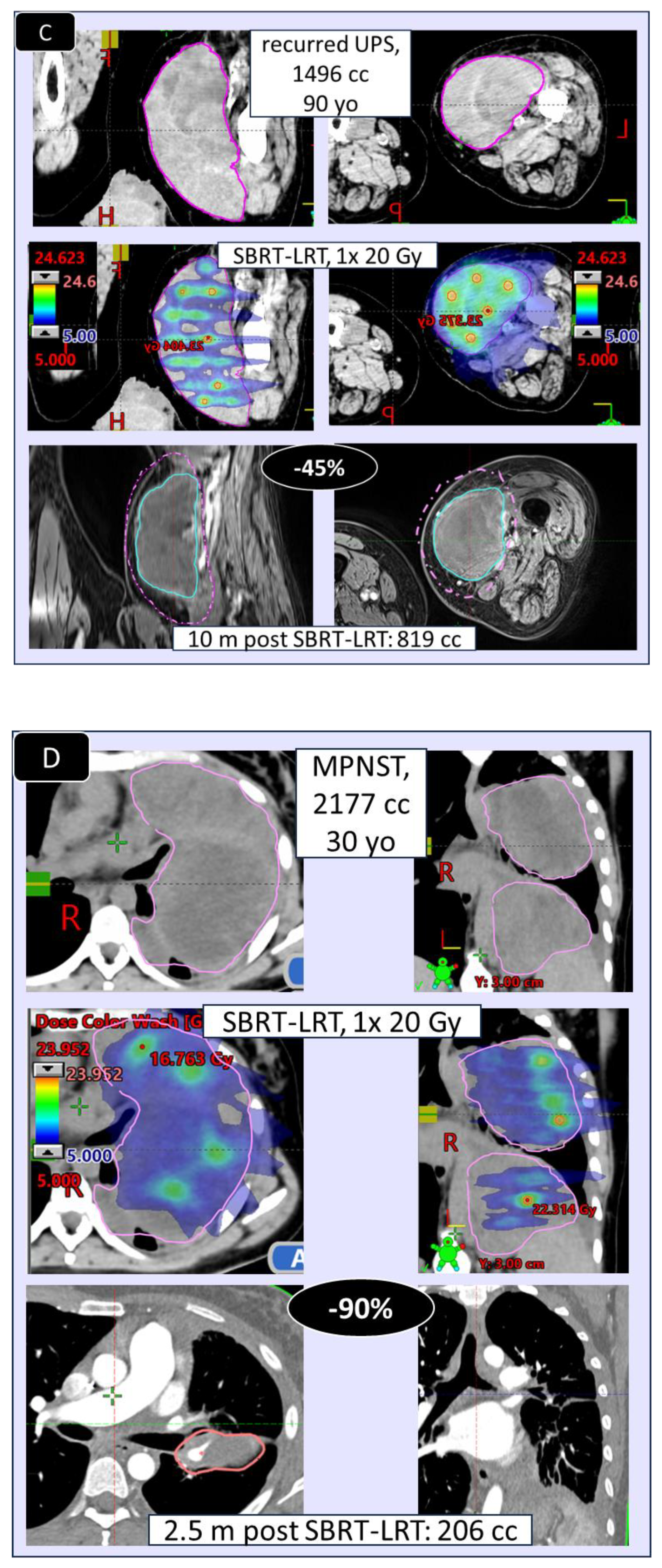
Figure 6) was found very comparable, encouraging to primarily go for the 1F SBRT-LRT regimen. Depicted in
Figure 7 A–D are four representative case illustrations before and after LRT using the 1 fraction SBRT-LRT regimen.
3.3. Toxicity
All patients completed the prescribed short-course LRT. Early tolerance was excellent (Grade 0–1), with only 2 cases of Grade 2–3 dermatitis due to tumors involving the skin; no late toxicity was assessed so far. Most patients felt some mild to moderate fatigue during a few days post LRT, several reported immediate better overall well-being.
4. Discussion
This update analysis of a single center cohort treated with palliative LRT represents -to our best knowledge- the first report on outcome comparison of different LRT regimens and across histopathologic entities. We found similar response rates in carcinomatous, melanomatous and sarcomatous lesions (~75% shrinkage), while the mean extent of shrinkage seemed higher in carcinoma and sarcoma (~50% of initial volume) as compared to melanoma (~28%), Fig. 5 - however, this is to take with caution considering the unbalanced and still small sample sizes. LRT reports on melanomatous lesions are very scant and limited to case reports, not allowing any comparative analysis with the own small subgroup.
Regarding LRT in sarcoma, besides the own here presented cohort of 26 patients with 31 lesions, we found two additional cohorts (53 and 15 cases) with bulky sarcomatous tumors treated with LRT [
10,
12] - taken together 94 patients treated for 110 lesions. After a median FU of 6-10 months, the rate of SD was >60% (difficult to compare, as different definitions of SD were used), with a high percentage of subjective benefit (PROM).
The previously reported duration of subjective and volumetric beneficial effects [
1] has been confirmed (>7 months), i.e. a life-long effect can be expected in most patients of such palliative cohorts.
In addition, we compared two different LRT regimens used. While the SBRT single fraction LRT (regimen A) represents the three-dimensional (3D) version of the original GRID thera-py, as also reported by Jiang et al and Dincer et al [
16,
17], the sib-LRT (B) represents an innovative version of a historic classic and still wide-spread palliative regimen, using homo-geneously calculated 5x 4-5Gy RT with a high-dose integrated boost (sib) as described by Duriseti et al [
18]. The sib-LRT regimen covers the entire tumor mass with an effective, broadly used palliative dose in 5 fractions, while -according to new immunological findings - small isolated hot spots (vertices) enforce protection of immunologically relevant cells in the microenvironment and increase building of neoantigens around the hot spots.
Comparing the two applied regimens (A) and B), different characteristics of the two sub-groups are to consider: the 1 fraction regimen (A) was initially mainly used for very large lesions (mean 1118 vs 733cc) and / or for previously irradiated tumors (46% vs 18%), Ta-ble 6. The very comparable response, despite these unbalanced features, encouraged us to routinely start LRT treatment with regimen (A), which is most convenient for palliative patients. The SBRT-LRT single fraction regimen may be supplemented by regimen (B) if needed – and vice versa, or by normo-fractionated external beam RT as used by several centers.
The presented update analysis may add two new findings in the field of knowledge:
similar volumetric response in carcinoma and sarcoma, and similar volumetric response following the two applied regimens.
In summary, the following clinical outcome characteristics following LRT can so far be drawn from literature and own analyses:
- ~80% of symptomatic patients experience fast subjective relief, in most cases life-long
- progressive disease / treatment failure in ~10%
- stable disease in ~10-20% (defined as +/-10% volume change)
- shrinkage (>10% shrinkage, partial to (rarely) complete response) in ~>70% of cas-es, with
o mean ~50% volume reduction (extent of shrinkage)
o shrinkage of 11-33%/34-66%/67-100% in ~1/3 of cases each
o complete response (CR) in ~5-<10%
o in ~15% regrowth of initially shrunk lesions to a larger than pre-treatment volume (Fig. 1,2)
o response to LRT independent of pre-therapeutic size of lesions [
2]
o response to LRT independent of previous RT vs RT-naïve lesions [
2]
o mostly fast onset of shrinkage (days to weeks) following LRT
o mean effect duration of >7 m, i.e. life-long benefit in palliative patients with large tumors
- similar probability of shrinkage/partial remission across the most frequent histologies
- similar extent of shrinkage in carcinomatous vs sarcomatous lesions, maybe lower extent of shrinkage in melanoma – further analyses on larger samples are required
- likely similar effectiveness of 1F SBRT-LRT vs 5F sib-LRT – further analyses on larger samples are required
5. Conclusions
LRT offers a highly effective and well-tolerated palliative approach for patients with large, inoperable tumors. This extended analysis confirms prior findings of rapid symptom relief and robust tumor response for >7 months.
In addition, LRT was found comparably effective in sarcomatous and carcinomatous lesions using a single fraction SBRT-LRT or 5 fraction sib-LRT. This is -to our best knowledge- the first clinical LRT report proving comparative response benefit across histologic subtypes and different LRT regimens.
Author Contributions
Conceptualization, G.S. and C.G.; methodology, G.S. and C.G.; treatment planning software, T.S., D.J., D.H.; formal analysis, G.S.; investigation, G.S. and C.G.; data curation, G.S., T.S.; writing—original draft preparation, G.S.; writing—review and editing, C.G., B.F.; supervision, C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SFRT |
Spatially Fractionated Radiation Therapy |
| LRT |
Lattice Radiation Therapy |
| SBRT-LRT |
Stereotactic Body Radiation Therapy-LRT |
| SIB |
Simultaneously Integrated Boost |
| SIB-LRT |
Simultaneously Integrated Boost-LRT |
| F |
Fraction (of radiation therapy) |
| PD |
Progressive Disease |
| CR |
Complete Response |
| SD |
Stable Disease |
| OS |
Overall survival |
| MLS |
myxoid liposarcoma |
| UPS |
undifferentiated pleomorphic sarcoma |
| MPNST |
malignant peripheral nerve sheet tumor) |
References
- Prezado Y, Grams M, Jouglar E, Martínez-Rovira I, Ortiz R, Joao Seco J, et al. Spatially fractionated radiation therapy: a critical review on current status of clinical and preclinical studies and knowledge gaps. Phys. Med. Biol. 2024;69;10TR02. [CrossRef]
- Studer G, Jeller D, Streller T, Huebner D, and Glanzmann G. Time-Related Outcome Following Palliative Spatially Fractionated Stereotactic Radiation Therapy (Lattice) of Large Tumors−A Case Series. ARO. 2024;9;101566. [CrossRef]
- Kinj R, Casutt A, Nguyen-Ngoc T, Mampuya A, Schiappacasse L, Bourhis J, et al. Salvage LATTICE radiotherapy for a growing tumour despite conventional radio chemo-therapy treatment of lung cancer. Clin Transl Radiat Oncol. 2022;39:100557. [CrossRef]
- Ferini G, Zagardo V, Viola A, Patanè D, Parisi S, Cuccia F, et al. The Promising Effects of Lattice Radiotherapy for Large, Fungating, or Ulcerating Breast Cancers: A Prospective Single-center Study. In Vivo. 2024;38(5):2484-2493. [CrossRef]
- Xu P, Wang S, Zhou J, Yuan K, Wang X, Li L, et al. Spatially fractionated radiotherapy (Lattice SFRT) in the palliative treatment of locally advanced bulky unresectable head and neck cancer. Clin Transl Radiat Oncol. 2024;30;48:100830. [CrossRef]
- Parisi S, Sciacca M, Critelli P, Ferrantelli G, Chillari F, Venuti V, et al. Lattice radiotherapy in inflammatory breast cancer: report of a first case treated with curative aim. Radiat Oncol J. 2024;42(2);160-165. [CrossRef]
- Amendola B, Perez NC, Wu X, Garcia-Serra A, Amendola MA. The Use of Lattice Radiotherapy (LRT) for Loco-Regionally Advanced Bulky Cervical Cancer. CUREUS, Abstract Published 03/06/2024.
- Liu TF, Liao B-C, Lin M-W, Chang Y-L, Yang W-C. Locally advanced thymoma treated with lattice radiotherapy: a case report. Ther Radiol Oncol. 2024;8:8. [CrossRef]
- Raiden B, Descamps C, Ferraris GA, Diaz Vazquez MF, Caussa L, Gilardi A, et al. Clinical Experience with Palliative Treatment of Bulky Tumors Using VMAT-Based Lattice Radiotherapy. RedJ. 2204;120;2S, ASTRO Poster 2512. [CrossRef]
- Ah-med SK, Petersen IA, Grams MP, Finley RR, Haddock MG, Owen D. Spatially Fractionated Radiation Therapy in Sarcomas: A Large Single-Institution Experience. Adv Radiat Oncol. 2023;4;9(3):101401. [CrossRef]
- Amarell K, Guo B, Cho YB, Ramalingam A, Stephans KL, et al. Spatially Fractionated Ra-diotherapy (SFRT) for Large Tumors: A Single Institutional Experience of Dosimetry and Clinical Outcomes. POSTER 2946. RadJ. 2024;120,2S;2946. [CrossRef]
- Majercakova K, Tejedor Aguilar N, Verdum JI, Vivancos Bargalló H, Vila Capel A, Soto MM, et al. Role of Spatially Fractionated Radiotherapy (LATTICE) Treatment in Inoperable Bulky Soft-Tissue Sarcomas. Cancers. 2025;17(4):624. [CrossRef]
- Iori F, Trojani V, Zamagni A, Ciammella P, Iori M, Botti A, Iotti C. Spatially Fractionated Radiation Therapy for Palliation in Patients With Large Cancers: A Retrospective Study. Adv Radiat Oncol. 2024;10(1):101665. [CrossRef]
- Snider JW, Mayr NA, Molitoris J, Chhabra AM, Mossahebi S, Griffin R, et al. The Radiosurgery Society Working Groups on GRID, LATTICE, Microbeam, and FLASH Radiotherapies: Advancements Symposium and Subsequent Progress Made. PRO. 2025; 15(3):300-307. [CrossRef]
- Li W, Piao M, Zhai L, Zhu Y, Lou F, Chen L, et al. Effectiveness and Safety of Lattice Radiotherapy in Treating Large Volume Tumors: A Systematic Review and Meta-analysis Based on Single-arm Clinical Studies. Balkan Med J 2025;42:311-20. [CrossRef]
- Jiang L, Li X, Zhang J, Li W, Dong F, Chen C, et al. Combined high-dose LATTICE radiation therapy (HDLRT) and immune checkpoint blockade for advanced bulky tumors: The concept and a case report. Front Oncol. 2021;10:548132. [CrossRef]
- Dincer N, Ugurluer G, Korkmaz L, Serkizyan A, Atalar B, Gungor G, et al. Magnetic resonance imaging-guided online adaptive lattice stereotactic body radiotherapy in voluminous liver metastasis: two case reports. Cureus.2022;14:e23980. [CrossRef]
- Duriseti S, Kavanaugh JA, Szymanski J, et al. LITE SABR M1: A phase I trial of lattice stereotactic body radiotherapy for large tumors. Radiother Oncol. 2022;167:317-32. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).