Submitted:
06 October 2025
Posted:
10 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Ethics Committee
2.2. Housing
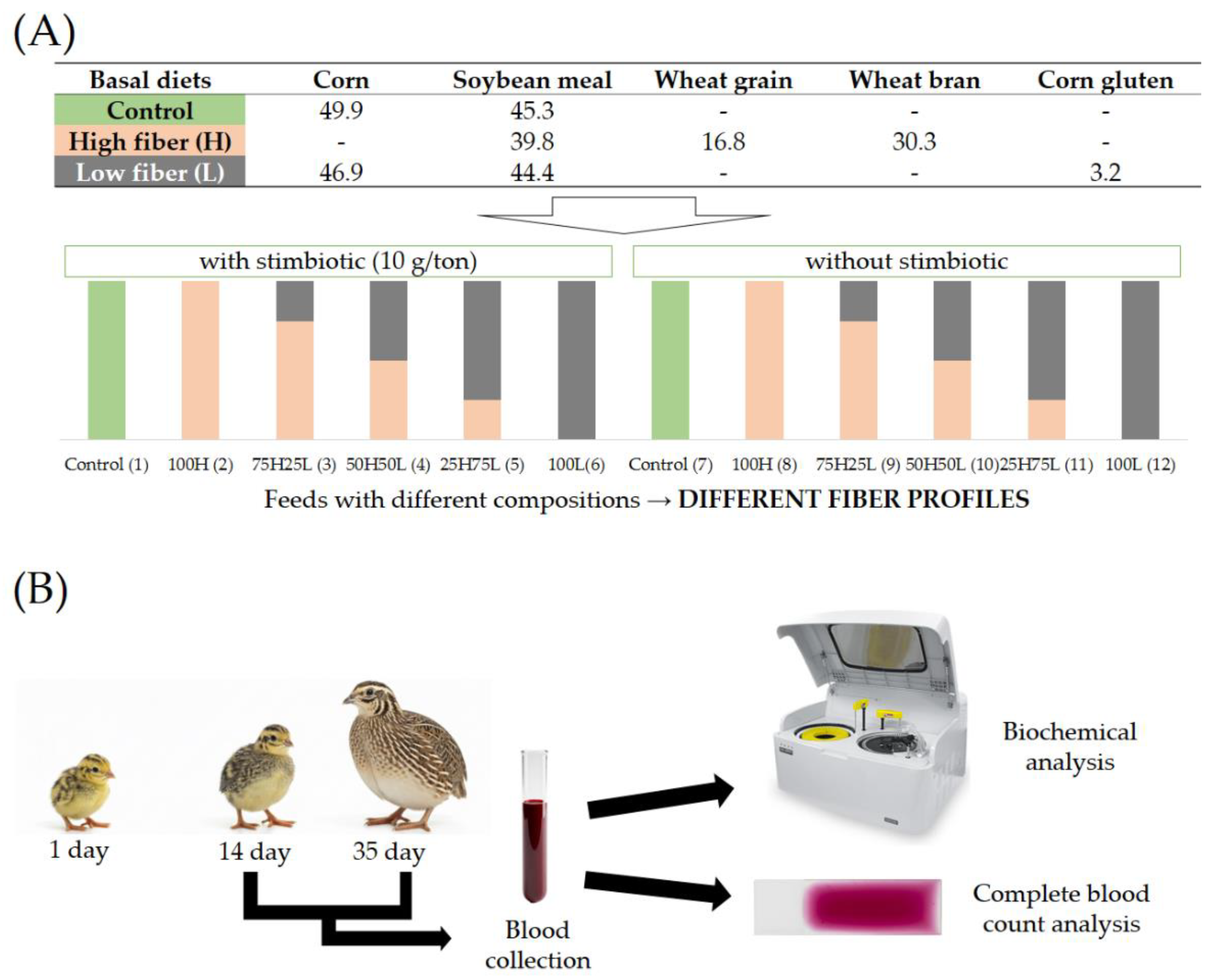
2.3. Animals, Experimental Design and Experimental Diets
2.4. Data Collection
2.5. Collection of Biological Material and Laboratory Analyses
2.6. Statistical Analysis
- Yijk = response variable
- μ = overall mean
- αi = effect of the i-th fiber profile (i = 1 to 6)
- βj = effect of the j-th stimbiotic level (j = 1 to 2)
- (αβ)ij = interaction effect between the i-th fiber profile and the j-th stimbiotic level
- ϵijk = random error term associated with each observation, assumed to be normally distributed with mean zero and constant variance
3. Results
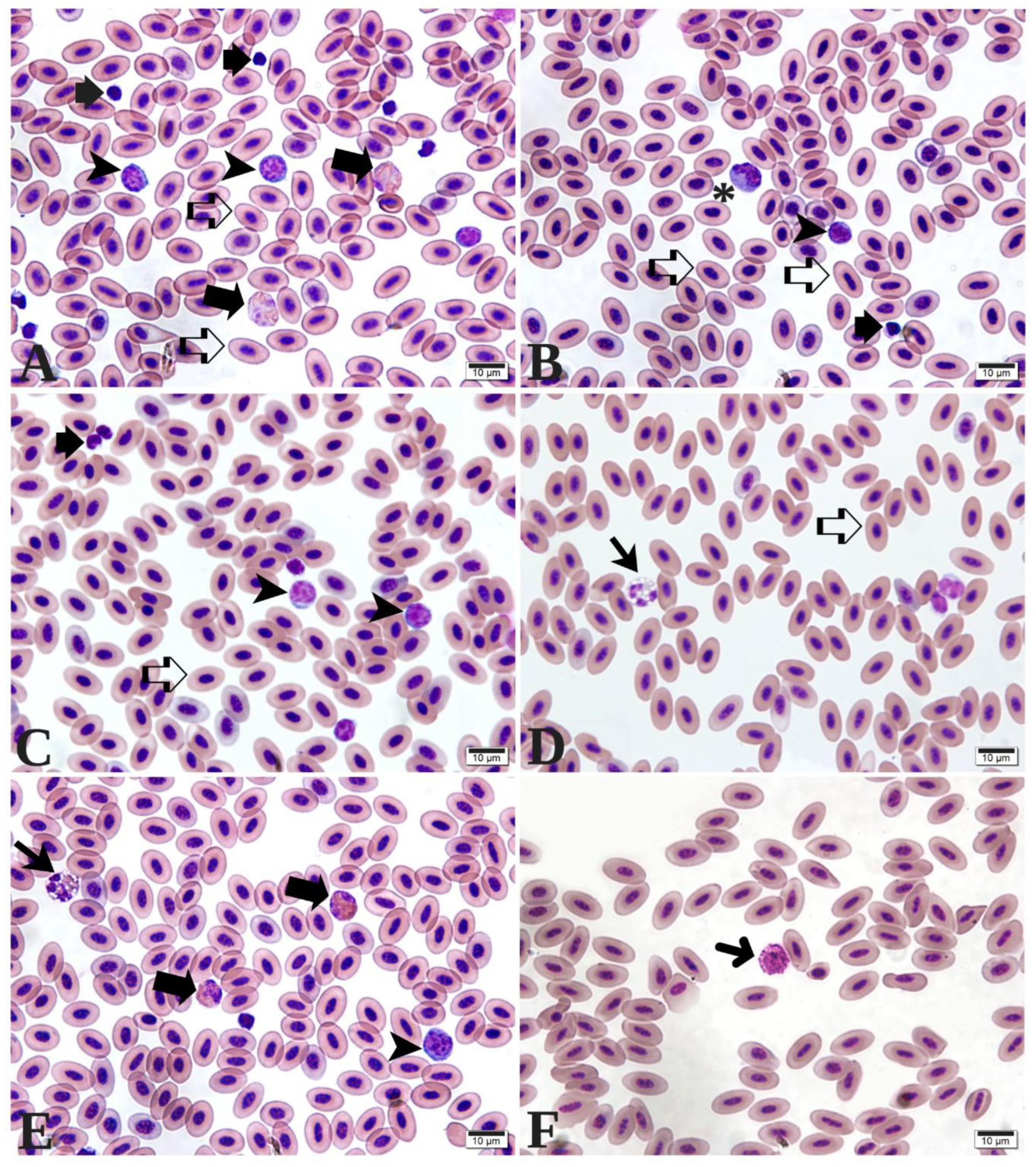
3.1. Hematological Parameters
3.2. Biochemical Parameters
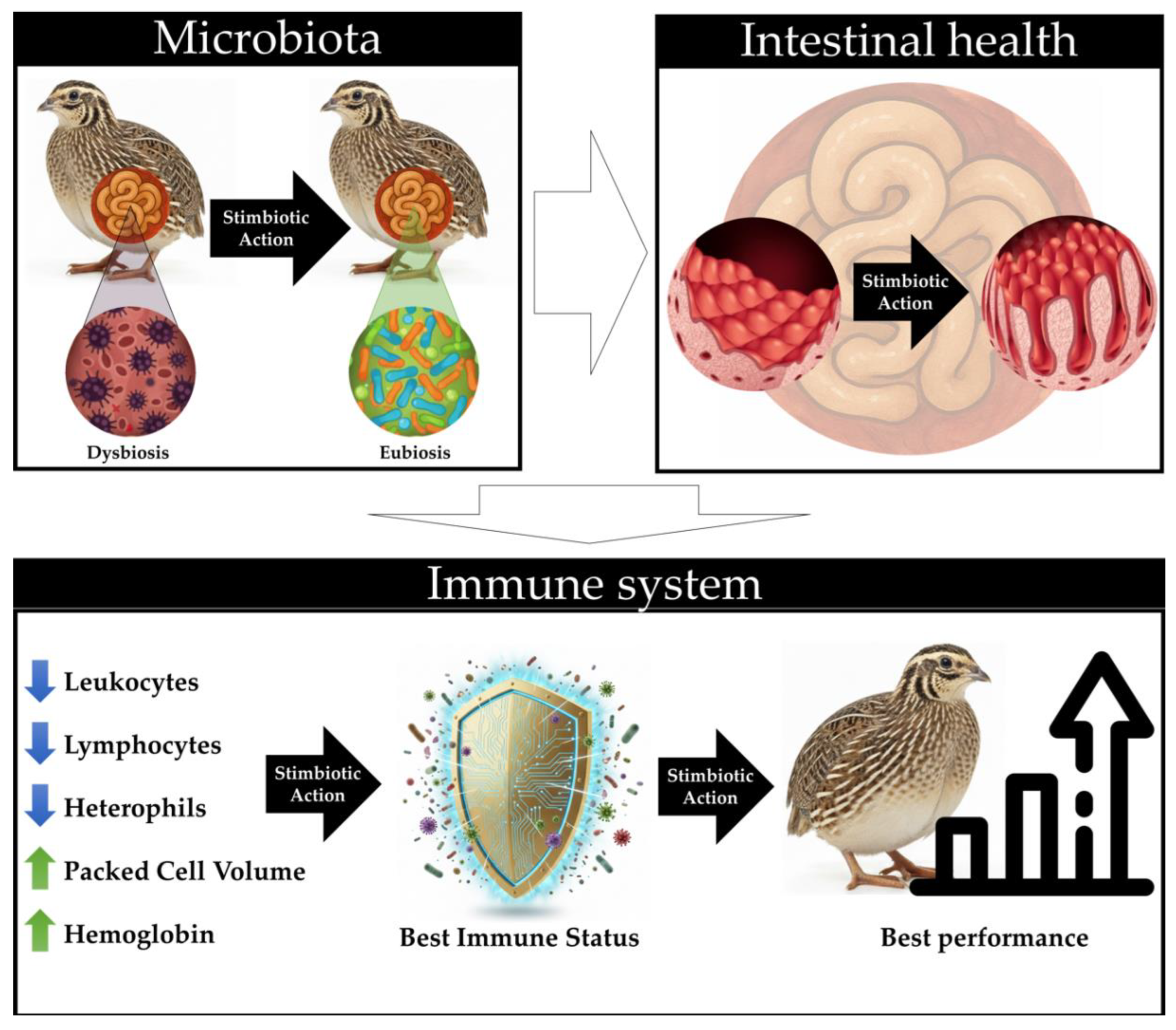
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullere, M.; Woods, M.J.; Van Emmenes, L.; Pieterse, E.; Homman, L.C.; Zotte, A.D. Hermetia illucens Larvae Reared on Diferent Substrates in Broiler Quail Diets: E_ect on Physicochemical and Sensory Quality of the Quail Meat. Animals. [CrossRef]
- Silva, J.H.V.; Jordão Filho, j.; Costa, F.G.P. ; Lacerda. P.B; Vargas, D.G.V; Lima, M.R. Exigências nutricionais de codornas. Revista Brasileira de Saúde e Produção Animal. [CrossRef]
- Rostagno, H.S; Albino, L.F.T; Calderano, A.A.; Hannas, M.I.; Sakomura, N.K.; Costa, F.G.P; Rocha, G.C.; Saraiva, A.; Abreu, M. L. T. Tabelas Brasileiras para Aves e Suínos: composição de alimentos e exigências nutricionais. 5th edn. (UFV: Viçosa. Brazil). 2024.
- Costa, F.G.P; Lima, A.V; Souza, P.E.L; Rodrigues, A.B; Nascimento, C.H; Cavalcante, D.T; Lima, M.R; Kaneko, IN. Eficácia de diferentes fontes de metionina em dietas de codornas europeias. Observatorio de la Economía Latinoamericana. [CrossRef]
- Annison, G. Relationship between the levels of soluble nonstarch polysaccharides and the apparent metabolizable energy of wheats assayed in broiler chickens. J. Agric. Food Chem 1991, 1252–1256. [Google Scholar] [CrossRef]
- Jha, R. , Fouhse, J.M., Tiwari, U.P., Li, L., Willing, B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci 2019. 6. 48. [CrossRef]
- Mateos, G.; Fondevila, G.; C´amara, L. The importance of the fibre fraction of the feed in non-ruminant diets. In The Value of Fibre: Engaging the Second Brain for Animal Nutrition, 1st ed.; González-Ortiz, G., Bedford, M.R., Bach Knudsen, K.E., Courtin, C.M., Classen, H.L., Eds.; Wageningen Academic Publishers: Wageningen, Netherlands, 2019; pp. 61–83. [Google Scholar] [CrossRef]
- Jha, R. , Berrocoso, J. Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- kasireddy, B.; Lourenco, J.; Gonzalez-ortiz, G.; Bedford, M.R.; Olukosi, O.A. Growth performance, nutrient utilization, gut integrity, short-chain fatty acids, and gene expression in Eimeria-challenged broilers receiving stimbiotics and wheat bran as an additional fiber source. Poultry Science 2025, 4877. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B. Challenges with nonfiber carbohydrate methods. Journal of Animal Science 2003, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M. , Cowieson, A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci Technol. [CrossRef]
- Macari, M.; Maiorka, A. Fisiologia das aves comerciais. (PROL Editora Gráfica: Jaboticabal. Brazil). 2017. 806p.
- Nguyen, H. , Bedford, M., Wu, S-B., Morgan, N.K. Soluble non-starch polysaccharide modulates broiler gastrointestinal tract environment. Poult. Sci, 2021; 100, 101183. [Google Scholar] [CrossRef]
- Jonchère, V.; Bussière, F.I.; Zemb, O.; Khaksar, V.; Cornaille, L.; Gambier, E.; Derouin-Tochon, F.; Hervo, F.; Schouler, C.; Rousseau, X.; Bedford, M.; Mignon, S.V.; Duclos, M.J.; Guabiraba, R.; Narcy, A. Differentiated short- and long-term impacts of a starter stimbiotic supplementation on gut health in broilers fed wheat and rye-based diets at homeostasis and under Eimeria tenella challenge. Animal Nutrition. [CrossRef]
- Toghyani, M.; Kim, E.; Macelline, S.P.; González-Ortiz, G.; Barekatain, R.; Liu, S.Y. Xylanase and stimbiotic supplementation improve broilers performance and nutrient digestibility across both wheat-barley and corn-based diets. Poultry Science 2025, 104, 105224. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Greenwood, W.; Santos, T.T. A new perspective on maximising the potential of dietary fibre, xylanase and gut health. ABVista 2020, 26-27, 2020. [Google Scholar]
- Davies, C.; Gonzalez-Ortiz, G.; Rinttila, T.; Apajalahti, J.; Alyassin, M.; Bedford, M.R. Stimbiotic supplementation and xylose-rich carbohydrates modulate broiler's capacity to ferment fibre. Front Microbiol. [CrossRef]
- Veluri, S.; Gonzalez-Ortiz, G.; Bedford, M.R.; Olukosi, O.A. Interactive effects of a stimbiotic supplementation and wheat bran inclusion in corn- or wheat-based diets on growth performance, ileal digestibility, and expression of nutrient transporters of broilers chickens. Poult Sci. 2024, 103, 103178. [Google Scholar] [CrossRef] [PubMed]
- Rajman, M.; Juráni, M.; Lamosová, D.; Macajová, M.; Sedlacková, M.; Kostál, L.; Vyboh, P. The effects of feed restriction on plasma biochemistry in growing meat type chickens (Gallus gallus). Comparative Biochemistry and Physiology Part A: Molecular e Integrative Physiolog. [CrossRef]
- Faria, P.P.D.; Cruz, L.C.F.; Sampaio, S.A.; Borges, K.F.; Minafra, C.S. Análises bioquímicas para frango de corte – revisão. Revista Eletrônica NutriTime 2021, 18, 06. [Google Scholar]
- Lee, J.H.; Lee, B.; Rousseau, X.; Gomes, G.A.; Oh, H.J.; Kim, Y.J.; Chang, S.Y.; An, J.W.; Go, Y.B.; Song, D.C.; Cho, H.A.; Cho, J.H. Stimbiotic supplementation modulated intestinal inflammatory response and improved broilers performance in an experimentally induced necrotic enteritis infection model. Journal of Animal Science and Biotechnology. [CrossRef]
- Ren, Y.; Tian, Y.; Hou, M.; Zhao, Y.; Li, J.; Aftab, U.; Rousseau, X.; Jiang, R.; Kang, X.; Tian, Y.; Gong, Y. Evaluation of stimbiotic on growth performance and intestinal development of broilers fed corn- or wheat-based diets. Poultry Science 2023, 102, 103094. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.H.V.; Costa, F.G.P. Tabelas para codornas japonesas e europeias. 2th edn. (FUNEP: Jabuticabal. Brazil). 2009. 110p.
- Jain N., C. Essentials of Veterinary Hematology. 1st. (Wiley: Pennsylvania. EUA). 1993.
- Weiss D., J. , Wardrop K. Schalm's Veterinary Hematology. 6nd ed. (WileyBlackwell: Nova Jersey. EUA). 2010. 1206p.
- Kerr M., G. . Exames Laboratoriais em Medicina Veterinária: bioquímica clínica e hematologia. 2nd ed. (Roca: São Paulo. Brazil) 2003. 465p.
- Schalm O., W. , Jain N. C., Carroll E. J. Scham’s Veterinary Hematology. 4nd ed. (Lea & Febiger: Philadelphia. EUA). 1986. 1221p.
- Onbaşılar, E.E.; Aksoy, F.T. Stress parameters and immune response of layers under different cage floor and density conditions. Livest. prod. Sci. 2005, 95, 255–263. [Google Scholar] [CrossRef]
- R Core Team. R: a language and environment for statistical computing (ver. 4.2.0).” (R Foundation for Statistical Compu-ting: Vienna. Austria). 2022. https://www.R-project.org/.
- Wang, H.; et al. Stimbiotic improves intestinal morphology and tight junction integrity in broilers fed corn- or wheat-based diets. Animals. [CrossRef]
- Martinez, I.; Brito, A.B.; Costa, M.A.; Garcia, A. Fermentação de polissacarídeos não amiláceos e sua aplicação na nutrição de aves. aviNews Brasil 2024. [Google Scholar]
- Thrall, M.A. ; Weiser. G.; Allison, R.,; Campbell, T.W. Hematologia e Bioquímica Clínica Veterinária. 2. ed. (Roca: São Paulo. Brazil). 2014.
- Garber, A.; PARRA, D. Estimbióticos: um elemento essencial das estratégias de resiliência nutricional. aviNews Brasil 2021. [Google Scholar]
- Mitchell, E.B.; Johns, J. Avian hematology and Related Disorders. Vet Clin Exot Anim. [CrossRef]
- Paul, M.S.; Paolucci, S.; Barjesteh, N. ; Wood. R.D.; Schat, R.A.; Sharif, S. Characterization of Chicken Thrombocyte Responses to Toll-Like Receptor Ligands. Plos One. [CrossRef]
- Claver, J. A.; Quaglia, A. I. E. Comparative Morphology, Development, and Function of Blood Cells in Nonmammalian Vertebrates. Journal of Exotic Pet Medicine. [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathogens. [CrossRef]
- Gross, W.B.; Siegel, H.S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Diseases 1983, 27, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.S.; Silva, P.L.; Guimarães, E.C.; Lellis, C.G.; Mundim, A.V. Variações fisiológicas, influência da idade e sexo no perfil bioquímico sanguíneo de aves da linhagem pesada de frango de corte na fase de recria. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 2019. [Google Scholar] [CrossRef]
- Evans, A.J.; Bannister, D.W.; Whitehead, C.C.; Siller, W.G.; Wight, P.A.L. Changes in plasma lipid and glucose levels during the onset of fatty liver and kidney syndrome in chicks. Res. Vet. Sci. [CrossRef]
- Silva, P.R.L.; Freitas Neto, O.C.; Larurentiz, A.C.; Junqueira, O.M.; Fagliari, J.J. Blood serum components and serum protein test of Hybro-PG broilers of different ages. Braz. J. Poult. Sci, 2007. [Google Scholar] [CrossRef]
- Choct, M. Managing gut health through nutrition. British Poultry Science. [CrossRef]
- Svihus, B. Function of the digestive system. Journal of Applied Poultry Research. [CrossRef]
- Yue, Y.; Luasiri, P.; Li, J. , Laosam, P.; Sangsawad, P. Research advancements on the diversity and host interactions of the chicken gut microbiome. Frontiers in Veterinary Science. [CrossRef]
- Chesson, A. Non-starch polysaccharide-degrading enzymes in poultry diets: Influence of ingredients on the selection of activities. World's Poultry Science Journal. [CrossRef]
- Cirule, D.; Krama, T.; Vrublevska, J.; Rantala, M.J; Krams, I. A rapid effect of handling on counts of white blood cells in a wintering passerine bird: a more practical measure of stress? Journal of Ornithology. [CrossRef]
- Grunkemeyer, V.L. Advanced diagnostic approaches and current management of avian hepatic disorders. Veterinary Clinics: Exotic Animal Practice. [CrossRef]
- Lumeij, J.T. Avian clinical biochemistry. In Clinical biochemistry. In Clinical biochemistry of domestic animals, 5th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: San Diego, USA, 1997; pp. 885–905. [Google Scholar] [CrossRef]
- Campbell, T. W. Veterinary hematology and clinical chemistry. 2. ed. (Wiley-Blackwell: Iowa, USA). 2012. 772p.
| Ingredients, % | With Stimbiotic | Without Stimbiotic | ||||||||||
| Control | 100H | 75H25L | 50H50L | 25H75L | 100L | Control | 100H | 75H25L | 50H50L | 25H75L | 100L | |
| Corn | 49.885 | 0.000 | 11.723 | 23.446 | 35.169 | 46.892 | 49.885 | 0.000 | 11.723 | 23.446 | 35.169 | 46.892 |
| Soybean meal | 45.331 | 39.758 | 40.913 | 42.068 | 43.223 | 44.378 | 45.331 | 39.758 | 40.913 | 42.068 | 43.223 | 44.378 |
| Whole wheat | 0.000 | 16.799 | 12.599 | 8.399 | 4.200 | 0.000 | 0.000 | 16.799 | 12.599 | 8.399 | 4.200 | 0.000 |
| Wheat bran | 0.000 | 30.337 | 22.753 | 15.169 | 7.584 | 0.000 | 0.000 | 30.337 | 22.753 | 15.169 | 7.584 | 0.000 |
| Corn gluten | 0.000 | 0.000 | 0.804 | 1.607 | 2.411 | 3.215 | 0.000 | 0.000 | 0.804 | 1.607 | 2.411 | 3.215 |
| Soybean oil | 1.544 | 10.100 | 8.145 | 6.190 | 4.235 | 2.280 | 1.544 | 10.100 | 8.145 | 6.190 | 4.235 | 2.280 |
| Dicalcium phosphate | 1.053 | 0.384 | 0.544 | 0.704 | 0.864 | 1.023 | 1.053 | 0.384 | 0.544 | 0.704 | 0.864 | 1.023 |
| Limestone | 1.110 | 1.484 | 1.395 | 1.306 | 1.218 | 1.129 | 1.110 | 1.484 | 1.395 | 1.306 | 1.218 | 1.129 |
| Salt | 0.393 | 0.389 | 0.386 | 0.384 | 0.382 | 0.379 | 0.393 | 0.389 | 0.386 | 0.384 | 0.382 | 0.379 |
| L-Lysine | 0.098 | 0.153 | 0.143 | 0.134 | 0.125 | 0.116 | 0.098 | 0.153 | 0.143 | 0.134 | 0.125 | 0.116 |
| DL-Methionine | 0.373 | 0.385 | 0.382 | 0.379 | 0.376 | 0.373 | 0.373 | 0.385 | 0.382 | 0.379 | 0.376 | 0.373 |
| L-Threonine | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mineral premix1 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 |
| Vitamin premix2 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Quantum Blue3 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| Signis4 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Inert | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| Total | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 |
| Calculated nutrients | ||||||||||||
| CP, % | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| ME, kcal/kg | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 |
| CF, % | 2.808 | 4.855 | 4.386 | 3.916 | 3.446 | 2.977 | 2.808 | 4.855 | 4.386 | 3.916 | 3.446 | 2.977 |
| NDF, % | 13.049 | 19.383 | 17.960 | 16.538 | 15.115 | 13.693 | 13.049 | 19.383 | 17.960 | 16.538 | 15.115 | 13.693 |
| MM, % | 3.333 | 4.052 | 3.895 | 3.738 | 3.582 | 3.425 | 3.333 | 4.052 | 3.895 | 3.738 | 3.582 | 3.425 |
| Starch, % | 34.519 | 21.041 | 24.075 | 27.110 | 30.144 | 33.178 | 34.519 | 21.041 | 24.075 | 27.110 | 30.144 | 33.178 |
| Total NSP, % | 10.269 | 15.100 | 14.031 | 12.961 | 11.892 | 10.822 | 10.269 | 15.100 | 14.031 | 12.961 | 11.892 | 10.822 |
| Insoluble NSP, % | 8.365 | 12.181 | 11.354 | 10.527 | 9.699 | 8.872 | 8.365 | 12.181 | 11.354 | 10.527 | 9.699 | 8.872 |
| Soluble NSP, % | 1.904 | 2.919 | 2.677 | 2.434 | 2.192 | 1.950 | 1.904 | 2.919 | 2.677 | 2.434 | 2.192 | 1.950 |
| Total AX, % | 3.405 | 6.749 | 5.993 | 5.238 | 4.483 | 3.727 | 3.405 | 6.749 | 5.993 | 5.238 | 4.483 | 3.727 |
| Soluble AX, % | 0.327 | 0.900 | 0.762 | 0.625 | 0.487 | 0.350 | 0.327 | 0.900 | 0.762 | 0.625 | 0.487 | 0.350 |
| Insoluble AX, % | 3.078 | 5.847 | 5.230 | 4.612 | 3.995 | 3.377 | 3.078 | 5.847 | 5.230 | 4.612 | 3.995 | 3.377 |
| EE, % | 4.731 | 12.495 | 10.726 | 8.957 | 7.189 | 5.420 | 4.731 | 12.495 | 10.726 | 8.957 | 7.189 | 5.420 |
| Calcium, % | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 | 0.850 |
| Available phosphorus, % | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 | 0.320 |
| Sodium, % | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 | 0.170 |
| Chlorine, % | 0.302 | 0.286 | 0.289 | 0.292 | 0.295 | 0.297 | 0.302 | 0.286 | 0.289 | 0.292 | 0.295 | 0.297 |
| Potassium, % | 0.989 | 1.127 | 1.094 | 1.060 | 1.027 | 0.994 | 0.989 | 1.127 | 1.094 | 1.060 | 1.027 | 0.994 |
| Digestible amino acids | ||||||||||||
| Lysine, % | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 | 1.370 |
| Methionine, % | 0.703 | 0.692 | 0.695 | 0.697 | 0.699 | 0.702 | 0.703 | 0.692 | 0.695 | 0.697 | 0.699 | 0.702 |
| Methionine + Cysteine, % | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 | 1.040 |
| Threonine, % | 0.856 | 0.787 | 0.803 | 0.819 | 0.835 | 0.852 | 0.856 | 0.787 | 0.803 | 0.819 | 0.835 | 0.852 |
| Tryptophan, % | 0.297 | 0.306 | 0.303 | 0.300 | 0.296 | 0.293 | 0.297 | 0.306 | 0.303 | 0.300 | 0.296 | 0.293 |
| Valine, % | 1.057 | 1.020 | 1.029 | 1.038 | 1.048 | 1.057 | 1.057 | 1.020 | 1.029 | 1.038 | 1.048 | 1.057 |
| Ingredients, % | With Stimbiotic | Without Stimbiotic | ||||||||||
| Control | 100H | 75H25L | 50H50L | 25H75L | 100L | Control | 100H | 75H25L | 50H50L | 25H75L | 100L | |
| Corn | 60.491 | 0.000 | 14.245 | 28.490 | 42.735 | 56.980 | 60.491 | 0.000 | 14.245 | 28.490 | 42.735 | 56.980 |
| Soybean meal | 34.809 | 28.153 | 29.773 | 31.393 | 33.012 | 34.632 | 34.809 | 28.153 | 29.773 | 31.393 | 33.012 | 34.632 |
| Whole wheat | 0.000 | 36.122 | 27.091 | 18.061 | 9.030 | 0.000 | 0.000 | 36.122 | 27.091 | 18.061 | 9.030 | 0.000 |
| Wheat bran | 0.000 | 3.761 | 2.821 | 1.880 | 0.940 | 0.000 | 0.000 | 3.761 | 2.821 | 1.880 | 0.940 | 0.000 |
| Corn gluten | 0.000 | 19.834 | 15.613 | 11.392 | 7.171 | 2.950 | 0.000 | 19.834 | 15.613 | 11.392 | 7.171 | 2.950 |
| Soybean oil | 2.055 | 9.628 | 7.929 | 6.231 | 4.532 | 2.833 | 2.055 | 9.628 | 7.929 | 6.231 | 4.532 | 2.833 |
| Dicalcium phosphate | 0.868 | 0.534 | 0.609 | 0.685 | 0.760 | 0.835 | 0.868 | 0.534 | 0.609 | 0.685 | 0.760 | 0.835 |
| Limestone | 0.937 | 1.114 | 1.074 | 1.033 | 0.993 | 0.953 | 0.937 | 1.114 | 1.074 | 1.033 | 0.993 | 0.953 |
| Salt | 0.345 | 0.263 | 0.280 | 0.298 | 0.315 | 0.333 | 0.345 | 0.263 | 0.280 | 0.298 | 0.315 | 0.333 |
| L-Lysine | 0.000 | 0.092 | 0.069 | 0.046 | 0.023 | 0.000 | 0.000 | 0.092 | 0.069 | 0.046 | 0.023 | 0.000 |
| DL-Methionine | 0.223 | 0.187 | 0.194 | 0.202 | 0.210 | 0.217 | 0.223 | 0.187 | 0.194 | 0.202 | 0.210 | 0.217 |
| L-Threonine | 0.059 | 0.100 | 0.088 | 0.077 | 0.065 | 0.054 | 0.059 | 0.100 | 0.088 | 0.077 | 0.065 | 0.054 |
| Mineral premix1 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 |
| Vitamin premix2 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Quantum Blue3 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| Signis4 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Inert | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| Total | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 |
| Calculated nutrients | ||||||||||||
| CP, % | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| ME, kcal/kg | 3050 | 3050 | 3050 | 3050 | 3050 | 3050 | 3050 | 3050 | 3050 | 3050 | 3050 | 3050 |
| CF, % | 2.540 | 4.020 | 3.693 | 3.366 | 3.039 | 2.712 | 2.540 | 4.020 | 3.693 | 3.366 | 3.039 | 2.712 |
| NDF, % | 13.084 | 16.736 | 15.968 | 15.200 | 14.432 | 13.664 | 13.084 | 16.736 | 15.968 | 15.200 | 14.432 | 13.664 |
| MM, % | 2.805 | 3.611 | 3.439 | 3.268 | 3.096 | 2.925 | 2.805 | 3.611 | 3.439 | 3.268 | 3.096 | 2.925 |
| Starch, % | 40.580 | 27.205 | 30.131 | 33.056 | 35.982 | 38.908 | 40.580 | 27.205 | 30.131 | 33.056 | 35.982 | 38.908 |
| Total NSP, % | 9.017 | 14.000 | 12.901 | 11.803 | 10.704 | 9.605 | 9.017 | 14.000 | 12.901 | 11.803 | 10.704 | 9.605 |
| Insoluble NSP, % | 7.555 | 11.641 | 10.748 | 9.856 | 8.964 | 8.071 | 7.555 | 11.641 | 10.748 | 9.856 | 8.964 | 8.071 |
| Soluble NSP, % | 1.462 | 2.359 | 2.153 | 1.946 | 1.740 | 1.534 | 1.462 | 2.359 | 2.153 | 1.946 | 1.740 | 1.534 |
| Total AX, % | 3.322 | 6.380 | 5.691 | 5.001 | 4.312 | 3.622 | 3.322 | 6.380 | 5.691 | 5.001 | 4.312 | 3.622 |
| Soluble AX, % | 0.275 | 0.800 | 0.675 | 0.550 | 0.425 | 0.300 | 0.275 | 0.800 | 0.675 | 0.550 | 0.425 | 0.300 |
| Insoluble AX, % | 3.047 | 5.577 | 5.013 | 4.450 | 3.886 | 3.322 | 3.047 | 5.577 | 5.013 | 4.450 | 3.886 | 3.322 |
| EE, % | 5.344 | 11.705 | 10.296 | 8.887 | 7.478 | 6.070 | 5.344 | 11.705 | 10.296 | 8.887 | 7.478 | 6.070 |
| Calcium, % | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 | 0.700 |
| Available phosphorus, % | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 | 0.270 |
| Sodium, % | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 | 0.150 |
| Chlorine, % | 0.278 | 0.244 | 0.251 | 0.258 | 0.266 | 0.273 | 0.278 | 0.244 | 0.251 | 0.258 | 0.266 | 0.273 |
| Potassium, % | 0.831 | 0.892 | 0.880 | 0.868 | 0.857 | 0.845 | 0.831 | 0.892 | 0.880 | 0.868 | 0.857 | 0.845 |
| Digestible amino acids | ||||||||||||
| Lysine, % | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 |
| Methionine, % | 0.510 | 0.471 | 0.479 | 0.488 | 0.497 | 0.506 | 0.510 | 0.471 | 0.479 | 0.488 | 0.497 | 0.506 |
| Methionine + Cysteine, % | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 | 0.800 |
| Threonine, % | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 |
| Tryptophan, % | 0.242 | 0.236 | 0.237 | 0.239 | 0.240 | 0.241 | 0.242 | 0.236 | 0.237 | 0.239 | 0.240 | 0.241 |
| Valine, % | 0.880 | 0.920 | 0.913 | 0.906 | 0.899 | 0.892 | 0.880 | 0.920 | 0.913 | 0.906 | 0.899 | 0.892 |
| Variables | Stimbiotic | Fiber | SEM | P-Value | ||||||||
| - | + | Control | 100H | 75H25L | 50H50L | 25H75L | 100L | S | F | S*F | ||
| Packed cell volume, L/L | 0.341 | 0.343 | 0.351 | 0.344 | 0.334 | 0.337 | 0.342 | 0.343 | 2.76 | 0.6586 | 0.6129 | 0.8699 |
| Erythrocyte count, ×10¹²/L | 2.49 | 2.39 | 2.45 | 2.41 | 2.29 | 2.44 | 2.46 | 2.60 | 0.39 | 0.2353 | 0.3883 | 0.7497 |
| Hemoglobin, g/dL | 6.61b | 7.35a | 7.16 | 7.02 | 6.86 | 6.87 | 6.91 | 7.08 | 0.75 | <0.001 | 0.8249 | 0.2448 |
| MCV, fL | 139 | 145.95 | 145.37 | 145.71 | 151.14 | 140.45 | 142.01 | 132.11 | 20.30 | 0.1555 | 0.2193 | 0.6958 |
| MCHC, g/dL | 19.41 | 21.04 | 20.44 | 20.34 | 20.52 | 20.24 | 20.22 | 20.63 | 1.37 | 0.0564 | 0.9600 | 0.0560 |
| Total platelets, ×10⁹/L | 39.26 | 35.96 | 45.87a | 31.69b | 34.22b | 35.07b | 40.37ab | 36.25b | 10.78 | 0.0848 | 0.0062 | 0.3595 |
| Total leukocyte count, ×10⁹/L | 8.81a | 6.70b | 8.07 | 7.49 | 6.12 | 7.42 | 8.91 | 8.44 | 3.69 | 0.0065 | 0.3626 | 0.1383 |
| Monocyte, ×10⁹/L | 0.02 | 0.02 | 0.04 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.05 | 0.3533 | 0.4431 | 0.4219 |
| Lymphocyte, ×10⁹/L | 5.85a | 4.79b | 5.25 | 5.07 | 4.68 | 5.11 | 6.45 | 5.31 | 2.48 | 0.0392 | 0.4652 | 0.0797 |
| Eosinophil, ×10⁹/L | 0.28 | 0.28 | 0.38 | 0.17 | 0.15 | 0.21 | 0.21 | 0.31 | 0.26 | 0.1043 | 0.1500 | 0.4244 |
| Heterophile, ×10⁹/L | 2.49a | 1.58b | 2.22 | 2.04 | 1.13 | 1.93 | 2.11 | 2.71 | 1.80 | 0.0162 | 0.2735 | 0.5212 |
| Basophil, ×10⁹/L | 0.19 | 0.17 | 0.18 | 0.19 | 0.15 | 0.14 | 0.12 | 0.08 | 0.13 | 0.0509 | 0.1763 | 0.3053 |
| H/L | 0.43 | 0.33 | 0.42 | 0.40 | 0.24 | 0.38 | 0.33 | 0.51 | 0.73 | 0.5346 | 0.3642 | 0.7329 |
| Variables | Stimbiotic | Fiber | SEM | P-Value | ||||||||
| - | + | Control | 100H | 75H25L | 50H50L | 25H75L | 100L | S | F | S*F | ||
| Packed cell volume, L/L | 0.403b | 0.428a | 0.423 | 0.427 | 0.404 | 0.401 | 0.415 | 0.427 | 5.15 | 0.0205 | 0.6528 | 0.7959 |
| Erythrocyte count, ×10¹²/L | 3.54 | 3.76 | 4.41 | 3.20 | 3.23 | 3.13 | 4.56 | 3.43 | 2.94 | 0.7117 | 0.5789 | 0.2184 |
| Hemoglobin, g/dL | 9.18b | 9.89a | 9.88 | 9.69 | 8.99 | 9.64 | 9.60 | 9.45 | 1.33 | 0.0117 | 0.5370 | 0.1136 |
| MCV, fL | 128.11 | 127.78 | 127.42 | 132.79 | 127.41 | 129.64 | 122.61 | 127.71 | 24.82 | 0.9482 | 0.9270 | 0.2774 |
| MCHC, g/dL | 22.33 | 22.66 | 21.89 | 23.04 | 22.31 | 24.07 | 21.67 | 21.97 | 3.82 | 0.6817 | 0.4941 | 0.1258 |
| Total platelets, ×10⁹/L | 35.98 | 32.79 | 37.69 | 38.50 | 33.66 | 27.70 | 33.57 | 35.16 | 12.37 | 0.2022 | 0.1832 | 0.8435 |
| Total leukocyte count, ×10⁹/L | 9.09a | 7.71b | 7.03 | 8.90 | 8.25 | 9.72 | 9.13 | 7.38 | 3.11 | 0.0386 | 0.1256 | 0.0803 |
| Monocyte, ×10⁹/L | 0.16 | 0.14 | 0.35 | 0.03 | 0.02 | 0.06 | 0.41 | 0.03 | 0.80 | 0.9282 | 0.5604 | 0.2698 |
| Lymphocyte, ×10⁹/L | 6.66a | 5.77b | 5.56 | 6.02 | 6.58 | 7.17 | 6.11 | 5.82 | 2.10 | 0.0437 | 0.3156 | 0.1298 |
| Eosinophil, ×10⁹/L | 0.48 | 0.46 | 0.30b | 0.78a | 0.34b | 0.57ab | 0.48ab | 0.37b | 0.38 | 0.8338 | 0.0055 | 0.1298 |
| Heterophile, ×10⁹/L | 1.74 | 1.36 | 1.04 | 1.76 | 1.27 | 1.82 | 2.43 | 1.05 | 1.76 | 0.2950 | 0.2155 | 0.2246 |
| Basophil, ×10⁹/L | 0.11 | 0.11 | 0.13 | 0.11 | 0.04 | 0.09 | 0.18 | 0.11 | 0.20 | 0.9000 | 0.5426 | 0.5995 |
| H/L | 0.26 | 0.24 | 0.19 | 0.29 | 0.19 | 0.25 | 0.40 | 0.18 | 0.84 | 0.6100 | 0.5350 | 0.9460 |
| Variables | Stimbiotic | Fiber | SEM | P-Value | ||||||||
| - | + | Control | 100H | 75H25L | 50H50L | 25H75L | 100L | S | F | S*F | ||
| Albumin, g/dL | 1.14 | 1.1 | 1.17 | 1.01 | 1.16 | 1.13 | 1.14 | 1.13 | 0.15 | 0.2573 | 0.0652 | 0.636 |
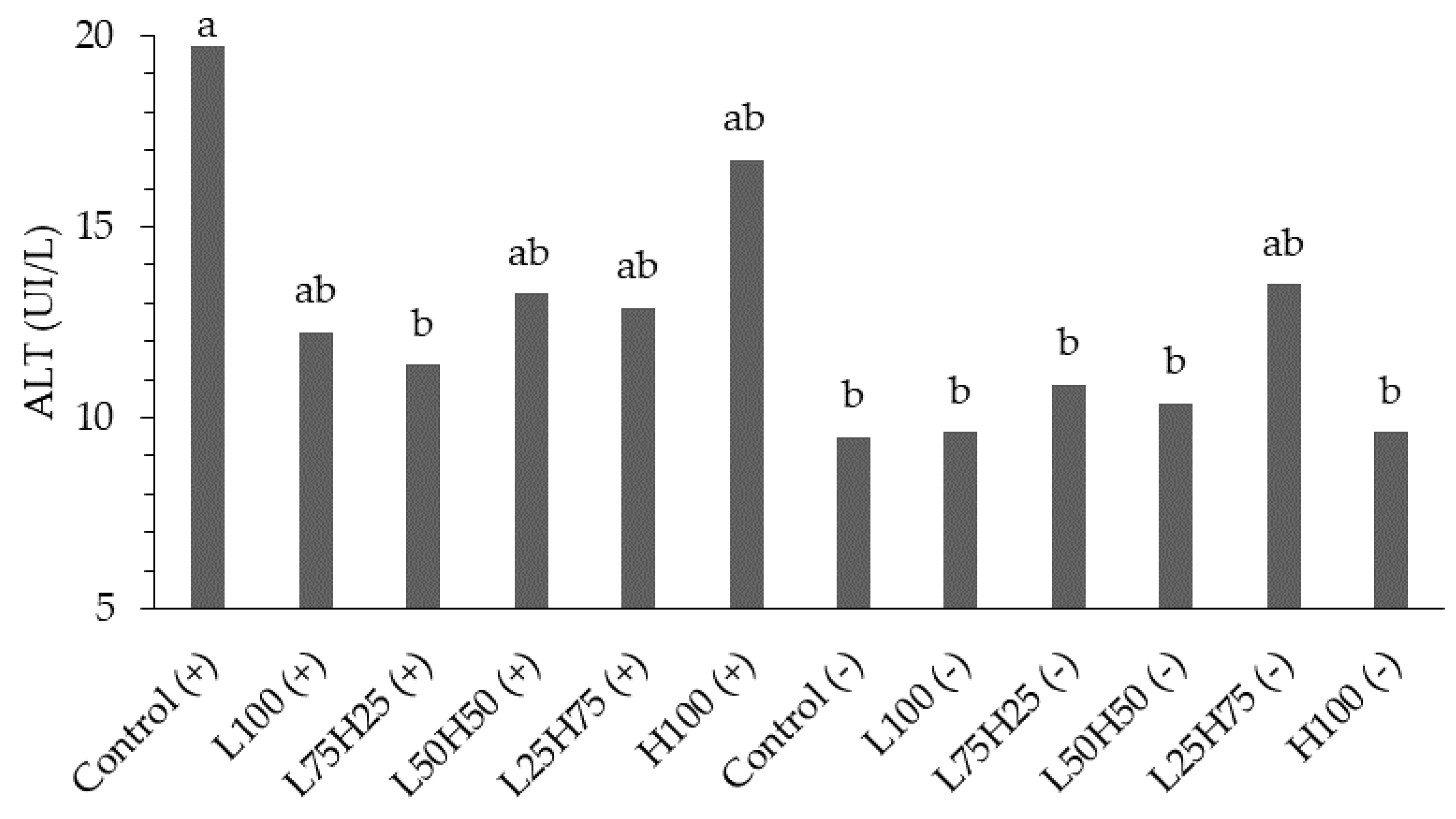
| ALT, UI/L | 10.58 | 14.38 | 14.6 | 13.19 | 13.19 | 11.81 | 11.13 | 10.94 | 4.52 | 0.5768 | 0.1662 | 0.0083 |
| AST, UI/L | 218.71 | 214.42 | 230.08 | 224.25 | 218.81 | 215.88 | 215.69 | 194.5 | 58.63 | 0.7208 | 0.6337 | 0.4337 |
| GGT, UI/L | 1.29 | 1.27 | 1.25 | 1.29 | 1.71 | 1.46 | 1.15 | 0.86 | 1.19 | 0.9018 | 0.4749 | 0.3318 |
| Total protein, g/dL | 2.94 | 2.88 | 2.98 | 2.76 | 2.96 | 2.95 | 2.91 | 2.94 | 0.34 | 0.3248 | 0.5286 | 0.2842 |
| Cholesterol, mg/dL | 149.45 | 140.68 | 136.08 | 142.74 | 157.26 | 152.46 | 146.29 | 138.56 | 29.22 | 0.105 | 0.2962 | 0.6456 |
| Triglycerides, mg/dL | 77.51b | 115.58a | 144.86a | 73.36b | 72.82b | 93.84b | 104.84ab | 90.07b | 46.91 | 0.0001 | 0.0003 | 0.3197 |
| Variables | Stimbiotic | Fiber | SEM | P-Value | ||||||||
| - | + | Control | 100H | 75H25L | 50H50L | 25H75L | 100L | S | F | S*F | ||
| Albumin, g/dL | 5.61 | 4.94 | 13.89 | 1.24 | 1.36 | 1.40 | 12.65 | 1.31 | 26.91 | 0.9048 | 0.5368 | 0.2791 |
| ALT, UI/L | 12.72 | 12.625 | 11.74 | 9.84 | 13.75 | 16.50 | 12.41 | 11.88 | 12.99 | 0.9393 | 0.7968 | 0.4774 |
| AST, UI/L | 198.69 | 212.96 | 206.18 | 204.79 | 201.88 | 199.44 | 199.86 | 222.13 | 52.29 | 0.1868 | 0.3386 | 0.0774 |
| GGT, UI/L | 37.72 | 89.94 | 15.10 | 36.53 | 45.07 | 137.76 | 94.89 | 53.89 | 139.21 | 0.0726 | 0.1520 | 0.9620 |
| Total protein, g/dL | 3.35 | 3.63 | 3.35 | 3.20 | 3.50 | 3.70 | 3.88 | 3.30 | 0.84 | 0.1013 | 0.1995 | 0.3563 |
| Cholesterol, mg/dL | 186.54 | 195.79 | 199.75 | 169.79 | 182.72 | 208.11 | 188.26 | 196.86 | 63.10 | 0.4795 | 0.6011 | 0.4710 |
| Triglycerides, mg/dL | 285.94 | 216.99 | 190.46 | 244.43 | 294.29 | 158.16 | 324.71 | 300.87 | 244.26 | 0.1749 | 0.3409 | 0.2480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





