Submitted:
04 October 2025
Posted:
08 October 2025
You are already at the latest version
Abstract
Background: Hexavalent chromium [Cr(VI)] and gamma radiation are environmental toxicants that cause DNA damage, oxidative stress, and endocrine disruption, with transgenerational impacts on offspring health. Preventive strategies against inherited toxic effects are lacking, prompting research into protective interventions such as phytopreparations. Methods: Adult male rats were exposed to Cr(VI) (180 mg/L in drinking water for 14 days) and/or a single gamma irradiation (0.2 Gy), with subgroups receiving stinging nettle (Urtica dioica) or burdock (Arctium lappa) seed oil (0.5 mL/day) prophylaxis before irradiation. After exposure, rats were bred and male first-generation (F1) offspring were evaluated at 16 months for serum testosterone and thyroxine (T4), oxidative stress markers (MDA, catalase, SOD), sperm concentration/morphology, and testicular histology. Group differences were analyzed via one-way ANOVA (p<0.05). Results: Parental exposure to Cr(VI) and gamma radiation caused significant reproductive and endocrine impairment in F1 males: testosterone and sperm concentration decreased, abnormal sperm morphology increased, and T4 levels were disrupted compared to controls. However, parental supplementation with nettle or burdock oil significantly mitigated these effects, improving offspring hormone levels and sperm quality. Notably, burdock oil co-treatment restored testosterone and T4 toward control values and reduced sperm abnormalities in the combined Cr(VI)+γ group, indicating preserved spermatogenesis. Conclusions: Phytopreparation prophylaxis (nettle and burdock oils) in exposed parents partially normalized hormonal and reproductive parameters in F1 offspring and preserved testicular structure. These findings highlight the potential of phytoprotective interventions to attenuate transgenerational toxicity from Cr(VI) and radiation, thereby safeguarding future generations.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Overview
2.2. Experimental Animals
2.3. Ethics and Regulatory Compliance
2.4. Interventions and Exposure Regimens
2.5. Mating and Offspring
2.6. Randomization, Allocation Concealment, and Blinding
2.7. Inclusion/Exclusion Criteria and Unit of Analysis
2.8. Outcome Measures
2.9. Housing, Husbandry, Monitoring, and Welfare
2.9.1. Justification:
2.9.2. Evaluation Methods
2.9.3. Statistical Analysis
- Family A (within γ only): Nettle vs None, Burdock vs None (γ context); and Nettle vs Burdock.
- Family B (within Cr(VI)+γ): Nettle vs None, Burdock vs None; and Nettle vs Burdock.Additionally, we report Control vs γ, γ vs Cr(VI)+γ (both “None”), and Control vs each oil+γ for context. These contrasts align to key biological questions (radiation effect; chromium add-on; phytoprotection within γ and within Cr(VI)+γ).
- Within each endpoint, all planned contrasts are adjusted by Benjamini–Hochberg (BH) FDR at q = 0.05, yielding q-values.
- Across endpoints, we control discovery rate hierarchically: first declare significance within primary endpoints at q = 0.05; secondary endpoints are interpreted exploratorily with BH at q = 0.10. We report both unadjusted p and q.
- For omnibus tests (Group effect), we report F/H statistics, df, and partial η² with 95% CIs.
2.9.4. Ethical Considerations
3. Results
- MDA, ng / ml:
- CAT, ng / ml:
- SOD, ng / ml:
- IL-10, pg/ml:
- IL-6, pg/ml:
- TNF-α, pg/ml:
- Teystosterone, ng / ml:
- Estradiol, pg / ml:
- Spermatozoa, 10 ^ 6/ml:
- Mobility, %:
- Abnormal sperm count, %:
- T3, nmol/L:
- T4, nmol/L:
- FT4, ng/ml:
- Cortisol, mcg / dl:
- Micronucleus, %:
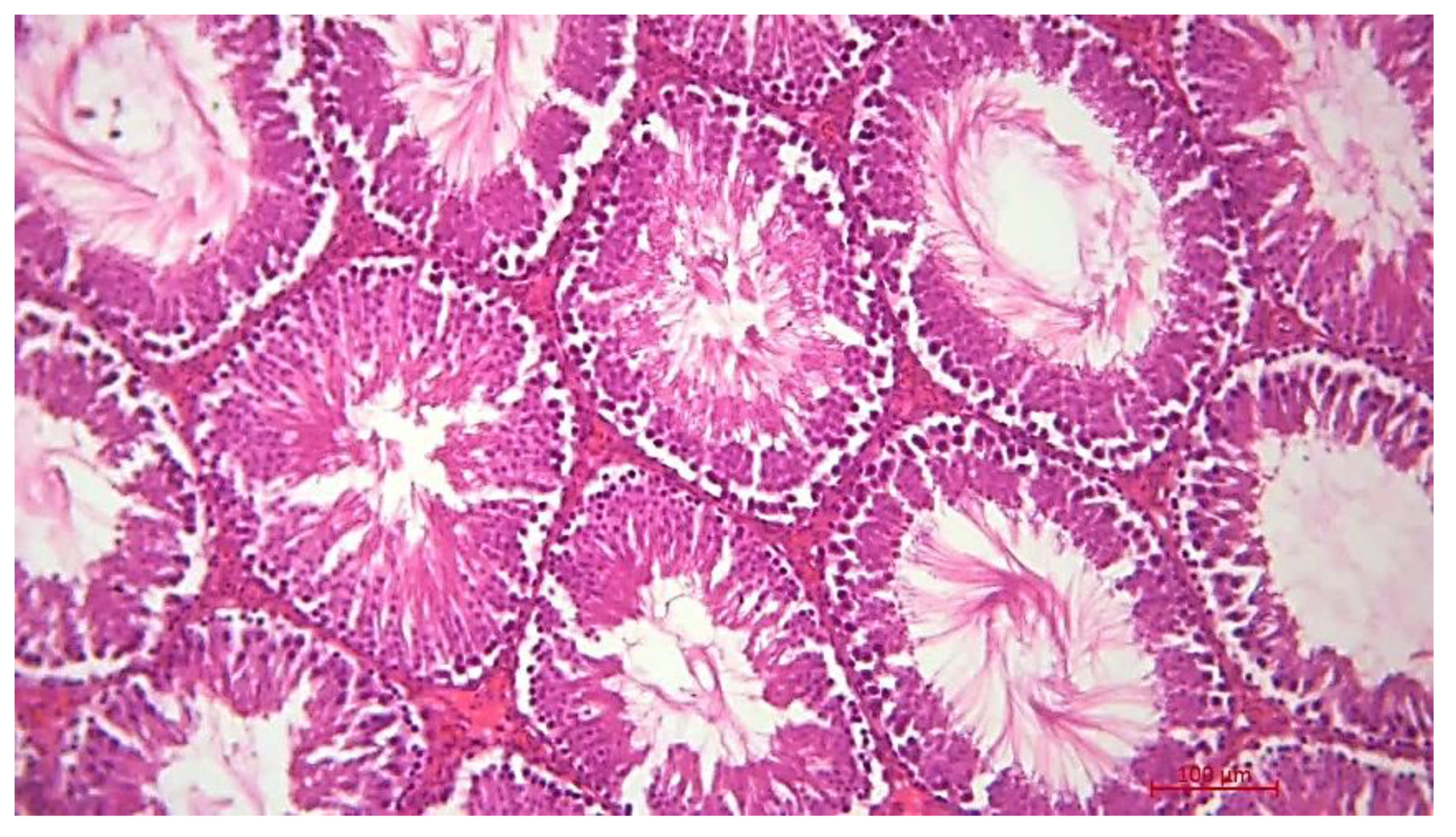
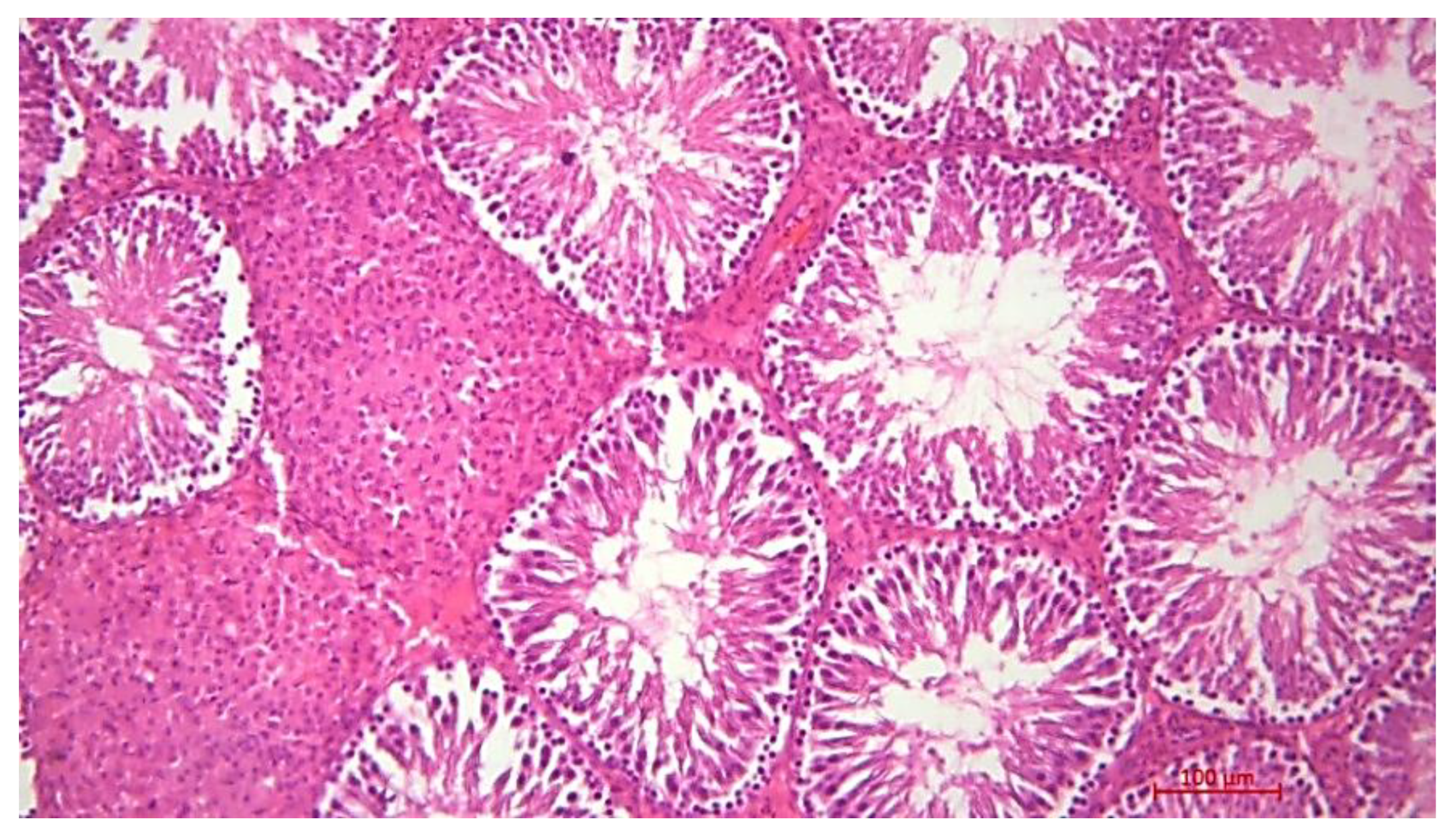
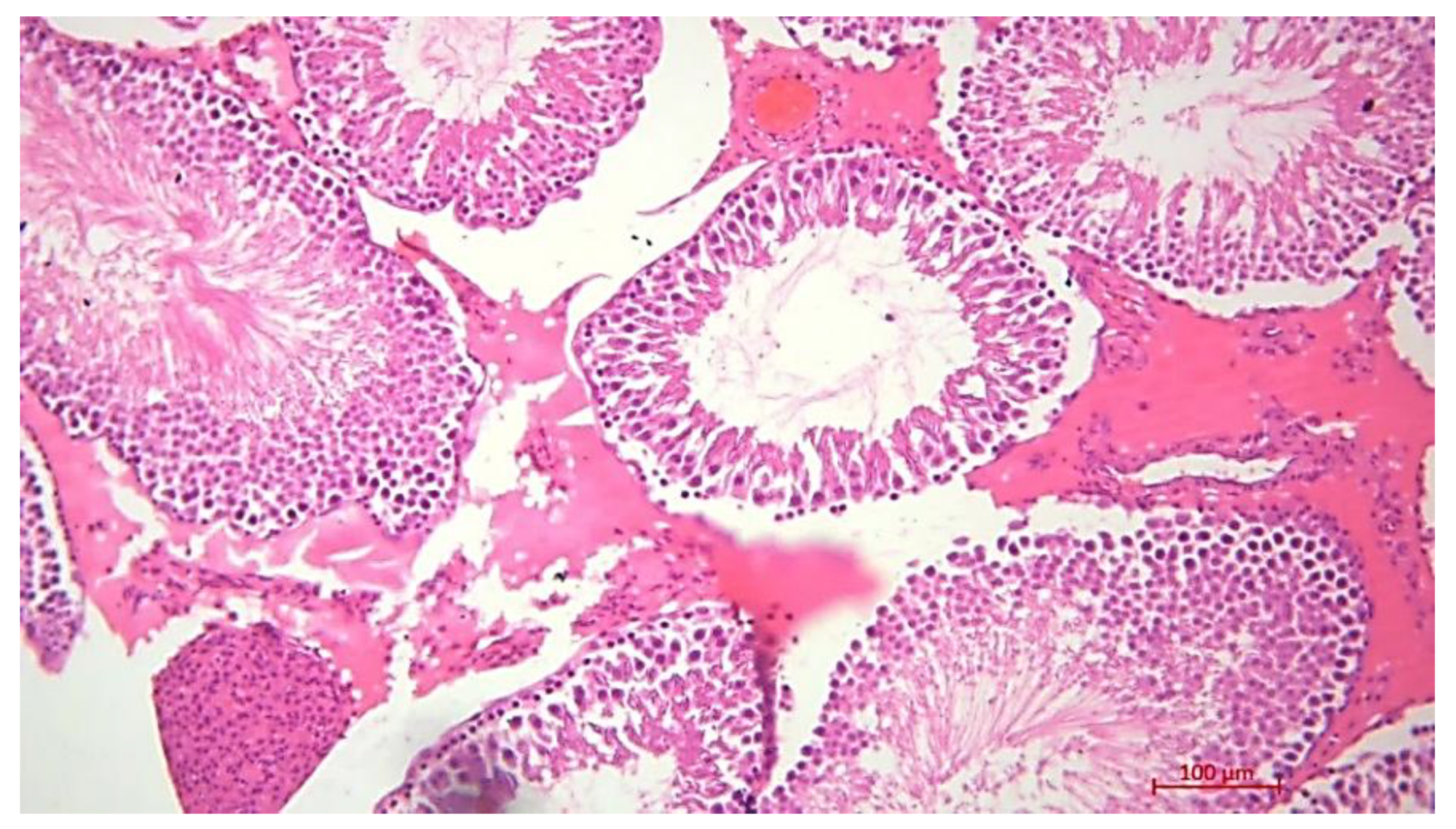
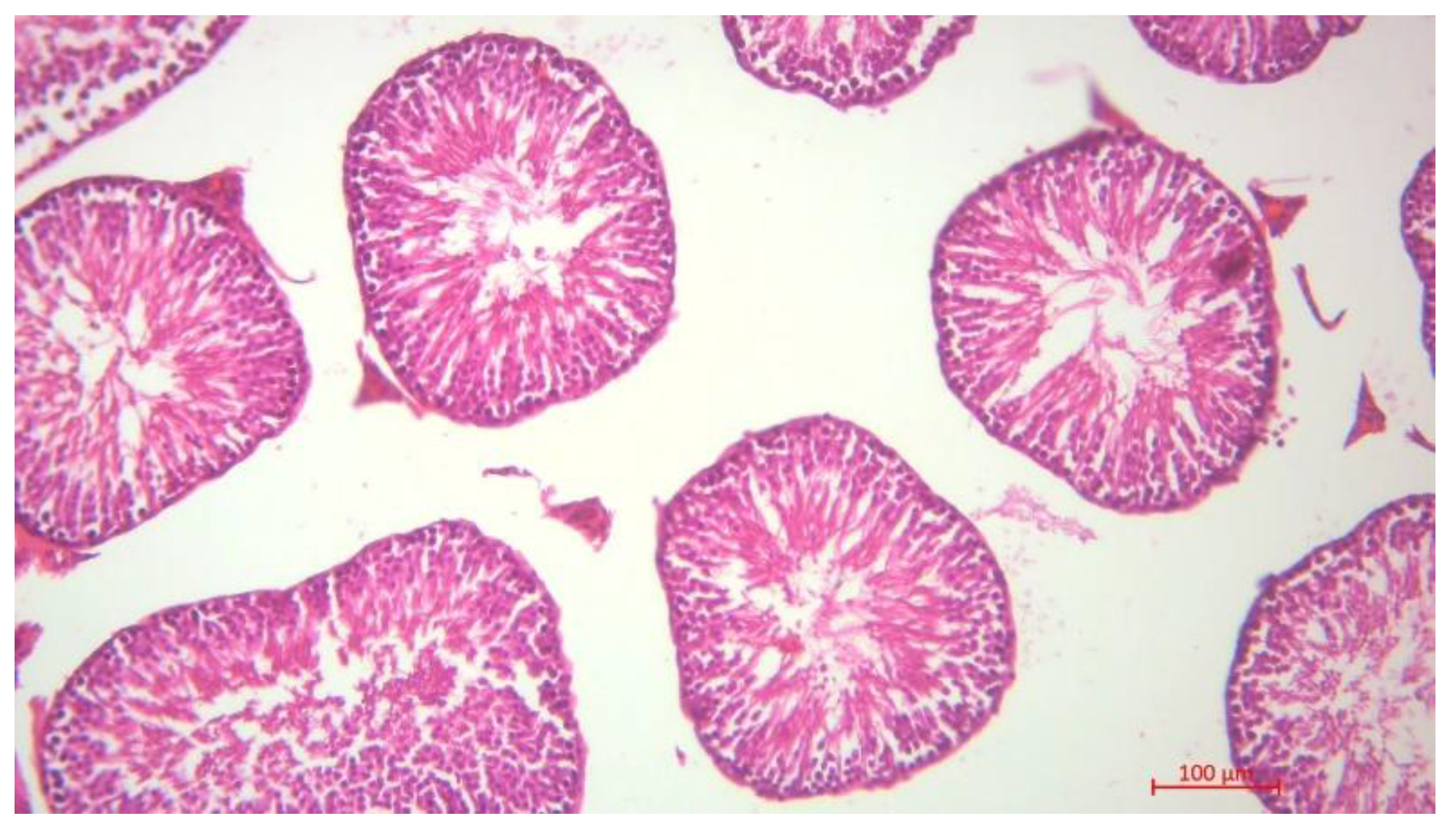
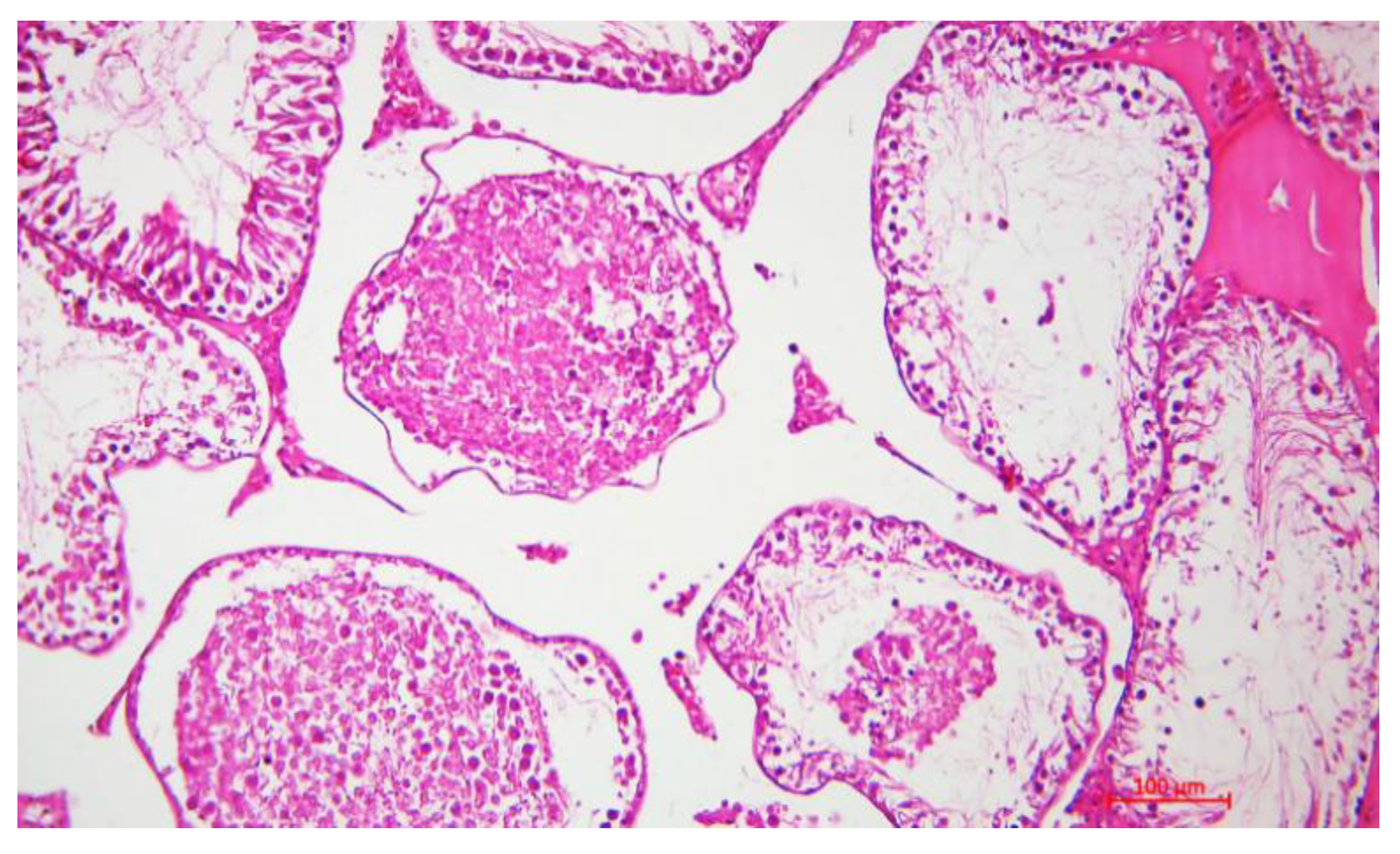
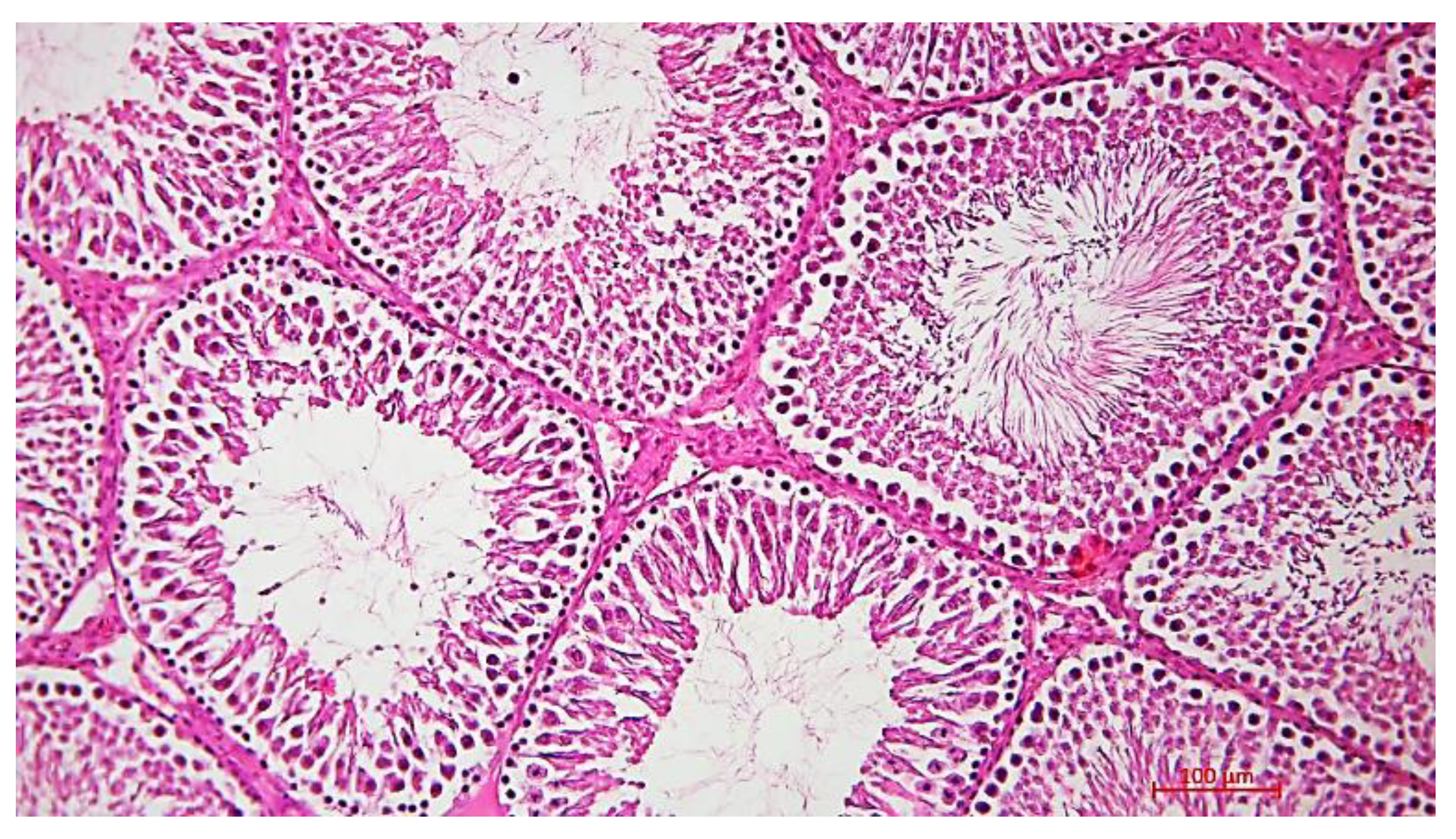
Histological Description of the Gonadal Condition in Males
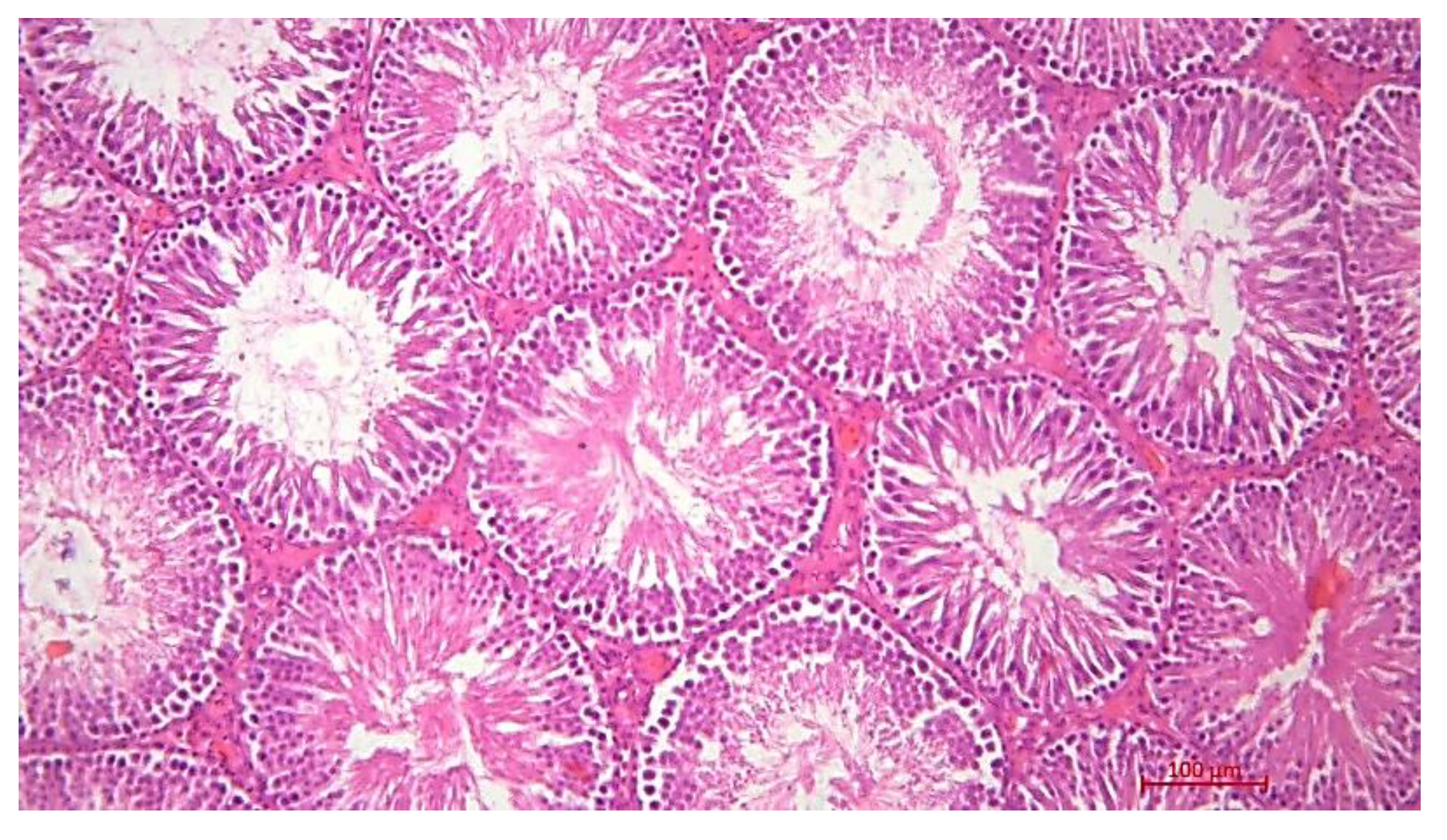
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Devi, VN. Sources and toxicological effects of some heavy metals—A mini review. J Toxicol Stud [Internet]. 2024 Feb 21 [cited 2025 Mar 18];2(1). Available from. [CrossRef]
- Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol [Internet]. 2021 Apr 13 [cited 2025 Mar 18];12. Available from. [CrossRef]
- Ionizing radiation and health effects [Internet]. Who.int. [cited 2025 Mar 18]. Available from: https://www.who.
- Chernobyl accident 1986 [Internet]. World-nuclear.org. [cited 2025 Mar 18]. Available from: https://world-nuclear.
- 7 The Chernobyl disasters Its effect on Belarus and Ukraine [Internet]. Unu.edu. [cited 2025 Mar 18]. Available from: https://archive.unu.edu/unupress/unupbooks/uu21le/uu21le0h.
- Tykhyi, V. Chernobyl sufferers in Ukraine and their social problems: short outline. Research Activities about the Radiological Consequences of the Chernobyl NPS Accident and Social Activities to Assist the Sufferers by the Accident, Kyoto. 1998; 27:235–45. Available from: https://www.rri.kyoto-u.ac.jp/NSRG/reports/kr21/kr21pdf/Tykhyi.
- Sharma P, Singh SP, Parakh SK, Tong YW. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered [Internet]. 2022;13(3):4923–38. Available from. [CrossRef]
- Duarte GT, Volkova PY, Fiengo Perez F, Horemans N. Chronic ionizing radiation of plants: An evolutionary factor from direct damage to non-target effects. Plants [Internet]. 2023;12(5). Available from. [CrossRef]
- Irsal M, Sutoro SG, Widiatmoko ME, Cahya I. Assessment Awareness and Knowledge of Apron to Protect Radiographer During Radiographic Examination. Futurity Medicine [Internet]. 2023 Dec. 30 [cited 2025 Mar. 19];2(4):10-6. Available from: https://futurity-medicine.com/index.
- Yin J, Ye Y, Gao Y, Xu Q, Su M, Sun S, et al. Low-dose ionizing radiation and male reproductive immunity: Elucidating subtle modulations and long-term health implications. Int J Mol Sci [Internet]. 2025;26(5). Available from. [CrossRef]
- Chen QY, Murphy A, Sun H, Costa M. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol Appl Pharmacol [Internet]. 2019;377(114636):114636. Available from. [CrossRef]
- Gelaye, Y. Public health and economic burden of heavy metals in Ethiopia: Review. Heliyon [Internet]. 2024;10(19): e39022. Available from. [CrossRef]
- Ukraine: Health Cluster bulletin #1 (January 2025) [Internet]. ReliefWeb. [cited 2025 Mar 18]. Available from: https://reliefweb.int/report/ukraine/ukraine-health-cluster-bulletin-1-january-2025.
- Guirandy N, Gagnaire B, Camilleri V, Cavalié I, Pierron F, Gonzalez P, et al. Multigenerational exposure to gamma radiation affects offspring differently over generations in zebrafish. Aquat Toxicol [Internet]. 2022;244(106101):106101. Available from. [CrossRef]
- Sangsuwan T, Mannervik M, Haghdoost S. Transgenerational effects of gamma radiation dose and dose rate on Drosophila flies irradiated at an early embryonal stage. Mutat Res Genet Toxicol Environ Mutagen [Internet]. 2022;881(503523):503523. Available from. [CrossRef]
- Kumar D, Seth CS. Photosynthesis, lipid peroxidation, and antioxidative responses of Helianthus annuus L. against chromium (VI) accumulation. Int J Phytoremediation [Internet]. 2021;24(6):1–10. Available from. [CrossRef]
- Gagnaire B, Arcanjo C, Cavalié I, Camilleri V, Simon O, Dubourg N, et al. Effects of gamma ionizing radiation exposure on Danio rerio embryo-larval stages - comparison with tritium exposure. J Hazard Mater [Internet]. 2021;408(124866):124866. Available from. [CrossRef]
- Lindeman LC, Kamstra JH, Ballangby J, Hurem S, Martín LM, Brede DA, et al. Gamma radiation induces locus specific changes to histone modification enrichment in zebrafish and Atlantic salmon. PLoS One [Internet]. 2019;14(2): e0212123. Available from. [CrossRef]
- Xue Q, Zhang L, Wang R, Xu J, Wang C, Gao S, et al. Hexavalent chromium reduces testosterone levels by impairing lipophagy and disrupting lipid metabolism homeostasis: Based on a metabolomic analysis. Toxicology [Internet]. 2024;508(153908):153908. Available from. [CrossRef]
- Shyam M, Sabina EP. Harnessing the power of Arctium lappa root: a review of its pharmacological properties and therapeutic applications. Natural Products and Bioprospecting 2024 14:1 2024; 14:1–27. [CrossRef]
- Iztleuov M, Iztleuov Y, Saparbayev S, Temirbayeva A, Medeuova R, Aleuova Z, Ismailova I, Imanbayev N. Effect of Burdock Root Oil on Oxidative Stress Induced by Isolated and Combined Use of Gamma Radiation and Hexavalent Chromium. Biomed Pharmacol J [Internet]. 2022 Mar 31 [cited 2025 Mar 18];15(1):421-32. Available from. [CrossRef]
- Sawicka E, Saczko J, Roik J, Kulbacka J, Piwowar A. Effect of Interaction between 17β-Estradiol, 2-Methoxyestradiol and 16α-Hydroxyestrone with Chromium (VI) on Ovary Cancer Line SKOV-3: Preliminary Study. Molecules [Internet]. 2020 Nov 9 [cited 2025 Mar 18];25(21):5214. Available from. [CrossRef]
- Błażewicz A, Wiśniewska P, Skórzyńska-Dziduszko K. Selected Essential and Toxic Chemical Elements in Hypothyroidism—A Literature Review (2001–2021). Int J Mol Sci [Internet]. 2021 Sep 20 [cited 2025 Mar 18];22(18):10147. Available from. [CrossRef]
- Rafi’i MR, Ja’afar MH, Mohammed Nawi A, Md Hanif SA, Md Asari SN. Association between toxic heavy metals and noncancerous thyroid disease: a scoping review. PeerJ [Internet]. 2025 Feb 11 [cited 2025 Mar 18];13: e18962. Available from. [CrossRef]
- Donayeva A, Kulzhanova D, Amanzholkyzy A, Abdelazim IA, Abilov T, Baubekov Z, et al. Relationship between vitamin D and adolescents’ hypothyroidism – a cross-sectional study. Prz Menopauzalny [Internet]. 2023;22(4):186–90. Available from. [CrossRef]
- Bharat, D. Burdock root: Health benefits, nutrition, uses for skin and hair, recipes, side effects [Internet]. Netmeds. 2022 [cited 2025 Mar 18]. Available from: https://www.netmeds.
- Al Zarzour RH, Kamarulzaman EE, Saqallah FG, Zakaria F, Asif M, Abdul Razak KN. Medicinal plants proposed nanocomposites for the management of endocrine disorders. Heliyon [Internet]. 2022;8(9): e10665. Available from. [CrossRef]
- Garofalo V, Condorelli RA, Cannarella R, Aversa A, Calogero AE, La Vignera S. Relationship between iron deficiency and thyroid function: A systematic review and meta-analysis. Nutrients [Internet]. 2023;15(22):4790. Available from. [CrossRef]
- Suhail N, Aftab T, Alruwaili A, Alruwaili D. Effects of multivitamin-mineral supplementation on chronic stress-induced oxidative damage in Swiss albino mice. Cureus [Internet]. 2024;16(6): e61896. Available from. [CrossRef]
- Song I-B, Han H-J, Kwon J. Immune-enhancing effects of gamma-irradiated sericin. Food Sci Biotechnol [Internet]. 2020;29(7):969–76. Available from. [CrossRef]
- Caputo L de S, Alves C de L, Laranjeira IM, Fonseca-Rodrigues D, da Silva Filho AA, Dias ACP, et al. Copaiba oil minimizes inflammation and promotes parenchyma re-epithelization in acute allergic asthma model induced by ovalbumin in BALB/c mice. Front Pharmacol [Internet]. 2024; 15:1356598. Available from. [CrossRef]
- Walcher L, Kistenmacher A-K, Sommer C, Böhlen S, Ziemann C, Dehmel S, et al. Low energy electron irradiation is a potent alternative to gamma irradiation for the inactivation of (CAR-)NK-92 cells in ATMP manufacturing. Front Immunol [Internet]. 2021; 12:684052. Available from. [CrossRef]
- Hussien SM, Rashed ER. Immuno-biochemical impacts of gamma irradiation in male rats: A dose-response study. Dose Response [Internet]. 2023;21(2):15593258231185461. Available from. [CrossRef]
- Boyko V, Savvi S, Korolevska A, Zhydetskyy V, Novikov Y, Bytiak S, et al. Surgical treatment of bening esophageal strictures after corrosive injuries. Georgian Med News [Internet]. 2018;(278):7–15. Available from: https://europepmc.org/article/med/29905537.
- Fukunaga H, Yokoya A, Prise KM. A brief overview of radiation-induced effects on spermatogenesis and oncofertility. Cancers (Basel) [Internet]. 2022;14(3):805. Available from. [CrossRef]
- Wdowiak A, Skrzypek M, Stec M, Panasiuk L. Effect of ionizing radiation on the male reproductive system. Ann Agric Environ Med [Internet]. 2019;26(2):210–6. Available from. [CrossRef]
- Li J, Liu J, Zhang Y, Qiu H, Zheng J, Xue J, et al. Effects of paternal ionizing radiation exposure on fertility and offspring’s health. Reprod Med Biol [Internet]. 2024;23(1): e12567. Available from. [CrossRef]
- Burdock extract effects on hormones and sperm in diabetic male mice [Internet]. Wisdomlib.org. 2024 [cited 2025 Mar 18]. Available from: https://www.wisdomlib.org/science/journal/the-malaysian-journal-of-medical-sciences/d/doc1425283.
- Govbakh IO, Zavodovskiy DO, Bulgakova N V., Sokołowska (Vereshchaka) I V., Maznychenko A V., Vasylenko DA. Nerve Conduction and Neuromuscular Transmission in C57Bl/6 Mice with Genetically Determined Peripheral Neuropathy. Neurophysiology [Internet]. 2019;51(4):248–52. Available from. [CrossRef]
- Wakeel A, Xu M, Gan Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int J Mol Sci [Internet]. 2020;21(3):728. Available from. [CrossRef]
- Surniyantoro HNE, Kisnanto T, Tetriana D, Yusuf D, Basri IKH, Lusiyanti Y. Study of immune response and malondialdehyde levels in irradiated rats supplemented with Curcuma xanthorriza Roxb extract. Asian Pac J Cancer Prev [Internet]. 2023;24(5):1717–23. Available from. [CrossRef]
- Shapoval LM, Dmytrenko O V., Sagach VF, Prylutska S V., Khrapatiy S V., Zavodovskyi DO, et al. Systemic Administrations of Water-Dispersible Single-Walled Carbon Nanotubes: Activation of NOS in Spontaneously Hypertensive Rats. Neurophysiology [Internet]. 2020;52(2):101–9. Available from. [CrossRef]
- Iztleuov M, Iztleuov Y, Yelubayeva A, Aleuova Z, Turganbaeva Z, Madikhan Z, et al. Effect of “nettle oil” on oxidative damage to the heart and lungs induced by radiation. Biomed Pharmacol J [Internet]. 2020;13(03):1495–504. Available from. [CrossRef]
| Group | Description |
| Control | No exposure to hexavalent chromium (Cr⁶⁺) or gamma (γ) radiation. |
| γ Gamma Radiation | Exposed to γ radiation only. 15 days after the beginning of the experiment, they were exposed to total single radiation at a dose of 0.2 Gy. together with the other groups. |
| γ + Nettle Oil | For 14 days before irradiation, Nettle Oil was obtained at a dose of 0.5 ml per rat through a probe intragastrically. On the 15th day of exposure. |
| γ + Burdock Oil | For 14 days before irradiation, Burdock Oil was obtained at a dose of 0.5 ml per rat through a probe intragastrically. On the 15th day of exposure. |
| Cr⁶ ⁺+ γ | For 14 days received hexavalent chromium orally with drinking water at a dose of 180 mg/l. On day 15of exposure |
| , Cr⁶ ⁺ + γ + Nettle Oil | was irradiated for 14 days orally with drinking water chromium at a dose of 180 mg/l and Nettle Oil 0.5 ml per rat through a probe. On day 15, 0.2 Gy of |
| Cr⁶ ⁺ + γ + Burdock Oil | was irradiated for 14 days orally with drinking water chromium at a dose of 180 mg/l and Burdock Oil 0.5 ml per rat through a probe. On the 15th day, the exposure was 0.2 Gy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




