Submitted:
05 October 2025
Posted:
06 October 2025
You are already at the latest version
Abstract
Keywords:
Introduction
2. Materials and Methods
2.1. Isolation and Culture of DPSCs
- Collagenase I: (Sigma-Aldrich, St. Louis, MO, USA).
- Dispase: (Gibco, Thermo Fisher Scientific, Waltham, MA, USA).
- Filter (70 µm): (Falcon, Corning Inc., Corning, NY, USA).
- α-MEM (Minimal essential medium Eagle – alpha modification): (Gibco, Thermo Fisher Scientific).
- Fetal bovine serum (FBS): (Gibco, Thermo Fisher Scientific).
- Penicillin–streptomycin: (Gibco, Thermo Fisher Scientific).
2.2. Phenotypic Characterization of DPSCs
- Bovine serum albumin (BSA): (Sigma-Aldrich).
- Antibodies: Ecto-5'-nucleotidase (CD73), Thy-1 (CD90), Endoglin (CD105), CD34, CD45.
- Flow cytometer: (BD Accuri C6 flow cytometer, BD Biosciences, San Jose, CA, USA).
- Data analysis software: (FlowJo v10, FlowJo LLC, Ashland, OR, USA).
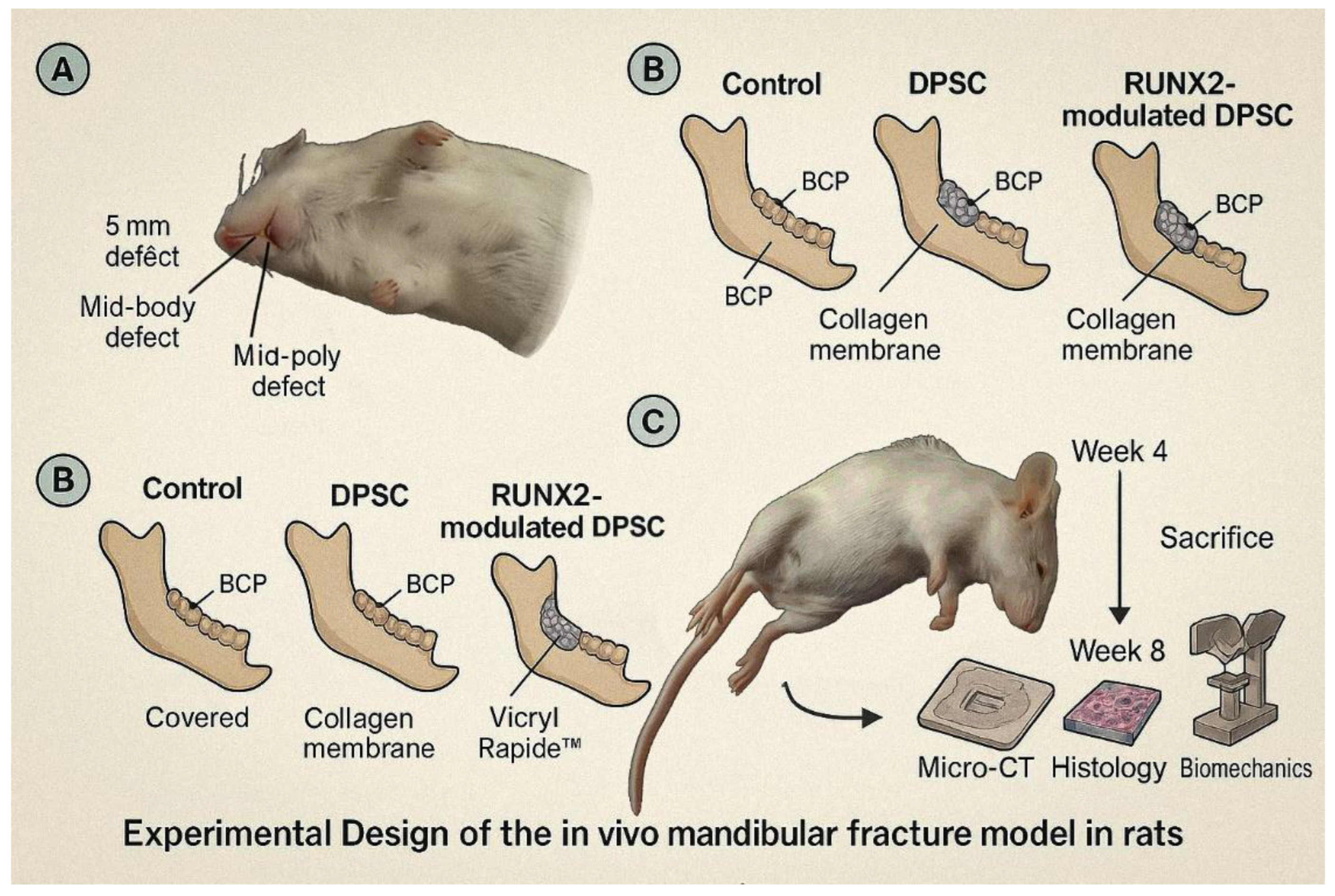
2.3. In Vivo Mandibular Fracture Model
- Isoflurane/O₂ anesthesia: (Baxter Healthcare, Deerfield, IL, USA).
- Micro-saw: (Fine Science Tools, Foster City, CA, USA).
- Biphasic calcium phosphate (BCP): (Berkeley Advanced Biomaterials Inc., Berkeley, CA, USA).
- RUNX2 lentivirus: (VectorBuilder Inc., Chicago, IL, USA).
- Resorbable collagen membrane: (Geistlich Pharma AG, Wolhusen, Switzerland).
- Vicryl Rapide™ sutures: (Ethicon, Johnson & Johnson, Somerville, NJ, USA).
- Buprenorphine (0.05 mg/kg): (Reckitt Benckiser, Slough, UK).
2.4. Genetic Marker Expression Analysis
- RNA extraction kit: (RNeasy Mini Kit, Qiagen, Hilden, Germany).
- cDNA synthesis kit: (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Thermo Fisher Scientific).
- SYBR Green assay kit: (PowerUp SYBR Green Master Mix, Applied Biosystems).
- SDS-PAGE: (Bio-Rad Laboratories, Hercules, CA, USA).
- PVDF membrane: (MilliporeSigma, Burlington, MA, USA).
- Chemiluminescence substrate: (SuperSignal™ Enhanced Chemiluminescence), (ECL), (Thermo Fisher Scientific).
- Paraformaldehyde (4% PFA): (Electron Microscopy Sciences, Hatfield, PA, USA).
- Triton X-100 (0.1%): (Sigma-Aldrich).
- BSA (5%): (Sigma-Aldrich).
- Primary antibodies (anti-RUNX2/OCN): (Abcam, Cambridge, UK).
- Secondary antibodies (Alexa Fluor): (Thermo Fisher Scientific).
- DAPI (4′,6-diamidino-2-phenylindole): (Sigma-Aldrich).
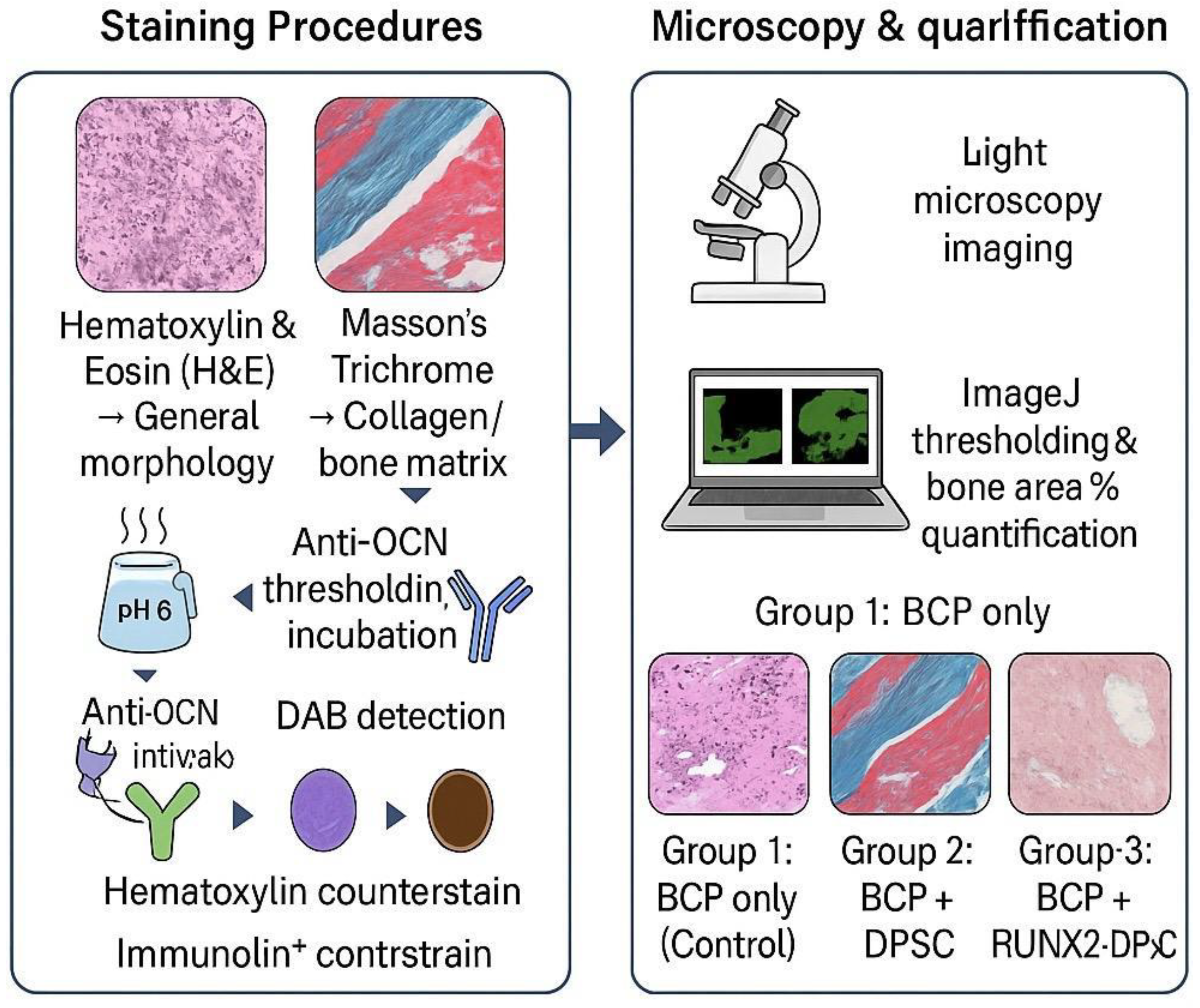
2.5. Histological and Immunohistochemical Evaluation
- Decalcification solution (10% EDTA): (Sigma-Aldrich).
- Hematoxylin and Eosin (H&E): (Sigma-Aldrich).
- Masson’s trichrome stain: (Polysciences, Warrington, PA, USA).
- Antigen retrieval buffer (citrate buffer pH 6): (Dako, Agilent Technologies, Santa Clara, CA, USA).
- Anti-Osteocalcin (OCN) antibody: (Abcam).
- DAB detection kit: (Dako).
- Hematoxylin counterstain: (same as above, Sigma-Aldrich implied)
- ImageJ software: (NIH, Bethesda, MD, USA).
2.6. Scaffold Preparation and Cell Seeding
- Biphasic calcium phosphate (BCP; 60/40 HA/β-TCP, 0.5–1 mm granules): (Berkeley Advanced Biomaterials Inc., Berkeley, CA, USA).
- α-MEM: (Gibco, Thermo Fisher Scientific).
2.7. Scaffold Design and Fabrication
- Type I collagen: (Corning).
- Polycaprolactone (PCL): (Sigma-Aldrich).
2.8. Methods
2.8.1. Isolation and Culture of DPSCs
2.8.2. Phenotypic Characterization of DPSCs
2.8.3. In Vivo Mandibular Fracture Model
- Control: defect + BCP only
- DPSC: defect + BCP + DPSCs (1×10⁶)
- RUNX2-modulated DPSC: defect + BCP + DPSCs transduced with RUNX2 lentivirus (MOI 50; VectorBuilder Inc., Chicago, IL, USA)
2.8.4. Genetic Marker Expression Analysis
- Real-Time Quantitative Reverse Transcription PCR (qRT-PCR): RNA extraction (RNeasy Mini Kit, Qiagen, Hilden, Germany), cDNA synthesis (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Thermo Fisher Scientific), and SYBR Green assays (PowerUp SYBR Green Master Mix, Applied Biosystems) for RUNX2, alkaline phosphatase (ALPL), Collagen type 1 alpha 1 (COL1A1), bone morphogenetic protein 2 (BMP2), and Osteocalcin (OCN); bone gamma-carboxyglutamic acid-containing protein (BGLAP) as internal control.
- Western Blot: Protein lysates separated by Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad Laboratories, Hercules, CA, USA), transferred to Polyvinylidene fluoride (PVDF; MilliporeSigma, Burlington, MA, USA), probed for RUNX2, COL1A1, and OCN, and visualized via Thermo Fisher Scientific’s SuperSignal™ Enhanced Chemiluminescence (ECL).
- Immunocytochemistry: Cells fixed Paraformaldehyde (4% PFA; Electron Microscopy Sciences, Hatfield, PA, USA), permeabilized (0.1% Triton X-100; Sigma-Aldrich), blocked (5% BSA; Sigma-Aldrich), incubated with anti-RUNX2/OCN (Abcam, Cambridge, UK), then Alexa Fluor secondary antibodies (Thermo Fisher Scientific); nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) [12,13].
2.8.5. Histological and Immunohistochemical Evaluation
2.1.6. Scaffold Preparation and Cell Seeding
2.1.7. Scaffold Design and Fabrication
2.1.8. Statistical Analysis
3. Results
3.1. Isolation and Phenotypic Characterization Confirms the Mesenchymal Stem Cell Identity of Cultured DPSCs
3.2. Sequential and Temporal Induction of Key Osteogenic Genes Confirmed by qRT-PCR Analysis
3.3. Western Blot and Immunocytochemistry Confirm Robust Elevation of RUNX2 and OCN Protein Expression
3.4. DPSC-BCP Scaffold Interactions: High Seeding Efficiency and Favorable Cell Adhesion
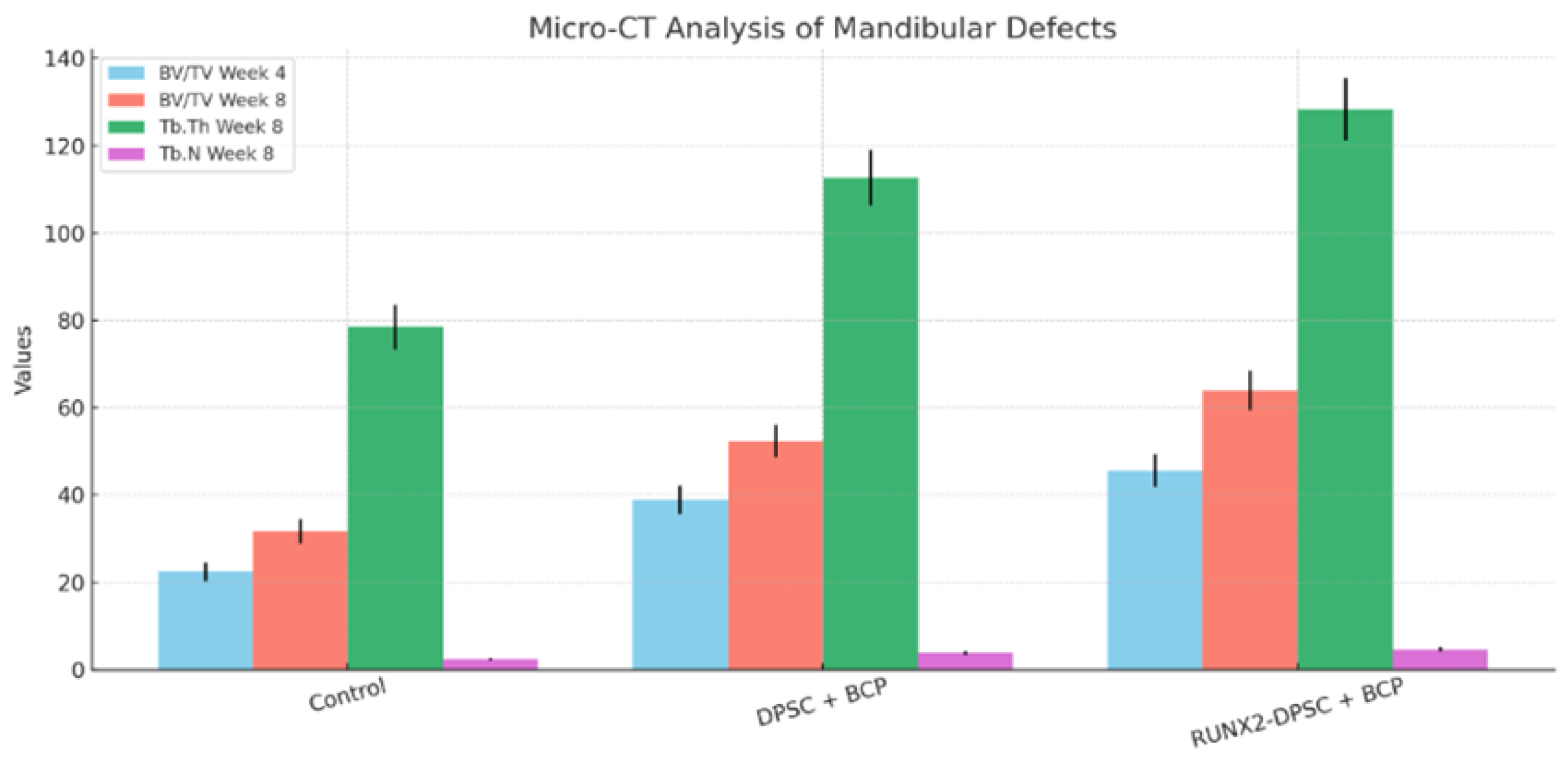
3.5. Micro-CT Morphometric Analysis Demonstrates Superior Bone Volume and Microarchitecture in DPSC-Treated Defects
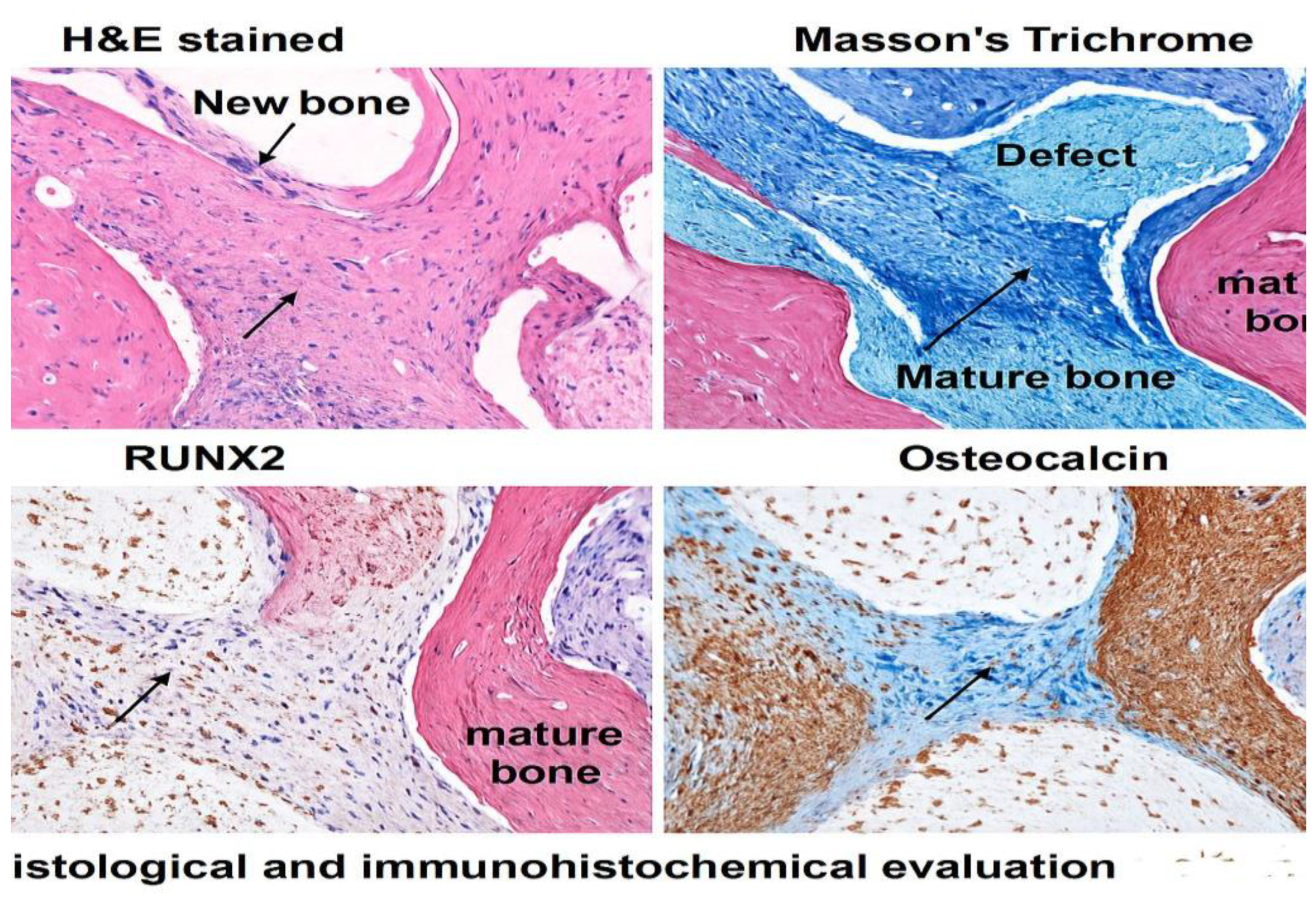
3.6. Histological and Immunohistochemical Evaluation Corroborates Enhanced Osteogenic Activity in DPSC and RUNX2-DPSC-Treated Defects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (DPSCs): Dental Pulp Stem Cells |
| (DFSCs): Dental Follicle Stem Cells |
| (PDLSCs): Periodontal Ligament Stem Cells |
| (SCAP): Stem Cells from the Apical Papilla |
| (HSV-1) Herpes Simplex Virus type 1 |
| (MSCs) Mesenchymal Stem Cells |
| (BM-MSCs) Bone Marrow derived Stem Cells |
| (RUNX2) Runt-Related transcription factor 2 |
| (COL1A1) Collagen Type 1 Alpha 1 |
| (SPP1) Secreted Phosphoprotein. Also known as Osteopontin |
| (BMPs) Bone Morphogenic Proteins |
| (WnT) Wingless-type MMTV integration site family |
| (FGF) Fibroblast Growth Factor |
| (DSPP) Dentin Sialophosphoprotein |
| (BCP) Biphasic Calcium Phosphate |
| RUNX2 – Runt-related Transcription Factor 2 |
| FGF – Fibroblast Growth Factor |
| MSC – Mesenchymal Stem Cells |
| CD73 – Ecto-5'-nucleotidase |
| CD90 – Thy-1 (Thymocyte Differentiation Antigen 1) |
| CD105 – Endoglin |
| CD34 – Hematopoietic Progenitor Cell Antigen |
| CD45 – Protein Tyrosine Phosphatase, Receptor Type C (Leukocyte Common Antigen) |
| BCP – Biphasic Calcium Phosphate |
| MOI – Multiplicity of Infection |
| α-MEM – Alpha Modification of Minimal Essential Medium Eagle |
| FBS – Fetal Bovine Serum |
| BSA – Bovine Serum Albumin |
| SDS-PAGE – Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| PVDF – Polyvinylidene Fluoride |
| ECL – Enhanced Chemiluminescence |
| PFA – Paraformaldehyde |
| DAPI – 4′,6-diamidino-2-phenylindole |
| EDTA – Ethylenediaminetetraacetic Acid |
| H&E – Hematoxylin and Eosin |
| IHC – Immunohistochemistry |
| HA – Hydroxyapatite |
| β-TCP – Beta-Tricalcium Phosphate |
| PCL – Polycaprolactone |
| qRT-PCR – Quantitative Reverse Transcription Polymerase Chain Reaction |
| ALPL – Alkaline Phosphatase |
| COL1A1 – Collagen Type 1 Alpha 1 |
| BMP2 – Bone Morphogenetic Protein 2 |
| OCN – Osteocalcin |
| BV/TV – Bone Volume / Total Volume |
| Tb.Th – Trabecular Thickness |
| Tb.N – Trabecular Number |
References
- Weissman, I. L. (2000). Stem cells: Units of development, units of regeneration, and units in evolution. Cell, 100(1), 157-168. [CrossRef]
- Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (n.d.). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences U.S.A. [CrossRef]
- Zhang, J., Ding, H., Liu, X., Sheng, Y., Liu, X., & Jiang, C. (2019). Dental Follicle Stem Cells: Tissue engineering and immunomodulation. Stem Cells and Development, 28(15), 986-994. [CrossRef]
- Zhu, W., & Liang, M. (2015). Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells International, 2015, 972313. [CrossRef]
- Liu, Q., Gao, Y., & He, J. (2023). Stem Cells from the Apical Papilla (SCAPs): Past, present, prospects, and challenges. Biomedicines, 11(7), 2047. [CrossRef]
- Thapliyal, G. K. (2006). Peterson's Principles of Oral & Maxillofacial Surgery. Med J Armed Forces India, 62(1), 89. [CrossRef]
- Panesar, K., & Susarla, S. M. (2021). Mandibular fractures: Diagnosis and management. Seminars in Plastic Surgery, 35(4), 238-249. [CrossRef]
- Frontiers in Medicine. (2024). Gene and Cell Therapy (Vol. 11). [CrossRef]
- Namjoynik, A., Islam, M. A., & Islam, M. (2023). Evaluating the efficacy of human dental pulp stem cells and scaffold combination for bone regeneration in animal models: A systematic review and meta-analysis. Stem Cell Research & Therapy, 14, 132. [CrossRef]
- Kwack, K. H., & Lee, H. W. (2022). Clinical potential of dental pulp stem cells in pulp regeneration: Current endodontic progress and future perspectives. Frontiers in Cell and Developmental Biology, 10, 857066. [CrossRef]
- Min, Q., Yang, L., Tian, H., Tang, L., Xiao, Z., & Shen, J. (2023). Immunomodulatory mechanism and potential application of dental pulp-derived stem cells in immune-mediated diseases. International Journal of Molecular Sciences, 24(9), 8068. [CrossRef]
- Fujii, Y., Kawase-Koga, Y., Hojo, H., et al. (2018). Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Research & Therapy, 9, 24. [CrossRef]
- Charoenlarp, P., Rajendran, A. K., & Iseki, S. (2017). Role of fibroblast growth factors in bone regeneration. Inflammation and Regeneration, 37, 10. [CrossRef]
- Oton-Gonzalez, L., Mazziotta, C., Iaquinta, M. R., Mazzoni, E., Nocini, R., Trevisiol, L., D'Agostino, A., Tognon, M., Rotondo, J. C., Martini, F. (2022). Genetics and epigenetics of bone remodeling and metabolic bone diseases. International Journal of Molecular Sciences, 23(3), 1500. [CrossRef]
- Dimitriou, R., & Giannoudis, P. V. (2013). The genetic profile of bone repair. Clinical Cases in Mineral and Bone Metabolism, 10(1), 19-21. [CrossRef]
- Wu, V., Helder, M. N., Bravenboer, N., Ten Bruggenkate, C. M., Jin, J., Klein-Nulend, J., & Schulten, E. A. J. M. (2019). Bone tissue regeneration in the oral and maxillofacial region: A review on the application of stem cells and new strategies to improve vascularization. Stem Cells International, 2019, 6279721. [CrossRef]
- Gómez-Barrena, E., Rosset, P., Müller, I., Giordano, R., Bunu, C., Layrolle, P., Konttinen, Y. T., & Luyten, F. P. (2011). Bone regeneration: Stem cell therapies and clinical studies in orthopaedics and traumatology. Journal of Cellular and Molecular Medicine, 15(6), 1266-1286. [CrossRef]
- Ferro, F., Spelat, R., & Baheney, C. S. (2014). Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. Methods in Molecular Biology, 1210, 91–115. [CrossRef]
- Polezhaev, L. V. (1972). Restoration of lost regenerative capacity of dental tissues. In Loss and Restoration of Regenerative Capacity in Tissues and Organs of Animals (pp. 141–152). Keterpress.
- Bai, X., Cao, R., Wu, D., Zhang, H., Yang, F., & Wang, L. (2023). Dental pulp stem cells for bone tissue engineering: A literature review. Stem Cells International, 2023, 7357179. [CrossRef]
- Ahmed, B., Ragab, M. H., Galhom, R. A., & Hassan, H. Y. (2023). Evaluation of dental pulp stem cells behavior after odontogenic differentiation induction by three different bioactive materials on two different scaffolds. BMC Oral Health, 23(1), 252. [CrossRef]
- Liu, T. M., & Lee, E. H. (2013). Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Engineering. Part B, Reviews, 19(3), 254–263. [CrossRef]
- Ryoo, H. M., Kang, H. Y., Lee, S. K., Lee, K. E., & Kim, J. W. (2010). RUNX2 mutations in cleidocranial dysplasia patients. Oral Diseases, 16(1), 55–60. [CrossRef]
- Sodek, J., Ganss, B., & McKee, M. D. (2000). Osteopontin. Critical Reviews in Oral Biology and Medicine, 11(3), 279–303. [CrossRef]
- Brunello, G., Zanotti, F., Scortecci, G., Sapoznikov, L., Sivolella, S., & Zavan, B. (2022). Dentin particulate for bone regeneration: An in vitro study. International Journal of Molecular Sciences, 23(16), 9283. [CrossRef]
- Parsegian, K. (2023). The BMP and FGF pathways reciprocally regulate odontoblast differentiation. Connective Tissue Research, 64(1), 53–63. [CrossRef]
- Nusse, R. (2008). Wnt signaling and stem cell control. Cell Research, 18(5), 523–527. [CrossRef]
- Choi, Y. S., Lee, J. Y., Suh, J. S., Lee, G., Chung, C. P., & Park, Y. J. (2013). The mineralization inducing peptide derived from dentin sialophosphoprotein for bone regeneration. Journal of Biomedical Materials Research. Part A, 101(2), 590–598. [CrossRef]
- Cox, R. F., & Morgan, M. P. (2013). Microcalcifications in breast cancer: Lessons from physiological mineralization. Bone, 53(2), 437-450. [CrossRef]
- Mastrolia, I., Foppiani, E. M., Murgia, A., Candini, O., Samarelli, A. V., Grisendi, G., Veronesi, E., Horwitz, E. M., & Dominici, M. (2019). Challenges in clinical development of mesenchymal stromal/stem cells: Concise review. Stem Cells Translational Medicine, 8(11), 1135–1148. [CrossRef]
- Rana, N., Suliman, S., Mohamed-Ahmed, S., Gavasso, S., Gjertsen, B. T., & Mustafa, K. (2022). Systemic and local innate immune responses to surgical co-transplantation of mesenchymal stromal cells and biphasic calcium phosphate for bone regeneration. Acta Biomaterialia, 141, 440–453. [CrossRef]
- Yamada, S., Shanbhag, S., & Mustafa, K. (2022). Scaffolds in periodontal regenerative treatment. Dental Clinics of North America, 66(1), 111-130. [CrossRef]
- Yamada, S., Yassin, M. A., Schwarz, T., Hansmann, J., & Mustafa, K. (2021). Induction of osteogenic differentiation of bone marrow stromal cells on 3D polyester-based scaffolds solely by subphysiological fluidic stimulation in a laminar flow bioreactor. Journal of tissue engineering, 12, 20417314211019375. [CrossRef]
- Shanbhag, S., Suliman, S., Pandis, N., Stavropoulos, A., Sanz, M., & Mustafa, K. (2019). Cell therapy for orofacial bone regeneration: A systematic review and meta-analysis. Journal of clinical periodontology, 46 Suppl 21, 162–182. [CrossRef]
| Gene | Day 0 | Day 7 (fold ± SD) | Day 14 (fold ± SD) | Day 21 (fold ± SD) | Notes |
| RUNX2 | 3.9 ± 0.4 | 4.2 ± 0.5 | 6.8 ± 0.6 | 5.9 ± 0.7 | Transcription factor — early/mid osteogenic marker. |
| ALPL (ALP) | 3.1 ± 0.4 | 3.5 ± 0.4 | 7.1 ± 0.8 | 4.5 ± 0.6 | Enzymatic marker of osteoblastic activity. |
| COL1A1 | 2.7 ± 0.3 | 2.8 ± 0.3 | 5.2 ± 0.5 | 3.9 ± 0.4 | Major bone matrix collagen. |
| BMP2 | 2.9 ± 0.4 | 3.1 ± 0.4 | 6.4 ± 0.7 | 4.2 ± 0.5 | Osteoinductive growth factor. |
| OCN (BGLAP) | 1.3 ± 0.2 | 1.5 ± 0.2 | 4.7 ± 0.6 | 7.8 ± 0.9 | Late osteogenic/mineralization marker. |
| Group | BV/TV (%) at Week 4 | BV/TV (%) at Week 8 | Tb.Th (µm) at Week 8 | Tb.N (1/mm) at Week 8 |
|---|---|---|---|---|
| Control (BCP only) | 22.4 ± 2.1 | 31.7 ± 2.8 | 78.5 ± 5.1 | 2.4 ± 0.3 |
| DPSC + BCP | 38.9 ± 3.2* | 52.3 ± 3.7* | 112.7 ± 6.4* | 3.8 ± 0.4* |
| RUNX2-DPSC + BCP | 45.6 ± 3.8*,† | 63.9 ± 4.5*,† | 128.3 ± 7.1*,† | 4.6 ± 0.5*,† |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





