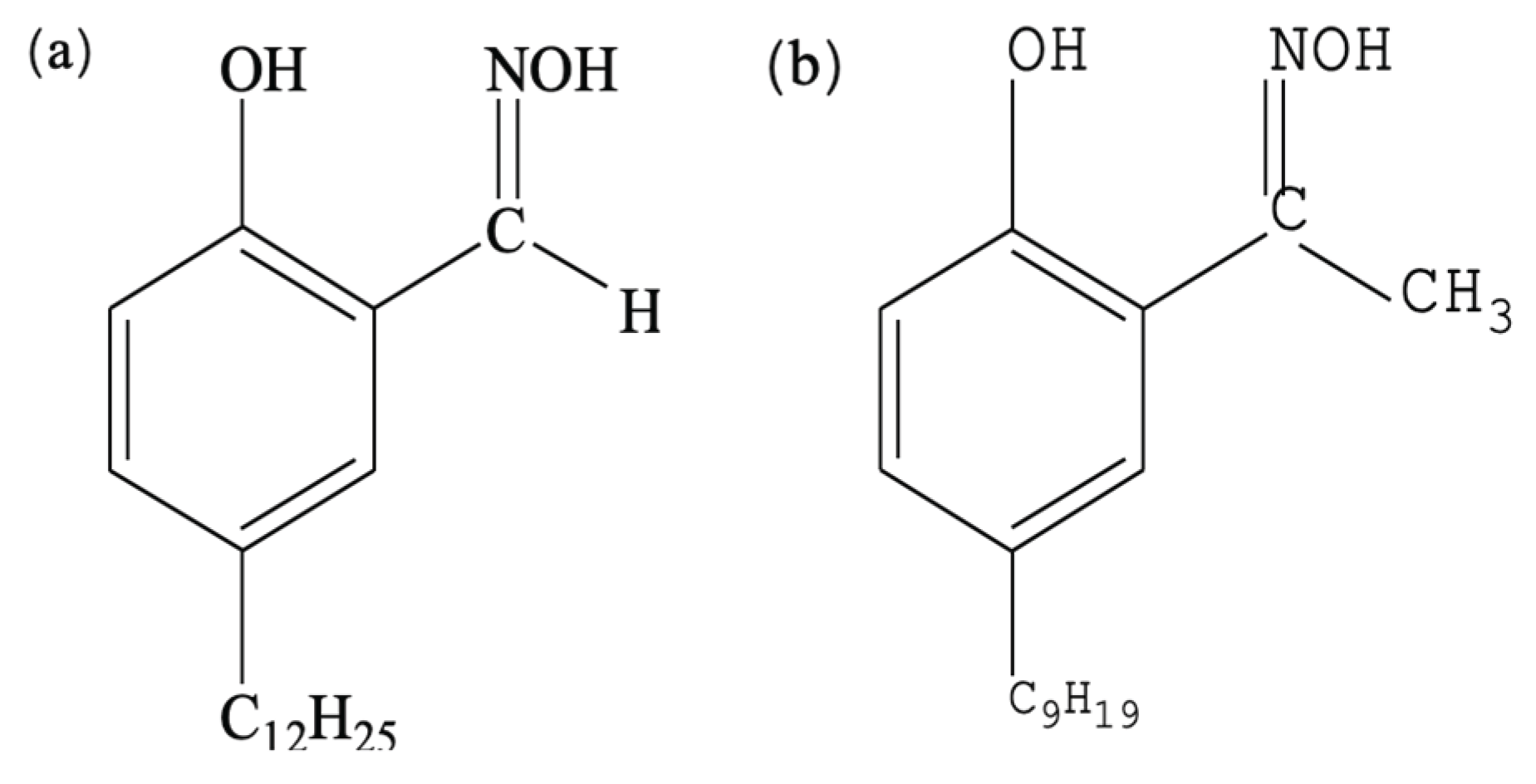
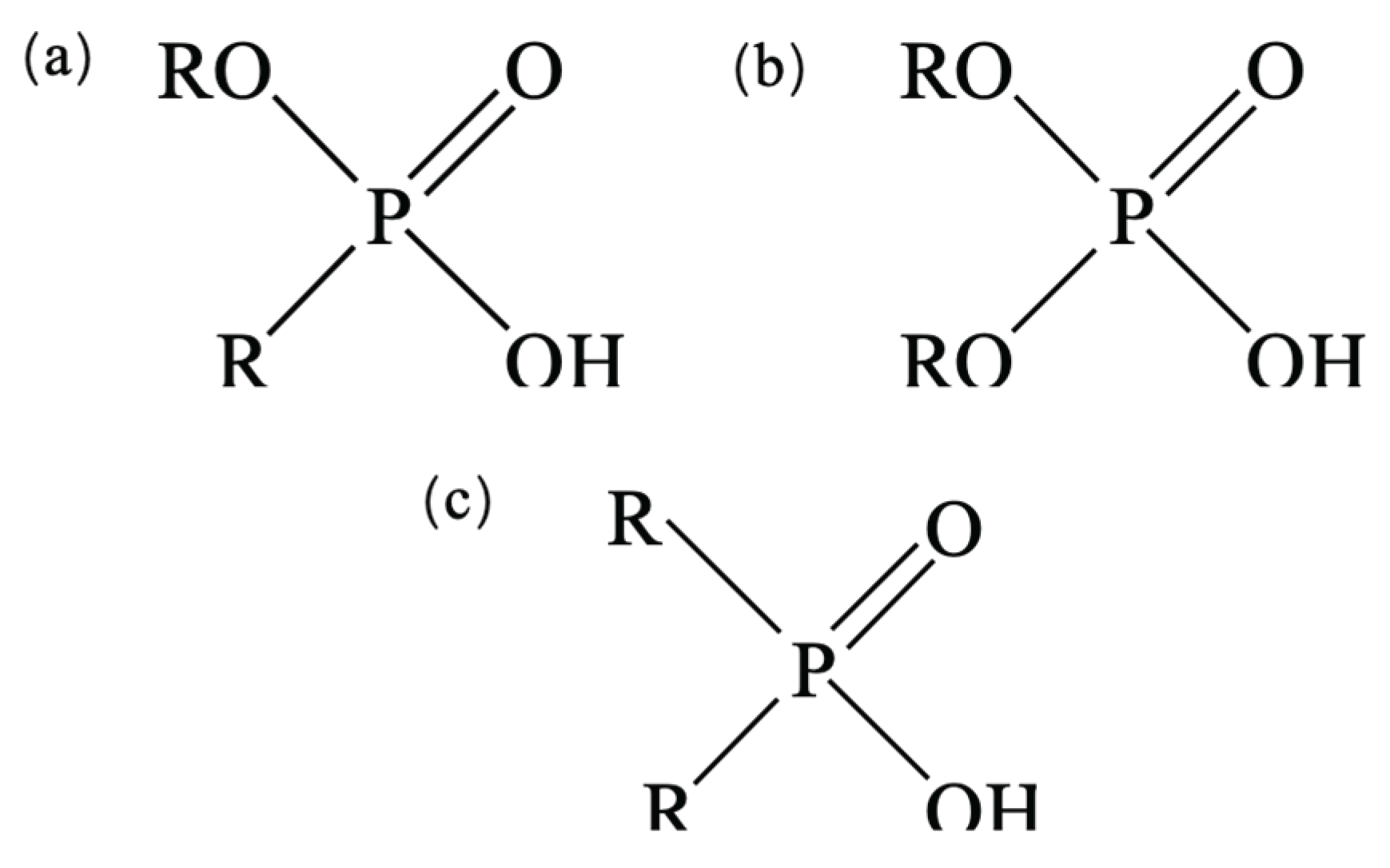
3.1. Synergistic Extraction Study of LIX984 with Three Organophosphorus Accelerators
LIX984 was synergized with three organophosphorus acid accelerators, P507, P204, and Cyanex272, to investigate the extraction characteristics of the mixed extractants under different conditions.
3.1.1. Comparison of the Synergistic Extraction of LIX984 with the Addition of Three Organophosphorus Acid Accelerators
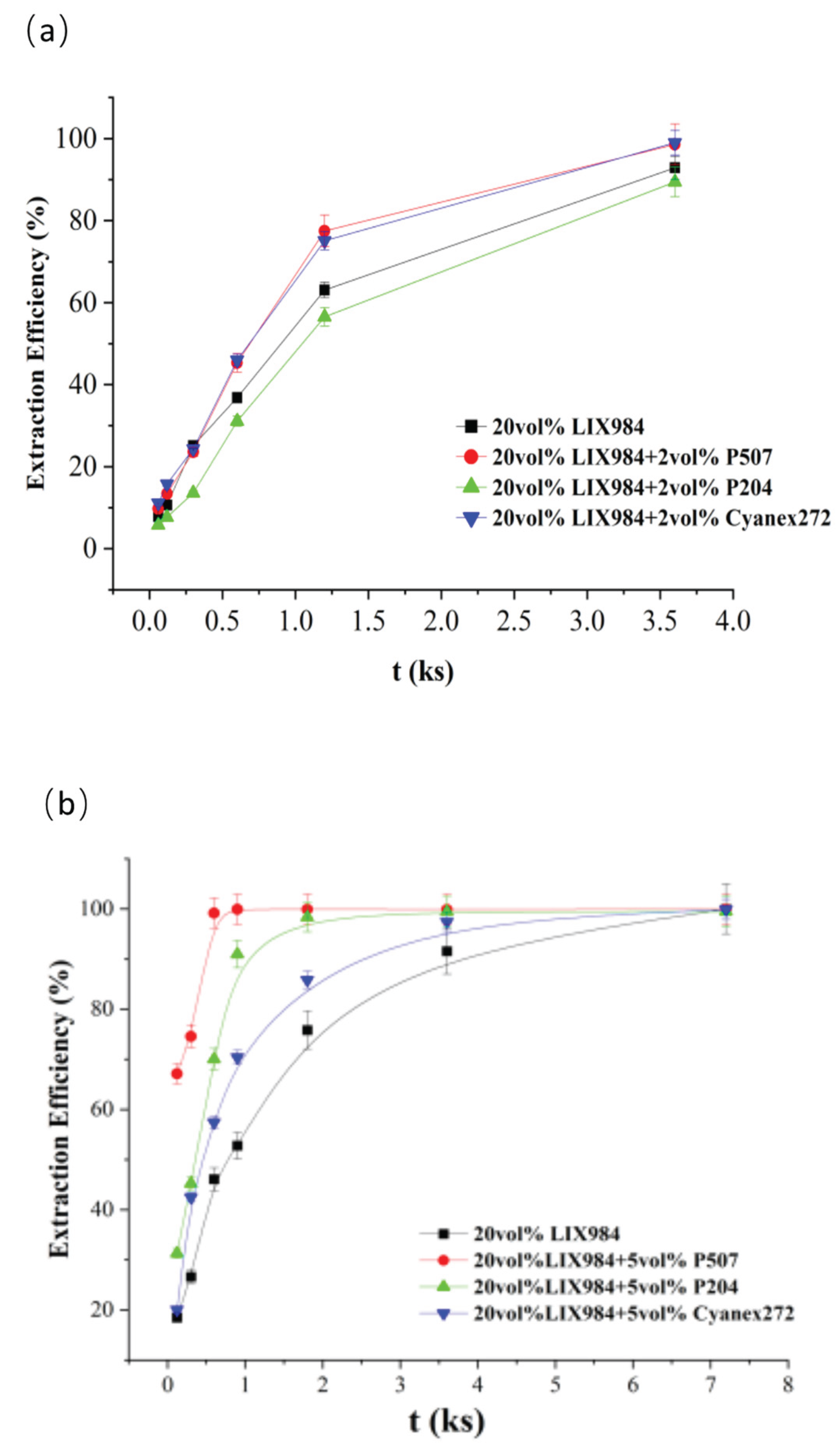
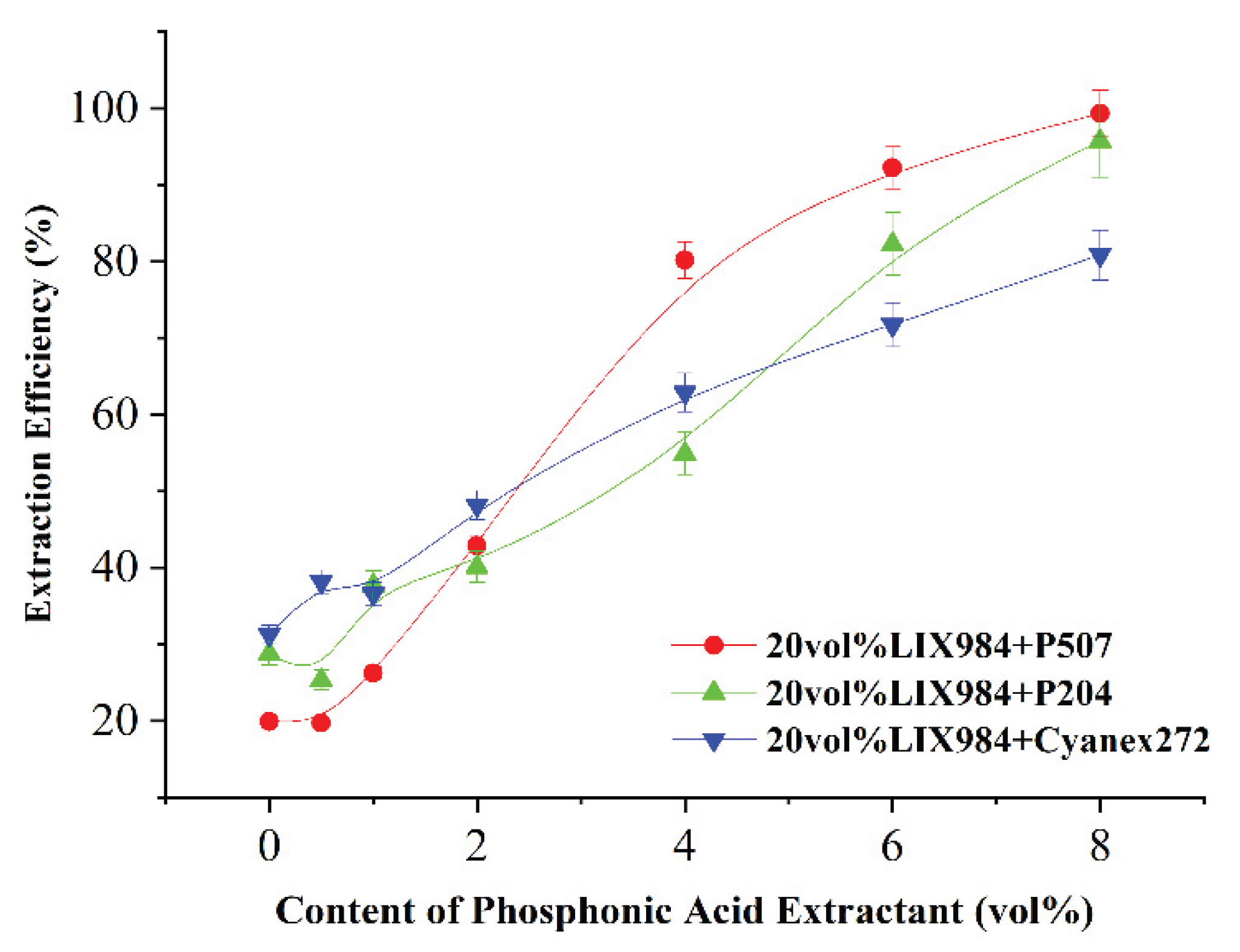
LIX984 at 20 vol% and three organophosphorus acid accelerators at two vol% or five vol% were selected to co-extract the simulated spent electroless nickel plating baths.
As can be seen from
Figure 3a,b, adding a small amount of organic phosphoric accelerator significantly increased the organic phase nickel extraction efficiency. When two vol% P507 or Cyanex272 were added, the extraction curve was significantly higher than that without the addition. After 20 min extraction, nickel extraction efficiencies increased from 63.1% to 77.5% and 75.1%, respectively, compared to the case without phosphoric accelerator, while two vol% P204 extraction efficiency was close to that without (see
Figure 3a); With the addition of 5 vol% P507, the extraction efficiency of nickel at 10 minutes was 99.2%, while the extraction efficiency of nickel from LIX984 without the addition of organic phosphoric promoter was still only 46.1%.P507 has the best co-extraction performance among the three organic phosphorus accelerators.
It has been observed that the kinetic extraction sequence of three phosphate extractors in different concentrations (2 vol% vs 5 vol%) is different. This may be due to their molecular structure and mechanism of action. At a lower concentration (2 vol%), since Cyanex272 exhibits better interphase activity, its kinetic rate is higher than P204; however, at a higher concentration (5 vol%) P204 was found to have better characteristics, which may be due to the enhanced synergistic effect between it and LIX984. From
Figure 3b, it can be seen that the LIX984+5 vol%P507 extraction system has the best extraction effect, followed by LIX984 + 5vol% P204 extraction system, and the worst is the LIX984 + 5vol%Cyanex272 extraction system. This extraction efficiency sequence correlates with the surface tension measurements that will be discussed later in
Section 3.3, where we demonstrate that lower surface tension values correspond to higher extraction efficiencies when the organophosphoric acid co-extractant addition ratio is 5 vol%.
3.1.2. Effect of Adding Organophosphate Accelerator on LIX984 Co-Extraction with Extraction Time
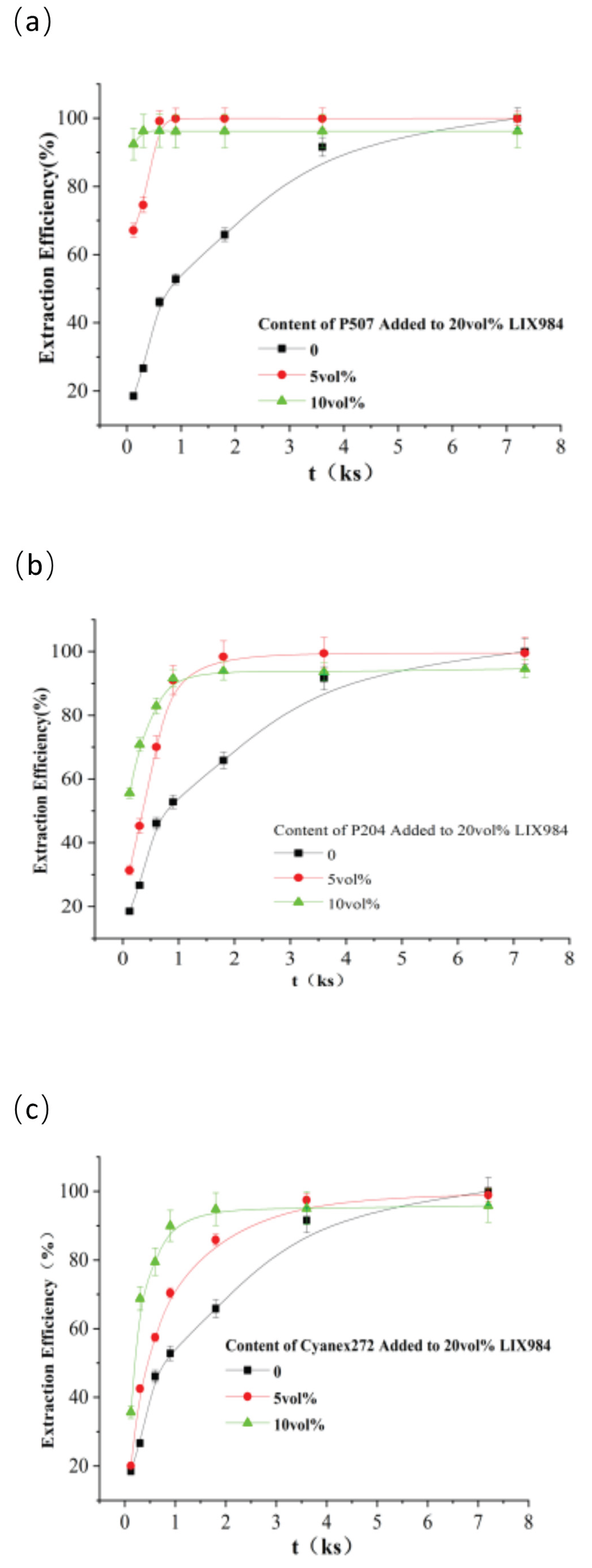
Figure 4 shows the nickel extraction efficiency versus time for 20 vol% LIX984 with different contents of organophosphorus accelerators P507, P204, and Cyanex272. The extraction rate of nickel by the mixed extractant increased significantly with a gradual increase in the level of the organophosphoric acid accelerator. After 10 minutes, the extraction efficiencies for nickel were 46.1 %, 99.2 %, and 96.3 %, respectively, when PC88 was added. Adding 5 vol% P507 resulted in a higher extraction efficiency than adding 10 vol%, as shown in
Figure 4(a). The addition of 5 and 10 vol% P204 in the organic phase increased nickel extraction efficiencies to 70.5% and 82.9% compared to 46.1% without addition (
Figure 4(b)); Adding 5 and 10 vol% Cyanex272 to the organic phase increased the extraction efficiency of nickel from 46.1% to 57.5% and 79.5% respectively, the extraction efficiency was higher when adding ten vol% Cyanex272 (
Figure 4(c)). Competitive extraction alone cannot fully explain this phenomenon. At a constant concentration of LIX984, the equilibrium extraction efficiency should theoretically be similar, but the observed decrease in efficiency at a higher concentration of a phosphoric acid-based extract (>5 vol.%) is most likely due to the Association of LIX984 with phosphoric acid-based extract molecules, which leads to a decrease in effective concentration. as a result of interaction with LIX984, the chemical medium around the extraction site changes and this also affects the interfacial properties of the system. In appropriate studies of binary extraction systems containing organic phosphoric acid, similar synergistic or antagonistic effects have been reported. A study by Narita et al. [
25] shows that D2EHPA can interact with LIX series extractors, affecting the extraction process. At higher concentrations, similar interactions are likely to occur with P507 and Cyanex272.
Extraction equilibrium was reached in 30 minutes with 5% P204, as opposed to 120 minutes without the accelerator. The addition of organophosphorus to LIX984 may play an essential role in the practical application of nickel extraction.
3.1.3. Effect of the Addition of Organophosphate Accelerators on the Efficiency of Nickel Extraction
The nickel extraction efficiency of LIX984 without the addition of organic phosphoric acid extractant was only about 30% when the extraction time was 5 minutes.
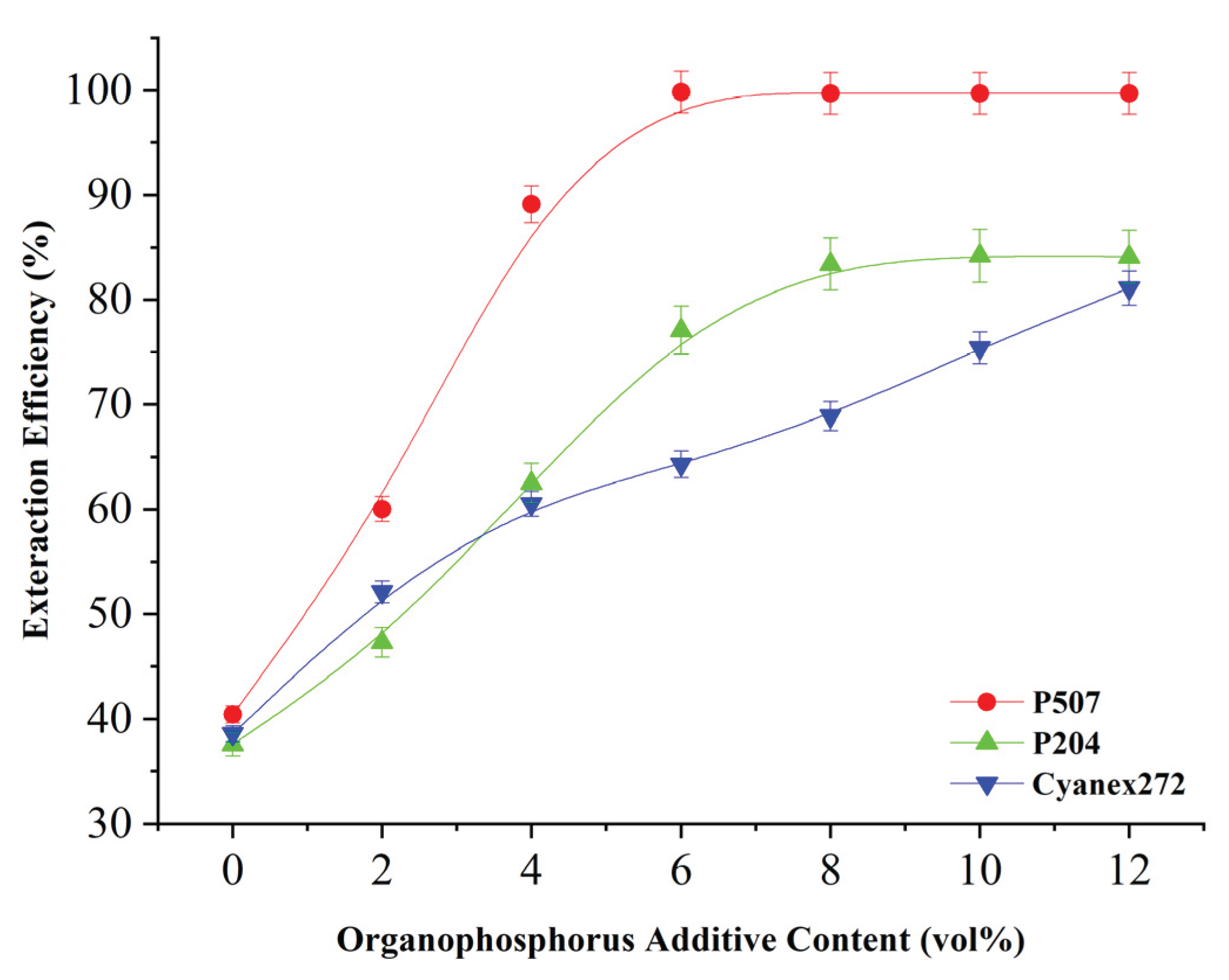
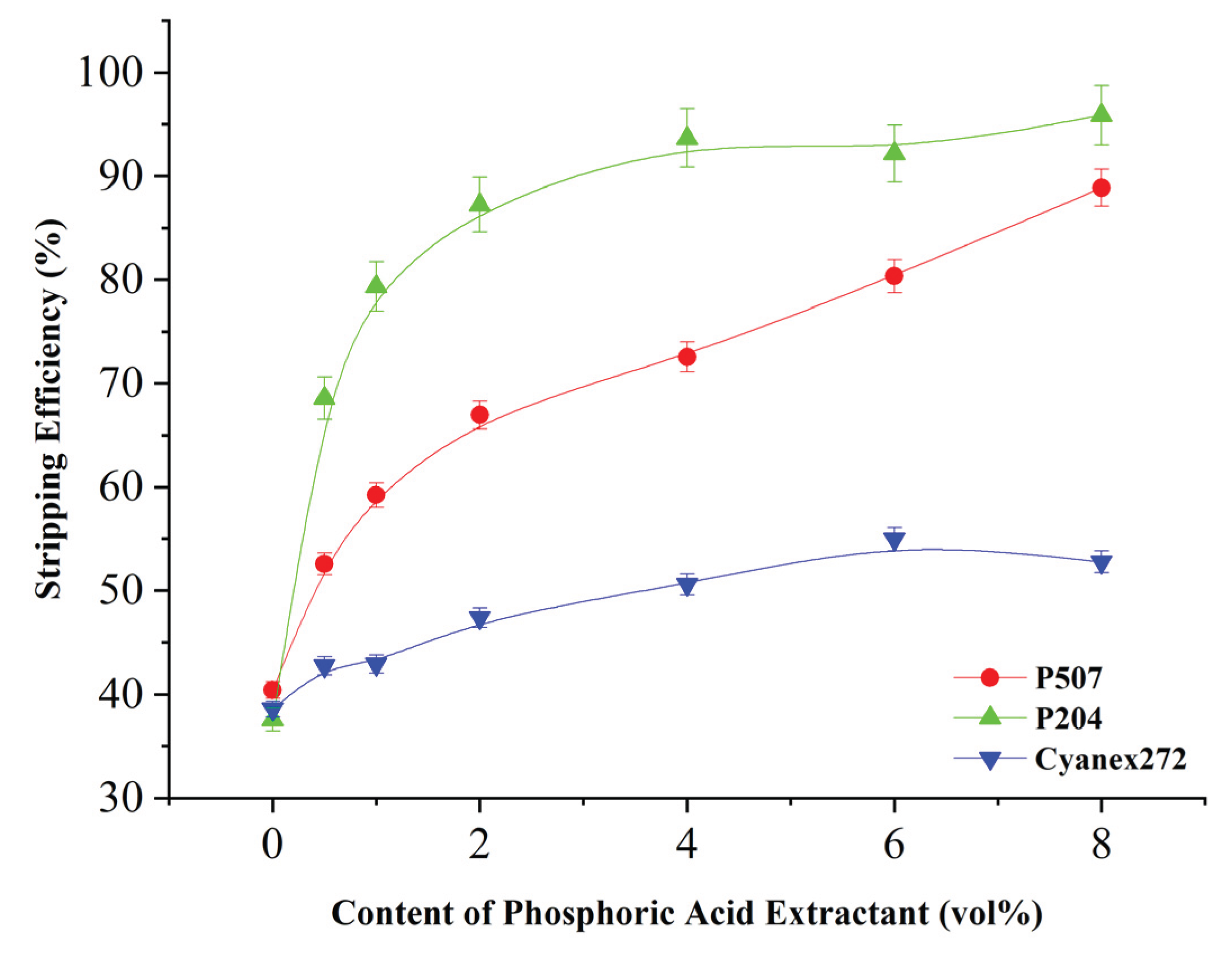
Figure 5 shows that with the addition of organic phosphoric acid extractant, nickel extraction efficiency increases rapidly, especially P507 and P204, when its additive amount of 6%, the extraction efficiency of nickel has achieved 99.3% and 95.8%, respectively, when adding Cyanex272, nickel extraction efficiency will also increase considerably when adding 8 vol%, nickel extraction efficiency from no additive when the 31.2% to 80.1%, but not as good as the co-extraction Effect of nickel by adding P507 and P204.
It has been found that organophosphate acid-based Acid extractors can increase extraction efficiency, probably due to their high selectivity towards nickel and ability to alter phase interface properties. Although the final equilibrium rate of extraction in different types of extractors does not differ much, their main advantage is that they significantly increase the kinetic rate of extraction, which is especially important in industrial applications, since the processing time directly affects economic feasibility.
To determine if there is a saturation effect and to determine the optimal dosing interval, a further study examined higher concentrations of organophosphate (as shown in
Figure 6). When the P507 supplement increased to 5 vol.%, the extraction efficiency steadily increased to 99.2% in just 10 minutes. However, when it increased to 8 vol.% and 10 vol.%, no significant changes were observed. At the time, it reached 99.3% and 99.5%, respectively, indicating that the acceleration effect was near the upper limit. For P204, the extraction efficiency is usually stabilized at about 8vol.%; and even if Cyanex272 is increased to 10 vol.%, will continue to increase steadily and there will be no obvious signs of saturation. It is assumed that the optimal range of use of P507 may be 4-6% volume, while P204 is suitable for 7-9% volume. Cyanex272 requires more than 10% volume to show effect. Once that limit is exceeded, adding more catalysts will not provide additional practical help, but will speed up the process. Therefore, this saturation characteristic can be caused by the limitation of the effective surface area of the catalytic interface or the fact that the active site in the extraction system has been fully exploited.
(Extraction time: 10 min, pH 6.5, simulated waste solution; PС88A: Rapid saturation at ~5 vol%; DEHРA: Plateaus at ~8 vol%; Cyanex272: Continues improving beyond 10 vol%)
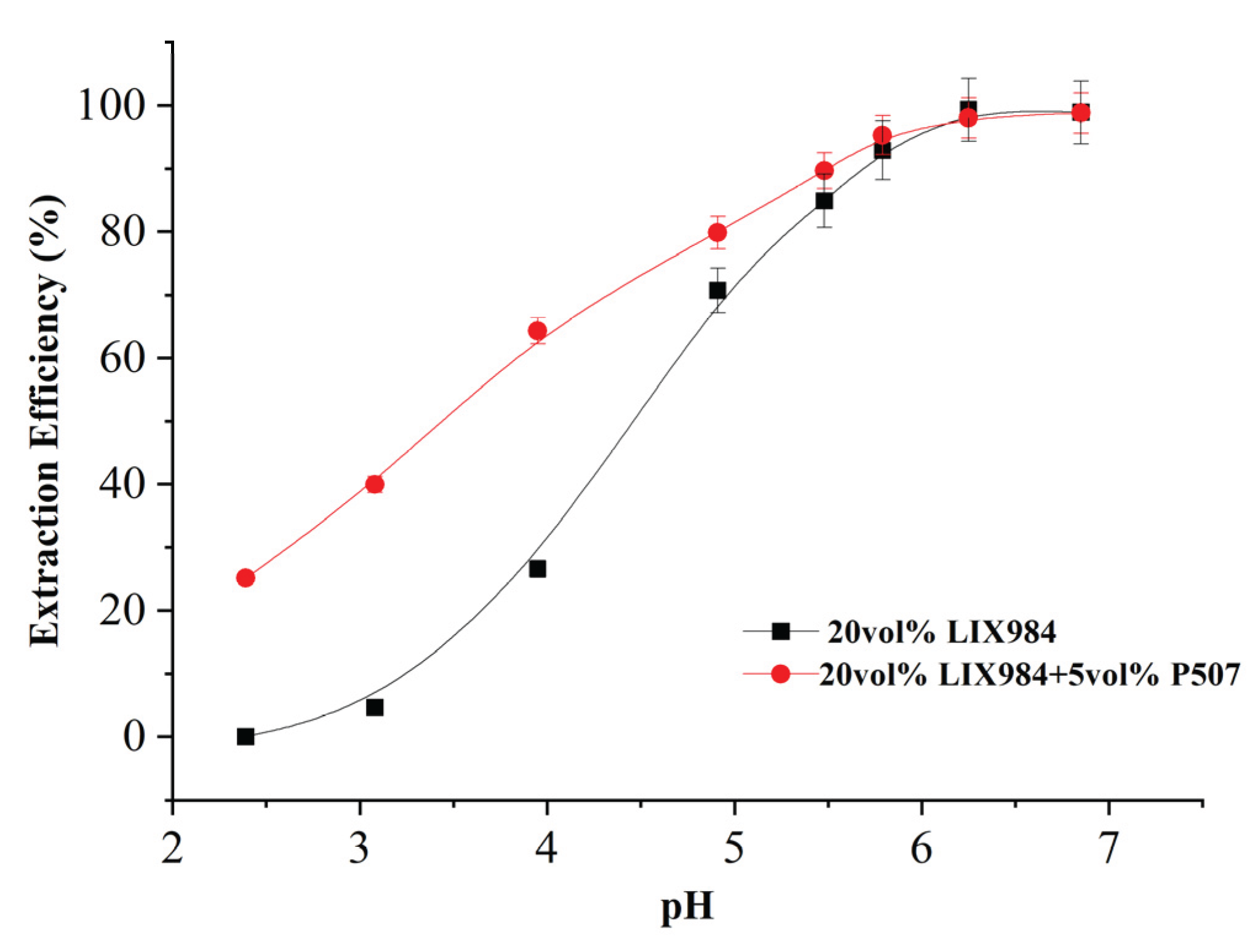
3.1.4. Nickel Extraction with P507 Organophosphate Accelerator Added to LIX984 at Different pHs
Figure 7 shows the extraction efficiencies of nickel in the simulated spent electroless nickel plating baths at different pH with no addition and 5 vol% P507 added to LIX984, respectively. It can be seen that the extraction efficiency of nickel was significantly improved with the addition of 5 vol% P507 when pH < 5; the improvement was not significant when pH > 5; and the extraction efficiency of close to 100% could be achieved in both cases at pH = 7. At pH=3.1, the extraction efficiency increased from 4.64% to 39.9% without addition.
3.3. Effect of Addition of Organophosphorus Acid Extractant to LIX984 on the Extraction and Stripping Rates of Nickel
3.3.1. Effect of the Addition of Organophosphorus Acid Extractant to LIX984 on the Extraction Rate of Nickel
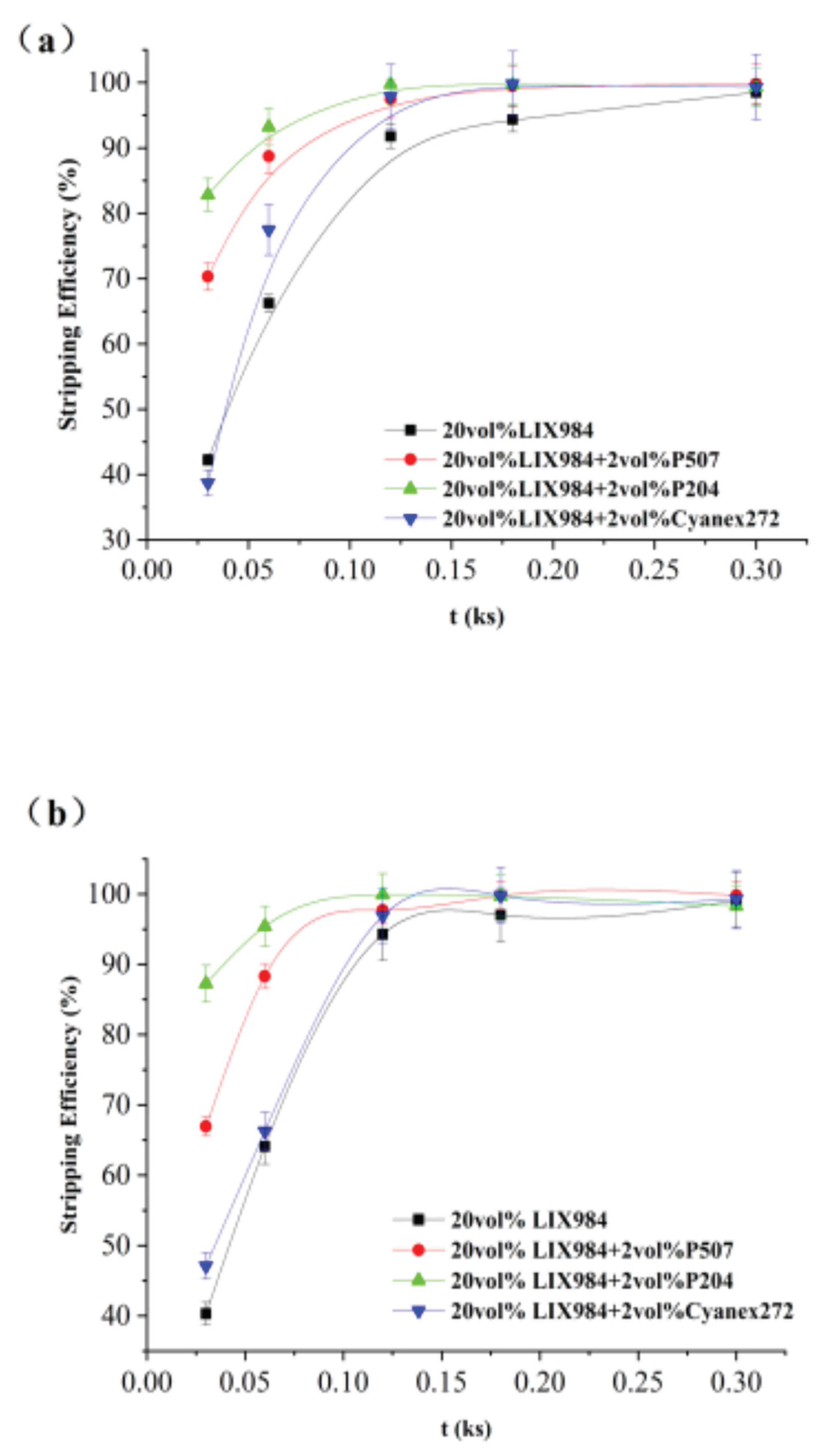
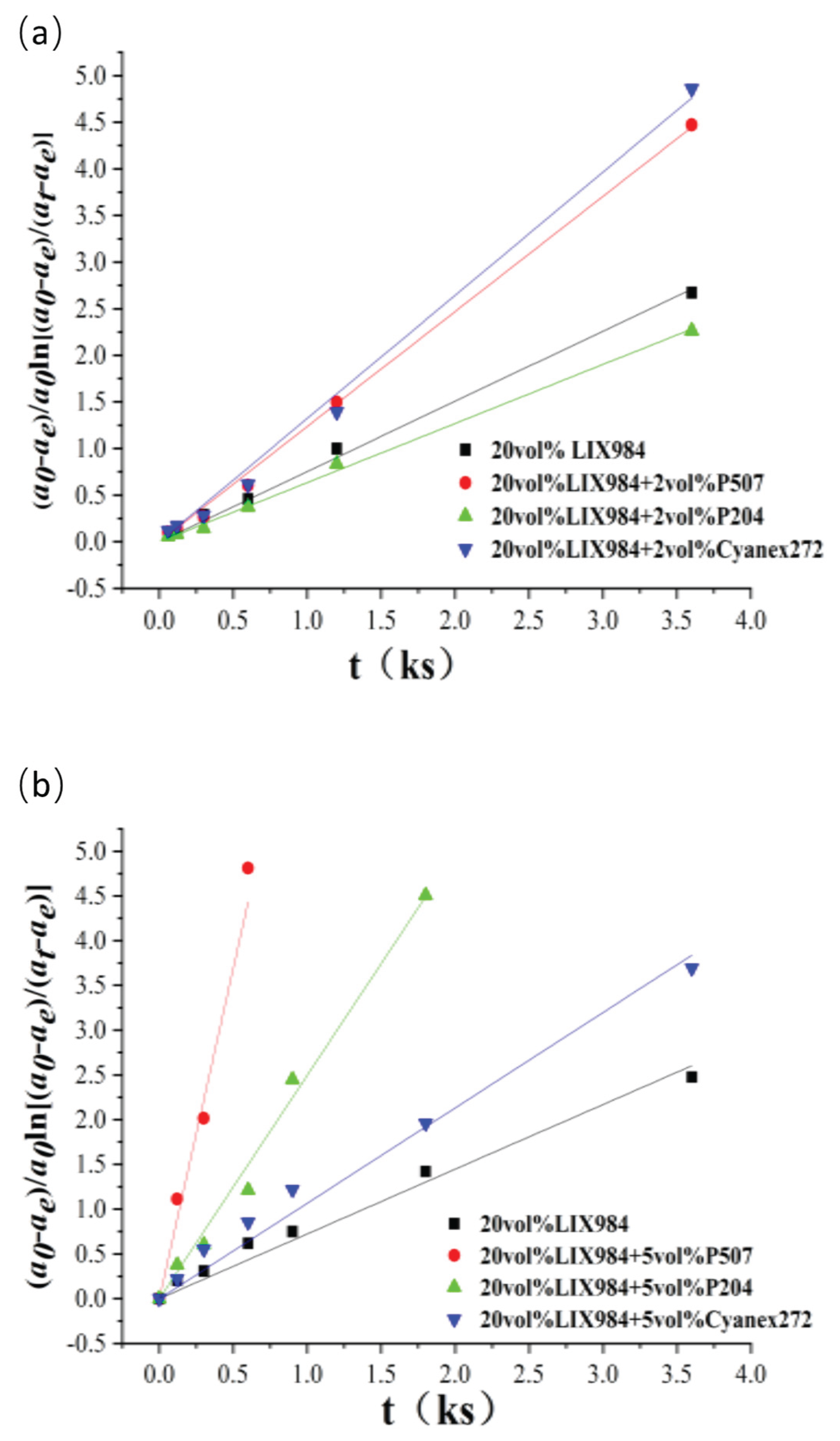
The organic phase was 20vol% LIX984 dissolved in D70 with 2vol% or 5vol% organic phosphoric acid accelerators P507, P204, and Cyanex272 for the co-extraction of nickel from spent electroless nickel plating baths; the aqueous phase was a simulated electroless nickel plating bath (as shown in section 2.1). The extraction rate of nickel from spent electroless nickel plating baths was analyzed, and the resulting data were plotted against time t using the least squares method according to Equation (3). (
Figure 10)
The corresponding slopes of each line can be obtained by fitting the resulting line in
Figure 10. This is the apparent rate constant of extraction, k
f, as shown in
Table 3.
Table 3.
Apparent rate constants
kf in
Figure 8(a).
Table 3.
Apparent rate constants
kf in
Figure 8(a).
| Organophosphate accelerator additions |
0 |
2vol%P507 |
2vol%P204 |
2vol%Cyanex272 |
| kf/ks-1 |
0.753 |
1.23 |
0.634 |
1.32 |
Table 4.
Apparent rate constants
kf in
Figure 8(b).
Table 4.
Apparent rate constants
kf in
Figure 8(b).
| Organophosphate accelerator additions |
0 |
5vol%P507 |
5vol%P204 |
5vol%Cyanex272 |
| kf/ks-1 |
0.723 |
7.80 |
2.50 |
1.07 |
It can be seen that the apparent extraction rate constant increased significantly with the addition of a small amount of organophosphorus accelerator than without, especially at 5 vol% addition. The apparent extraction rate constants kf of extractions with 2 vol% addition of P507 and Cyanex272 increased by 1.64 and 1.76 times, respectively, compared with that without addition of organophosphorus accelerator, and the apparent extraction rate constants with 5 vol% addition of P507, P204, and Cyanex272 increased by 10.8, 3.45 and 1.47 times, respectively. It was 1.5 to 10 times higher than that obtained without adding the organophosphorus accelerators, which had an apparent accelerating effect on the extraction equilibrium. Among the three organophosphorus accelerators, the accelerating Effect of P507 on reaction rate is more obvious: 2 vol% of P507 can accelerate 1.64 times, and 5 vol% of P507 can accelerate up to 10.8 times, which has significant economic benefits in practical applications.
3.3.2. Effect of Addition of Organophosphate Accelerator to LIX984 on Stripping Rate of Nickel
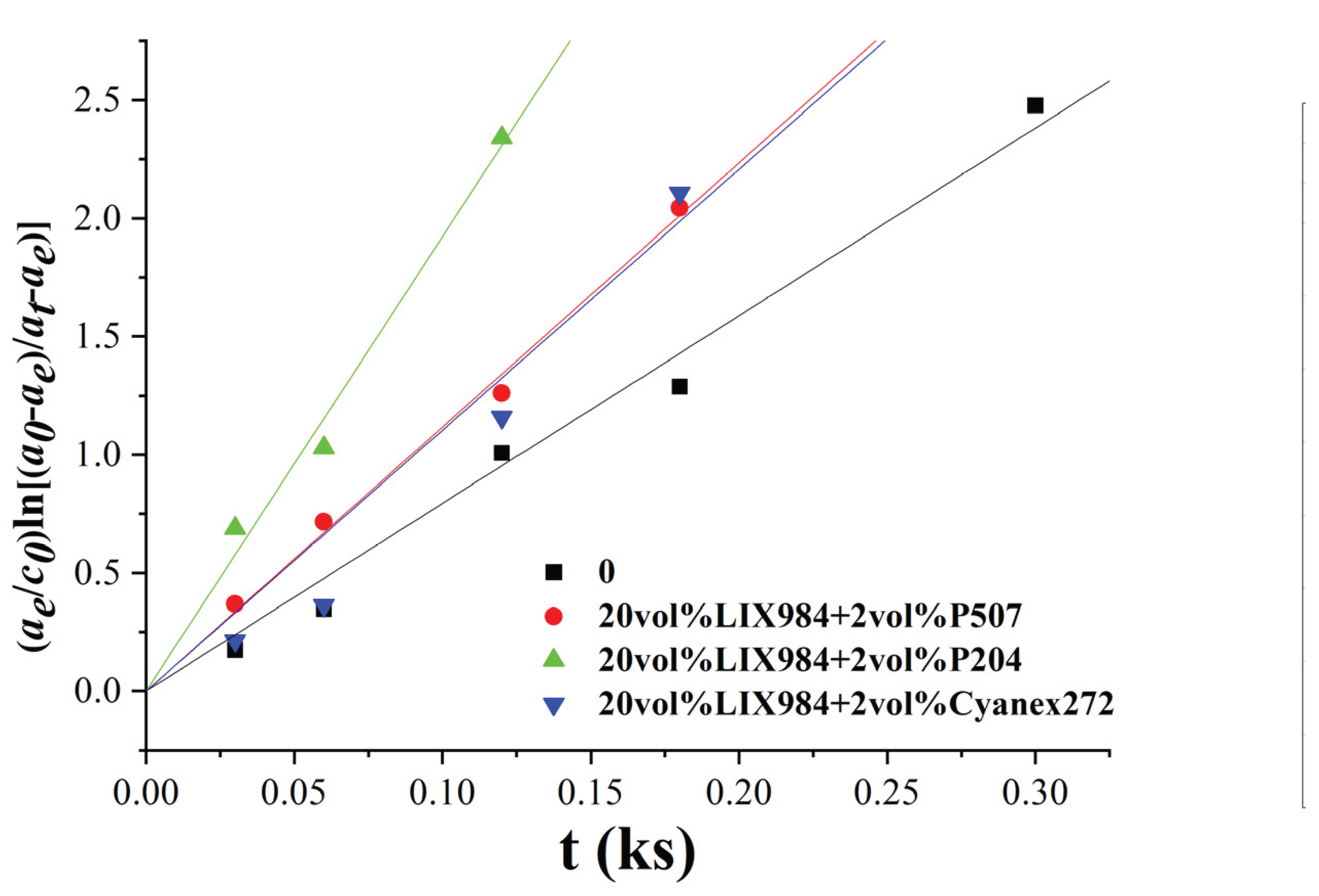
To investigate the Effect of adding 20 vol% LIX984 as an organophosphorus acid accelerator on the stripper effect, a 1 mol/L sulphuric acid solution was chosen to perform the stripper experiments on the extracted organic phases. The metal concentrations of the extracted aqueous phases of the stripper extracts were determined by varying the extraction oscillation time. The data obtained were fitted by the least squares method according to Equation (4) to obtain
Figure 11, the slope of which is the apparent rate constant of the stripping k
b.
The corresponding slopes of each line can be obtained by fitting the resulting line from
Figure 11, which is the apparent rate constant of stripping, k
b, as shown in
Table 5.
As can be seen in
Figure 10, the apparent extraction rate constant k
b of the extractions with the addition of 2 vol% P507, P204, and Cyanex272 increased by a factor of 1.41, 2.42, and 1.34, respectively, compared to that without the addition of the organophosphoric acid co-extractant. After addition, the apparent rate constant of the stripping k
b increased by 1.5 to 3 times, respectively, compared to that without the addition of the organophosphoric acid co-extractant, which had a particular accelerating effect on the stripping equilibrium. The accelerating Effect of P507 and Cyanex272 was close to that of P204, while the accelerating Effect of P204 was more prominent, and the Comparison of the apparent rate constant of the stripping, k
b, showed that the accelerating Effect on the extraction was more than two times when 2 vol% P204 was added to LIX984.
Three types of complexes can be formed when a small amount of organophosphorus accelerator is added to the LIX984 extractant: organophosphorus complex, LIX984 complex, and LIX984 and organophosphorus co-complex. The study in
Section 3.4 shows that organic phosphoric acid extractant alone shows low extraction efficiency for nickel from spent electroless nickel plating baths (less than 10% at pH<5 as shown in
Figure 12). Therefore, the main nickel complex is still formed by LIX984 with nickel. The basic complex formed from LIX984 and nickel involves the Coordination of nitrogen and oxygen donor atoms in the components aldocyma (LIX860) and ketocyma (LIX84). Spectroscopic studies by Narita et al. [
25] have shown that nickel and these ligands form square plane complexes, and the metal coordinates with two molecules of the extract. When an organophosphate extract is present, it can initially form intermediate complexes at the phase interface, promoting the transition of nickel to the organic phase and eventually forming a more stable complex of LIX984. It can be inferred that Ni
2+ and organophosphoric acid molecules form the corresponding complex at the interface, and this complex enters the organic phase and reacts with hydroxamic acid molecules, followed by the formation of hydroxamic acid complexes with the planar tetragonal structure of Ni(II) [
25], which improves the overall extraction rate. Thus, organophosphorus accelerators, as phase transfer catalysts, significantly accelerate the extraction reaction [
26].
Tanaka et al. [
23] discussed the interfacial pressure between the organic and aqueous phases without and with the addition of an organophosphoric acid accelerator during the extraction of metallic nickel with LIX84I. The interfacial area under extraction equilibrium conditions does not increase significantly with the addition of a small amount of P204. In contrast, dynamic interfacial activity is an essential factor influencing droplet diffusion [
11]. The dynamic interfacial activity of the organophosphorus accelerator is higher than that of LIX984, and the droplet dispersion of the LIX984 extractant with the addition of the organophosphorus accelerator is significantly better than that of the extractant without LIX984. Therefore, after the addition of the organophosphoric acid extractant to LIX984, the interfacial area between the organic phase and the aqueous phase will increase accordingly, and under this condition, the extraction and stripping efficiency of metals will be significantly improved. Studies have reported that [
30] the addition of 2% P507 or P204 to the hydroxyoxime extractant LIX984 leads to a significant improvement in the dispersion of the droplets, which also corroborates our experimental results.
In summary, under the existing experimental conditions, the organophosphorus accelerator mainly played the phase transfer catalyst and dispersant role in the extraction process, resulting in a significant increase in the extraction rate.
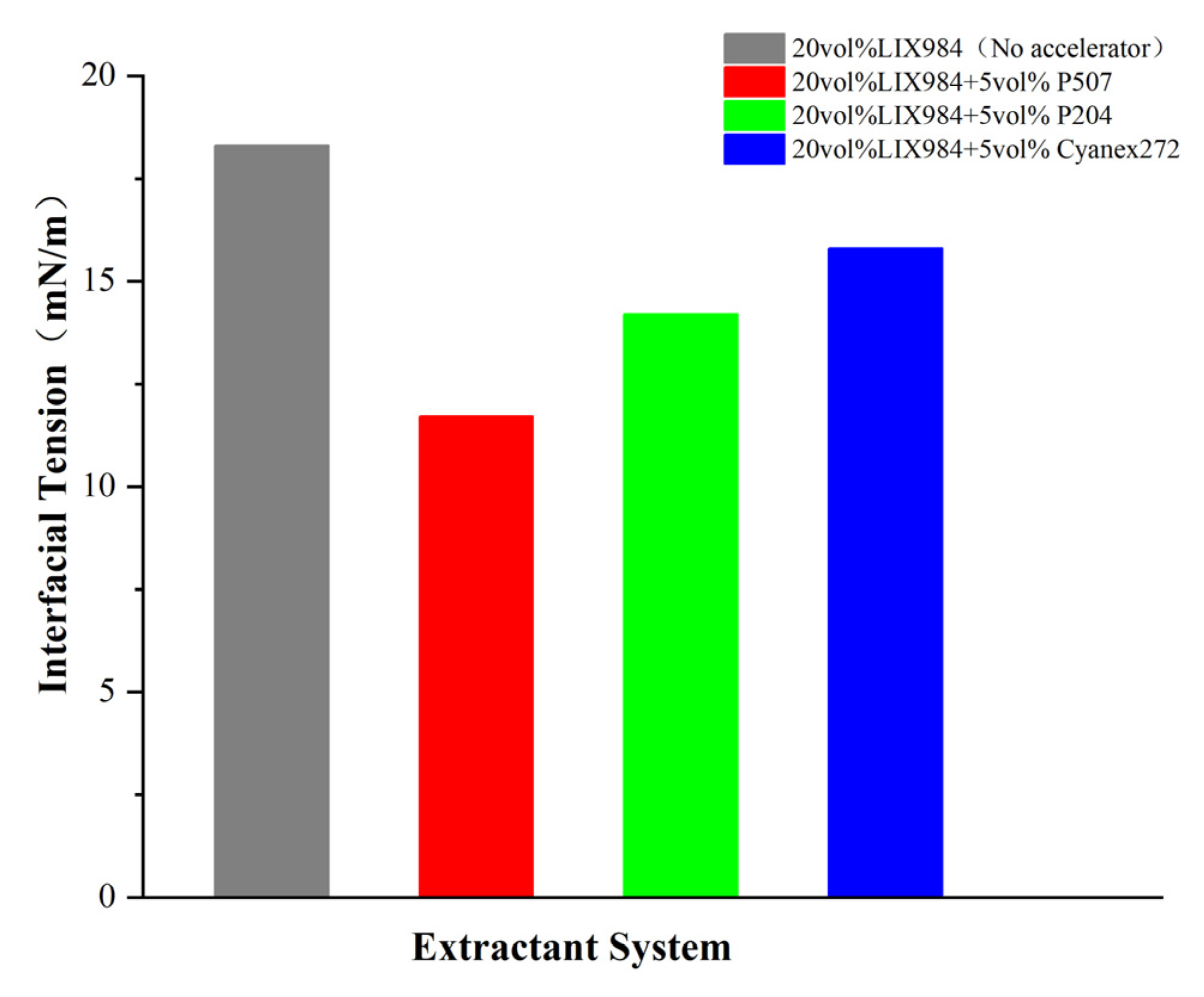
In order to analyze the mechanism of superior operation of P507 as an accelerator, we performed measurements of interphase tension in various extraction systems (
Figure 12). The results showed that the surface tension between the aqueous phase (liquid waste simulation) and the organic phase was significantly reduced from 18.3 mN/m of pure LIX984 to 11.7 mN/m containing 50% P507. It was also noted that the surface tension of P204 and Cyanex272 systems was reduced to 14.2 mN/M and 15.8 mN/m, respectively. This reduction in surface tension is associated with an excellent accelerating extraction effect. At the same time, IR analysis of the extracted organic phase showed that there is a characteristic shift between the telescopic range P=O (shift from 1230 cm
⁻1 to 1205 cm
⁻1) and P-O-C oscillations (shift from 1050 cm
-1 to 1030 cm
⁻1) in the P507 system, indicating a specific interaction between organophosphorus accelerator and metal⁻ert984 complex. These spectral phenomena support the proposed catalytic phase transfer mechanism, that is, the formation of intermediate complexes reduces the energy barrier for extraction and promotes the transfer of metals from the liquid to the phase interface.
3.3.3. Comparative Performance Analysis with Other Established Nickel Extractants
After perfecting the characteristics of the improved LIX984 system, the results are compared with the nickel extraction facilities described in the Tanaka et al. [
10,
11,
12] studies on the extraction of nickel from chemical nickel solution show that LIX84I with a similar structure to LIX984 can achieve an extraction efficiency of about 95% after 30 minutes at a pH value of 6.5. The use of the optimized system (volume ratio 20% LIX984 +5% P507) takes only 10 minutes under similar pH conditions, and the extraction efficiency reaches 99.2%, indicating a significant improvement in extraction dynamics.
The dithiophosphatic acid-based extract Cyanex301 reportedly provides a high degree of nickel extraction-over 98%, but usually the mating conditions are more acidic and demanding than in our system. The advantage of the mixed extract system is faster processing, effective removal of conventional sulfuric acid and the ability to selectively separate nickel while simultaneously removing iron and zinc impurities.
Table 6.
Comparative performance of various nickel extractant systems.
Table 6.
Comparative performance of various nickel extractant systems.
| Extractant System |
Extraction Efficiency (%) |
Extraction Time to >95% (min) |
Stripping Efficiency (%) |
pH range for optimal extraction |
| LIX84I |
95 |
30 |
95 |
6-7 |
| Cyanex301 |
98 |
40 |
92 |
5-6 |
| LIX984 (this study) |
91 |
120 |
90 |
6-7 |
| LIX984 + 5 vol% P507 (this study) |
99.2 |
10 |
97 |
6-7 |
| LIX984 + 5 vol% P204 (this study) |
97 |
30 |
99.8 |
6-7 |
| LIX984 + 5 vol% Cyanex272 (this study) |
96 |
40 |
94 |
6-7 |
According to this comparative analysis, the LIX984 Plus system with organophosphate extract has excellent operational characteristics. Among them, the combination of LIX984 and P507 stands out in particular. with the maintenance of high extraction and evaporation efficiency, performance in extraction dynamics become more visible.
3.4. Extraction of Iron, Zinc, and Nickel from Spent Electroless Nickel Plating Baths by Three Organic Phosphoric Acid Extractants
The goal of this section is to explore whether an organophosphate extract can target the removal of impurities like iron and zinc to achieve the goal of nickel extraction and understand how this selectivity manifests itself, which is especially important for building an efficient two-step extraction process. In this process, an organophosphate extract with a lower pH value must first be used to remove impurities, and then the organophosphate system LIX984 is used to perform nickel extraction tasks under conditions of higher pH value.
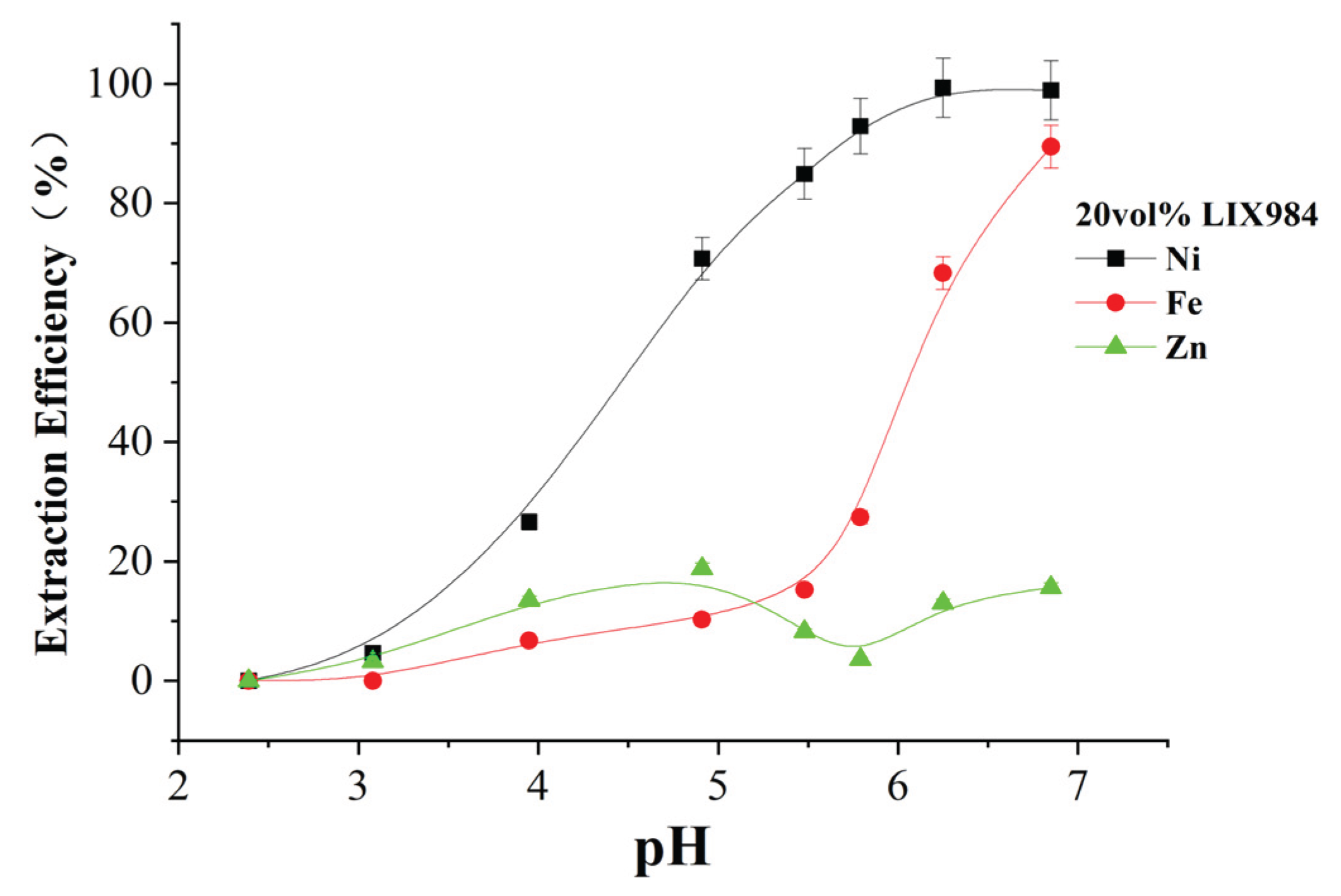
Figure 13 shows the extraction efficiency of 20 vol% LIX984 for iron, zinc, and nickel in spent electroless nickel plating baths.
Figure 11 shows that LIXIL984 can recover high amounts of iron and low amounts of zinc during nickel recovery at different pH levels. For example, at pH=6, although the nickel extraction efficiency is high at 99.3%, the iron extraction efficiency is also high at 68.3% and the zinc extraction rate is 13.0%. This indicates that the separation of nickel, iron, and zinc by LIX984 is low. Therefore, other extractants should be used to remove the iron and zinc contaminants from spent electroless nickel plating baths first before extracting and recovering nickel. To avoid iron deposition at pH above 3, existing freshly prepared solutions have been used in experiments. During the extraction process, the pH value is carefully monitored. In experiments with higher pH values, measurement of turbidity and calculation of mass balance confirmed the absence of deposition phenomena. The complex agents (lactic acid and propionic acid) found in the solution of the waste galvanizing Bath have a significant effect on the stability of the iron in the solution even at slightly higher pH values.
The organic phase was configured using three organic phosphoric acid extractants, P507, P204, and Cyanex272, dissolved in D70, each added at 10 vol%. The aqueous phase used simulated spent electroless nickel plating baths containing nickel, iron, and zinc with the actual waste solution. The pH of the waste solution was adjusted, and the three organic phosphoric acid extractants reacted with the aqueous phase. The extracted aqueous phase's nickel, iron, and zinc content was determined.
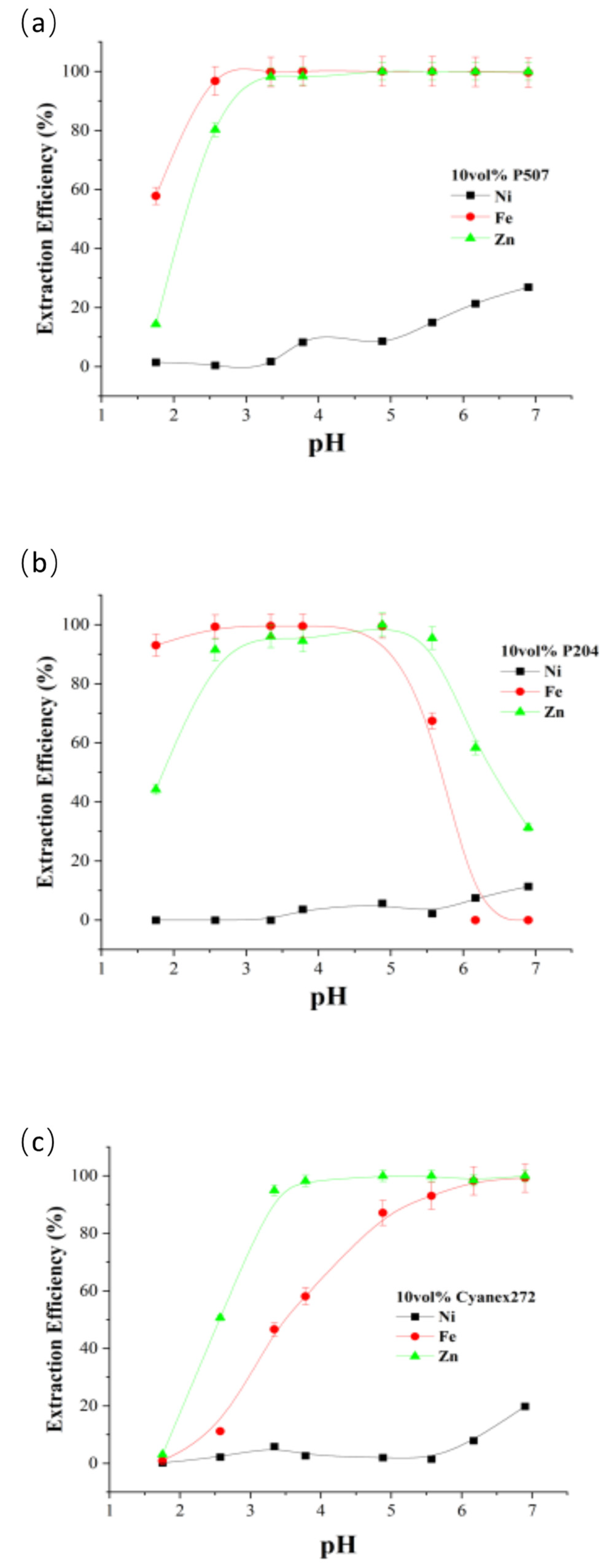
Figure 14 and
Figure 15 show iron, zinc, and nickel extraction efficiencies from simulated and actual spent electroless nickel plating baths at different aqueous phase pH conditions for 10 vol% P507, P204, and Cyanex272.
As can be seen from
Figure 12, the extraction efficiencies of all three organophosphorus extractants were significantly higher for iron and zinc than for nickel. When pH<4, the extraction efficiency of nickel was almost 0. The extraction efficiency of nickel increased only when the pH of the waste solution was higher than 6. When the pH of the aqueous phase was higher than 5, the extraction efficiency of P204 for iron and zinc decreased. For the three organophosphoric acid extractants, P507, P204, and Cyanex272, the highest separation of nickel, iron, and zinc was achieved in the simulated spent electroless nickel plating baths at pH 3.3, 3.8, and 5.6, respectively. Preliminary studies have shown that a sample containing a deionized nickel waste bath in addition to nickel contains elements other than iron and zinc, such as copper, lead and chromium, the concentration of which in the sample is negligible. The high concentration of sodium and phosphorus-containing anions listed in
Table 1 affects only the ionic strength and buffer capacity of the solution relative to PH, rather than directly affecting the efficiency of nickel extraction.
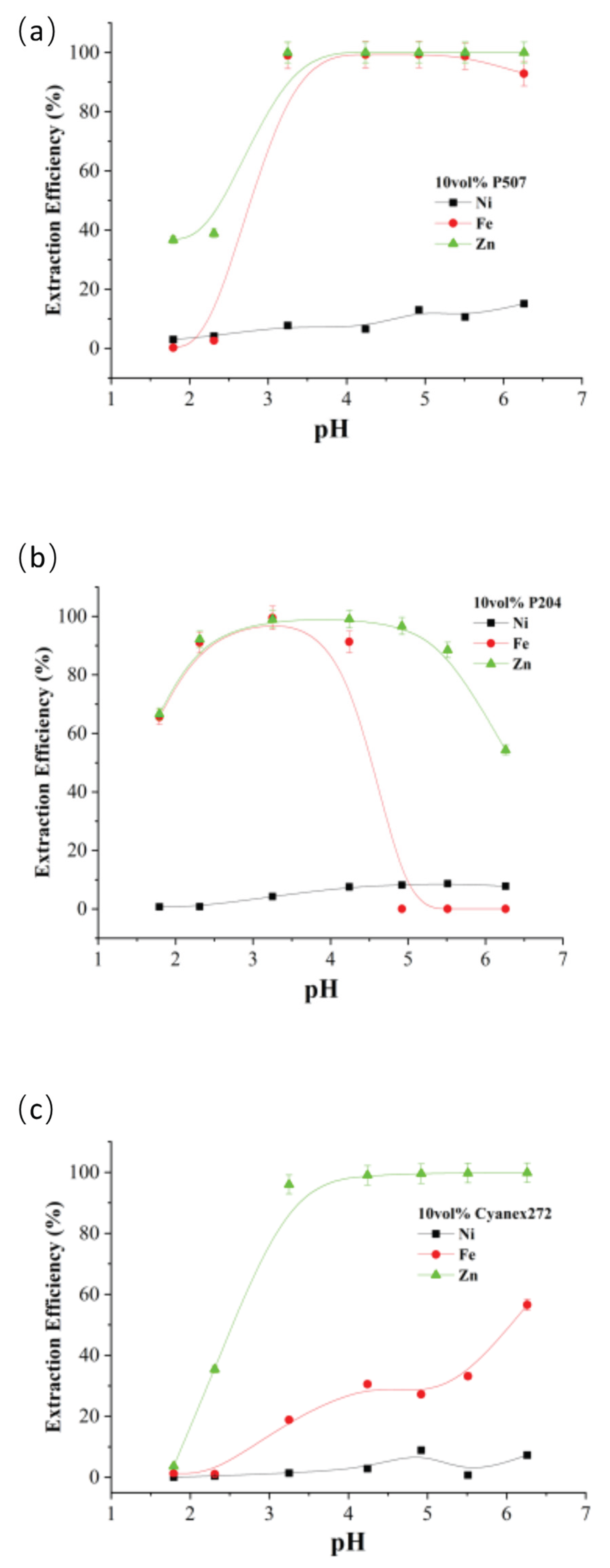
As can be seen from
Figure 13, the three organophosphorus acid extractants P507, P204, and Cyanex272 achieved the highest separation of nickel, iron, and zinc at pH 3.3, 4.9, and 4.9, respectively, in the actual spent electroless nickel plating baths.
Generally speaking, the pH of spent electroless nickel plating baths is usually between 4 and 6, under which the extraction rate of organic phosphoric acid extractant for iron and zinc is more than 99%, which can achieve satisfactory results [
13]. Nickel is not extracted under this condition, so the organic phosphoric acid extractant should be synergized with LIX984 to have a good extraction effect. This property is important in studying nickel recovery from spent electroless nickel plating baths.
Based on our experimental data from
Figure 14 and
Figure 15,
Table 7 presents the distribution coefficients(D)and separation factors(β)for the three organophosphorus extractants at their optimal pH conditions. Calculated according to Equations (4) and (5).
Very low values of βNi / Fe and βNi / Zn (<0.001) indicate that the organophosphate extract has significant selectivity over Fe2+ and Zn2+ relative to Ni2+ PRI under optimal pH conditions. The results of the verification of the two-stage separation scheme have also become verifiable. In the second extraction step (pH 6-7), when using the organophosphate accelerator LIX984 plus, after iron and zinc removal, the βNi/Fe ratio increases to >100, and the selectivity of nickel extraction also becomes very high.