1. Introduction
Gastric cancer continues to be one of the leading global malignancies, with 1 million new cases and 769,000 deaths during 2020, which placed it as the fifth most common cancer and fourth leading cause of cancer-related deaths worldwide [
1]. The disease maintains its position as a significant health burden in East Asia and Eastern Europe regions despite ongoing screening progress and multimodal treatment approaches, and perioperative care improvements [
2,
3]. Surgical resection stands as the primary treatment for curative purposes, and distal gastrectomy serves as the standard surgical method for removing tumors found in the distal stomach [
2,
3,
4].
The surgical treatment of gastric cancer has received equal emphasis on oncological radicality and functional preservation according to international guidelines from the past decades [
2,
3,
5,
6,
7]. The reconstruction method after distal gastrectomy has become a key determinant of both short- and long-term outcomes. The main goal of reconstruction surgery is to achieve food passage through the digestive system while reducing postoperative complications and minimizing both nutritional deficiencies and reflux problems [
4,
5,
8]. The best method for reconstruction remains unclear because surgeons and patients achieve different results in their practices throughout the world.
The most ‘’physiological,, reconstruction method, Billroth I (gastroduodenostomy) maintains the natural food pathway while producing lower bile reflux rates and better nutritional outcomes and superior long-term life quality [
9,
10]. The treatment method has certain restrictions because tumors located in specific areas and duodenal involvement, as well as technical difficulties after extensive lymph node removal.
By contrast, Billroth II and Roux-en-Y reconstructions are widely applied alternatives, particularly when Billroth I is not feasible [
10,
11]. The Billroth II procedure is easy to perform, yet it leads to higher chances of duodenogastric reflux and remnant gastritis, while Roux-en-Y is recommended for minimizing bile reflux and enhancing postoperative function. The techniques result in longer surgical procedures and Roux-en-Y stasis syndrome and other particular complications [
4,
5].
Research studies have investigated which reconstruction methods provide the most beneficial advantages. The results of extensive multicenter research in Korea showed that Billroth I and Billroth II surgeries produce different treatment results for patients [
10]. The results of nationwide surveys indicate that surgical methods continue to vary across different regions of the country [
11]. The selection and success rates of reconstruction methods seem to be influenced by patient demographics and healthcare systems, according to survival data from Asian and Western European patient groups [
9].
Multiple studies using randomized controlled trials and meta-analyses have investigated this matter, with their findings disagreeing about postoperative complications and nutritional outcomes, and life quality [
4,
5,
6,
7,
8,
10,
11]. Most of the available data originate from East Asian populations, with relatively few prospective studies from European centers. Research has thoroughly examined perioperative morbidity, while the literature contains limited information about combined short-term and mid-term complications and functional and nutritional results.
Given this ongoing controversy, the selection of the optimal reconstruction after distal gastrectomy remains an unresolved question. Billroth I is often preferred when technically feasible, yet the true advantages of Roux-en-Y over Billroth II or Billroth I are still debated, especially in non-Asian cohorts.
Therefore, the prospective observational cohort study aimed to compare the short- and mid-term surgical outcomes, postoperative complications, and functional parameters between Billroth I and Billroth II/Roux-en-Y reconstruction following distal gastrectomy in gastric cancer patients.
2. Materials and Methods
2.1. Study Design and Setting
The study took place at the General Surgical Clinic I within Emergency County Hospital in Târgu Mureș, Romania, from October 2021 through December 2024. The research followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for cohort study reporting in its design and process.
2.2. Patient Population
Patients were eligible if they were diagnosed with histologically confirmed gastric adenocarcinoma located in the distal stomach, distal gastric body, or multicentric tumors confined to the lower body and distal region, and underwent curative-intent distal gastrectomy. The study included patients who met three criteria: being at least 18 years old, with tumors located in the distal or lower gastric region, and being able to undergo elective surgery for curative purposes. The study excluded patients who needed total gastrectomy for proximal gastric or esophagogastric junction tumors, those with distant metastases, patients who underwent palliative or emergency procedures, and patients with missing follow-up information.
The reconstruction decision occurred during surgery based on three main factors, which included oncological needs and both anatomical possibilities and personal surgeon choices. Billroth I (gastroduodenostomy) was performed when adequate resection margins could be achieved and sufficient duodenal mobility allowed the creation of a safe, tension-free anastomosis. The surgical team chose Bill-Roth II or Roux-en-Y (gastrojejunostomy) procedures when they needed to remove more stomach tissue or when the duodenum was involved, or when the surgeon wanted to perform gastrojejunostomy reconstruction to ease postoperative recovery and minimize anastomotic stress. The two reconstruction methods follow international gastric cancer surgery guidelines [
2,
3] as standard recommendations for clinical practice. The researchers used inverse probability of treatment weighting (IPTW) to reduce allocation bias in their non-randomized study by making groups statistically equal through their baseline characteristics.
2.3. Surgical Technique
All patients underwent subtotal distal gastrectomy with curative intent, performed according to oncological principles of gastric cancer surgery. The extent of lymphadenectomy was classified as D1+ or D2, following the Japanese Gastric Cancer Association (JGCA) guidelines [
2]. The choice of surgical approach was determined by the operating surgeon and institutional practice standards. Reconstruction methods were applied as follows:
Billroth I surgical procedure involved gastroduodenostomy through end-to-end or end-to-side anastomosis between the stomach remnant and duodenum when a safe tension-free anastomosis can be achieved.
Billroth II (gastrojejunostomy) procedure with Braun enteroenterostomy as an option based on surgeon preference for patients who needed a longer resection margin or had limited duodenal mobility.
The Roux-en-Y procedure (gastrojejunostomy with Roux limb) serves as an antecolic Roux-en-Y gastro-jejunostomy with standardized limb length for patients who need an alternative method to minimize bile reflux and achieve secure tension-free reconstruction.
The surgical team of experienced gastrointestinal surgeons performed all reconstructions according to intraoperative results and individual patient needs for oncological and anatomical conditions.
2.4. Data Collection and Variables
The patient information included age, sex, BMI, ASA class, comorbidities, weight change during 3–6 months, and CRP levels. The variables related to tumors included the location, its size, pathological T and N stage, margin status, and treatment with neoadjuvant therapy. The study included four operative variables: operative time, intraoperative blood loss, extent of lymphadenectomy, and type of anastomosis. The study evaluated postoperative results through three recovery metrics and four categories of adverse events and their severity levels according to Clavien–Dindo classification and three early outcome measures. The assessment of mid-term functional results took place at 3 months and 6 months by evaluating PPI use and Los Angeles classification of reflux esophagitis with bile reflux gastritis severity and Sigstad score for dumping syndrome evaluation.
2.5. Outcome Measures
The main study outcome measured the occurrence of severe postoperative complications, which were defined as Clavien–Dindo grade III or higher complications during the first 90 days after surgery. The research team assessed secondary outcomes through evaluations of total illness rates and particular postoperative issues, healing indicators, and functional outcomes, which included PPI medication use, esophagitis, bile reflux gastritis, dumping syndrome, and death rates at 30 days and 90 days post-surgery.
2.6. Statistical Analysis
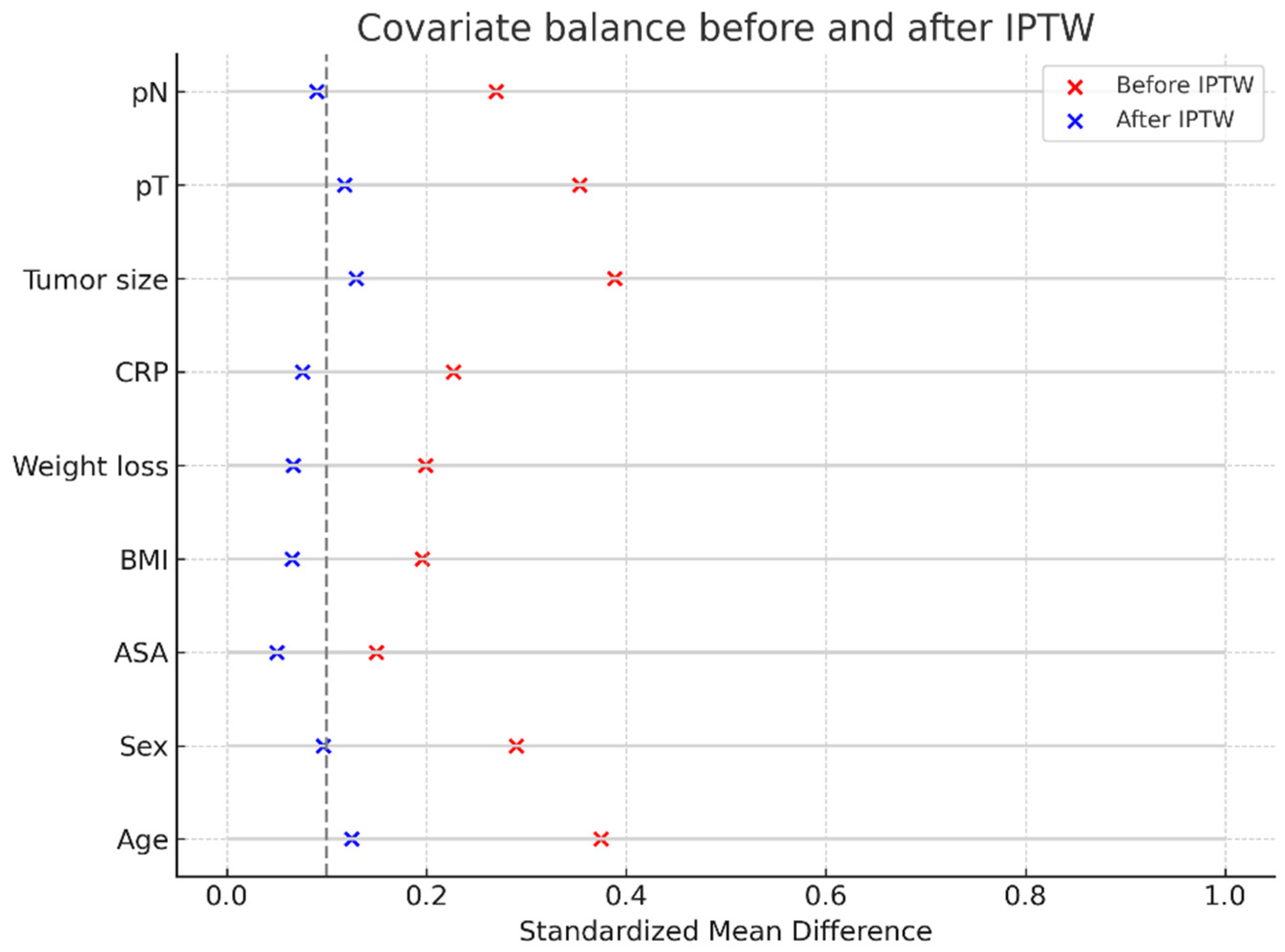
The research used means and standard deviations (SD) to present continuous data for normally distributed variables, while medians with interquartile ranges (IQR) for variables that did not follow a normal distribution. The researchers conducted independent-sample t-tests or Mann–Whitney U tests for group comparison analysis. Categorical variables were expressed as numbers and percentages and compared using χ2 or Fisher’s exact tests. The study employed inverse probability of treatment weighting (IPTW) to address confounding through propensity score estimation based on baseline covariates. The researchers employed Standardized mean differences (SMDs) to evaluate covariate balance at two time points: before and after adjustment. The researchers applied logistic regression for their binary outcome analysis and linear or mixed-effects models for their continuous and time-dependent data. The studies reported their effect sizes through odds ratios (ORs) and mean differences, and regression coefficients with 95% confidence intervals (CIs). All analyses were performed using EasyMedStat (SAS, France) software. Statistical significance was set at p < 0.05 (two-tailed).
3. Results
3.1. Patient Characteristics
A total of 150 patients were included, with 72 undergoing Billroth I and 78 undergoing Billroth II/Roux-en-Y reconstruction. The mean age of the cohort was 61.5 ± 10.8 years, with a male predominance of 60.7%. No significant differences were observed between groups with respect to ASA class distribution, BMI, weight loss, CRP, tumor size, pT or pN stage, or neoadjuvant therapy. The R1 resection rate was 9.3% overall, with a slightly higher frequency in Billroth II/Roux-en-Y. Covariate balance was confirmed following IPTW adjustment.
(Table 1, Figure 1)
3.2. Short-Term Surgical Outcomes
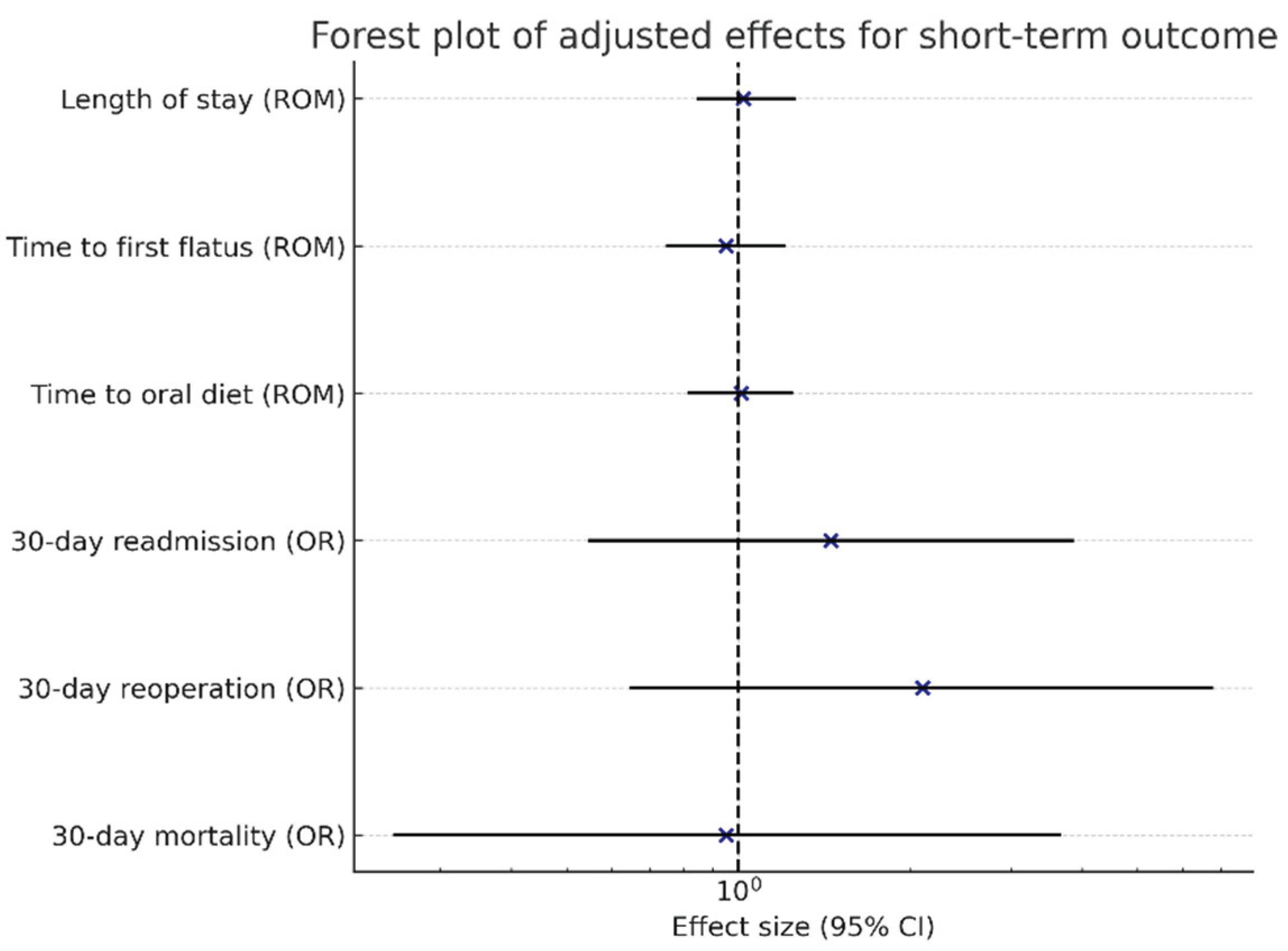
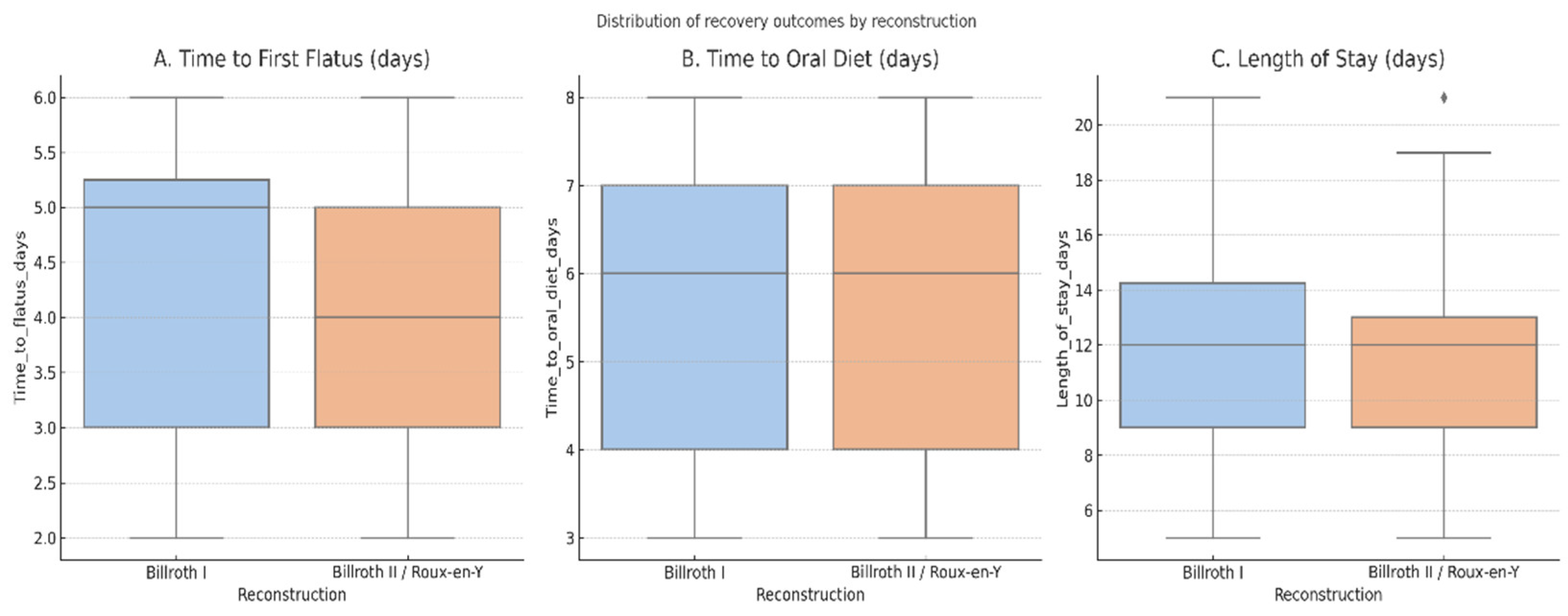
The median time to first flatus was 4 days, time to oral diet 6 days, and hospital stay 12 days. There were no significant differences between reconstruction groups in any of these recovery parameters. Thirty-day readmission occurred in 11.3% of patients, reoperation in 5.3%, and 30-day mortality in 4.0%. Ninety-day mortality was 5.3% overall. Adjusted analyses did not demonstrate significant treatment-related effects across recovery endpoints or adverse events.
(Table 2, Figure 2 and Figure 3)
3.3. Mid-Term Functional Outcomes
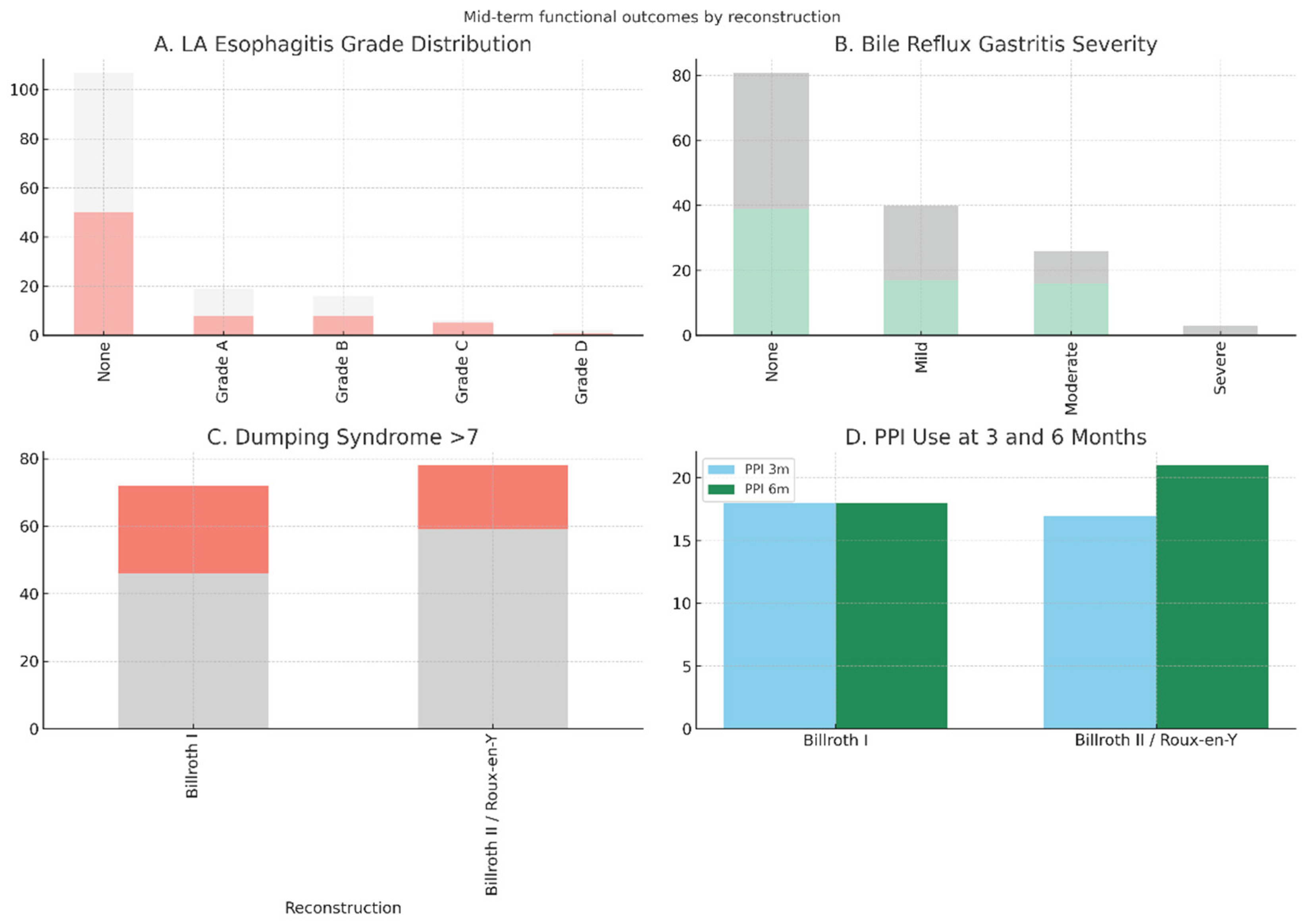
At 3 and 6 months, approximately one-quarter of patients required PPI therapy. LA esophagitis was identified in 28.7% overall, predominantly Grades A and B, with no significant intergroup differences. Bile reflux gastritis was more frequent after Billroth II/Roux-en-Y, including severe forms in 3.8% of cases. Dumping syndrome, defined as a Sigstad score >7, occurred in 30.0% of patients, with higher prevalence in Billroth I (36.1% vs. 24.4%).
(Table 3, Figure 4)
3.3. Postoperative Complications
Overall morbidity was 32.7%. The most common events were wound infection (7.3%), pulmonary complications (8.7%), and intra-abdominal abscess (4.7%). Duodenal stump leak was observed exclusively in the Billroth II/Roux-en-Y group. Reoperation was required in 5.3% of patients. Complication severity according to Clavien–Dindo classification was comparable between groups, with 19.3% experiencing Grade II, 12.7% Grade III, and 8.7% Grade V events.
(Table 4 and Table 5)
4. Discussion
This prospective observational cohort study compared short- and mid-term surgical outcomes between Billroth I and Billroth II/Roux-en-Y reconstruction following distal gastrectomy for gastric cancer. The study produced four essential results: (i) The three reconstruction methods showed no significant differences in postoperative recovery times for flatus production, oral diet return, and hospital stay duration. (ii) The rates of major postoperative complications and 30-/90-day mortality showed no significant differences between the reconstruction groups after risk factor adjustment. (iii) The two reconstruction methods produced different functional results during the mid-term period because Billroth II/Roux-en-Y patients experienced more bile reflux gastritis, while Billroth I patients developed dumping syndrome more frequently. (iv) The study found that reflux esophagitis rates (Los Angeles classification) were equivalent between all treatment groups.
The research findings confirm previous studies showing Billroth I reconstruction leads to better digestive passage, increasing the chance of dumping syndrome, while Billroth II and Roux-en-Y procedures minimize duodenogastric reflux that creates problems with bile stasis and Roux-related complications [
13,
14,
15,
16,
18,
20,
23,
25,
30,
32]. In a large multicenter Korean analysis, Kang et al. demonstrated differences in perioperative morbidity between Billroth I and Billroth II [
10], findings echoed in our cohort, although our IPTW-adjusted analysis suggests no significant early advantage for either method. The South Korean nationwide survey data revealed reconstruction methods followed distinct patterns because surgeons selected different techniques and patients had different characteristics [
11].
Several randomized controlled trials have directly compared Billroth I and Roux-en-Y. Kimura et al. found no survival difference at 5 years, but nutritional recovery was superior after Billroth I [
18]. Nakamura et al. and Takiguchi et al. demonstrated that long-term quality of life and functional outcomes were better after Billroth I, though Roux-en-Y reduced bile reflux [
24,
30]. The study results support these patterns because nutritional recovery (indirectly assessed through early recovery) showed no substantial differences, yet Billroth II/Roux-en-Y patients developed bile reflux gastritis more often. This is consistent with the findings of Tokunaga et al. [
32] and Maehara et al. [
33], who stressed that bile reflux develops as a chronic condition after gastrojejunostomy reconstruction surgeries.
Multiple studies have tried to answer this question through their meta-analyses. Zong et al. [
13] and Xiong et al. [
14] reported that Roux-en-Y reconstruction reduced reflux symptoms but at the cost of increased operative complexity. The Cochrane review [
19] showed no survival benefit and no major complication variations between the two procedures, while it did detect functional outcome variations through bile reflux and dumping symptoms. The study contributes European data to the Asian-dominant research field, which supports the practice of tailoring reconstruction methods to each patient rather than following a single standard approach.
Interestingly, we observed a higher proportion of dumping syndrome in the Billroth I group. This observation is in agreement with Yang et al. [
23] and Hirao et al. [
25], who reported more frequent postprandial symptoms in Billroth I reconstructions. The direct flow of chyme from the stomach to the duodenum following Billroth I surgery leads to faster intestinal movement, which increases the risk of dumping. Conversely, bile reflux was more common in Billroth II/Roux-en-Y, which reflects altered bile flow dynamics and has been extensively reported in both Asian RCTs [
18,
24,
26] and Western cohorts [
27].
The study benefits from its prospective design, predefined endpoints and statistical adjustment through IPTW, which reduces bias from non-random reconstruction method distribution. The study provides a complete evaluation through its assessment of short-term surgical complications together with mid-term functional and endoscopic results, which exceeds the scope of numerous previous retrospective studies.
Nonetheless, some limitations must be acknowledged. The study conducted at a single location restricts the ability to apply its findings to other settings. The research study monitored participants for six months to assess functional results, yet future studies need to follow participants for longer periods to determine nutritional effects, oncologic outcomes, and survival rates. The IPTW adjustment method decreased baseline imbalances, yet non-randomized studies face ongoing difficulties in removing all residual confounding effects. The study had a limited number of patients who received endoscopic evaluation between 3–6 months, which could have resulted in underdiagnosis of reflux-related pathologies.
Our research confirms that different reconstruction methods have their own strengths and weaknesses, which prevent any single method from being the best for all applications. Billroth I remain preferable when technically feasible, given its physiological passage and lower bile reflux risk, but surgeons must weigh this against the higher risk of dumping syndrome. The Billroth II/Roux-en-Y procedures should be used for patients who need extensive resection areas or have restricted duodenal movement. Future studies need to conduct multicenter European randomized controlled trials (RCTs) that follow patients for extended periods to evaluate nutritional results, oncologic outcomes, functional recovery, and patient-reported results for determining the best reconstruction method in gastric cancer surgery.
5. Conclusions
In this prospective cohort study, short-term surgical outcomes and major postoperative morbidity did not differ significantly between Billroth I and Billroth II/Roux-en-Y reconstructions after distal gastrectomy for gastric cancer. The two surgical approaches produced different functional results during the mid-term period because Billroth I patients developed dumping syndrome more often than Billroth II/Roux-en-Y patients, who experienced bile reflux gastritis. The research demonstrates that both methods continue to be effective while following established guidelines because surgeons select reconstruction techniques based on surgical conditions and individual patient needs.
Author Contributions
Conceptualization: C.D.C. and V.O.B.; Methodology: C.D.C., V.O.B., and M.B.; Software and Formal Analysis: C.D.C. and C.N.; Investigation and Data Curation: C.D.C., V.O.B., M.B., C.N., and R.P.C.; Resources: V.O.B. and C.M.; Writing—Original Draft Preparation: C.D.C. and V.O.B.; Writing—Review and Editing: M.B., C.N., and R.P.C.; Visualization: C.D.C. and V.O.B.; Supervision and Project Administration: C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the G.E. Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureş,. This study was not supported by any medical device company or other institution.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Emergency County Hospital Tg.Mures (Decision No. 30104/07.10.2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study based on the decision of the Institutional Ethics Committee of Emergency County Hospital, Tg.Mures (Decision No. 30104/07.10.2021).
Data Availability Statement
The data that support the findings of this study are available from the main author, C.C., upon request,
catalin.cosma@umfst.ro.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT 5 for English editing and refining the clarity of methodological descriptions. The authors have reviewed and edited all AI-assisted content and take full responsibility for the scientific integrity and accuracy of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASA— |
American Society of Anesthesiologists |
| BI— |
Billroth I |
| BII/RY— |
Billroth II/Roux-en-Y |
| BMI— |
Body Mass Index |
| BRG— |
Bile Reflux Gastritis |
| CI— |
Confidence Interval |
| CONUT— |
Controlling Nutritional Status |
| CRP— |
C-Reactive Protein |
| IPTW— |
Inverse Probability of Treatment Weighting |
| IQR— |
Interquartile Range |
| JGCA— |
Japanese Gastric Cancer Association |
| LOS— |
Length of Stay |
| OR— |
Odds Ratio |
| PPI— |
Proton Pump Inhibitor |
| QoL— |
Quality of Life |
| RCT— |
Randomized Controlled Trial |
| SD— |
Standard Deviation |
| SMD— |
Standardized Mean Difference |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [CrossRef]
- National Comprehensive Cancer Network. Gastric Cancer (Version 2.2025). NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 9 September 2025).
- Digklia, A.; Wagner, A.D. Advanced Gastric Cancer: Current Treatment Landscape and Future Perspectives. World J. Gastroenterol. 2016, 22, 2403–2414. [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.T.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [CrossRef]
- Ajani, J.A.; In, H.; Sano, T.; Gaspar, L.E.; Hart, L.; Almhanna, K.; Bang, Y.J.; Benson, A.; Das, P.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 1286–1312. [CrossRef]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric Cancer. Lancet 2016, 388, 2654–2664. [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 700–713. [CrossRef]
- Strong, V.E.; Wu, A.-W.; Selby, L.V.; Gonen, M.; Hsu, M.; Song, K.Y.; Park, C.H.; Coit, D.G.; Brennan, M.F. Differences in Gastric Cancer Survival between the U.S. and China. J. Surg. Oncol. 2015, 112, 31–37. [CrossRef]
- Kang, K.-C.; Cho, G.S.; Han, S.U.; Kim, W.; Kim, H.-H.; Kim, M.-C.; Hyung, W.J.; Ryu, S.Y.; Ryu, S.W.; Lee, H.J.; Song, K.Y.; Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Comparison of Billroth I and Billroth II Reconstructions after Laparoscopy-Assisted Distal Gastrectomy: A Retrospective Analysis of Large-Scale Multicenter Results from Korea. Surg. Endosc. 2011, 25, 1953–1961. [CrossRef]
- Oh, J.; Park, Y.K. Clinicopathological Features and Surgical Treatment of Gastric Cancer in South Korea: Results of 2009 Nationwide Survey on Surgically Treated Gastric Cancer Patients. J. Gastric Cancer 2011, 11, 69–77. [CrossRef]
- Kim, H.H.; Hyung, W.J.; Cho, G.S.; Kim, M.C.; Han, S.U.; Kim, W.; Ryu, S.W.; Lee, H.J. Morbidity and Mortality of Laparoscopic Gastrectomy versus Open Gastrectomy for Gastric Cancer: Interim Report of a Phase III Trial (KLASS). Ann. Surg. 2010, 251, 417–420. [CrossRef]
- Zong, L.; Chen, P. Billroth I vs. Billroth II vs. Roux-en-Y Following Distal Gastrectomy: A Meta-Analysis Based on 15 Studies. Hepatogastroenterology 2011, 58, 1413–1424. [CrossRef]
- Xiong, J.J.; Altaf, K.; Javed, M.A.; Nunes, Q.M.; Huang, W.; Mai, G.; Tan, C.; Liu, X.-B. Roux-en-Y versus Billroth I Reconstruction after Distal Gastrectomy for Gastric Cancer: A Meta-Analysis. World J. Gastroenterol. 2013, 19, 1124–1134. [CrossRef]
- He, L.; Zhao, Y. Is Roux-en-Y or Billroth-II Reconstruction the Preferred Choice for Gastric Cancer Patients Undergoing Distal Gastrectomy when Billroth I is not Applicable? A Meta-Analysis. Medicine (Baltimore) 2019, 98, e17093. [CrossRef]
- Inokuchi, M.; Kojima, K.; Yamada, H.; Kato, K.; Hayashi, M.; Motoyama, K.; Sugihara, K. Long-Term Outcomes of Roux-en-Y and Billroth I Reconstruction after Laparoscopic Distal Gastrectomy. Gastric Cancer 2013, 16, 67–73. [CrossRef]
- Kinoshita, T.; Honda, M.; Matsuki, A.; Enomoto, N.; Aizawa, M.; Nunobe, S.; Yabusaki, H.; Abe, T.; Hiki, N. Billroth-I vs Roux-en-Y after Distal Gastrectomy: Comparison of Long-Term Nutritional Status and Survival in a Multicenter Study. Ann. Gastroenterol. Surg. 2020, 4, 142–150. [CrossRef]
- Kimura, Y.; Mikami, J.; Yamasaki, M.; Hirao, M.; Imamura, H.; Fujita, J.; et al. Comparison of 5-Year Postoperative Outcomes after Billroth I and Roux-en-Y Reconstruction following Distal Gastrectomy: Results from an RCT. Ann. Gastroenterol. Surg. 2021, 5, 93–101. [CrossRef]
- Nishizaki, D.; Ganeko, R.; Hoshino, N.; Hida, K.; Obama, K.; Furukawa, T.A. Roux-en-Y versus Billroth-I Reconstruction after Distal Gastrectomy for Gastric Cancer. Cochrane Database Syst. Rev. 2021, CD012998. [CrossRef]
- Chen, Y.-X.; Huang, Q.-Z.; Wang, P.-C.; Zhu, Y.-J.; Chen, L.-Q.; Wu, C.-Y.; Wang, J.-T.; Chen, J.-X.; Ye, K. Short- and Long-Term Outcomes of Roux-en-Y and Billroth II with Braun Reconstruction in Total Laparoscopic Distal Gastrectomy. World J. Surg. Oncol. 2023, 21, 361. [CrossRef]
- Min, J.S.; Kim, E.H.; Park, S.; Kim, H.I.; Hyung, W.J. Optimal Reconstruction after Distal Gastrectomy: A Systematic Review and Meta-Analysis. J. Gastric Cancer 2022, 22, e9. [CrossRef]
- Yan, Y.; Zhao, J.; Chen, X.; Zhao, G.; Zhao, Z. Optimal Reconstruction after Laparoscopic Distal Gastrectomy: A Systematic Review and Network Meta-Analysis. Surg. Innov. 2022, 29, 589–603. [CrossRef]
- Yang, K.; Zhang, W.-H.; Liu, K.; Chen, X.-Z.; Zhou, Z.-G.; Hu, J.-K. Comparison of Quality of Life between Billroth-I and Roux-en-Y Anastomosis after Distal Gastrectomy: A Randomized Controlled Trial. Sci. Rep. 2017, 7, 11245. [CrossRef]
- Nakamura, M.; Nakamori, M.; Ojima, T.; Iwahashi, M.; Horiuchi, T.; Kobayashi, Y.; et al. RCT Comparing Long-Term QoL for Billroth I versus Roux-en-Y after Distal Gastrectomy. Br. J. Surg. 2016, 103, 337–347. [CrossRef]
- Hirao, M.; Takiguchi, S.; Imamura, H.; Yamamoto, K.; Kurokawa, Y.; Fujita, J.; et al. Comparison of Billroth I and Roux-en-Y Reconstruction after Distal Gastrectomy for Gastric Cancer: One-Year Postoperative Effects (RCT). Ann. Surg. Oncol. 2013, 20, 1591–1597. [CrossRef]
- Imamura, H.; Takiguchi, S.; Yamamoto, K.; Hirao, M.; Fujita, J.; Yano, M.; et al. Morbidity and Mortality Results from an RCT Comparing Billroth I and Roux-en-Y after Distal Gastrectomy. World J. Surg. 2012, 36, 632–637. [CrossRef]
- Tran, T.B.; Worhunsky, D.J.; Squires, M.H.; Jin, L.X.; Spolverato, G.; Votanopoulos, K.I.; et al. To Roux or Not to Roux: A Comparison between Roux-en-Y and Billroth II Reconstruction following Partial Gastrectomy. Gastric Cancer 2016, 19, 994–1001. [CrossRef]
- Imai, Y.; Hata, T.; Miyake, M.; Kanno, T.; Toyama, H.; Motoi, F.; et al. Comparison of the Gastric Microbiome in Billroth I and Roux-en-Y Reconstructions after Distal Gastrectomy. Sci. Rep. 2022, 12, 10754. [CrossRef]
- Wei, T.; Wu, Z.; Chen, Y.; Li, Y.; Pang, F.; Shan, F.; Li, Z.; Ji, J. Comparison of Uncut Roux-en-Y and Billroth II with Braun Anastomosis after Distal Gastrectomy. Front. Surg. 2024, 11, 1390876. [CrossRef]
- Takiguchi, S.; Yamamoto, K.; Hirao, M.; Imamura, H.; Fujita, J.; Yano, M.; Kobayashi, K.; Kimura, Y.; Kurokawa, Y.; Mori, M.; Doki, Y. A Comparison of Postoperative Quality of Life and Dysfunction after Billroth I and Roux-en-Y Reconstruction following Distal Gastrectomy for Gastric Cancer: Results from a Multi-Institutional RCT. Gastric Cancer 2012, 15, 198–205. [CrossRef]
- Fujiya, K.; Kawamura, T.; Omae, K.; Makuuchi, R.; Irino, T.; Tokunaga, M.; Tanizawa, Y.; Bando, E.; Terashima, M. Impact of Malnutrition after Gastrectomy for Gastric Cancer on Long-Term Survival. Ann. Surg. Oncol. 2018, 25, 974–983. [CrossRef]
- Tokunaga, M.; Makuuchi, R.; Miki, Y.; Tanizawa, Y.; Bando, E.; Kawamura, T.; et al. Late Complications and Nutritional Impact of Billroth I vs Roux-en-Y. World J. Surg. 2015, 39, 1803–1810. [CrossRef]
- Maehara, Y.; Kakeji, Y.; Oda, S.; et al. Long-Term Functional Outcomes after Distal Gastrectomy with Billroth I or Roux-en-Y. Surg. Today 2012, 42, 255–260. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).