Submitted:
02 October 2025
Posted:
03 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Zona Pellucida Composition and Its Evolutionary Origin
3. Zona Pellucida Maturation
3.1. Zona Pellucida Formation During Ovarian Development
3.2. Synthesis and Polymerization of ZP Proteins
3.3. Fertilization and ZP Modifications
3.4. Journey Through the Oviduct
3.5. Role in Oocyte Fertilization, Embryo Protection, and Embryo Implantation
3.6. Specificity of Sperm Binding
3.7. Assisted Hatching: Rationale, Technique, and Clinical Outcomes
4. Clinical Implications and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Acrosome-intact |
| AR | Acrosome-reacted |
| ARTs | Assisted Reproductive Techniques |
| EVs | Extracellular vesicles |
| GAGs | Heparin-like glycosaminoglycans |
| GEFs | Genuine empty follicle syndrome |
| HSPs | Heat shock proteins |
| IVM | in vitro matured |
| OF | Oviductal fluid |
| OVGP1 | Oviductal glycoprotein 1 |
| NTR | N-terminal region |
| TGM2 | Transglutaminase 2 |
| ZP | Zona pellucida |
References
- Wassarman, P.M. Zona Pellucida Glycoproteins. J Biol Chem. 2008, 283, 24285–24289. [Google Scholar] [CrossRef]
- Bokhove, M.; Jovine, L. Structure of Zona Pellucida Module Proteins. In Current Topics in Developmental Biology [Internet]; Elsevier, 2018; pp. 413–442, [cited 2025 Sept 9]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0070215318300395.
- Clark, G.F. The molecular basis of mouse sperm–zona pellucida binding: a still unresolved issue in developmental biology. REPRODUCTION 2011, 142, 377–381. [Google Scholar] [CrossRef]
- De La Fuente, D.; Maroto, M.; Cajas, Y.N.; Canon-Beltran, K.; Fernandez-Gonzalez, R.; Munoz-Maceda, A.; et al. Oviductin sets the species-specificity of the mammalian zona pellucida. eLife 2025, 13, RP101338. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Emori, C.; Wiseman, B.; Fahrenkamp, D.; Dioguardi, E.; Zamora-Caballero, S.; et al. ZP2 cleavage blocks polyspermy by modulating the architecture of the egg coat. Cell 2024, 187, 1440–1459.e24. [Google Scholar] [CrossRef]
- Goudet, G.; Mugnier, S.; Callebaut, I.; Monget, P. Phylogenetic Analysis and Identification of Pseudogenes Reveal a Progressive Loss of Zona Pellucida Genes During Evolution of Vertebrates1. Biol Reprod. 2008, 78, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Rico, M.J.; Jiménez-Movilla, M.; Llop, E.; Pérez-Oliva, A.B.; Ballesta, J.; Gutiérrez-Gallego, R.; et al. Hamster Zona Pellucida Is Formed by Four Glycoproteins: ZP1, ZP2, ZP3, and ZP4. J Proteome Res. 2009, 8, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Stetson, I.; Avilés, M.; Moros, C.; García-Vázquez, F.A.; Gimeno, L.; Torrecillas, A.; et al. Four glycoproteins are expressed in the cat zona pellucida. Theriogenology 2015, 83, 1162–1173. [Google Scholar] [CrossRef]
- Tpfer-Petersen, E.; Ekhlasi-Hundrieser, M.; Tsolova, M. Glycobiology of fertilization in the pig. Int J Dev Biol. 2008, 52, 717–736. [Google Scholar] [CrossRef]
- Wiesak, T.; Wasielak, M.; Złotkowska, A.; Milewski, R. Effect of vitrification on the zona pellucida hardening and follistatin and cathepsin B genes expression and developmental competence of in vitro matured bovine oocytes. Cryobiology 2017, 76, 18–23. [Google Scholar] [CrossRef]
- Gupta, S.K. Human Zona Pellucida Glycoproteins: Binding Characteristics With Human Spermatozoa and Induction of Acrosome Reaction. Front Cell Dev Biol. 2021, 9, 619868. [Google Scholar] [CrossRef]
- Vanroose, G.; Nauwynck, H.; Soom, A.V.; Ysebaert, M.T.; Charlier, G.; Oostveldt, P.V.; et al. Structural Aspects of the Zona Pellucida of In Vitro-Produced Bovine Embryos: A Scanning Electron and Confocal Laser Scanning Microscopic Study1. Biol Reprod. 2000, 62, 463–469. [Google Scholar] [CrossRef]
- Avilés, M.; Gutiérrez-Adán, A.; Coy, P. Oviductal secretions: will they be key factors for the future ARTs? MHR Basic Sci Reprod Med. 2010, 16, 896–906. [Google Scholar] [CrossRef]
- Litscher, E.S.; Wassarman, P.M. Zona Pellucida Proteins, Fibrils, and Matrix. Annu Rev Biochem. 2020, 89, 695–715. [Google Scholar] [CrossRef]
- Moros-Nicolás, C.; Chevret, P.; Jiménez-Movilla, M.; Algarra, B.; Cots-Rodríguez, P.; González-Brusi, L.; et al. New Insights into the Mammalian Egg Zona Pellucida. Int J Mol Sci. 2021, 22, 3276. [Google Scholar] [CrossRef] [PubMed]
- Michelmann, H.; Rath, D.; Töpfer-Petersen, E.; Schwartz, P. Structural and Functional Events on the Porcine Zona Pellucida During Maturation, Fertilization and Embryonic Development: a Scanning Electron Microscopy Analysis. Reprod Domest Anim. 2007, 42, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Okumura, H.; Mizuno, A.; Iwamoto, E.; Sakuma, R.; Nishio, S.; Nishijima Kichi et, a.l. New insights into the role of microheterogeneity of ZP3 during structural maturation of the avian equivalent of mammalian zona pellucida. Mishra B, editor. PLOS ONE. 2023, 18, e0283087. [Google Scholar] [CrossRef]
- Gardner, A.J.; Evans, J.P. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev. 2006, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, H.H.; Refai, F.H.; Avella, M.A. The molecular mechanisms mediating mammalian fertilization. Development. 2019, 146, dev176966. [Google Scholar] [CrossRef]
- Nishio, S.; Emori, C.; Wiseman, B.; Fahrenkamp, D.; Dioguardi, E.; Zamora-Caballero, S.; et al. ZP2 cleavage blocks polyspermy by modulating the architecture of the egg coat. Cell. 2024, 187, 1440–1459.e24. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Litscher, E.S. Female fertility and the mammalian egg’s zona pellucida. Histol Histopathol. 2024, 39, 1273–1284. [Google Scholar]
- Li, R.; Albertini, D.F. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef]
- Dai, C.; Hu, L.; Gong, F.; Tan, Y.; Cai, S.; Zhang, S.; et al. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med. 2019, 21, 431–440. [Google Scholar] [CrossRef]
- Sun, L.; Fang, X.; Chen, Z.; Zhang, H.; Zhang, Z.; Zhou, P.; et al. Compound heterozygous ZP1 mutations cause empty follicle syndrome in infertile sisters. Hum Mutat. 2019, 40, 2001–2006. [Google Scholar] [CrossRef]
- Liu, M.; Shen, Y.; Zhang, X.; Wang, X.; Li, D.; Wang, Y. Novel biallelic loss-of-function variants in ZP1 identified in an infertile female with empty follicle syndrome. J Assist Reprod Genet. 2020, 37, 2151–2157. [Google Scholar] [CrossRef]
- Zhang, Z.; Shangguan, T.; Li, Y.Y.; He, W. Infertility due to Lack of Zona Pellucida Caused by a Compound Heterozygous Mutation in ZP1 Gene. Reprod Dev Med. 2018, 2, 183–186. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, C.; Zhang, X.; Zhang, H.; Lu, Q.; Wang, C.; et al. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med. 2020, 24, 8557–8566. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Dioguardi, E.; Nishio, S.; Villa, A.; Han, L.; Matsuda, T.; et al. Molecular basis of egg coat cross-linking sheds light on ZP1-associated female infertility. Nat Commun. 2019, 10, 3086. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zeng, Y.; Chen, H.; Zhou, Z.; Fu, J.; Sang, Q.; et al. A novel homozygous variant in ZP2 causes abnormal zona pellucida formation and female infertility. J Assist Reprod Genet. 2021, 38, 1239–1245. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Liu, Q.; Liu, W.; Yan, X.; Zhu, X.; et al. Mutations in ZP4 are associated with abnormal zona pellucida and female infertility. J Clin Pathol. 2022, 75, 201–204. [Google Scholar] [CrossRef]
- Tumova, L.; Zigo, M.; Sutovsky, P.; Sedmikova, M.; Postlerova, P. Ligands and Receptors Involved in the Sperm-Zona Pellucida Interactions in Mammals. Cells. 2021, 10, 133. [Google Scholar] [CrossRef]
- Litscher, E.S.; Wassarman, P.M. Zona Pellucida Proteins, Fibrils, and Matrix. Annu Rev Biochem. 2020, 89, 695–715. [Google Scholar] [CrossRef] [PubMed]
- Chothia, C.; Gough, J.; Vogel, C.; Teichmann, S.A. Evolution of the Protein Repertoire. Science. 2003, 300, 1701–1703. [Google Scholar] [CrossRef]
- Spargo, S.C.; Hope, R.M. Evolution and Nomenclature of the Zona Pellucida Gene Family. Biol Reprod. 2003, 68, 358–362. [Google Scholar] [CrossRef]
- Goudet, G.; Mugnier, S.; Callebaut, I.; Monget, P. Phylogenetic Analysis and Identification of Pseudogenes Reveal a Progressive Loss of Zona Pellucida Genes During Evolution of Vertebrates1. Biol Reprod. 2008, 78, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.M.; Tian, H.F.; Hu, Q.M.; Meng, Y.; Xiao, H.B. Evolution and multiple origins of zona pellucida genes in vertebrates. Biol Open. 2018, 7, bio036137. [Google Scholar] [CrossRef]
- Bleil, J.D.; Greve, J.M.; Wassarman, P.M. Identification of a secondary sperm receptor in the mouse egg zona pellucida: Role in maintenance of binding of acrosome-reacted sperm to eggs. Dev Biol. 1988, 128, 376–385. [Google Scholar] [CrossRef]
- Lefievre, L. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004, 19, 1580–1586. [Google Scholar] [CrossRef]
- Izquierdo-Rico, M.J.; Jiménez-Movilla, M.; Llop, E.; Pérez-Oliva, A.B.; Ballesta, J.; Gutiérrez-Gallego, R.; et al. Hamster Zona Pellucida Is Formed by Four Glycoproteins: ZP1, ZP2, ZP3, and ZP4. J Proteome Res. 2009, 8, 926–941. [Google Scholar] [CrossRef]
- Stetson, I.; Izquierdo-Rico, M.J.; Moros, C.; Chevret, P.; Lorenzo, P.L.; Ballesta, J.; et al. Rabbit zona pellucida composition: A molecular, proteomic and phylogenetic approach. J Proteomics. 2012, 75, 5920–5935. [Google Scholar] [CrossRef]
- Stetson, I.; Avilés, M.; Moros, C.; García-Vázquez, F.A.; Gimeno, L.; Torrecillas, A.; et al. Four glycoproteins are expressed in the cat zona pellucida. Theriogenology. 2015, 83, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M.; Litscher, E.S. The Mouse Egg’s Zona Pellucida. In Current Topics in Developmental Biology [Internet]; Elsevier, 2025; pp. 331–356, [cited 2025 Sept 10]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0070215318300036.
- Bleil, J.D.; Wassarman, P.M. Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein’s sperm receptor activity.
- Dunbar, B.; Avery, S.; Lee, V.; Prasad, S.; Schwahn, D.; Schwoebel, E.; et al. The mammalian zona pellucida: its biochemistry, immunochemistry, molecular biology, and developmental expression. Reprod Fertil Dev. 1994, 6(, 331. [Google Scholar] [CrossRef]
- Bousquet, D.; Léveillé, M.C.; Roberts, K.D.; Chapdelaine, A.; Bleau, G. The cellular origin of the zona pellucida antigen in the human and hamster. J Exp Zool. 1981, 215, 215–218. [Google Scholar] [CrossRef]
- Pelletier, C.; Keefe, D.L.; Trimarchi, J.R. Noninvasive polarized light microscopy quantitatively distinguishes the multilaminar structure of the zona pellucida of living human eggs and embryos. Fertil Steril. 2004, 81, 850–856. [Google Scholar] [CrossRef]
- Suzuki, H.; Yang, X.; Foote, R.H. Surface alterations of the bovine oocyte and its investments during and after maturation and fertilization in vitro. Mol Reprod Dev. 1994, 38, 421–430. [Google Scholar] [CrossRef]
- Familiari, G.; Nottola, S.A.; Macchiarelli, G.; Micara, G.; Aragona, C.; Motta, P.M. Human zona pellucida during in vitro fertilization: An ultrastructural study using saponin, ruthenium red, and osmium-thiocarbohydrazide. Mol Reprod Dev. 1992, 32, 51–61. [Google Scholar] [CrossRef]
- Coy, P.; Cánovas, S.; Mondéjar, I.; Saavedra, M.D.; Romar, R.; Grullón, L.; et al. Oviduct-specific glycoprotein and heparin modulate sperm–zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc Natl Acad Sci. 2008, 105, 15809–15814. [Google Scholar] [CrossRef]
- Travnickova, I.; Hulinska, P.; Sladek, Z.; Skowronski, M.T.; Machatkova, M. Changes of the zona pellucida patterns during oocyte maturation, fertilization and embryo development in mammals: mini-review. Med J Cell Biol. 2022, 10, 23–28. [Google Scholar] [CrossRef]
- Oikawa, T.; Yanagimachi, R.; Nicolson, G.L. SPECIES DIFFERENCES IN THE LECTIN-BINDING SITES ON THE ZONA PELLUCIDA OF RODENT EGGS. Reproduction. 1975, 43, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Wright, G.J. Find and fuse: Unsolved mysteries in sperm–egg recognition. PLOS Biol. 2020, 18, e3000953. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: its site and role in fertilization†. Biol Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Litscher, E.S. Zona Pellucida Genes and Proteins: Essential Players in Mammalian Oogenesis and Fertility. Genes. 2021, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- La Spina, F.A.; Puga Molina, L.C.; Romarowski, A.; Vitale, A.M.; Falzone, T.L.; Krapf, D.; et al. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev Biol. 2016, 411, 172–182. [Google Scholar] [CrossRef]
- Kang, I.; Koo, M.; Yoon, H.; Park, B.S.; Jun, J.H.; Lee, J. Ovastacin: An oolemma protein that cleaves the zona pellucida to prevent polyspermy. Clin Exp Reprod Med. 2023, 50, 154–159. [Google Scholar] [CrossRef]
- Avella, M.A.; Baibakov, B.; Dean, J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J Cell Biol. 2014, 205, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Rankin, T.; Familari, M.; Lee, E.; Ginsberg, A.; Dwyer, N.; Blanchette-Mackie, J.; et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development. 1996, 122, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Lu, Y.; Miao, Y.; Dai, X.; Zhang, Y.; Xiong, B. Transglutaminase 2 crosslinks zona pellucida glycoprotein 3 to prevent polyspermy. Cell Death Differ. 2022, 29, 1466–1473. [Google Scholar] [CrossRef]
- Coy, P.; García-Vázquez, F.A.; Visconti, P.E.; Avilés, M. Roles of the oviduct in mammalian fertilization. REPRODUCTION. 2012, 144, 649–660. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci. 2011, 108, 4892–4896. [Google Scholar] [CrossRef]
- Muro, Y.; Buffone, M.G.; Okabe, M.; Gerton, G.L. Function of the Acrosomal Matrix: Zona Pellucida 3 Receptor (ZP3R/sp56) Is Not Essential for Mouse Fertilization1. Biol Reprod [Internet] 2012, 86. [cited 2025 Sept 10]. Available from: https://academic.oup.com/biolreprod/article-lookup/doi/10.1095/biolreprod.111.095877. [CrossRef]
- Algarra, B.; Maillo, V.; Avilés, M.; Gutiérrez-Adán, A.; Rizos, D.; Jiménez-Movilla, M. Effects of recombinant OVGP1 protein on in vitro bovine embryo development. J Reprod Dev. 2018, 64, 433–443. [Google Scholar] [CrossRef]
- Mondéjar, I.; Avilés, M.; Coy, P. The human is an exception to the evolutionarily-conserved phenomenon of pre-fertilization zona pellucida resistance to proteolysis induced by oviductal fluid. Hum Reprod. 2013, 28, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Avilés, M.; El-Mestrah, M.; Jaber, L.; Castells, M.T.; Ballesta, J.; Kan, F.W.K. Cytochemical demonstration of modification of carbohydrates in the mouse zona pellucida during folliculogenesis. Histochem Cell Biol. 2000, 113, 207–219. [Google Scholar] [CrossRef]
- Avilés, M.; Okinaga, T.; Shur, B.D.; Ballesta, J. Differential expression of glycoside residues in the mammalian zona pellucida. Mol Reprod Dev. 2000, 57, 296–308. [Google Scholar] [CrossRef]
- Fang, X.; Bang, S.; Tanga, B.; Seo, C.; Zhou, D.; Seong, G.; et al. Oviduct epithelial cell-derived extracellular vesicles promote the developmental competence of IVF porcine embryos. Mol Med Rep. 2023, 27, 122. [Google Scholar] [CrossRef]
- Miyashita, N.; Akagi, S.; Somfai, T.; Hirao, Y. Serum-free spontaneously immortalized bovine oviduct epithelial cell conditioned medium promotes the early development of bovine in vitro fertilized embryos. J Reprod Dev. 2024, 70, 42–48. [Google Scholar] [CrossRef]
- Kan, F.W.K.; Roux, E.; Bleau, G. Immunolocalization of Oviductin in Endocytic Compartments in the Blastomeres of Developing Embryos in the Golden Hamster1. Biol Reprod. 1993, 48, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Balastegui-Alarcón, M.; Moros-Nicolás, C.; Ballesta, J.; Izquierdo-Rico, M.J.; Chevret, P.; Avilés, M. Molecular Evolution of the Ovgp1 Gene in the Subfamily Murinae. Animals. 2024, 15, 55. [Google Scholar] [CrossRef]
- Moros-Nicolás, C.; Fouchécourt, S.; Goudet, G.; Monget, P. Genes Encoding Mammalian Oviductal Proteins Involved in Fertilization are Subjected to Gene Death and Positive Selection. J Mol Evol. 2018, 86, 655–667. [Google Scholar] [CrossRef]
- Simintiras, C.A.; Fröhlich, T.; Sathyapalan, T.; Arnold, G.J.; Ulbrich, S.E.; Leese, H.J.; et al. Modelling aspects of oviduct fluid formation in vitro. Reproduction. 2017, 153, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cerezales, S.; Ramos-Ibeas, P.; Acuña, O.S.; Avilés, M.; Coy, P.; Rizos, D.; et al. The oviduct: from sperm selection to the epigenetic landscape of the embryo†. Biol Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef]
- Almiñana, C.; Corbin, E.; Tsikis, G.; Alcântara-Neto, A.S.; Labas, V.; Reynaud, K.; et al. Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk. Reproduction. 2017, 154, 253–268. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.; Schmaltz, L.; Caldas, E.; Blache, M.C.; Mermillod, P.; Almiñana, C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology 2020, 155, 240–255. [Google Scholar] [CrossRef]
- Fiorentino, G.; Merico, V.; Zanoni, M.; Comincini, S.; Sproviero, D.; Garofalo, M.; et al. Extracellular vesicles secreted by cumulus cells contain microRNAs that are potential regulatory factors of mouse oocyte developmental competence. Mol Hum Reprod. 2024, 30, gaae019. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Peng, H.; Ward, S.M.; Hennig, G.W.; Zheng, H.; et al. Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport. Proc Natl Acad Sci. 2021, 118, e2102940118. [Google Scholar] [CrossRef]
- Murayama, Y.; Yoshida, K.; Takahashi, H.; Mizuno, J.; Akaishi, K.; Inui, H. Softening of the mouse zona pellucida during oocyte maturation. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) [Internet], Osaka; IEEE, 2013; pp. 6834–6837, [cited 2025 Sept 10]. Available from: http://ieeexplore.ieee.org/document/6611127/.
- Kolbe, T.; Holtz, W. Differences in proteinase digestibility of the zona pellucida of in vivo and in vitro derived porcine oocytes and embryos. Theriogenology. 2005, 63, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Lamas-Toranzo, I.; Fonseca Balvís, N.; Querejeta-Fernández, A.; Izquierdo-Rico, M.J.; González-Brusi, L.; Lorenzo, P.L.; et al. ZP4 confers structural properties to the zona pellucida essential for embryo development. eLife. 2019, 8, e48904. [Google Scholar] [CrossRef] [PubMed]
- Scully, D.M.; Xia, T.; Musina, G.R.; McCown, M.A.; Umezu, K.; Kircher, B.K.; et al. Region-specific roles of oviductal motile cilia in oocyte/embryo transport and fertility. Biol Reprod. 2025, 112, 651–662. [Google Scholar] [CrossRef]
- Gupta, S.K.; Srinivasan, V.A.; Suman, P.; Rajan, S.; Nagendrakumar, S.B.; Gupta, N.; et al. Contraceptive Vaccines Based on the Zona Pellucida Glycoproteins for Dogs and Other Wildlife Population Management. Am J Reprod Immunol. 2011, 66, 51–62. [Google Scholar] [CrossRef]
- Burkart, A.D.; Xiong, B.; Baibakov, B.; Jiménez-Movilla, M.; Dean, J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J Cell Biol. 2012, 197, 37–44. [Google Scholar] [CrossRef]
- Maddirevula, S.; Coskun, S.; Al-Qahtani, M.; Aboyousef, O.; Alhassan, S.; Aldeery, M.; et al. ASTL is mutated in female infertility. Hum Genet. 2022, 141, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Von Wiegen, N.; Behl, C.; Körschgen, H. Crossing the barrier or how regulation of ovastacin controls fertilization and translates into clinical phenotypes. iScience. 2025, 28, 112976. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Mysteries and unsolved problems of mammalian fertilization and related topics. Biol Reprod. 2022, 106, 644–675. [Google Scholar] [CrossRef]
- Kuske, M.; Floehr, J.; Yiallouros, I.; Michna, T.; Jahnen-Dechent, W.; Tenzer, S.; et al. Limited proteolysis by acrosin affects sperm-binding and mechanical resilience of the mouse zona pellucida. Mol Hum Reprod. 2021, 27, gaab022. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, T.V.; Dang, H.N.T.; Nguyen, Q.H.V. Formation of Blastocysts From Zona Pellucida–Free Oocytes: A Case Report on a Modified Technique in In Vitro Fertilization. Singh M, editor. Case Rep Obstet Gynecol. 2025, 2025, 5247242. [Google Scholar] [CrossRef]
- Clark, G.F. The role of carbohydrate recognition during human sperm-egg binding. Hum Reprod. 2013, 28, 566–577. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, L.; Liu, Z.; Teng, Y.; Li, M.; Peng, X.; et al. Effect of blastocyst development on hatching and embryo implantation. Theriogenology. 2024, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Satouh, Y.; Nishimasu, H.; Kurabayashi, A.; Morita, J.; Fujihara, Y.; et al. Structural and functional insights into IZUMO1 recognition by JUNO in mammalian fertilization. Nat Commun. 2016, 7, 12198. [Google Scholar] [CrossRef]
- Swann, C.A.; Cooper, S.J.B.; Breed, W.G. Molecular evolution of the carboxy terminal region of the zona pellucida 3 glycoprotein in murine rodents. Reproduction. 2007, 133, 697–708. [Google Scholar] [CrossRef]
- Pang, P.C.; Chiu, P.C.N.; Lee, C.L.; Chang, L.Y.; Panico, M.; Morris, H.R.; et al. Human Sperm Binding Is Mediated by the Sialyl-Lewisx Oligosaccharide on the Zona Pellucida. Science. 2011, 333, 1761–1764. [Google Scholar] [CrossRef]
- Algarra, B.; Han, L.; Soriano-Úbeda, C.; Avilés, M.; Coy, P.; Jovine, L.; et al. The C-terminal region of OVGP1 remodels the zona pellucida and modifies fertility parameters. Sci Rep. 2016, 6, 32556. [Google Scholar] [CrossRef] [PubMed]
- Priel, E.; Priel, T.; Szaingurten-Solodkin, I.; Wainstock, T.; Perets, Y.; Zeadna, A.; et al. Zona pellucida shear modulus, a possible novel non-invasive method to assist in embryo selection during in-vitro fertilization treatment. Sci Rep. 2020, 10, 14066. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Cao, X.; Liu, S.Y.; Dong, X. Laser-assisted hatching and clinical outcomes in frozen-thawed cleavage-embryo transfers of patients with previous repeated failure. Lasers Med Sci. 2019, 34, 1137–1145. [Google Scholar] [CrossRef]
- Priel, E.; Mittelman, B.; Efraim, L.; Priel, T.; Szaingurten-Solodkin, I.; Har-Vardi, I. Hyperelastic models for the human zona pellucida and their implications on shear modulus estimation in the clinical practice. Sci Rep. 2024, 14, 31411. [Google Scholar] [CrossRef]
- Vani, V.; Vasan, S.S.; Adiga, S.K.; Varsha, S.R.; Seshagiri, P.B. Molecular regulators of human blastocyst development and hatching: Their significance in implantation and pregnancy outcome. Am J Reprod Immunol. 2023, 89, e13635. [Google Scholar] [PubMed]
- Guo, J.; Lu, W.F.; Liang, S.; Choi, J.W.; Kim, N.H.; Cui, X.S. Peroxisome proliferator-activated receptor δ improves porcine blastocyst hatching via the regulation of fatty acid oxidation. Theriogenology 2017, 90, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lee, J.M.; Kim, S.K.; Youm, H.W.; Jee, B.C. Associations of post-warming embryo or blastocyst development with clinical pregnancy in vitrified embryo or blastocyst transfer cycles. Clin Exp Reprod Med. 2020, 47, 140–146. [Google Scholar] [CrossRef]
- Michailov, Y.; Friedler, S.; Saar-Ryss, B. Methods to improve frozen-thawed blastocyst transfer outcomes- the IVF laboratory perspective. J IVF-Worldw [Internet] 2025, 1. [cited 2025 Sept 10]. Available from: https://jivfww.scholasticahq.com/article/87541-methods-to-improve-frozen-thawed-blastocyst-transfer-outcomes-the-ivf-laboratory-perspective. [CrossRef]
- Yang, W.; Wang, Q.; Zhang, B.; Leung, R.K.K.; Deng, K.; Geng, S.; et al. Association between hatching status and pregnancy outcomes in single blastocyst transfers: a retrospective cohort analysis. J Assist Reprod Genet. 2025, 42, 1707–1715. [Google Scholar] [CrossRef]
- Mahdavinezhad, F.; Kazemi, P.; Fathalizadeh, P.; Sarmadi, F.; Hashemi, E.; Hajarian, H.; et al. In vitro versus In vivo: Development-, Apoptosis-, and Implantation-Related Gene Expression in Mouse Blastocyst. Iran J Biotechnol. 2019, 17, 90–97. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, Y.T.; Ding, K.; Xue, F.R.; Wang, X.Y.; Yang, Q.; et al. Assisted Hatching Treatment of Piezo-Mediated Small Hole on Zona Pellucida in Morula Stage Embryos Improves Embryo Implantation and Litter Size in Mice. Front Cell Dev Biol. 2021, 9, 746104. [Google Scholar] [CrossRef]
- Liu, Y.; Jones, C.; Coward, K. The Current Practice of Assisted Hatching for Embryos in Fertility Centres: a General Survey. Reprod Sci. 2022, 29, 2664–2673. [Google Scholar] [CrossRef]
- Syrkasheva, A.G.; Dolgushina, N.V.; Romanov AYu Burmenskaya, O.V.; Makarova, N.P.; Ibragimova, E.O.; et al. Cell and genetic predictors of human blastocyst hatching success in assisted reproduction. Zygote. 2017, 25, 631–636. [Google Scholar] [CrossRef]
- Batista, M.R.; Diniz, P.; Torres, A.; Murta, D.; Lopes-da-Costa, L.; Silva, E. Notch signaling in mouse blastocyst development and hatching. BMC Dev Biol. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, T.; Liu, Y.; Hou, Z.; Wu, K.; Cao, Y.; et al. The critical role of ZP genes in female infertility characterized by empty follicle syndrome and oocyte degeneration. Fertil Steril. 2021, 115, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Yuan, G.; Yang, J.; Liu, X.; Chen, S.; et al. A compound heterozygous mutation in ZP1 and two novel heterozygous cis mutations in ZP3 causes infertility in women presenting with empty follicle syndrome. J Ovarian Res. 2025, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Kodani, M.; Emori, C.; Kiyozumi, D.; Mori, M.; Fujihara, Y.; et al. CRISPR/Cas9-Mediated Genome Editing Reveals Oosp Family Genes are Dispensable for Female Fertility in Mice. Cells 2020, 9, 821. [Google Scholar] [CrossRef]
- Dioguardi, E.; Stsiapanava, A.; Fahrenkamp, E.; Han, L.; De Sanctis, D.; Inzunza, J.; et al. Structural basis of ZP2-targeted female nonhormonal contraception. Proc Natl Acad Sci. 2025, 122, e2426057122. [Google Scholar] [CrossRef]
- Avella, M.A.; Baibakov, B.A.; Jimenez-Movilla, M.; Sadusky, A.B.; Dean, J. ZP2 peptide beads select human sperm in vitro, decoy mouse sperm in vivo, and provide reversible contraception. Sci Transl Med [Internet] 2016, 8. [cited 2025 Sept 10]. Available from: https://www.science.org/doi/10.1126/scitranslmed.aad9946.
- Zhang, B.; Qu, G.; Nan, Y.; Zhou, E.M. Ovarian Oxidative Stress Induced Follicle Depletion After Zona Pellucida 3 Vaccination Is Associated With Subfertility in BALB/c Mice. Front Vet Sci. 2022, 9, 814827. [Google Scholar] [CrossRef]
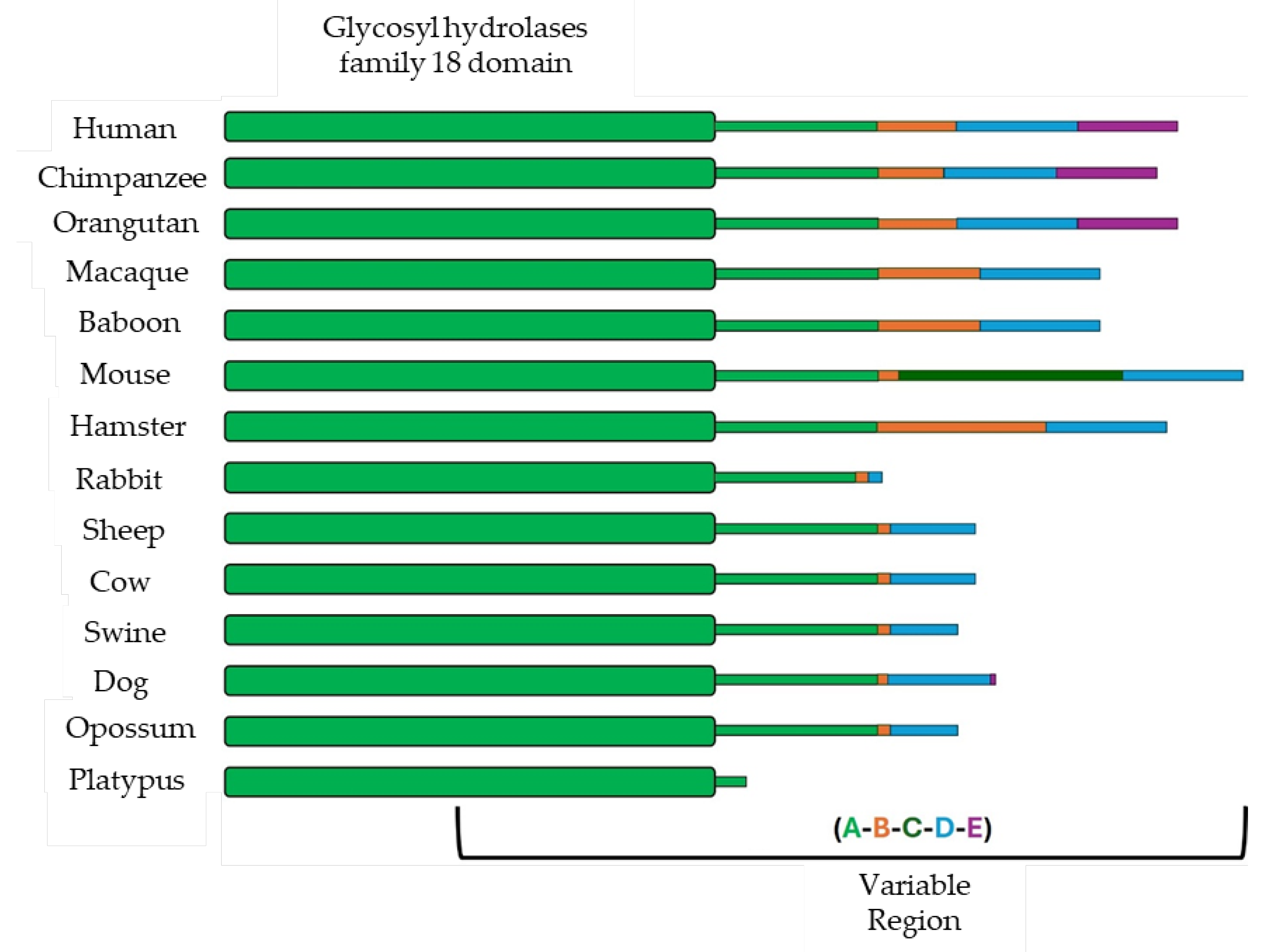
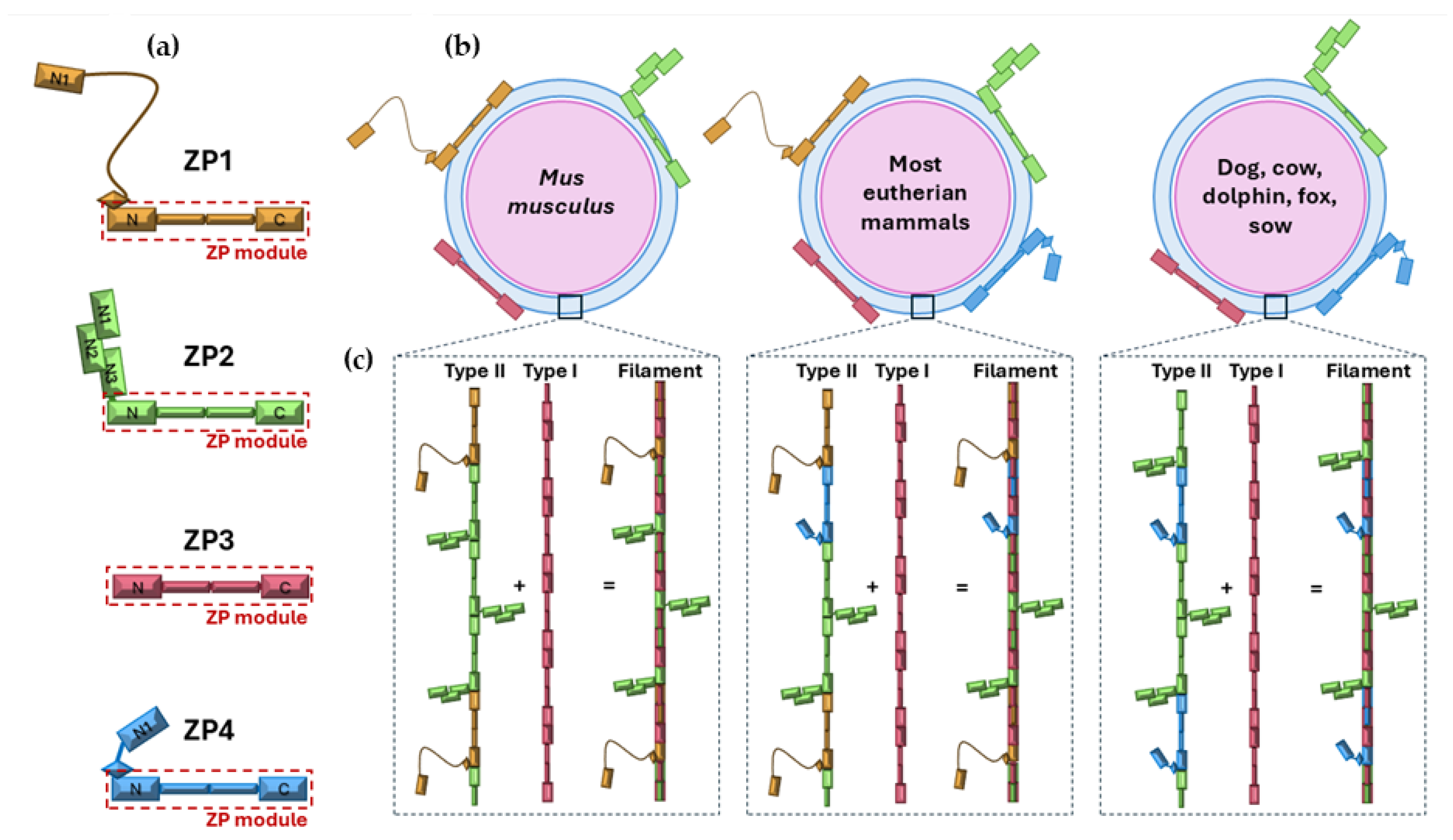
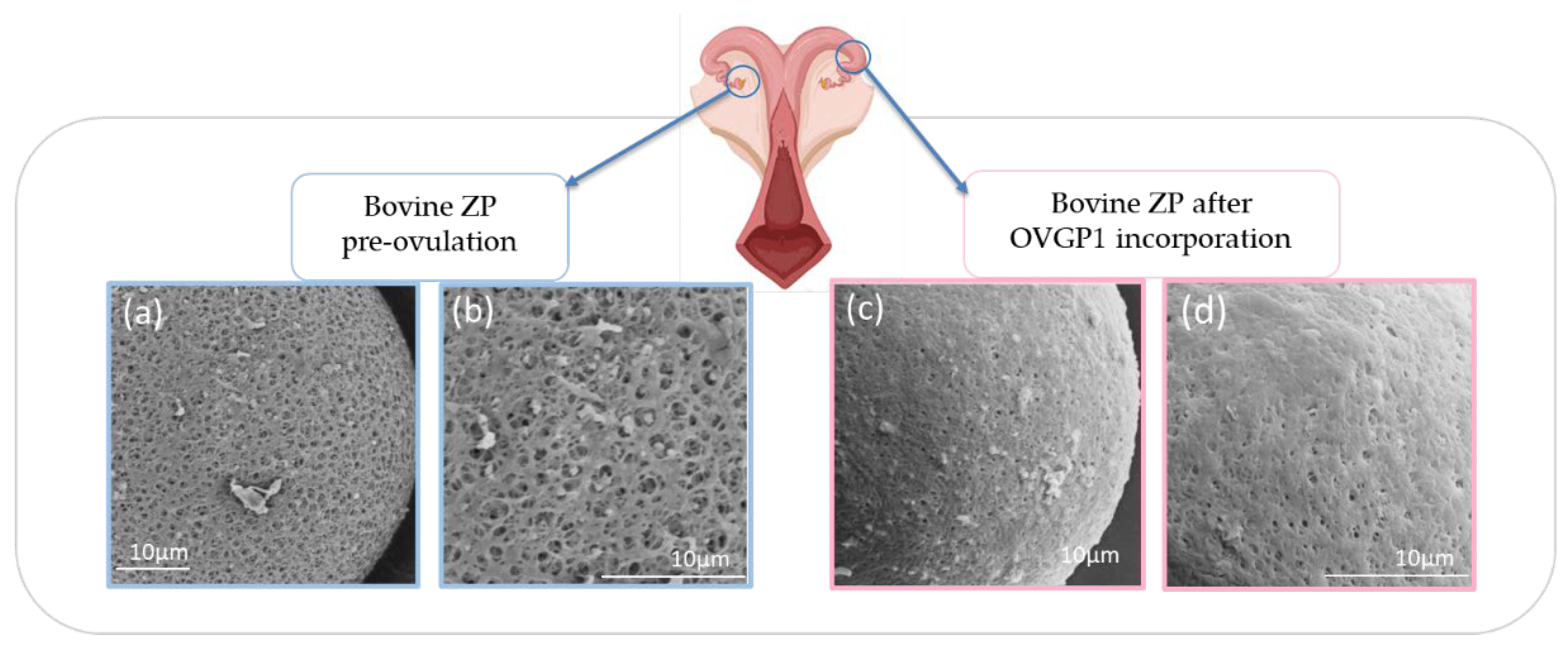
| Group of mammals | ZP1 | ZP2 | ZP3-1a | ZP3-1b | ZP3-1c | ZP3-2 | ZP4 | ZPAX | ZPY |
|---|---|---|---|---|---|---|---|---|---|
| Mus musculus | ✓ | ✓ | ✓ | ||||||
| Most eutherian mammals | ✓ | ✓ | ✓ | ✓ | |||||
| Dog, cow, sow, dolphin, fox | ✓ | ✓ | ✓ | ||||||
| American marsupials | ✓ | ✓ | ✓ | ✓ | |||||
| Australian marsupials | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Monotremes | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Immature Oocytes | Mature Oocytes | Day 3 Embryos | |
|---|---|---|---|
| Layer 1 | |||
| Thickness (µm) | 10.5 ± 2.3 | 9.8 ± 2.1ᵇ | 7.9 ± 1.9 |
| Retardance (nm) | 3.26 ± 1.27 | 2.84 ± 1.07 | 3.00 ± 0.86 |
| Layer 2 | |||
| Thickness (µm) | 3.4 ± 0.6 | 3.7 ± 0.9 | 3.6 ± 1.2 |
| Retardance (nm) | 0.21 ± 0.07ᵃ | 0.24 ± 0.05ᶜ | 0.33 ± 0.29 |
| Layer 3 | |||
| Thickness (µm) | 6.5 ± 2.1 | 6.1 ± 1.7ᵇ | 3.7 ± 1.4 |
| Retardance (nm) | 0.91 ± 0.24 | 0.95 ± 0.23ᵇ | 0.78 ± 0.27 |
| Total Zona | |||
| Thickness (µm) | 20.4 ± 2.4 | 19.5 ± 2.2ᵇ | 15.2 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





