Submitted:
02 October 2025
Posted:
03 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
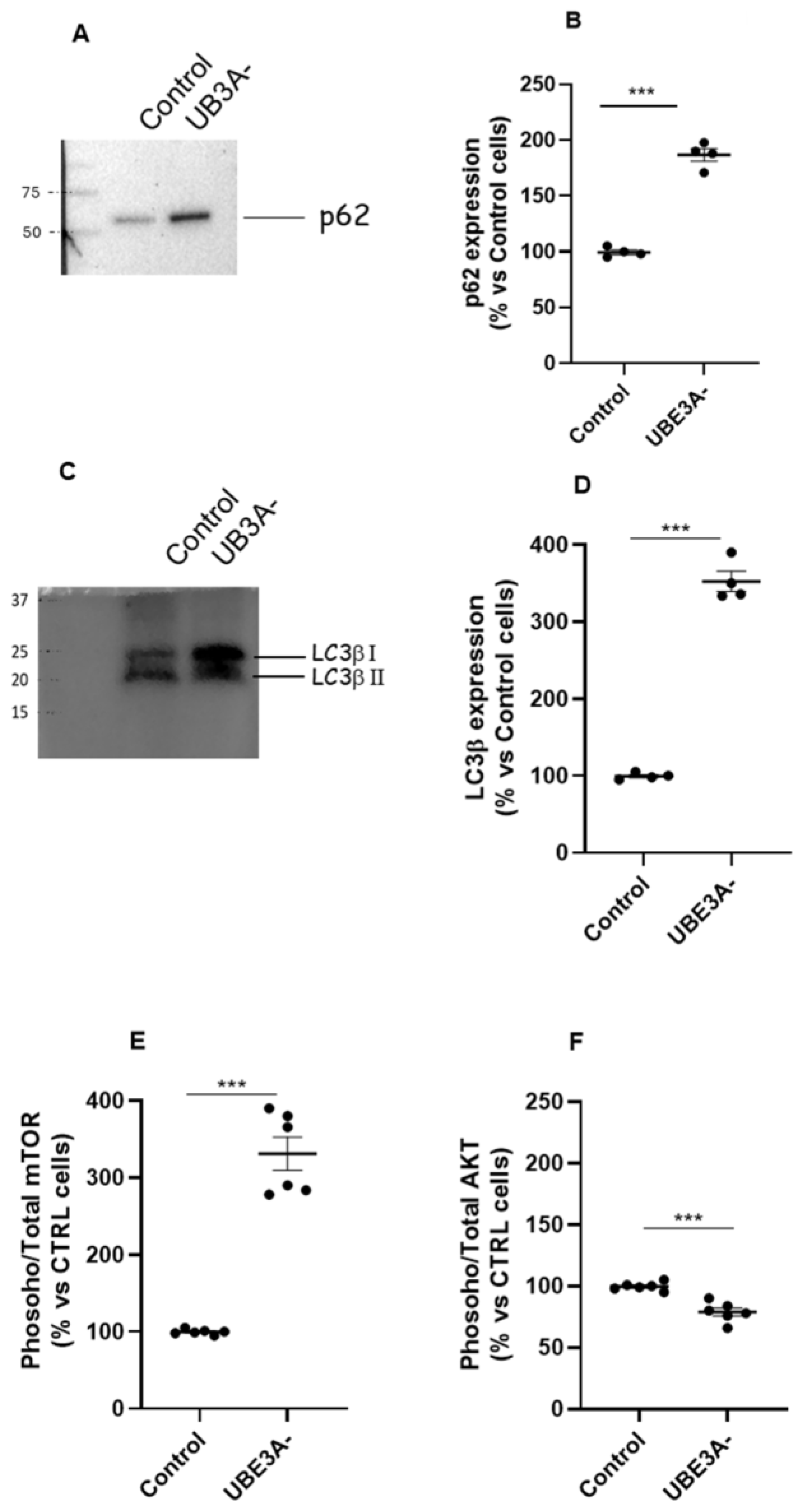
2.1. Involvement of the Autophagy System in the AS Model
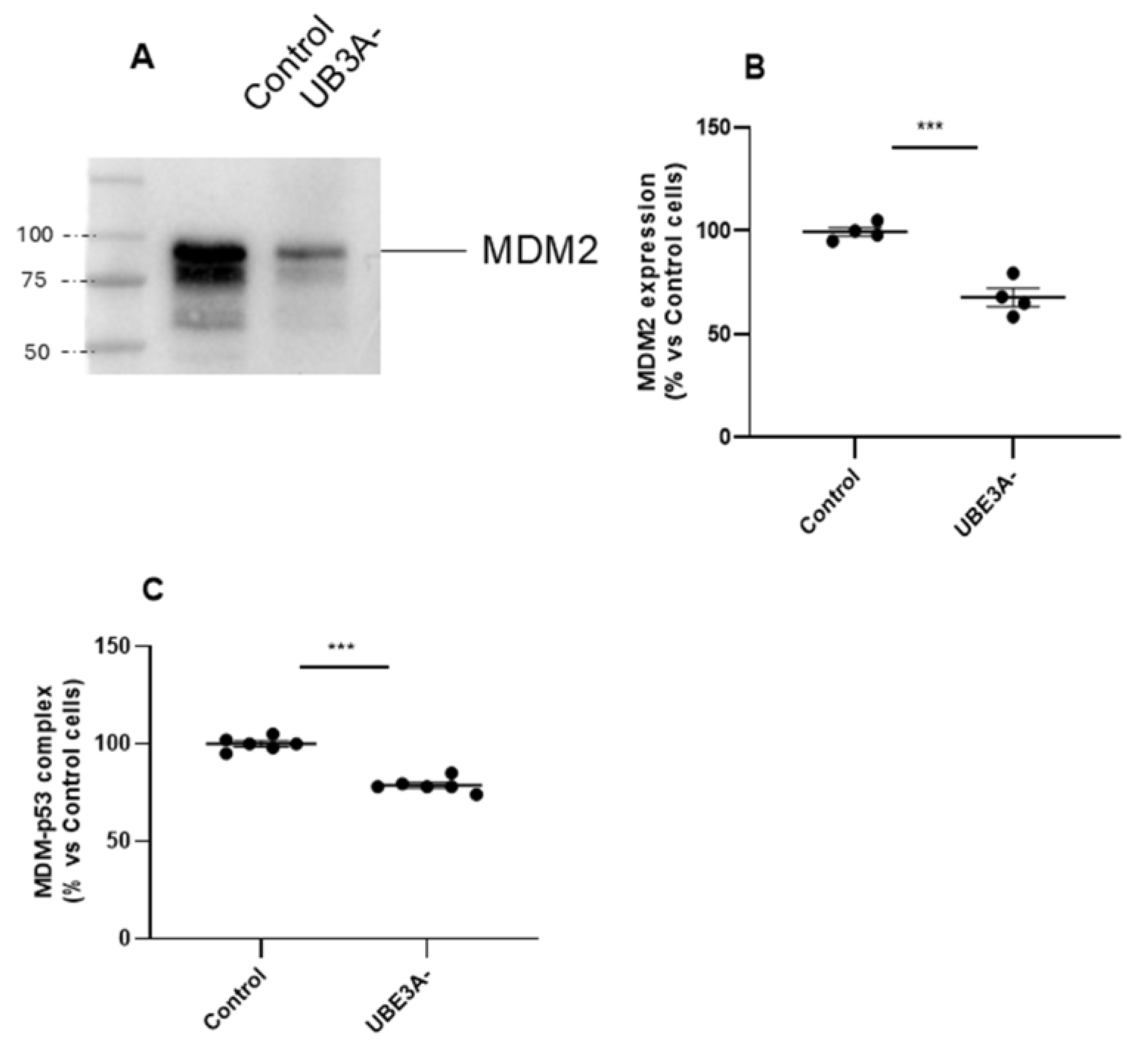
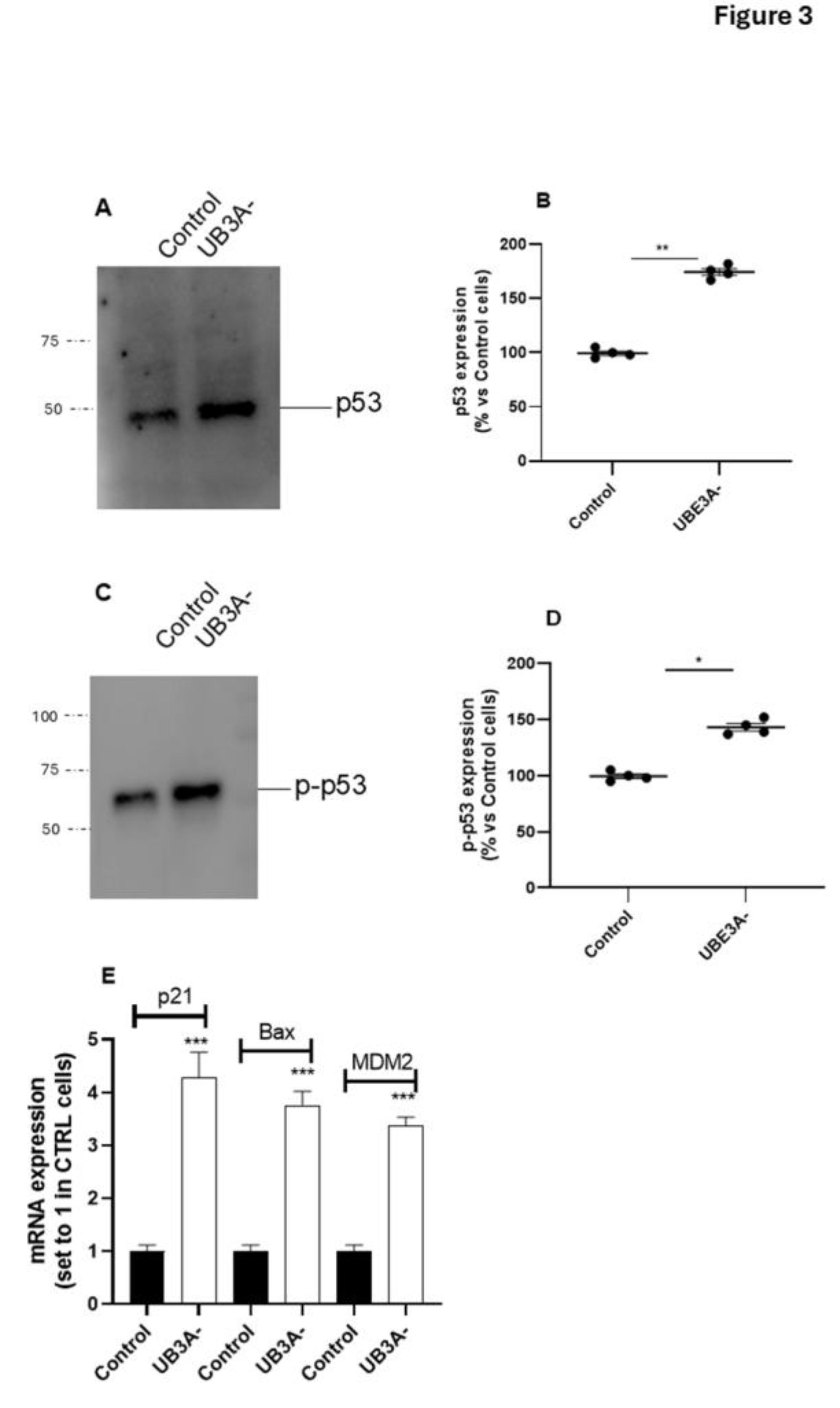
2.2. Involvement of the UPS in the AS Model
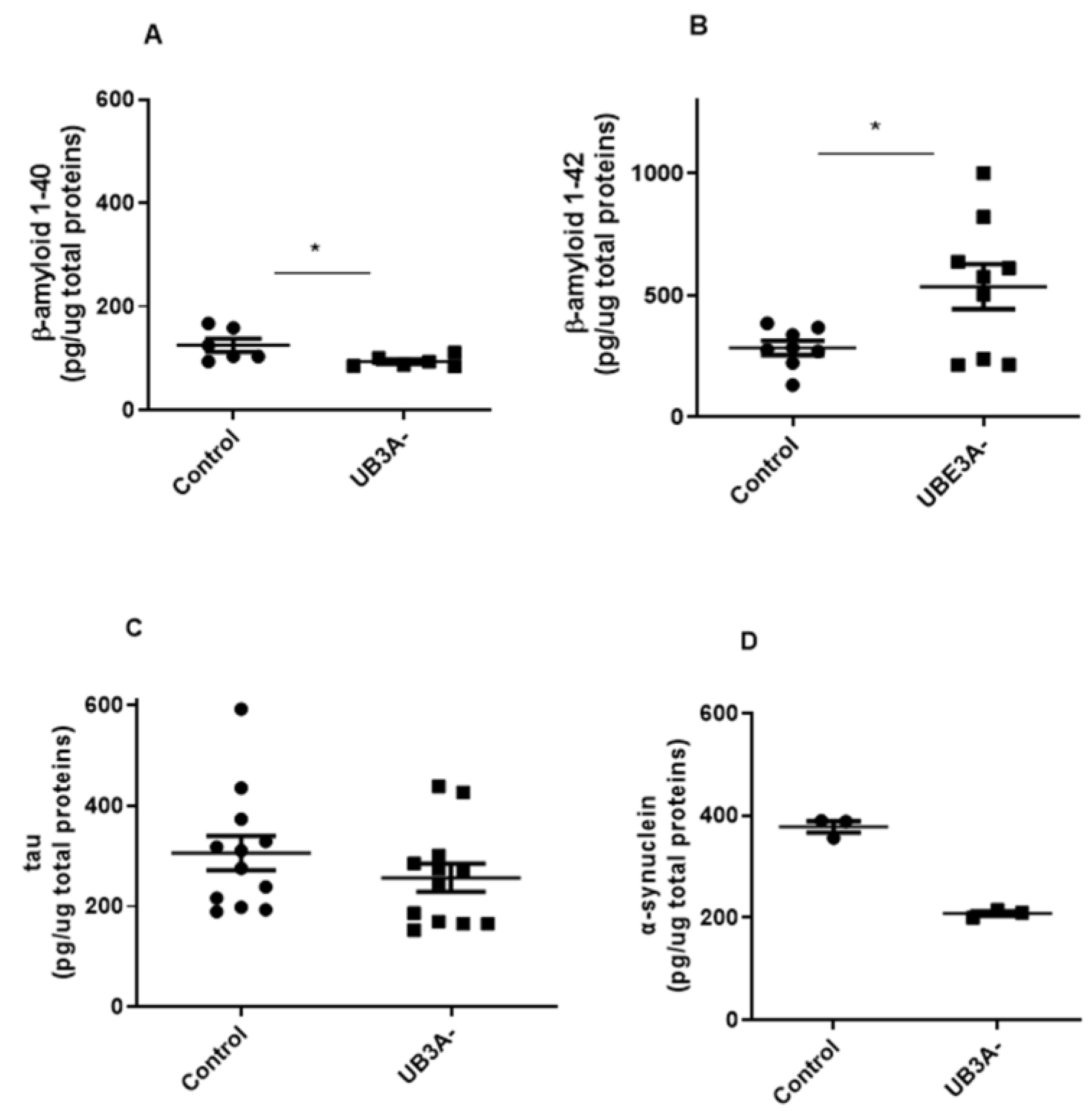
2.3. Misfolded Protein Accumulation in the AS Models
3. Discussion
4. Materials and Methods
4.1. Neuronal Cell Cultures
4.2. Mouse Model of AS
4.3. Samples Lysate Preparation for Immunoenzymatic Assays
4.4. Protein Expression by Western Blot Analysis
4.5. MDM2-p53 Complex in AS Models
4.6. Total and Phosphorylated AKT/mTOR in AS Models
4.7. RNA Extraction and RealTime PCR Analysis
4.8. Misfolded Protein Accumulation in Angelman Syndrome Models
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS Angelman Syndrome | AS Angelman Syndrome |
|
UBE3A Ubiquitin-Protein ligase E3A UPS The ubiquitin-proteasome system JNK c-Jun-N-terminal Kinase ASD autism spectrum disorder AMPK-mTOR mammalian target of rapamycin MDM2 murine double minute 2 AKT protein kinase B PI3K phosphoinositide 3-kinase Aβ Amyloid-β α-syn α-synuclein |
UBE3A Ubiquitin-Protein ligase E3A UPS The ubiquitin-proteasome system JNK c-Jun-N-terminal Kinase ASD autism spectrum disorder |
References
- de Almeida, J.F.M.; Tonazzini, I.; Daniele, S. Molecular aspects of Angelman Syndrome: Defining the new path forward. Biomol. Biomed. 2025, 25, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, M.; Baronchelli, F.; Fossati, M. Multiscale spatio-temporal dynamics of UBE3A gene in brain physiology and neurodevelopmental disorders. Neurobiol. Dis. 2024, 201, 106669. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, R.B. The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021, 28, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Đukić, A.; Lulić, L.; Thomas, M.; Skelin, J.; Saidu, N.E.B.; Grce, M.; Banks, L.; Tomaić, V. HPV Oncoproteins and the Ubiquitin Proteasome System: A Signature of Malignancy? Pathogens 2020, 9, 133. [Google Scholar] [CrossRef]
- Kawabe, H.; Brose, N. The role of ubiquitylation in nerve cell development. Nat. Rev. Neurosci. 2011, 12, 251–268. [Google Scholar] [CrossRef]
- Kuang, E.; Qi, J.; Ronai, Z. Emerging roles of E3 ubiquitin ligases in autophagy. Trends Biochem. Sci. 2013, 38, 453–460. [Google Scholar] [CrossRef]
- Aria, F.; Pandey, K.; Alberini, C.M. Excessive Protein Accumulation and Impaired Autophagy in the Hippocampus of Angelman Syndrome Modeled in Mice. Biol. Psychiatry 2022, 94, 68–83. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Jia, Y.; Hao, X.; Lin, W.J.; Tran, J.; Lynch, G.; Baudry, M.; Bi, X. UBE3A-mediated p18/LAMTOR1 ubiquitination and degradation regulate mTORC1 activity and synaptic plasticity. eLife 2018, 7. [Google Scholar] [CrossRef]
- Hao, X.; Sun, J.; Zhong, L.; Baudry, M.; Bi, X. UBE3A deficiency-induced autophagy is associated with activation of AMPK-ULK1 and p53 pathways. Exp. Neurol. 2023, 363, 114358–114358. [Google Scholar] [CrossRef]
- Chaudhary, P.; Proulx, J.; Park, I.-W. Ubiquitin-protein ligase E3A (UBE3A) mediation of viral infection and human diseases. Virus Res. 2023, 335, 199191. [Google Scholar] [CrossRef]
- Perry, M.E. The Regulation of the p53-mediated Stress Response by MDM2 and MDM4. Cold Spring Harb. Perspect. Biol. 2009, 2, a000968–a000968. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Yue, J.; Gao, M.; Zhang, J.; Huang, Y.; Xiong, H.; Li, X.; Wang, Y.; Yuan, Y.; et al. Unlocking the potential of LHPP: Inhibiting glioma growth and cell cycle via the MDM2/p53 pathway. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2024, 1871, 167509. [Google Scholar] [CrossRef]
- Barmaki, H.; Nourazarian, A.; Khaki-Khatibi, F. Proteostasis and neurodegeneration: a closer look at autophagy in Alzheimer's disease. Front. Aging Neurosci. 2023, 15, 1281338. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Daniele, S.; Martini, C. Potential biomarkers and novel pharmacological targets in protein aggregation-related neurodegenerative diseases. Biochem. Pharmacol. 2017, 131, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ciryam, P.; Tartaglia, G.G.; Morimoto, R.I.; Dobson, C.M.; Vendruscolo, M. Widespread Aggregation and Neurodegenerative Diseases Are Associated with Supersaturated Proteins. Cell Rep. 2013, 5, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Brezic, N.; Gligorevic, S.; Sic, A.; Knezevic, N.N. Protein Misfolding and Aggregation as a Mechanistic Link Between Chronic Pain and Neurodegenerative Diseases. Curr. Issues Mol. Biol. 2025, 47, 259. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Shukla, M.; Narayan, M. Proteostasis and Its Role in Disease Development. Cell Biochem. Biophys. 2024, 83, 1725–1741. [Google Scholar] [CrossRef]
- Bhat, K.P.; Yan, S.; Wang, C.-E.; Li, S.; Li, X.-J. Differential ubiquitination and degradation of huntingtin fragments modulated by ubiquitin-protein ligase E3A. Proc. Natl. Acad. Sci. 2014, 111, 5706–5711. [Google Scholar] [CrossRef]
- Mishra, A.; Dikshit, P.; Purkayastha, S.; Sharma, J.; Nukina, N.; Jana, N.R. E6-AP Promotes Misfolded Polyglutamine Proteins for Proteasomal Degradation and Suppresses Polyglutamine Protein Aggregation and Toxicity. J. Biol. Chem. 2008, 283, 7648–7656. [Google Scholar] [CrossRef]
- Mishra, A.; Godavarthi, S.K.; Maheshwari, M.; Goswami, A.; Jana, N.R. The Ubiquitin Ligase E6-AP Is Induced and Recruited to Aggresomes in Response to Proteasome Inhibition and May Be Involved in the Ubiquitination of Hsp70-bound Misfolded Proteins. J. Biol. Chem. 2009, 284, 10537–10545. [Google Scholar] [CrossRef]
- Kühnle, S.; Mothes, B.; Matentzoglu, K.; Scheffner, M. Role of the ubiquitin ligase E6AP/UBE3A in controlling levels of the synaptic protein Arc. Proc. Natl. Acad. Sci. 2013, 110, 8888–8893. [Google Scholar] [CrossRef]
- Mezzena, R.; Masciullo, C.; Antonini, S.; Cremisi, F.; Scheffner, M.; Cecchini, M.; Tonazzini, I. Study of adhesion and migration dynamics in ubiquitin E3A ligase (UBE3A)-silenced SYSH5Y neuroblastoma cells by micro-structured surfaces. Nanotechnology 2020, 32, 025708. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Armstrong, D.; Albrecht, U.; Atkins, C.M.; Noebels, J.L.; Eichele, G.; Sweatt, J.; Beaudet, A.L. Mutation of the Angelman Ubiquitin Ligase in Mice Causes Increased Cytoplasmic p53 and Deficits of Contextual Learning and Long-Term Potentiation. Neuron 1998, 21, 799–811. [Google Scholar] [CrossRef]
- Brooks, C.L.; Li, M.; Gu, W. Mechanistic Studies of MDM2-mediated Ubiquitination in p53 Regulation. J. Biol. Chem. 2007, 282, 22804–22815. [Google Scholar] [CrossRef]
- Chao, C.; Hergenhahn, M.; Kaeser, M.D.; Wu, Z.; Saito, S.; Iggo, R.; Hollstein, M.; Appella, E.; Xu, Y. Cell Type- and Promoter-specific Roles of Ser18 Phosphorylation in Regulating p53 Responses. J. Biol. Chem. 2003, 278, 41028–41033. [Google Scholar] [CrossRef]
- Greenblatt, M.S.; Bennett, W.P.; Hollstein, M.; Harris, C.C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. . 1994, 54, 4855–78. [Google Scholar]
- Cummings, K.A. Protein Accumulation and Impaired Autophagy Underlie Cognitive Dysfunction in Angelman Syndrome. Biol. Psychiatry 2023, 94, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Pérez-Rojas, J.M.; Hernández-Damián, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Tran, J.; O’nEal, P.; Baudry, M.; Bi, X. mTORC1–S6K1 inhibition or mTORC2 activation improves hippocampal synaptic plasticity and learning in Angelman syndrome mice. Cell. Mol. Life Sci. 2016, 73, 4303–4314. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Moreno, S.; Baudry, M.; Bi, X. Imbalanced Mechanistic Target of Rapamycin C1 and C2 Activity in the Cerebellum of Angelman Syndrome Mice Impairs Motor Function. J. Neurosci. 2015, 35, 4706–4718. [Google Scholar] [CrossRef]
- Bi, X.; Sun, J.; Baudry, M. Yin-and-Yang of mTORC1/C2 in Angelman syndrome mice. Oncotarget 2015, 6, 13844–13845. [Google Scholar] [CrossRef]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.L.; Levine, A.J. The p53 pathway: positive and negative feedback loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Mendrysa, S.M.; Ghassemifar, S.; Malek, R. p53 in the CNS: Perspectives on Development, Stem Cells, and Cancer. Genes Cancer 2011, 2, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Li, H.; Pan, X.; Wang, Y. New Insights into the Roles of p53 in Central Nervous System Diseases. Int. J. Neuropsychopharmacol. 2023, 26, 465–473. [Google Scholar] [CrossRef]
- Barmaki, H.; Nourazarian, A.; Khaki-Khatibi, F. Proteostasis and neurodegeneration: a closer look at autophagy in Alzheimer's disease. Front. Aging Neurosci. 2023, 15, 1281338. [Google Scholar] [CrossRef]
- Frackowiak, J.; Mazur-Kolecka, B. Intraneuronal accumulation of amyloid-β peptides as the pathomechanism linking autism and its co-morbidities: epilepsy and self-injurious behavior — the hypothesis. Front. Mol. Neurosci. 2023, 16, 1160967. [Google Scholar] [CrossRef]
- Nair, L.D.V.; Sivanesan, S.K.; Kumar, D.S. Synucleins As Biomarkers of Severity in Autism Spectrum Disorder. Cureus 2024, 16. [Google Scholar] [CrossRef]
- Elgersma, Y. A molecular tightrope. Nature 2015, 526, 50–51. [Google Scholar] [CrossRef]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Yi, J.J.; Berrios, J.; Newbern, J.M.; Snider, W.D.; Philpot, B.D.; Hahn, K.M.; Zylka, M.J. An Autism-Linked Mutation Disables Phosphorylation Control of UBE3A. Cell 2015, 162, 795–807. [Google Scholar] [CrossRef]
- Tonazzini, I.; Van Woerden, G.M.; Masciullo, C.; Mientjes, E.J.; Elgersma, Y.; Cecchini, M. The role of ubiquitin ligase E3A in polarized contact guidance and rescue strategies in UBE3A-deficient hippocampal neurons. Mol. Autism 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Copping, N.A.; Silverman, J.L. Abnormal electrophysiological phenotypes and sleep deficits in a mouse model of Angelman Syndrome. Mol. Autism 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Bonfanti, E.; Daniele, S.; Zappelli, E.; Lecca, D.; Martini, C.; Trincavelli, M.L.; Abbracchio, M.P. The ubiquitin ligase Mdm2 controls oligodendrocyte maturation by intertwining mTOR with G protein-coupled receptor kinase 2 in the regulation of GPR17 receptor desensitization. Glia 2015, 63, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Zappelli, E.; Daniele, S.; Vergassola, M.; Ceccarelli, L.; Chelucci, E.; Mangano, G.; Durando, L.; Ragni, L.; Martini, C. A specific combination of nutraceutical Ingredients exerts cytoprotective effects in human cholinergic neurons. PharmaNutrition 2022, 22. [Google Scholar] [CrossRef]
- Daniele, S.; Costa, B.; Zappelli, E.; Da Pozzo, E.; Sestito, S.; Nesi, G.; Campiglia, P.; Marinelli, L.; Novellino, E.; Rapposelli, S.; et al. Combined inhibition of AKT/mTOR and MDM2 enhances Glioblastoma Multiforme cell apoptosis and differentiation of cancer stem cells. Sci. Rep. 2015, 5, 9956. [Google Scholar] [CrossRef]
- Costa, B.; Bendinelli, S.; Gabelloni, P.; Da Pozzo, E.; Daniele, S.; Scatena, F.; Vanacore, R.; Campiglia, P.; Bertamino, A.; Gomez-Monterrey, I.; et al. Human Glioblastoma Multiforme: p53 Reactivation by a Novel MDM2 Inhibitor. PLOS ONE 2013, 8, e72281. [Google Scholar] [CrossRef]
- Baldacci, F.; Daniele, S.; Piccarducci, R.; Giampietri, L.; Pietrobono, D.; Giorgi, F.S.; Nicoletti, V.; Frosini, D.; Libertini, P.; Gerfo, A.L.; et al. Potential Diagnostic Value of Red Blood Cells α-Synuclein Heteroaggregates in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6451–6459. [Google Scholar] [CrossRef]
- Daniele, S.; Pietrobono, D.; Fusi, J.; Iofrida, C.; Chico, L.; Petrozzi, L.; Gerfo, A.L.; Baldacci, F.; Galetta, F.; Siciliano, G.; et al. α-Synuclein Aggregates with β-Amyloid or Tau in Human Red Blood Cells: Correlation with Antioxidant Capability and Physical Exercise in Human Healthy Subjects. Mol. Neurobiol. 2017, 55, 2653–2675. [Google Scholar] [CrossRef]
- El-Agnaf, O.M.A.; Salem, S.A.; Paleologou, K.E.; Curran, M.D.; Gibson, M.J.; Court, J.A.; Schlossmacher, M.G.; Allsop, D. Detection of oligomeric forms of α-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006, 20, 419–425. [Google Scholar] [CrossRef]
- Daniele, S.; Baldacci, F.; Piccarducci, R.; Palermo, G.; Giampietri, L.; Manca, M.L.; Pietrobono, D.; Frosini, D.; Nicoletti, V.; Tognoni, G.; et al. α-Synuclein Heteromers in Red Blood Cells of Alzheimer’s Disease and Lewy Body Dementia Patients. J. Alzheimer's Dis. 2021, 80, 885–893. [Google Scholar] [CrossRef]
| Protein | Coating antibody | Primary antibody | Secondary antibody |
| Aβ1-42 | #44-344, Invitrogen Rabbit polyclonal antibody Dilution 1:1000 |
#sc-28365, Santa Cruz Biotechnology Inc Mouse monoclonal antibody Dilution 1:200 |
#31430, Invitrogen Goat anti-mouse IgG (HRP) Dilution 1:5000 |
| Aβ1-40 | #sc-53828, Santa Cruz Antibody β-Amyloid AB 1-40 (coating), mouse monoclonal (aa 1-200 APP695) Dilution 1:100 |
# 512700 Invitrogen rabbit monoclonal antibody Dilution 1:1000 |
#A0545, Sigma-Aldrich Goat anti-rabbit IgG (HRP) Dilution 1:5000 |
| t-tau | #sc-32274, Santa Cruz Biotechnology Inc Mouse monoclonal antibody (recognizing C-terminal) Dilution 1:100 |
ab109392, abcam Rabbit polyclonal antibody Dilution 1:1000 |
#A0545, Sigma-Aldrich Goat anti-rabbit IgG (HRP) Dilution 1:5000 |
| α-syn | NBP2-15365, Alpha-Synuclein Antibody Rabbit polyclonal antibody Dilution 1:100 |
# sc-12767, Santa Cruz Mouse monoclonal antibody Dilution 1:200 |
#31430, Invitrogen Goat anti-mouse IgG (HRP) Dilution 1:5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





