Submitted:
01 October 2025
Posted:
02 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
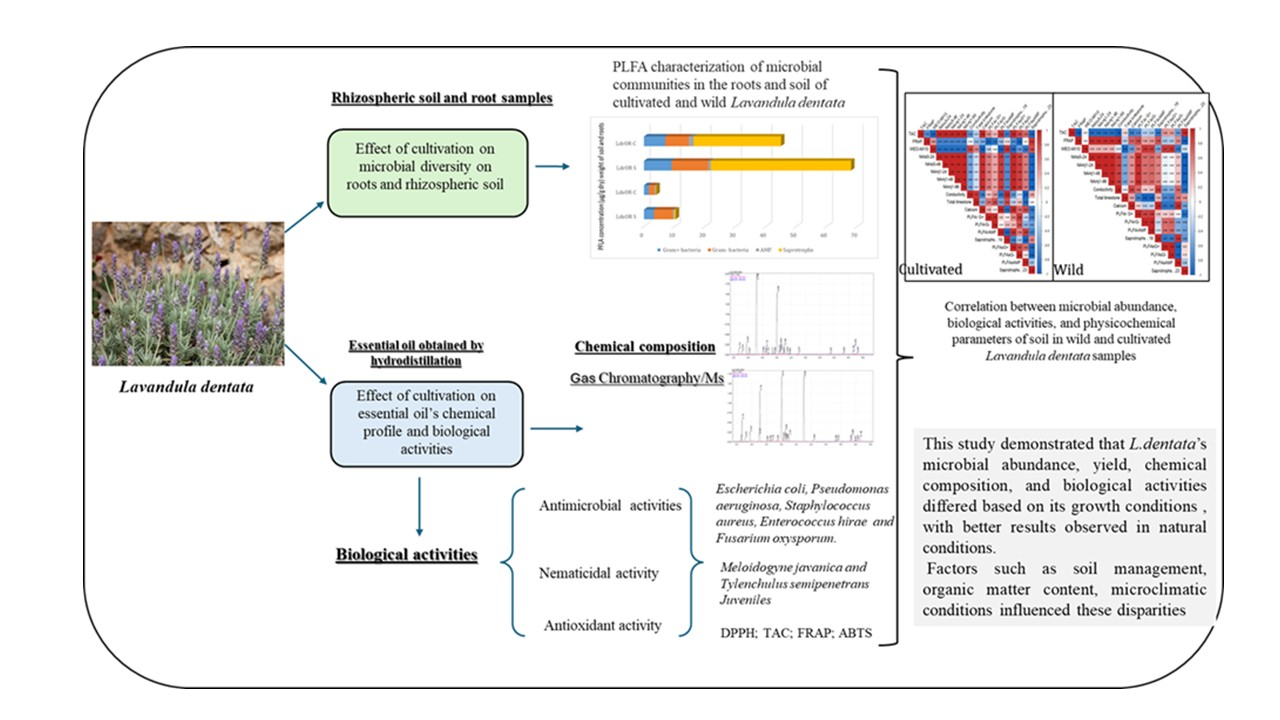
2. Results
2.1. Essential Oil Composition
2.2. Soil Physicochemical Properties
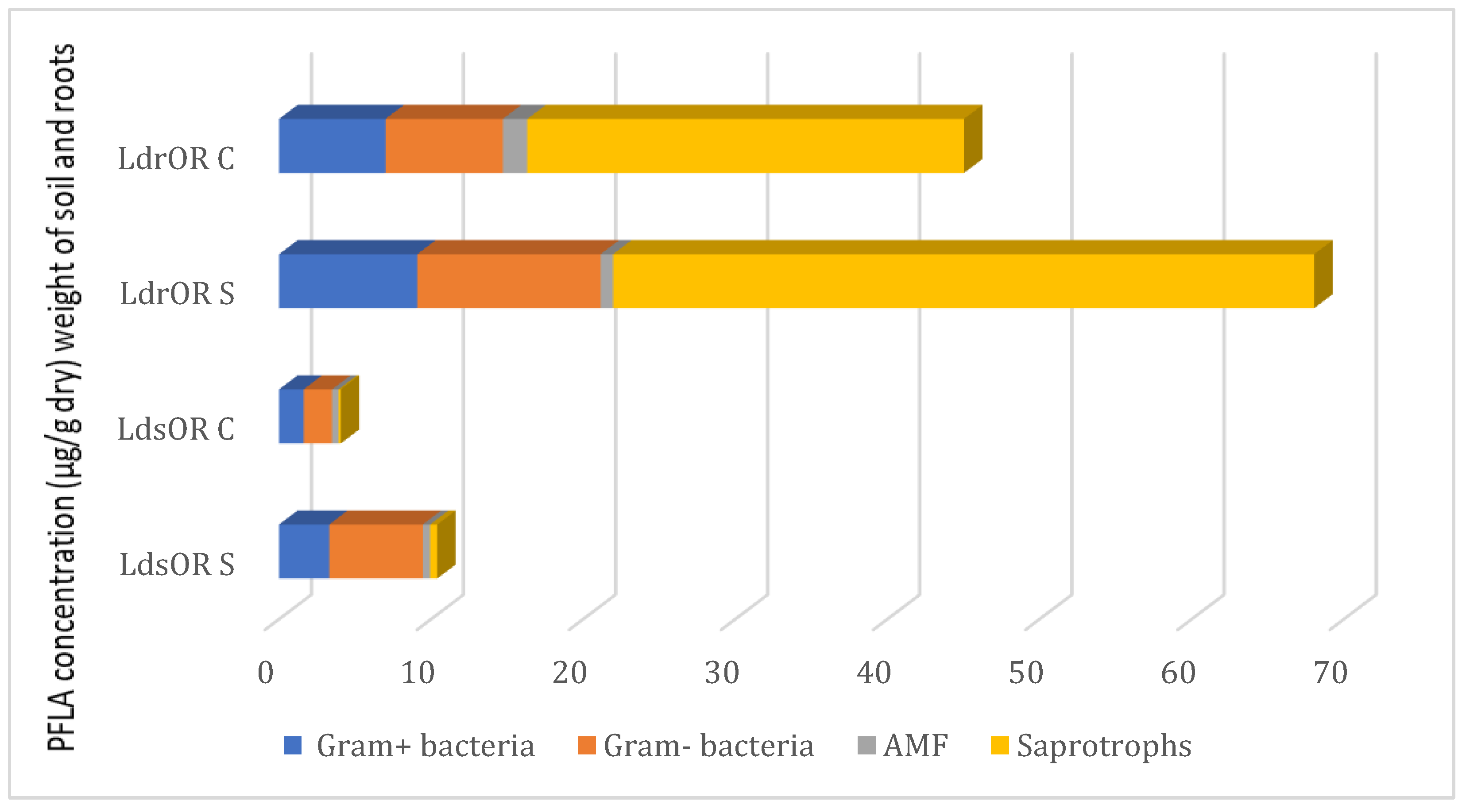
2.3. Microbial Diversity in Soil and Roots
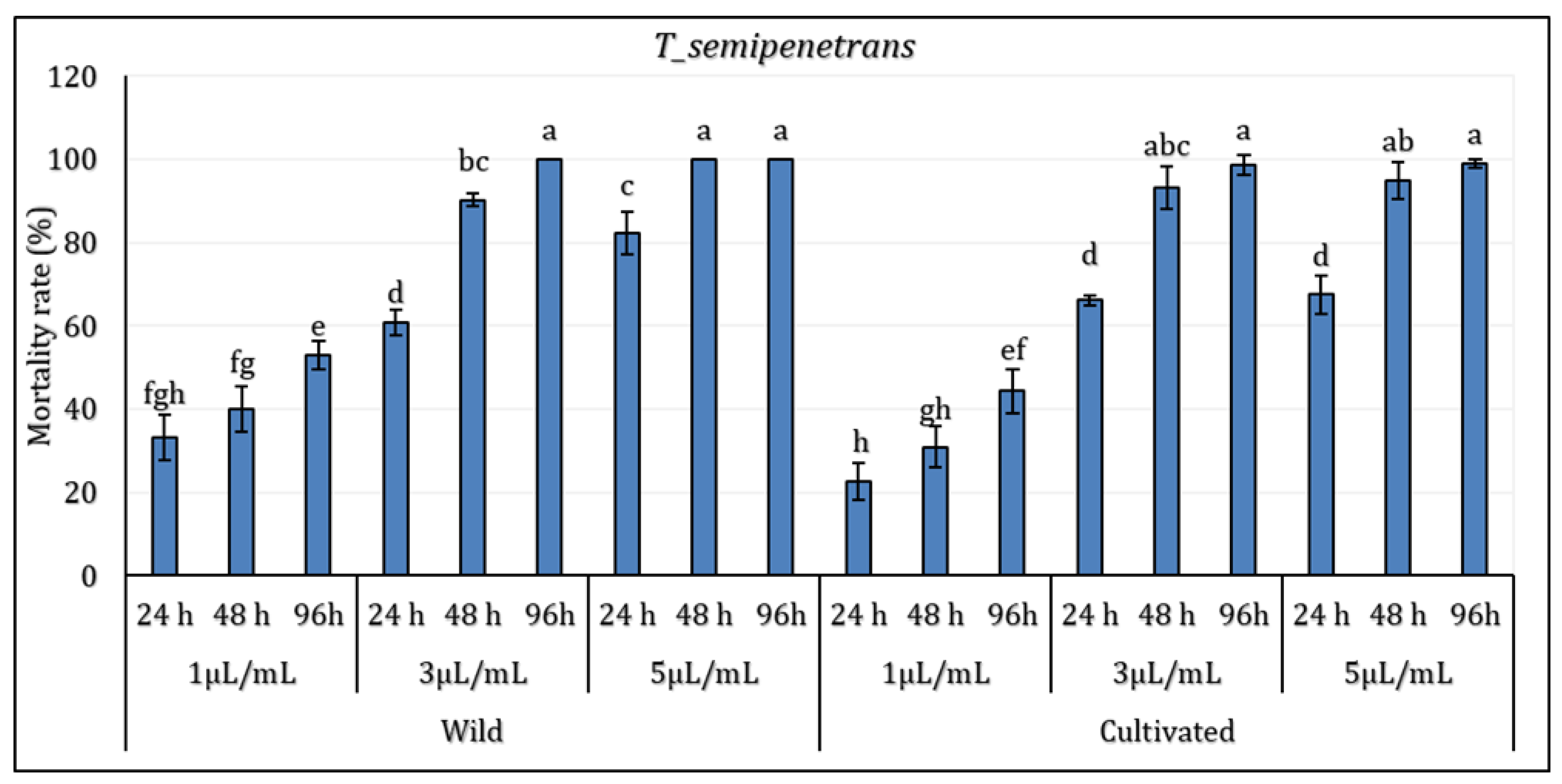
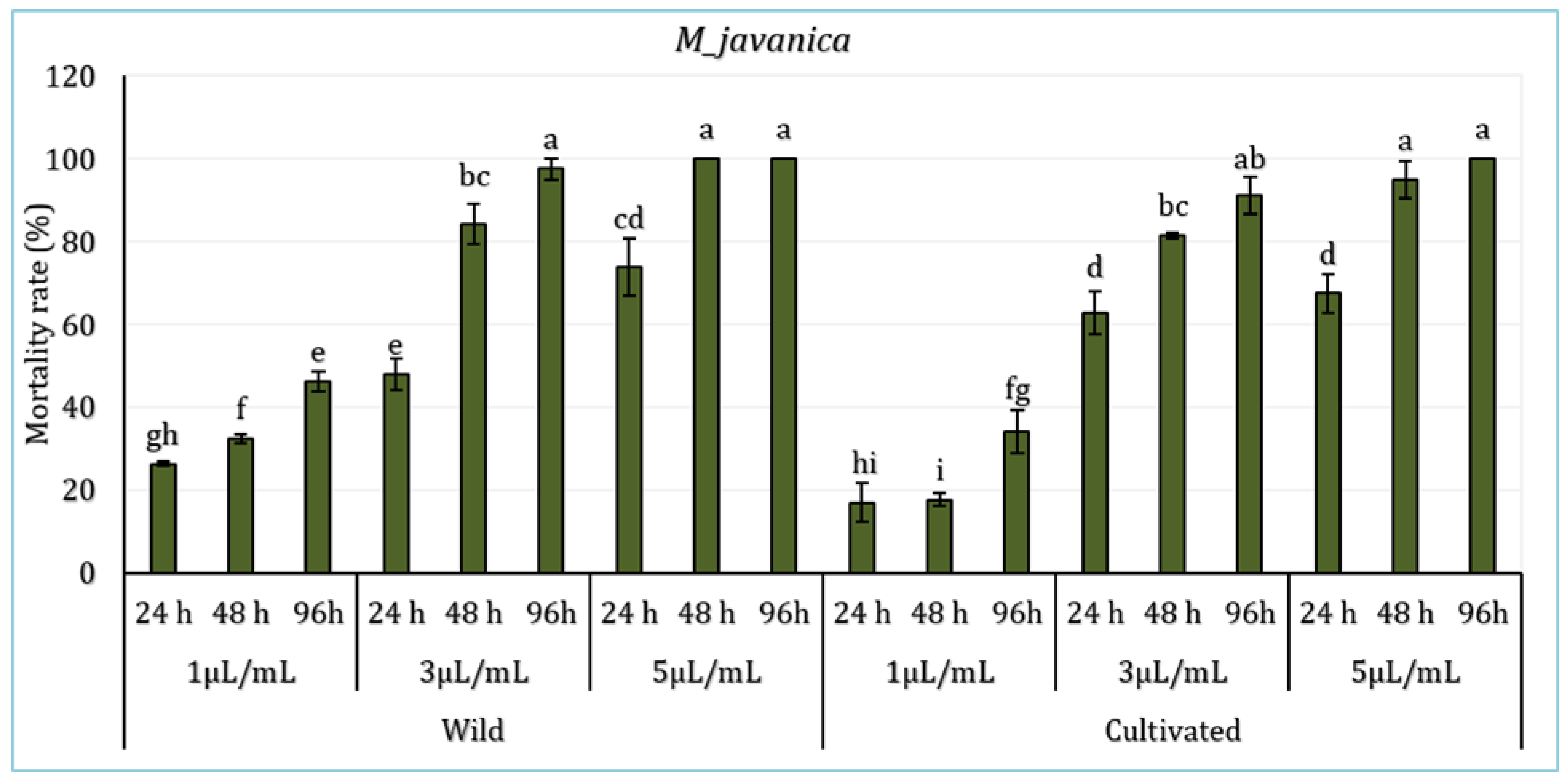
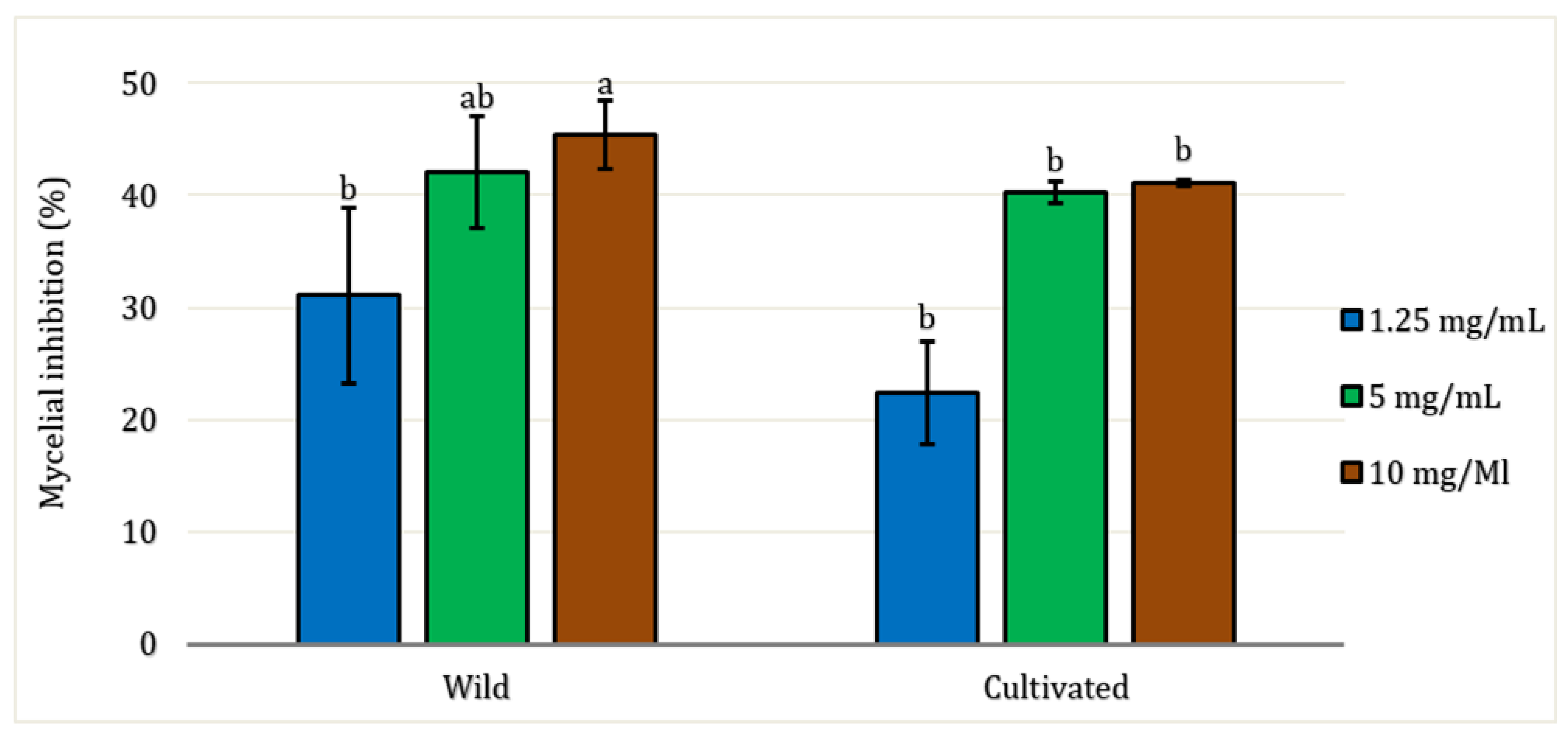
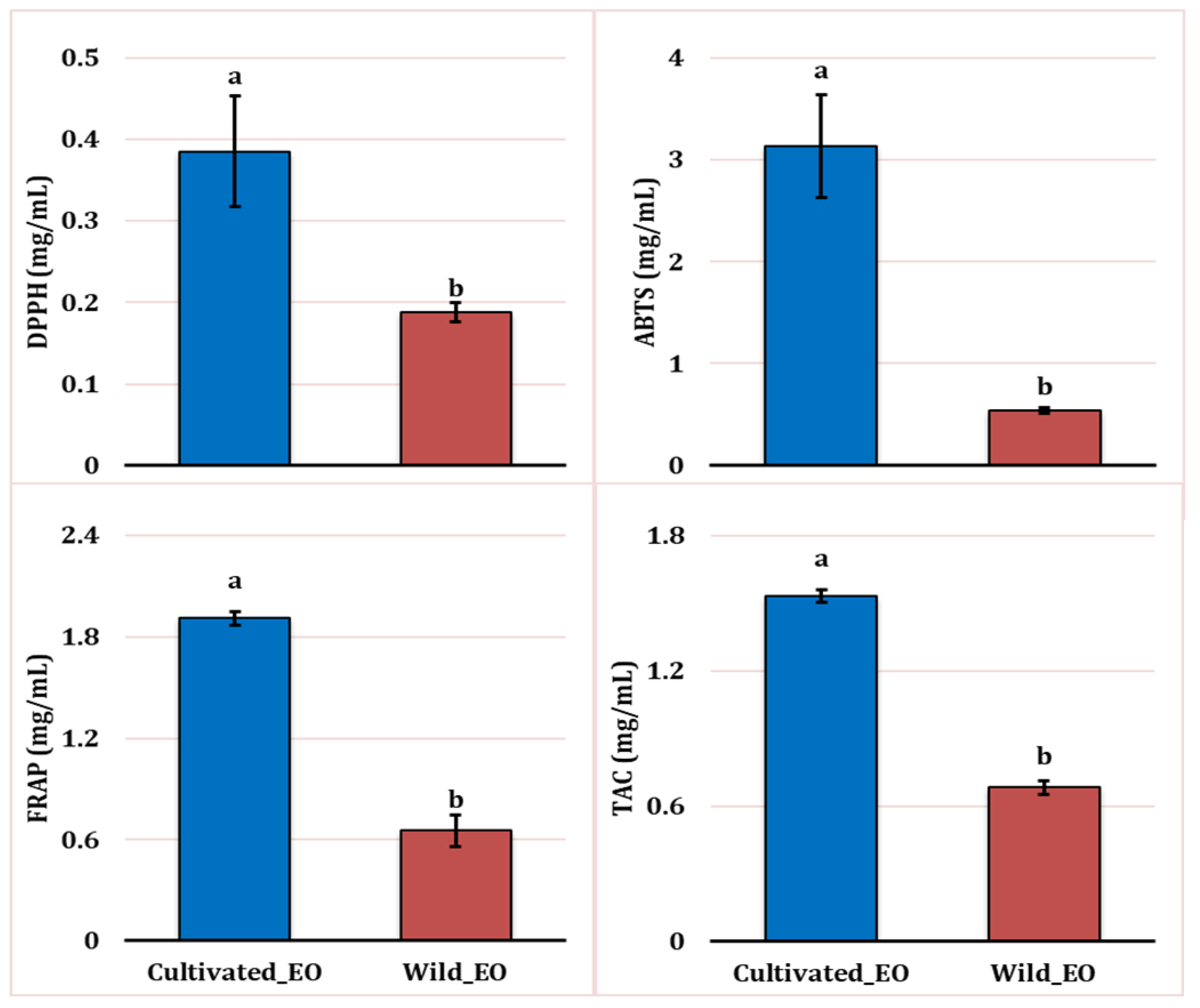
2.4. Biological Activities
- Nematicidal Activity:
3.6. Heatmap Correlation Analysis
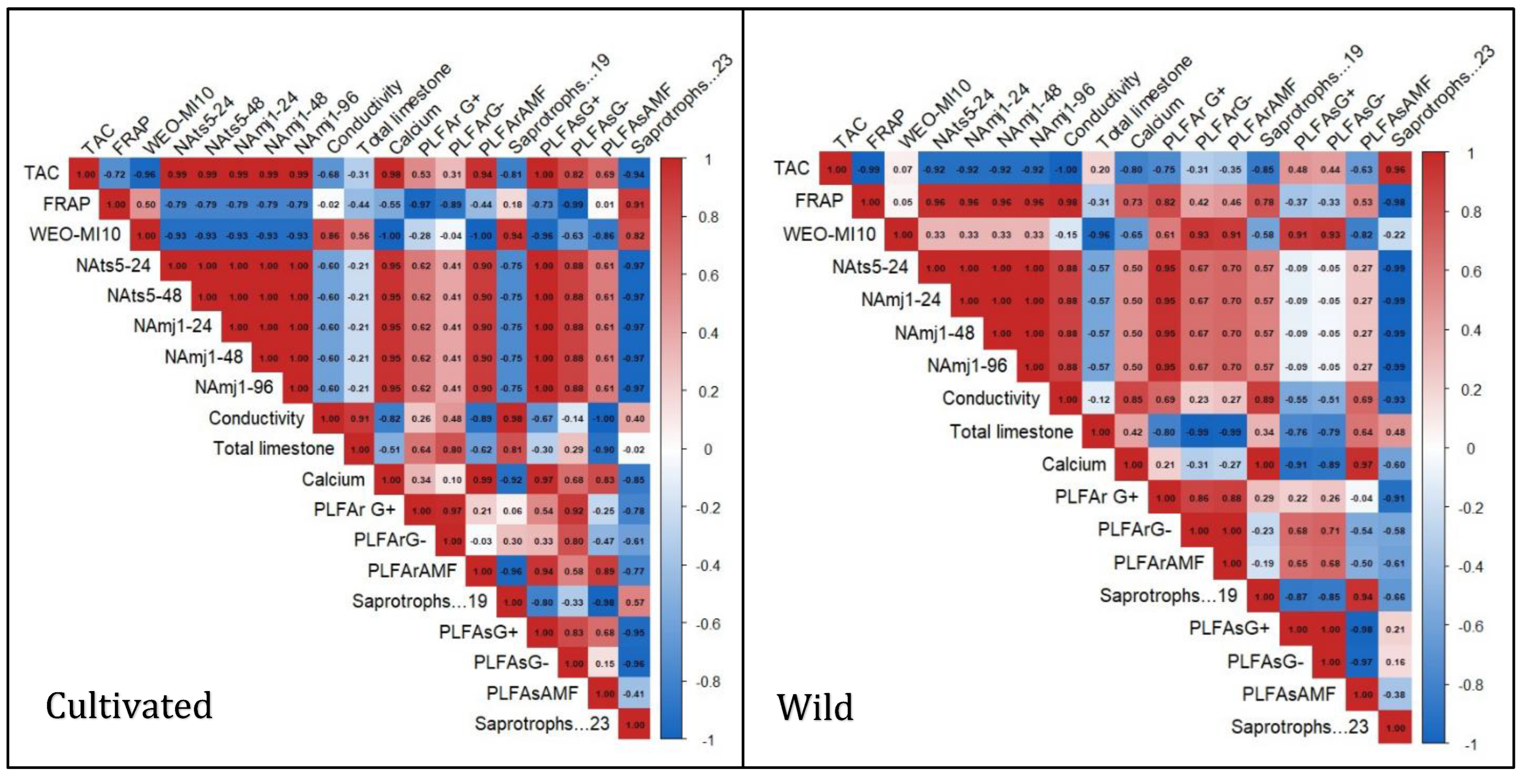
3. Discussion
4. Materials and Methods
4.1. Study Site and Sampling
4.2. Soil Physicochemical Properties Analysis
4.3. Essential Oils Extraction and GC-MS Analysis:
4.4. Analysis of Soil Fatty Acids
4.5. Biological Activity Assessment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaytor, D. A. (1937). A taxonomic study of the genus Lavandula. Botanical Journal of the Linnean Society, 51(338), 153-204.Author 1, A.; Author 2, B. Title of the chapter. In Book Title, 2nd ed.; Editor 1, A., Editor 2, B., Eds.; Publisher: Publisher Location, Country, 2007; Volume 3, pp. 154–196.
- Lamrani-Alaoui, M., & Hassikou, R. (2018). Rapid risk assessment to harvesting of wild medicinal and aromatic plant species in Morocco for conservation and sustainable management purposes. Biodiversity and Conservation, 27(10), 2729-2745.Author 1, A.B.; Author 2, C. Title of Unpublished Work. Abbreviated Journal Name year, phrase indicating stage of publication (submitted; accepted; in press).
- González-Coloma, A., Delgado, F., Rodilla, J. M., Silva, L., Sanz, J., & Burillo, J. (2011). Chemical and biological profiles of Lavandula luisieri essential oils from western Iberia Peninsula populations. Biochemical Systematics and Ecology, 39(1), 1-8.
- Touati, B., Chograni, H., Hassen, I., Boussaïd, M., Toumi, L., & Brahim, N. B. (2011). Chemical composition of the leaf and flower essential oils of Tunisian Lavandula dentata L.(Lamiaceae). Chemistry & Biodiversity, 8(8), 1560-1569.
- Gallotte, P., Fremondière, G., Gallois, P., Bernier, J. P. B., Buchwalder, A., Walton, A., ... & Fopa-Fomeju, B. (2020). Lavandula angustifolia mill. And Lavandula x intermedia emeric ex loisel: lavender and lavandin. In Medicinal, Aromatic and Stimulant Plants (pp. 303-311). Cham: Springer International Publishing.
- Rezaei, M. N., Dornez, E., Jacobs, P., Parsi, A., Verstrepen, K. J., & Courtin, C. M. (2014). Harvesting yeast (Saccharomyces cerevisiae) at different physiological phases significantly affects its functionality in bread dough fermentation. Food microbiology, 39, 108-115.
- Algieri, F., Rodriguez-Nogales, A., Vezza, T., Garrido-Mesa, J., Garrido-Mesa, N., Utrilla, M. P., ... & Galvez, J. (2016). Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. Journal of ethnopharmacology, 190, 142-158.
- Wagner, L. S., Sequin, C. J., Foti, N., & Campos-Soldini, M. P. (2021). Insecticidal, fungicidal, phytotoxic activity and chemical composition of Lavandula dentata essential oil. Biocatalysis and Agricultural Biotechnology, 35, 102092.
- Zuzarte, M., Vale-Silva, L., Gonçalves, M. J., Cavaleiro, C., Vaz, S., Canhoto, J., ... & Salgueiro, L. (2012). Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. European Journal of Clinical Microbiology & Infectious Diseases, 31(7), 1359-1366.
- Toda, M., & Matsuse, R. (2020). Endocrinological effect of lavender aromatherapy on stressful visual stimuli. Contemporary Clinical Trials Communications, 17, 100547.
- Zhang, S., Zhang, L., Zou, H., Qiu, L., Zheng, Y., Yang, D., & Wang, Y. (2021). Effects of light on secondary metabolite biosynthesis in medicinal plants. Frontiers in plant science, 12, 781236.
- Selwal, N., Rahayu, F., Herwati, A., Latifah, E., Suhara, C., Suastika, I. B. K., ... & Wani, A. K. (2023). Enhancing secondary metabolite production in plants: Exploring traditional and modern strategies. Journal of agriculture and food research, 14, 100702.
- Akachoud, O., Bouamama, H., Laruelle, F., Facon, N., Broudi, S. E., Houssayni, S., ... & Qaddoury, A. (2024). The developmental stage and arbuscular mycorrhizal symbiosis influence the essential oil yield, chemical profile, and biological activities in Thymus pallidus, T. satureioides, and Lavandula dentata. Industrial Crops and Products, 220, 119188.
- Hamilton, A. C. (2004). Medicinal plants, conservation and livelihoods. Biodiversity & Conservation, 13(8), 1477-1517.
- Abbad, A., Belaqziz, R., Bekkouche, K., & Markouk, M. (2011). Influence of temperature and water potential on laboratory germination of two Moroccan endemic thymes: Thymus maroccanus Ball. and Thymus broussonetii Boiss. Afr. J. Agric. Res, 6(20), 4740-4745.
- El-Bakkal, S. E., Zeroual, S., Elouazkiti, M., Mansori, M., Bouamama, H., Zehhar, N., & El-Kaoua, M. (2020). Comparison of yield chemical composition and biological activities of essential oils obtained from thymus pallidus and thymus satureioides Coss. grown in wild and cultivated conditions in Morocco. Journal of Essential Oil Bearing Plants, 23(1), 1-14.
- Lubbe, A., Verpoorte, R., 2011. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 34, 785–801.
- Carrión, V. J., Perez-Jaramillo, J., Cordovez, V., Tracanna, V., De Hollander, M., Ruiz-Buck, D., ... & Raaijmakers, J. M. (2019). Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science, 366(6465), 606-612.
- De Vries, F. T., Griffiths, R. I., Knight, C. G., Nicolitch, O., & Williams, A. (2020). Harnessing rhizosphere microbiomes for drought-resilient crop production. Science, 368(6488), 270-274.
- Liu, H., Brettell, L. E., Qiu, Z., & Singh, B. K. (2020). Microbiome-mediated stress resistance in plants. Trends in plant science, 25(8), 733-743.
- Zhang, S., Li, M., Cui, X., & Pan, Y. (2023). Effect of different straw retention techniques on soil microbial community structure in wheat–maize rotation system. Frontiers in Microbiology, 13, 1069458.
- Akachoud, O., Bouamama, H., Facon, N., Laruelle, F., Zoubi, B., Benkebboura, A., ... & Lounès-Hadj Sahraoui, A. (2022). Mycorrhizal inoculation improves the quality and productivity of essential oil distilled from three aromatic and medicinal plants: Thymus satureioides, Thymus pallidus, and Lavandula dentata. Agronomy, 12(9), 2223.
- Khmelevtsova, L. E., Sazykin, I. S., Azhogina, T. N., & Sazykina, M. A. (2022). Influence of agricultural practices on bacterial community of cultivated soils. Agriculture, 12(3), 371.
- Pandey, A., & Singh, S. (2016). Aloe vera: A systematic review of its industrial and ethno-medicinal efficacy. International Journal of Pharmaceutical Research & Allied Sciences, 5(1), 21-33.
- Lerotholi, L., Chaudhary, S. K., Combrinck, S., & Viljoen, A. (2017). Bush tea (Athrixia phylicoides): A review of the traditional uses, bioactivity and phytochemistry. South African Journal of Botany, 110, 4-17.
- Thakur, M., Bhattacharya, S., Khosla, P. K., & Puri, S. (2019). Improving production of plant secondary metabolites through biotic and abiotic elicitation. Journal of Applied Research on Medicinal and Aromatic Plants, 12, 1-12.
- Martins, R. D. P., Gomes, R. A. D. S., Malpass, A. C. G., & Okura, M. H. (2019). Chemical characterization of Lavandula dentata L. essential oils grown in Uberaba-MG. Ciência Rural, 49, e20180964.
- El Abdali, Y., Agour, A., Allali, A., Bourhia, M., El Moussaoui, A., Eloutassi, N., ... & Bouia, A. (2022). Lavandula dentata L.: phytochemical analysis, antioxidant, antifungal and insecticidal activities of its essential oil. Plants, 11(3), 311.
- Belcadi, H., Aknouch, A., Chraka, A., Kassout, J., Lachkar, M., Mouhib, M., & Mansour, A. I. (2024). Moroccan Lavandula dentata L. essential oil: γ-Irradiation effect on the chemical composition and antibacterial activity. Scientific African, 23, e02087.
- Sile, I., Krizhanovska, V., Nakurte, I., Mezaka, I., Kalane, L., Filipovs, J., ... & Kronberga, A. (2022). Wild-grown and cultivated Glechoma hederacea L.: Chemical composition and potential for cultivation in organic farming conditions. Plants, 11(6), 819.
- Abdellaoui, M., Derouich, M., & El-Rhaffari, L. (2020). Essential oil and chemical composition of wild and cultivated fennel (Foeniculum vulgare Mill.): A comparative study. South African Journal of Botany, 135, 93-100.
- Atyane, L. H., Lagram, K., Ben El Caid, M., Lachheb, M., Salaka, L., Serghini, M. A., & Elmaimouni, L. (2016, November). Study of the influence of geographical origin and environment conditions on the three secondary metabolites of Moroccan saffron by UV-visible spectrometry. In V International Symposium on Saffron Biology and Technology: Advances in Biology, Technologies, Uses and Market 1184 (pp. 267-272).
- Kumar, S., Saini, R., Suthar, P., Kumar, V., & Sharma, R. (2022). Plant secondary metabolites: Their food and therapeutic importance. In Plant secondary metabolites: Physico-chemical properties and therapeutic applications (pp. 371-413). Singapore: Springer Nature Singapore.
- Chrysargyris, A., & Tzortzakis, N. (2025). Nitrogen, Phosphorus, and Potassium Requirements to Improve Portulaca oleracea L. Growth, Nutrient and Water Use Efficiency in Hydroponics. Agronomy, 15(1), 111.
- Villette, J., Cuéllar, T., Verdeil, J. L., Delrot, S., & Gaillard, I. (2020). Grapevine potassium nutrition and fruit quality in the context of climate change. Frontiers in Plant Science, 11, 123.
- Willy, D. K., Muyanga, M., Mbuvi, J., & Jayne, T. (2019). The effect of land use change on soil fertility parameters in densely populated areas of Kenya. Geoderma, 343, 254-262.
- Niinemets, Ü., Seufert, G., Steinbrecher, R., & Tenhunen, J. D. (2002). A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean evergreen Quercus species. New Phytologist, 153(2), 257-275.
- Erbaş, S., Kucukyumuk, Z., Baydar, H., Erdal, İ., & Sanlı, A. (2017). Effects of different phosphorus doses on nutrient concentrations as well as yield and quality characteristics of lavandin (Lavandula× intermedia Emeric ex Loisel. var. Super). Turkish Journal of Field Crops, 22(1), 32-38.
- Amanuel, W., Yimer, F., & Karltun, E. (2018). Soil organic carbon variation in relation to land use changes: the case of Birr watershed, upper Blue Nile River Basin, Ethiopia. Journal of Ecology and Environment, 42(1), 16.
- Murindangabo, Y. T., Kopecký, M., Hoang, T. N., Bernas, J., Parajuli, T., Dhakal, S., ... & Shrestha, A. K. (2023). Comparative analysis of soil organic matter fractions, lability, stability ratios, and carbon management index in various land use types within bharatpur catchment, Chitwan District, Nepal. Carbon Balance and Management, 18(1), 21.
- Gerke, J. (2022). The central role of soil organic matter in soil fertility and carbon storage. Soil Systems, 6(2), 33.
- Hoffland, E., Kuyper, T. W., Comans, R. N., & Creamer, R. E. (2020). Eco-functionality of organic matter in soils. Plant and Soil, 455(1), 1-22.
- Chaouqi, S., Moratalla-López, N., Alonso, G. L., Lorenzo, C., Zouahri, A., Asserar, N., ... & Guedira, T. (2023). Effect of soil composition on secondary metabolites of moroccan saffron (Crocus sativus L.). Plants, 12(4), 711.
- Blanch, J. S., Peñuelas, J., & Llusià, J. (2007). Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiologia Plantarum, 131(2), 211-225.
- Ormeño, E., Goldstein, A., & Niinemets, Ü. (2011). Extracting and trapping biogenic volatile organic compounds stored in plant species. TrAC Trends in Analytical Chemistry, 30(7), 978-989.
- Koeduka, T., Fridman, E., Gang, D. R., Vassao, D. G., Jackson, B. L., Kish, C. M., ... & Pichersky, E. (2006). Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proceedings of the National Academy of Sciences, 103(26), 10128-10133.
- Hazrati, S., Mousavi, Z., Mollaei, S., Sedaghat, M., Mohammadi, M., Pignata, G., & Nicola, S. (2024). Optimizing Nitrogen Fertilization to Maximize Yield and Bioactive Compounds in Ziziphora clinopodioides. Agriculture, 14(10), 1690.
- Santoyo, G., Urtis-Flores, C. A., Loeza-Lara, P. D., Orozco-Mosqueda, M. D. C., & Glick, B. R. (2021). Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology, 10(6), 475.
- Sasse, J., Martinoia, E., & Northen, T. (2018). Feed your friends: do plant exudates shape the root microbiome. Trends in plant science, 23(1), 25-41.
- Olanrewaju, O. S., Ayangbenro, A. S., Glick, B. R., & Babalola, O. O. (2019). Plant health: feedback effect of root exudates-rhizobiome interactions. Applied microbiology and biotechnology, 103(3), 1155-1166.
- Andreote, F. D., Gumiere, T., & Durrer, A. (2014). Exploring interactions of plant microbiomes. Scientia agrícola, 71, 528-539.
- Potthoff, M., Steenwerth, K. L., Jackson, L. E., Drenovsky, R. E., Scow, K. M., & Joergensen, R. G. (2006). Soil microbial community composition as affected by restoration practices in California grassland. Soil Biology and Biochemistry, 38(7), 1851-1860.
- Yang, W., Yan, Y., Jiang, F., Leng, X., Cheng, X., & An, S. (2016). Response of the soil microbial community composition and biomass to a short-term Spartina alterniflora invasion in a coastal wetland of eastern China. Plant and Soil, 408(1), 443-456.
- Manral, V., Bargali, K., Bargali, S. S., & Shahi, C. (2020). Changes in soil biochemical properties following replacement of Banj oak forest with Chir pine in Central Himalaya, India. Ecological Processes, 9(1), 30.
- Shao, W., Li, M., Wu, Y., Ma, X., Song, Q., Zhang, Y., ... & Dong, J. (2022). Identification of varied soil hydraulic properties in a seasonal tropical rainforest. Catena, 212, 106104.
- Chiba, A., Uchida, Y., Kublik, S., Vestergaard, G., Buegger, F., Schloter, M., & Schulz, S. (2021). Soil bacterial diversity is positively correlated with decomposition rates during early phases of maize litter decomposition. Microorganisms, 9(2), 357.
- Zhou, J., Guan, D., Zhou, B., Zhao, B., Ma, M., Qin, J., ... & Li, J. (2015). Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biology and Biochemistry, 90, 42-51.
- Huang, Q., Wang, J., Wang, C., & Wang, Q. (2019). The 19-years inorganic fertilization increased bacterial diversity and altered bacterial community composition and potential functions in a paddy soil. Applied Soil Ecology, 144, 60-67.
- Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G., ... & Fierer, N. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME journal, 4(10), 1340-1351.
- Wang, N., Zhang, T., Li, Y., Cong, A., Lian, J., & Feng, K. (2025). Integrated application of fertilization increased maize (Zea mays L.) yield by improving soil quality, particularly under limited water conditions in a semi-arid sandy area. Agricultural Water Management, 309, 109334.
- Fang, X., Wang, M., Zhou, X., Wang, H., Wang, H., & Xiao, H. (2022). Effects of growth years on ginsenoside biosynthesis of wild ginseng and cultivated ginseng. BMC genomics, 23(1), 325.
- Kokkoris, V., Hamel, C., & Hart, M. M. (2019). Mycorrhizal response in crop versus wild plants. PloS one, 14(8), e0221037.
- Jackson, L. E., Miller, D., & Smith, S. E. (2002). Arbuscular mycorrhizal colonization and growth of wild and cultivated lettuce in response to nitrogen and phosphorus. Scientia Horticulturae, 94(3-4), 205-218.
- Rillig, M. C., Sosa-Hernández, M. A., Roy, J., Aguilar-Trigueros, C. A., Vályi, K., & Lehmann, A. (2016). Towards an integrated mycorrhizal technology: harnessing mycorrhiza for sustainable intensification in agriculture. Frontiers in Plant Science, 7, 1625.
- Hontoria, C., García-González, I., Quemada, M., Roldán, A., & Alguacil, M. M. (2019). The cover crop determines the AMF community composition in soil and in roots of maize after a ten-year continuous crop rotation. Science of the Total Environment, 660, 913-922.
- Strom N, Hu W, Haarith D, Chen S, Bushley K. 2020. Interactions between soil properties, fungal communities, the soybean cyst nematode, and crop yield under continuous corn and soybean monoculture. Applied Soil Ecology 147: 103388.
- Yuan, H., Ma, Q., Ye, L., & Piao, G. (2016). The traditional medicine and modern medicine from natural products. Molecules, 21(5), 559.
- Zhai, X., Jia, M., Chen, L., Zheng, C. J., Rahman, K., Han, T., & Qin, L. P. (2017). The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Critical reviews in microbiology, 43(2), 238-261.
- Zhou, J. Y., Li, X., Zheng, J. Y., & Dai, C. C. (2016). Volatiles released by endophytic Pseudomonas fluorescens promoting the growth and volatile oil accumulation in Atractylodes lancea. Plant Physiology and Biochemistry, 101, 132-140.
- Zheng, L. P., Tian, H., Yuan, Y. F., & Wang, J. W. (2016). The influence of endophytic Penicillium oxalicum B4 on growth and artemisinin biosynthesis of in vitro propagated plantlets of Artemisia annua L. Plant Growth Regulation, 80(1), 93-102.
- Wang, X. M., Yang, B., Ren, C. G., Wang, H. W., Wang, J. Y., & Dai, C. C. (2015). Involvement of abscisic acid and salicylic acid in signal cascade regulating bacterial endophyte-induced volatile oil biosynthesis in plantlets of Atractylodes lancea. Physiologia plantarum, 153(1), 30-42.
- Chen, L., & Liu, Y. (2024). The function of root exudates in the root colonization by beneficial soil rhizobacteria. Biology, 13(2), 95.
- Kashid, S., Joshi, K., More, S., Shinde, A., & Nene, S. (2023). Enhanced Productivity of Fragrance Compounds: Biotransformation of d-limonene Using Whole Cell Immobilization of Pseudomonas putida and Rhodococcus erythropolis. Journal of The Institution of Engineers (India): Series E, 104(1), 83-93.
- Epand, R. M., Walker, C., Epand, R. F., & Magarvey, N. A. (2016). Molecular mechanisms of membrane targeting antibiotics. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1858(5), 980-987.
- Gachkar, L., Yadegari, D., Rezaei, M. B., Taghizadeh, M., Astaneh, S. A., & Rasooli, I. (2007). Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food chemistry, 102(3), 898-904.
- Su, J., Chen, J., Liao, S., Li, L., Zhu, L., & Chen, L. (2012). Composition and biological activities of the essential oil extracted from a novel plant of Cinnamomum camphora Chvar. Borneol. J Med Plants Res, 6(18), 3487-3494.
- Leite-Sampaio, N. F., Gondim, C. N. F., de Souza, C. E. S., & Coutinho, H. D. (2022). Antibiotic potentiating action of α-PINENE and borneol against EPEC and ETEC sorotypes. Microbial Pathogenesis, 162, 105371.
- Yu, H., Ren, X., Yang, F., Xie, Y., Guo, Y., Cheng, Y., & Yao, W. (2022). Antimicrobial and anti-dust mite efficacy of Cinnamomum camphora chvar. Borneol essential oil using pilot-plant neutral cellulase-assisted steam distillation. Letters in Applied Microbiology, 74(2), 258-267.
- Ma, R., Lu, D., Wang, J., Xie, Q., & Guo, J. (2023). Comparison of pharmacological activity and safety of different stereochemical configurations of borneol: L-borneol, D-borneol, and synthetic borneol. Biomedicine & Pharmacotherapy, 164, 114668.
- Soković, M., Glamočlija, J., Marin, P. D., Brkić, D., & Van Griensven, L. J. (2010). Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules, 15(11), 7532-7546.
- Rosato, A., Vitali, C., De Laurentis, N., Armenise, D., & Milillo, M. A. (2007). Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine, 14(11), 727-732.
- Farhanghi, A., Aliakbarlu, J., Tajik, H., Mortazavi, N., Manafi, L., & Jalilzadeh-Amin, G. (2022). Antibacterial interactions of pulegone and 1, 8-cineole with monolaurin ornisin against Staphylococcus aureus. Food Science & Nutrition, 10(8), 2659-2666.
- Sun, Y., Cai, X., Cao, J., Wu, Z., & Pan, D. (2018). Effects of 1, 8-cineole on carbohydrate metabolism related cell structure changes of Salmonella. Frontiers in microbiology, 9, 1078.
- Chen, J., Tang, C., Zhou, Y., Zhang, R., Ye, S., Zhao, Z., ... & Yang, D. (2020). Anti-inflammatory property of the essential oil from Cinnamomum camphora (Linn.) Presl leaves and the evaluation of its underlying mechanism by using metabolomics analysis. Molecules, 25(20), 4796.
- Patterson, A. D., Carlson, B. A., Li, F., Bonzo, J. A., Yoo, M. H., Krausz, K. W., ... & Hatfield, D. L. (2013). Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chemical research in toxicology, 26(7), 1088-1096.
- Hachlafi, N. E., Aanniz, T., Menyiy, N. E., Baaboua, A. E., Omari, N. E., Balahbib, A., ... & Bouyahya, A. (2023). In vitro and in vivo biological investigations of camphene and its mechanism insights: a review. Food Reviews International, 39(4), 1799-1826.
- Ruberto, G., & Baratta, M. T. (2000). Antioxidant activity of selected essential oil components in two lipid model systems. Food chemistry, 69(2), 167-174.
- Badawy, M. E., Marei, G. I. K., Rabea, E. I., & Taktak, N. E. (2019). Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pesticide Biochemistry and Physiology, 158, 185-200.
- Zhang, Y., Tian, Z., Huang, T., Lei, L., & Zuo, Z. (2025). Sesquiterpene emissions from four chemotypes of Cinnamomum camphora in different seasons. Industrial Crops and Products, 225, 120505.
- Ortiz de Elguea-Culebras, G., Sánchez-Vioque, R., Berruga, M. I., Herraiz-Peñalver, D., González-Coloma, A., Andrés, M. F., & Santana-Méridas, O. (2018). Biocidal potential and chemical composition of industrial essential oils from Hyssopus officinalis, Lavandula× intermedia var. super, and Santolina chamaecyparissus. Chemistry & Biodiversity, 15(1), e1700313.
- D’Addabbo, T., Laquale, S., Argentieri, M. P., Bellardi, M. G., & Avato, P. (2021). Nematicidal activity of essential oil from lavandin (Lavandula× intermedia Emeric ex Loisel.) as related to chemical profile. Molecules, 26(21), 6448.
- Uludamar, E. B. K. (2023). Screening of the nematicidal potential of some essential oils against the Columbia root-knot nematode, Meloidogyne chitwoodi. Çukurova Tarım ve Gıda Bilimleri Dergisi, 38(2), 345-350.
- Sarri, K., Mourouzidou, S., Ntalli, N., & Monokrousos, N. (2024). Recent advances and developments in the nematicidal activity of essential oils and their components against root-knot nematodes. Agronomy, 14(1), 213.
- Dutta, A., Mandal, A., Kundu, A., Malik, M., Chaudhary, A., Khan, M. R., ... & Singh, A. (2021). Deciphering the behavioral response of Meloidogyne incognita and Fusarium oxysporum toward mustard essential oil. Frontiers in Plant Science, 12, 714730.
- Padilla-Montaño, N., de León Guerra, L., & Moujir, L. (2021). Antimicrobial activity and mode of action of celastrol, a nortriterpen quinone isolated from natural sources. Foods, 10(3), 591.
- Caballero-Gallardo, K., Olivero-Verbel, J., Nayive, P. B., & Stashenko, E. E. (2014). Chemical composition and bioactivity of Piper auritum and P. multiplinervium essential oils against the red flour beetle, Tribolium castaneum (Herbst). Boletín Latinoamericano y del Caribe de Plantas Medicinales y aromáticas, 13(1), 10-19.
- Zore, G. B., Thakre, A. D., Jadhav, S., & Karuppayil, S. M. (2011). Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine, 18(13), 1181-1190.
- de Macêdo Andrade, A. C., Rosalen, P. L., Freires, I. A., Scotti, L., Scotti, M. T., Aquino, S. G., & de Castro, R. D. (2018). Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-β-pinene enantiomers against Candida spp. Current topics in medicinal chemistry, 18(29), 2481-2490.
- Richards, L. A. (Ed.). (1954). Diagnosis and improvement of saline and alkali soils (No. 60). US Government Printing Office.
- Aubert, G. (1978). Méthodes d’analyse des Sols Edition CRDP Marseille.
- Barbano, D. M., Clark, J. L., Dunham, C. E., & Flemin, R. J. (1990). Kjeldahl method for determination of total nitrogen content of milk: collaborative study. Journal of the Association of Official Analytical Chemists, 73(6), 849-859.
- Olsen, S. R., Watanabe, F. S., & Bowman, R. A. (1983). Evaluation of fertilizer phosphate residues by plant uptake and extractable phosphorus. Soil Science Society of America Journal, 47(5), 952-958.
- Michel-Dewez, N., & Ek, C. (1982). Méthode rapide de caractérisation des dolomies et calcaires magnésiens: la gaz-volumétrie. Bulletin de la Société Géographique de Liège, 18.
- Brown, J. G., & Lilleland, O. M. U. N. D. (1946). Rapid determination of potassium and sodium in plant materials and soil extracts by flame photometry.
- Güllüce, M., Sökmen, M., Daferera, D. I. M. I. T. R. A., Aǧar, G., Özkan, H., Kartal, N. U. K. E. T., ... & Şahin, F. (2003). In vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L. Journal of agricultural and food chemistry, 51(14), 3958-3965.
- Greco, N., & D'Addabbo, T. (1990). Efficient procedure for extracting Tylenchulus semipenetrans from citrus roots. Journal of nematology, 22(4), 590.
- Taylor, A. L., & Sasser, J. N. (1978). Biology, identification and control of root-knot nematodes. North Carolina State University Graphics, 111.
- Yang, Y. M., Liu, P., Dong, H., Zhang, W. T., & Hu, X. Q. (2020). Pathogen identification of Eupatorium adenophorum root-knot nematode disease in Yunnan Province.
- 109. Hooper.
- Mc Donnell, R., Yoo, J., Patel, K., Rios, L., Hollingsworth, R., Millar, J., & Paine, T. (2016). Can essential oils be used as novel drench treatments for the eggs and juveniles of the pest snail Cornu aspersum in potted plants?. Journal of pest science, 89(2), 549-555.
- Zoubi, B., Mokrini, F., Amer, M., Cherki, G., Rafya, M., Benkebboura, A., ... & Qaddoury, A. (2023). Eco-friendly management of the citrus nematode Tylenchulus semipenetrans using some aromatic and medicinal plants. Archives of Phytopathology and Plant Protection, 56(1), 66-86.
- Chacón, C., Bojórquez-Quintal, E., Caamal-Chan, G., Ruíz-Valdiviezo, V. M., Montes-Molina, J. A., Garrido-Ramírez, E. R., ... & Ruiz-Lau, N. (2021). In vitro antifungal activity and chemical composition of Piper auritum Kunth essential oil against Fusarium oxysporum and Fusarium equiseti. Agronomy, 11(6), 1098.
- Quiroga et al. (2019).
- Zatar, N. A., Abu-Eid, M. A., & Eid, A. F. (1999). Spectrophotometric determination of nitrite and nitrate using phosphomolybdenum blue complex. Talanta, 50(4), 819-826.
- Berker, K. I., Güçlü, K., Tor, İ., & Apak, R. (2007). Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta, 72(3), 1157-1165.

| Area (%) | |||||
| Components | RT | IK | IK* | Wild EO | Cultivated EO |
| Tricyclene | 2.114 | 927 | 926 | - | 0.37 |
| α -Pinene | 2.173 | 937 | 919 | 2.01 | 5.83 |
| Camphene | 2.279 | 955 | 964 | 1.17 | 1.33 |
| β-Pinene | 2.444 | 984 | 981 | 3.97 | 2.59 |
| D-Limonene | 2.747 | 1035 | 1036 | - | 2.02 |
| Eucalyptol | 2.799 | 1043 | 1033 | 23.01 | 14.71 |
| γ- Terpinene | 2.994 | 1076 | 1062 | 0.48 | 0.27 |
| L-Fenchone | 3.092 | 1092 | 1094 | 0.35 | 0.29 |
| Linalool | 3.118 | 1096 | 1098 | 0.38 | - |
| β-fenchol | 3.17 | 1105 | 1117 | 1.45 | 0.84 |
| Camphor | 3.346 | 1135 | 1143 | 0.46 | 0.45 |
| Borneol | 3.509 | 1162 | 1165 | 49.47 | 32.83 |
| Camphene hydrate | 3.542 | 1168 | 1150 | - | 0.88 |
| Pinocarvone | 3.57 | 1173 | 1168 | 1.54 | 0.88 |
| Crypton | 3.624 | 1182 | 1188 | 1.22 | |
| Terpinen-4-ol | 3.671 | 1190 | 1189 | 3.10 | 3.70 |
| Myrtenol | 3.758 | 1205 | 1202 | 3.61 | 2.68 |
| Cis-Verbenol | 3.833 | 1218 | 1216 | 0.37 | - |
| Carveol | 3.894 | 1229 | 1229 | 0.27 | - |
| Isobornyl acetate | 4.237 | 1291 | 1286 | - | 24.45 |
| Terpinyl acetate | 4.564 | 1351 | 1352 | - | 0.69 |
| β-caryophyllène | 5.296 | 1494 | 1428 | 0.25 | 0.30 |
| β -selinene | 5.345 | 1504 | 1485 | 0.82 | 0.43 |
| α -Selinene | 5.434 | 1522 | 1494 | 0.35 | - |
| Caryophyllene oxide | 5.803 | 1599 | 1581 | 0.73 | 0.38 |
| Cubenol | 6.024 | 1646 | 1642 | 0.32 | 0.35 |
| β-Eudesmol | 6.136 | 1670 | 1654 | 3.79 | 1.79 |
| Monoterpene hydrocarbons | 7.65 | 12.43 | |||
| Oxygenated monoterpenes | 84.06 | 83.68 | |||
| Hydrocarbon sesquiterpenes | 1.43 | 0.73 | |||
| Oxygenated sesquiterpenes | 4.85 | 2.54 | |||
| Wild L. dentata | Cultivated L. dentata | |
| pH water | 7.63 | 6.63 |
| pH KCl Conductivity ( µs/cm) |
7.27 107.97 |
6.13 1513 |
| Organic matter (g/kg) | 38.90 | 21.67 |
| Organic carbon (g/kg) | 22.60 | 12.6 |
| Nitrogen (g/kg) | 3.64 | 2.82 |
| Carbon/Nitrogen | 6.20 | 4.46 |
| Total limestone (g/kg) | 10.70 | 54.04 |
| Calcium (g/kg) | 11.50 | 17.55 |
| Potassium (g/kg) | 0.53 | 1.22 |
| Sodium (g/kg) | 0.01 | 0.03 |
| Phosphorus (g/kg) | 0.03 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





