1. Introduction
Volatile organic compounds (VOCs) are primarily byproducts of human activities, such as transportation and industrial production [
1,
2]. Large quantities of VOCs are released into the atmosphere, where they contribute to the formation of fine particulate matter (PM2.5) and ozone photochemical pollutants, posing significant risks to both environmental quality and human health [
3,
4]. Various emission control technologies have been developed to mitigate VOC emissions, including adsorption, condensation, membrane separation, photocatalytic degradation, biodegradation, direct combustion, catalytic oxidation, and plasma treatment [
5,
6,
7]. Among these, plasma technology has gained widespread recognition as an efficient method for toluene removal due to its low energy consumption and absence of secondary pollution. Research on plasma-based purification of atmospheric pollutants has advanced rapidly, establishing plasma technology as a prominent focus in both domestic and international academic communities [
8].
In recent years, cold plasma technology has emerged as a versatile approach for catalyst preparation. This technique introduces energy into hybrid systems, generating high-energy electrons, photons, ions, and free radicals, thereby creating a highly reactive environment that enables catalysts to exhibit high activity at low temperatures [
9,
10,
11]. Dielectric barrier discharge (DBD) plasma is a widely used plasma source that employs high-frequency, high-voltage power to generate a strong electric field across dielectric barriers, inducing gas discharge and plasma formation, which facilitates gas excitation and initiates chemical reactions. Compared with traditional thermal plasma sources, DBD operates as a non-thermal plasma system, characterized by a low bulk gas temperature while simultaneously producing high-energy electrons. In catalyst modification, DBD plasma promotes the uniform aggregation of particles and enhances the interaction between metal nanoparticles and support materials [
12,
13].
Compared with the catalysts prepared by traditional high-temperature calcination, the catalysts treated by plasma exhibit lower activation energy and higher product selectivity [
14]. The researchers prepared a CuO/γ-Al
2O
3 catalyst via impregnation at ambient conditions and applied it in a non-thermal plasma DBD reactor. By leveraging the synergy between plasma and catalyst, methane conversion and methanol selectivity improved significantly. Compared to the plasma-only system, using the 5CA catalyst with 5% copper loading increased methanol selectivity from 27% to 37%, while also enhancing methane conversion. This improvement is mainly due to the catalyst-promoted charge deposition and optimization of the gas-phase reaction environment [
15]. Liu
et al. [
13] synthesized a Ni/Ga
2O
3 catalyst via cold plasma, achieving 13.8% CO₂ conversion and 56% methanol selectivity at 300 °C and 5 MPa—outperforming catalysts prepared by calcination or chemical reduction. The improved performance resulted from smaller Ni nanoparticles (4.12 nm) due to the low thermal impact of plasma, which suppressed agglomeration and increased active site density. Continuous high-energy electron bombardment also strengthened the Ni–Ga
2O
3 interfacial interaction, enhancing catalytic activity. Our research group synthesized nano-hydroxyapatite by hydrolyzing calcium citrate with ammonia under different hydrothermal temperatures and showed its effectiveness as a support for silver catalysts in toluene removal. Tuning the synthesis temperature precisely controlled the HAP crystalline structure, surface-adsorbed oxygen content, and Ag⁰/Ag⁺ ratio. This nanostructured HAP layer enhanced reactant adsorption and diffusion while effectively confining and stabilizing silver nanoparticles, significantly improving catalytic activity and long-term stability [
16]. A series of novel active materials composed of HAP supported Ni(acac)
2 complexes exhibit high activity in the CO₂ methanation reaction. The optimal grafting temperature is 30 °C, achieving a Ni loading of up to 4.6 wt%. Compared with impregnated Ni/HAP catalysts, the grafting method improves nickel dispersion and reducibility, resulting in smaller particle sizes (3.2–6.3 nm). The grafted Ni(acac)₂/HAP catalyst shows excellent performance, with the optimized Ni(acac)₂/HAP-(5) sample reaching a T
50 of 313 °C and 84% CO₂ conversion at 400 °C [
17].
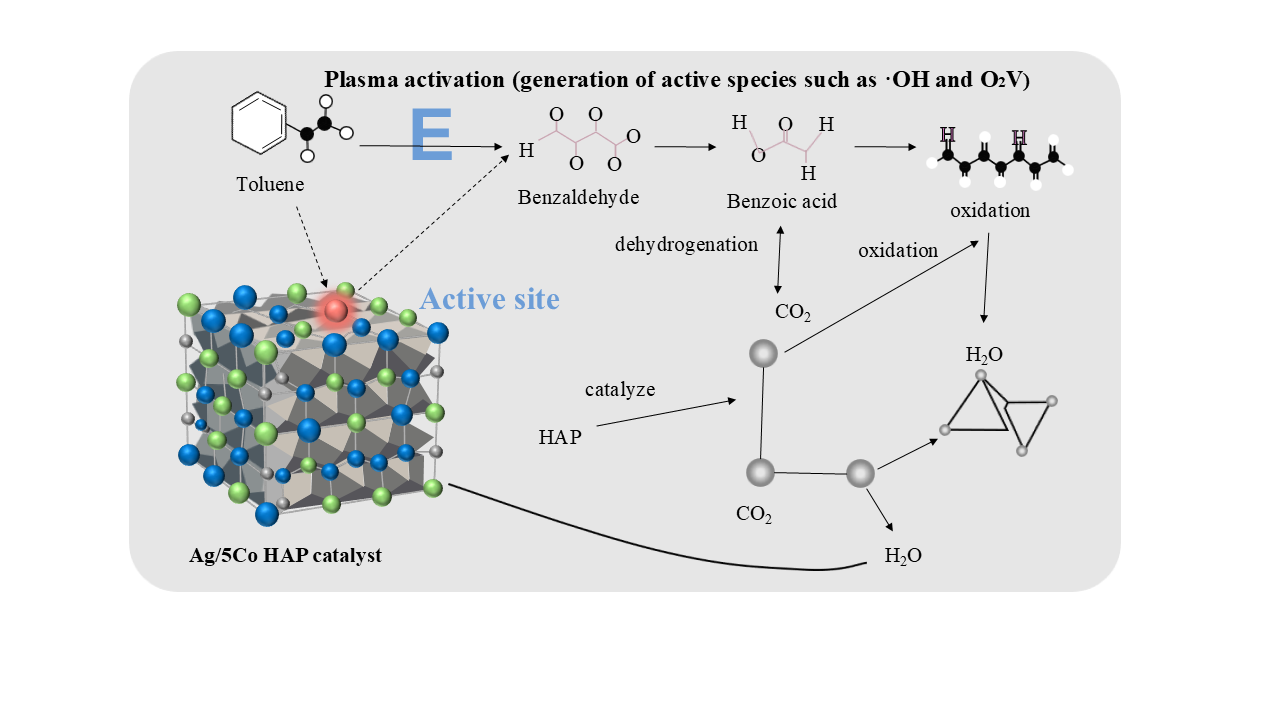
Previous studies prepared silver-cobalt bimetallic catalysts using equal-volume impregnation and systematically evaluated their performance in toluene oxidation. Coupling plasma technology with the 3Ag/15Co/HA catalyst achieved efficient toluene removal at low temperatures and significantly enhanced CO₂ selectivity, due to plasma-promoted surface-active oxygen species that boost catalytic oxidation [
18]. This study reports the synthesis of a 5Co-HA support via the sol-gel method, followed by silver loading through wet impregnation to form the Ag/5Co-HA catalyst. The catalyst was then modified with DBD plasma treatment to produce Ag/5Co-HA-P. Characterizations show that plasma treatment improves the distribution and reactivity of surface oxygen species and significantly enhances low-temperature catalytic oxidation of toluene.
2. Materials and Methods
2.1. Preparation of the Carrier
The HA and 5Co-HA carriers were synthesized using the sol-gel method. For HA, 0.9444 g of calcium nitrate was dissolved in 20 mL of deionized water. As for 5Co-HA, a mixture of 0.875 g calcium nitrate and 0.057 g cobalt nitrate was used to achieve a 5% Co³⁺/(Co³⁺+Ca²⁺) molar ratio. Then, 0.2352 g of phosphoric acid was added to each solution. Based on the stoichiometric Ca:P ratio of 1.67 in hydroxyapatite, the solutions were stirred thoroughly on a magnetic stirrer at constant temperature. Aqueous ammonia was slowly added dropwise until the pH reached 10. After stirring for another 30 min, the gels were aged for 24 h. The resulting precursors were freeze-dried and then calcined at 400 °C for 2 h in a muffle furnace to obtain pure HA and 5% Co-doped HA.
2.2 Preparation of Catalysts
(1) Preparation of Ag/HA catalyst
The Ag/HA catalyst with a theoretical Ag loading of 3 wt% was prepared via the impregnation method. First, a precise amount of AgNO3 was weighed and dissolved in distilled water, after which the solution was uniformly deposited onto the pre-prepared HA support. The resulting mixture was dried in an oven and subsequently calcined at 300 °C for 2 h under a 5% H2/Ar atmosphere to obtain the 3% Ag/HA catalyst, hereafter referred to as Ag/HA.
(2) Preparation of Ag/5Co-HA catalyst
Impregnate 3%Ag onto a 5% Co-HA support. Place the resulting mixture in an oven for drying, followed by the same reduction procedure as described previously, to obtain the 3%Ag/5% Co-HA catalyst, abbreviated as Ag/5Co-HA.
(3) Preparation of Ag/2.88%Co/HA Catalyst
The 3%Ag/2.88Co/HA catalyst was prepared as follows. First, a 2.88% Co/HA catalyst was synthesized by impregnating cobalt onto the HA support, followed by calcination at 400 °C for 2 h in a muffle furnace, based on the measured cobalt loading in the Co-HA sample. Then, 3% silver was loaded onto the 2.88%Co/HA support via incipient wetness impregnation. The sample was reduced under the same conditions as above to obtain the final catalyst, referred to as Ag/2.88Co/HA.
(4) Preparation of Ag/5%Co-HA-P Catalyst
The impregnated Ag/5Co-HA was dried in an oven, after which 0.4 g of the precursor sample was accurately weighed and introduced into a plasma-assisted bioreactor. Reduction was carried out under low-temperature plasma using 5% H2/Ar as the working gas at a total flow rate of 40 mL/min. The input power was set to 160 W, and the reduction was conducted for 10 min, yielding the 3%Ag/5%Co-HA-160-10 min catalyst, hereafter referred to as Ag/5Co-HA-P.
(5) Preparation of Ag/2.88%Co/HA-P Catalyst
Dry the impregnated 2.88%Co/HA in an oven, then weigh out 0.4 g of the precursor sample and transfer it into a plasma-assisted preparation reactor. The same treatment procedure as described above is applied to obtain the 3%Ag/2.88%Co/HA-P catalyst, hereafter referred to as Ag/2.88Co/HA-P.
2.3 Sample Characterization
For physical and chemical characterizations, including XRD, BET, and XPS, please refer to the supplementary materials.
2.4 Catalyst Activity Evaluation
To investigate the differences between the catalyst prepared by plasma treatment in a 5% H
2/Ar atmosphere and that obtained via conventional high-temperature calcination, catalytic toluene oxidation was carried out over all the aforementioned samples. The plasma treatment catalyst device is in the supplement material
Figure S1. The toluene oxidation experiments were performed in a fixed-bed microreactor using 100 mg of catalyst. Toluene vapor was introduced via the N
2 bubbling method, and the reaction gas mixture consisted of oxygen and nitrogen, with a total flow rate of 130 mL/min, corresponding to a space velocity of 78,000 mL/(g·h). The inlet toluene concentration was maintained at 610 ppm, and the reaction temperature was varied from 90 °C to 300 °C.
Toluene conversion was calculated based on the difference between inlet and outlet concentrations. The toluene conversion rate (X
toluene) and CO
2 selectivity (S
CO2) are calculated according to the following equations:
where C
in and C
out denote the toluene concentrations at the inlet and outlet, respectively.
The inlet and outlet concentrations of toluene are denoted by Cin and Cout, respectively, while CCO2 represents the CO2 concentration at the outlet.
The fundamental formula for calculating the activation energy using the FWO method (Flynn–Wall–Ozawa method) is as follows:
β denotes the heating rate, Tα represents the temperature (in K) corresponding to a fixed conversion rate, Ea refers to the apparent activation energy.
3. Results
3.1. Structure and Morphology of the Catalyst
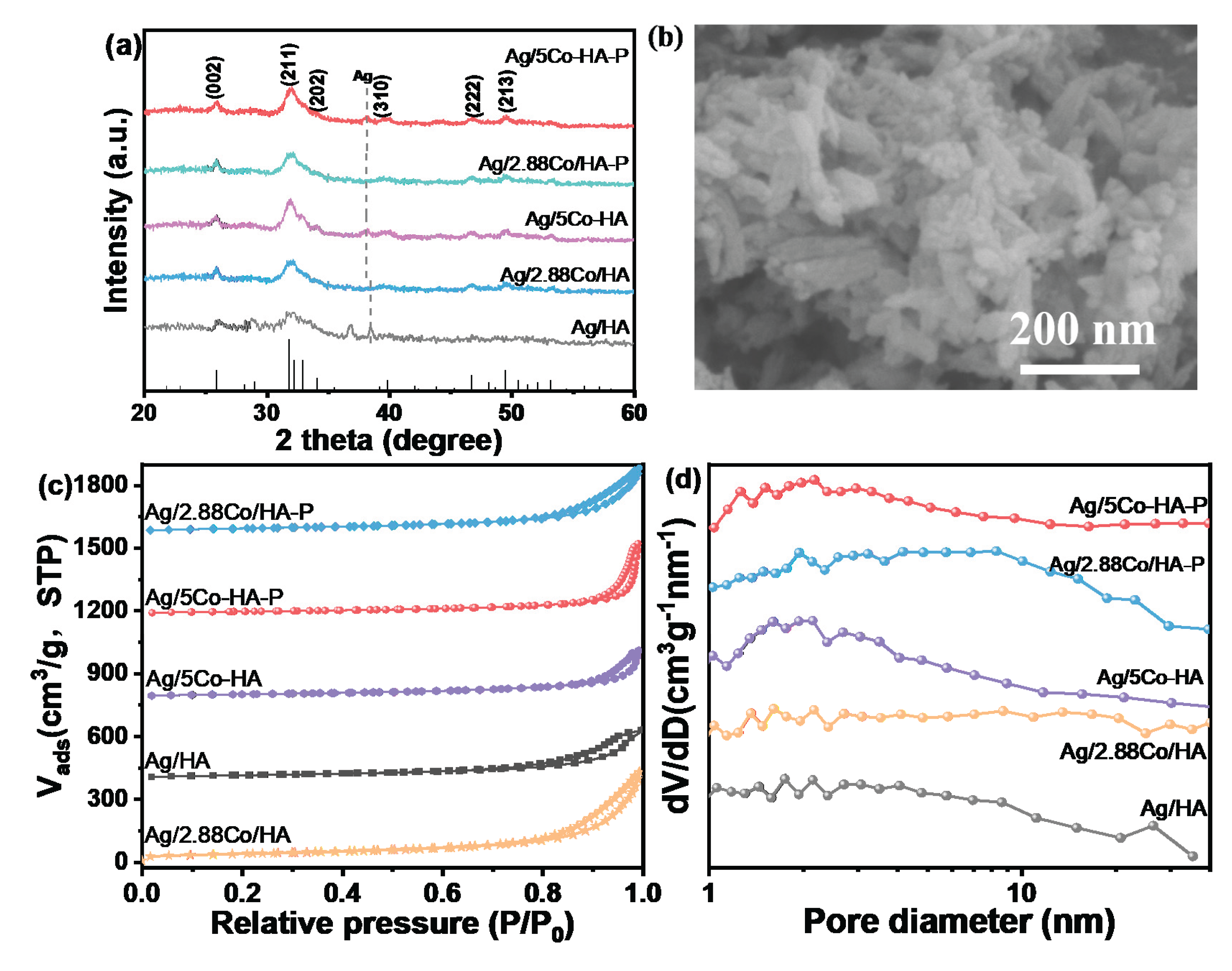
To investigate the influence of DBD plasma treatment on the structural characteristics and physicochemical properties of catalysts, all catalyst samples were systematically characterized using XRD. As shown in Supplementary Material S2(a), both the HA support and 5Co-HA exhibit the typical crystalline structure of HA.
Figure 1(a) presents the XRD patterns of the catalysts following high-temperature calcination, as well as before and after DBD plasma treatment. The diffraction peaks observed at 2
θ = 25.9° and 31.9° are attributed to the (002) and (211) crystal planes of HA as referenced in the standard JCPDS card (No. 09-0423), confirming that all samples exhibit the characteristic crystalline structure of HA. In comparison, the XRD peaks of plasma-treated catalysts are broader and exhibit lower intensities, whereas those subjected to high-temperature calcination display sharper peaks with higher intensities, suggesting superior crystallinity in the latter. Furthermore, no distinct diffraction peaks corresponding to metallic Co were detected in any of the XRD patterns, irrespective of the treatment method, indicating a high degree of dispersion of cobalt species on the catalyst support [
19].
Figure 1(b) presents the morphological and structural characteristics of the Ag/5Co-HA-P catalyst synthesized via the sol-gel method. The microstructure was analyzed using SEM, which reveals a distinct rod-like morphology. The incorporation of Ag and Co does not significantly alter the overall structural framework of the support. For comparison, the SEM images of the pristine HA-P support are provided in the supplementary materials. Support S1 exhibits a disordered lamellar structure, and no substantial morphological changes are observed following Co doping. The specific surface area, pore volume, and pore size of each catalyst were systematically characterized using a physical adsorption instrument. As shown in
Figure 2(c), the N
2 adsorption-desorption isotherms of all catalysts display typical Type IV behavior with H3-type hysteresis loops occurring in the P/P₀ = 0.8–1.0 range, indicating the presence of mesoporous structures [
20]. Based on the adsorption branch data, the pore size distribution was further determined using the BJH model. Notably, for the plasma-treated catalysts, the hysteresis loop size remained largely unchanged, suggesting that plasma treatment has no significant impact on the mesoporous architecture of the catalysts.
As is known from
Table 1, the specific surface area of the Co-HA catalyst prepared by the sol-gel method is lower than that of the HA catalyst and the catalysts prepared by the impregnation method. This indicates that during the preparation process, some Co species occupy the pore structure of the support. The Ag/5Co-HA-P catalyst exhibits the smallest specific surface area. This is because, after DBD treatment, the atoms at the grain boundaries of small crystals possess relatively high energy. Due to this elevated energy state, these atoms tend to migrate under plasma activation and subsequently deposit onto the surfaces of larger crystals. This process promotes the coalescence of small crystallites into larger ones-commonly referred to as grain growth. As grain growth proceeds, the crystal packing becomes denser, resulting in a reduction in internal porosity. Consequently, the overall specific surface area of the catalyst further decreases [
21].
The pore size distribution of the catalysts follows the order: Ag/5Co-HA-P > Ag/2.88Co/HA-P > Ag/5Co-HA > Ag/HA, indicating that plasma-assisted synthesis leads to larger pore sizes and higher pore volumes. These structural features facilitate the formation of lattice defects and oxygen vacancies, thereby creating more active sites and enhancing the catalytic oxidation performance of toluene. Under a 5% H2/Ar reducing atmosphere, plasma-synthesized catalysts consistently exhibit greater pore volumes and larger pore sizes compared to those prepared by conventional methods. The specific surface area increases from 63.70 cm2/g for the pure HA support to 71.32 cm2/g for the 5Co-HA support, which can be attributed to the successful incorporation of Co3+ into the HA lattice. Furthermore, the addition of Ag improves the redox properties of the catalyst, primarily due to the synergistic interaction between Ag and Co.
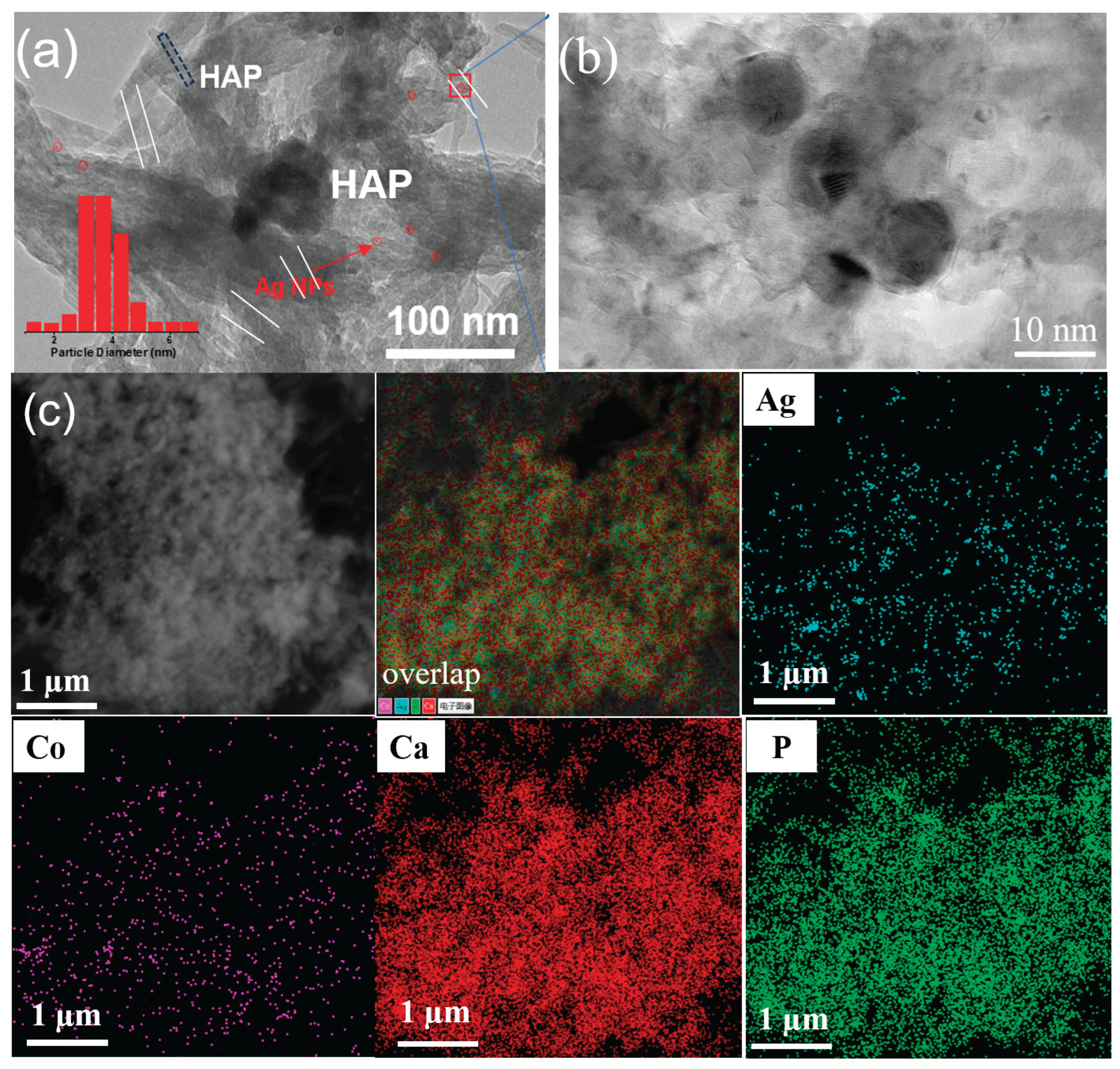
3.2. TEM Analysis
Figure 2(a–b) presents TEM images of the Ag/5Co-HA-P and Ag/5Co-HA catalysts. It is evident that plasma treatment did not significantly alter the catalyst morphology, the HAP support retained its rod-like layered structure. Following the deposition of Ag and Co, a limited number of small-sized nanoparticles were observed to be dispersed on the surface of the support, with minimal aggregation. The average size of Ag nanoparticles is about 3.35 ± 0.15 nm. Energy-dispersive X-ray spectroscopy (EDS) analysis of the Ag/5Co-HA-P catalyst further revealed a uniform distribution of Ag and Co elements across the HAP support, demonstrating excellent dispersibility of the metal components.
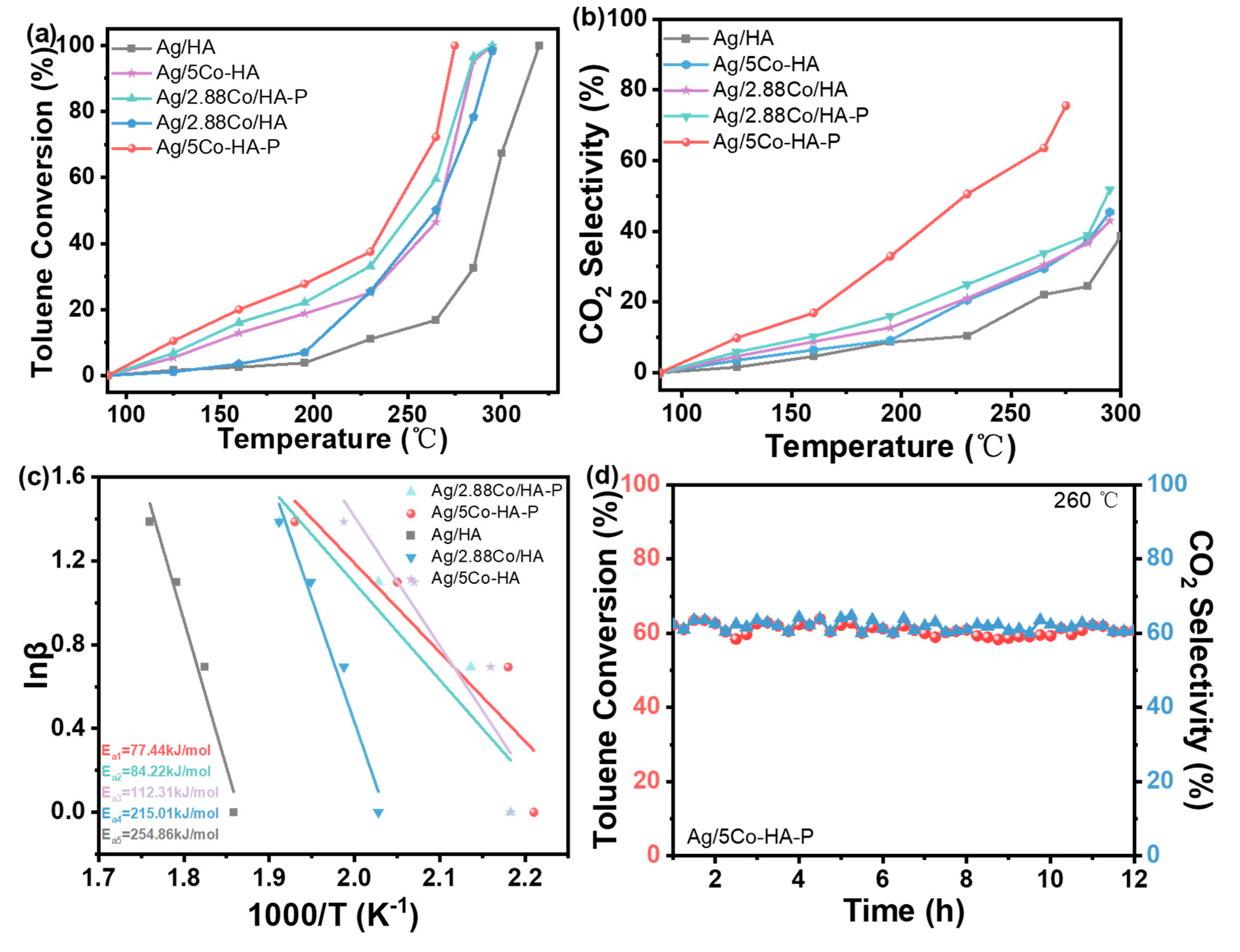
3.3. Catalyst Activity
The catalytic oxidation performance of toluene over catalysts prepared by different loading methods reveals that unmodified Ag/HA achieved complete toluene conversion (T
100) at 315 °C. In comparison, the incorporation of the transition metal Co significantly enhanced low-temperature catalytic activity. Among the tested catalysts, Ag/5Co-HA-P, subjected to plasma treatment, exhibited the highest performance, achieving 100% toluene conversion at 275 °C. ICP-OES analysis showed that the actual Co loading in 5%Co-HA was 2.88%. For a more accurate comparison, a support with precisely 2.88% Co loading (2.88%Co-HA) was prepared via impregnation, followed by calcination in a muffle furnace. Subsequently, 3% Ag was loaded by wet impregnation, and the sample was treated using DBD plasma to yield the Ag/2.88Co-HA-P catalyst. When compared with Ag/5Co-HA-P, the latter demonstrated superior low-temperature activity, particularly when treated under a 5% H
2/Ar plasma atmosphere. This improvement is attributed to the reducing environment promoting the formation of additional active sites during plasma treatment, thereby enhancing catalytic performance. As summarized in supplementary materials
Table S2, Ag/5Co-HA-P maintained 90% toluene conversion at 270 °C under conditions of low toluene concentration and high GHSV, demonstrating excellent catalytic stability and efficiency. As shown in
Figure 3(b), the CO
2 selectivity of the Ag/5Co-HA-P catalyst is approximately 20% higher than that of the Ag/HA catalyst. This finding suggests that the synergistic interaction between the metal and plasma enhances the generation of reactive oxygen species within the system. These highly oxidative species facilitate the deep oxidation of toluene, promoting a more complete conversion to CO
2.
To further evaluate the catalytic activity, kinetic studies were conducted. At 20% toluene conversion, the Flynn-Wall-Ozawa (FWO) method was employed to establish a linear relationship between 1/T and lnβ under different heating rates, enabling the calculation of the apparent activation energy. As shown in
Figure 3(c), the Ag/5Co-HA-P catalyst exhibited the lowest activation energy of 77.44 kJ/mol, significantly lower than that of the other samples. This indicates that Ag/5Co-HA-P possesses a reduced reaction energy barrier, facilitating the catalytic oxidation of toluene. The kinetic results are in excellent agreement with its superior catalytic performance.
3.4. Catalyst Stability Test
Under specific reaction conditions, stability represents a critical parameter for assessing the catalytic performance in toluene oxidation. Given that the Ag/5Co-HA-P catalyst treated with 5% H
2/Ar plasma demonstrates superior catalytic activity, a 12-hour stability test was performed at 260 °C, with results presented in
Figure 3(d). The data show that the catalyst maintains consistent toluene conversion and CO
2 generation rates throughout the test, indicating excellent catalytic durability.
4. Discussion
4.1. H2-TPR Analysis
Figure 4(a) presents the H
2-TPR profiles of the catalysts treated with DBD plasma. Reduction peaks observed in the temperature range of 100-300 ℃ are attributed to the reduction of silver species on the catalyst surface, specifically corresponding to the conversion of Ag
2O to Ag
0 [
22]. For the Ag/HA catalyst, the Ag
0 reduction peak appears at 272 ℃, whereas in the Ag/5Co-HA-P catalyst, this peak shifts to 222 ℃, indicating enhanced reducibility of the silver species. The H
2-TPR profile of the Ag/5Co-HA catalyst displays two well-defined reduction peaks at 456 ℃ and 563 ℃, which correspond to the two-step reduction of Co
3O
4: the first peak at approximately 456 ℃ is associated with the reduction of Co
3+ to Co
2+, followed by a more intense peak at around 563 ℃, assigned to the subsequent reduction of Co
2+ to Co
0 [
23]. In contrast, for the Ag/5Co-HA-P catalyst, the cobalt reduction peak shifts forward to 412 ℃, suggesting a lower reduction temperature and thus improved reducibility of the cobalt species. This shift implies that the interaction between the active components and the HAP support has been effectively optimized in the plasma-treated sample, thereby facilitating the reduction process and enhancing catalytic performance [
25]. Generally, a lower reduction peak temperature reflects weaker metal-support interaction and higher reducibility of the metal species. Among the four samples, the Ag/5Co-HA-P catalyst exhibits the lowest reduction peak temperature and the highest H
2 consumption, as indicated by the area under the reduction peak, demonstrating its superior reducibility. This enhanced reduction capability facilitates the formation of a greater number of oxygen vacancies, which play a crucial role in promoting the catalytic oxidation of toluene [
24]. Typically, a lower reduction peak temperature corresponds to improved catalyst reducibility, further confirming that plasma treatment significantly enhances the redox properties of the catalyst.
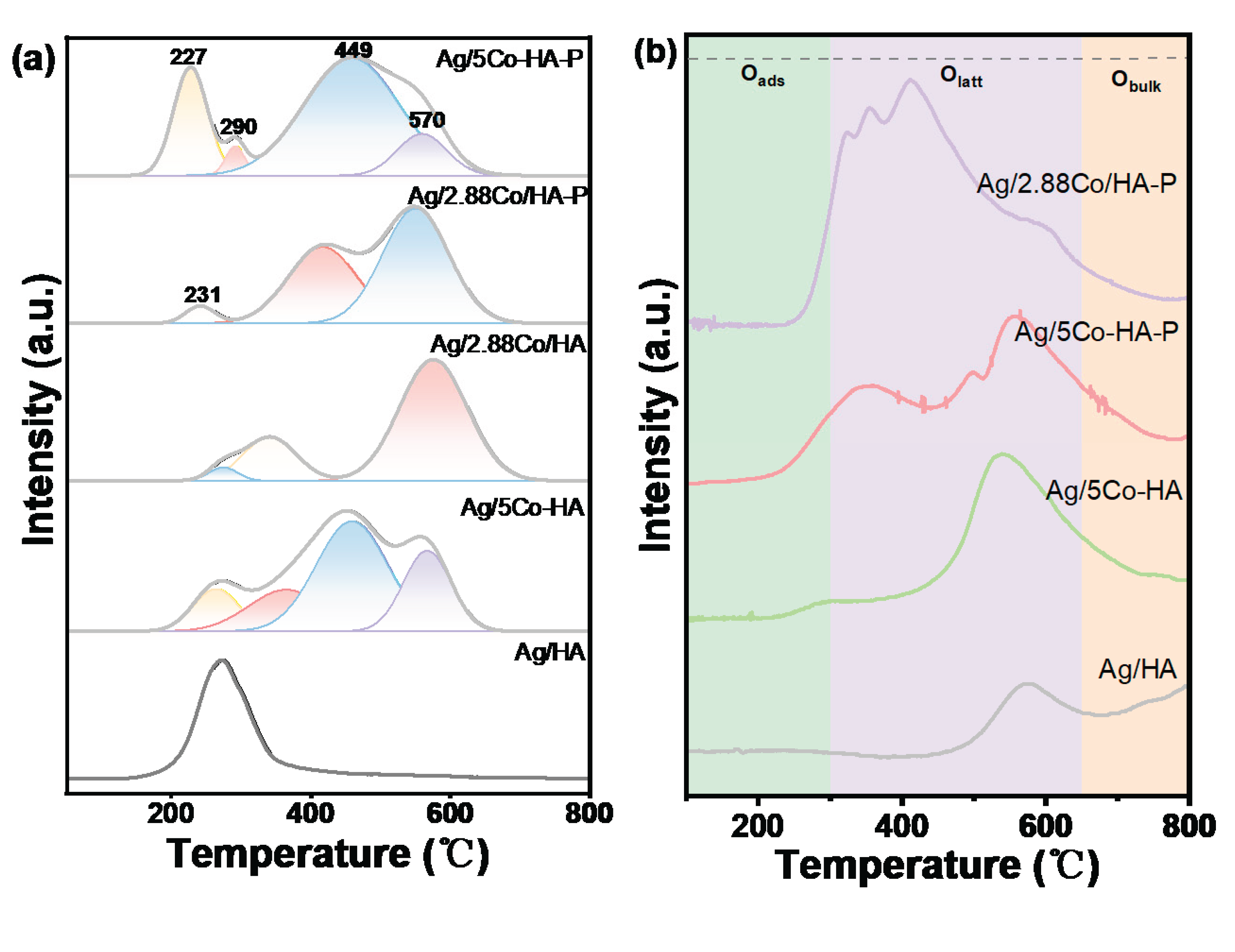
4.2. O2-TPD Analysis
To elucidate the chemical characteristics of oxygen species in the sample, O
2-TPD experiments were conducted on the DBD plasma-treated catalyst over a temperature range of 100-800 °C. As illustrated in
Figure 4(b), the oxygen desorption profile can be deconvoluted into three distinct temperature regions: the low-temperature peak below 350 °C is assigned to the desorption of chemisorbed oxygen from surface oxygen vacancies; the medium-temperature peak between 350 °C and 600 °C primarily corresponds to the release of surface lattice oxygen; and the high-temperature peak above 600 °C is attributed to the evolution of bulk lattice oxygen [
26]. According to prior studies, oxygen species desorbing within the 350–600 °C range exhibit relatively high reactivity and are critically involved in the catalytic oxidation of volatile organic compounds (VOCs) [
27].
The increased lattice oxygen (Olatt) content on the surface of the Ag/5Co-HA-P catalyst can be attributed to the strong interaction between Ag and the Co-HAP support, which effectively modulates the catalyst's surface structure. Meanwhile, plasma treatment facilitates the formation of additional oxygen vacancies, thereby promoting the desorption of Olatt and accelerating the catalytic oxidation of toluene. Comparative analysis of lattice oxygen desorption peak areas during temperature-programmed experiments reveals that the DBD-treated Ag/5Co-HA-P catalyst exhibits the largest peak area, indicating the highest concentration of chemisorbed oxygen. This demonstrates superior oxygen adsorption capacity and enhanced oxygen mobility, resulting in improved reducibility. Given that chemisorbed oxygen typically arises from the adsorption and activation of gaseous O2 at oxygen vacancies on metal oxides, it is reasonable to infer a high density of oxygen vacancies in the Ag/5Co-HA-P catalyst. This conclusion is in excellent agreement with the H2-TPR characterization results.
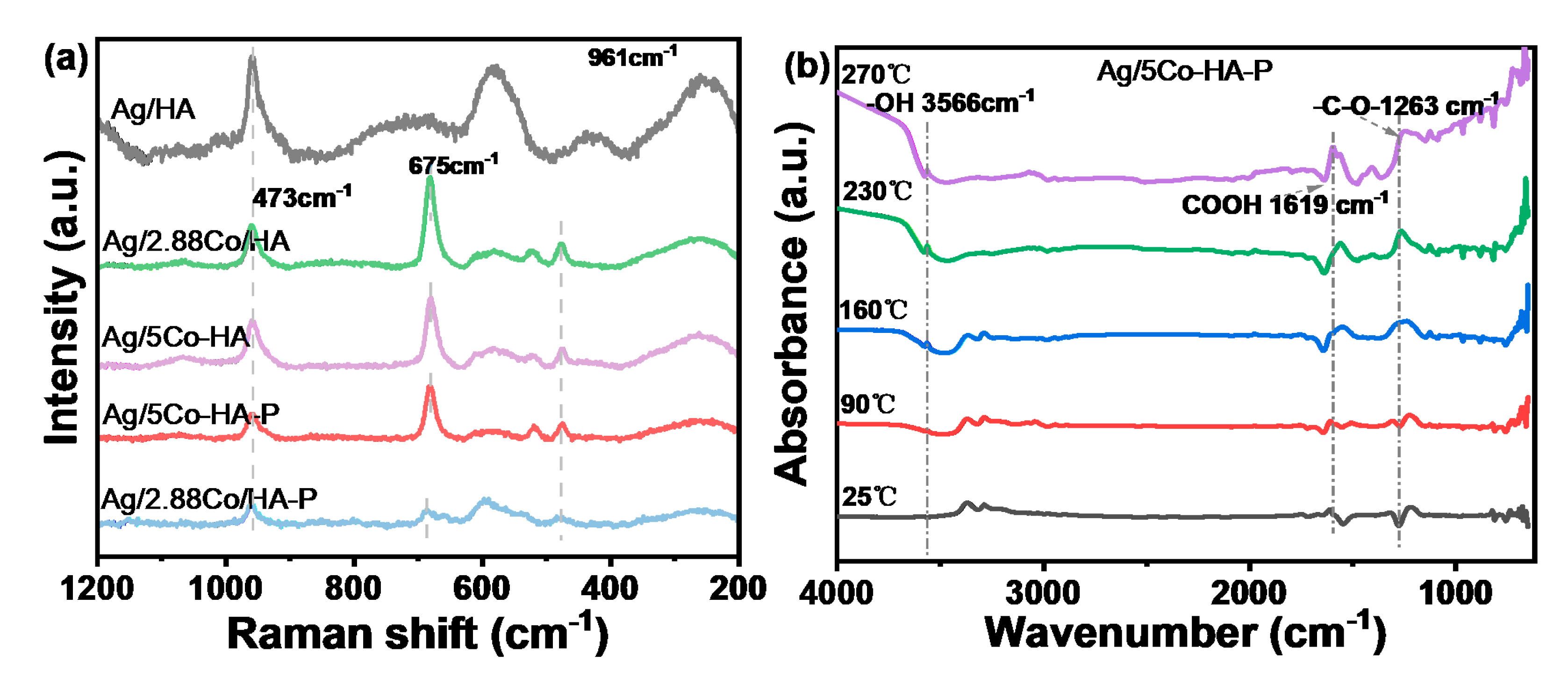
4.3. Raman Analysis
Figure 6(a) presents the Raman spectra of HAP and various catalysts supported on HAP. As observed, all catalysts display a prominent characteristic absorption peak at 961 cm
-1, corresponding to the symmetric stretching vibration mode (V₁) of the PO
43- group in HAP [
28]. Notably, upon loading of the active components, the intensity of this peak is reduced across all samples, suggesting a decrease in the crystallinity of the HAP support as a result of the impregnation process. The peak at 473 cm
-1 is assigned to the E
2g vibration mode, while the signal at 675 cm
-1 is associated with the A
1g vibration mode of octahedrally coordinated Co
3+ in Co
3O
4-both are characteristic Raman-active modes of Co
3O
4. Furthermore, the incorporation of Ag weakens the Co–O bond strength, thereby attenuating the interaction between cobalt and oxygen ions [
29].
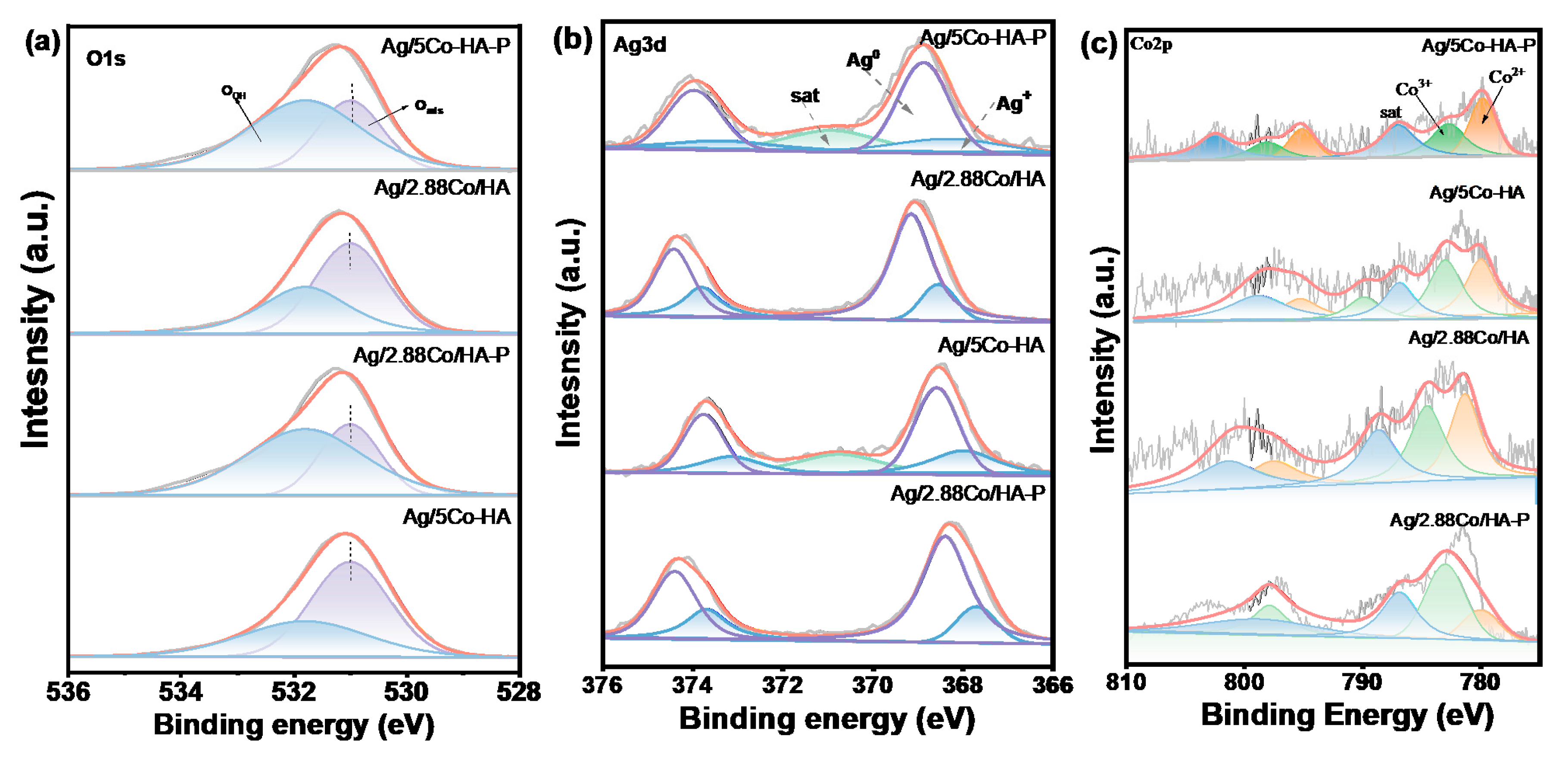
4.4. XPS Analysis
To further investigate the surface structure of the samples, X-ray photoelectron spectroscopy (XPS) was employed to characterize each catalyst. As shown in
Figure 5(a), the O1s XPS spectra of the catalysts can be deconvoluted into two components: a lower binding energy range of 531.0-531.4 eV and a higher range of 531.7-533.8 eV, which are assigned to adsorbed water molecules (O
OH), as well as surface lattice oxygen (O
latt) and surface adsorbed oxygen (O
ads), respectively. The surface adsorbed oxygen species include O
-, O
2-, and O
22-, among others [
30]. As shown in
Table 2, the relative content of adsorbed oxygen and oxygen vacancies is quantified by the O
ads/(O
ads+O
OH) ratio. The Ag/5Co-HA-P catalyst exhibits an O
ads ratio of 1.6, significantly higher than that of other comparative catalysts. This enhancement can be attributed to the synergistic anchoring effect between Ag and Co at the interface, which promotes the stable dispersion of metal nanoparticles on the HAP support and consequently facilitates the formation of additional oxygen vacancies. Previous studies have established that oxygen vacancies play a crucial role in the catalytic oxidation of VOCs. Notably, a higher concentration of oxygen vacancies is positively correlated with superior oxidation activity. The abundance of oxygen vacancies in this catalyst not only enhances the adsorption and activation of O
2 molecules but also accelerates the decomposition and migration of the harmful by-product O
3. As a result, more reactive lattice oxygen and adsorbed oxygen species are generated, thereby significantly improving the toluene degradation performance of the catalyst [
31].
To investigate the valence state transformations of Ag nanoparticles and the evolution of the chemical environment on the catalyst surface during plasma-assisted synthesis, XPS was employed to characterize catalysts prepared under different atmospheric conditions. As shown in
Figure 5(b), the Ag 3d spectra of all samples exhibit two distinct peaks corresponding to Ag
+ and Ag
0 species, indicating that silver was not fully reduced to its metallic state during plasma treatment. The binding energies of Ag 3d
5/2 were observed at 367.7 eV (assigned to Ag
+) and 368.4 eV (assigned to Ag
0), reflecting two chemically distinct silver states. Variations in the relative content of Ag
0 across samples can be attributed to differences in catalyst preparation methods. Notably, the Ag/5Co-HA-P catalyst exhibited over a 1% increase in Ag
0 content compared to other samples. A higher proportion of metallic silver provides more accessible active sites for the catalytic oxidation of toluene, thereby enhancing catalytic performance. This enhancement is likely due to the effective interaction between high-energy electrons generated during DBD plasma treatment in a 5% H
2/Ar atmosphere and the surface silver species, which promotes the reduction of Ag⁺ to Ag⁰ [
32,
33].
As shown in
Figure 5(c), the Co 2p energy spectrum exhibits two primary peaks at binding energies of 795.1 eV and 780.1 eV, which are assigned to the spin-orbit splitting components of Co 2p
3/2 and Co 2p
1/2, respectively—characteristic features of the Co 2p electronic structure. Additionally, two distinct satellite peaks at 802.5 eV and 787.1 eV are clearly observed, corresponding to shake-up satellite transitions, consistent with previously reported data [
34,
35]. The Co³⁺ content serves as a key indicator of oxygen vacancy concentration in the material. As indicated in
Table 2, the Ag/5Co-HA-P catalyst exhibits a Co
3+/Co
2+ ratio of 1.05, suggesting a relatively high concentration of Co
3+ species, thereby providing abundant adsorption and reaction sites for reactive oxygen species. This may be attributed to the enhanced formation of Co
3+ during the reduction process of the support. Previous studies have demonstrated that Co
3+ cations act as the principal active centers in the catalytic oxidation of toluene; thus, a higher Co
3+ content increases the density of active sites and enhances the catalyst’s oxidative performance toward toluene [
36].
4.5. In-Situ DRIFTS Spectra
Figure 6(b) presents the in-situ diffuse reflectance infrared Fourier transform spectroscopy (In-situ DRIFTS) of the Ag/5Co-HA-P catalyst recorded at various temperatures, aimed at analyzing the evolution of surface functional groups during thermal treatment. At 25 °C, no distinct absorption peak is observed at 3566 cm
-1, indicating relatively high transmittance. As the temperature increases, the absorbance at this wavenumber progressively decreases, reflecting a corresponding increase in the infrared absorption intensity of hydroxyl groups. This trend can be attributed to enhanced formation or surface exposure of hydroxyl species induced by elevated temperatures, thereby strengthening their interaction with incident infrared radiation [
37]. At 25 °C, the adsorption peak at 1619 cm
-1 is relatively high, indicating weak infrared absorption by carboxyl groups. As the temperature increases to 90 °C and 160 °C, the absorbance at this wavenumber remains high, and the characteristic absorption of carboxyl groups is not clearly observed. However, when the temperature reaches 230 °C and 270 °C, the at 1619 cm
-1 decreases significantly, suggesting enhanced infrared absorption by carboxyl groups. This change may result from surface oxidation reactions or functional group rearrangements under high-temperature conditions, leading to an increased concentration of carboxyl groups or a more infrared-active vibrational mode. At 25 °C and 90 °C, the adsorption peak at 1263 cm
-1 is also relatively high, indicating weak absorption by the -C-O- bond. Although a slight decrease in absorbance is observed at 160 °C, the value remains high. At 230 °C and 270 °C, the transmittance at 1263 cm
-1 drops markedly, demonstrating significantly enhanced infrared absorption by the -C-O- bond. Analysis of the temperature-dependent trend suggests that elevated temperatures may promote the formation of -C-O- containing functional groups or structural reorganization, thereby enhancing the infrared responsiveness of this bond [
38,
39].
Plasma rapidly generates highly reactive species such as ·OH and O· at room temperature, effectively breaking the benzene ring and side chain of toluene and producing reactive intermediates. The catalyst then selectively oxidizes these intermediates into CO₂ and H
2O by adsorbing active species, supplying lattice oxygen, and lowering the reaction energy barrier [
40]. The results of In-situ DRIFTS and Raman spectroscopy indicate that the reaction mechanism over the Ag/5Co-HA-P catalyst during toluene catalytic oxidation proceeds as follows:
The tolyl group (C
6H
5CH
2·) can react with O
- or OH·, leading to oxidation at the carbon atom of its methyl group.
Under the strong oxidation of hydroxyl radicals (OH·), the benzene ring of phenyl radicals (C6H5·) undergoes a ring-opening reaction, which leads to the breakage of C=C and generates aliphatic dicarboxylic acid intermediates (such as adipic acid, HOOC(CH2)4COOH, denoted as intermediate 4).
Under the synergistic action of O
-, OH·, and Co
3+, the C-C bonds in aliphatic dicarboxylic acids are progressively cleaved, yielding low-molecular-weight carboxylic acids such as acetic acid (CH
3COOH) and formic acid (HCOOH).
5. Conclusions
In conclusion, plasma treatment enables precise control over catalyst structure and electronic properties under mild, eco-friendly conditions, significantly improving activity, selectivity, and stability. It also simplifies synthesis and reduces energy consumption. Characterization shows that plasma-treated catalysts exhibit higher hydrogen consumption and richer oxygen vacancies, enhancing toluene adsorption. Kinetic analysis reveals low activation energy, favoring low-temperature catalytic oxidation of toluene. Among tested catalysts, Ag/5Co-HA-P demonstrates excellent low-temperature activity and cycling stability, achieving 100% toluene conversion at 275 °C with 75.5% CO2 selectivity. The low activation energy aligns well with high catalytic performance. Comparative studies indicate that Ag/5Co-HA-P performs best due to effective anchoring of cobalt species in the hydroxyapatite lattice, which stabilizes low-valent cobalt during reduction and promotes efficient toluene oxidation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Shu-Yao Zhang and Qiang Chen; Data curation, Si-Wen Pan; Methodology, Hui Zhu; Supervision, Yu-Xin Miao; Writing – original draft, Shu-Yao Zhang, Xue-Min Wang, En-Peng Deng and Ya-Ni Zhang; Writing – review & editing, Shu-Yao Zhang and Yu-Xin Miao.
Funding
This research received no external funding.
Data Availability Statement
The dataset will be made available upon request by the authors. The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (22272112), Major Incubation Project of Shenyang Normal University (ZD202303).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Zhang, H.; Jin, X.; Wang, X.; Shao, S.; Tu, C.; Guo, Y., Q Dai. Synergistic oxidation of multicomponent VOCs over Pt0 and Mo clusters engineered CeO2 via facilitated H/O spillover: Boosting chlorine removal and hydrolysis oxidation. Appl. Catal. B: Environ. Energy 2025, 125684. [CrossRef]
- Zhang, M.; Liu, L.; Ding, L.; Fang, N., Y Chu. Modulating surface properties of catalysts to promote the oxidation of multi-component VOCs. Journal of Environmental Chemical Engineering, 2025, 13(5): 118118. [CrossRef]
- Guo, W.; Hao, Z.; Wang, Q.; Zhang, Y.; Chen, T.; Gong, Y.; Zhang, Y.; Wang, P., S Zhan. Targeted regulation of d-band center in LaCo1-xNixO3 perovskite toward Sabatier-optimized catalytic oxidation of VOCs. Applied Catalysis B: Environment and Energy, 2025, 378, 125640.
- Wu, L.; Liu, Y.; Jia, Y.; Sun, Q.; Feng, Y.; Hou, Z.; Wang, Z.; Wei, L.; Wei, Z.; Jing, L.; Deng, J., H Dai. A novel strategy for enhancing resistance to chlorine, water, and sulfur oxide of the Pt/Co-ZSM-5 catalyst by synergistic coupling of acidity and redox sites for the oxidation of multicomponent VOCs. Applied Catalysis B: Environment and Energy, 2025, 378, 125557. [CrossRef]
- Gao, H.; Song, Z.; Mao, Y.; Fan, Y.; Li, R.; Chen, X.; Liu, W.; Zhang, J.; Huang, Z., X Zhang. Tight coupling of oxygen vacancies and acidity on α-MnO2 through cerium doping engineering for efficient removal of multi-component VOCs. Applied Catalysis B: Environment and Energy, 2025, 362: 124745. [CrossRef]
- Hou, Z.; An, X.; Zhu, K.; Tang, Q.; Lan, H.; Liu, H., J Qu. Revealing the pore size-dependent sorption mechanism of toluene and cetane in porous carbon by nuclear magnetic resonance. Environmental Science & Technology, 2023, 57(12): 5003-5012. [CrossRef]
- Fan, Y.; Song, Z.; Gao, H.; Guo, R.; Zhang, M.; Zhang, J.; Chen, X.; Liu, W.; Huang, Z., X Zhang. Adjustment mechanism of Co3+ performance in Co3O4-LaOx catalyst for high-efficiency catalytic combustion of toluene. Separation and Purification Technology, 2025, 354, 128941. [CrossRef]
- Zhang, L.; Zou, Z.; Lei, Z., Y Jia. Research on the mechanism of synergistic treatment of VOCs–O3 by low temperature plasma catalysis technology. Plasma Chemistry and Plasma Processing, 2023, 43(6): 1651-1672. [CrossRef]
- Du Z, Lin X. Research progress in treatment of VOCs by dielectric barrier plasma cooperating catalyst. IOP Conference Series Earth and Environmental Science, 2020, 508(1): 012132.
- Sun, T.; He, B.; Pan, G.; Guo, Y.; Wang, K.; Jia, W.; Guo, Y.; Tang, P., G Chen. Engineering NiFe2O4 decorated with IrO2 on plasma-treated iron foam for enhanced electrocatalytic hydrogen evolution. Journal of colloid and interface science, 2025, 696, 137843. [CrossRef]
- Mahaleh, M.A.; Nilkar, M.; Leus, K.; Abednatanzi, S.; Deng, M., P Van Der Voort, R Morent, N De Geyter. Pyridine-covalent triazine framework (py-CTF) as a metal-free catalyst for effective toluene abatement in post-plasma catalytic systems. Plasma Chemistry and Plasma Processing, 2025, 45(4): 1205-1232.
- Li, S.; Xin, Z.; Luo, Y.; Liao, G.; Li, Q.; Zhang, K.; Tehrani, Z.; Tan, R., Z Feng. Enhancing direct borohydride fuel cell performance via low-temperature plasma pretreatment of cobalt hydroxide catalysts. Ionics, 2025, 31(5): 4591-4602.
- Liu, Z.; Wang, L.; Jin, Z., Z Wang. Enhanced CO2 hydrogenation to methanol via plasma-treated Ni/Ga2O3 catalysts. Russian Journal of Applied Chemistry, 2025, 98(1): 32-42. [CrossRef]
- Wang, J.; Yu, H., H Liang. Activity and stability of Ni/magnesium slag catalysts enhanced by plasma pretreatment for toluene steam reforming. Journal of Analytical and Applied Pyrolysis, 2025, 187, 106996.
- Golubev, O.V., AL Maksimov Plasma-assisted catalytic decomposition of carbon dioxide. Russian Journal of Applied Chemistry, 2022, 95(5): 617-630.
- Deng, E.P.; Zhang, S.Y.; Zhu, H.; Li, J.H.; Wang, X.M.; Zhang, Y.N.; Chen, Q.; Lu, P., YX Miao. Facile hydrothermal synthesis of hydroxyapatite nanosheets as highly "active" supports for stabilizing silver nanoparticles in toluene oxidation. Applied Catalysis A, General, 2025, 705, 120438.
- Berroug, N.; Gutiérrez-Ortiz, M.A., J R González Velasco, Z Boukha. Grafting of Ni(acac)2 complex on hydroxyapatite support for suitable speciation of Ni and intensification of the CO2 methanation reaction. Journal of Industrial and Engineering Chemistry, 2025, 149, 764-777. [CrossRef]
- Wang, X.; Li, J.; Lu, P.; Liu, S.; Zhang, S.; Deng, E.; Miao, Y., Z Zhao. A novel Co coordinated highly dispersed nano Ag/HAP catalysts in enhanced toluene catalytic oxidation with non-thermal plasma. Plasma Chemistry and Plasma Processing, 2025, 45(4): 1191-1204. [CrossRef]
- Bristy, N.S.; Kawsar, M., M S Hossain. Effects of different types of modifiers on structural variation of nano-hydroxyapatite for efficient application. Nanoscale advances, 2025, 7(17), 5133-5160.
- Liu, M.; Wu, X.; Liu, S.; Gao, Y.; Chen, Z.; Ma, Y.; Ran, R., D Weng. Study of Ag/CeO2 catalysts for naphthalene oxidation: Balancing the oxygen availability and oxygen regeneration capacity. Applied Catalysis B: Environmental, 2017, 219, 231-240. [CrossRef]
- Liu, H.; Chen, X.; Qiu, S.; Wei, Q.; Qin, Y.; Ji, L., J Xu. Insight into enhanced catalytic performance of alkali-treated Al2O3 supported Ag catalyst for toluene oxidation. Fuel, 2025, 393, 134987.
- Liu, S.; Liu, Y.; Tang, D.; Miao, Y.; Cao, Z., Z Zhao. Synergy of NTP-La1-xAgxMn1-yCoyO3-δ hybrid for soot catalytic combustion at low temperature. Plasma Chemistry and Plasma Processing, 2021, 41(4): 1009-1019. [CrossRef]
- Wang, C.; Hua, W.; Chai, G.; Zhang, C., Y Guo. Insights into the morphological effect of Co3O4 crystallite on catalytic oxidation of vinyl chloride. Catalysts, 2019, 9(5): 408.
- Chen, H.; Wei, G.; Liang, X.; Liu, P.; He, H.; Xi, Y., J Zhu. The distinct effects of substitution and deposition of Ag in perovskite LaCoO3 on the thermally catalytic oxidation of toluene. Applied Surface Science, 2019, 489, 905-912.
- Lei, N.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Maik, H.; Kovarik, L.; Datye, A.K., Y Wang. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science, 2017, 358(6369): 1419-1423. [CrossRef]
- Zhai, X.; Li, L.; Song, S.; Zhang, J., J Ma. Influence of MnO2 crystal existing form on its catalytic performance for toluene oxidation. Fuel, 2023, 334: 126780.
- Qin, F.; Feng, Q.; Li, X.; Lu, W.; Ma, D.; Huang, X.; Liu, Z.; Zhang, X.; Zuo, C.; Huang, Y.; Zhu, H., S Zhang. Palygorskite-supported bimetallic ZIFs-derived cobalt oxides with coexisting cobalt defects and oxygen vacancies for enhanced antibiotic degradation. Journal of Environmental Chemical Engineering, 2025, 13(2): 115611. [CrossRef]
- Carvalho, D.C.; Pinheiro, L.G.; Campos, A.; Millet, E.R.; de Sousa, F.F.; Filho, J.M., GD Saraiva. Characterization and catalytic performances of copper and cobalt-exchanged hydroxyapatite in glycerol conversion for 1-hydroxyacetone production Applied Catalysis A, General, 2014, 471, 39-49. [CrossRef]
- Sharmin, M.; Band gap tuning, J.P., n-type to p-type transition and ferrimagnetic properties of Mg doped α-Fe2O3 nanostructured thin films. Journal of Alloys and Compounds, 2020, 818, 152850.
- Yao, X.; Zhanh, J.; Liang, X., C Long. Niobium doping enhanced catalytic performance of Mn/MCM-41 for toluene degradation in the NTP-catalysis system. Chemosphere. 2019, 230, 479-487.
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Sanchez, M.C., G Tyuliev. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Applied Catalysis B: Environmental, 2018, 225, 340-353. [CrossRef]
- Guo, H.; Guo, T.; Zhao, M.; Zhang, Y.; Shangguan, W., Y Liao. Improving benzene catalytic oxidation on Ag/Co3O4 by regulating the chemical states of Co and Ag. Journal of Environmental Science, 2024, 143, 201-212. [CrossRef]
- Zhu, B.; Yan, Y.; Li, M.; Li, X.S.; Liu, J.L., YM Zhu. Low temperature removal of toluene over Ag/CeO2/Al2O3 nanocatalyst in an atmospheric plasma catalytic system. Plasma Processes and Polymers, 2018, 15(8): 1700215.
- Wang, J.; Wu, Z.; Zhao, J.; Sun, M.; Ma, X.; Abudula, A., G Guan. Enhanced catalytic oxidation of toluene over amorphous cubic structured manganese oxide-based catalysts promoted by functionally designed Co-Fe nanowires. Catalysis Science & Technology, 2024, 14(10): 2806–2816. [CrossRef]
- Liu, W.; Liu, R.; Zhang, H.; Song, Z., X Zhang. Fabrication of Co3O4 nanospheres and their catalytic performances for toluene oxidation: The distinct effects of morphology and oxygen species. Applied Catalysis A: General. 2020, 597, 117539. [CrossRef]
- Zhu, Z.; Lu, G.; Zhang, Z.; Guo, Y.; Guo, Y., Y Wang. Highly Active and stable Co3O4/ZSM-5 catalyst for propane oxidation: Effect of the preparation method. ACS Catalysis, 2013, 3(6): 1154-1164.
- Zhao, Y., J Zhang. Microstrain and grain-size analysis from diffraction peak width and graphical derivation of high-pressure thermomechanics. Journal of Applied Crystallography, 2008, 41(6): 1095-1108.
- Li, D.; Qiu, R.; Moskowitz, B.M.; Jiang, Z.; Gu, H.; Wen, Q.; Wachs, I.E., M Zhu. Nature of the active sites and reaction mechanism during methanol steam reforming over Cu/ZnO: An isotopic modulated excitation diffuse reflectance infrared fourier transform spectroscopy study. Journal of the American Chemical Society, 2025, 147(27): 24040-24049.
- Ewald, C.; Saito, N.; Weimar, U., N Barsan. Role of potassium loading in ZnO-based gas sensors under NO2 exposure–operando diffuse reflectance infrared Fourier transform spectroscopic study. Sensors and Actuators: B. Chemical, 2023, 393, 134321. [CrossRef]
- Chen, Y.; Zhu, B.; Sun, L.; Du, X.; Shi, C.; Dong, Y.; Ren, Y.; Wang, X.; Liu, Y., H Zhou. Insights into interaction mechanism of plasma, catalysis and thermal processes during toluene oxidation in a packed dielectric barrier discharge plasma reactor. Fuel, 2025, 396, 135309. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).