Submitted:
26 September 2025
Posted:
30 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Generation of Milk By-Products and Wastes: Case of Whey
| Total solids | Protein | Fat | Lactose | Ash | COD (g/L) | BOD (g/L) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Milk | Cattle | 11.8-13 | 3.0-3.9 | 1.3-5.4 | 4.2-5.6 | 0.6-0.8 | [48,49,50] | ||
| Buffalo | 15.7-17.2 | 2.7-4.7 | 5.3-9.0 | 3.2-4.9 | 0.8-0.9 | [49] | |||
| Goat | 11.9-16.3 | 2.5-5.2 | 3.0-7.2 | 3.2-5.0 | 0.7-0.9 | [49,50,51,52] | |||
| Sheep | 18.1-20.0 | 4.5-7.0 | 5.0-9.0 | 4.1-5.9 | 0.8-1.0 | [49] | |||
| Human | 1.1-1.4 | 3.5-3.8 | 6.6 | 0.1-0.2 | [50,51] | ||||
| Whey | Cattle | 6.6 | 0.8 | 0.2 | 5.0 | 50-102 | 27-60 | [53,54] | |
| Buffalo | 6.0-7.0 | 0.7-0.9 | 0.1-0.8 | 4.2-5.0 | [4,28] | ||||
| Goat | 5.1 | 0.43 | 1.2 | 4.1 | [53] | ||||
| Sheep | 9.5 | 1.75 | 1.5 | 3.7 | [53] |
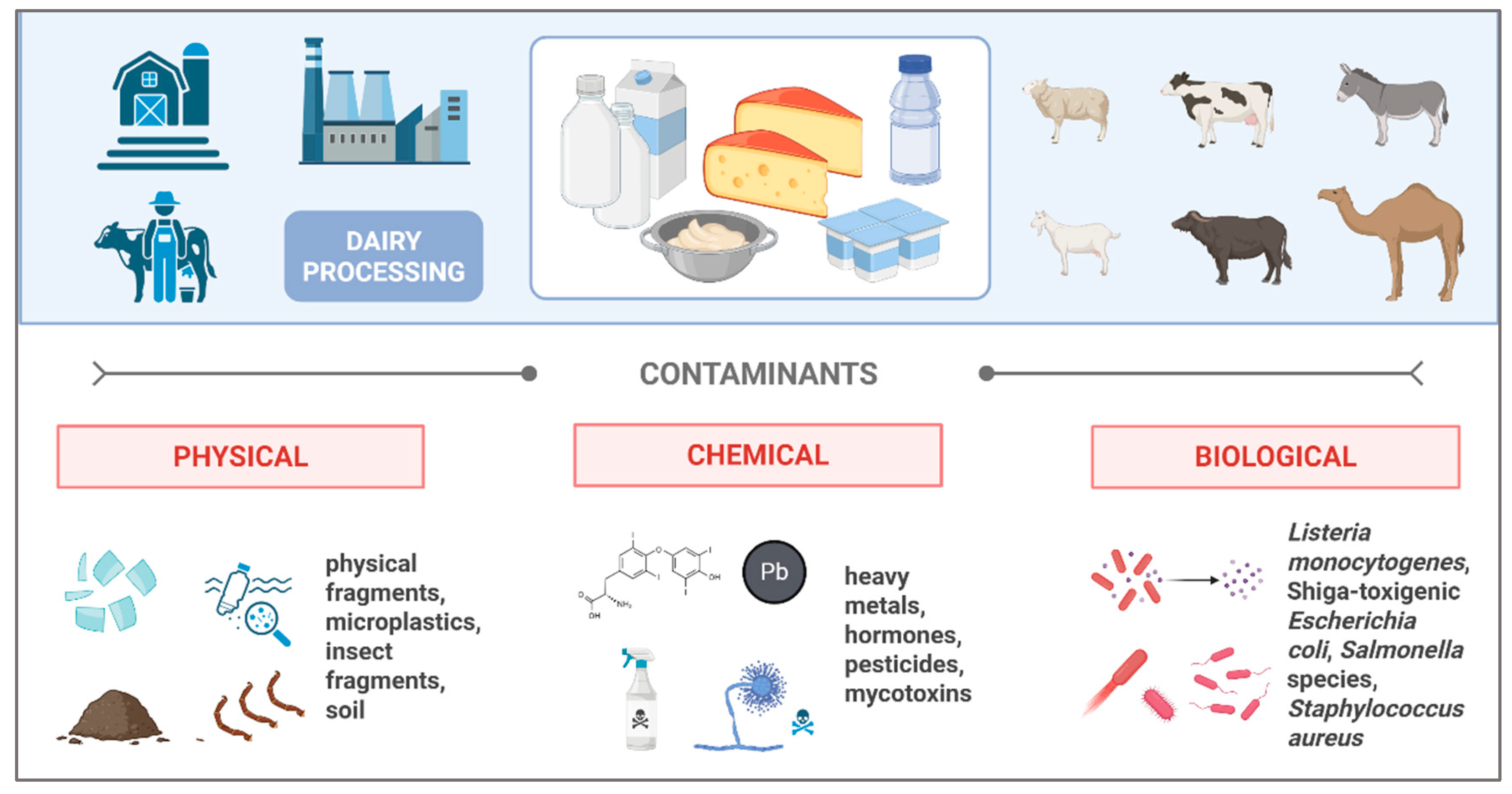
3. Challenges Related to the Safety of Whey Derived from Milk Processing
4. Fractionation of Whey for the Recovery of Bioactive Compounds
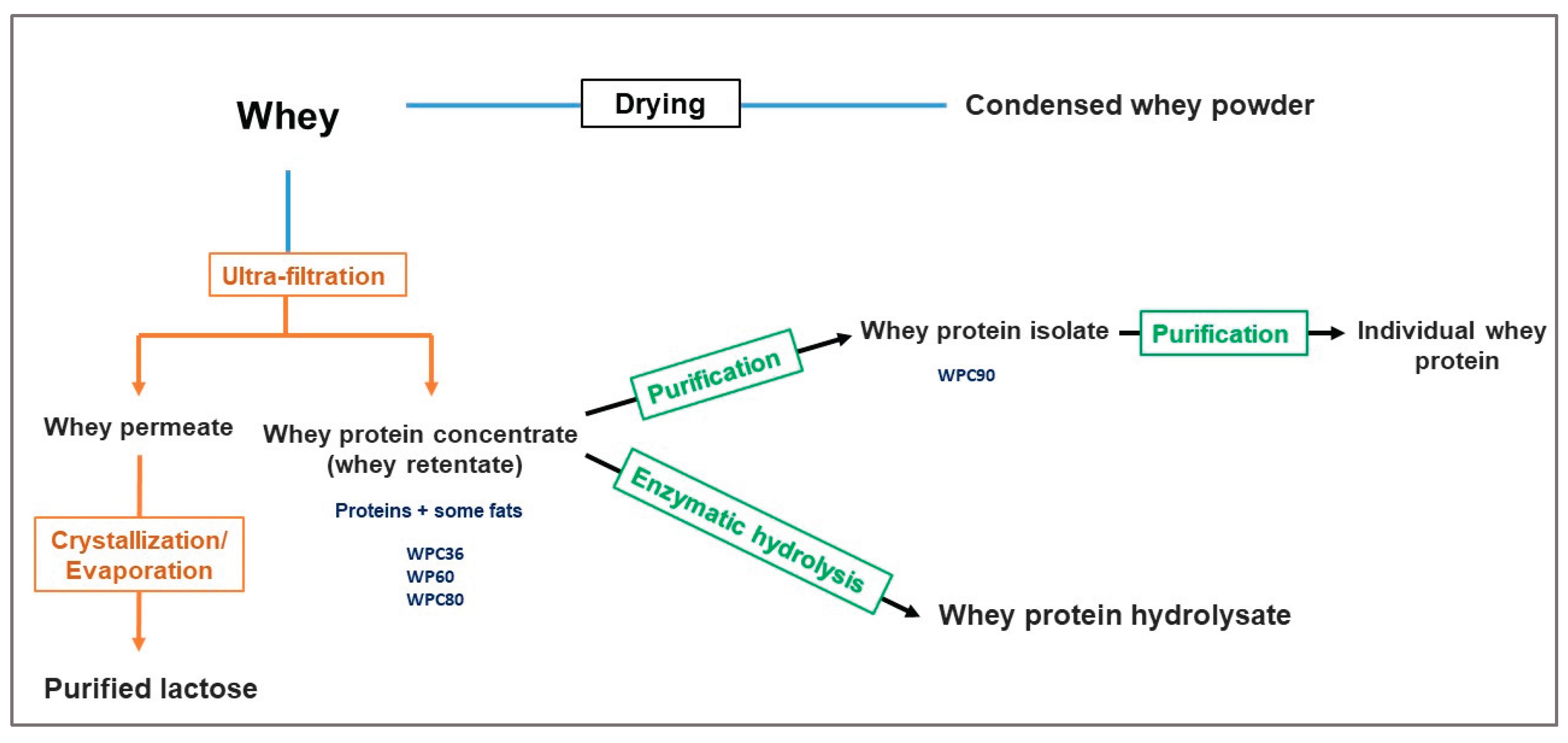
| Total solids | Protein | Fat | Lactose | Ash | Reference | |
|---|---|---|---|---|---|---|
| Cheese whey (%) | 6.3-30.9 | 0.7-14.2 | 0.1-12 | 3.7-4.9 | 0.6-0.7 | [5,116] |
| Second cheese whey (%) | 6.0-7.0 | 0.7-0.9 | 0.1-0.8 | 4.2-5.0 | n.d. | [4] |
| Sweet whey (%) | 6.1-6.6 | 0.78-1.7 | 0.05-0.1 | 4.2-5.3 | 0.5-0.7 | [5,117,118] |
| Acid whey (%) | 6.2-8.7 | 0.5-1.8 | 0.07-0.4 | 3.9-5.1 | 0.7-1.8 | [5,118,119,120] |
| Whey powder (%) | n.d. | 11.0-14.5 | 1.0-1.5 | 63.0-75.0 | 8.2-8.8 | [5] |
| Demineralized whey (%) | n.d. | 11.0-15.0 | 0.5-1.8 | 70.0-80.0 | 1.0-7.0 | [5] |
| Cheese whey permeate (%) | 5.2-6.0 | 0.1-0.4 | <0.01 | 4.7 | 0.52 | [121,122,123] |
| WPC34 (%) | n.d. | 34.0-36.0 | 3.0-4.5 | 47.7-52.0 | 6.1-8.0 | [5,124] |
| WPC60 (%) | n.d. | 56.3-62.0 | 1.0-9.0 | 25.0-30.0 | 4.0-6.0 | [5,124] |
| WPC80 (%) | n.d. | 76.0-82.0 | 4.0-8.3 | 4.0-8.0 | 0.9-4.0 | [5,124] |
| WPI (%) | n.d. | 90.0-92.0 | <1.5 | 0.5-1.0 | 2.0-8.0 | [5,117,125] |
5. Utilisation of Whey in the Generation of Functional Foods
6. Challenges in Introducing Whey in the Food Industry
7. Utilisation of Whey in Non-Food Production: Metabolite Production
7.7. Organic Acids
7.2. Polymers
7.3. Ethanol
7.4. Methane
7.5. Peptides
7.6. Other Metabolites (e.g Vitamins, Glycerol, Volatile Fatty Acids)
8. Conclusion and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ntuli, V.; Sibanda, T.; Elegbeleye, J.A.; Mugadza, D.T.; Seifu, E.; Buys, E.M. Chapter 30 - Dairy production: microbial safety of raw milk and processed milk products. In Present Knowledge in Food Safety, 1st ed; edited by Knowles, M. E., Anelich, L. E., Boobis, A. R., B. Popping M.E.; Academic Press, 2023, 439-454.
- Food and Agriculture Organization Corporate Statistical Database (FAOSATAT). Available online: https://www.fao.org/faostat/ (accessed on June 2025).
- Fancello, F.; Zara, G.; Hatami, F.; Scano, E.A.; Mannazzu, I. Unlocking the potential of second cheese whey: A comprehensive review on valorisation strategies. Reviews in Environmental Science and Bio/Technology 2024, 23(2), pp.411-441. [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Guo, M.; G. Wang. Chapter1 History of Whey Production and Whey Protein Manufacturing. In Whey Protein Production Chemistry, Functionality, and Applications; edited by Guo. M; John Wiley & Sons Lt, 2019, 1-12.
- García-Casas, V.E.; Seiquer, I.; Pardo, Z.; Haro, A.; Recio, I.; Olías, R. Antioxidant Potential of the Sweet Whey-Based Beverage Colada after the Digestive Process and Relationships with the Lipid and Protein Fractions. Antioxidants 2022, 11, 1827. [Google Scholar] [CrossRef]
- Zielińska, D.; Marciniak-Lukasiak, K.; Karbowiak, M.; Lukasiak, P. Effects of Fructose and Oligofructose Addition on Milk Fermentation Using Novel Lactobacillus Cultures to Obtain High-Quality Yogurt-like Products. Molecules 2021, 26, 5730. [Google Scholar] [CrossRef]
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Majcher, M.A. The use of sour and sweet whey in producing compositions with pleasant aromas using the mold Galactomyces geotrichum: identification of key odorants. Journal of Agricultural and Food Chemistry 2020, 68(39), 10799-10807. [CrossRef]
- Kurnick, J.V.; Michellim, M.G.G.; Yada, R.Y.; Junior, B.R.D.C.L.; Tribst, A.A.L. Development of value-added beverages using sheep and goat cheese whey and secondary whey. International Dairy Journal 2024, 152, 105886. [Google Scholar] [CrossRef]
- Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Gonou-Zagou, Z.; Kopsahelis, N. Trametes versicolor as a natural source of bioactive compounds for the production of whey protein films with functional properties: A holistic approach to valorize cheese whey. Waste and Biomass Valorization 2022, 13(9), 3989–3998. [Google Scholar] [CrossRef]
- Moatsou, G.; Moschopoulou, E. CHEESE and WHEY: The Outcome of Milk Curdling. Foods 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food structure 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Alesi, A.; Vittori, S.; Sagratini, G.; Caprioli, G. Development of functional whey cheese enriched in vitamin D3: nutritional composition, fortification, analysis, and stability study during cheese processing and storage. International Journal of Food Sciences and Nutrition 2021, 72(6), 746–756. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Bella, A.; Viegas, J.; Gomes, D.M.; Henriques, M.H.; Pereira, C.J. Use of ultrafiltrated cow's whey for the production of whey cheese with Kefir or probiotics. Journal of the Science of Food and Agriculture 2021, 101(2), 555–563. [Google Scholar] [CrossRef]
- Drężek, K.; Kozłowska, J.; Detman, A.; Mierzejewska, J. Development of a Continuous System for 2-Phenylethanol Bioproduction by Yeast on Whey Permeate-Based Medium. Molecules 2021, 26, 7388. [Google Scholar] [CrossRef]
- Drężek, K.; Sobczyk, M.K.; Kállai, Z.; Detman, A.; Bardadyn, P.; Mierzejewska, J. Valorisation of Whey Permeate in Sequential Bioprocesses towards Value-Added Products–Optimisation of Biphasic and Classical Batch Cultures of Kluyveromyces marxianus. International Journal of Molecular Sciences 2023, 24, 7560. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.M.; Esmael, M.E.; Farrag, A.A.; Ibrahim, M.I. Structural assessment of the bioplastic (poly-3-hydroxybutyrate) produced by Bacillus flexus Azu-A2 through cheese whey valorization. International Journal of Biological Macromolecules 2021, 190, 319–332. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Isipato, M.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. Control of fermentation duration and pH to orient biochemicals and biofuels production from cheese whey. Bioresource Technology 2019, 289, 121722. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-fermentative hydrogen production from cheese whey: Engineering of a mixed culture process in a semi-continuous, tubular photo-bioreactor. International Journal of Hydrogen Energy 2023, 48(55), 21038–21054. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazadeh-Bari, M.; Alizadeh-Khaledabad, M.; Rezaei-Mokarram, R.; Sowti-Khiabani, M. Fermentation Optimization for Co-production of Postbiotics by Bifidobacterium lactis BB12 in Cheese Whey. Waste and Biomass Valorization 2021, 12(11), 5869–5884. [Google Scholar] [CrossRef]
- Solieri, L.; Valentini, M.; Cattivelli, A.; Sola, L.; Helal, A.; Martini, S.; Tagliazucchi, D. Fermentation of whey protein concentrate by Streptococcus thermophilus strains releases peptides with biological activities. Process Biochemistry 2022, 121, 590–600. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Lopes, A.C.A.; Pereira, R.A.; Cardoso, P.G.; Duarte, W.F. Selection of potentially probiotic Kluyveromyces lactis for the fermentation of cheese whey–based beverage. Annals of Microbiology 2019, 69(13), 1361–1372. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Valorisation of sweet whey by fermentation with mixed yoghurt starter cultures with focus on galactooligosaccharide synthesis. International Dairy Journal 2021, 119, 105068. [Google Scholar] [CrossRef]
- Trejo-Flores, P.G.; Santiago-Rodríguez, L.A.; Domínguez-Espinosa, M.E.; Cruz-Salomón, A.; Velázquez-Jiménez, P.E.; Hernández-Méndez, J.M.E.; Morales-Ovando, M.A.; Cruz-Salomón, K.d.C.; Hernández-Cruz, M.d.C.; Vázquez-Villegas, P.T.; Cruz-Rodríguez, R.I. Sustainable Ice Cream Base: Harnessing Mango Seed Kernel (Mangifera indica L. var. Tommy Atkins) Waste and Cheese Whey. Sustainability 2023, 15, 14583. [Google Scholar] [CrossRef]
- El-Aidie, S.A.M.; Khalifa, G.S.A. Innovative applications of whey protein for sustainable dairy industry: Environmental and technological perspectives—A comprehensive review. Comprehensive Reviews in Food Science and Food Safety 2024, 23(2), 13319. 10.1111/1541-4337.13319.
- Hameed, A.; Anwar, M.J.; Perveen, S.; Amir, M.; Naeem, I.; Imran, M.; Hussain, M.; Ahmad, I.; Afzal, M.I.; Inayat, S.; Awuchi, C.G. Functional, industrial and therapeutic applications of dairy waste materials. International Journal of Food Properties 2023, 26(1), 1470–1496. [Google Scholar] [CrossRef]
- Lindsay, M.J.; Walker, T.W.; Dumesic, J.A.; Rankin, S.A.; Huber, G.W. Production of monosaccharides and whey protein from acid whey waste streams in the dairy industry. Green Chemistry 2018, 20(8), 1824–1834. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. Sustainable Approaches in Whey Cheese Production: A Review. Dairy 2023, 4(2), 249–270. [Google Scholar] [CrossRef]
- Wherry, B.; Barbano, D.M.; Drake, M.A. Use of acid whey protein concentrate as an ingredient in nonfat cup set-style yogurt. Journal of Dairy Science 2019, 102(10), 8768–8784. [Google Scholar] [CrossRef]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited review: Acid whey trends and health benefits. Journal of Dairy Science 2021, 104(2), 1262–1275. [Google Scholar] [CrossRef]
- Menchik, P.; Zuber, T.; Zuber, A.; Moraru, C.I. Short communication: Composition of coproduct streams from dairy processing: Acid whey and milk permeate. Journal of Dairy Science 2019, 102(5), 3978–3984. [Google Scholar] [CrossRef]
- Hejtmánková, A.; Pivec, V.; Trnková, E.; Dragounová, H. Differences in the composition of total and whey proteins in goat and ewe milk and their changes throughout the lactation period. Czech Journal of Animal Science 2012, 57(7), 323–331. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Comprehensive Reviews in Food Science and Food Safety 2018, 17(1), 137–164. [Google Scholar] [CrossRef]
- Broersen, K. Milk Processing Affects Structure, Bioavailability and Immunogenicity of β-lactoglobulin. Foods 2020, 9(7), 874. [Google Scholar] [CrossRef]
- Bhutto, R.A.; Fan, Y.; Kang, L.; Wang, M.; Iqbal, S.; Yi, J. Bovine α-lactalbumin: Source, extraction, techno-functional properties, and applications as a (nano-) delivery system for nutraceuticals. Trends in Food Science & Technology 2024, 146, 104381. [Google Scholar]
- Rocha, J.M.; Guerra, A. On the valorization of lactose and its derivatives from cheese whey as a dairy industry by-product: an overview. European Food Research and Technology 2020, 246(11), 2161–2174. [Google Scholar] [CrossRef]
- Roselli, M.; Onesti, R.; Boi, C.; Bandini, S. Recovery of lactose from acid whey by nanofiltration: An experimental study. Separation and Purification Technology 2025, 353, 128303. [Google Scholar]
- ALKaisy, Q.H.; Al-Saadi, J.S.; Al-Rikabi, A.K.J.; Altemimi, A.B.; Hesarinejad, M.A.; Abedelmaksoud, T.G. Exploring the health benefits and functional properties of goat milk proteins. Food Science & Nutrition 2023, 11(10), 5641–5656. [Google Scholar] [CrossRef]
- Salah, A.; Sany, H.; El-Sayed, A.E.K.B.; El-Bahbohy, R.M.; Mohamed, H.I.; Amin, A. Growth Performance and Biochemical Composition of Desmodesmus sp. Green Alga Grown on Agricultural Industries Waste (Cheese Whey). Water, Air, & Soil Pollution 2023, 234(12), 770. [Google Scholar] [CrossRef]
- Borba, K.K.S.; Gadelha, T.S.; Sant’Ana, A.M.S.; Pacheco, M.T.B.; Pinto, L.S.; Madruga, M.S.; Medeiros, A.N.; Bessa, R.J.B.; Alves, S.P.A.; Magnani, M.; Pimentel, T.C. Fatty acids, essential amino acids, minerals and proteins profile in whey from goat cheese: Impacts of raising system. Small Ruminant Research 2022, 217, 106842. [Google Scholar] [CrossRef]
- Lambrini, K.; Aikaterini, F.; Konstantinos, K.; Christos, I.; Ioanna, P.V.; Areti, T. Milk nutritional composition and its role in human health. Journal of Pharmacy and Pharmacology 2021, 9, 8–13. [Google Scholar]
- Zain, S.M.; Behkami, S.; Bakirdere, S.; Koki, I.B. Milk authentication and discrimination via metal content clustering – A case of comparing milk from Malaysia and selected countries of the world. Food Control 2016, 66, 306–314. [Google Scholar] [CrossRef]
- Rako, A.; Kalit, M.T.; Kalit, S.; Soldo, B.; Ljubenkov, I. Nutritional characteristics of Croatian whey cheese (Bračka skuta) produced in different stages of lactation. LWT 2018, 96, 657–662. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Castro, N.; Morales-delaNuez, A.; Sánchez-Macías, D.; Assunção, P.; Capote, J.; Argüello, A. Farm and factory production of goat cheese whey results in distinct chemical composition. Journal of Dairy Science 2009, 92(10), 4792–4796. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.G.; Vegarud, G.E.; Skeie, S. Seasonal and regional variation in the composition of whey from Norwegian Cheddar-type and Dutch-type cheeses. International Dairy Journal 2002, 12(7), 621–629. [Google Scholar] [CrossRef]
- Lievore, P.; Simões, D.R.; Silva, K.M.; Drunkler, N.L.; Barana, A.C.; Nogueira, A.; Demiate, I.M. Chemical characterisation and application of acid whey in fermented milk. Journal of Food Science and Technology 2015, 52(4), 2083–2092. [Google Scholar] [CrossRef]
- Bandara, T.A.; Munasinghe-Arachchige, S.P.; Gamlath, C.J. Fermented whey beverages: A review of process fundamentals, recent developments and nutritional potential. International Journal of Dairy Technology 2023, 76(4), 737–757. [Google Scholar] [CrossRef]
- Cheruiyot, E.K.; Bett, R.C.; Amimo, J.O.; Mujibi, F.D. Milk Composition for Admixed Dairy Cattle in Tanzania. Frontiers in Genetics 2018, 9, 142. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk From Different Species—A Review. Frontiers in Nutrition 2020, 7, 577759. [Google Scholar] [CrossRef]
- New Zealand Food Composition Database 2024, N.Z.F.C.D.D. Manual, Editor.: The New Zealand Institute for Plant & Food Research Limited and Ministry of Health. Available online: https://www.foodcomposition.co.nz/ (accessed on 12 June 2025).
- El-Hatmi, H.; Jrad, Z.; Salhi, I.; Aguibi, A.; Nadri, A.; Khorchani, T. Comparison of composition and whey protein fractions of human, camel, donkey, goat and cow milk. Dairy/Mljekarstvo 2015, 65(3), 159–167. [Google Scholar] [CrossRef]
- Manuelian, C.L.; Penasa, M.; Visentin, G.; Zidi, A.; Cassandro, M.; De Marchi, M. Mineral composition of cow milk from multibreed herds. Animal Science Journal 2018, 89(11), 1622–1627. [Google Scholar] [CrossRef]
- Anand, S.; Som Nath, K.; Chenchaiah, M. Chapter 22 Whey and Whey Products. In Milk and Dairy Products in Human Nutrition; Edited by Park, Y.W.; G.F.W. John Wiley & Sons Ltd, Haenlein, 2013; 477-497.
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Science of The Total Environment 2013, 445-446, 385–396. [Google Scholar] [CrossRef]
- Hebishy, E.; Yerlikaya, O.; Mahony, J.; Akpinar, A.; Saygili, D. Microbiological aspects and challenges of whey powders – I thermoduric, thermophilic and spore-forming bacteria. International Journal of Dairy Technology, 2023, 76(4), 779–800. [Google Scholar] [CrossRef]
- Ballom, K.F.; Tsai, H.C.; Taylor, M.; Tang, J.; Zhu, M.J. Stability of Listeria monocytogenes in non-fat dry milk powder during isothermal treatment and storage. Food Microbiology 2020, 87, 103376. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; Yousef, A.E. Natural Antimicrobials Suitable for Combating Desiccation-Resistant Salmonella enterica in Milk Powder. Microorganisms 2021, 9(2), 421. [Google Scholar] [CrossRef] [PubMed]
- Jandová, M.; Měřička, P.; Fišerová, M.; Landfeld, A.; Paterová, P.; Hobzová, L.; Jarkovská, E.; Kacerovský, M.; Houška, M. Bacillus cereus as a major cause of discarded pasteurized human banked milk: a single human milk bank experience. Foods 2021, 10(12), 2955. [Google Scholar] [CrossRef]
- Sonnier, J.L.; Karns, J.S.; Lombard, J.E.; Kopral, C.A.; Haley, B.J.; Kim, S.W.; Van Kessel, J.A.S. Prevalence of Salmonella enterica, Listeria monocytogenes, and pathogenic Escherichia coli in bulk tank milk and milk filters from US dairy operations in the National Animal Health Monitoring System Dairy 2014 study. Journal of Dairy Science 2018, 101(3), 1943–1956. [Google Scholar] [CrossRef] [PubMed]
- Adzitey, F.; Awini Tibile, B.; Addy, F.; Adu-Bonsu, G.; Atsu Amagloh, A.S.; Noyoro, E.J.; Tsigbey, V.E. Occurrence, antimicrobial susceptibility and genomic characterization of Salmonella enterica isolated from milk and related sources. Cogent Food & Agriculture 2025, 11(1), 2486330. [Google Scholar]
- Shimojima, Y.; Kodo, Y.; Soeda, K.; Koike, H.; Kanda, M.; Hayashi, H.; Nishino, Y.; Fukui, R.; Kuroda, S.; Hirai, A.; Suzuki, J. Prevalence of Cereulide-Producing Bacillus cereus in Pasteurized Milk. Shokuhin eiseigaku zasshi. Journal of the Food Hygienic Society of Japan 2020, 61(5), 178–182. [Google Scholar] [CrossRef]
- Proroga, Y.T.; Capuano, F.; Castellano, S.; Giordano, A.; Mancusi, A.; Delibato, E.; Dumontet, S.; Pasquale, V. Occurrence and toxin gene profile of Bacillus cereus in dairy products. Journal of Microbiology, Biotechnology & Food Sciences 2019, 9(1). [Google Scholar]
- Montone, A.M.I.; Capuano, F.; Mancusi, A.; Di Maro, O.; Peruzy, M.F.; Proroga, Y.T.R.; Cristiano, D. Exposure to Bacillus cereus in water buffalo mozzarella cheese. Foods 2020, 9(12), 1899. [Google Scholar] [CrossRef] [PubMed]
- Di Pinto, A.; Bonerba, E.; Bozzo, G.; Ceci, E.; Terio, V.; Tantillo, G. Occurence of potentially enterotoxigenic Bacillus cereus in infant milk powder. European Food Research and Technology 2013, 237(2), 275–279. [Google Scholar] [CrossRef]
- Tirloni, E.; Stella, S.; Bernardi, C.; Mazzantini, D.; Celandroni, F.; Ghelardi, E. Identification and Pathogenic Potential of Bacillus cereus Strains Isolated from a Dairy Processing Plant Producing PDO Taleggio Cheese. Microorganisms 2020, 8(6), 949. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, G.; Islam, M.S.; Liu, K.; Xue, S.; Song, Z.; Ye, Y.; Zhou, Y.; Shi, Y. , Wei, S.; Zhou, R. Combine thermal processing with polyvalent phage LPEK22 to prevent the Escherichia coli and Salmonella enterica contamination in food. Food Research International 2023, 165, 112454. [Google Scholar] [CrossRef]
- D'Incecco, P.; Limbo, S.; Hogenboom, J.A.; Pellegrino, L. Novel technologies for extending the shelf life of drinking milk: Concepts, research trends and current applications. LWT 2021, 148, 111746. [Google Scholar] [CrossRef]
- Tsermoula, P.; Khakimov, B.; Nielsen, J.H.; Engelsen, S.B. WHEY - The waste-stream that became more valuable than the food product. Trends in Food Science & Technology 2021, 118, 230–241. [Google Scholar] [CrossRef]
- Krivohlavek, A.; Palac Bešlić, I.; Jurak, G.; Gavran, M.; Mandić Andačić, I.; Ivešić, M.; Šikić, S.; Vitale, K.; Štefančić, M.; Žuntar, I.; Oštarić, F. Heavy Metals and Pesticide Residues in Small Farm Cheese Production in Croatia—Challenge between Quality and Quantity. Sustainability 2024, 16(4), 1356. [Google Scholar] [CrossRef]
- Almášiová, S.; Toman, R.; Pšenková, M.; Tančin, V.; Jančo, I. Toxic Elements in Sheep Milk, Whey, and Cheese from the Environmentally Burdened Area in Eastern Slovakia and Health Risk Assessment with Different Scenarios of Their Consumption. Toxics 2024, 12(7), 467. [Google Scholar] [CrossRef]
- Duan, J.; Cheng, Z.; Bi, J.; Xu, Y. Residue behavior of organochlorine pesticides during the production process of yogurt and cheese. Food Chemistry 2018, 245, 119–124. [Google Scholar] [CrossRef]
- Power, C.; Danaher, M.; Sayers, R.; O’Brien, B.; Whelan, M.; Furey, A.; Jordan, K. Investigation of the persistence of rafoxanide residues in bovine milk and fate during processing. Food Additives & Contaminants: Part A 2013, 30(6), 1087–1095. [Google Scholar] [CrossRef]
- Mohammadi, M.; Shadnoush, M.; Sohrabvandi, S.; Yousefi, M.; Khorshidian, N.; Mortazavian, A.M. Probiotics as potential detoxification tools for mitigation of pesticides: a mini review. International Journal of Food Science and Technology 2020, 56(5), 2078–2087. [Google Scholar]
- Yuan, S.; Yang, F.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W. Ultrasonic stimulation of milk fermentation: effects on degradation of pesticides and physiochemical, antioxidant, and flavor properties of yogurt. Journal of the Science of Food and Agriculture 2022, 102(14), 6612–6622. [Google Scholar] [CrossRef]
- Yuan, S.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W. Research on the Mechanism of Ultrasound To Enhance the Biodegradation of Profenofos by Lactiplantibacillus plantarum. ACS Agricultural Science & Technology 2023, 3(6), 535–542. [Google Scholar] [CrossRef]
- Chiesa, L.M.; DeCastelli, L.; Nobile, M.; Martucci, F.; Mosconi, G.; Fontana, M.; Castrica, M.; Arioli, F.; Panseri, S. Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT 2020, 131, 109783. [Google Scholar] [CrossRef]
- Giraldo, J.; Althaus, R.L.; Beltrán, M.C.; Molina, M.P. Antimicrobial activity in cheese whey as an indicator of antibiotic drug transfer from goat milk. International Dairy Journal 2017, 69, 40–44. [Google Scholar] [CrossRef]
- Escobar Gianni, D.; Pelaggio, R.; Cardozo, G.; Moreno, S.; De Torres, E.; Rey, F.; Martínez, I.; Suarez Veirano, G.; Olazabal, L. Transfer of β-lactam and tetracycline antibiotics from spiked bovine milk to Dambo-type cheese, whey, and whey powder. Food Additives & Contaminants: Part A 2023, 40(7), 824–837. [Google Scholar]
- Di Rocco, M.; Scollard, J.; Sayers, R.; Furey, A.; Danaher, M.; Jordan, K.; Lourenco, A. Migration of Cefquinome Antibiotic Residues from Milk to Dairy Products. Dairy 2021, 2(4), 658–670. [Google Scholar] [CrossRef]
- László, N.; Lányi, K.; Laczay, P. LC-MS study of the heat degradation of veterinary antibiotics in raw milk after boiling. Food Chemistry 2018, 267, 178–186. [Google Scholar] [CrossRef]
- Yang, C.; Xie, J.; Gowen, A.; Xu, J.L. Machine learning driven methodology for enhanced nylon microplastic detection and characterization. Scientific Reports 2024, 14(1), 3464. [Google Scholar] [CrossRef]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A.; Poitevin, E. Detection and characterization of small-sized microplastics (≥ 5 µm) in milk products. Scientific Reports 2021, 11(1), 24046. [Google Scholar] [CrossRef]
- Abedi, D.; Niari, M.H.; Ramavandi, B.; De-la-Torre, G.E.; Renner, G.; Schmidt, T.C.; Dobaradaran, S. Microplastics and phthalate esters in yogurt and buttermilk samples: characterization and health risk assessment. Journal of Environmental Health Science and Engineering 2025, 23(1), 14. [Google Scholar] [CrossRef]
- Visentin, E.; Manuelian, C.L.; Niero, G.; Benetti, F.; Perini, A.; Zanella, M.; Pozza, M.; De Marchi, M. Characterization of microplastics in skim-milk powders. Journal of Dairy Science 2024, 107(8), 5393–5401. [Google Scholar] [CrossRef]
- Ling, X.; Cheng, J.; Yao, W.; Qian, H.; Ding, D.; Yu, Z.; Xie, Y.; Yang, F. Identification and Visualization of Polystyrene Microplastics/Nanoplastics in Flavored Yogurt by Raman Imaging. Toxics 2024, 12(5), 330. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Antimicrobial activity of a biosurfactant produced by Bacillus licheniformis strain M104 grown on whey. Brazilian Archives of Biology and Technology 2013, 56, 259–268. [Google Scholar] [CrossRef]
- La Storia, A.; Di Giuseppe, F.A.; Volpe, S.; Oliviero, V.; Villani, F.; Torrieri, E. Physical properties and antimicrobial activity of bioactive film based on whey protein and Lactobacillus curvatus 54M16 producer of bacteriocins. Food Hydrocolloids 2020, 108, 105959. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, H.; Tian, B.; Jiang, B.; Xu, J.; Li, D.; Feng, Z.; Liu, C. Novel edible coating with antioxidant and antimicrobial activities based on whey protein isolate nanofibrils and carvacrol and its application on fresh-cut cheese. Coatings 2019, 9(9), 583. [Google Scholar] [CrossRef]
- Mantovani, R.A.; de Figueiredo Furtado, G.; Netto, F.M.; Cunha, R.L. Assessing the potential of whey protein fibril as emulsifier. Journal of Food Engineering 2018, 223, 99–108. [Google Scholar] [CrossRef]
- İspirli, H.; Dertli, E. Production of lactose derivative hetero-oligosaccharides from whey by glucansucrase E81 and determination of prebiotic functions. LWT 2021, 137, 110471. [Google Scholar] [CrossRef]
- Limnaios, A.; Tsevdou, M.; Zafeiri, E.; Topakas, E.; Taoukis, P. Cheese and Yogurt By-Products as Valuable Ingredients for the Production of Prebiotic Oligosaccharides. Dairy 2024, 5(1), 78–92. [Google Scholar] [CrossRef]
- Chen, G.Q.; Qu, Y.; Gras, S.L.; Kentish, S.E. Separation Technologies for Whey Protein Fractionation. Food Engineering Reviews 2023, 15(3), 438–465. [Google Scholar] [CrossRef]
- Wang, Z.L.; Tang, X.; Wang, M.; She, Y.X.; Yang, B.R.; Sheng, Q.H.; Abd El-Aty, A.M. β-Lactoglobulin separation from whey protein: A comprehensive review of isolation and purification techniques and future perspectives. Journal of Dairy Science 2024, 107(12), 11785–11795. [Google Scholar] [CrossRef]
- Almécija, M.C.; Ibáñez, R.; Guadix, A.; Guadix, E.M. Effect of pH on the fractionation of whey proteins with a ceramic ultrafiltration membrane. Journal of Membrane Science 2007, 288(1-2), 28–35. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Ng, A.Q.Q.; Chew, J.W. Understanding single-protein fouling in micro-and ultrafiltration systems via machine-learning-based models. Industrial & Engineering Chemistry Research 2023, 62(19), 7610–7621. [Google Scholar]
- Ratnaningsih, E.; Reynard, R.; Khoiruddin, K.; Wenten, I.G.; Boopathy, R. Recent Advancements of UF-Based Separation for Selective Enrichment of Proteins and Bioactive Peptides—A Review. Applied Sciences 2021, 11(3), 1078. [Google Scholar] [CrossRef]
- Argenta, A.B.; Scheer, A.D.P. Membrane separation processes applied to whey: A review. Food Reviews International 2020, 36(5), 499–528. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends in Food Science & Technology 2015, 42(1), 44–63. [Google Scholar] [CrossRef]
- Mazzei, R.; Szymczak, A.M.; Drioli, E.; Al-Fageeh, M.; Aljohi, M.A.; Giorno, L. High purity of α-lactalbumin from binary protein mixture by charged UF membrane far from the isoelectric point to limit fouling. Applied Sciences 2021, 11(19), 9167. [Google Scholar] [CrossRef]
- Arahman, N.; Rosnelly, C.M.; Yusni, Y.; Fahrina, A.; Silmina, S.; Ambarita, A.C.; Bilad, M.R.; Gunawan, P.; Rajabzadeh, S.; Takagi, R.; Matsuyama, H. Ultrafiltration of α-Lactalbumin Protein: Acquaintance of the Filtration Performance by Membrane Structure and Surface Alteration. Polymers 2021, 13(21), 3632. [Google Scholar] [CrossRef]
- Dlask, O.; Václavíková, N. Electrodialysis with ultrafiltration membranes for peptide separation. Chemical Papers 2018, 72(2), 261–271. [Google Scholar]
- Kentish, S.E.; Chen, G.Q. Membrane Applications in Dairy Science. In Handbook of Membrane Separations, 3rd ed; Edited by Kentish S. E., Chen G. Q.; CRC Press, Boca Raton 2023, pp. 263-294.
- Talebi, S.; Kee, E.; Chen, G.Q.; Bathurst, K.; Kentish, S.E. Utilisation of salty whey ultrafiltration permeate with electrodialysis. International Dairy Journal 2019, 99, 104549. [Google Scholar] [CrossRef]
- Radosavljević, J.; Stanić-Vučinić, D.; Stojadinović, M.; Radomirović, M.; Simović, A.; Radibratović, M.; Veličković, T.Ć. Application of Ion Exchange and Adsorption Techniques for Separation of Whey Proteins from Bovine Milk. Current Analytical Chemistry 2022, 18(3), 341–359. [Google Scholar] [CrossRef]
- Besselink, T.; Janssen, A.E.M.; Boom, R.M. Isolation of bovine serum albumin from whey using affinity chromatography. International Dairy Journal 2015, 41, 32–37. [Google Scholar] [CrossRef]
- Gurgel, P.V.; Carbonell, R.G.; Swaisgood, H.E. Fractionation of whey proteins with a hexapeptide ligand affinity resin. Bioseparation 2000, 9(6), 385–392. [Google Scholar] [CrossRef]
- El-Sayed, M.M.H.; Chase, H.A. Purification of the two major proteins from whey concentrate using a cation-exchange selective adsorption process. Biotechnology Progress 2010, 26(1), 192–199. [Google Scholar]
- Marciniak, A.; Suwal, S.; Touhami, S.; Chamberland, J.; Pouliot, Y.; Doyen, A. Production of highly purified fractions of α-lactalbumin and β-lactoglobulin from cheese whey using high hydrostatic pressure. Journal of Dairy Science 2020, 103(9), 7939–7950. [Google Scholar]
- Irazoqui, J.M.; Santiago, G.M.; Mainez, M.E.; Amadio, A.F.; Eberhardt, M.F. Enzymes for production of whey protein hydrolysates and other value-added products. Applied Microbiology and Biotechnology 2024, 108(1), 354. [Google Scholar] [CrossRef]
- Eberhardt, A.; López, E.C.; Marino, F.; Mammarella, E.J.; Manzo, R.M.; Sihufe, G.A. Whey protein hydrolysis with microbial proteases: Determination of kinetic parameters and bioactive properties for different reaction conditions. International Journal of Dairy Technology 2021, 74(3), 489–504. [Google Scholar] [CrossRef]
- Ambrosi, V.; Polenta, G.; Gonzalez, C.; Ferrari, G.; Maresca, P. High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innovative Food Science & Emerging Technologies 2016, 38, 294–301. [Google Scholar] [CrossRef]
- Jakopović, K.L.; Cheison, S.C.; Kulozik, U.; Božanić, R. Comparison of selective hydrolysis of α-lactalbumin by acid Protease A and Protease M as alternative to pepsin: potential for β-lactoglobulin purification in whey proteins. Journal of Dairy Research 2019, 86(1), 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hinnenkamp, C.; Ismail, B.P. A proteomics approach to characterizing limited hydrolysis of whey protein concentrate. Food Chemistry 2021, 350, 129235. [Google Scholar] [CrossRef]
- Kruchinin, A.; Barkovskaya, I.; Illarionova, E.; Bolshakova, E.; Turovskaya, S.; Galstyan, A. Effect of enzymatic degradation of proteins on technological properties of whey powdered products. International Journal of Dairy Technology 2025, 78(2), 70005. [Google Scholar] [CrossRef]
- Jrad, Z.; Oussaief, O.; Khorchani, T.; El-Hatmi, H. Microbial and enzymatic hydrolysis of dromedary whey proteins and caseins: techno-functional, radical scavenging, antimicrobial properties and incorporation in beverage formulation. Journal of Food Measurement and Characterization 2020, 14(1), 1–10. [Google Scholar] [CrossRef]
- Kaminarides, S.; Zagari, H.; Zoidou, E. Effect of whey fat content on the properties and yields of whey cheese and serum. Journal of the Hellenic Veterinary Medical Society 2020, 71(2), 2149–2156. [Google Scholar] [CrossRef]
- Tunick, M.H. Chapter 1. Whey Protein Production and Utilization: A Brief History. In Whey Processing, Functionality and Health Benefits, 1st ed; Edited by Onwulata C.I., Huth P. J.; John Wiley & Sons, Ltd. 2008; pp. 1-13.
- Giroux, H.J.; Veillette, N.; Britten, M. Use of denatured whey protein in the production of artisanal cheeses from cow, goat and sheep milk. Small Ruminant Research 2018, 161, 34–42. [Google Scholar] [CrossRef]
- Lavelli, V.; Beccalli, M.P. Cheese whey recycling in the perspective of the circular economy: Modeling processes and the supply chain to design the involvement of the small and medium enterprises. Trends in Food Science & Technology 2022, 126, 86–98. [Google Scholar] [CrossRef]
- Macedo, A.; Azedo, D.; Duarte, E.; Pereira, C. Valorization of Goat Cheese Whey through an Integrated Process of Ultrafiltration and Nanofiltration. Membranes 2021, 11(7), 477. [Google Scholar] [CrossRef]
- O'Donoghue, L.T.; Murphy, E.G. Nondairy food applications of whey and milk permeates: Direct and indirect uses. Comprehensive Reviews in Food Science and Food Safety 2023, 22(4), 2652–2677. [Google Scholar] [CrossRef] [PubMed]
- Jooyandeh, H.; Minhas, K.S. Utilization of fermented whey protein concentrate and whey permeate in beard loaf making. Journal of Food and Bioprocess Engineering 2021, 4(2), 186–192. [Google Scholar]
- Reale, E.; Govindasamy-Lucey, S.; Johnson, M.E.; Jaeggi, J.J.; Molitor, M.; Lu, Y.; Lucey, J.A. Effects of the depletion of whey proteins from unconcentrated milk using microfiltration on the yield, functionality, and nutritional profile of Cheddar cheese. Journal of Dairy Science 2020, 103(11), 9906–9922. [Google Scholar] [CrossRef]
- Melnikova, E.; Bogdanova, E.; Paveleva, D. Chemical composition, functional and technological (processing) properties of whey ingredients. In IOP Conference Series: Earth and Environmental Science, 845 (1), 012017; IOP Publishing Ltd, 2021. IOP Publishing.
- Kelly, P. Chapter 3 - Manufacture of Whey Protein Products: Concentrates, Isolate, Whey Protein Fractions and Microparticulated. In Whey Proteins; Edited by H.C. Deeth, N. Bansal; Academic Press, 2019; pp. 97-122.
- Abadía-García, L.; Castaño-Tostado, E.; Ozimek, L.; Romero-Gómez, S.; Ozuna, C.; Amaya-Llano, S.L. Impact of ultrasound pretreatment on whey protein hydrolysis by vegetable proteases. Innovative Food Science & Emerging Technologies 2016, 37, 84–90. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Jia, J.; Kuang, C.; Yang, H. Effect of ultrasonic pretreatment on whey protein hydrolysis by alcalase: Thermodynamic parameters, physicochemical properties and bioactivities. Process Biochemistry 2018, 67, 46–54. [Google Scholar] [CrossRef]
- Rivas-Vela, C.I.; Amaya-Llano, S.L.; Castaño-Tostado, E.; Castillo-Herrera, G.A. Protein Hydrolysis by Subcritical Water: A New Perspective on Obtaining Bioactive Peptides. Molecules 2021, 26(21), 6655. [Google Scholar] [CrossRef]
- Bella, K.; Pilli, S.; Rao, P.V. A comparison of ultrasonic, ozone, and enzyme pre-treatments on cheese whey degradation for enhancement of anaerobic digestion. Journal of Environmental Management 2023, 340, 117960. [Google Scholar] [CrossRef] [PubMed]
- Bella, K.; Pilli, S.; Rao, P.V. and Tyagi, R.D. Bio-conversion of whey lactose using enzymatic hydrolysis with β-galactosidase: an experimental and kinetic study. Environmental Technology 2024, 45(6), 1234–1247. [Google Scholar]
- Marwaha, S.S.; Kennedy, J.F. Whey—pollution problem and potential utilization. International Journal of Food Science and Technology 1988, 23(4), 323–336. [Google Scholar]
- Fernández-Gutiérrez, D.; Veillette, M.; Giroir-Fendler, A.; Ramirez, A.A.; Faucheux, N.; Heitz, M. Biovalorization of saccharides derived from industrial wastes such as whey: a review. Reviews in Environmental Science and Bio/Technology 2017, 16, 147–174. [Google Scholar] [CrossRef]
- Mehra, R.; Kumar, H.; Kumar, N.; Ranvir, S.; Jana, A.; Buttar, H.S.; Telessy, I.G.; Awuchi, C.G.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P. Whey proteins processing and emergent derivatives: An insight perspective from constituents, bioactivities, functionalities to therapeutic applications. Journal of Functional Foods 2021, 87, 104760. [Google Scholar] [CrossRef]
- Barba, F.J. An Integrated Approach for the Valorization of Cheese Whey. Foods 2021, 10(3), 564. [Google Scholar] [CrossRef]
- Padilla, B.; Frau, F.; Ruiz-Matute, A.I.; Montilla, A.; Belloch, C.; Manzanares, P.; Corzo, N. Production of lactulose oligosaccharides by isomerisation of transgalactosylated cheese whey permeate obtained by β-galactosidases from dairy Kluyveromyces. Journal of Dairy Research 2015, 82(3), 356–364. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, K.; Zhao, F.; Kurahara, L.H.; Li, X.; Yamashita, T.; Hashimoto, T.; Matsuda, Y.; Sun, Z.; Zhang, H.; Hirano, K. Lactulose modulates the structure of gut microbiota and alleviates colitis-associated tumorigenesis. Nutrients 2022, 14(3), 649. [Google Scholar] [CrossRef]
- Seo, Y.H.; M. Sung,; Han, J.-I. Lactulose production from cheese whey using recyclable catalyst ammonium carbonate. Food Chemistry 2016, 197, 664–669. [Google Scholar]
- de Albuquerque, T.L.; Gomes, S.D.L.; D’Almeida, A.P.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; Rocha, M.V.P. Immobilization of β-galactosidase in glutaraldehyde-chitosan and its application to the synthesis of lactulose using cheese whey as feedstock. Process Biochemistry 2018, 73, 65–73. [Google Scholar] [CrossRef]
- de Freitas, M.D.F.M.; Hortêncio, L.C.; de Albuquerque, T.L.; Rocha, M.V.P.; Gonçalves, L.R.B. Simultaneous hydrolysis of cheese whey and lactulose production catalyzed by β-galactosidase from Kluyveromyces lactis NRRL Y1564. Bioprocess and Biosystems Engineering 2020, 43(4), 711–722. [Google Scholar] [CrossRef]
- Wu, L.; Xu, C.; Li, S.; Liang, J.; Xu, H.; Xu, Z. Efficient production of lactulose from whey powder by cellobiose 2-epimerase in an enzymatic membrane reactor. Bioresource Technology 2017, 233, 305–312. [Google Scholar] [CrossRef]
- Karim, A.; Aider, M. Sustainable Valorization of Whey by Electroactivation Technology for In Situ Isomerization of Lactose into Lactulose: Comparison between Electroactivation and Chemical Processes at Equivalent Solution Alkalinity. ACS Omega 2020, 5(14), 8380–8392. [Google Scholar] [CrossRef]
- Duan, F.; Zhao, R.; Yang, J.; Xiao, M.; Lu, L. Integrated Utilization of Dairy Whey in Probiotic β-Galactosidase Production and Enzymatic Synthesis of Galacto-Oligosaccharides. Catalysts 2021, 11(6), 658. [Google Scholar] [CrossRef]
- Limnaios, A.; Tsevdou, M.; Tsika, E.; Korialou, N.; Zerva, A.; Topakas, E.; Taoukis, P. Production of Prebiotic Galacto-Oligosaccharides from Acid Whey Catalyzed by a Novel β-Galactosidase from Thermothielavioides terrestris and Commercial Lactases: A Comparative Study. Catalysts 2023, 13(10), 1360. [Google Scholar]
- Orrego, D.; Klotz-Ceberio, B. Enzymatic Synthesis of Galacto-Oligosaccharides from Concentrated Sweet Whey Permeate and Its Application in a Dairy Product. Applied Sciences 2022, 12(20), 10229. [Google Scholar] [CrossRef]
- Hackenhaar, C.R.; Spolidoro, L.S.; Flores, E.E.E.; Klein, M.P.; Hertz, P.F. Batch synthesis of galactooligosaccharides from co-products of milk processing using immobilized β-galactosidase from Bacillus circulans. Biocatalysis and Agricultural Biotechnology 2021, 36, 102136. [Google Scholar]
- Aguilar-Raymundo, V.G.; Ramírez-Murillo, J.I.; Barajas-Ramírez, J.A. Assessing the yield, physicochemical, sensory characteristics, and acceptance of queso fresco added with whey cheese. International Journal of Food Science and Technology 2022, 57(9), 6038–6045. [Google Scholar]
- Motamedzadegan, A.; Rahmani, S.; Reza kasaai, M.; Amiri, Z.R. Physicochemical and sensory characteristics of foam mat dried ricotta cheese as a function of raw material composition and drying temperature. Journal of Food Processing and Preservation 2022, 46(5), 16510. [Google Scholar]
- Marnotes, N.G.; Pires, A.F.; Díaz, O.; Cobos, A.; Pereira, C.D. Sheep’s and Goat’s Frozen Yoghurts Produced with Ultrafiltrated Whey Concentrates. Applied Sciences 2021, 11(14), 6568. [Google Scholar]
- Moschopoulou, E.; Dernikos, D.; Zoidou, E. Ovine ice cream made with addition of whey protein concentrates of ovine-caprine origin. International Dairy Journal 2021, 122, 105146. [Google Scholar] [CrossRef]
- Lai, G.; Addis, M.; Caredda, M.; Fiori, M.; Dedola, A.S.; Furesi, S.; Pes, M. Development and Characterization of a Functional Ice Cream from Sheep Milk Enriched with Microparticulated Whey Proteins, Inulin, Omega-3 Fatty Acids, and Bifidobacterium BB-12®. Dairy 2024, 5(1), 134–152. [Google Scholar] [CrossRef]
- Rojas, O.E.; Cuervo, L.V.; Serrato, J.C. Sustainable production of lactic acid from cheese whey using Co-cultures and enzymatic hydrolysis. Journal of Chemical Technology & Biotechnology 2025, 100(9), 1940–1947. [Google Scholar]
- Dishan, A.; Gönülalan, Z. Lacticaseibacillus paracasei AD22 Stress Response in Brined White Cheese Matrix: In Vitro Probiotic Profiles and Molecular Characterization. Probiotics and Antimicrobial Proteins 2025, 17(3), 1725–1738. [Google Scholar] [CrossRef]
- Del Toro-Barbosa, M.; Uribe-Velázquez, T.; Hurtado-Romero, A.; Rosales-De la Cruz, M.F.; Carrillo-Nieves, D.; Garcia-Amezquita, L.E.; García-Cayuela, T. Evaluation of GABA-Producing Fermented Whey Formulations: From Strain Selection to Raspberry-Enriched Beverages with Psychobiotic Potential. Foods 2025, 14(16), 2762. [Google Scholar]
- Skryplonek, K.; Dmytrów, I.; Mituniewicz-Małek, A. Probiotic fermented beverages based on acid whey. Journal of Dairy Science 2019, 102(9), 7773–7780. [Google Scholar] [CrossRef]
- Islam, M.Z.; Tabassum, S.; Harun-ur-Rashid, M.; Vegarud, G.E.; Alam, M.S.; Islam, M.A. Development of probiotic beverage using whey and pineapple (Ananas comosus) juice: Sensory and physico-chemical properties and probiotic survivability during in-vitro gastrointestinal digestion. Journal of Agriculture and Food Research 2021, 4, 100144. [Google Scholar]
- Dinkçi, N.; Akdeniz, V.; Akalın, A.S. Probiotic Whey-Based Beverages from Cow, Sheep and Goat Milk: Antioxidant Activity, Culture Viability, Amino Acid Contents. Foods 2023, 12(3), 610. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting attributes. LWT 2022, 160, 113269. [Google Scholar] [CrossRef]
- Shukla, V.; Villarreal, M.; Padilla-Zakour, O.I. Consumer Acceptance and Physicochemical Properties of a Yogurt Beverage Formulated with Upcycled Yogurt Acid Whey. Beverages 2024, 10(1), 18. [Google Scholar] [CrossRef]
- Cunha, D.S.; Coelho, M.C.; Ribeiro, S.C.; Silva, C.C. Application of Enterococcus malodoratus SJC25 for the Manufacture of Whey-Based Beverage Naturally Enriched with GABA. Foods 2022, 11(3), 447. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey as a bio-preservation ingredient. LWT 2023, 174, 114427. [Google Scholar] [CrossRef]
- Luz, C.; Rodriguez, L.; Romano, R.; Mañes, J.; Meca, G. A natural strategy to improve the shelf life of the loaf bread against toxigenic fungi: The employment of fermented whey powder. International Journal of Dairy Technology 2020, 73(1), 88–97. [Google Scholar]
- Ferreyra, L.S.; Verdini, R.A.; Soazo, M.; Piccirilli, G.N. Impact of whey protein addition on wheat bread fermented with a spontaneous sourdough. International Journal of Food Science and Technology 2021, 56(9), 4738–4745. [Google Scholar]
- Pořízka, J.; Slavíková, Z. , Bidmonová, K., Vymětalová, M. and Diviš, P. Physiochemical and Sensory Properties of Bread Fortified with Wheat Bran and Whey Protein Isolates. Foods 2023, 12(13), 2635. [Google Scholar] [CrossRef]
- Andreou, V.; Chanioti, S.; Xanthou, M.Z.; Katsaros, G. Incorporation of Acid Whey Yogurt By-Product in Novel Sauces Formulation: Quality and Shelf-Life Evaluation. Sustainability 2022, 14(23), 15722. [Google Scholar] [CrossRef]
- Ozel, B.; McClements, D.J.; Arikan, C.; Kaner, O.; Oztop, M.H. Challenges in dried whey powder production: Quality problems. Food Research International 2022, 160, 111682. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; O’Connor, J.; Cudmore, M.; Dos Santos, D. Caking behaviour of food powder binary mixes containing sticky and non-sticky powders. Journal of Food Engineering 2017, 204, 73–79. [Google Scholar] [CrossRef]
- Saraç, M.G.; Türker, D.A.; Dogan, M. Determination of morphological structure and powder flow characteristics of commercially important powdered milk products. GIDA - Journal of Food 2021, 46(1), 119–133. [Google Scholar]
- Sahoo, T.K.; Jayaraman, G. Co-culture of Lactobacillus delbrueckii and engineered Lactococcus lactis enhances stoichiometric yield of d-lactic acid from whey permeate. Applied Microbiology and Biotechnology 2019, 103(14), 5653–5662. [Google Scholar] [CrossRef]
- Mejia-Gomez, C.E.; Balcázar, N. Isolation, characterisation and continuous culture of Lactobacillus spp. and its potential use for lactic acid production from whey. Food Science and Technology 2020, 40, 1021–1028. [Google Scholar] [CrossRef]
- Sharma, A.; Mukherjee, S.; Tadi, S.R.R.; Ramesh, A.; Sivaprakasam, S. Kinetics of growth, plantaricin and lactic acid production in whey permeate based medium by probiotic Lactobacillus plantarum CRA52. LWT 2021, 139, 110744. [Google Scholar] [CrossRef]
- Lech, M. Optimisation of protein-free waste whey supplementation used for the industrial microbiological production of lactic acid. Biochemical Engineering Journal 2020, 157, 107531. [Google Scholar] [CrossRef]
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.; Fabbricino, M. Repeated-Batch Fermentation of Cheese Whey for Semi-Continuous Lactic Acid Production Using Mixed Cultures at Uncontrolled pH. Sustainability 2019, 11(12), 3330. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Z.; Xu, Q.; Qian, Z.; Liu, J.; Ouyang, J. Valorization of dairy waste for enhanced D-lactic acid production at low cost. Process Biochemistry 2018, 71, 18–22. [Google Scholar] [CrossRef]
- Louasté, B.; Eloutassi, N. Succinic acid production from whey and lactose by Actinobacillus succinogenes 130Z in batch fermentation. Biotechnology Reports 2020, 27, 00481. [Google Scholar] [CrossRef]
- Uysal, U. , Hamamcı, H. Succinic acid production from cheese whey via fermentation by using alginate immobilized Actinobacillus succinogenes. Bioresource Technology Reports 2021, 16, 100829. [Google Scholar] [CrossRef]
- Banger, G.; Kaya, K.; Omwene, P.; Shakoory, S.; Karagündüz, A.; Keskinler, B.; Nikerel, E. Delactosed Whey Permeate as Substrate for Succinic Acid Fermentation by Actinobacillus succinogenes. Waste and Biomass Valorization 2021, 12(10), 5481–5489. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Marciniak, P.; Moraczewski, K.; Rytlewski, P.; Czaplicki, S.; Zadernowska, A. Cheese whey mother liquor as dairy waste with potential value for polyhydroxyalkanoate production by extremophilic Paracoccus homiensis. Sustainable Materials and Technologies 2022, 33, 00449. [Google Scholar] [CrossRef]
- Bosco, F.; Cirrincione, S.; Carletto, R.; Marmo, L.; Chiesa, F.; Mazzoli, R.; Pessione, E. PHA Production from Cheese Whey and “Scotta”: Comparison between a Consortium and a Pure Culture of Leuconostoc mesenteroides. Microorganisms 2021, 9(12), 2426. [Google Scholar] [CrossRef]
- Das, S.; Majumder, A.; Shukla, V.; Suhazsini, P.; Radha, P. Biosynthesis of Poly(3-hydroxybutyrate) from Cheese Whey by Bacillus megaterium NCIM 5472. Journal of Polymers and the Environment 2018, 26(11), 4176–4187. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Verardo, V.; Martín-García, B.; Oliviero, M.; Baselice, M.; Di Pierro, P.; Sorrentino, A.; Viscardi, S.; Marileo, L.; Sacchi, R. Bioconversion of Cheese Whey and Food By-Products by Phaeodactylum tricornutum into Fucoxanthin and n-3 Lc-PUFA through a Biorefinery Approach. Marine Drugs 2023, 21(3), 190. [Google Scholar] [CrossRef]
- Donzella, S.; Fumagalli, A.; Arioli, S.; Pellegrino, L.; D’Incecco, P.; Molinari, F.; Speranza, G.; Ubiali, D.; Robescu, M.S.; Compagno, C. Recycling Food Waste and Saving Water: Optimization of the Fermentation Processes from Cheese Whey Permeate to Yeast Oil. Fermentation 2022, 8(7), 341. [Google Scholar] [CrossRef]
- Jang, E.J.; Padhan, B.; Patel, M.; Pandey, J.K.; Xu, B.; Patel, R. Antibacterial and biodegradable food packaging film from bacterial cellulose. Food Control 2023, 153, 109902. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Preparation and characterization of bacterial cellulose produced from fruit and vegetable peels by Komagataeibacter hansenii GA2016. International Journal of Biological Macromolecules 2020, 162, 1597–1604. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Papadaki, A.; Stamatiou, A.; Ladakis, D.; Eriotou, E.; Kopsahelis, N. A comprehensive bioprocessing approach to foster cheese whey valorization: on-site β-galactosidase secretion for lactose hydrolysis and sequential bacterial cellulose production. Fermentation 2021, 7(3), 184. [Google Scholar] [CrossRef]
- Lotfy, V.F.; Basta, A.H.; Abdel-Monem, M.O.; Abdel-Hamed, G.Z. Utilization of bacteria in rotten Guava for production of bacterial cellulose from isolated and protein waste. Carbohydrate Polymer Technologies and Applications 2021, 2, 100076. [Google Scholar] [CrossRef]
- Rollini, M.; Musatti, A.; Cavicchioli, D.; Bussini, D.; Farris, S.; Rovera, C.; Romano, D.; De Benedetti, S.; Barbiroli, A. From cheese whey permeate to Sakacin-A/bacterial cellulose nanocrystal conjugates for antimicrobial food packaging applications: a circular economy case study. Scientific Reports 2020, 10(1), 21358. [Google Scholar] [CrossRef]
- Chalermthai, B.; Chan, W.Y.; Bastidas-Oyanedel, J.R.; Taher, H.; Olsen, B.D.; Schmidt, J.E. Preparation and Characterization of Whey Protein-Based Polymers Produced from Residual Dairy Streams. Polymers 2019, 11(4), 722. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, J.; Chen, D.; Shi, Z.; Wang, L. Bioconversion of cheese whey into a hetero-exopolysaccharide via a one-step bioprocess and its applications. Biochemical Engineering Journal 2020, 161, 107701. [Google Scholar] [CrossRef]
- Carrero-Puentes, S.; Fuenmayor, C.; Jiménez-Pérez, C.; Guzmán-Rodríguez, F.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; Alatorre-Santamaría, S.; García-Garibay, M.; Cruz-Guerrero, A. Development and characterization of an exopolysaccharide-functionalized acid whey cheese (requesón) using Lactobacillus delbrueckii ssp. bulgaricus. Journal of Food Processing and Preservation 2022, 46(6), 16095. [Google Scholar] [CrossRef]
- Antunes, S.; Freitas, F.; Alves, V.D.; Grandfils, C.; Reis, M.A. Conversion of cheese whey into a fucose- and glucuronic acid-rich extracellular polysaccharide by Enterobacter A47. Journal of Biotechnology 2015, 210, 1–7. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Bastos, R.; Coelho, E.; Coimbra, M.A.; Silva, C.C. Exopolysaccharide (EPS) Produced by Leuconostoc mesenteroides SJC113: Characterization of Functional and Technological Properties and Application in Fat-Free Cheese. Macromol 2024, 4(3), 680–696. [Google Scholar] [CrossRef]
- Macedo, M.G.; Lacroix, C.; Champagne, C.P. Combined Effects of Temperature and Medium Composition on Exopolysaccharide Production by Lactobacillusrhamnosus RW-9595M in a Whey Permeate Based Medium. Biotechnology Progress 2002, 18(2), 167–173. [Google Scholar] [CrossRef]
- Pendón, M.D.; Madeira Jr, J.V.; Romanin, D.E.; Rumbo, M.; Gombert, A.K.; Garrote, G.L. A biorefinery concept for the production of fuel ethanol, probiotic yeast, and whey protein from a by-product of the cheese industry. Applied Microbiology and Biotechnology 2021, 105(9), 3859–3871. [Google Scholar] [CrossRef]
- Das, B.; Sarkar, S.; Maiti, S.; Bhattacharjee, S. Studies on production of ethanol from cheese whey using Kluyveromyces marxianus. Materials Today: Proceedings 2016, 3(10), 3253–3257. [Google Scholar] [CrossRef]
- Zou, J.; Chang, X. Past, present, and future perspectives on whey as a promising feedstock for bioethanol production by yeast. Journal of Fungi 2022, 8(4), 395. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, F.C.; de Faria, J.T.; da Silva, M.F.; de Souza Oliveira, R.P.; Converti, A. Cheese whey permeate fermentation by Kluyveromyces lactis: a combined approach to wastewater treatment and bioethanol production. Environmental Technology 2020, 41(24), 3210–3218. [Google Scholar] [CrossRef]
- Colacicco, M.; De Micco, C.; Macrelli, S.; Agrimi, G.; Janssen, M.; Bettiga, M.; Pisano, I. Process scale-up simulation and techno-economic assessment of ethanol fermentation from cheese whey. Biotechnology for Biofuels and Bioproducts 2024, 17(1), 124. [Google Scholar] [CrossRef]
- Zhang, R.; Li, F.; Liu, X.; Zhou, X.; Jiang, K. Valorization of Cheese Whey Powder by Two-Step Fermentation for Gluconic Acid and Ethanol Preparation. Applied Biochemistry and Biotechnology 2024, 196(8), 5391–5402. [Google Scholar] [CrossRef]
- Zou, J.; Chen, X.; Hu, Y.; Xiao, D.; Guo, X.; Chang, X.; Zhou, L. Uncoupling glucose sensing from GAL metabolism for heterologous lactose fermentation in Saccharomyces cerevisiae. Biotechnology Letters 2021, 43(8), 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Pasotti, L.; Zucca, S.; Casanova, M.; Micoli, G.; Cusella De Angelis, M.G.; Magni, P. Fermentation of lactose to ethanol in cheese whey permeate and concentrated permeate by engineered Escherichia coli. BMC Biotechnology 2017, 17(1), 48. [Google Scholar] [CrossRef] [PubMed]
- Farkas, C.; Rezessy-Szabó, J.M.; Gupta, V.K.; Bujna, E.; Pham, T.M.; Pásztor-Huszár, K.; Friedrich, L.; Bhat, R.; Thakur, V.K.; Nguyen, Q.D. Batch and Fed-Batch Ethanol Fermentation of Cheese-Whey Powder with Mixed Cultures of Different Yeasts. Energies 2019, 12(23), 4495. [Google Scholar]
- Okamoto, K.; Nakagawa, S.; Kanawaku, R.; Kawamura, S. Ethanol Production from Cheese Whey and Expired Milk by the Brown Rot Fungus Neolentinus lepideus. Fermentation 2019, 5(2), 49. [Google Scholar] [CrossRef]
- Ramos, L.R.; de Menezes, C.A.; Soares, L.A.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Controlling methane and hydrogen production from cheese whey in an EGSB reactor by changing the HRT. Bioprocess and Biosystems Engineering 2020, 43(4), 673–684. [Google Scholar] [CrossRef] [PubMed]
- Mainardis, M.; Flaibani, S.; Trigatti, M.; Goi, D. Techno-economic feasibility of anaerobic digestion of cheese whey in small Italian dairies and effect of ultrasound pre-treatment on methane yield. Journal of Environmental Management 2019, 246, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Treu, L.; Tsapekos, P.; Peprah, M.; Campanaro, S.; Giacomini, A.; Corich, V.; Kougias, P.G.; Angelidaki, I. Microbial profiling during anaerobic digestion of cheese whey in reactors operated at different conditions. Bioresource Technology 2019, 275, 375–385. [Google Scholar] [CrossRef]
- Zolfaghari, S.; Hashemi, S.S.; Karimi, K.; Sadeghi, M. Valorization of cheese whey to eco-friendly food packaging and biomethane via a biorefinery. Journal of Cleaner Production 2022, 366, 132870. [Google Scholar] [CrossRef]
- Ghosh, B.C.; Prasad, L.; Saha, N. Enzymatic hydrolysis of whey and its analysis. Journal of food science and technology 2017, 54, 1476–1483. [Google Scholar] [CrossRef]
- Rosseto, M.; Rigueto, C.V.T.; Gomes, K.S.; Krein, D.D.C.; Loss, R.A.; Dettmer, A.; Richards, N.S.P.D.S. Whey filtration: a review of products, application, and pretreatment with transglutaminase enzyme. Journal of the Science of Food and Agriculture 2024, 104(6), 3185–3196. [Google Scholar] [CrossRef]
- Martín-del-Campo, S.T.; Martínez-Basilio, P.C.; Sepúlveda-Álvarez, J.C.; Gutiérrez-Melchor, S.E.; Galindo-Peña, K.D.; Lara-Domínguez, A.K.; Cardador-Martínez, A. Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants 2019, 8(6), 158. [Google Scholar] [CrossRef]
- Zapata Bustamante, S.; Sepulveda Valencia, J.U.; Correa Londono, G.A.; Durango Restrepo, D.L.; Gil Gonzalez, J.H. Hydrolysates from ultrafiltrated double-cream cheese whey: Enzymatic hydrolysis, antioxidant, and ACE-inhibitory activities and peptide characterization. Journal of Food Processing and Preservation 2021, 45(10), 15790. [Google Scholar] [CrossRef]
- Le Maux, S.; Nongonierma, A.B.; Barre, C.; FitzGerald, R.J. Enzymatic generation of whey protein hydrolysates under pH-controlled and non pH-controlled conditions: Impact on physicochemical and bioactive properties. Food Chemistry 2016, 199, 246–251. [Google Scholar] [CrossRef]
- Elbarbary, H.A.; Ejima, A.; Sato, K. Generation of antibacterial peptides from crude cheese whey using pepsin and rennet enzymes at various pH conditions. Journal of the Science of Food and Agriculture 2019, 99(2), 555–563. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Liu, J.; Meng, Z.; Huang, A.; Xu, F.; Wang, X. Identification, characterization and in vitro activity of hypoglycemic peptides in whey hydrolysates from rubing cheese by-product. Food Research International 2023, 164, 112382. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.M.; Lee, B.H.; Park, B.J.; Kim, S.H.; Oh, D.H. Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Science and Biotechnology 2018, 27(6), 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Mazorra-Manzano, M.A.; Robles-Porchas, G.R.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Martínez-Porchas, M.; García-Sifuentes, C.O.; González-Córdova, A.F.; Vallejo-Córdoba, B. Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity. Fermentation 2020, 6(1), 19. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Pérez-Escalante, E.; Castañeda-Ovando, A.; Contreras-López, E.; Cruz-Guerrero, A.E.; Regal-López, P.; Cardelle-Cobas, A.; González-Olivares, L.G. ACE-Inhibitory Activity of Whey Proteins Fractions Derived of Fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102. Foods 2023, 12(12), 2416. [Google Scholar] [CrossRef]
- Hati, S.; Patel, N.; Sakure, A.; Mandal, S. Influence of Whey Protein Concentrate on the Production of Antibacterial Peptides Derived from Fermented Milk by Lactic Acid Bacteria. International Journal of Peptide Research and Therapeutics 2018, 24(1), 87–98. [Google Scholar] [CrossRef]
- Dineshbhai, C.K.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Patil, G.B.; Mankad, M.; Liu, Z.; Hati, S. Exploring the potential of Lactobacillus and Saccharomyces for biofunctionalities and the release of bioactive peptides from whey protein fermentate. Food Bioscience 2022, 48, 101758. [Google Scholar] [CrossRef]
- Gutiérrez-Cortés, C.; Suarez, H.; Buitrago, G.; Nero, L.A.; Todorov, S.D. Enhanced Bacteriocin Production by Pediococcus pentosaceus 147 in Co-culture With Lactobacillus plantarum LE27 on Cheese Whey Broth. Frontiers in Microbiology 2018, 9, 2952. [Google Scholar] [CrossRef] [PubMed]
- Kocabaş, D.S.; Lyne, J.; Ustunol, Z. Hydrolytic enzymes in the dairy industry: Applications, market and future perspectives. Trends in Food Science & Technology 2022, 119, 467–475. [Google Scholar] [CrossRef]
- de Divitiis, M.; Ami, D.; Pessina, A.; Palmioli, A.; Sciandrone, B.; Airoldi, C.; Regonesi, M.E.; Brambilla, L.; Lotti, M.; Natalello, A.; Brocca, S. Cheese-whey permeate improves the fitness of Escherichia coli cells during recombinant protein production. Biotechnology for Biofuels and Bioproducts 2023, 16(1), 30. [Google Scholar]
- Bianchi, G.; Pessina, A.; Ami, D.; Signorelli, S.; de Divitiis, M.; Natalello, A.; Lotti, M.; Brambilla, L.; Brocca, S.; Mangiagalli, M. Sustainable production of a biotechnologically relevant β-galactosidase in Escherichia coli cells using crude glycerol and cheese whey permeate. Bioresource Technology 2024, 406, 131063. [Google Scholar] [CrossRef]
- Nascimento, M.F.; Barreiros, R.; Oliveira, A.C.; Ferreira, F.C.; Faria, N.T. Moesziomyces spp. cultivation using cheese whey: new yeast extract-free media, β-galactosidase biosynthesis and mannosylerythritol lipids production. Biomass Conversion and Biorefinery 2024, 14(5), 6783–6796. [Google Scholar] [CrossRef]
- Bosso, A.; Setti, A.C.I.; Tomal, A.B.; Guemra, S.; Morioka, L.R.I.; Suguimoto, H.H. Substrate consumption and beta-galactosidase production by Saccharomyces fragilis IZ 275 grown in cheese whey as a function of cell growth rate. Biocatalysis and Agricultural Biotechnology 2019, 21, 101335. [Google Scholar] [CrossRef]
- El-Naga, M.Y.A.; Khan, M.A.; Abu-Hussien, S.H.; Mahdy, S.M.; Al-Farga, A.; Hegazy, A.A. Optimizing lipase production by Bacillus subtilis on cheese whey and evaluating its antimicrobial, antibiofilm, anti virulence and biosafety properties. Scientific Reports 2025, 15(1), 11087. [Google Scholar] [CrossRef] [PubMed]
- Knob, A.; Izidoro, S.C.; Lacerda, L.T.; Rodrigues, A.; de Lima, V.A. A novel lipolytic yeast Meyerozyma guilliermondii: Efficient and low-cost production of acid and promising feed lipase using cheese whey. Biocatalysis and Agricultural Biotechnology 2020, 24, 101565. [Google Scholar] [CrossRef]
- Hausjell, J.; Miltner, M.; Herzig, C.; Limbeck, A.; Saracevic, Z.; Saracevic, E.; Weissensteiner, J.; Molitor, C.; Halbwirth, H.; Spadiut, O. Valorisation of cheese whey as substrate and inducer for recombinant protein production in E. coli HMS174(DE3). Bioresource Technology Reports 2019, 8, 100340. [Google Scholar]
- Alizadeh Behbahani, B.; Jooyandeh, H.; Falah, F.; Vasiee, A. Gamma-aminobutyric acid production by Lactobacillus brevis A3: Optimization of production, antioxidant potential, cell toxicity, and antimicrobial activity. Food Science & Nutrition 2020, 8(10), 5330–5339. [Google Scholar]
- Karimian, E.; Moayedi, A.; Khomeiri, M.; Aalami, M.; Mahoonak, A.S. Application of high-GABA producing Lactobacillus plantarum isolated from traditional cabbage pickle in the production of functional fermented whey-based formulate. Journal of Food Measurement and Characterization 2020, 14(6), 3408–3416. [Google Scholar] [CrossRef]
- De Giorgi, S.; Raddadi, N.; Fabbri, A.; Toschi, T.G.; Fava, F. Potential use of ricotta cheese whey for the production of lactobionic acid by Pseudomonas taetrolens strains. New Biotechnology 2018, 42, 71–76. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, Y.; Cao, M.; Zhang, W.; Lü, C.; Yang, C.; Gao, C.; Xu, P.; Ma, C. Efficient 2,3-butanediol production from whey powder using metabolically engineered Klebsiella oxytoca. Microbial Cell Factories 2020, 19(1), 162. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods 2023, 12(3), 658. [Google Scholar] [CrossRef]
- Ruchala, J.; Andreieva, Y.A.; Tsyrulnyk, A.O.; Sobchuk, S.M.; Najdecka, A.; Wen, L.; Kang, Y.; Dmytruk, O.V.; Dmytruk, K.V.; Fedorovych, D.V.; Sibirny, A.A. Cheese whey supports high riboflavin synthesis by the engineered strains of the flavinogenic yeast Candida famata. Microbial Cell Factories 2022, 21(1), 161. [Google Scholar] [CrossRef] [PubMed]
- Charalampia, D.; Antonios, K.E.; Rafaela, M.; Skiadaresis, A.; Haralabos, K.; Yanniotis, S.; Charalampia, D. Using cheese whey for the production of carotenoids, ergosterol and novel functional foods of industrial interest though a series of optimized bio-and chemical-processes. Journal of Agriculture, Environment and Biotechnology 2019, 4(2), 529–538. [Google Scholar] [CrossRef]
- Sahoo, A.; Mahanty, B.; Daverey, A. and Dutta, K. Nattokinase production from Bacillus subtilis using cheese whey: Effect of nitrogen supplementation and dynamic modelling. Journal of Water Process Engineering 2020, 38, 101533. [Google Scholar] [CrossRef]
- Velez, M.E.V.; da Luz, J.M.R.; da Silva, M.D.C.S.; Cardoso, W.S.; de Souza Lopes, L.; Vieira, N.A.; Kasuya, M.C.M. Production of bioactive compounds by the mycelial growth of Pleurotus djamor in whey powder enriched with selenium. LWT 2019, 114, 108376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





