1. Introduction
Curcumin, the principal bioactive compound in the spice turmeric, has attracted global attention in recent decades after its discovery in 1870 due to its potential health benefits and therapeutic properties [
1]. Promising results from both preclinical and clinical studies suggest that it possesses antioxidant, anti-inflammatory, and anti-carcinogenic effects [
2]. However, the clinical evidence remains mixed, often limited by issues such as low bioavailability, small sample sizes, and variability in study design. Despite these challenges, curcumin-containing products are widely used in food supplements, traditional medicine, and, increasingly, in pharmaceutical applications [
3]. Curcumin is poorly soluble in water, which significantly limits its bioavailability. In addition to its low aqueous solubility, curcumin also exhibits poor gastrointestinal absorption, undergoes rapid Phase II metabolism in the liver and intestinal wall, and is quickly eliminated from the body [
4].
These pharmacokinetic limitations result in low plasma and tissue concentrations after oral administration. Various strategies have been developed to improve its bioavailability, including complexation with carrier systems such as cyclodextrins, liposomes, micelles, or nanoparticles [
5,
6,
7]. Co-administration with fatty foods and the use of bioenhancers such as piperine - a compound found in black pepper that not only improves absorption but also inhibits hepatic metabolism - can further enhance systemic availability and therapeutic potential [
8].
Millions of people worldwide consume curcumin, either as dietary supplements or as an ingredient in functional foods. There is international consensus that orally administered unmodified curcumin is safe across a wide range of doses. Toxicity studies on unmodified curcumin have established an Acceptable Daily Intake (ADI) of 3 mg/kg body weight per day, derived from a No-Observed-Adverse-Effect Level (NOAEL) of 250–320 mg/kg body weight per day by applying an uncertainty factor of 100 [
9].
JECFA has derived an extremely conservative ADI from a NOAEL from a No-Observed-Adverse-Effect Level (NOAEL) of 250–320 mg/kg body weight per day by applying an uncertainty factor of 100. While the authors concluded on the next higher dose level as the NOAEL JECFA has used some minor effects of questionable toxicological relevance for the ADI [
10]. For an average 60 kg person, the ADI of 3 mg/kg body weight per day corresponds to approximately 180 mg per day.
However, it is important to recognize that safety data derived from unmodified curcumin may not directly apply to formulations with enhanced bioavailability. This concern came to the forefront in 2023, when several cases of hepatotoxicity were reported and appeared to be associated with the consumption of curcumin [
11].
The use of dietary supplements containing turmeric, curcumin or CAVACURMIN® has increasingly come under scrutiny, as regulators cannot exclude the possibility that (high) doses of curcumin—particularly those with enhanced bioavailability—may exert off-target or even toxic effects. However, the data base of reported human cases is limited and complex. A clear relationship to bioavailability enhancement could neither be proven nor refuted. Consequently, the Heads of Food Safety Agencies (HoA) requested, in a letter dated June 6, 2024, that the safety of CAVACURMIN® be evaluated through an Article 8 procedure under EC Regulation No 1925/2006 (First report of the HOA working group "Food supplements", BVL June 6, 2024).
For this reason, products designed to increase the bioavailability of curcuminoids must undergo a thorough safety assessment, especially given the wide variety of technologies employed and their potential specific effects related to the active compound curcumin.
Conducting a toxicity study for CAVACURMIN® which is currently sold as food supplement is essential for both consumer protection and scientific and regulatory evaluation. A systematic investigation of its potential toxic effects would help define clear limits for safe intake and provide a basis for evidence-based recommendations. This is particularly important as curcumin is increasingly explored as a future potential therapeutic agent in conditions such as cancer, Alzheimer’s disease, and inflammatory disorders. Without a sound toxicological assessment, clinical trials could be jeopardized, and the development of curcumin-based therapeutics could stall.
Regulatory authorities, including the European Food Safety Authority (EFSA) and the US Food and Drug Administration (FDA), require comprehensive safety data before a substance can be classified as safe or approved for therapeutic use. The present study addresses this regulatory and scientific gap by evaluating the subacute oral toxicity profile of CAVACURMIN® in Wistar rats. Using two dose levels (2000 and 3500 mg/kg/day) and including both vehicle and gamma-cyclodextrin controls, this investigation aimed to:
Identify any treatment-related systemic or local adverse effects over a 28-day period.
Assess potential biochemical or liver weight changes, with interpretation in the context of historical control ranges.
Establish an appropriate high dose for subsequent OECD 408-compliant 90-day studies.
Clarify if gamma-cyclodextrin which makes up a major part of the formulation may be responsible for any observed effect and should be examined as 2nd control group in the sub chronic study.
2. Materials and Methods
2.1. Characterisation of the Test Item
The identity of the test item, CAVACURMIN® (Batch No.: N385030522), was confirmed upon delivery by comparing the test item name, batch number, and other provided data with the label specifications. CAVACURMIN® was received as an orange, solid powder containing 18.3 % curcuminoids (a mixture of CAS 458-37-7, CAS 22608-11-3, and CAS 24939-16-0) and 73 % .gamma.-cyclodextrin (CAS 17465-86-0). Impurities were not included in the final formulation. The material was stored at 2–8 °C, protected from light, and had an expiry date of March 2025. Routine hygienic procedures were deemed sufficient to ensure personnel safety.
2.2. Characterisation of the Control Substance
The second control group (C2) received CAVAMAX® W8 (Batch No.: 801278), consisting of 98.7 % gamma-cyclodextrin (CAS 17465-86-0). The substance was stored at 2–8 °C and protected from light. The expiry date was February 2025. Standard hygienic protocols were followed to assure safe handling.
2.3. Characterisation of the Vehicle
The vehicle used in the study was a 1 % carboxymethylcellulose (CMC) solution prepared using carboxymethylcellulose (Sigma-Aldrich, Batch No.: SLCK1957) and aqua ad injectionem (Deltamedica, Batch No.: 2210342). CMC was a solid stored at room temperature (expiry: 18 May 2027), and aqua ad injectionem was a liquid stored at room temperature (expiry: September 2025). Both were handled under standard hygienic conditions.
2.4. Preparation of the Test Item and Control Formulations
Formulations of the test item and control substance were freshly prepared each administration day. Each formulation was suspended in 1 % CMC, which was selected based on the test item's properties and prepared weekly. Weighed test item or control substance was combined with vehicle to achieve target concentrations and stirred until visually homogenous. During administration, formulations were kept under continuous magnetic stirring. The vehicle alone served as the control item.
2.5. Test System
The study used Wistar rats (Crl: WI[Han]) sourced from Charles River (Sulzfeld, Germany). Both male and female animals were used (non-pregnant and nulliparous females). Animals were 7–8 weeks old at treatment initiation. Body weights at group allocation were 239–269 g for males (mean: 252.85 g) and 155–175 g for females (mean: 166.45 g). Animals were bred under SPF conditions, and the study was conducted in an AAALAC-accredited facility, with ethical approval from the Bavarian animal welfare authority.
2.6. Housing and Feeding Conditions
Animals were housed in polysulphone cages (type IV) in groups of five per sex per group. Environmental conditions included a temperature of 22 ± 3 °C, relative humidity of 40–70 %, and a 12 h light/dark cycle. Ten air changes per hour were maintained. Animals had ad libitum access to Altromin 1324 diet and acidified tap water (pH ~2.8). Food, water, and bedding were certified and records were archived. Animals acclimatised for 7 days prior to treatment.
2.7. Number and Sex of Animals
A total of 40 animals (20 males and 20 females) were used, with five animals per sex per group.
2.8. Preparation of the Animals
Prior to treatment, animals underwent detailed clinical observation and were weighed. Only healthy animals were selected. Animals were randomised into groups using IDBS E-WorkBook 21.9.0 to ensure homogenous body weight distribution.
2.9. Experimental Groups and Doses
Four groups of 5 animals for each sex were established (total 40): two control groups (C1: vehicle; C2: CAVAMAX® W8 at 2555 mg/kg bw/day), a low dose (CAVACURMIN LD: 2000 mg/kg bw/day), and a high dose (HD: 3500 mg/kg bw/day) group. Doses were administered twice daily (2 x 10 mL/kg bw) for 28 consecutive days.
2.10. Administration of Doses
Formulations were administered by oral gavage twice daily with a 4–6-hour interval. Individual dosing volumes were based on the most recent body weights.
2.11. Body Weight and Food Consumption
Body weights were recorded pre-treatment, and on Days 1, 8, 15, 22, 28, and at necropsy. Food consumption was recorded for intervals Day 1–8, 8–15, 15–22, and 22–28.
2.12. Clinical Observations
Clinical signs were assessed daily, including morbidity and mortality checks (twice daily on weekdays, once on weekends/public holidays). Observations were timed to detect peak effects.
2.13. Haematology, Clinical Biochemistry, Pathology, and Organ Weights
At study end, following overnight fasting, blood from the abdominal aorta was collected in EDTA tubes for haematology analysis using an ADVIA®120 (Siemens) and in serum separator tubes for biochemical analysis using an Olympus AU 480 (Beckman Coulter). All surviving animals were euthanised on Day 29 under anaesthesia (ketamine/xylazin) and subjected to gross necropsy, including examination of external and internal organs. Macroscopic abnormalities and all livers were preserved in 4 % neutral-buffered formaldehyde. Animals that died or were euthanised prematurely were also necropsied. The wet weight of livers from all animals was recorded immediately after necropsy.
2.14. Evaluation of Results
Body weight gain and food consumption were calculated weekly. Data were presented in summary tables or individual listings. Toxicological and pathological results were recorded in compliance with SOPs, either on paper or via the Ascentos® System (version 1.3.4, Pathology Data Systems Ltd.).
2.15. Guidelines and Archiving
This study followed the procedures as indicated in the following internationally accepted guidelines and recommendations:
OECD Guidelines for the Testing of Chemicals,
Section 4, No. 408, "Repeated Dose 90-Day Oral Toxicity Study in Rodents," adopted 25 June 2018 [
1].
Commission Regulation (EC) No. 440/2008, L 142, Annex Part B, 30 May 2008 [
2].
Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes [
3].
The national animal protection law, which governs the protection of animals used in experimental and scientific procedures [
4].
All original data generated during the conduct of the study, including raw data and the final report, are stored in the archives of Eurofins Medical Device Testing Munich GmbH (Robert-Koch-Str. 3a, 82152 Planegg, Germany) for a period of 10 years.
3. Results
3.1. Mortality
To determine whether CAVACURMIN® induces mortality or moribund conditions at the tested dose levels, mortality was monitored throughout the 28-day study. Four groups were evaluated: C1 (vehicle), C2 (CAVAMAX® W8 at 2555 mg/kg bw/day), LD (2000 mg/kg bw/day CAVACURMIN® = 366 mg/kg bw/day curcuminoids), and HD (3500 mg/kg bw/day CAVACURMIN® = 640.5 mg/kg bw/day curcuminoids) administered twice daily via oral gavage.
All animals survived to scheduled sacrifice except one female in the LD group (Animal No. 34), which was euthanised in a moribund state on Day 8. Clinical signs in this animal included abnormal breathing, hunched posture, half-closed eyelids, reduced spontaneous activity, slow movement, and pronounced piloerection. These signs first appeared on Day 8. Macroscopic necropsy revealed thoracic cavity filled with brown, pasty fluid, and adhesions between the heart, lungs, and thymus.
Although no histopathology was performed, the presence of fluid—presumably the test item—in the thoracic cavity could suggest a test item-related incident. However, given the isolated occurrence, lack of similar findings in the HD group, and absence of supportive histopathological data, the death was considered incidental and likely due to a pre-existing congenital defect or a gavage error. All other animals showed no abnormalities across any organ system (see Supplementary Table S1).
3.2. Clinical Observations
To determine whether administration of the test items results in observable clinical signs indicative of systemic or local toxicity, daily clinical observations were conducted. No systemic toxicity was observed in this study.
3.2.1. Local Toxicity
Minor local reactions were observed in control animals: one male in the C1 group (Animal No. 2) presented with a superficial head scratch, and one male in the C2 group (No. 10) exhibited transient clinical signs—abnormal breathing, wasp waist, hunched posture, half eyelid closure, and mild-to-moderate piloerection—which resolved within 3 days. These findings were considered incidental.In contrast, local signs post-dosing were common in the curcumin-treated groups and appeared to be related to the test item:
- LD group: Increased salivation was observed in 2/5 males and 1/5 females. Bedding displacement was noted in 3/5 males and 1/5 females.
- HD group: Increased salivation occurred in all males (5/5) and 3/5 females. Bedding displacement was observed in all animals (5/5 males and 5/5 females).
These signs appeared immediately after gavage and were transient, indicating a local irritant or sensory effect of CAVACURMIN® rather than systemic toxicity.
3.2.2. Clinical Signs in Female and Male Animals
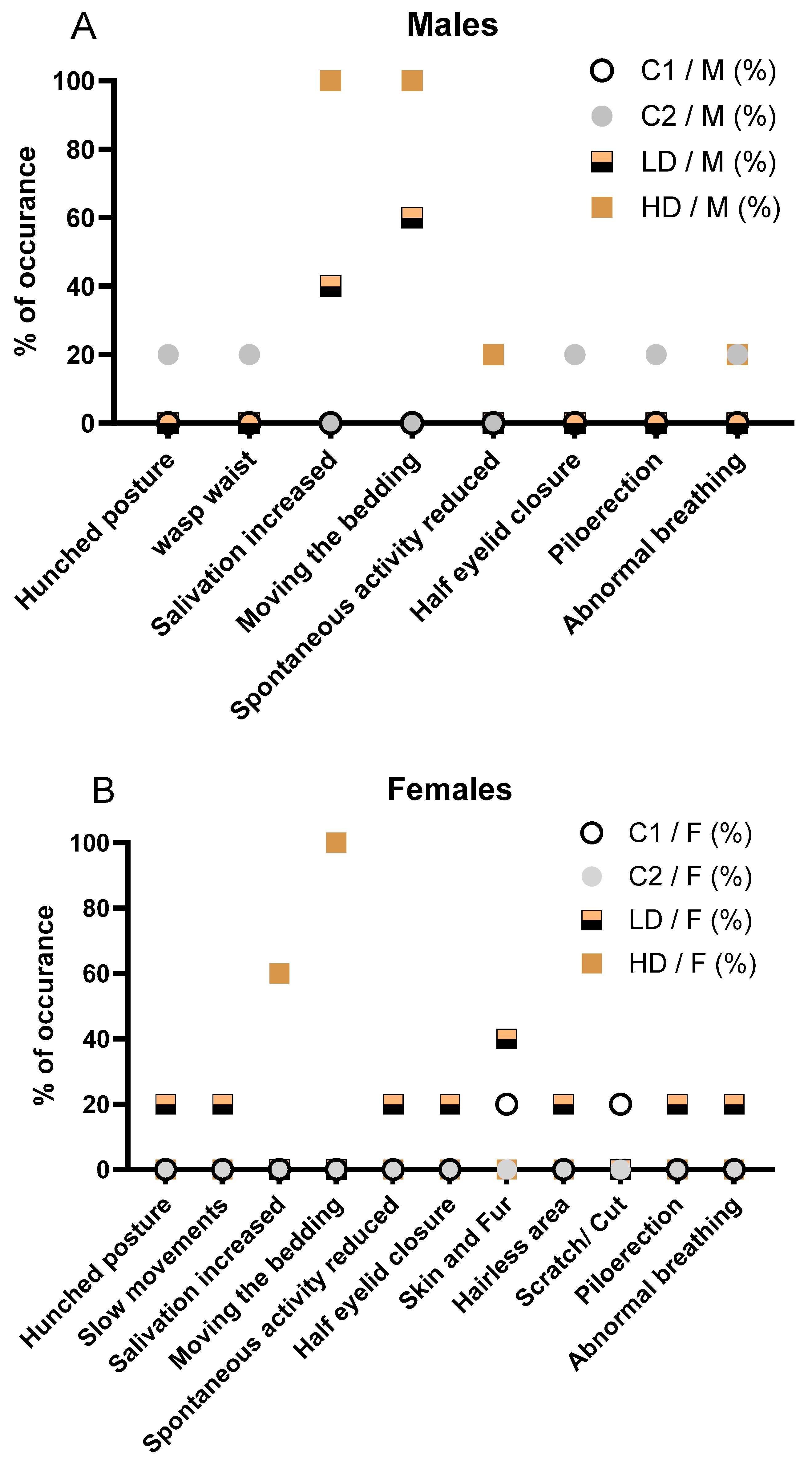
Throughout the 28-day observation period, the incidence of clinical signs increased with dose level, with no signs observed in the C2 control group and only a single minor event (a superficial scratch) noted in 1/5 animals (20 %) in the C1 control group. In the low dose CAVACURMIN® (LD) group, 2/5 females (40 %) exhibited clinical signs, while all animals (5/5, 100 %) in the high dose (HD) CAVACURMIN® group were affected.
In detail, in male animals, the incidence and severity of clinical signs increased with dose level. No clinical signs were observed in the C1 control group. One animal (1/5, 20 %) in the C2 control group displayed multiple transient signs including hunched posture, wasp waist, half eyelid closure, piloerection, and abnormal breathing. These signs were short-lived and considered unrelated to treatment. In the low dose CAVACURMIN® (LD) group, 3 out of 5 males (60 %) exhibited clinical signs. Most prominent were signs related to local irritation, aversion behaviour or activity stimulation, including increased bedding-moving behaviour (3/5, 60%) and increased salivation (2/5, 40 %), both observed shortly after administration and resolving spontaneously (
Fig. 1A).
All animals in the male high dose (HD) CAVACURMIN® group (5/5, 100 %) displayed clinical signs. The most frequent were increased salivation (100 %), bedding-moving behaviour (100 %), and activity stimulation-related signs. One male also showed slightly reduced spontaneous activity and abnormal breathing, suggesting mild transient effects (
Fig. 1A).
Signs observed in the female LD group included hunched posture, slow movements, reduced spontaneous activity, piloerection, half eyelid closure, and minor skin alterations (hairless area). These events were infrequent and isolated to individual animals (
Figure 1B). The females in the HD group predominantly exhibited increased salivation (3/5, 60 %) and bedding-moving behaviour (5/5, 100 %), both occurring shortly after dosing. These findings are consistent with a local irritant response to the test item. Importantly, no systemic toxicity-related signs such as hunched posture or reduced activity were recorded in this group (
Figure 1B).
No animals in any group displayed musculoskeletal or pulmonary abnormalities beyond the isolated cases in the LD group. The absence of clinical signs in the C2 group supports the interpretation that the observed signs in the treated groups are test item-related and dose-dependent but largely limited to local reactions rather than systemic toxicity.
The observed salivation and bedding-moving behavior in both LD and HD groups were immediate post-dose events, likely reflecting a local or sensory irritant effect of curcumin, rather than systemic toxicity. No musculoskeletal, dermatological, or ocular signs were observed in treated animals, and isolated signs such as reduced spontaneous activity or abnormal breathing were rare and not dose dependent. Overall, the pattern in male animals mirrors that seen in females, with a clear dose-dependent increase in non-systemic, transient clinical signs suggestive of local irritation. The detailed description of all clinical observations is also available in Supplementary Table S2A and S2B).
(A) Incidence of clinical signs in male animals, stratified by treatment group ((C1: vehicle; C2: CAVAMAX® W8 at 2555 mg/kg bw/day), a low CAVACURMIN® dose (LD: 2000 mg/kg bw/day), and a high CAVACURMIN® dose (HD: 3500 mg/kg bw/day) group. Doses were administered twice daily (2 x 10 mL/kg bw) for 28 consecutive days (
Table 1). (B) Equivalent data for female animals. Signs included increased salivation, bedding-moving behaviour, piloerection, hunched posture, and abnormal breathing. Data reflect non-systemic, transient signs consistent with local irritant effects of the test item.
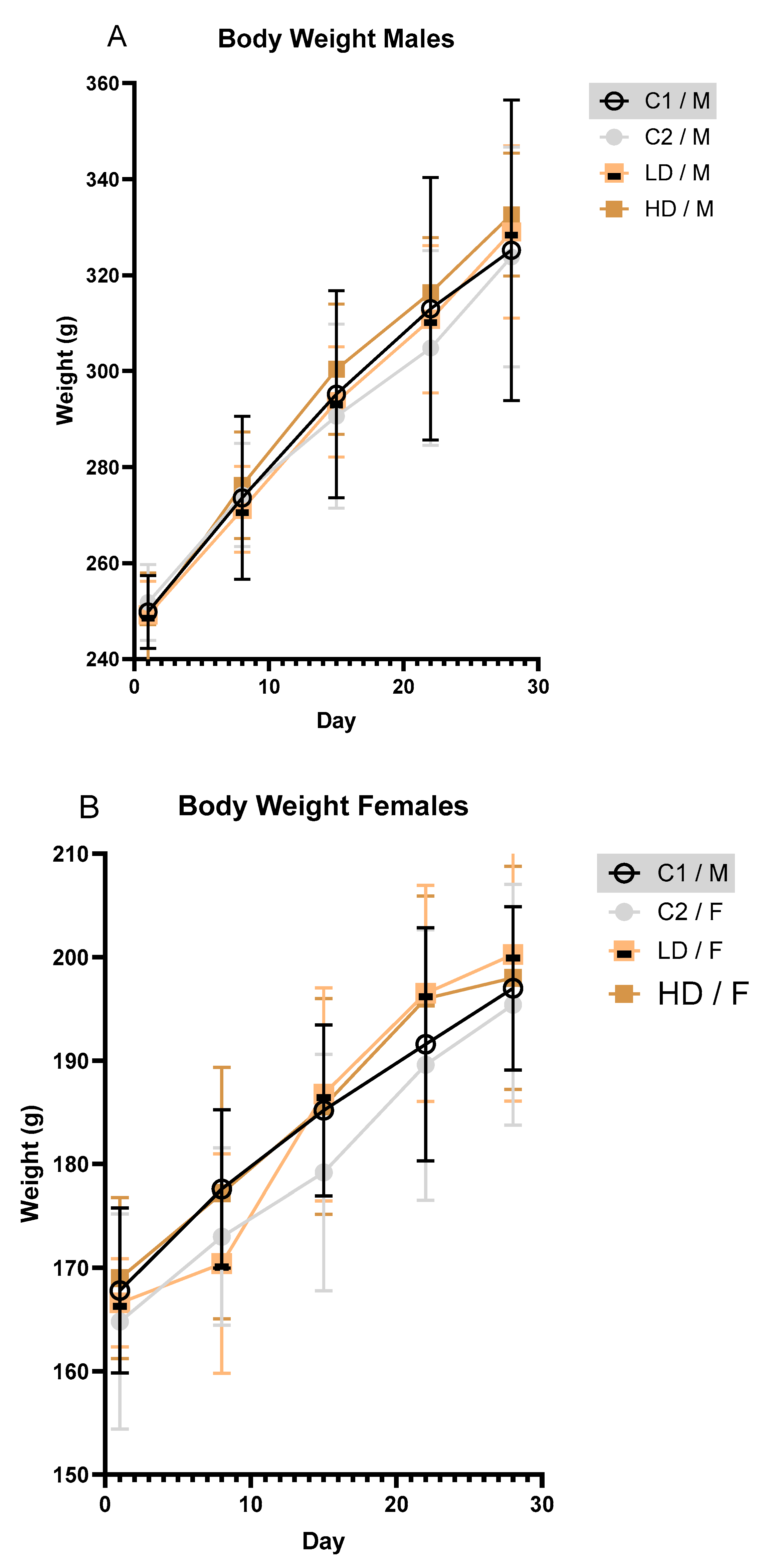
3.2.3. Body Weight
To investigate whether and how the exposure to the test item affects body weight over time in male and female rodents, body weights were recorded at regular intervals. Mean body weight increased steadily across all groups during the study. No toxicologically significant differences were observed between treated and control animals of either sex (
Fig. 2A, B).
3.2.4. Food Consumption
To assess whether the test item influences feeding behaviour or food intake during the observation period, mean daily food consumption was recorded and compared in one animal of each group. Food consumption was not affected by the test item. Average daily intake in treated animals (both sexes) was comparable to controls throughout the study (Supplementary Table S3).
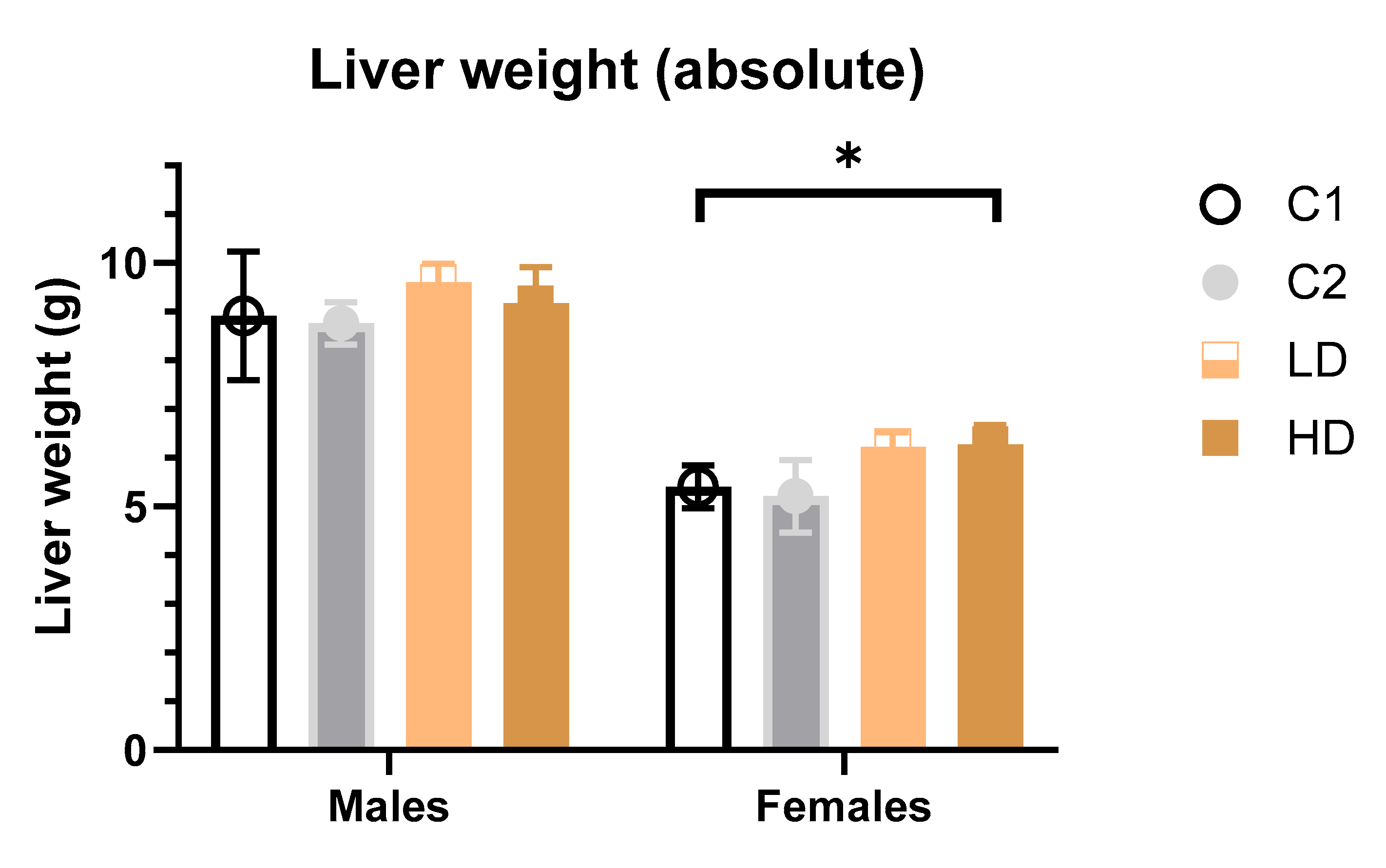
3.2.5. Organ Weight
To determine whether the test item alters absolute or relative liver weights—were recorded and analysed.Absolute weight: In female HD animals, a significant increase (~14 % vs. control C1) in both absolute and relative liver weights was detected. A similar but non-significant increase was noted in the LD group. All values remained within the historical control range, and no such effect was seen in the C2 control group, suggesting the finding may be test item-related but not toxicologically relevant (
Fig. 3)
. In male animals, no statistically significant differences in liver weights were observed in animals across dose groups (
Fig. 3).
Relative weight: The results of the multiple comparisons (Tukey HSD) against the C1 control group, for both males and females did not show statistically significant differences in liver weight ratio compared to C1 (Supplementary Table S4).
3.2.6. Pathology
To identify whether the test item induces any gross pathological changes in major organs or tissues, macroscopic evaluations were conducted at necropsy.
Males (C1/M, C2/M, LD/M, HD/M): No macroscopic abnormalities were observed in any of the 20 male rats across all dose groups. All examined organs and tissues—including brain, spinal cord, heart, lungs, liver, kidneys, gastrointestinal tract, lymphatic tissues, and reproductive organs—were normal in appearance.
Females (C1/F, C2/F, LD/F, HD/F): Most female animals (19/20) showed no macroscopic abnormalities, including in gastrointestinal, urogenital, or nervous system tissues of females.
In summary, no gross pathological abnormalities were detected in any animals that survived to their scheduled sacrifice, in either treated or control groups (Supplementary Table S1).
3.2.7. Haematology
To determine whether the test item affects haematological parameters in a dose-dependent manner, standard haematology analyses were performed at the end of the treatment period.No statistically significant differences were observed in any haematological parameters in male animals. For WBCs, despite the large effect size in both sexes, the difference was not significantly different due to the large variability among the animals. In females, a dose-dependent but statistically non-significant increase in platelet count (PLT) was observed (LD: +4 %; HD: +14 % vs. control C1). Two HD females had values above the historical control range. Although potentially test item-related, the low magnitude of the change and lack of statistical significance indicated no toxicological relevance.
3.2.8. Clinical Biochemistry (Blood Biomarkers)
To evaluate potential organ-specific toxicological effects, a panel of standard serum biochemistry parameters in blood was assessed on Day 29. These parameters provide insight into the functional status of key organs, particularly the liver and kidneys. The presence of Alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) in blood serve as sensitive indicators of hepatocellular injury, while alkaline phosphatase (AP) is associated with cholestatic liver damage and bone turnover. Creatinine (Crea) and urea are widely used markers of renal function, reflecting glomerular filtration and nitrogen metabolism, respectively. Total protein (TP) and albumin (ALB) offer additional information on nutritional and hepatic synthetic status, as well as systemic inflammation.
The measured values for each group, along with their standard deviations and percentage deviation from control, are summarized below and in Supplementary Table S4.
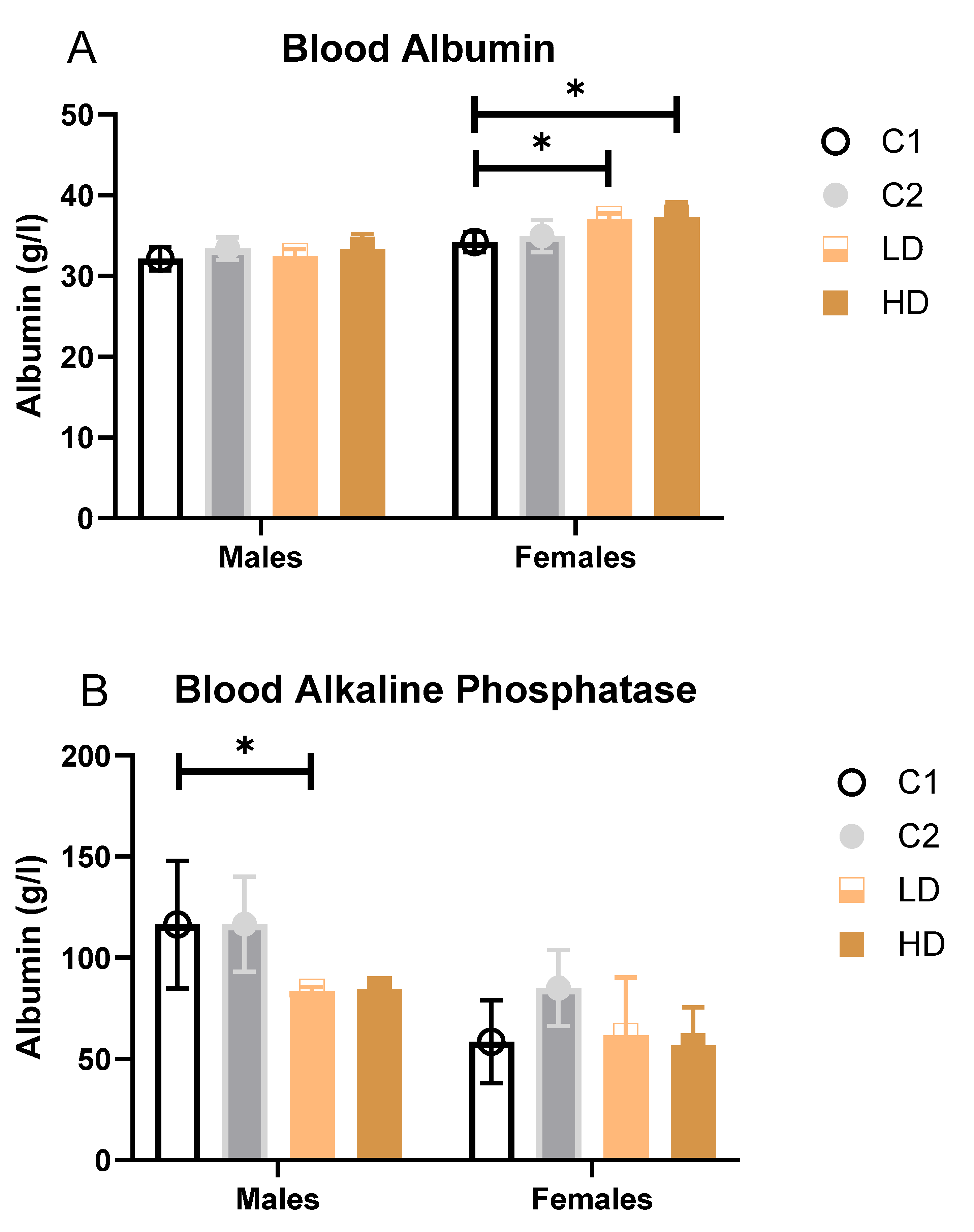
Parameters that showed statistically significant differences between groups (Blood Albumin, Alkaline phosphatase and Urea) are additionally illustrated as bar graphs to facilitate visual comparison.
Liver markers: ALAT decreased significantly in C2 and LD and showed a trend across groups, suggesting a degree of hepatoprotection rather than injury. Albumin increased in females of both dose groups (LD: +8 %; HD: +9 % vs. control C1). While some values exceeded the historical control range, the change was not considered toxicologically significant (
Figure 4A).
Alkaline Phosphatase (AP): A statistically significant decrease was observed in LD males (−28 % vs. C1); however, all individual values remained within the historical range (
Figure 4B).
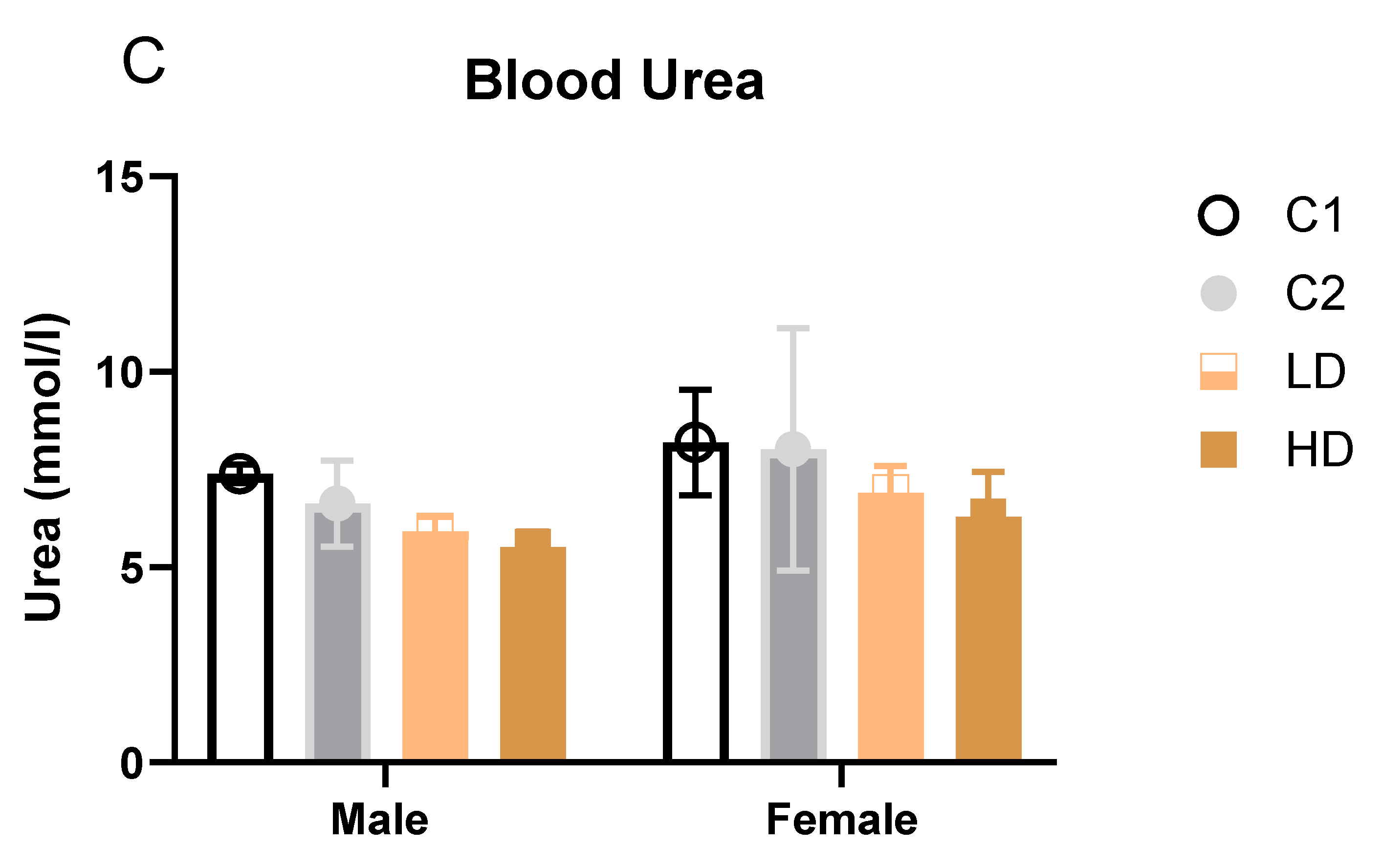
Renal markers: Males showed statistically significant decreases in both dose groups (LD: −20 %; HD: −25 %). One HD male value was below the historical range. Females showed a non-significant trend toward decreased urea levels (LD: −16 %; HD: −23 %). All individual values remained within historical limits, except one animal in group C2 (considered incidental) (
Figure 4C). Creatinine remained unchanged. The dose-dependent reduction in blood urea, in the absence of creatinine changes or renal pathology, may reflect improved renal function and a favourable shift in nitrogen metabolism rather than an adverse effect.
Proteins: Total protein was relatively stable, with no concern for protein loss or synthesis impairment.
For all other evaluated parameters not specifically mentioned, no sex-related differences were observed.
A structured summary and interpretation of all clinical biochemical parameters measured on Day 29 (mean ± SD, N=5 per group), with statistical annotations and percentage deviations vs control is available as Supplementary Table S4.
4. Discussion
This 28-day oral toxicity study demonstrates that CAVACURMIN®, a gamma-cyclodextrin-complexed curcumin formulation with markedly enhanced bioavailability, was well tolerated in male and female Wistar rats at daily doses up to 3500 mg/kg. No systemic toxicity, adverse effects on survival, or meaningful changes in body weight, food intake, haematology, or gross pathology were observed.
The main clinical findings - transient salivation and bedding displacement - were dose-dependent, appeared immediately after gavage, and resolved spontaneously within minutes. These signs are consistent with local sensory or irritant effects rather than systemic toxicity, a pattern reported previously for nanoparticle and micellar curcumin preparations [
12,
13].
A small increase in absolute liver weight (~14 %) was observed in high-dose females. However, values remained within historical control ranges and were not accompanied by elevated liver enzymes or pathological changes. Such small liver weight increases have also been reported in subchronic studies involving THERACURMIN® and other bioavailable curcumin formulations and were typically interpreted as adaptive, non-adverse responses such as hepatic enzyme induction [
13,
14,
15]. Similar liver weight increases were also reported for conventional (low bioavailability) curcumin formulations, which in general have also yielded neutral toxicological profiles.
Three rodent studies—including a two-generation reproductive study at high dietary doses (10,000–15,000 ppm) and two others using ethanolic turmeric extracts and histomorphological assessment —have reported similar small increases in liver weight. In all three these cases, liver weights generally remained within historical control ranges, were not accompanied by hepatocellular injury or significant enzyme elevations, and were therefore interpreted as adaptive changes (likely reflecting hepatocellular hypertrophy or metabolic enzyme induction) rather than adverse toxicological effects [
14,
15,
16].
The modest increase in serum albumin observed in CAVACURMIN®-treated females, in the absence of liver pathology or enzyme elevations, may reflect a beneficial modulation of protein metabolism rather than an adverse effect. Similarly, the modest decrease in alkaline phosphatase, observed without accompanying pathology or liver enzyme elevations, may indicate a favourable modulation of hepatic or bone metabolism. The dose-dependent reduction in blood urea, in the absence of creatinine changes or renal pathology, may reflect improved renal function and a favourable shift in nitrogen metabolism. For all other evaluated parameters not specifically mentioned, no sex-related differences were observed.
Serum biochemistry changes included a dose-dependent reduction in urea levels in male rats and a modest, statistically significant increase in serum albumin in females. Both parameters remained within historical control ranges and were not associated with clinical signs or pathological findings, rendering them of uncertain toxicological significance. It can be suggested that these findings are suggest positive favourable pharmacological properties, including enhanced nitrogen retention, which supports anabolic and tissue-maintenance processes, alongside a mild anti-inflammatory modulation that may contribute to improved recovery and overall physiological resilience without overt immunosuppression. Interestingly, a similar biochemical profile was previously observed in a 90-day curcumin-piperine study, where decreased urea and increased albumin were attributed to enhanced nitrogen retention and mild anti-inflammatory modulation [
17].
Haematological evaluations revealed a non-significant trend toward increased platelet counts (PLT) in LD and HD females. In contrast, no meaningful changes in haematology were observed in male animals.
Importantly, there were no CAVACURMIN®-related effects on mortality, systemic clinical signs, body weight, food consumption, or gross pathology. The absence of relevant differences between the two control groups (vehicle vs gamma-cyclodextrin) further suggests that the carrier substance does not contribute to any observed effects, supporting the use of a single control group in future studies.
As this was a dose-range finding study, the relatively small group size, limited 28-day duration, and omission of histopathology were intentional design features; while these constrain definitive long-term conclusions, they are appropriate for establishing suitable dose levels for subsequent chronic studies.
Overall, these results align with previous preclinical work showing that curcumin - whether in conventional or enhanced-bioavailability form - has a wide safety margin. The present study fulfils OECD 408 dose-range finding objectives and identifies 3500 mg/kg/day as an appropriate top dose for a subsequent 90-day toxicity study, which will include full histopathological evaluation to establish a definitive NOAEL for regulatory submissions.
5. Conclusions
This 28-day dose-range finding study in male and female Wistar rats demonstrated that CAVACURMIN® was well tolerated at daily doses of 2000 and 3500 mg/kg body weight. Statistically significant changes included a modest increase in serum albumin in low- and high-dose females, and a decrease in serum urea in low- and high-dose males. All values remained within historical control ranges and were unaccompanied by adverse clinical signs, organ pathology, or other indicators of systemic toxicity, suggesting these shifts are non-adverse and potentially reflect favourable physiological modulation.
No treatment-related effects were observed on mortality, overall clinical condition, body weight progression, food consumption, gross pathology, or organ weights in either sex. No meaningful differences were detected between the vehicle and gamma-cyclodextrin control groups, indicating that a single control group may be sufficient in future studies.
Based on these findings, 3500 mg/kg body weight/day is an appropriate top dose for a subsequent OECD 408-compliant 90-day repeated-dose oral toxicity study. That investigation will incorporate full histopathological evaluation and expanded endpoints to establish a definitive no-observed-adverse-effect level (NOAEL) to support regulatory submissions and the continued clinical development of CAVACURMIN®.
Funding
Funding for the Study was provided by Wacker Chemie AG, Gisela-Stein-Straße 1, 81671 München, Germany.
Materials Availability and Data Availability Statements
CAVACURMIN® is commercially available. The data are mostly shown in the text and the supplementary information.
Author Contributions (CRediT taxonomy)
Conceptualization, Heiko Zipp and Sandra Schmid; Formal analysis, Gerald Muench; Investigation, Natascha Rivera; Validation, Gerald Muench; Writing – original draft, Heiko Zipp and Gerald Muench; Writing – review & editing, Heiko Zipp, Marco Kellert and Gerald Muench.
Animal Ethics Statement
The following study intended for publication was notified to the Government of Upper Bavaria: 28-Day Dose Range Finding Oral Toxicity Study in Wistar Rats with CAVACURMIN® – 2024, BSL Munich Study No.: 2200461 Reference number: ROB-55.2-2532.Vet_03-18-63 This study only required notification to the authority; no special approval was necessary.
Acknowledgments
We thank BSL BIOSERVICE Scientific Laboratories Munich GmbH for conducting the biosafety testing and the report, this publication is based on.
Conflicts of Interest
M.K., H.Z are employees of Wacker Chemie AG.
References
- Daube, F.W. Ueber Curcumin den Farbstoff der Curcuma-Wurzel. Inaugural Dissertation, Freiburg i. B., 1870.
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Woltje, K. Curcumin and Its Role in Health and Disease; Nutrition and Diet Research Progress; Nova Science Publishers: Hauppauge, NY, USA, 2023. [Google Scholar]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutrients 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.X.; Mackie, A.R.; Ettelaie, R.; Niaz, T.; Murray, B.S. Enhancement of curcumin bioaccessibility: An assessment of possible synergistic effect of γ-cyclodextrin metal–organic frameworks with micelles. Food Res. Int. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Chuah, A.M.; Jacob, B.; Jie, Z.; Ramesh, S.; Mandal, S.; Puthan, J.K.; Deshpande, P.; Vaidyanathan, V.V.; Gelling, R.W.; Patel, G.; et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chem. 2014, 156, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, M.; Guzowska, M.; Gazi, O.; Urbański, J.; Bieganowski, P. Curcumin dispersed with colloidal nanoparticles inhibits enteric virus replication. Antivir. Res. 2025, 237, 106140. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Makino, Y. Novel curcumin oral delivery systems. Anticancer Res. 2013, 33, 2807–2821. [Google Scholar] [PubMed]
- Dadhaniya, P.; Patel, C.; Muchhara, J.; Bhadja, N.; Mathuria, N.; Vachhani, K.; Soni, M.G. Safety assessment of a solid lipid curcumin particle preparation: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2011, 49, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Ganiger, S.; Malleshappa, H.N.; Krishnappa, H.; Rajashekhar, G.; Rao, V.R.; Sullivan, F. A two-generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Li, Y.J.; Dellinger, A.; Navarro, V.; Bonkovsky, H.; Fontana, R.J.; Gu, J.; Barnhart, H.; Phillips, E.; Lammert, C.; et al. Clinical features, outcomes, and HLA risk factors associated with nitrofurantoin-induced liver injury. J. Hepatol. 2023, 78, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- El-Akabawy, G.; El-Sherif, N.H.; Othman, A.I.; El-Missiry, M.A. Ethanolic extract of Curcuma longa ameliorates metabolic syndrome–induced hepatic and renal injury in rats. Egypt. Liver J. 2023, 13, 8. [Google Scholar] [CrossRef]
- Salama, A.; Elmongy, N.; Elhady, M. Curcumin alleviates hepatotoxicity induced by lead acetate via activation of Nrf2/HO-1 pathway. Pathophysiology 2018, 25, 197–204. [Google Scholar] [CrossRef]
- Ganiger, S.; Malleshappa, H.N.; Krishnappa, H.; Rajashekhar, G.; Rao, V.R.; Sullivan, F. A two-generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).