Submitted:
25 September 2025
Posted:
26 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Study population
3.2. Indicators of puberty
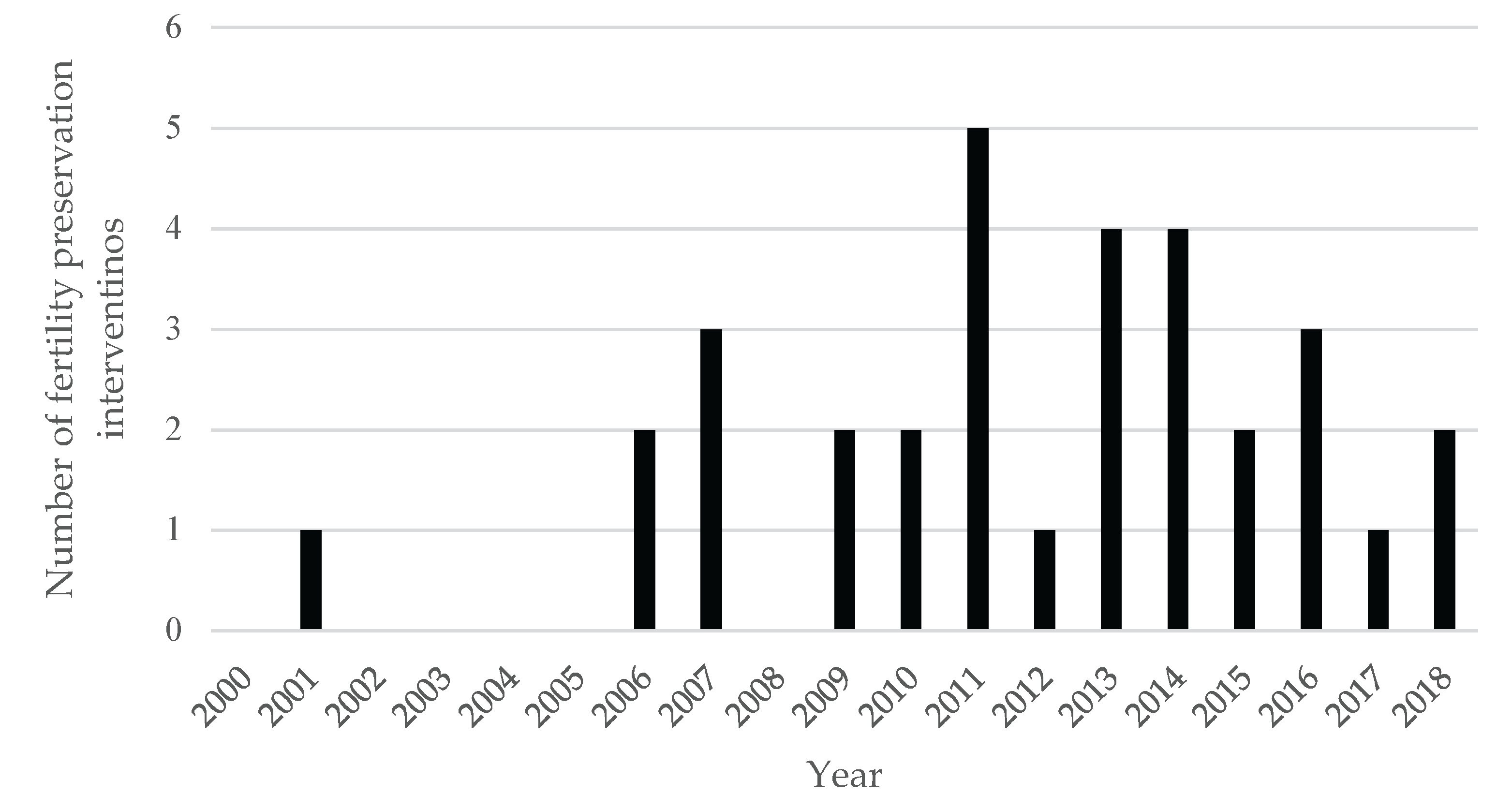
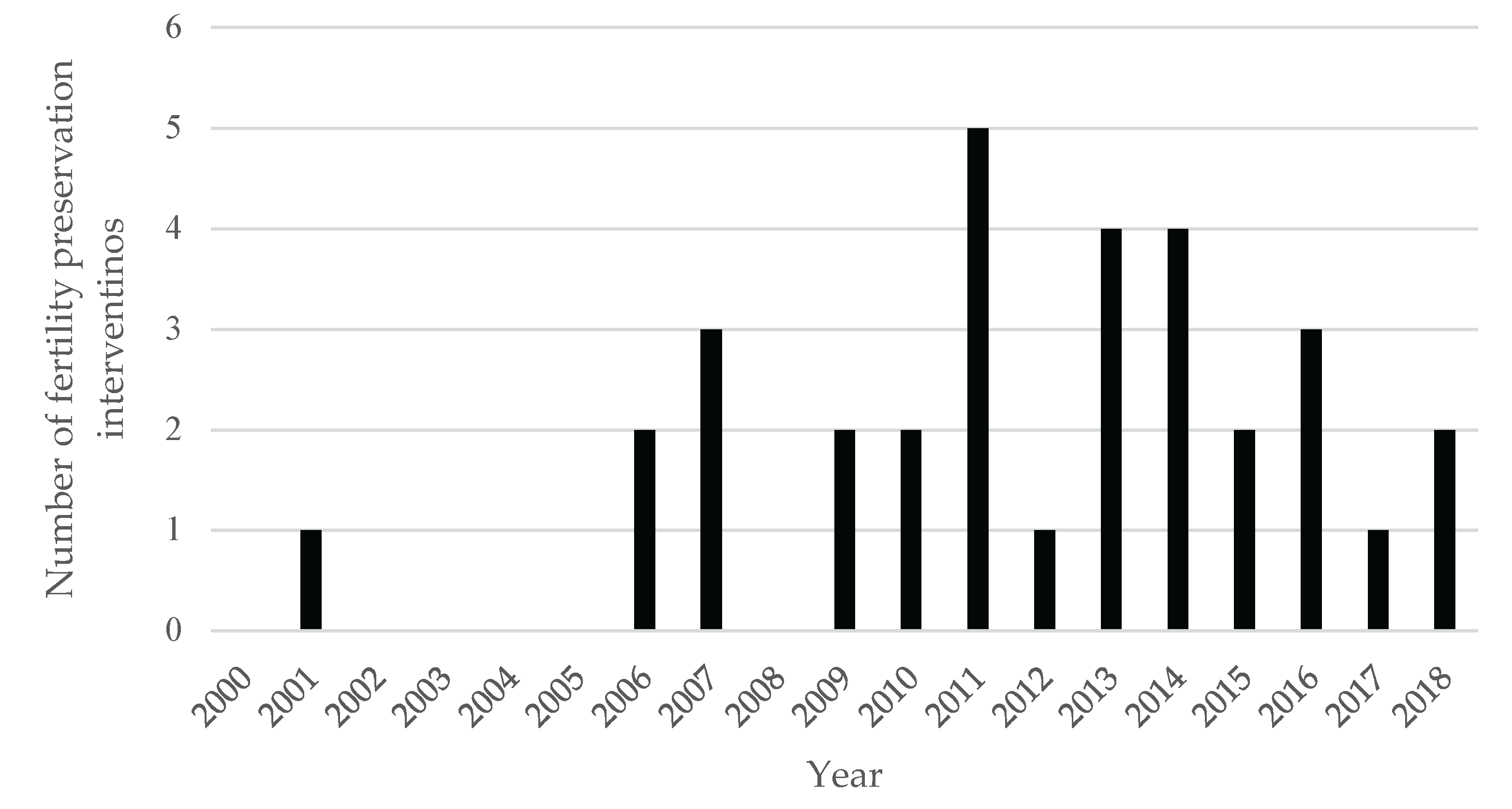
3.3. Fertility preservation
4. Discussion
4.1. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMH | Anti-Müllerian Hormone |
| CCS | Childhood Cancer Survivors |
| COH | Controlled Ovarian Hyperstimulation |
| HPG | Hypothalamic-Pituitary-Gonadal Axis |
| HSCT | Hematopoietic Stem Cell Transplantation |
| NHL | Non-Hodgkin Lymphoma |
| OC | Oocyte Cryopreservation |
| OTC | Ovarian Tissue Cryopreservation |
| PVS | Penile Vibratory Stimulation |
| POI | Premature Ovarian Insufficiency |
| QoL | Quality of Life |
| SC | Sperm Cryopreservation |
| TESE | Testicular Sperm Extraction |
| TTC | Testicular Tissue Cryopreservation |
References
- Calaminus, G.; Langer, T.; Willich, N.; Beck, J.D. Lebensqualität Und Spätfolgen Bei Kindern Und Jugendlichen Mit Krebserkrankungen. Onkologe 2000, 6, 868–877.
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.M.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; De Moor, J.S.; Hartigan, D.B.; et al. Survivors of Childhood Cancer in the United States: Prevalence and Burden of Morbidity. Cancer Epidemiol Biomarkers Prev 2015, 24, 653–663, doi:10.1158/1055-9965.EPI-14-1418.
- Bitsko, M.J.; Cohen, D.; Dillon, R.; Harvey, J.; Krull, K.; Klosky, J.L. Psychosocial Late Effects in Pediatric Cancer Survivors: A Report From the Children’s Oncology Group. Pediatr Blood Cancer 2016, 63, 337–343, doi:10.1002/pbc.25773.
- Zebrack, B.J.; Chesler, M.A. Quality of Life in Childhood Cancer Survivors. Psychooncology 2002, 11, 132–141, doi:10.1002/pon.569.
- Meacham, L.R.; Burns, K.; Orwig, K.E.; Levine, J. Standardizing Risk Assessment for Treatment-Related Gonadal Insufficiency and Infertility in Childhood Adolescent and Young Adult Cancer: The Pediatric Initiative Network Risk Stratification System. J Adolesc Young Adult Oncol 2020, 9, 662–666, doi:10.1089/jayao.2020.0012.
- Green, D.M.; Liu, W.; Kutteh, W.H.; Ke, R.W.; Shelton, K.C.; Sklar, C.A.; Chemaitilly, W.; Pui, C.H.; Klosky, J.L.; Spunt, S.L.; et al. Cumulative Alkylating Agent Exposure and Semen Parameters in Adult Survivors of Childhood Cancer: A Report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014, 15, 1215–1223, doi:10.1016/S1470-2045(14)70408-5.
- Ritenour, C.W.M.; Seidel, K.D.; Leisenring, W.; Mertens, A.C.; Wasilewski-Masker, K.; Shnorhavorian, M.; Sklar, C.A.; Whitton, J.A.; Stovall, M.; Constine, L.S.; et al. Erectile Dysfunction in Male Survivors of Childhood Cancer-A Report from the Childhood Cancer Survivor Study. J Sex Med 2016, 13, 945–954, doi:10.1016/j.jsxm.2016.03.367.
- Romerius, P.; Ståhl, O.; Moëll, C.; Relander, T.; Cavallin-Ståhl, E.; Wiebe, T.; Giwercman, Y.L.; Giwercman, A. Hypogonadism Risk in Men Treated for Childhood Cancer. Clin Endocrinol Metab 2009, 94, 4180–4186, doi:10.1210/jc.2009-0337.
- Claessens, J.J.M.; Penson, A.; Bronkhorst, E.M.; Kremer, L.C.M.; van Dulmen-den Broeder, E.; van der Heiden-van der Loo, M.; Tissing, W.J.E.; van der Pal, H.J.H.; Blijlevens, N.M.A.; van den Heuvel-Eibrink, M.M.; et al. Desire for Children among Male Survivors of Childhood Cancer: A DCCSS LATER Study. Cancer 2023, 129, 1432–1442, doi:10.1002/cncr.34685.
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of Male Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J Clin Oncol 2010, 28, 332–339, doi:10.1200/JCO.2009.24.9037.
- Green, D.M.; Sklar, C.A.; Boice, J.D.; Mulvihill, J.J.; Whitton, J.A.; Stovall, M.; Yasui, Y. Ovarian Failure and Reproductive Outcomes after Childhood Cancer Treatment: Results from the Childhood Cancer Survivor Study. J Clin Oncol 2009, 27, 2374–2381, doi:10.1200/JCO.2008.21.1839.
- Bath, L.E.; Critchley, H.O.D.; Chambers, S.E.; Anderson, R.A.; Kelnar, C.J.H.; Wallace, W.H.B. Ovarian and Uterine Characteristics after Total Body Irradiation in Childhood and Adolescence: Response to Sex Steroid Replacement. BJOG: Int. J. Obstet. Gynaecol. 1999, 106, 1265–1272, doi:10.1111/j.1471-0528.1999.tb08180.x.
- Torella, M.; Riemma, G.; De Franciscis, P.; Verde, M. La; Colacurci, N. Serum Anti-Müllerian Hormone Levels and Risk of Premature Ovarian Insufficiency in Female Childhood Cancer Survivors: Systematic Review and Network Meta-Analysis. Cancers (Basel) 2021, 13, 1–13, doi:10.3390/cancers13246331.
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of Female Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J Clin Oncol 2009, 27, 2677–2685, doi:10.1200/JCO.2008.20.1541.
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The Impact of Cancer on Subsequent Chance of Pregnancy: A Populationbased Analysis. Hum Reprod 2018, 33, 1281–1290, doi:10.1093/humrep/dey216.
- Rendtorff, R.; Hohmann, C.; Reinmuth, S.; Müller, A.; Dittrich, R.; Beyer, M.; Wickmann, L.; Keil, T.; Henze, G.; Borgmann-Staudt, A. Hormone and Sperm Analyses after Chemo- and Radiotherapy in Childhood and Adolescence. Klin Padiatr 2010, 222, 145–149, doi:10.1055/s-0030-1249658.
- Hohmann, C.; Borgmann, A.; Keil, T. Re: Induced Abortions in Danish Cancer Survivors: A Population-Based Cohort Study. . 2011;103(8):698. J Natl Cancer Inst 2011, 103, 698, doi:doi:10.1093/jnci/djr063.
- Donnez, J.; Martinez-Madrid, B.; Jadoul, P.; Van Langendonckt, A.; Demylle, D.; Dolmans, M.M. Ovarian Tissue Cryopreservation and Transplantation: A Review. Hum. Reprod. Update. 2006, 12, 519–535, doi:10.1093/humupd/dml032.
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-Induced Damage to Ovary: Mechanisms and Clinical Impact. Future Oncol. 2016, 12, 2333–2344, doi:10.2217/fon-2016-0176.
- Meistrich, M. Male Gonadal Toxicity. Pediatr Blood Cancer 2009, 53, 261–266, doi:10.1002/pbc.22004.
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int J Mol Sci 2019, 20, 1–17, doi:10.3390/ijms20215342.
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of Chemotherapy-Induced Human Ovarian Aging Double Strand DNA Breaks and Microvascular Compromise. Aging 2011, 3, 782–793.
- Poganitsch-Korhonen, M.; Masliukaite, I.; Nurmio, M.; Lähteenmäki, P.; Van Wely, M.; Van Pelt, A.M.M.; Jahnukainen, K.; Stukenborg, J.B. Decreased Spermatogonial Quantity in Prepubertal Boys with Leukaemia Treated with Alkylating Agents. Leukemia 2017, 31, 1460–1463, doi:10.1038/leu.2017.76.
- Ginsberg, J.P. New Advances in Fertility Preservation for Pediatric Cancer Patients. Curr. Opin. Pediatr 2011, 23, 9–13, doi:10.1097/MOP.0b013e3283420fb6.
- Tharmalingam, M.D.; Matilionyte, G.; Wallace, W.H.B.; Stukenborg, J.B.; Jahnukainen, K.; Oliver, E.; Goriely, A.; Lane, S.; Guo, J.; Cairns, B.; et al. Cisplatin and Carboplatin Result in Similar Gonadotoxicity in Immature Human Testis with Implications for Fertility Preservation in Childhood Cancer. 18:374. BMC Med 2020, 18, doi:10.1186/s12916-020-01844-y.
- Zhou, B.; Kwan, B.; Desai, M.J.; Nalawade, V.; Henk, J.; Viravalli, N.; Murphy, J.D.; Nathan, P.C.; Ruddy, K.J.; Shliakhtsitsava, K.; et al. Association of Platinum-Based Chemotherapy with Live Birth and Infertility in Female Survivors of Adolescent and Young Adult Cancer. Fertil Steril 2024, 121, 1020–1030, doi:10.1016/j.fertnstert.2024.01.039.
- Chow, E.J.; Stratton, K.L.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Donaldson, S.S.; Ginsberg, J.P.; Kenney, L.B.; Levine, J.M.; Robison, L.L.; et al. Pregnancy after Chemotherapy in Male and Female Survivors of Childhood Cancer Treated between 1970 and 1999: A Report from the Childhood Cancer Survivor Study Cohort. Lancet Oncol 2016, 17, 567–576, doi:10.1016/S1470-2045(16)00086-3.
- Reinmuth, S.; Hohmann, C.; Rendtorff, R.; Balcerek, M.; Holzhausen, S.; Müller, A.; Henze, G.; Keil, T.; Borgmann-Staudt, A. Impact of Chemotherapy and Radiotherapy in Childhood on Fertility in Adulthood: The FeCt - Survey of Childhood Cancer Survivors in Germany. J Cancer Res Clin Oncol 2013, 139, 2071–2078, doi:10.1007/s00432-013-1527-9.
- Wallace, W.H.B.; Thomson, A.B.; Kelsey, T.W. The Radiosensitivity of the Human Oocyte. Hum. Reprod. 2003, 18, 117–121, doi:10.1093/humrep/deg016.
- Larsen, E.C.; Schmiegelow, K.; Rechnitzer, C.; Loft, A.; Müller, J.; Andersen, A.N. Radiotherapy at a Young Age Reduces Uterine Volume of Childhood Cancer Survivors. Acta Obstet Gynecol Scand 2004, 83, 96–102, doi:10.1111/j.1600-0412.2004.00332.x.
- De Felice, F.; Marchetti, C.; Marampon, F.; Cascialli, G.; Muzii, L.; Tombolini, V. Radiation Effects on Male Fertility. Andrology 2019, 7, 2–7, doi:10.1111/andr.12562.
- Koustenis, E.; Pfitzer, C.; Balcerek, M.; Reinmuth, S.; Zynda, A.; Stromberger, C.; Hohmann, C.; Keil, T.; Borgmann-Staudt, A. Impact of Cranial Irradiation and Brain Tumor Location on Fertility: A Survey. Klin Padiatr 2013, 225, 320–324, doi:10.1055/s-0033-1353206.
- Wasilewski-Masker, K.; Seidel, K.D.; Leisenring, W.; Mertens, A.C.; Shnorhavorian, M.; Ritenour, C.W.; Stovall, M.; Green, D.M.; Sklar, C.A.; Armstrong, G.T.; et al. Male Infertility in Long-Term Survivors of Pediatric Cancer: A Report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2014, 8, 437–447, doi:10.1007/s11764-014-0354-6.
- Thomas-Teinturier, C.; El Fayech, C.; Oberlin, O.; Pacquement, H.; Haddy, N.; Labbé, M.; Veres, C.; Guibout, C.; Diallo, I.; De Vathaire, F. Age at Menopause and Its Influencing Factors in a Cohort of Survivors of Childhood Cancer: Earlier but Rarely Premature. Hum. Reprod. 2013, 28, 488–495, doi:10.1093/humrep/des391.
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility Preservation and Post-Treatment Pregnancies in Post-Pubertal Cancer Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1664–1678, doi:10.1016/j.annonc.2020.09.006.
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous Grafting of Cryopreserved Prepubertal Rhesus Testis Produces Sperm and Offspring. Science 2019, 363, 1314–1319, doi:doi:10.1126/science.aav2914.
- Goossens, E.; Jahnukainen, K.; Mitchell, R.T.; Van Pelt, A.M.M.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.A.; et al. Fertility Preservation in Boys: Recent Developments and New Insights. Hum Reprod Open 2020, 2020, 1–18, doi:10.1093/hropen/hoaa016.
- Gül Siraz, Ü.; Hatipoğlu, N. Fertility Preservation Methods in Childhood and Adolescence Cancers: A Review. J Pediatr Acad 2021, 2, 91–96, doi:10.51271/jpea-2021-0145.
- Bahadur, G.; Ling, K.L.E.; Hart, R.; Ralph, D.; Riley, V.; Wafa, R.; Ashraf, A.; Jaman, N.; Oyede, A.W. Semen Production in Adolescent Cancer Patients. Hum. Reprod. 2002, 17, 2654–2656.
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001, doi:10.1200/JCO. 2018.78.1914.
- Poirot, C.; Abirached, F.; Prades, M.; Coussieu, C.; Bernaudin, F.; Piver, P. Induction of Puberty by Autograft of Cryopreserved Ovarian Tissue. Lancet 2012, 379, 588, doi:doi:10.1016/S0140-6736(11)61781-9.
- Matthews, S.; Picton, H.; Ernst, E.; Andersen, C. Successful Pregnancy in a Woman Previously Suffering from β-Thalassemia Following Transplantation of Ovarian Tissue Cryopreserved before Puberty. Minerva Ginecol. 2018, 70, 432–435.
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsépélidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live Birth after Autograft of Ovarian Tissue Cryopreserved during Childhood. Hum. Reprod. 2015, 30, 2107–2109, doi:10.1093/humrep/dev128.
- Kinderonkologische Erkrankungen. In Indikation und Durchführung fertilitätsprotektiver Maßnahmen bei onkologischen und nicht-onkologischen Erkrankungen; Balcerek, M., von Wolff, M., Borgmann-Staudt, A., Eds.; FertiPROTEKT: Kiel, Germany, 2020; pp. 117–131 ISBN 9783883121291.
- Mossa, B.; Schimberni, M.; Di Benedetto, L.; Mossa, S.; Schimberni, M. Ovarian Transposition in Young Women and Fertility Sparing. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3418–3425.
- Irtan, S.; Orbach, D.; Helfre, S.; Sarnacki, S. Ovarian Transposition in Prepubescent and Adolescent Girls with Cancer. Lancet Oncol. 2013, 14, e601–e608, doi:10.1016/S1470-2045(13)70288-2.
- Cobo, A.; García-Velasco, J.A.; Coello, A.; Domingo, J.; Pellicer, A.; Remohí, J. Oocyte Vitrification as an Efficient Option for Elective Fertility Preservation. Fertil Steril 2016, 105, 755–764, doi:10.1016/j.fertnstert.2015.11.027.
- von Wolff, M.; Bruckner, T.; Strowitzki, T.; Germeyer, A. Fertility Preservation: Ovarian Response to Freeze Oocytes Is Not Affected by Different Malignant Diseases—an Analysis of 992 Stimulations. J Assist Reprod Genet 2018, 35, 1713–1719, doi:10.1007/s10815-018-1227-0.
- Dolmans, M.M.; Taylor, H.S.; Rodriguez-Wallberg, K.A.; Blumenfeld, Z.; Lambertini, M.; von Wolff, M.; Donnez, J. Utility of Gonadotropin-Releasing Hormone Agonists for Fertility Preservation in Women Receiving Chemotherapy: Pros and Cons. Fertil Steril 2020, 114, 725–738, doi:10.1016/j.fertnstert.2020.08.011.
- Blumenfeld, Z. Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy. Clin Med Insights Reprod Health 2019, 13, 1, doi:10.1177/1179558119870163.
- Diesch, T.; von der Weid, N.X.; Szinnai, G.; Schaedelin, S.; De Geyter, C.; Rovó, A. Fertility Preservation in Pediatric and Adolescent Cancer Patients in Switzerland: A Qualitative Cross-Sectional Survey. Cancer Epidemiol 2016, 44, 141–146, doi:10.1016/j.canep.2016.08.013.
- Terenziani, M.; Spinelli, M.; Jankovic, M.; Bardi, E.; Hjorth, L.; Haupt, R.; Michel, G.; Byrne, J. Practices of Pediatric Oncology and Hematology Providers Regarding Fertility Issues: A European Survey. Pediatr Blood Cancer 2014, 61, 2054–2058, doi:10.1002/pbc.25163.
| Variable | n | Percentage (%)1 |
| Sex | ||
| Female | 247 | 44.7 |
| Male | 305 | 55.3 |
| Survival, recurrence, and radiation status | ||
| Alive | 441 | 79.9 |
| Deceased | 111 | 20.1 |
| Recurrence (min. 1) | 124 | 22.5 |
| Radiation | 162 | 29.3 |
| Extracranial Radiation | 105 | 19.0 |
| Cranial Radiation | 57 | 10.3 |
| Variable | n | Percentage (%)1 |
| Total (female) | 75 | 30.4 |
| AMH | 4 | 1.6 |
| FSH | 73 | 29.6 |
| Estradiol | 72 | 29.1 |
| Hormonally active | 23 | 30.62 |
| Total (male) | 89 | 29.2 |
| FSH | 88 | 28.9 |
| Testosterone | 82 | 26.9 |
| Hormonally active | 18 | 20.22 |
| Variable | n | Percentage (%)1 |
| Fertility preservation (female) | ||
| no | 239 | 96.8 |
| yes | 8 | 3.2 |
| OCT | 6 | 2.4 |
| OC | 3 | 1.2 |
| Fertility preservation (male) | ||
| no | 277 | 90.8 |
| yes | 28 | 9.2 |
| SC via masturbation | 27 | 8.9 |
| SC via TESE | 1 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





