1. Introduction
Cranioplasty is a well-established surgical procedure performed to repair cranial defects using either autologous or synthetic cranial grafts [1]. Autologous bone graft or the synthetic prostheses are secured to the skull using specific devices [2]. Synthetic flaps are often employed where autologous flaps are unavailable, such as after infection, osteolysis or tumor invasion. Over time, various synthetic materials have been used throughout the history of cranioplasty, but no consensus has yet emerged regarding the best material. Research continues to explore and develop the ideal reconstruction material [3].
Currently, the most used alloplastic materials for cranioplasty include titanium, acrylics such as Polymethyl methacrylate (PMMA), polymers like polyether ether ketone (PEEK), and bioceramics such as porous hydroxyapatite (PHA). These materials have enabled the creation of patient-specific, custom-made 3D prostheses, which provide superior fit, improved aesthetic results, better protection of neural structures, and reduced operative time [4,5,12].
Notably, PHA exhibits a high degree of osseointegration, making it a promising choice for cranial repair [5,6,7,8]. However, PHA prosthesis appear more prone to displacement compared to other materials. This may be due to initial fragility, which precludes the use of traditional plates and screws until full osseointegration occurs -typically over a period of 6 to 12 months. PHA implants are delivered with predefined anchorage holes defined in number and position by the surgeon during the preoperative planning phase. Additional holes may be drilled intraoperatively if needed. In Europe, the only manufacturer-approved fixation method remains non-absorbable silk sutures (CustomBone Service, FinCeramica Faenza S.p.A, Italy) which may not offer optimal implant stability.
The ideal combination of prosthesis and fixation device must ensure mechanical stability, protection of underlying neural tissue, promote early bone healing, and be safe, reliable, and user-friendly. It should also minimize imaging artifacts, foreign body reactions, and ensure a satisfactory cosmetic outcome [11,12]. Over the years, fixation methods have evolved alongside advancements in surgical tools, anatomical understanding, and growing emphasis on cosmesis and infection prevention. Multiple traditional in addition to newer techniques are available, each with pros and cons [11].
Titanium-based device (e.g., clamp-like systems or plates and screws) and resorbable polyglycolic acid plates and screws are the most used alternatives. However, their combination with PHA implants is associated with increased risk of prosthetic fracture and migration [8,9,10,25].
A clamp-like fixation system made of PEEK, such as the Cranial LOOP® (NEOS Surgery S.L., Barcelona, Spain), has been proposed as an alternative, offering enhanced flexibility and reducing mechanical stress on the fragile implant [19].
In the United States, the combination of PEEK OPTIMA® clamps (Cranial LOOP®) with customized PHA implants (CustomizedBone, FinCeramica Faenza S.p.A, Italy) has received FDA clearance. Nonetheless, further evidence is needed to confirm its long-term safety and efficacy.
This study aims to address this gap by presenting a case series of patients treated with customized PHA implants fixed using Cranial LOOP® systems, with long-term clinical and radiological follow-up.
2. Materials and Methods
The authors present a consecutive series of patients who underwent cranioplasty with a PHA custom made prosthesis combined with cranial LOOP® system at a single institution, the Lausanne University Hospital, (CHUV), Switzerland, between January and December 2019. All patients presented with conditions that requires temporary removal of a bone flap, such as traumatic brain injury, space-occupying cerebral infarction, cranial malformation, or cerebral tumor. Post-operative cerebral CT scans were obtained on the second postoperative day and during follow-up visits at 6 and 12 months, depending on the underlying pathology. Clinical data, including information such as wound healing and cosmetic outcomes, were systematically recorded. Aesthetic outcome was measured using the Global Aesthetic Improvement Scale Assessment (GAIS). The aesthetic results were compared to the previous bone flap/prosthesis implanted [30]. Postoperative imaging was evaluated for complications, including prosthesis displacement, epidural hematoma, and CSF leak formation. All participants provided informed consent, and the study protocol was approved by the competent Ethics Review Board (CER-VD).
2.1. Patient Population
A total of seven patients is included in this study.
Table 1 summarizes the patients’ characteristics. The mean age is 38.8 years (range 10-61 years), with 43% female and 57% male participants. Trauma is the most common initial pathology, accounting for 72% of cases (5 patients). Similarly, infection of a previous bone flap or prosthesis is the most frequent indication for PHA cranioplasty (5 patients, 72% of cases). The mean follow-up duration is 42 months (range 6-60 months).
2.2. Device Description
Cranial Loop™ (CL) is a fixation system designed to secure cranial bone flaps following craniotomy. It consists of a lower platform with cable ties, an upper platform with toothed slots, a grip handle for controlled tightening, and an applier ensuring safe and repeatable closure. The device, made of biocompatible PEEK OPTIMA™, does not require specialized instrumentation for placement or removal.
The implantation process involves inserting the lower platform in the epidural space, placing the bone flap between the platforms, and securing it by pulling the handle while guiding the upper platform with the applier. Tension should not exceed 40 N to prevent structural failure. Stability is confirmed by testing for movement after fixation. Removal involves lifting the upper platform with forceps and ensuring no fragments remain in the surgical site.
Contraindications include infection, bone disease, lack of adequate tissue coverage, sinus penetration, and intolerance to PEEK. The manufacturer recommends using at least three CLs per craniotomy for optimal fixation. Being non-ferromagnetic, CL does not interfere with MRI imaging. System integrity should be monitored postoperatively, with replacement as needed.
2.3. PHA Prosthesis Fixation: Surgical Procedure
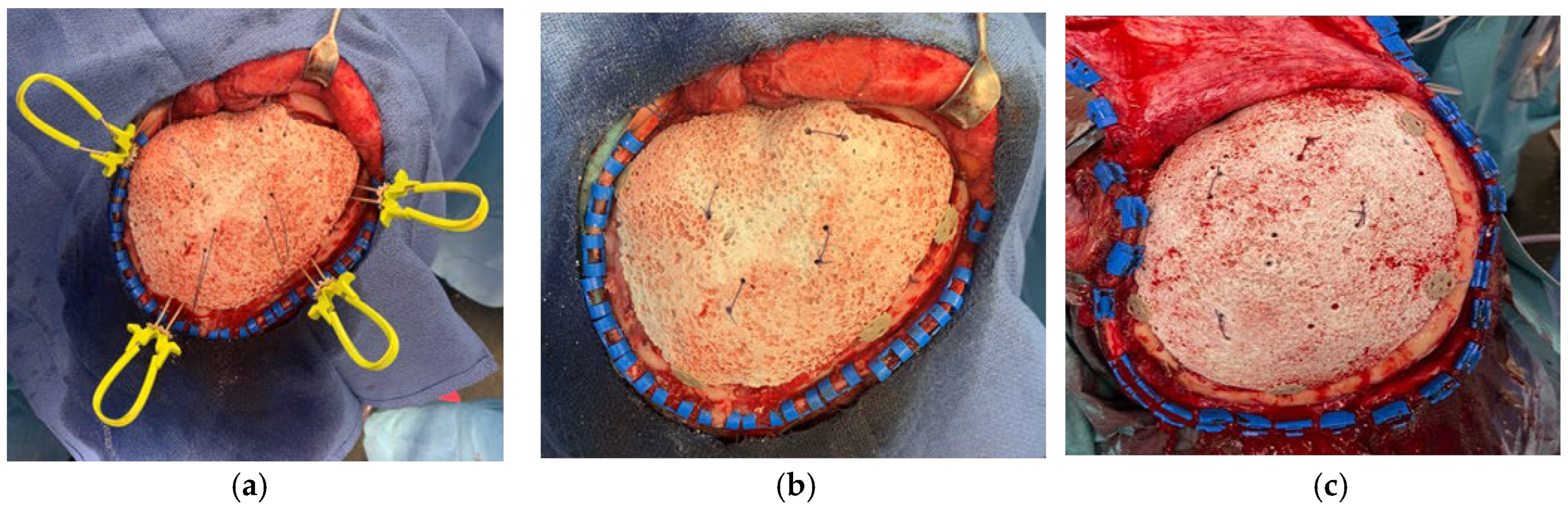
For the fixation of the PHA prosthesis, at least three Cranial Loop™ (CL) devices are employed to ensure stable and secure positioning within the cranial defect. Additional CLs may be used depending on the prosthesis size and anatomical considerations.
The fixation procedure begins with the careful placement of the lower platform of each CL into the epidural space beneath the craniotomy margin, ensuring minimal manipulation to avoid dural injury. Once the prosthesis is positioned within the cranial defect, the upper platform is placed epicranially and secured by gradually applying tension using the applier. The double-locking mechanism of the CL prevents loosening and maintains stable fixation throughout the postoperative healing period.
The cable ties are then trimmed using surgical scissors, taking advantage of the device’s self-cutting feature to ensure a clean and flush fit. To confirm adequate fixation, gentle pressure is applied to the prosthesis to assess resistance to displacement. The strategic distribution of the CLs around the prosthesis allows for optimized biomechanical stability without requiring predefined fixation slots, offering superior intraoperative adaptability compared to traditional suturing techniques.
Figure 1.
Intra-operative images show the prosthesis implantation: (a) 4 Cranial LOOP are positioned around the prosthesis; (b,c) The cranial LOOP are clamped and the cable ties removed, the external platform shows its low profile.
Figure 1.
Intra-operative images show the prosthesis implantation: (a) 4 Cranial LOOP are positioned around the prosthesis; (b,c) The cranial LOOP are clamped and the cable ties removed, the external platform shows its low profile.
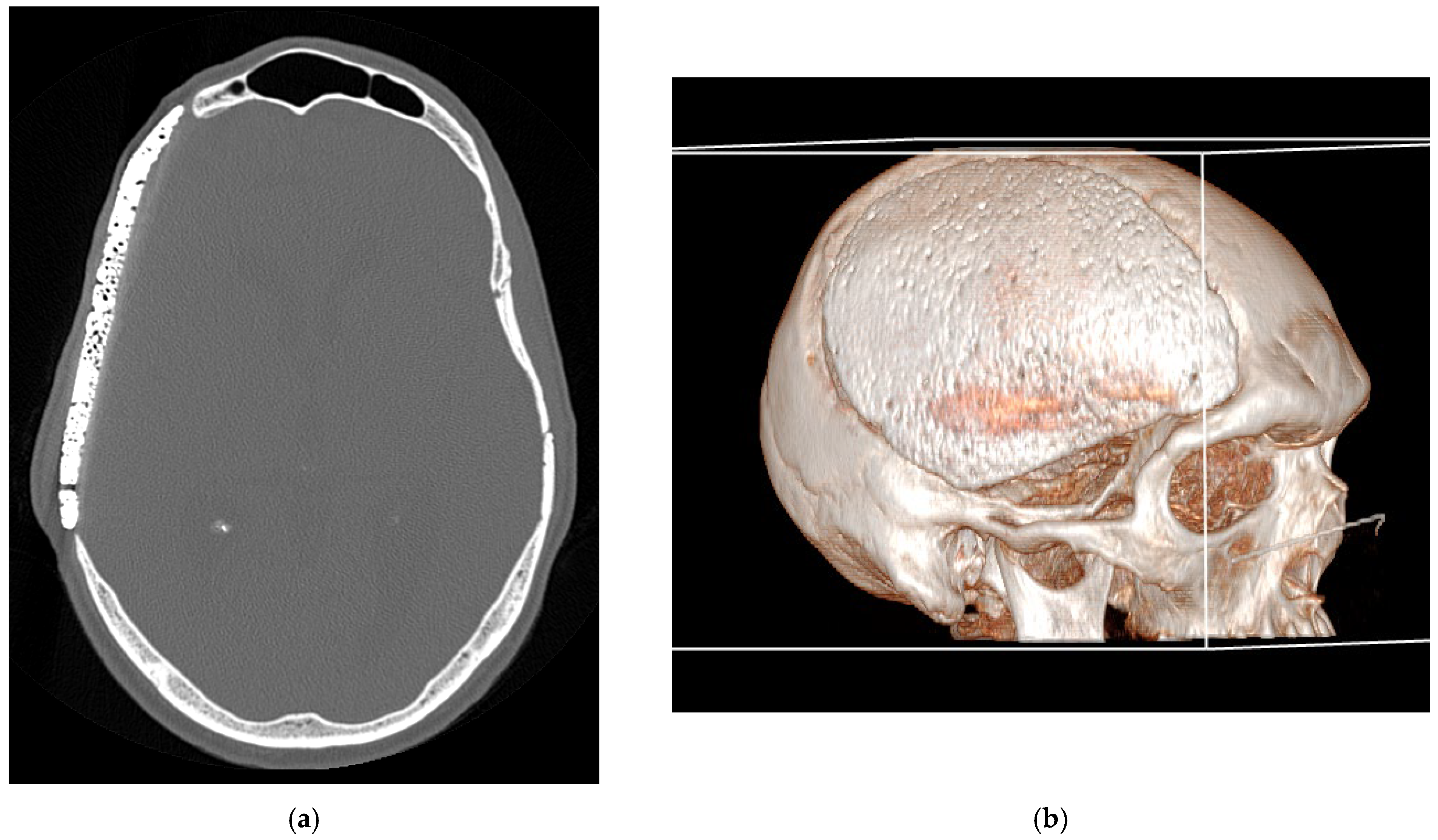
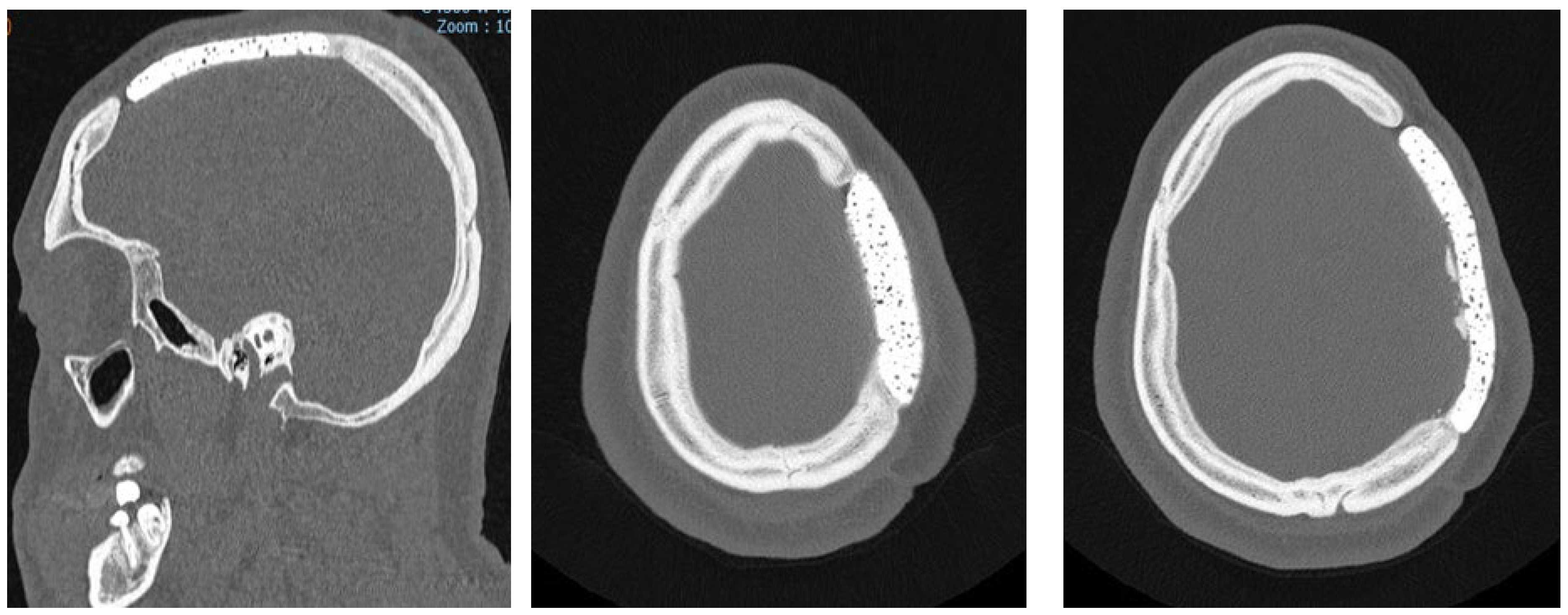
Figure 2.
Bone CT-scan at 48h post-operatively: (a) axial bone CT image showing satisfactory placement of the cranioplasty flap; (b) 3D reconstruction of the CT scan.
Figure 2.
Bone CT-scan at 48h post-operatively: (a) axial bone CT image showing satisfactory placement of the cranioplasty flap; (b) 3D reconstruction of the CT scan.
The Cranial Loop™ system provides a reliable and adjustable method for securing CustomBone hydroxyapatite prostheses in cranioplasty, ensuring robust fixation while preserving flexibility for intraoperative modifications. Its non-ferromagnetic nature allows for safe postoperative imaging, and its design minimizes the need for supplementary fixation hardware.
2.4. Case Example
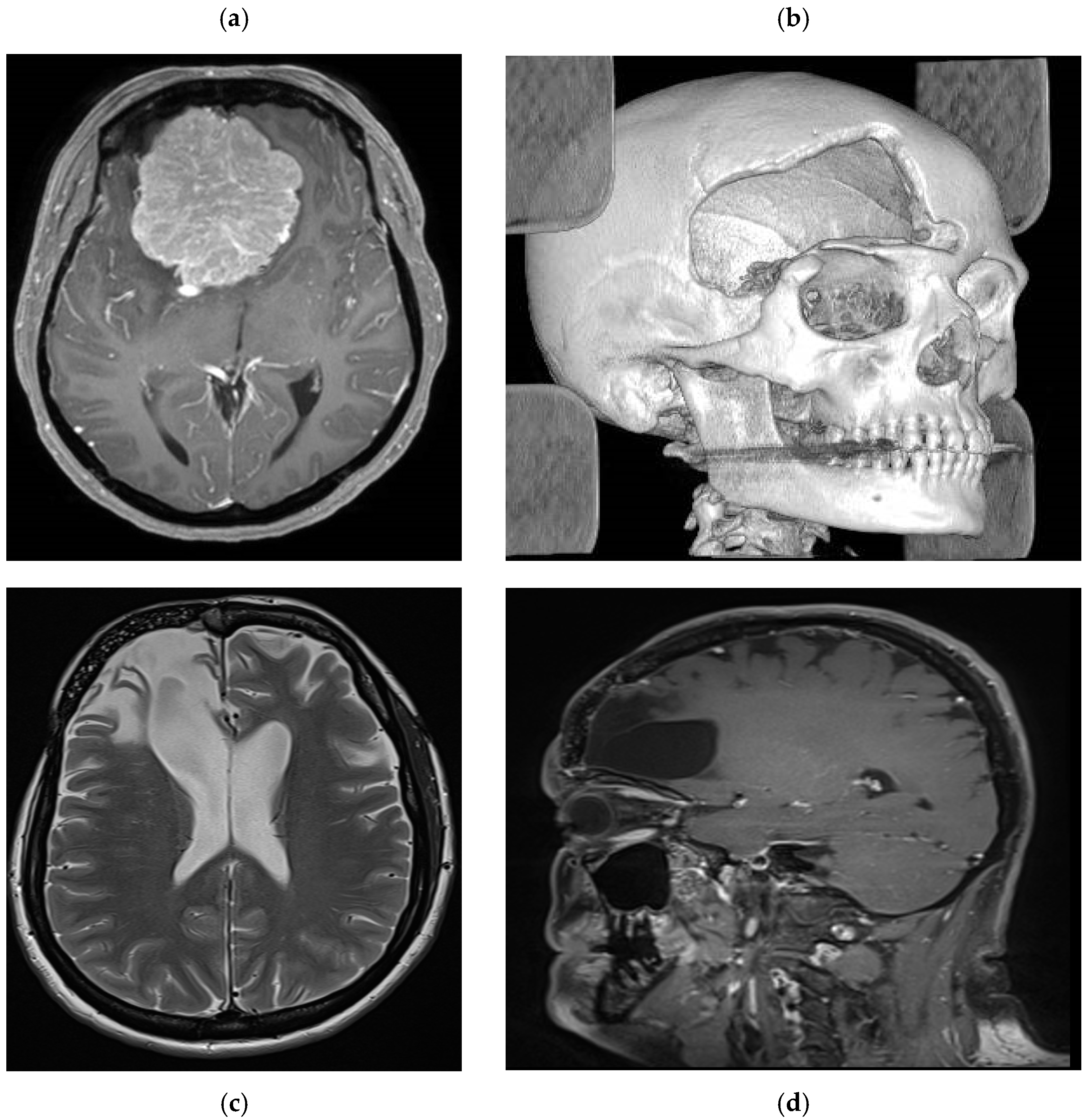
Patient number 1 is a 48-year-old female who underwent surgery for a large olfactory groove meningioma using a right frontotemporal craniotomy (
Figure 3). One month later, she underwent reoperation following postoperative infection (frontal sinus cranialization and bone flap removal). Intraoperative samples were positive for S. epidermidis, and she received appropriate antibiotic treatment for 6 weeks. Due to the location of the bone flap, a custom-made prosthesis was proposed to the patient to achieve the best aesthetic result. Once the treatment was completed, a fine-cut CT scan was performed to plan the 3D custom bone prosthesis, which was implanted 2 months after the end of the treatment. The aesthetic result was satisfactory, with no visible bony gap and good frontal symmetry. Long-term radiological follow-up was performed due to the risk of meningioma recurrence. At 5 years, no signs of fracture or displacement were observed (
Figure 3), and a satisfactory aesthetic result was reported (
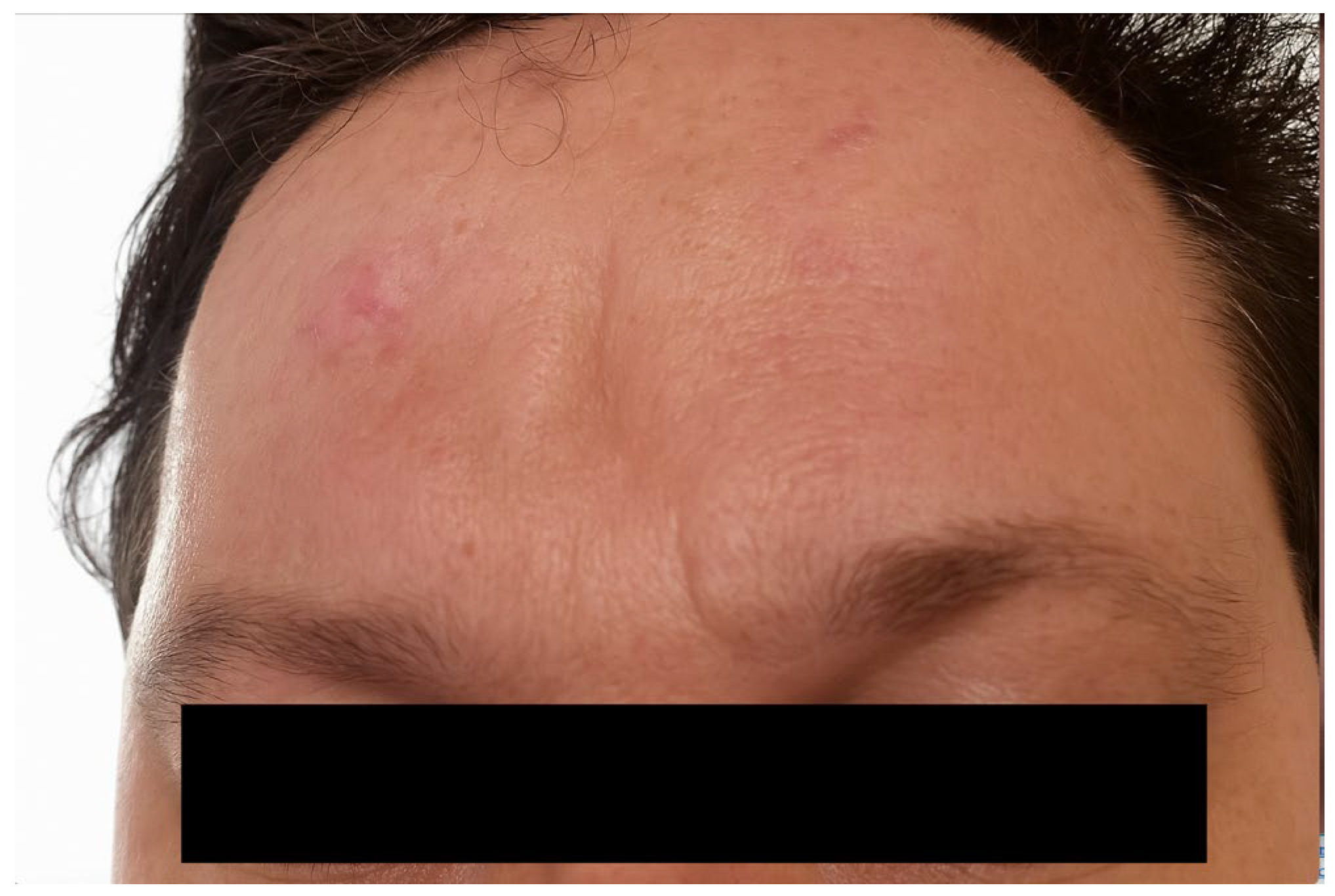
Figure 4).
Figure 3.
Case example MR images. (a) preoperative axial T1 weighted with gadolinium showing a large olfactory groove meningioma. (b) postoperative CT scan with 3D reconstruction showing a large aesthetic defect in the right frontal region. (c) Postoperative axial T2 weighted and (d) T1 weighted sagittal images with gadolinium showing the prosthesis perfectly fitting the cranial defect with imaging artifact.
Figure 3.
Case example MR images. (a) preoperative axial T1 weighted with gadolinium showing a large olfactory groove meningioma. (b) postoperative CT scan with 3D reconstruction showing a large aesthetic defect in the right frontal region. (c) Postoperative axial T2 weighted and (d) T1 weighted sagittal images with gadolinium showing the prosthesis perfectly fitting the cranial defect with imaging artifact.
Figure 4.
Case example: 5 years postoperative picture showing the satisfactory aesthetic result in the right frontal region.
Figure 4.
Case example: 5 years postoperative picture showing the satisfactory aesthetic result in the right frontal region.
3. Results
Clinical Results
All seven patients in this study underwent successful cranioplasty procedures with custom made PHA prostheses securing using the Cranial Loop fixation system.
The postoperative course was uneventful in all cases, with no instances of acute complications such as wound dehiscence, infection, hematoma, or cerebrospinal fluid leakage. Immediate postoperative CT scans confirmed correct prosthesis positioning, with no evidence of displacement or misalignment in all cases (
Figure 2a).
Radiological assessments, while performed, demonstrated progressive osseointegration, with bone remodeling visible at the prosthesis margins (
Figure 5).
Figure 5.
Progressive osseointegration with bone remodeling visible at the prosthesis margins.
Figure 5.
Progressive osseointegration with bone remodeling visible at the prosthesis margins.
Long-term clinical follow-up (mean duration: 42 months) revealed no cases of secondary displacement, prosthesis fracture, or foreign body reaction. The PHA implants maintained their structural integrity, and no patient required revision surgery. Cosmetic outcomes were rated as excellent by all patients, with restoration of normal cranial contour and no palpable irregularities at the surgical site.
Cosmetic outcomes were rated according to the GAIS. Satisfactory results were reported in all patients and an aesthetic improvement in two cases where osteolysis and displacement were present with the previous flap. Subjective evaluation of cranial symmetry, contour restoration, and skin adaptation over the prosthesis was performed at each follow-up visit. All patients exhibited a natural cranial contour with no visible or palpable irregularities at the surgical site. Compared to the traditional silk suture fixation method, no differences in cosmetic results were noted. Additionally, the absence of soft tissue irritation and minimal palpability of the fixation devices contributed to overall patient satisfaction. No patient reported aesthetic dissatisfaction or the need for secondary correction.
4. Discussion
PHA has been proposed as a novel biocompatible material for custom made prosthesis. Due to its molecular and physicochemical properties, it exhibits enhanced osseointegration capacity [2]. To achieve complete osteointegration close contact between the prosthesis margins and the rest of the theca is required. If this is not the case, the osteoblastic colonization process may be compromised [3] [4]. Furthermore, PHA prostheses have been shown to reduce bacterial biofilms formation of, thereby lowering infection rates [4] [5]. However, PHA prostheses are associated with a slightly higher rate of displacement compared to other materials [4]. A recent multicentric European retrospective study involving 494 patients reported fractures in 1-6 % of patients and displacement in 0.4-4,5% [6] [7]. Their mechanical fragility in the early postoperative period necessitates reliable fixation methods to prevent displacement and optimize osseointegration. Several fixation devices, such as non-absorbable sutures and titanium plates, have historically been employed to mitigate complications after cranioplasty.
Non-absorbable sutures, while manufacturer-validated for fixing PHA custom made implants, may occasionally provide insufficient stability, leading to implant dislocation (depression or protrusion) [4].
Alternative fixation systems have been explored, included titanium-based devise as Craniofix® (B Braun Aesculap, Tuttlingen, Germany), which improve stability but carry risks of prostheses fractures, due to its initial fragility, and significant imaging artifacts, and are incompatible with PHA [8] [3] [4] [5].
There is some evidence from outside Europe of the use of titanium screws of different lengths to fix titanium miniplates to HA flaps (APACERAM, supplied by Pentax, Co, Ltd. Japan). Pull-out resistance tests determined that fixation strengths were directly dependent on screw length but HA implants also have a tendency to break when screwed in [9].
Polyglycolic acid plates and screws offer resorbable options, but their mechanical stability diminishes over time, and their implantation is technically challenging, with reported risk of intracranial migration (10-14% of cases) and accidental dural tears [10] [11] [12] [13]. To address these limitations, PEEK fixation systems have emerged as a promising alterna tive solution for securing PHA prosthesis. These systems, including Cranial LOOP® (NEOS Surgery S.L., Barcelona, Spain), offer several advantages: they require no additional instrumentation, provide enhanced stability, are practical and efficient to implant, and do not cause imaging artifacts [14] [11] [15] [16]. Previous reports by the authors highlighted the successful use of Cranial LOOP® in combination with PHA prosthesis in complex cases, including pediatric patients [15].
The Cranial Loop system offers an innovative alternative by providing stable, non-metallic fixation without need for additional instrumentation. The findings of this study confirm the safety and efficacy of the Cranial Loop system for PHA prostheses fixation. No intraoperative complication was encountered, and all patients achieved stable long-term outcomes without prostheses displacement. Furthermore, the absence of imaging artifacts facilitates postoperative surveillance, making the Cranial Loop™ system particularly advantageous for patients requiring long-term follow-up with MRI (
Figure 3).
Cosmetic outcomes are a crucial aspect of cranioplasty, as patient satisfaction is significantly influenced by the restoration of cranial symmetry. The subjective evaluation of postoperative aesthetics by both surgeons and patients using the GAIS demonstrated that Cranial Loop™ fixation provided results comparable to those achieved with traditional silk suture fixation. The absence of device palpability under the scalp, together with optimal contour restoration, contributed to the high satisfaction rates observed in this study. Moreover, the minimally invasive nature of the device allowed for uniform soft tissue adaptation, minimizing visible irregularities. The ability to securely fix the prosthesis without excessive hardware projection or contour disturbances represents a major advantage in aesthetic neurosurgery. Compared to alternative fixation methods, the Cranial Loop™ system offers several advantages:
Ease of implantation: The device can be applied without specialized tools, reducing operative time and technical complexity.
Minimally invasive profile: The low-profile design prevents soft tissue irritation and facilitates patient comfort.
Flexibility in placement: Unlike pre-drilled fixation holes, the system allows for intraoperative adjustments to optimize positioning.
Enhanced safety: The biocompatible PEEK material eliminates concerns related to metal ion release and associated inflammatory responses.
These advantages align with recent literature advocating for non-metallic fixation techniques in cranioplasty, in fact compared to titanium devices (clamps, plates and screws), Cranial Loop® provides greater flexibility, ensuring a secure grip without risking damage to the PHA prosthesis. Additionally, titanium is associated with metal ions leaching and local inflammation, which can result in chronic infection and cranioplasty failure, particularly in paediatric patients [3] [4]. Cranial Loop® also minimize imaging artifacts on CT and MRI.
The use of Cranial Loop® in combination with PHA prosthesis has been reported in the literature only once before by the authors [12]. In this series, satisfactory safety and mechanical performance of Cranial Loop® with PHA prosthesis were observed over a mean follow-up of 42 months. This exceeds the time frame during which PHA prostheses are expected to fulfill their primary function (6-12 months for solid bone healing in the craniotomy gap) [17].
From a safety perspective, no complications or adverse events were reported in this cohort, either intraoperative or during follow-up. All patients demonstrated satisfactory imaging and aesthetic outcomes.
5. Conclusions
Cranial Loop® in combination with customized PHA implants, appears to be a reliable and effective solution for cranioplasty. In this series of patients treated with PHA prosthesis secured by Cranial Loop®, the authors observed the absence of device related complications and excellent clinical and radiologic outcomes at five years of follow-up.
Cranial Loop® demonstrated ease of implantation without requiring specific planning during prosthesis design. Its use facilitates PHA fixation while reducing surgery duration.
In summary, this study demonstrates that the combination of Cranial Loop® and PHA prostheses provides good mechanical performances and excellent safety profile in cranioplasty. Nevertheless, larger series and prospective studies are necessary to validate these findings.
Author Contributions
Conceptualization, methodology, review and editing, Dr Rodolfo Maduri.; investigation, data curation, Dr Nathalie Zimmerman; writing—original draft preparation, project administration, Dr Alberto Vandenbulcke. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee of the Canton of Vaud (CER-VD, project ID 2024-02281, date of approval 09.01.2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
Dr Rodolfo Maduri is consultant for FinCeramica Faenza S.p.A, Italy.
References
- Klinger, D.R.; Madden, C.; Beshay, J.; White, J.; Gambrell, K.; Rickert, K. Autologous and acrylic cranioplasty: a review of 10 years and 258 cases. World Neurosurg. 2014, 82, e525–530. [Google Scholar] [CrossRef] [PubMed]
- Zaed, I.; Cardia, A.; Stefini, R. From Reparative Surgery to Regenerative Surgery: State of the Art of Porous Hydroxyapatite in Cranioplasty. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Martini, L.; Staffa, G.; Giavaresi, G.; Salamanna, F.; Parrilli, A.; Serchi, E.; Pressato, D.; Arcangeli, E.; Fini, M. Long-term results following cranial hydroxyapatite prosthesis implantation in a large skull defect model. Plast. Reconstr. Surg. 2012, 129, 625e–635e. [Google Scholar] [CrossRef] [PubMed]
- Morselli, C.; Zaed, I.; Tropeano, M.P.; Cataletti, G.; Iaccarino, C.; Rossini, Z.; Servadei, F. Comparison between the different types of heterologous materials used in cranioplasty: a systematic review of the literature. J. Neurosurg. Sci. 2019, 63, 723–736. [Google Scholar] [CrossRef]
- Iaccarino, C.; Mattogno, P.P.; Zanotti, B.; Bellocchi, S.; Verlicchi, A.; Viaroli, E.; Pastorello, G.; Sgulo, F.; Ghadirpour, R.; Servadei, F. Septic complication following porous hydroxyapatite cranioplasty: prosthesis retention management. J. Neurosurg. Sci. 2018, 62, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Zaed, I.; Safa, A.; Spennato, P.; Mottolese, C.; Chibbaro, S.; Cannizzaro, D.; Faggin, R.; Frassanito, P.; Maduri, R.; Messerer, M.; et al. A Multicentric European Clinical Study on Custom-Made Porous Hydroxyapatite Cranioplasty in a Pediatric Population. Front. Surg. 2022, 9, 848620. [Google Scholar] [CrossRef]
- Zaed, I.; Rossini, Z.; Faedo, F.; Fontanella, M.M.; Cardia, A.; Servadei, F. Long-term follow-up of custom-made porous hydroxyapatite cranioplasty in adult patients: a multicenter European study. Can we trust self-reported complications? J. Neurosurg. Sci. 2022, 66, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Messina, R.; Speranzon, L.; de Gennaro, L.; Nigri, E.M.; Dibenedetto, M.; Bozzi, M.T.; Delvecchio, C.; Signorelli, F. Early osteointegration in “one-step” resection and reconstruction using porous hydroxyapatite custom implants for skull-infiltrating tumors: a monocentric prospective series. Acta Neurochir (Wien) 2024, 166, 470. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Development of titanium fixation screw for hydroxyapatite osteosynthesis (APACERAM). Surg. Neurol. 2008, 70, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Minabe, T.; Kato, T.; Kishi, K. Assessment of the RIVET fixation system for cranioplasty using the pull-out technique. J. Craniomaxillofac Surg. 2015, 43, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Camarini, E.T.; Tomeh, J.K.; Dias, R.R.; da Silva, E.J. Reconstruction of frontal bone using specific implant polyether-ether-ketone. J. Craniofac Surg. 2011, 22, 2205–2207. [Google Scholar] [CrossRef] [PubMed]
- Yaremchuk, M.J.; Posnick, J.C. Resolving controversies related to plate and screw fixation in the growing craniofacial skeleton. J. Craniofac Surg. 1995, 6, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Persing, J.A.; Posnick, J.; Magge, S.; Spinelli, H.M.; Wolfe, S.A.; Munro, I.; Mulliken, J.B. Cranial plate and screw fixation in infancy: an assessment of risk. J. Craniofac Surg. 1996, 7, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Islam Aboulfetouh, W.A.Y. Cranial Bone Flap Fixation: Comparison of Titanium-Based Device (Skull Fix) and PEEK-Based Device (Cranial Loop): Technical Report. Med. J. Cairo Univ./Med. J. Cairo Univ. 2019, 87, 377–384. [Google Scholar] [CrossRef]
- Vandenbulcke, A.M., R. . Use of PEEK clamps to fix hydroxyapatite cranial implants: a pediatric case report. Prog. Progress. Neurosci. 2022, 7, 27–31. [Google Scholar]
- Asencio-Cortes, C.; Salgado-Lopez, L.; Munoz-Hernandez, F.; de Quintana-Schmidt, C.; Rodriguez-Rodriguez, R.; Alvarez-Holzapfel, M.J.; Conesa, G. Long-Term Safety and Performance of a Polymeric Clamplike Cranial Fixation System. World Neurosurg. 2019, 126, e758–e764. [Google Scholar] [CrossRef] [PubMed]
- Frydman, G.H.; Marini, R.P.; Bakthavatchalu, V.; Biddle, K.E.; Muthupalani, S.; Vanderburg, C.R.; Lai, B.; Bendapudi, P.K.; Tompkins, R.G.; Fox, J.G. Local and Systemic Changes Associated with Long-term, Percutaneous, Static Implantation of Titanium Alloys in Rhesus Macaques (Macaca mulatta). Comp. Med. 2017, 67, 165–175. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).