Submitted:
23 September 2025
Posted:
25 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
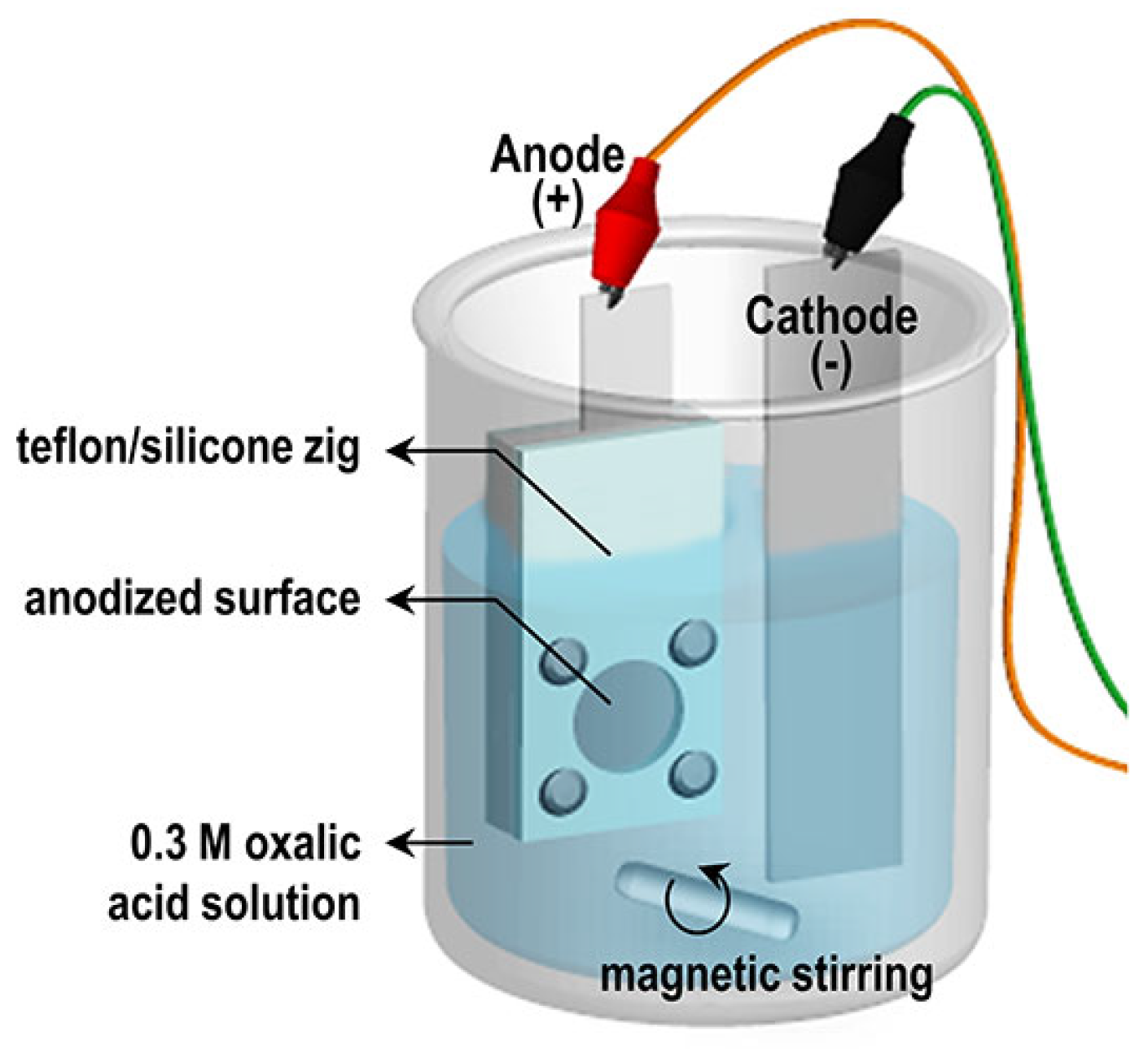
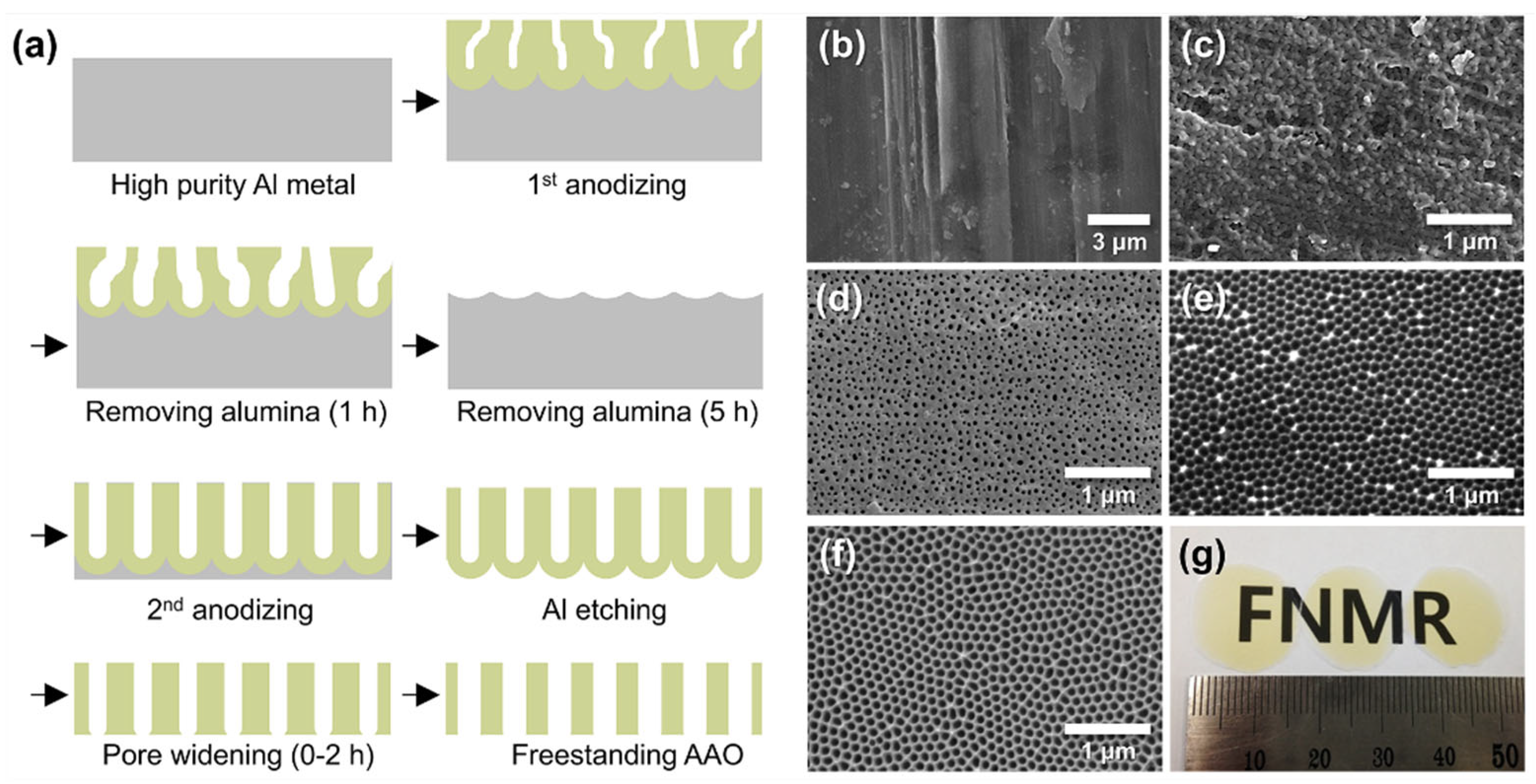
2.1. Anodizing and Pore Widening of Al
2.2. Synthesis of AAO Film on Textured Silicon Substrates
2.3. Surface Treatments: Pore Widening, Annealing, and Boiling-Water Conversion
2.4. Characterization
3. Results and Discussion
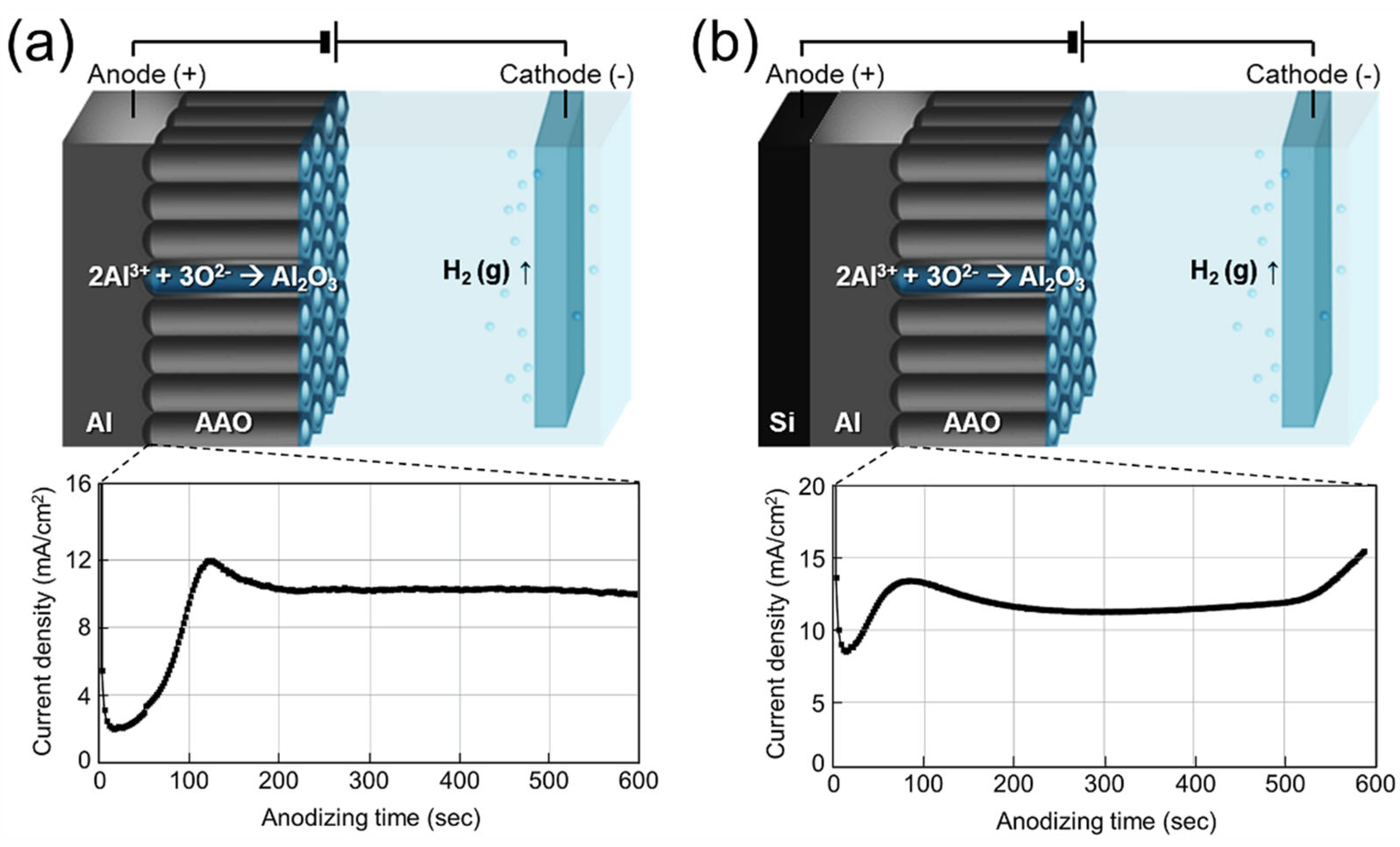
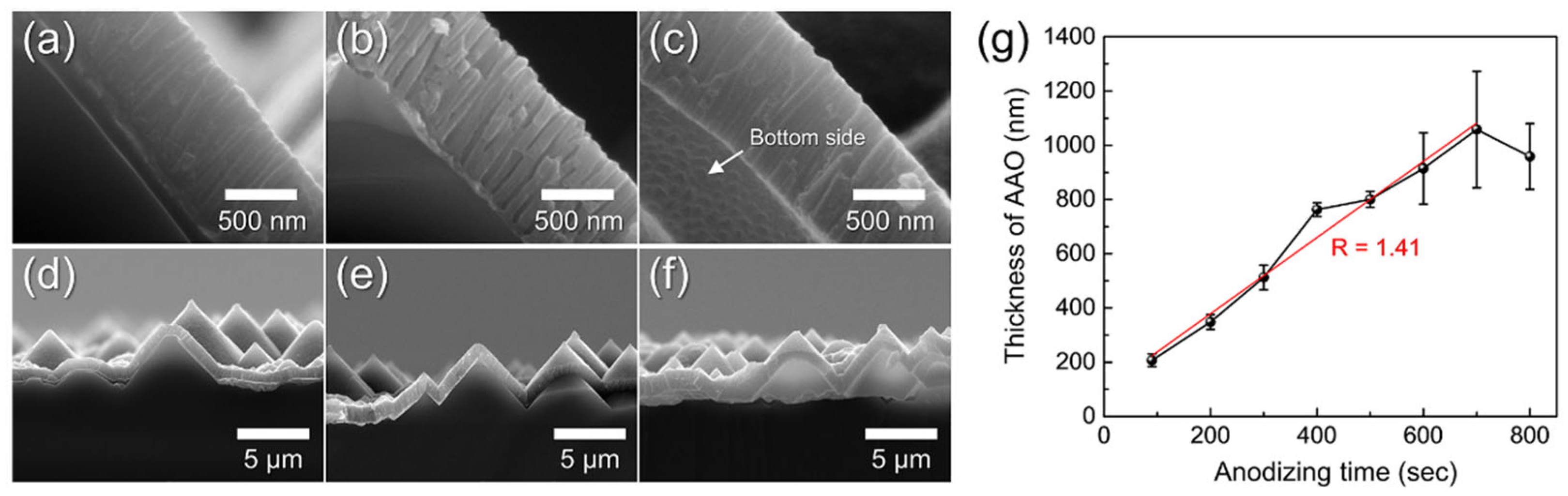
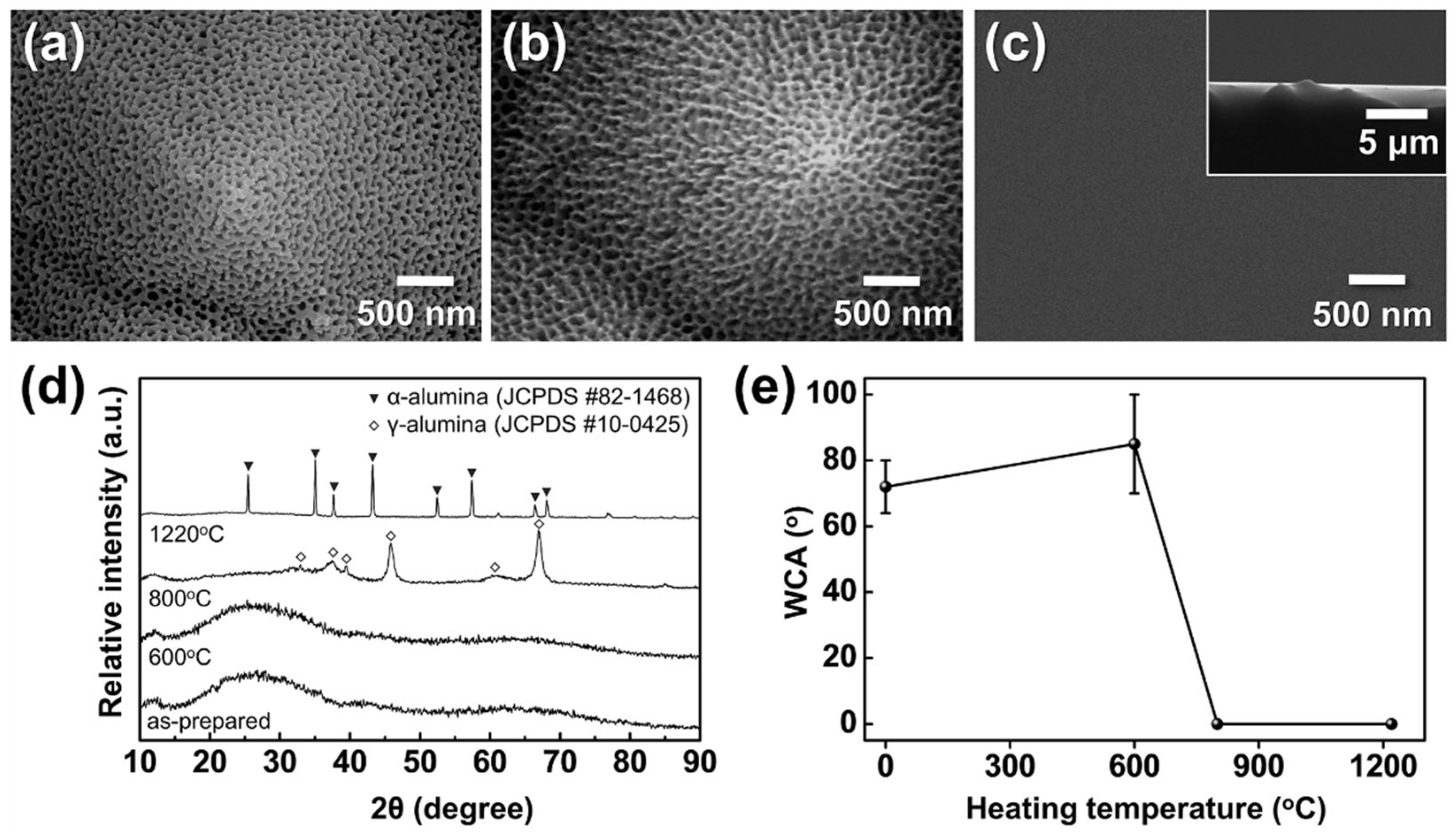
3.1. Anodizing of Al Foil and Al on Textured Silicon Wafers
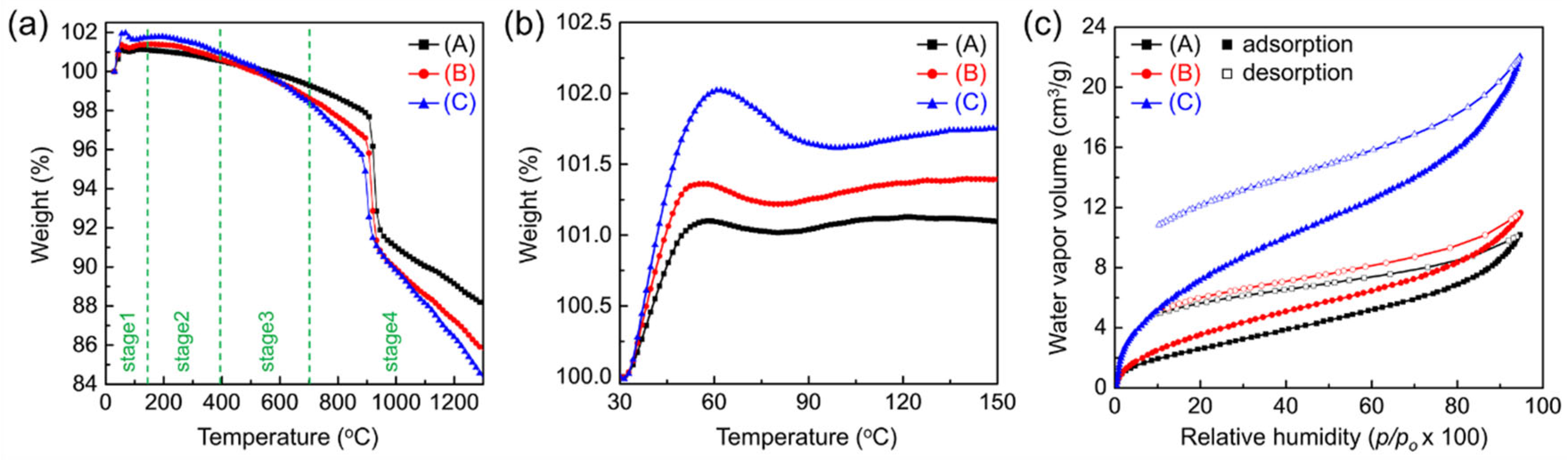
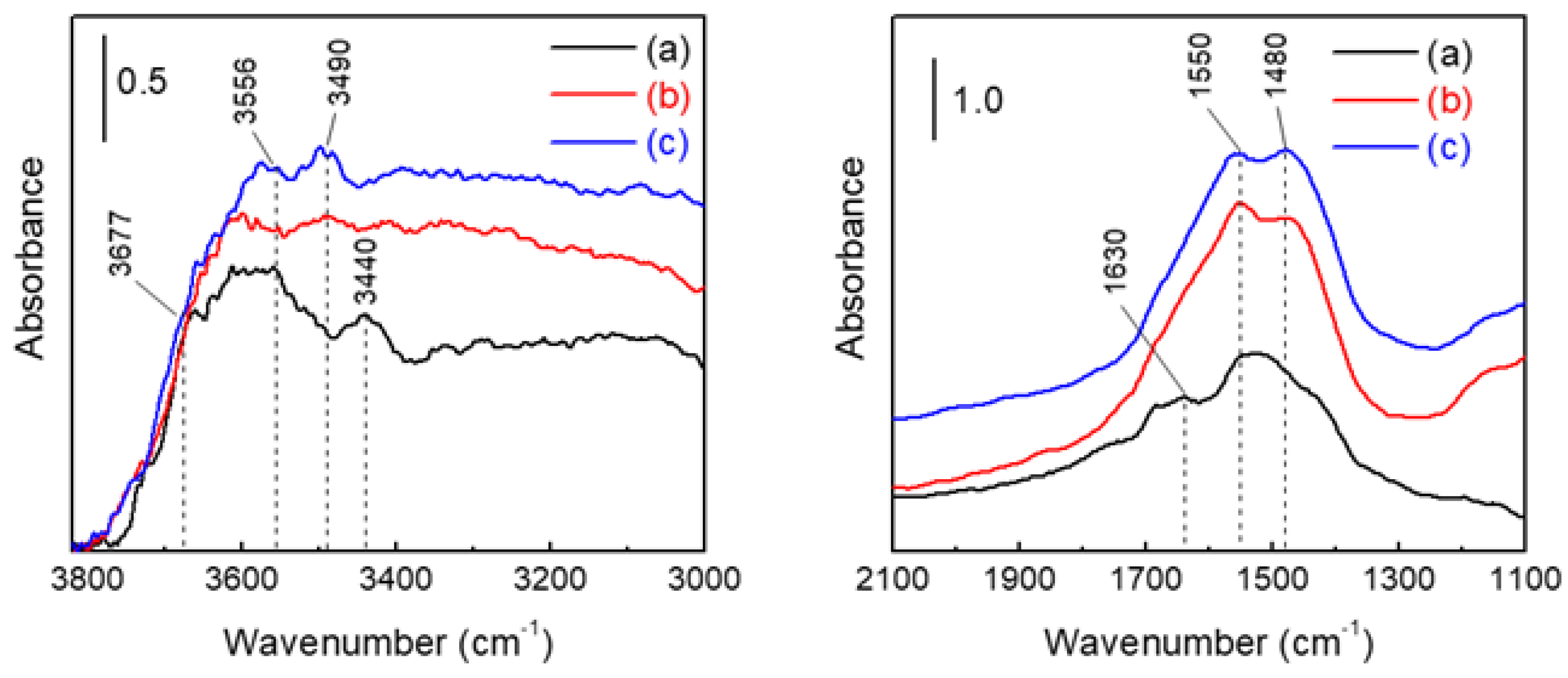
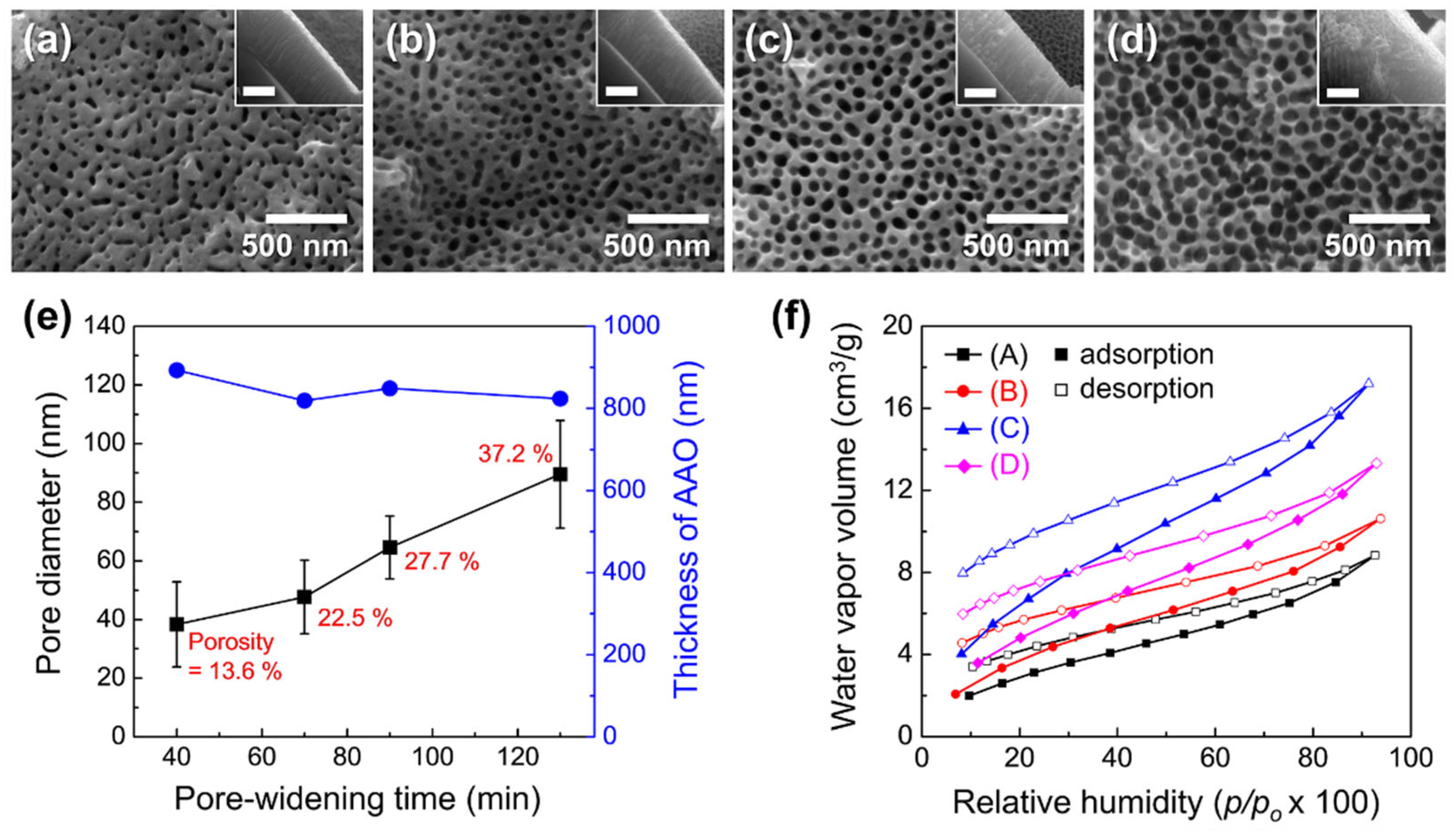
3.2. Fabrication of Freestanding AAO Films from Al Foil and Their Water Vapor Adsorption Characteristics
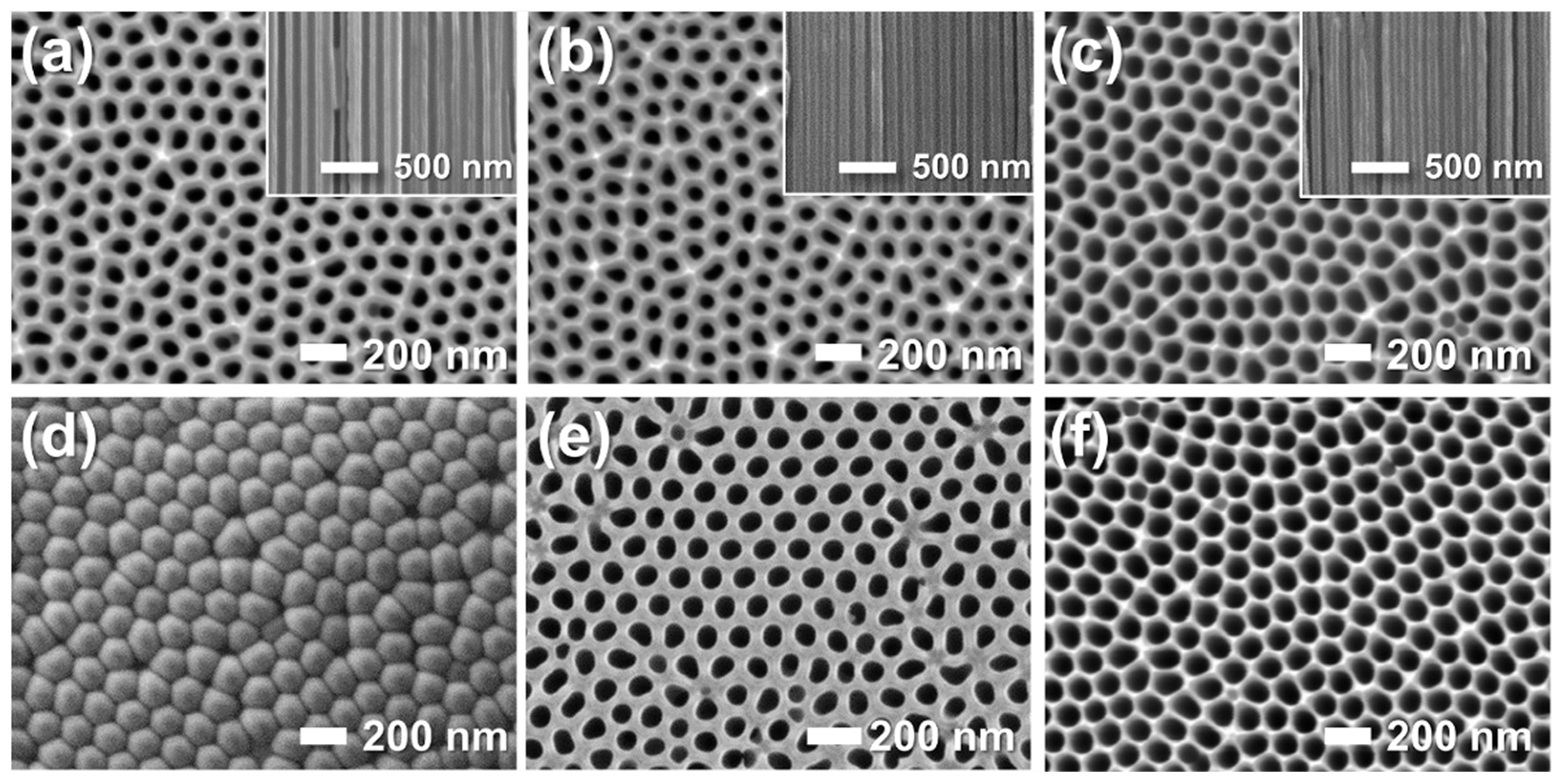
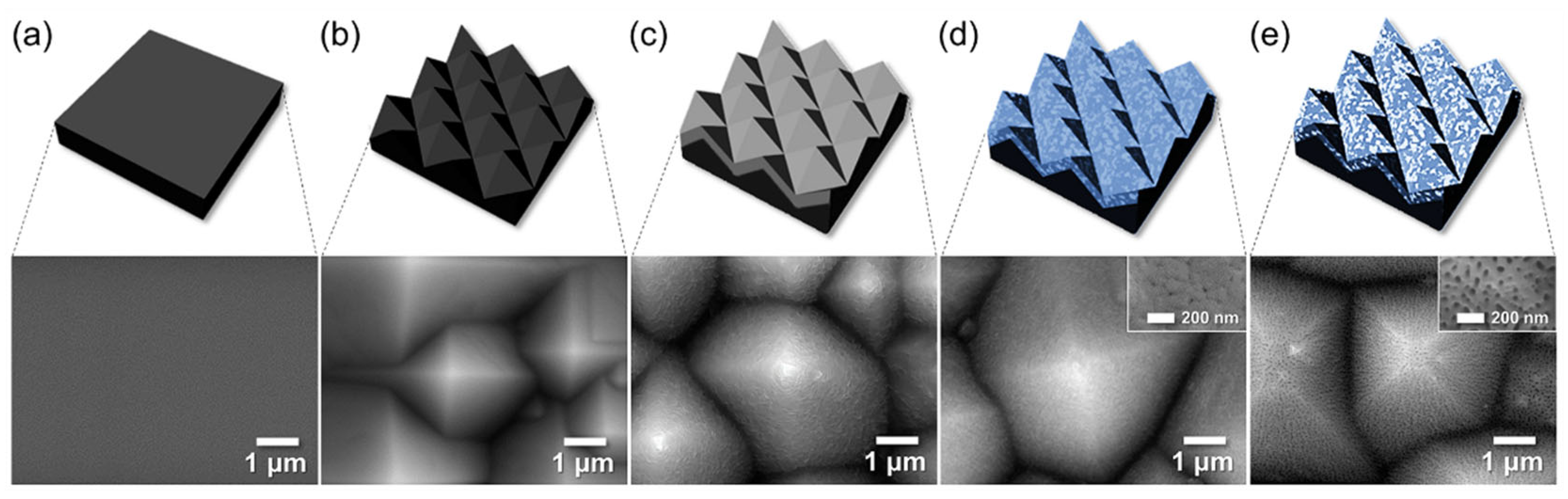
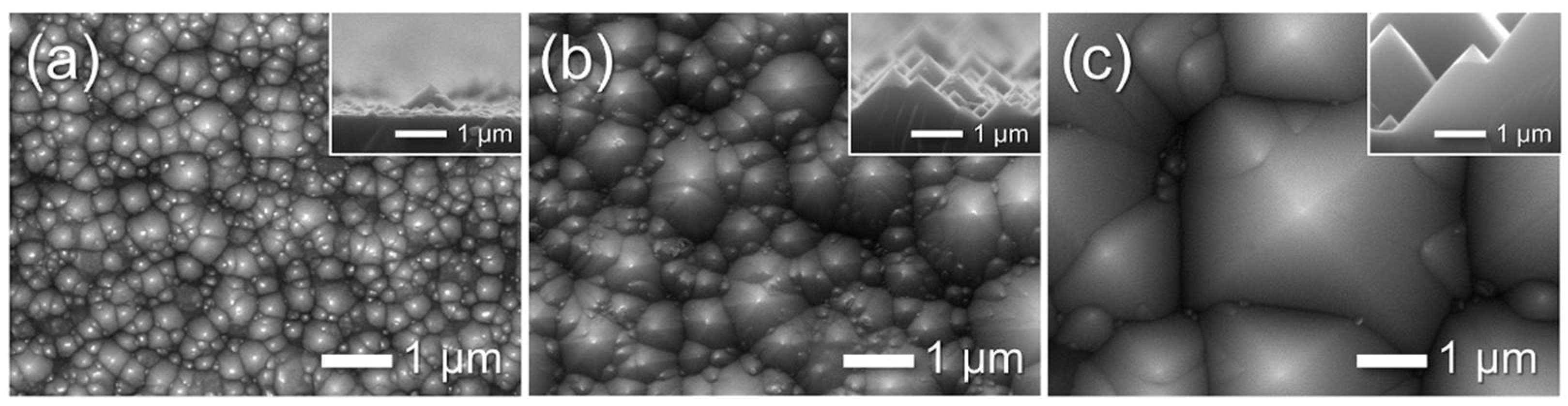
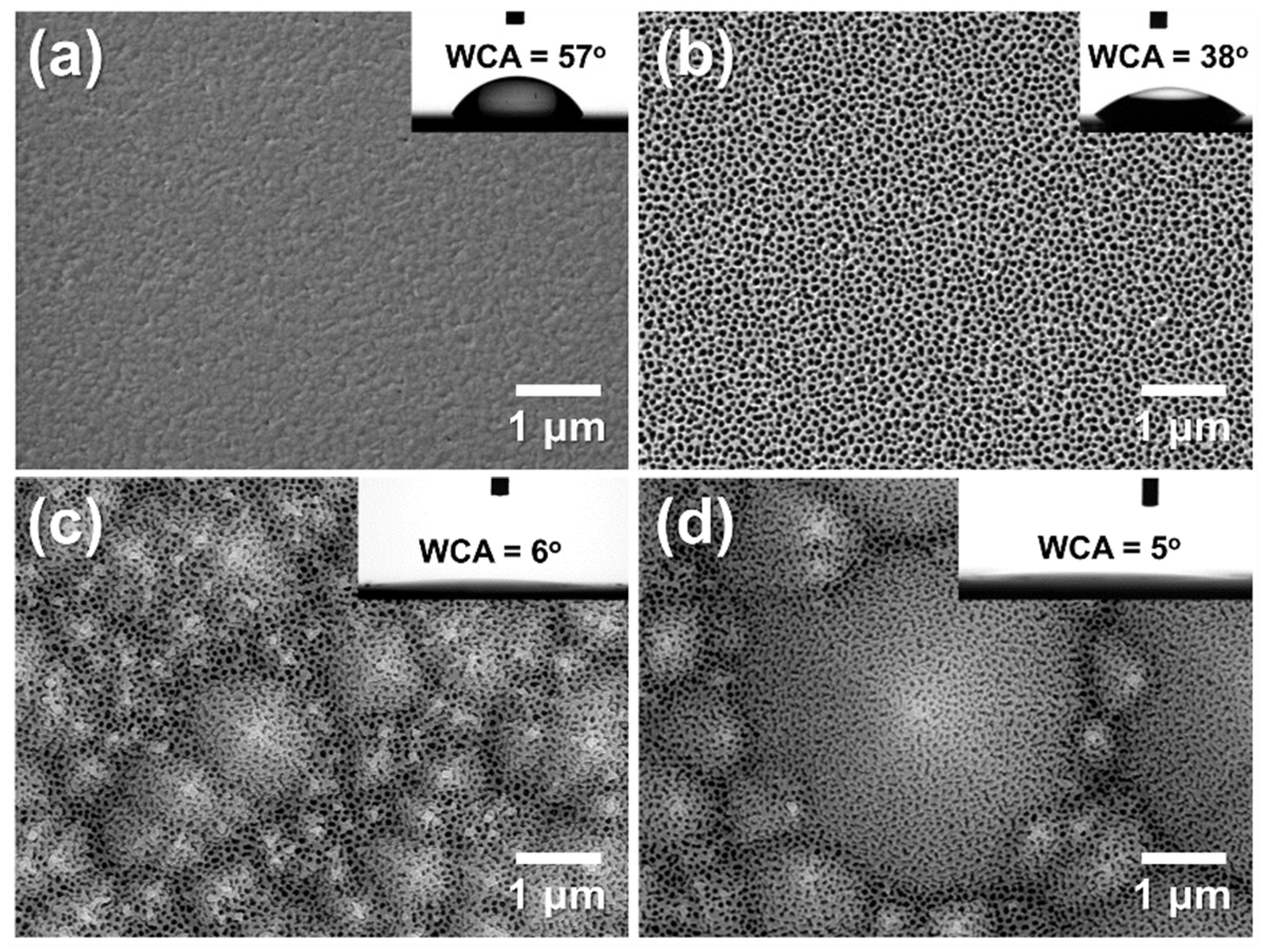
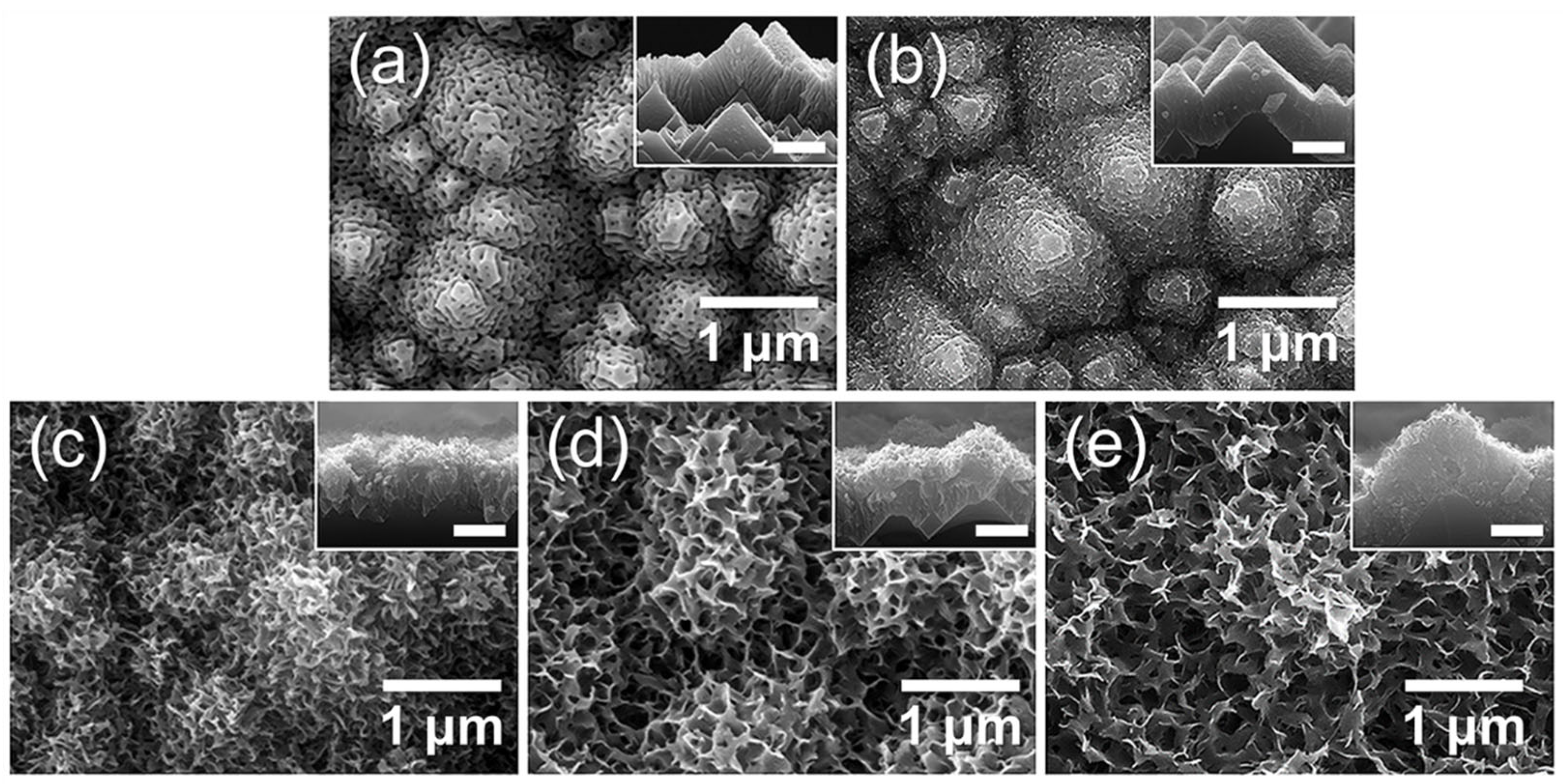
3.3. Fabrication of AAO on Textured Silicon Wafers
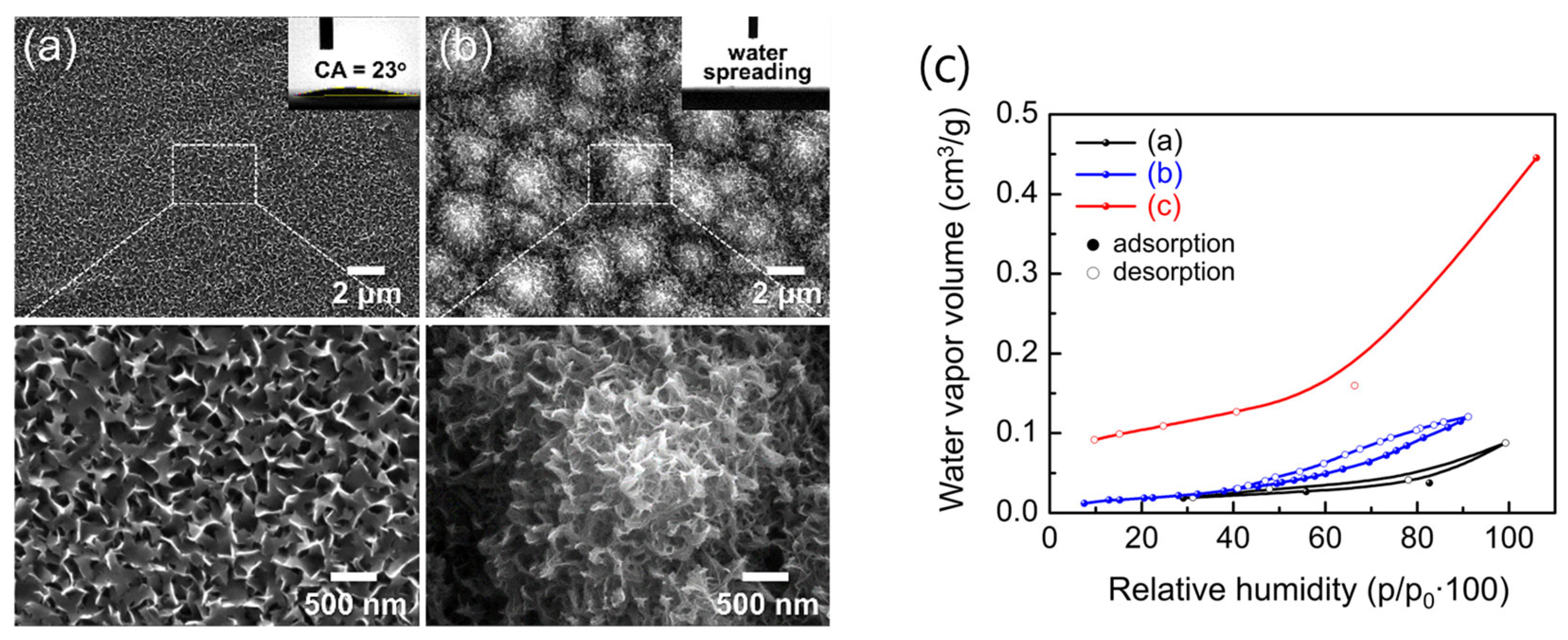
3.4. Vapor and Aqueous-Phase H₂O Responses of Hierarchical AAO/t-Si Surfaces
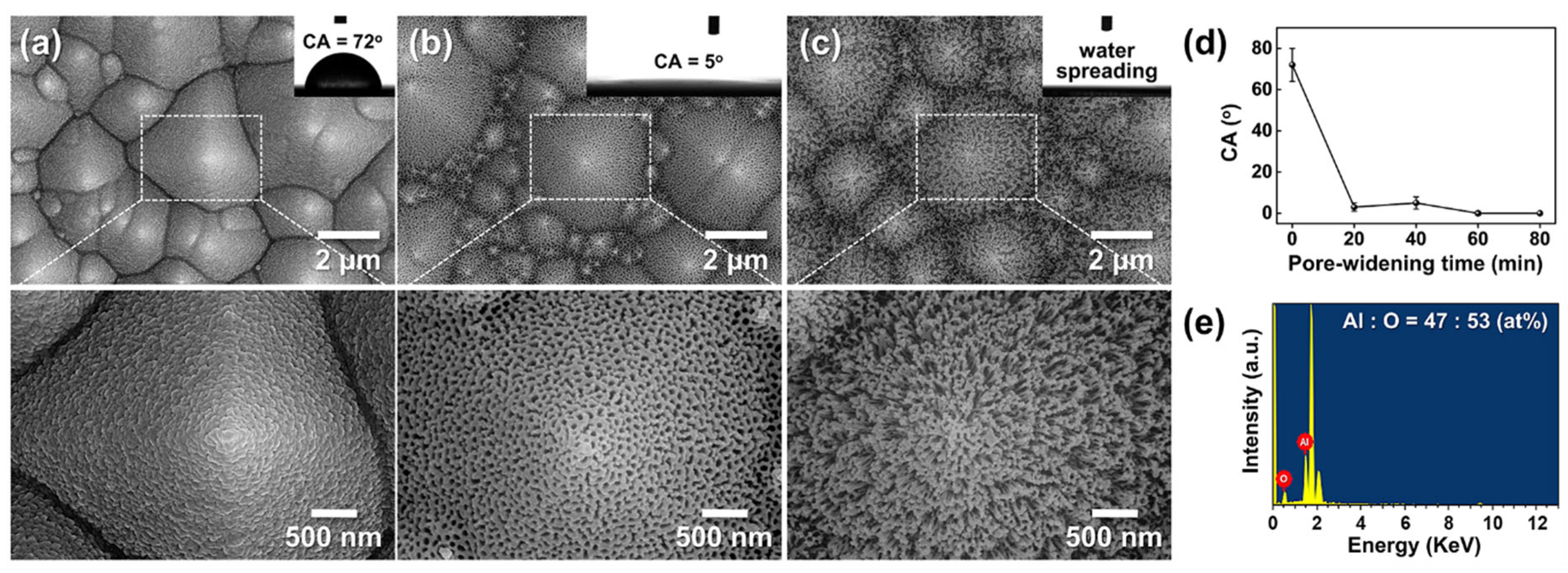
3.5. Hydrophobic Surface Modification of Hierarchical Al₂O₃/Si via PFOTS Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anagnostou, D.E.; Chryssomallis, M.T.; Braaten, B.D.; Ebel, J.L.; Sepúlveda, N. Reconfigurable UWB antenna with RF-MEMS for on-demand WLAN rejection. IEEE Trans. Antennas Propag. 2014, 62, 602–608. [Google Scholar] [CrossRef]
- Chakraborty, A.; Gupta, B.; Sarkar, B.K. Design, fabrication and characterization of miniature RF MEMS switched capacitor based phase shifter. Microelectron. J. 2014, 45, 1093–1102. [Google Scholar] [CrossRef]
- Solgaard, O.; Godil, A.A.; Howe, R.T.; Lee, L.P.; Peter, Y.-A.; Zappe, H. Optical MEMS: From micromirrors to complex systems. J. Microelectromech. Syst. 2014, 23, 517–538. [Google Scholar] [CrossRef]
- Huang, Y.; Vasan, A.S.S.; Doraiswami, R.; Osterman, M.; Pecht, M. MEMS reliability review. IEEE Trans. Device Mater. Reliab. 2012, 12, 482–493. [Google Scholar] [CrossRef]
- Moraja, M.; Amiotti, M.; Kullberg, R.C. Hermetic sealing challenges for MEMS packages. IEEE Trans. Adv. Packag. 2002, 25, 260–267. [Google Scholar]
- Previti, M.; Gilleo, K. Moisture ingress and getter solutions. In Proceedings of the International Symposium on Advanced Packaging Materials Processes; Properties and Interfaces (IEEE Cat. No.01TH8562), IEEE: Piscataway, NJ, USA, 2001; pp. 201–206. [Google Scholar]
- Komvopoulos, K. Surface engineering and microtribology for microelectromechanical systems. Wear 1996, 200, 305–327. [Google Scholar] [CrossRef]
- Miller, W.M. MEMS reliability and testing. In Proceedings of the 1999 MEMS Reliability Conference, Chicago, IL, USA, 23–24 October 1999. [Google Scholar]
- Srivastava, N.C.; Eames, I.W. A review of adsorbents and adsorbates in solid–vapour adsorption heat pump systems. Appl. Therm. Eng. 1998, 18, 707–714. [Google Scholar] [CrossRef]
- Ramesham, R.; Kullberg, R.C. Review of vacuum packaging and maintenance of MEMS and the use of getters therein. J. Micro/Nanolithogr. MEMS MOEMS 2009, 8, 031307. [Google Scholar]
- Wu, C.-S.; Liao, J.-Y.; Fang, S.-Y.; Chiang, A.S. Flexible and transparent moisture getter film containing zeolite. Adsorption 2010, 16, 69–74. [Google Scholar] [CrossRef]
- Chuntonov, K.; Setina, J. Reactive getters for MEMS applications. Vacuum 2016, 123, 42–48. [Google Scholar] [CrossRef]
- Busca, G. The surface of transitional aluminas: A critical review. Catal. Today 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Loeb, G.I.; Schrader, M.E. Modern Approaches to Wettability: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Tavakoli, A.H.; Browning, N.D.; Arslan, I.; Gates, B.C.; Dixon, D.A.; Glezakou, V.-A. Amorphous alumina nanoparticles: Structure, surface energy, and thermodynamic phase stability. J. Phys. Chem. C 2013, 117, 17123–17130. [Google Scholar] [CrossRef]
- McHale, J.; Navrotsky, A.; Perrotta, A. Effects of increased surface area and chemisorbed H₂O on the relative stability of nanocrystalline γ-Al₂O₃ and α-Al₂O₃. J. Phys. Chem. B 1997, 101, 603–613. [Google Scholar] [CrossRef]
- Hass, K.C.; Schneider, W.F.; Curioni, A.; Andreoni, W. The chemistry of water on alumina surfaces: Reaction dynamics from first principles. Science 1998, 282, 265–268. [Google Scholar] [CrossRef]
- Wippermann, S.; Schmidt, W.G.; Thissen, P.; Grundmeier, G. Dissociative and molecular adsorption of water on α-Al₂O₃ (0001). Phys. Status Solidi C 2010, 7, 137–140. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 3393–3403. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, F.; Hao, J.; Liu, W. Stable biomimetic super-hydrophobic engineering materials. J. Am. Chem. Soc. 2005, 127, 15670–15671. [Google Scholar] [CrossRef]
- Fu, X.; He, X. Fabrication of super-hydrophobic surfaces on aluminum alloy substrates. Appl. Surf. Sci. 2008, 255, 1776–1781. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Farzaneh, M. Superhydrophobic Coatings with Reduced Ice Adhesion. J. Adhes. Sci. Technol. 2009, 23, 1215–1237. [Google Scholar] [CrossRef]
- Saleema, N.; Farzaneh, M.; Paynter, R.W.; Sarkar, D.K. Prevention of Ice Accretion on Aluminum Surfaces by Enhancing Their Hydrophobic Properties. J. Adhes. Sci. Technol. 2011, 25, 27–40. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.; Gallant, D.; Paynter, R.; Chen, X.-G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Interfaces 2011, 3, 4775–4781. [Google Scholar] [CrossRef]
- Ye, J.; Yin, Q.; Zhou, Y. Superhydrophilicity of anodic aluminum oxide films: From “honeycomb” to “bird’s nest”. Thin Solid Films 2009, 517, 6012–6015. [Google Scholar] [CrossRef]
- Kim, Y.; et al. Robust Superhydrophilic/Hydrophobic Surface Based on Self-Aggregated Al₂O₃ Nanowires by Single-Step Anodization and Self-Assembly Method. ACS Appl. Mater. Interfaces 2012, 4, 5074–5078. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Zhong, R.; Tsyntsaru, N.; Celis, J.-P. Surface wettability of macroporous anodized aluminum oxide. ACS Appl. Mater. Interfaces 2013, 5, 3224–3233. [Google Scholar] [CrossRef]
- Kiyoharu, T.; Noriko, K.; Tsutomu, M. Super-Water-Repellent Al₂O₃ Coating Films with High Transparency. J. Am. Ceram. Soc. 1997, 80, 1040–1042. [Google Scholar]
- Kiyoharu, T.; Noriko, K.; Tsutomu, M. Formation Process of Super-Water-Repellent Al₂O₃ Coating Films with High Transparency by the Sol–Gel Method. J. Am. Ceram. Soc. 1997, 80, 3213–3216. [Google Scholar]
- Jafari, R.; Farzaneh, M. Fabrication of superhydrophobic nanostructured surface on aluminum alloy. Appl. Phys. A 2011, 102, 195–199. [Google Scholar] [CrossRef]
- Cho, H.; Kim, D.; Lee, C.; Hwang, W. A simple fabrication method for mechanically robust superhydrophobic surface by hierarchical aluminum hydroxide structures. Curr. Appl. Phys. 2013, 13, 762–767. [Google Scholar] [CrossRef]
- Feng, L.; Che, Y.; Liu, Y.; Qiang, X.; Wang, Y. Fabrication of superhydrophobic aluminium alloy surface with excellent corrosion resistance by a facile and environment-friendly method. Appl. Surf. Sci. 2013, 283, 367–374. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Zuo, Z.; et al. Fabrication and Anti-icing Property of Coral-like Superhydrophobic Aluminum Surface. Appl. Surf. Sci. 2015. [CrossRef]
- Kim, J.; Jun, S.; Lee, J.; Godinez, J.; You, S.M. Effect of Surface Roughness on Pool Boiling Heat Transfer of Water on a Superhydrophilic Aluminum Surface. J. Heat Transf. 2017, 139, 101501. [Google Scholar] [CrossRef]
- Sharma, C.S.; Combe, J.; Giger, M.; Emmerich, T.; Poulikakos, D. Growth Rates and Spontaneous Navigation of Condensate Droplets Through Randomly Structured Textures. ACS Nano 2017, 11, 1673–1682. [Google Scholar] [CrossRef]
- Parin, R.; et al. Nano-structured aluminum surfaces for dropwise condensation. Surf. Coat. Technol. 2018, 348, 1–12. [Google Scholar] [CrossRef]
- Ngo, C.-V.; Chun, D.-M. Control of laser-ablated aluminum surface wettability to superhydrophobic or superhydrophilic through simple heat treatment or water boiling post-processing. Appl. Surf. Sci. 2018, 435, 974–982. [Google Scholar] [CrossRef]
- Zubel, I.; Kramkowska, M. The effect of isopropyl alcohol on etching rate and roughness of (100) Si surface etched in KOH and TMAH solutions. Sens. Actuators A Phys. 2001, 93, 138–147. [Google Scholar] [CrossRef]
- Papet, P.; Nichiporuk, O.; Kaminski, A.; Rozier, Y.; Kraiem, J.; Lelievre, J.F.; Chaumartin, A.; Fave, A.; Lemiti, M. Pyramidal texturing of silicon solar cell with TMAH chemical anisotropic etching. Sol. Energy Mater. Sol. Cells 2006, 90, 2319–2328. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chemical Reviews 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Sousa, C.T.; et al. Nanoporous alumina as templates for multifunctional applications. Applied Physics Reviews 2014, 1. [Google Scholar] [CrossRef]
- Yang, Y.; et al. Anodic alumina template on Au/Si substrate and preparation of CdS nanowires. Solid State Commun. 2002, 123, 279–282. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B.; Gösele, U. Self-ordering regimes of porous alumina: the 10 porosity rule. Nano Lett. 2002, 2, 677–680. [Google Scholar] [CrossRef]
- Mata-Zamora, M.; Saniger, J. Thermal evolution of porous anodic aluminas: a comparative study. Revista mexicana de física 2005, 51, 502–509. [Google Scholar]
- Mardilovich, P.P.; Govyadinov, A.N.; Mukhurov, N.I.; Rzhevskii, A.M.; Paterson, R. New and modified anodic alumina membranes Part I. Thermotreatment of anodic alumina membranes. Journal of Membrane Science 1995, 98, 131–142. [Google Scholar] [CrossRef]
- Yang, S.G.; et al. Stability of anodic aluminum oxide membranes with nanopores. Physics Letters A 2003, 318, 440–444. [Google Scholar] [CrossRef]
- Al-Abadleh, H.A.; Grassian, V. FT-IR study of water adsorption on aluminum oxide surfaces. Langmuir 2003, 19, 341–347. [Google Scholar] [CrossRef]
- Loeb, G.I.; Schrader, M.E. Modern approaches to wettability: theory and applications. (Springer Science & Business Media, 2013).
- Fubini, B.; Della Gatta, G.; Venturello, G. Energetics of adsorption in the alumina—water system microcalorimetric study on the influence of adsorption temperature on surface processes. Journal of Colloid and Interface Science 1978, 64, 470–479. [Google Scholar] [CrossRef]
- Hass, K.C.; Schneider, W.F.; Curioni, A.; Andreoni, W. The chemistry of water on alumina surfaces: Reaction dynamics from first principles. science 1998, 282, 265–268. [Google Scholar] [CrossRef]
- Verdaguer, A.; Sacha, G.M.; Bluhm, H.; Salmeron, M. Molecular Structure of Water at Interfaces: Wetting at the Nanometer Scale. Chemical Reviews 2006, 106, 1478–1510. [Google Scholar] [CrossRef]
- Thomas, A.C.; Richardson, H.H. Growth of Thin Film Water on α-Al2O3 (0001): An FTIR Study. The Journal of Physical Chemistry C 2008, 112, 20033–20037. [Google Scholar] [CrossRef]
- Choi, J.; et al. Perfect two-dimensional porous alumina photonic crystals with duplex oxide layers. Journal of Applied Physics 2003, 94, 4757–4762. [Google Scholar] [CrossRef]
- Lujun, Y.; Maojun, Z.; Haibin, L.; Li, M.; Wenzhong, S. High-performance humidity sensors based on high-field anodized porous alumina films. Nanotechnology 2011, 22, 379501. [Google Scholar]
- Ono, S.; Ichinose, H.; Masuko, N. The high resolution observation of porous anodic films formed on aluminum in phosphoric acid solution. Corrosion Science 1992, 33, 841–850. [Google Scholar] [CrossRef]
- Xiong, G.; et al. Effect of Atomic Layer Deposition Coatings on the Surface Structure of Anodic Aluminum Oxide Membranes. The Journal of Physical Chemistry B 2005, 109, 14059–14063. [Google Scholar] [CrossRef] [PubMed]
- Eng, P.J.; et al. Structure of the Hydrated α-Al<sub>2</sub>O<sub>3</sub> (0001) Surface. Science 2000, 288, 1029. [Google Scholar] [CrossRef]
- Liu, X.; Truitt, R.E. DRFT-IR Studies of the Surface of γ-Alumina. Journal of the American Chemical Society 1997, 119, 9856–9860. [Google Scholar] [CrossRef]
- Chatterjee, M.; Enkhtuvshin, D.; Siladitya, B.; Ganguli, D. Hollow Alumina Microspheres From Boehmite Sols. J Mater Sci 1998, 33, 4937–4942. [Google Scholar] [CrossRef]
- Young, L. Anodic oxide films. (London; New York: Academic Press, 1961).
- Emmer, I.; Hajek, Z.; Řepa, P. Surface adsorption of water vapor on hydrated layers of Al2O3. Surface Science 1985, 162, 303–309. [Google Scholar] [CrossRef]
- Park, B.K.; Lee, Y.S.; Koo, K.K. Preparation of highly porous aluminum hydroxide gels by hydrolysis of an alumi-num sulfate and mineralizer. Journal of Ceramic Processing Research 2010, 11, 64–68. [Google Scholar]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. European Journal of Inorganic Chemistry 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Wenzel, R.N. RESISTANCE OF SOLID SURFACES TO WETTING BY WATER. Industrial & Engineering Chemistry 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. The Journal of Physical and Colloid Chemistry 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Leem, J.W.; Dudem, B.; Yu, J.S. Biomimetic nano/micro double-textured silicon with outstanding antireflective and super-hydrophilic surfaces for high optical performance. RSC Advances 2017, 7, 33757–33763. [Google Scholar] [CrossRef]
- Bico, J.; Tordeux, C.; Quéré, D. Rough wetting. EPL (Europhysics Letters) 2001, 55, 214. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discussions of the Faraday Society 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Transactions of the Faraday Society 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Redon, R.; et al. Contact angle studies on anodic porous alumina. Journal of colloid and interface science 2005, 287, 664–670. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: a review of materials and mechanisms. Sensor letters 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Henderson, M.A. The interaction of water with solid surfaces: fundamental aspects revisited. Surface Science Reports 2002, 46, 1–308. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Gan, Y. Characterization of critically cleaned sapphire single-crystal substrates by atomic force microscopy, XPS and contact angle measurements. Applied Surface Science 2013, 274, 405–417. [Google Scholar] [CrossRef]
- Spooner, R.C. Water Sealing of Detached Aluminium Oxide Anodic Film. Nature 1956, 178, 1113–1114. [Google Scholar] [CrossRef]
- Hart, R. A study of boehmite formation on aluminium surfaces by electron diffraction. Trans. Faraday Soc. 1954, 50, 269–273. [Google Scholar] [CrossRef]
- Seo, Y.I.; Lee, Y.J.; Kim, D.-G.; Lee, K.H.; Kim, Y.D. Mechanism of aluminum hydroxide layer formation by surface modification of aluminum. Applied Surface Science 2010, 256, 4434–4437. [Google Scholar] [CrossRef]
- Hozumi, A.; Kim, B.; McCarthy, T.J. Hydrophobicity of Perfluoroalkyl Isocyanate Monolayers on Oxidized Aluminum Surfaces. Langmuir 2009, 25, 6834–6840. [Google Scholar] [CrossRef]
- Tadanaga, K.; Katata, N.; Minami, T. Formation Process of Super-Water-Repellent Al2O3 Coating Films with High Transparency by the Sol–Gel Method. Journal of the American Ceramic Society 1997, 80, 3213–3216. [Google Scholar] [CrossRef]
- Łodziana, Z.; Topsøe, N.-Y.; Nørskov, J.K. A negative surface energy for alumina. Nature materials 2004, 3, 289. [Google Scholar] [CrossRef]
- Kim, S.; Cheung, E.; Sitti, M. Wet Self-Cleaning of Biologically Inspired Elastomer Mushroom Shaped Microfibrillar Adhesives. Langmuir 2009, 25, 7196–7199. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




