1. Introduction
The transition to a zero CO2 emissions economy is accelerating the development of new energy production technologies. In December 2019, the European Commission introduced the European Green Deal (European Parliament, 2022), with the aim of making Europe climate-neutral by 2050.
Critical raw materials (CRMs) are key materials for renewable energy technologies and electric mobility. Given the decline of scarce but essential raw materials (Mishra et al., 2022), (Krausmann et al., 2009), (Henckens, 2021), (Calvo et al., 2017) such as indium, tin, lithium, cobalt, magnesium, rare earths, copper, etc. and the difficulty in obtaining them, the proposal for recycling and recovery of critical metals arises; however, currently, most of these resources have low recovery rates at the end of their useful lives. The consumption of CRMs is particularly relevant in the development of electric mobility, since the production of lithium-ion batteries requires the consumption of a significant amount of metals (MacKinsey Co, 2023). Therefore, it is essential to develop technologies for the recovery of the metals present in these batteries (Heath et al., 2022) (Huang et al., 2018).
Various methods have been employed for metal recovery from waste and by-products. Pyrometallurgy is a commonly used technique, offering the retrieval of high-purity alloys. However, this energy-intensive approach involves high temperatures, and there is a risk of metal losses in slag, resulting in lower recovery rates compared to other methods (Dias et al., 2022). On the other hand, hydrometallurgical recovery is also prevalent for metal separation in aqueous medium (Vieceli et al., 2021). This method achieves higher metal recovery rates with lower energy consumption, yet further research is needed to mitigate the environmental impact associated with hydrometallurgical processes (Chabhadiya et al., 2021). However, there is a continuous need to explore new extraction agents that are more selective and less environmentally harmful (Ilyas et al., 2023). Particularly, deep eutectic solvents have emerged as a green alternative to traditional solvents due to their advantages. They are derived from renewable raw materials, easily accessible, cost-effective, reusable, energy-efficient in synthesis, low in toxicity, biodegradable, and thermally stable (Prabhune & Dey, 2023), (Palluzzi et al., 2023). DES’s have become more widely used in the last 5 years as an alternative to pyro- and hydrometallurgical methods for the extraction of lithium, cobalt, nickel, and manganese (Kovačević et al., 2024) (Biniaz et al., 2025) (Zanoletti et al., 2025)(Dar et al., 2025).
Deep eutectic solvents are derived from blending two or three substances with specific compositions, wherein the individual components possess higher melting points than the resulting mixture. This mixture comprises a well-matched combination of a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) (Prabhune & Dey, 2023). The reduction in the melting point is attributed to the extensive intermolecular hydrogen bonding and the charge delocalization (Smith et al., 2014). There are two types of DESs: hydrophilic and hydrophobic. Both types of DESs have been previously used for metal recovery in solution (Martín et al., 2023). They have been described as leaching agents (Abbott et al., 2004),(Thompson et al., 2022), and hydrophobic DESs have been widely employed as extraction agents by creating biphasic systems with hydrophilic compounds like water, for instance (Tereshatov et al., 2016),(Ola & Matsumoto, 2019). Recently Xue K et al., 2023 (Xue et al., 2023) have studied the lithium extraction from aqueous medium using hydrophobic deep eutectic solvents. The hydrophobicity of DESs depends on the chemical nature of the eutectic mixture, i.e., the HBA and the HBD.
The hydrophobic DES obtained in this study from Aliquat 336 and L-Menthol has previously been utilized for the extraction of various metals such as Li, Co, Ni, etc (Milevskii et al., 2022) (Kozhevnikova et al., 2022). However, despite the conducted studies, it is necessary to delve deeper into the understanding and the effect of the different parameters influencing the selective extraction of cobalt using the 3 Aliquat 336:7 L-menthol DES as the extraction agent.
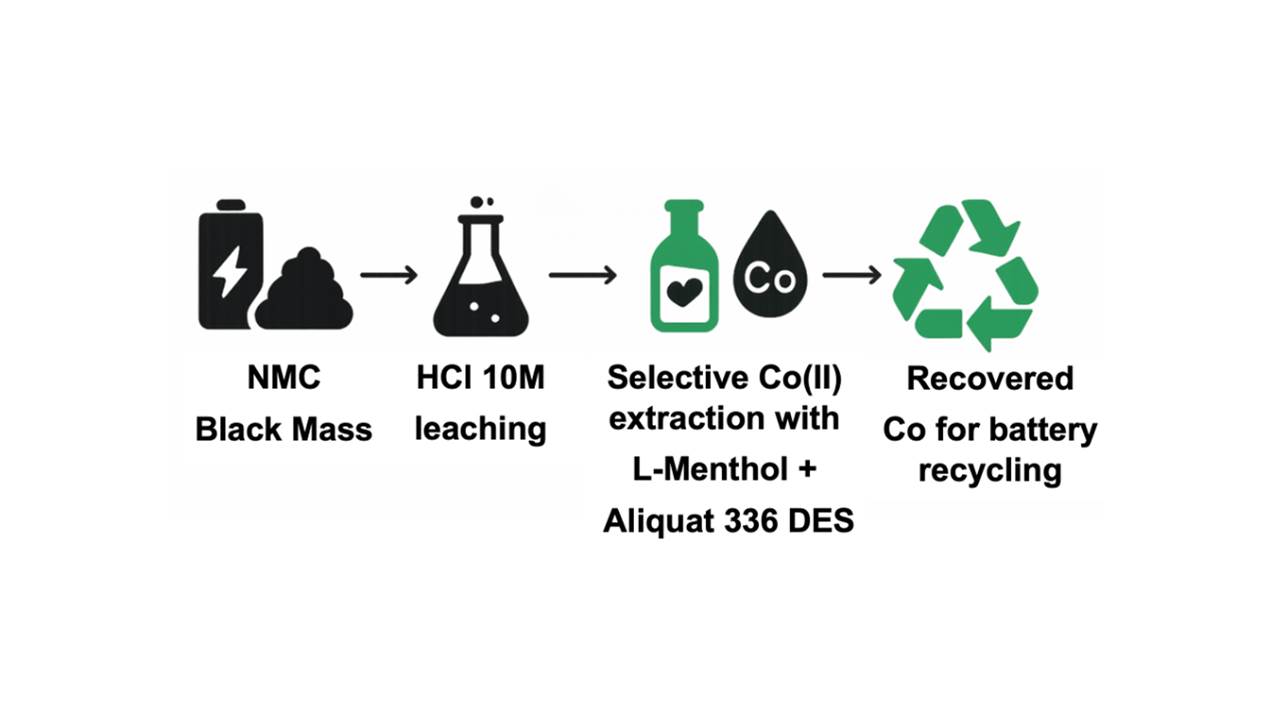
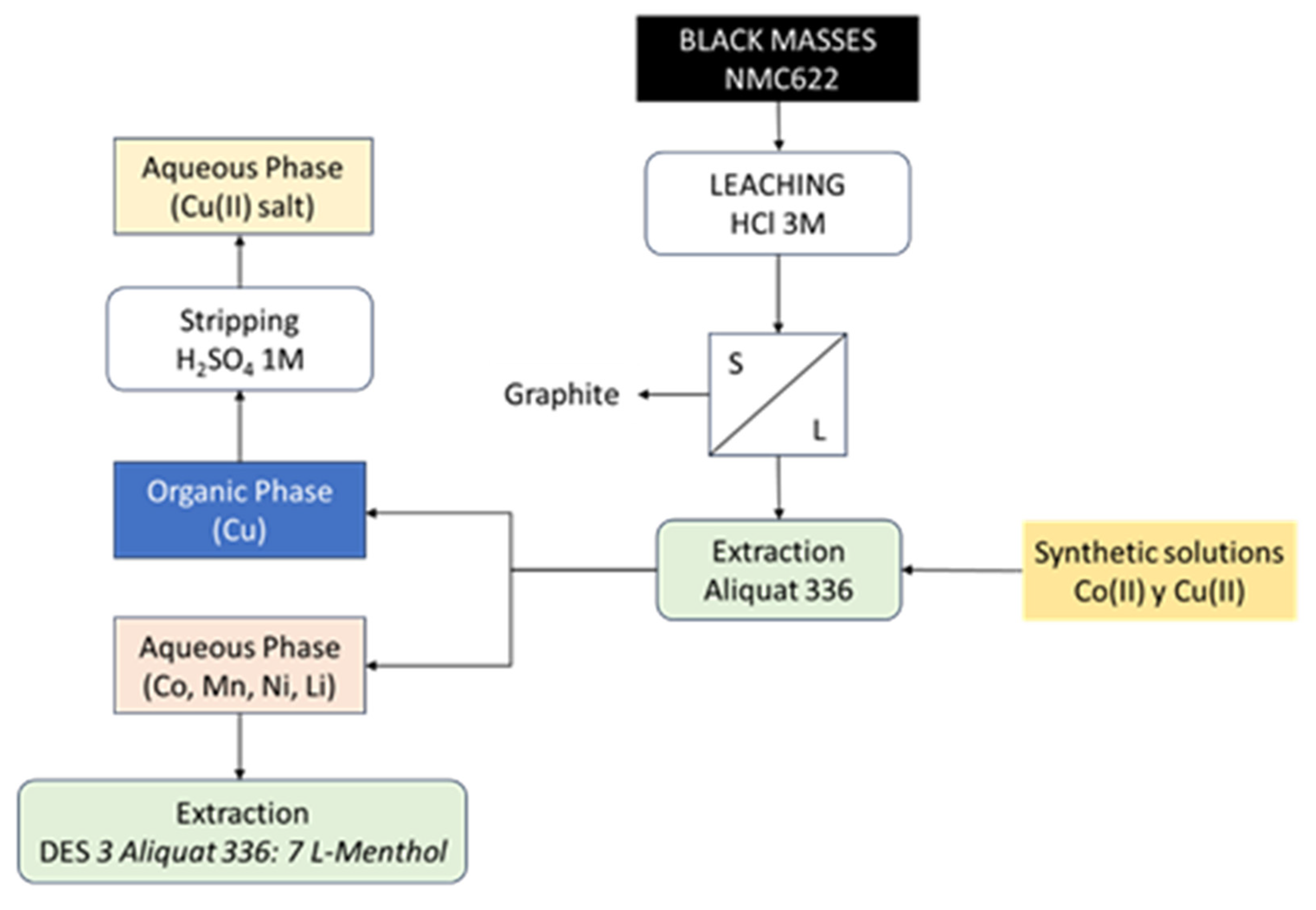
In thE present work, the DES is employed to investigate and optimize the parameters influencing cobalt extraction, utilizing a synthetic Co(II) solution for this purpose, and subsequently, the optimal conditions are applied to the selective extraction and separation of Co(II) from solutions obtained from the hydrochloric acid leaching of five black masses (black mass is a black material obtained from the processing of used batteries after dismantling and crushing them) originating from NMC 622-type lithium-ion batteries. The optimization of the Co/Cu separation conditions is carried out, achieving the separation of Cu(II) using Aliquat 336 in kerosene.
2. Results and Discussion
2.1. Characterization of DES
2.1.1. Fourier-Transform Infrared Spectroscopy
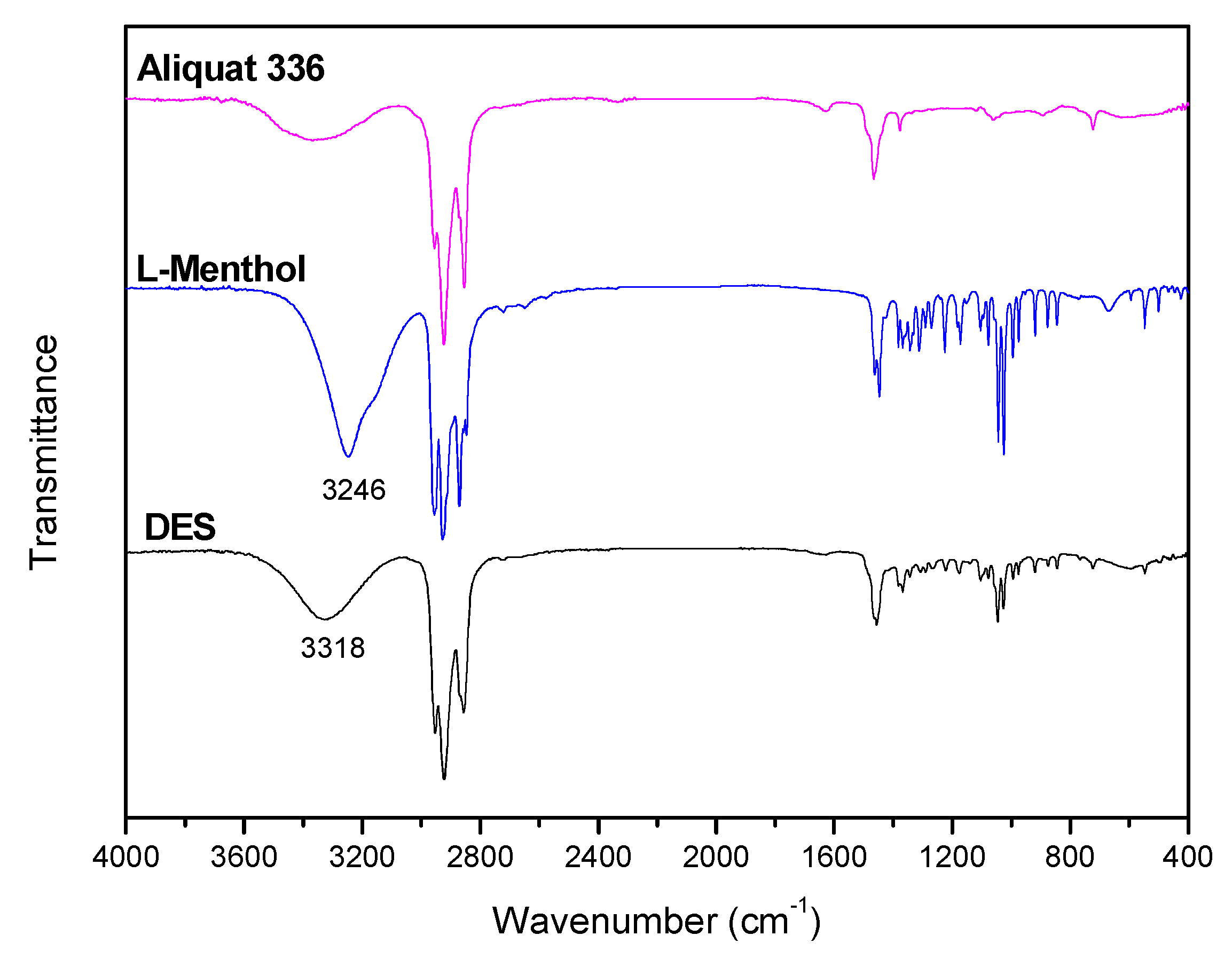
DES is formed by hydrogen bonds between Aliquat 336 and L-Menthol. FTIR spectra of Aliquat 336, L-Menthol and DES are shown in
Figure 1. This Figure shows the presence of OH vibration peak at 3246 cm
-1 for L-Menthol. When DES is formed OH vibration of the DES is shifted to 3318 cm
-1. This shift in OH vibration (72 cm
-1) confirms the presence of hydrogen bonds between L-Menthol (HBD) and Aliquat 336 (HBA) when DES is obtained.
2.1.2. Differential Scanning Calorimetry (DSC) and Thermogravimetry (TGA) of DES
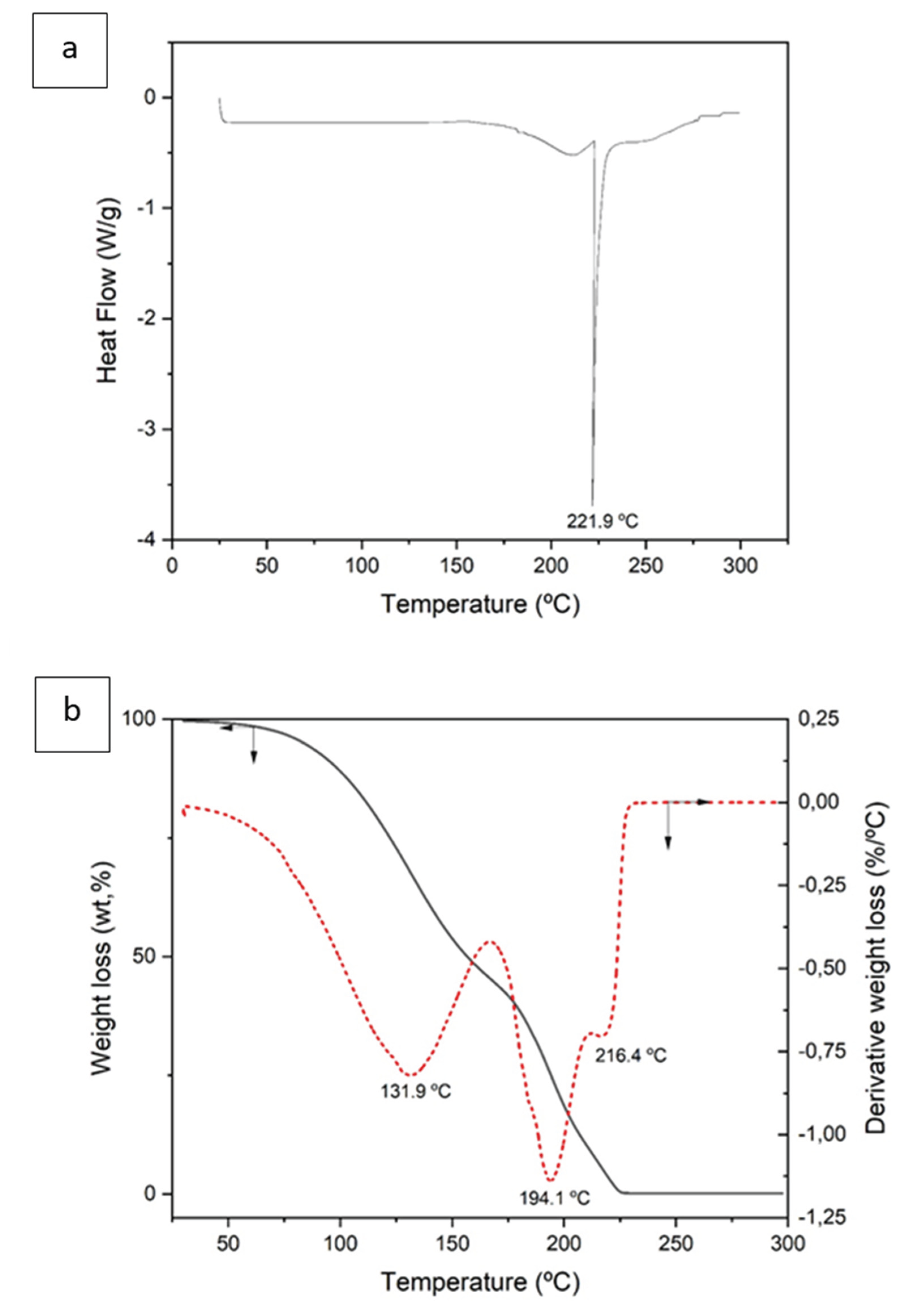
Figure 2a shows the DSC curve obtained after the formation of DES. An endothermic peak at 223.1ºC with an associated enthalpy of 329.4 J/g is observed, which may correspond to the decomposition of the organic compounds that form the DES (L-Menthol and Aliquat 336).
Figure 2b shows mass loss as a function of temperature (TGA) as well as the derivative of the above curve. The TGA curve shows that at 225ºC the total decomposition of the compound occurs, in agreement with the DSC curve data. The total mass loss is 99.6%.
In the derivative curve, three peaks are observed at 131.9ºC, 194.1ºC and 216.4ºC respectively. At the temperature range between ambient and 166ºC, a mass loss of 53.81% is observed, which could correspond to the loss of coordination water as well as to the loss of mass corresponding to the initial decomposition of L-menthol (Trivedi, M.K., 2015) (Phaechamud et al., 2016).
Between 166ºC and 211ºC the mass loss is 36.4%, which could correspond to the partial decomposition of the organic compounds formINF the DES, essentially to the final decomposition of L-menthol together with the initial decomposition of Aliquat 336 (Vera et al., 2019) (Sellami et al., 2021), giving rise to other intermediate organic compounds of lower molecular weight.
Finally, between 211ºC and 228ºC there is a mass loss of 9.4%, which would correspond to the decomposition of the intermediate organic products that could have been generated in the previous decomposition reaction, which come essentially from the previous decomposition of Aliquat 336.
2.1.3. Nuclear Magnetic Resonance Spectroscopy
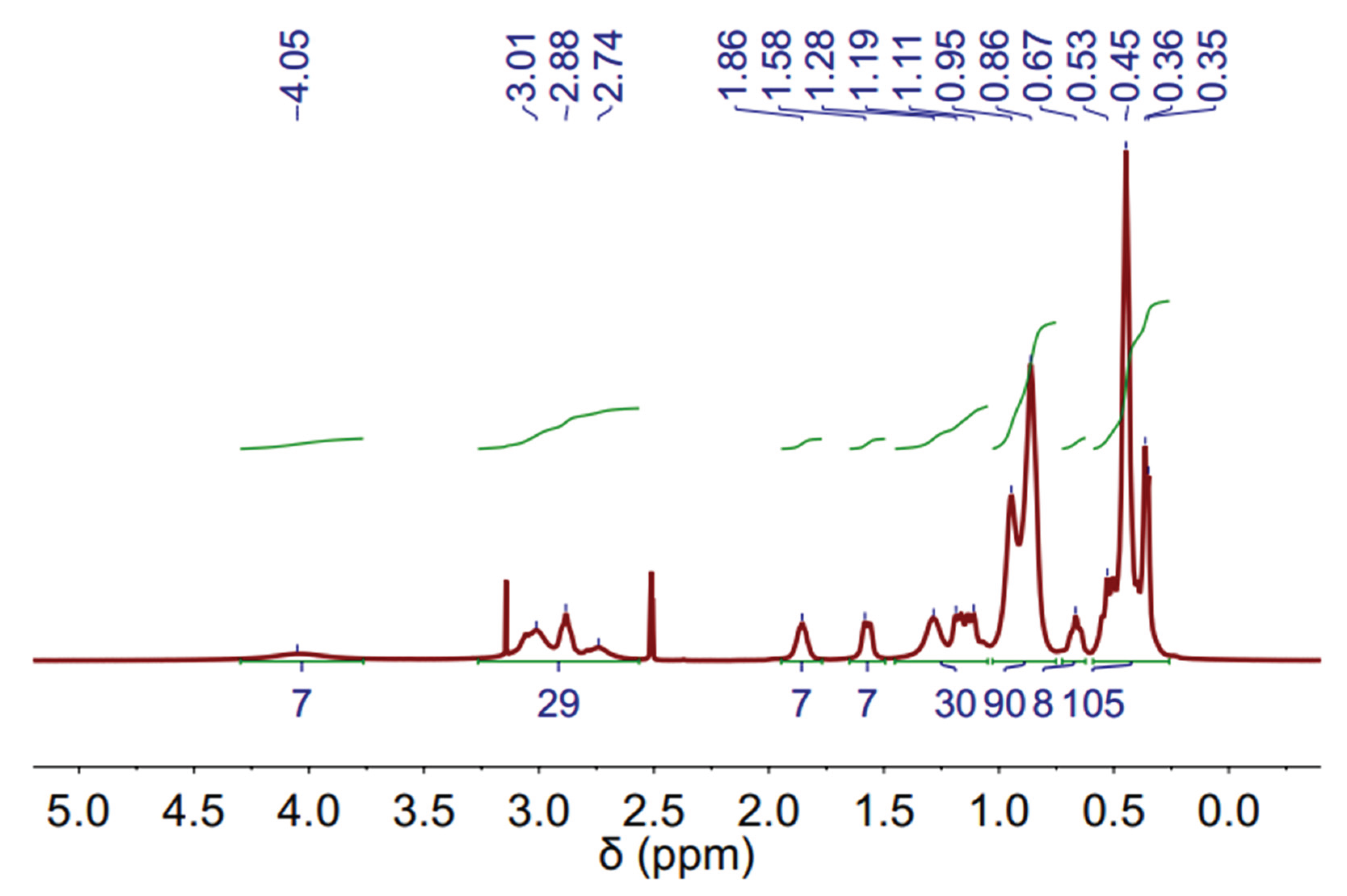
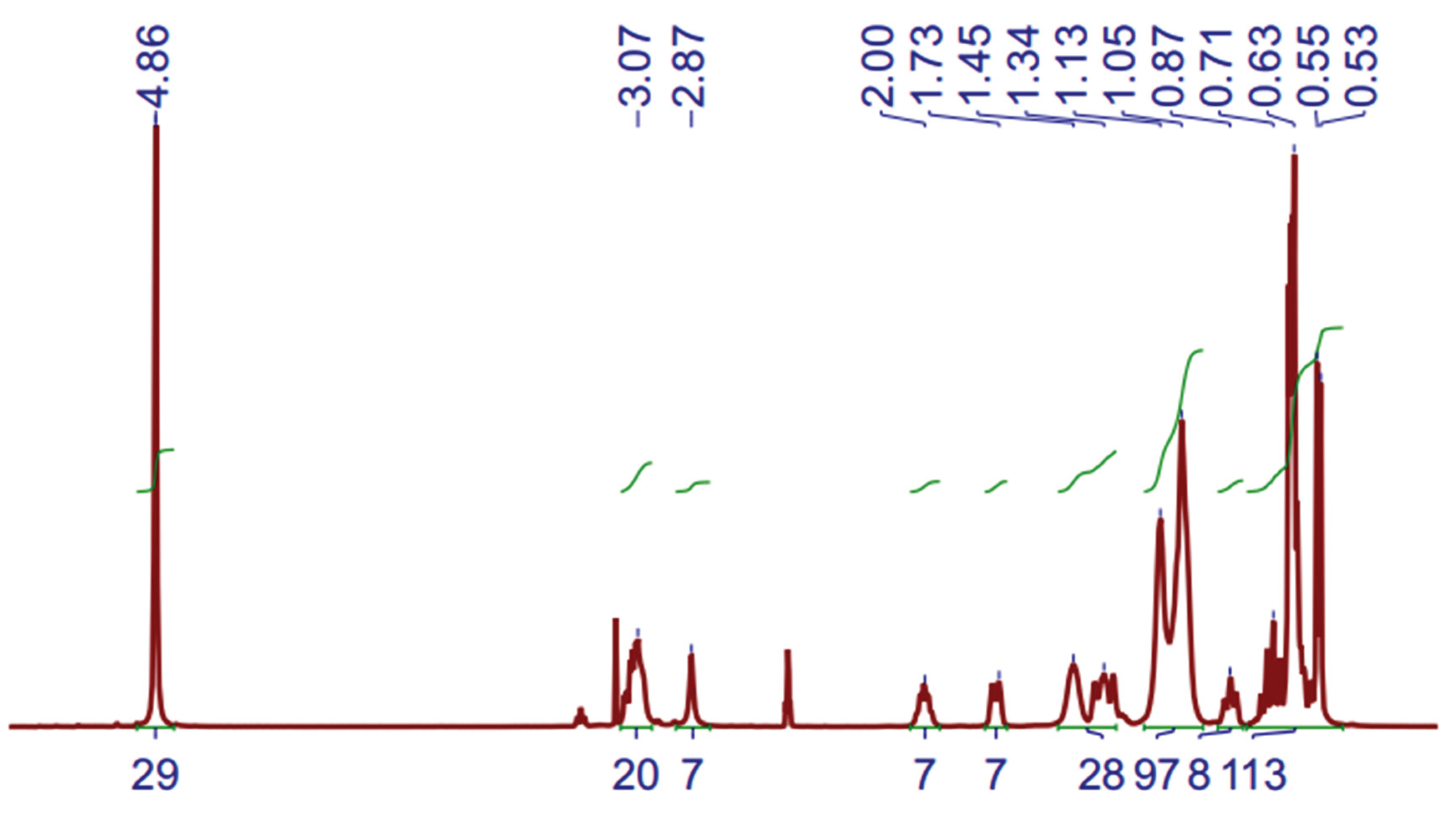
The
1H-NMR (Proton Nuclear Magnetic Resonance) spectra of DES (3 Aliquat:7 L-Menthol) is depicted in
Figure 4. The measurements were performed at 60 ºC to decrease the sample viscosity and improve the spectra resolution. Not all peaks can be clearly assigned, however, some peaks related to Aliquat-336 and L-Menthol can be observed.
Figure 3.
1H-NMR spectrum of DES (3 Aliquat 336:7 L-Menthol) (500 MHz, DMSO-d6) at 60 ºC.
Figure 3.
1H-NMR spectrum of DES (3 Aliquat 336:7 L-Menthol) (500 MHz, DMSO-d6) at 60 ºC.
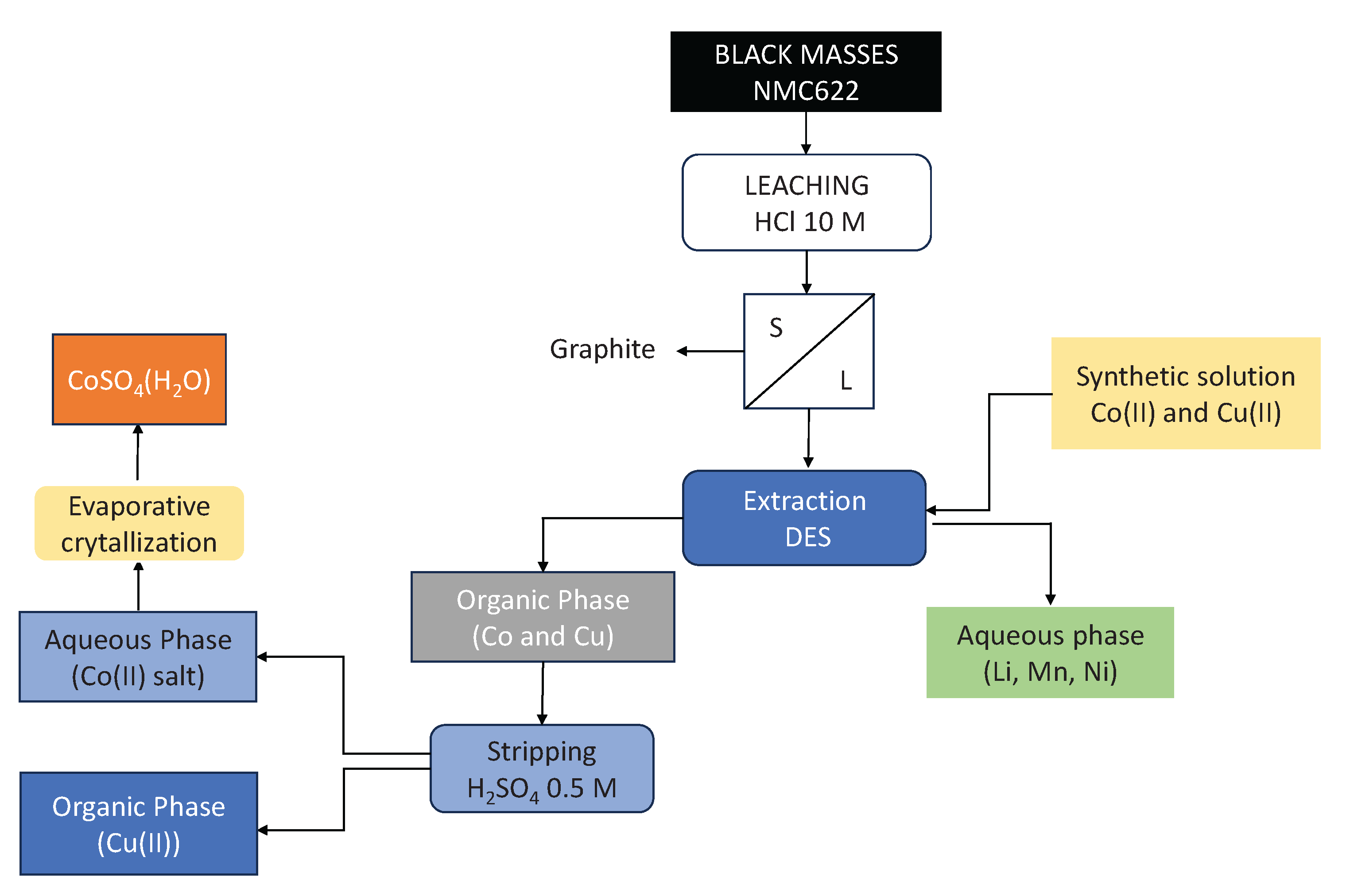
Figure 4.
Scheme of the studied process.
Figure 4.
Scheme of the studied process.
2.2. Extraction Experiments with Synthetic Co(II) Solutions
Figure 4 shows a schem of the studied process, starting from both synthetic solutions and black masses.
2.2.1. Equilibrium Time
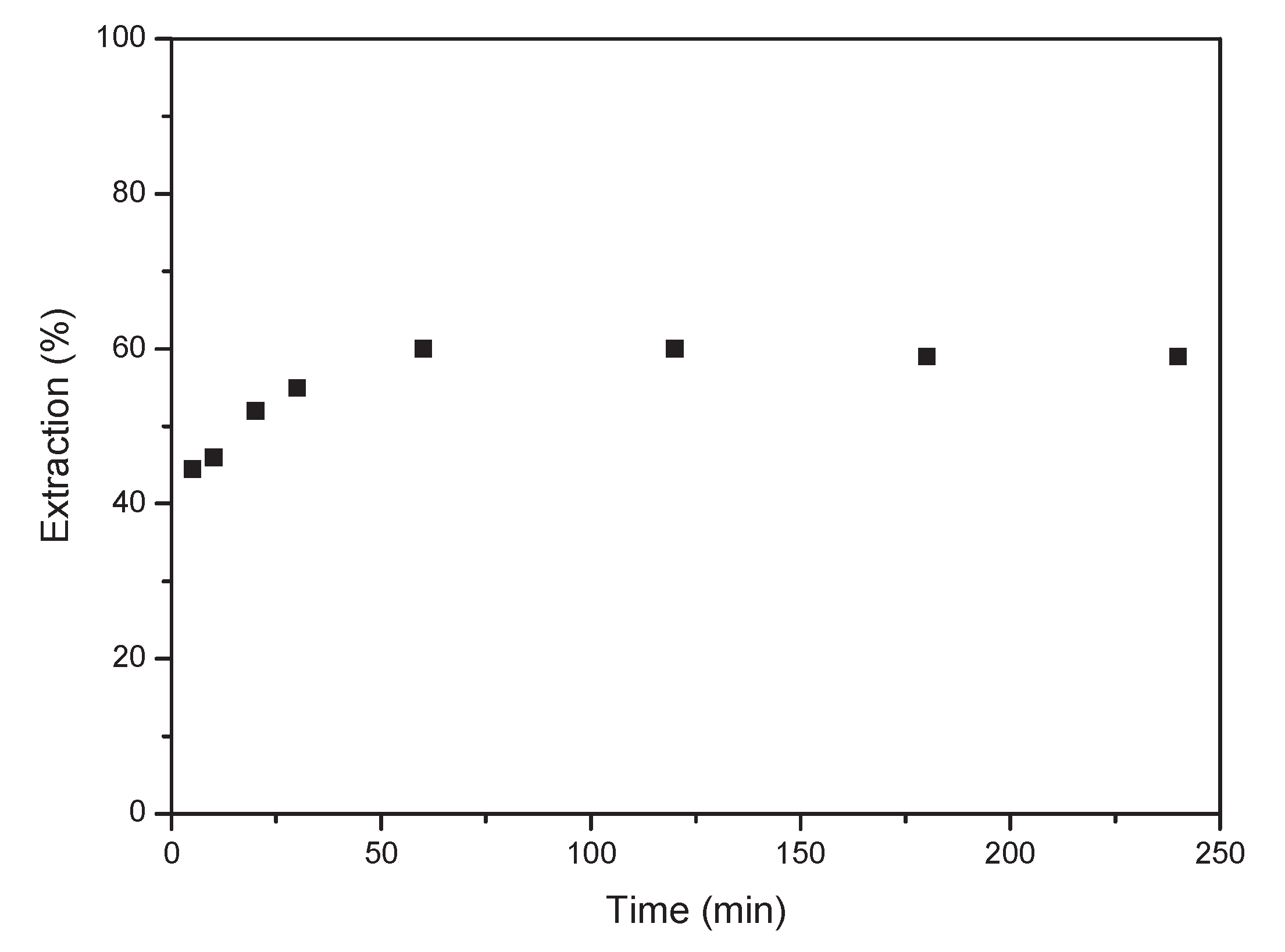
Initially, the equilibrium time for Co extraction with the deep eutectic solvent was investigated. A series of experiments were conducted in which an aqueous solution containing 1 g/L of Co(II) at a concentration of 6 M HCl was brought into contact with DES.. The ratio between the aqueous phase and THE ORGANIC phase was 1. As shown in
Figure 5, equilibrium was reached at 60 minutes of reaction, with the extraction percentage remaining constant thereafter. The extraction percentages were around 60%, remaining consistent at longer time.
Cobalt distribution coefficients have been studied using equation (2) at different reaction times (
Table 1). The distribution coefficient values range between 1 and 1.44, indicating that cobalt is distributed, in general, similarly between the aqueous and organic (DES) phases. The distribution coefficient values range from 1 to 1.44, indicating that cobalt is generally distributed similarly between the aqueous and organic phases (DES), with no significant difference when the reaction time varies.
2.2.2. Influence of Co(II) Concentration
The effect of the initial Co(II) concentration in the solution was studied for an aqueous phase where the initial (II) concentration ranged from 1 g/L to 6 g/L. The rest of the conditions were kept constant. The results show that the Co(II) extraction, calculated according to equation (1), is independent of its initial concentration, with extraction percentage values ranging between 55% and 59% for all concentrations studies (
Table 2). These findings indicate that an increase in cobalt concentration in the solution does not influence the extraction percentage, indicating the absence of specimen contained metal-polynuclear species in its composition (Alguacil et al., 2019), (Sastre et al., 2004).
2.2.3. Effect of HCl Concentration in the Aqueous Phase Solution
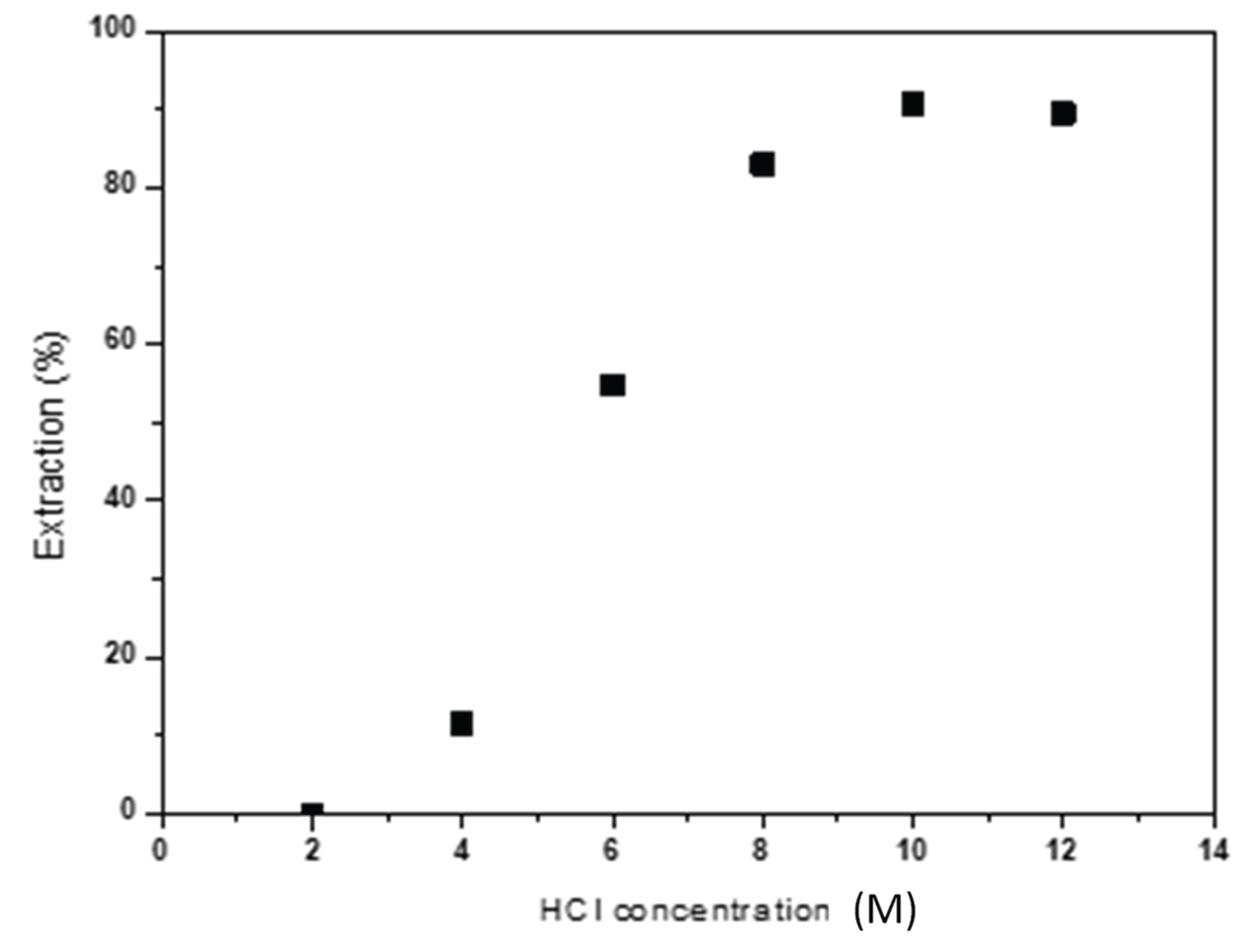
The influence of HCl concentration on Co(II) extraction has been also studied. Extraction tests were conducted varying the acid concentration between 2 and 12M. The obtained results are collected in
Figure 6. It is observed that an increase in HCl concentration enhances Co(II) extraction, reaching an extraction percentage of 90% for HCl concentrations of 10 M.
Table 3 shows cobalt distribution coefficients (D
Co). The increase in HCl concentration raises the cobalt distribution coefficient between the organic and aqueous phases, reaching the highest values for an HCl concentration of 10 M.
Therefore, the extraction of Co(II) is favored in strongly acidic media with high concentrations of Cl- ions, which promote the formation of cobalt tetrachloride (CoCl42-) (Rybka and Regel-Rosocka, 2012).
2.2.4. Influence of Cl- Concentration (NaCl Addition)
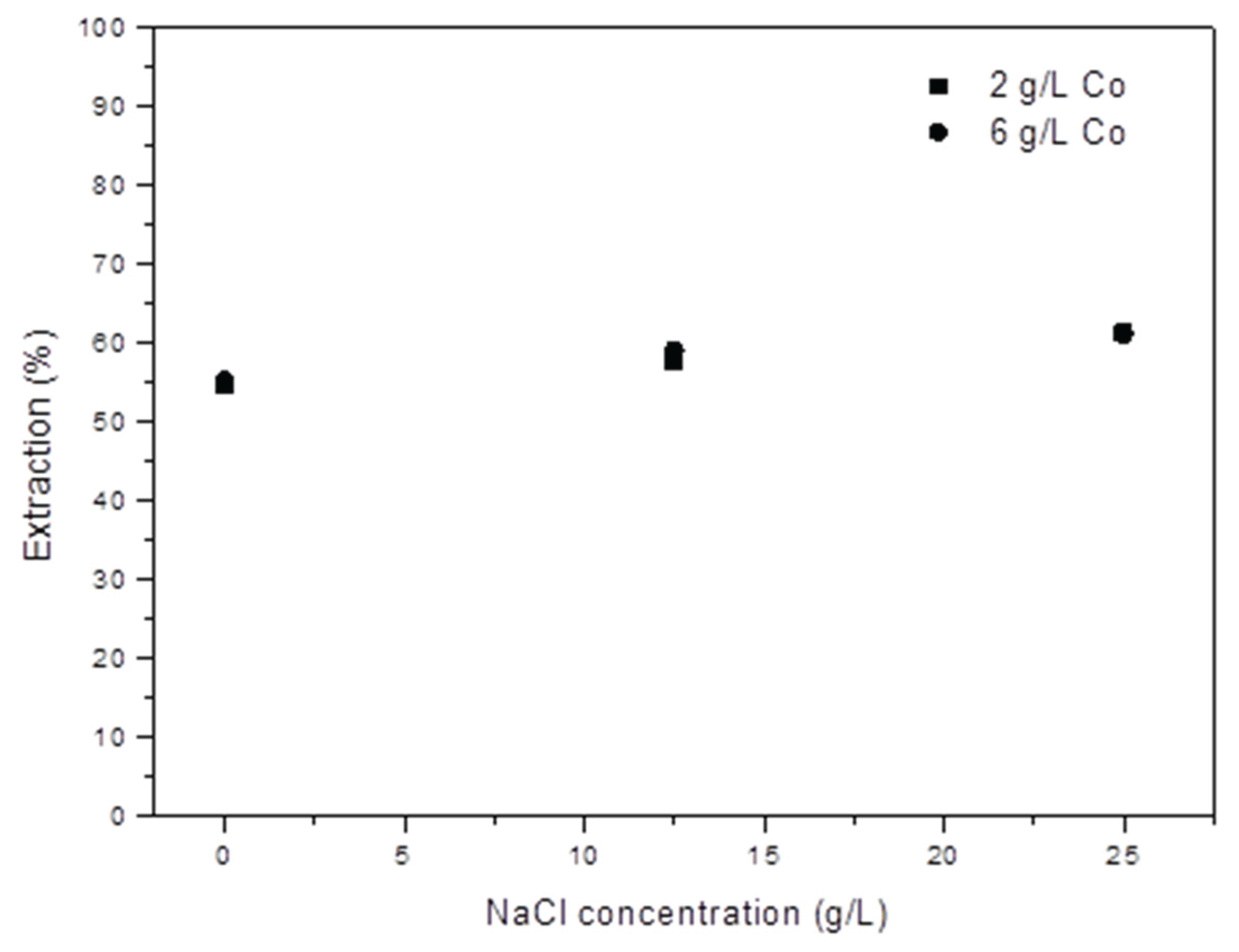
The influence of chloride ions on extraction has been studied. For this purpose, various concentrations of NaCl were added into the solution, and extraction tests were conducted using two initial Co(II) concentrations (2 and 6 g/L). The NaCl concentration was varied between 12.5 and 25 g/L. The obtained results demonstrate that the addition of NaCl hardly affects the Co extraction percentage, as evident in
Figure 7.
Table 4 presents the Co extraction percentages and distribution coefficients obtained for each test. These coefficients remain unchanged, both with the initial cobalt concentration in the aqueous solution and with the added NaCl concentration in the solution.
2.2.5. Two-Stage Co(II) Extraction
To enhance Co extraction yields, a two-stage extraction was carried out. In the second stage, the aqueous phase was retained and mixed with a fresh organic phase.
Table 5 presents the results for each individual stage, as well as the total extraction percentage. The study was conducted for two initial Co concentrations, 2 and 6 g/L. Both concentrations exhibited a similar behavior: in the first extraction stage, around 60% of the cobalt in solution was extracted, and in the second stage, the extraction percentages slightly decreased to 50%.
As observed in
Table 5, after two stages, the cobalt extraction percentages are approximately 80% and independent of the initial Co(II) concentration in the solution. These percentages are slightly lower than those obtained with higher HCl concentrations (see section 3.2.3).
2.2.6. Influence of Phase Relationship
Various phase ratios have been studied and their influence on the extraction process investigated. The results obtained are shown in
Table 6. It is observed that an increase in the aqueous-organic ratio from 0.33 to 2 reduces the recovery percentages and the distribution coefficient values, decreasing from 73% to 52%, respectively.
3.2.7. Nuclear Magnetic Resonance Spectroscopy of DES After Extraction Process
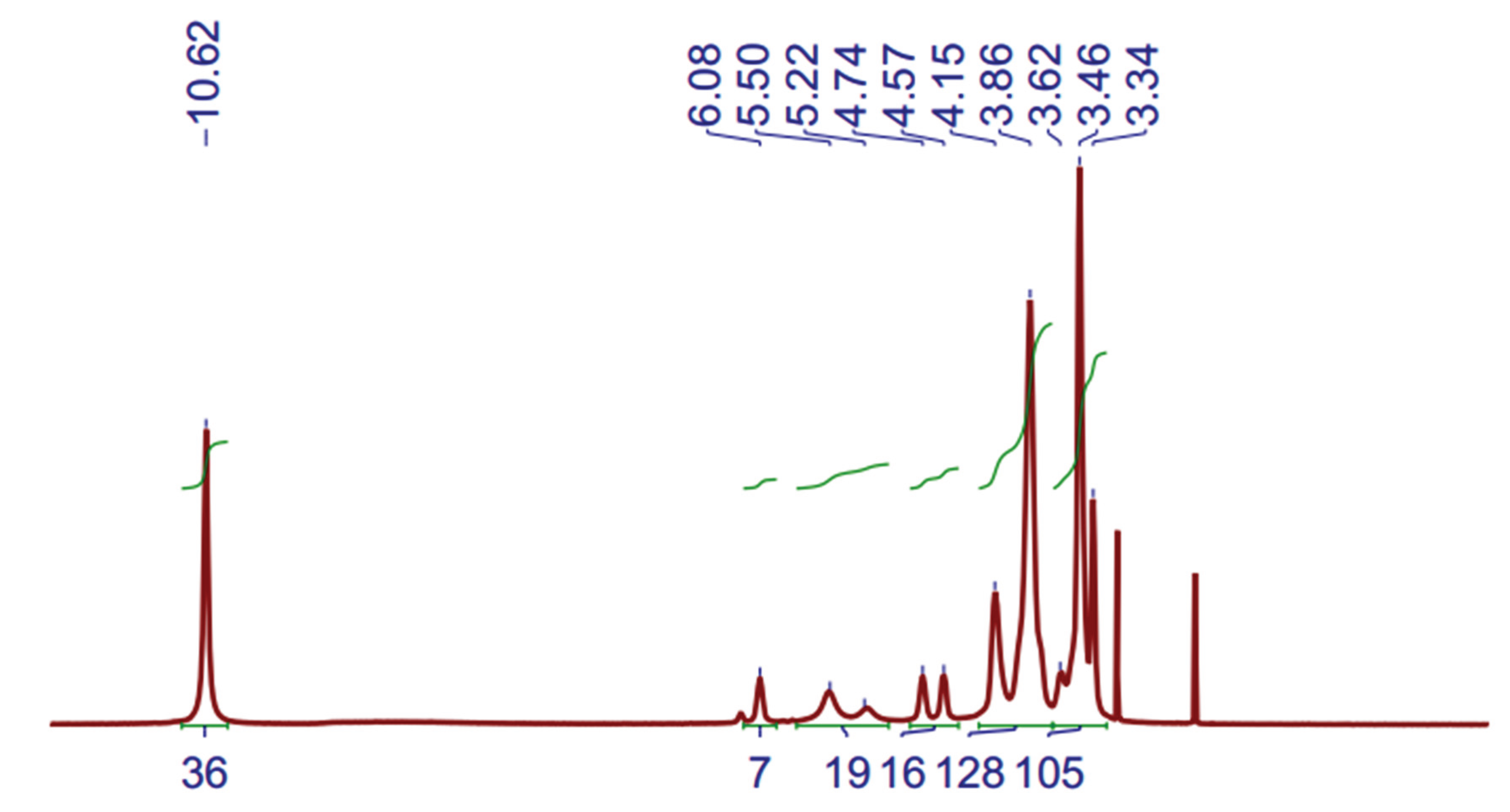
1H-NMR was measured in the organic phases (DES-phase) after cobalt extraction process, and results are shown in
Figure 6.
Figure 8.
1H-NMR spectra of DES-Co after extraction process, (500 MHz, DMSO-d6) at 60 ºC.
Figure 8.
1H-NMR spectra of DES-Co after extraction process, (500 MHz, DMSO-d6) at 60 ºC.
The presence of Co(II) causes the signals to shift toward lower fields, compared to DES displacements without Co(II) (
Figure 4).
2.3. Stripping Experiments
The stripping experiments were conducted by loading the organic phase with a solution of 2 g/L of Co(II) and stripping it with various acids (HCl and H2SO4) at different concentrations. Concentrations of 0.01 and 0.1 M of HCl, as well as 0.01, 0.5, 1, 3, and 5 M of H2SO4, were used. The experiments were carried out during 60 minutes at 25°C. The organic phase-to-re-extraction phase ratio was set at 1.
Table 7 shows the obtained results using HCl. For both concentrations, it is possible to recover 100% of the cobalt existing in the organic phase, indicating that the HCl concentration does not influence the stripping of Co(II).
In the case of H
2SO
4, the obtained results demonstrate that increasing acid concentration decreases the recovery percentages. It is 100% for a concentration 0.01M H
2SO
4 and 58% when the H
2SO
4 concentration is increased to 5 M (
Table 8).
2.3.1. Nuclear Magnetic Resonance Spectroscopy of DES After Stripping Process
As can be seen in
Figure 9,
1H-NMR measurements have also been carried out on the organic phases (DES-phase) from cobalt stripping process.
The presence of Co(II) also causes a slight displacement of the signals towards lower fields, if compared to the displacements of the DES without Co(II) (
Figure 4), but since the Co(II) content is lower than in the sample of
Figure 9, the deshielding is lower.
In order to obtain Co(II) salts, the stripping aqueous phases obtained with 0.01 M and 0.5 M H2SO4 (where the Co(II) stripping percentages are 100% and 84% respectively) were evaporated and dried at 80°C for 24 hours. The obtained solids were characterized using X-ray diffraction (XRD).
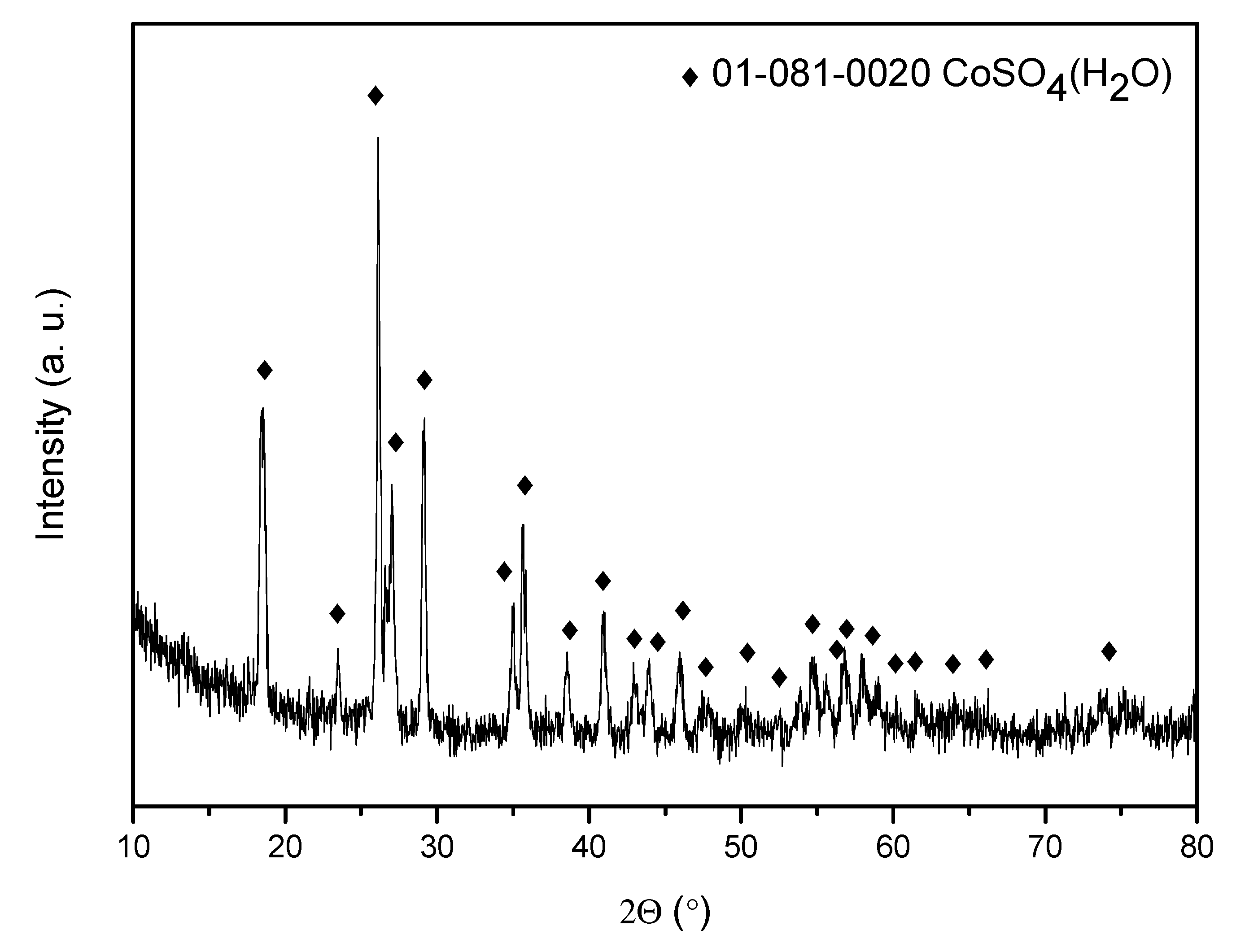
Figure 10a shows the diffractogram of the salt obtained using a stripping phase with a concentration of 0.01M H
2SO
4. It can be observed that the major component corresponds to cobalt chloride hexahydrate, with minor presence of cobalt sulfate monohydrate. However, when using a stripping aqueous phase with a concentration of 0.5 M H
2SO
4, the obtained salt corresponds to cobalt sulfate monohydrate (
Figure 10b).
2.4. Regeneration of the DES
An important aspect in liquid-liquid extraction is studying the regeneration of the organic phase. For this purpose, two complete cycles of extraction and stripping were carried out maintaining the same organic phase.
Table 9 displays the extraction stripping percentages as well as the cobalt distribution coefficients using initial Co(II) concentrations of 2 and 6 g/L. It is observed that after the first cycle of extraction and stripping, the DES loses efficiency, decreasing the extraction percentage by approximately half, regardless of the cobalt concentration used in the test.
2.5. Optimal Conditions for Cobalt Recovery
The obtained results indicate that the optimal conditions for Co(II) extraction are achieved in a single extraction and stripping stage with an initial concentration 6 g/L Co(II), 10 M HCl, aqueous-to-organic ratio of 1/1, temperature 25°C, and equilibrium time 60 min. Stripping using 0.5 M H
2SO
4 at temperature of 25°C with an equilibrium time of 60 min provides the best results.
Table 10 displays the extraction and stripping percentages as well as the distribution coefficients under the optimal conditions. Recovery percentages of Co(II) in the stripping stage reach 91%, and in the re-extraction stage, reach 84%.
2.5. Optimal Conditions for Cobalt Recovery
The obtained results indicate that the optimal conditions for Co(II) extraction are achieved in a single extraction and stripping stage with an initial concentration 6 g/L Co(II), 10 M HCl, aqueous-to-organic ratio of 1/1, temperature 25°C, and equilibrium time 60 min. Stripping using 0.5 M H
2SO
4 at temperature of 25°C with an equilibrium time of 60 min provides the best results.
Table 10 displays the extraction and stripping percentages as well as the distribution coefficients under the optimal conditions. Recovery percentages of Co(II) in the extraction stage reach 91%, and in the stripping stage, they reach 84%.
2.6. Extraction of Co(II) in Black Masses of NMC 622 Batteries
To investigate the selectivity of the DES against other metals present in leaching solutions of black masses from NMC 622-type lithium-ion batteries, extraction experiments were conducted under the optimal conditions described in section 2.5.
Table 11 displays the concentrations of different metals in the solutions obtained after leaching the black masses.
Table 12 and
Table 13 show the extraction and stripping percentages for each analyzed element for studied black masses.
Table 12 shows that the extraction is selective for cobalt and copper, with extraction percentages > 80% for Co(II) and > 83% for Cu(II) in all the studied samples. Ni and Li are not extracted in any of the samples.
In the stripping process (
Table 13) using 0.5 M H
2SO
4, a higher selectivity for Co(II) over Cu(II) is observed, with stripping percentages > 76% for Co(II) and < 57% for Cu(II).
The evaporation of the stripping aqueous phases leads to the formation of cobalt sulfate monohydrate majority salt as revealed by its X-ray diffraction diagram (
Figure 11). The figure shows the XRD diagram for sample BM6 as an example. For the other samples, the diagram is identical to that for sample BM6.
Based on the results, the process conditions should be optimized to improve selective recovery of Co(II) versus Cu(II).
2.7. Optimization of the Extraction Conditions of the Co/Cu Separation Process
2.7.1. Separation of Cu(II) from Synthetic Solutions and Black Masses Leaching Solutions Containing Co(II), Mn(II), Ni(II) and Li(I).
The
Figure 12 depicts a scheme of the studied process.
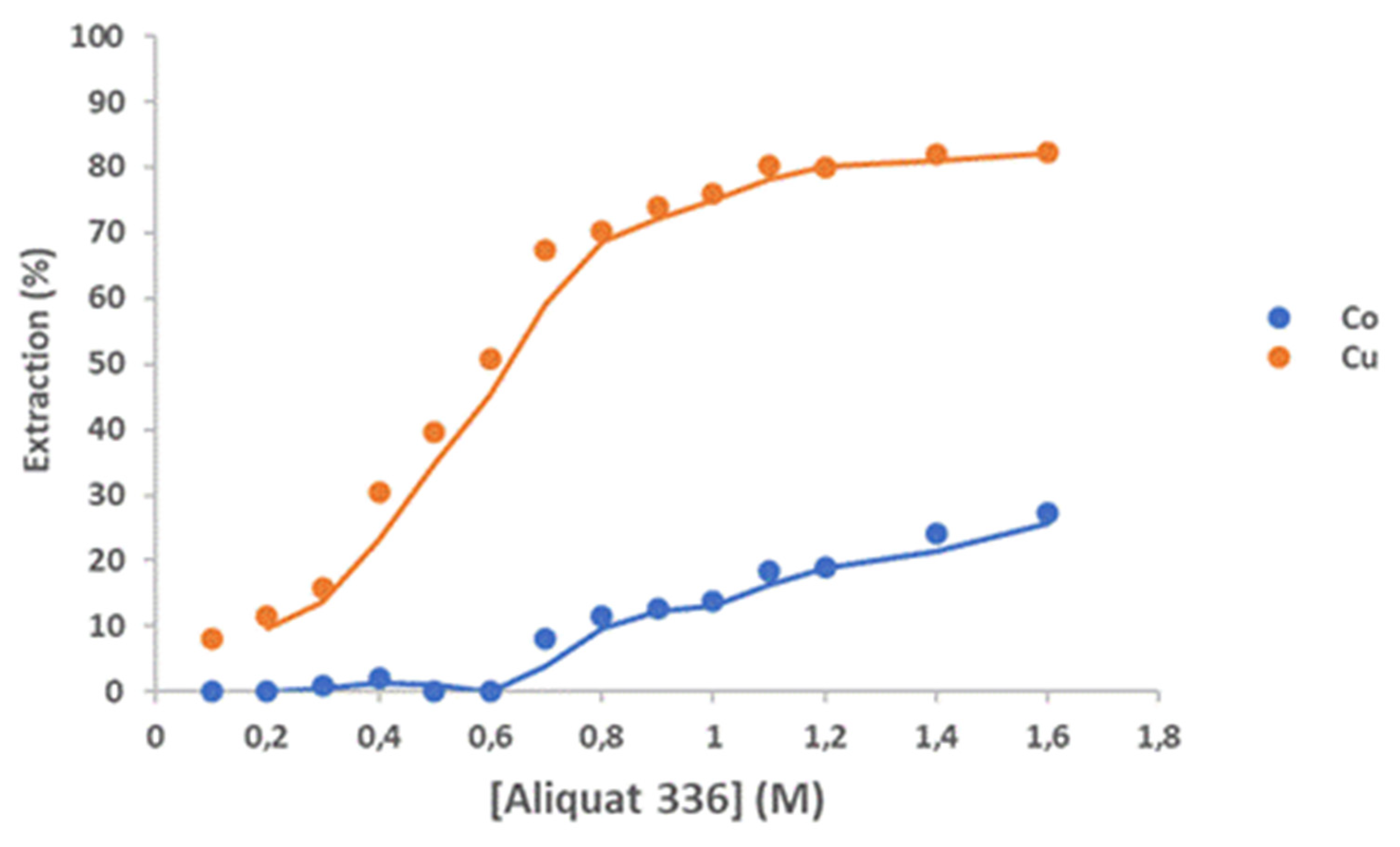
In HCl solution, metal ions form complexes with chloride ions. To utilize the difference in complex formation tendency among the metals ions, Aliquat 336 with 10% v/v decanol as a modifier was employed for selective extraction of Cu(II) from solution.
Figure 13 shows that the extraction percentage of Cu(II) increased from 8.2 to 82.3% as Aliquat 336 concentration increased from 0.1 to 1.6 M. When Aliquat 336 concentration was 0.6 M, Co(II) began to be co-extracted with Cu(II) (50.9%) and 27.3% of Co(II) was extracted by 1.6 M Aliquat 336. On the other hand, when Aliquat 336 concentration was 0.7 M, 8% of Co(II) was extracted with 67.5% of Cu(II). The extraction of Cu(II) from 3 M HCl solution by Aliquat 336 can be represented as (Nguyen and Lee, 2020):
where R = C8 aliphatic and R’= methyl.
2.7.1.1. Equilibrium Time
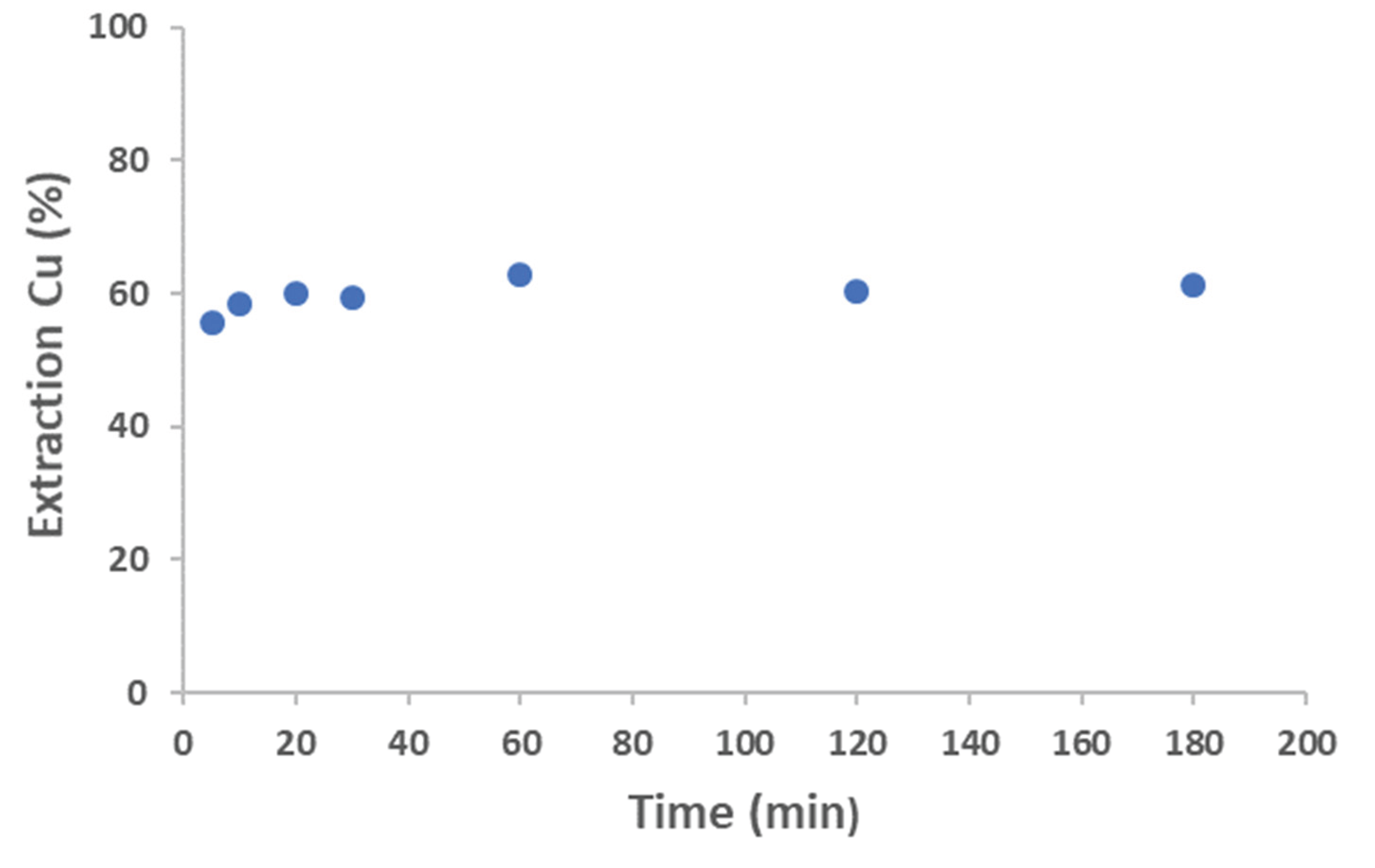
Initially, the equilibrium time for Cu extraction with Aliquat 336 was investigated. A series of experiments were conducted in which an aqueous solution containing 5 g/L of Co(II) and 6 g/L of Cu(II) in 3 M HCl was brought into contact. The ratio between the aqueous phase and the organic phase was 1. As shown in
Figure 14, equilibrium was reached at 60 minutes of reaction, with the extraction percentage remaining constant thereafter.
2.7.2.2. Extraction Stages
Table 14 shows that 4 stages of extraction are required for complete extraction of Cu(II) from the aqueous solution containing 5 g/L of Co(II) and 6 g/L of Cu(II), obtaining 97.5% Cu(II) extraction with the 4 stages using 0.7 M Aliquat 336 and 95.1 with 0.7 M Aliquat 336. Using 0.6 M of Aliquat the Co(II) extraction is zero.
2.7.2.3. Copper Stripping
The stripping experiments were conducted by loading the organic phase with a aqueous solution of 5 g/L of Co(II) y 6 g/L of Cu(II) in the same conditions than the previous essays (organic phase: Aliquat 336 0.7 M in kerosene with 10% decanol) and stripping it with H2SO4 1 M. The reactions were carried out during 60 minutes at a temperature of 25°C. The organic phase-to-stripping phase ratio was set at 1. Experimental results indicated that Cu(II) was completely stripped by 1 M H2SO4 solution.
The copper extraction process studied has been applied to the recycling of Li-ion batteries NMC 622, using aqueous solutions from the leachate of two black masses of these batteries in 3 M HCl.
Table 15 displays the concentrations of the studied metals in the solutions obtained after leaching the two black masses.
Table 16 and
Table 17 show the extraction percentages for two leached black masses using Aliquat 336 0.6 M or 0.7 M in kerosene.
Table 16 shows that 4 stages of extraction are required for complete extraction of Cu(II) from the aqueous solution from black mass BM5 obtaining 99.5% Cu(II) extraction using 0.6 M Aliquat 336.
Table 17 shows that 4 stages of extraction are sufficient to extract Cu(II) using 0.7 M Aliquat 336 as extraction agent, obtaining 99.8% Cu(II) extraction.
In the case of sample TUC2, 4 stages of extraction are necessary to extract copper, reaching an extraction of 100% of copper with Aliquat 336 of 0.7 M concentration as an extraction agent. This percentage is the same when the extraction agent is used at a concentration of 0.6 M (
Table 16).
2.7.2.4. Cu(II) Extraction in 3 M HCl Medium
The Cu(II) in solution was extracted in 3 M HCl medium with an organic phase Aliquat 336 0.6 M in kerosene with 10% decanol. The aqueous-to-organic ratio was 1/1, the temperature 25ºC and equilibrium time 60 min.
Table 18 knows that 2 stages of extraction are required for complete extraction of Cu(II) from the aqueous solution from black mass BM5 (in medium 3 M HCL) obtaining 92% Cu(II) extraction using 0.6 M Aliquat 336 as extracted agent.
In the case of the TUC2 sample with two extraction stages, 94% of the copper existing in the aqueous leaching phase is removed. It can be seen in the table that a minority percentage of Co(II) is also extracted.
It was chosen 2 extraction stages to extract the copper in solution
instead of 4 phases carried out previously (
Table 18) to avoid the extraction of Co, and still maintaining a good percentage of copper removal.
The stripping experiments of Cu(II) contained in the organic phases were conducted with H2SO4 1 M. The reactions were carried out during 60 minutes at 25°C. The organic phase-to-tripping phase ratio was set at 1.
Table 19 shows that Cu(II) can be stripped by 1 M H
2SO
4 solution in the two studied black mass samples.
2.7.4.5. Recovery of Co(II) Using DES 3 Aliquat 336: 7 L-Menthol
Once the Cu(II) in solution has been removed, the extraction of the Co(II) contained in solution is carried out. This metal was recovered in 10 M HCl medium (for enhance the formation of anionic complexes of Co(II)) with the DES 3 Aliquat 336: 7 L-Menthol. To carry out the test in 10 M HCl medium, the samples (in 3 M HCl medium) are concentrated in HClcc in a ratio of 1/5: sample/HClcc. The aqueous-to-organic ratio was 1/1, the temperature 25ºC and equilibrium time 60 min.
Table 20 shows that the extraction percentages is 93% for Co(II) in the two the
studied samples being the concentration of copper in the aqueous extraction phases practically negligible (0.003 and 0.5*10-3 g/L) due to its elimination in the previous phase of the process.
Table 21 shows that experimental results indicated that Co(II) can be stripped by 0.5 M H
2SO
4 solution in the two studied black mass samples, with stripping percentages > 82% and > 71% respectively.
3. Materials and Methods
3.1. Chemical Reagents
The aqueous solutions were prepared with the following reagents: cobalt(II) sulfate heptahydrate (CoSO4·7H2O) and copper(II) sulfate pentahydrate (CuSO4·5H2O), supplied by Sigma Aldrich and hydrochloric acid 37% (HCl) supplied by Panreac AppliChem. DES was prepared using Aliquat 336 ([CH3(CH2)7]3NCH3Cl) and L-menthol 99% (C10H20O) supplied by Thermo Fisher Scientific.
3.2. Synthesis and Characterization of DES
The synthesis of DES (3 Aliquat:7 L-Menthol) was carried out by mixing the two respective components (Aliquat 336:L-menthol, molar ratio 3:7) under continuous stirring (at 300 rpm) during 30 minutes at 60 ºC. The components were placed in an Erlenmeyer flask and immersed in a silicone oil bath with immersion thermostat (model DIGITERM TFT-200, J.P. SELECTA, Scharlab S.L.). The reaction was maintained until a clear and homogenous liquid was obtained (Martín et al., 2024). The formation of DES was studied by Fourier-transform infrared spectroscopy (FTIR) (Prabhune & Dey, 2023).
The infrared spectra of the DES synthesized as well as the inicial reagents, were obtained using a Nicolet iS50 FT-IR Spectrometer of Thermo Scientific™, operated in Attenuated Total Reflection (ATR) mode, in the range from 4000 to 400 cm-1, with spectral resolution of 4 cm-1 and accumulation of 64 scans.
The thermal analysis study of the DES synthesized (differential scanning calorimetry (DSC) and thermogravimetry (TGA)) was carried out in a differential scanning calorimeter model DSC 25 and a thermogravimetric analyzer model of TGA 550 of TA instrument.
1H-NMR spectra of DES synthesized and the DES-Co were conducted using a Bruker Avance DRX500 spectrometer operating at 500 MHz. The NMR parameters employed included a 30° pulse, an acquisition time of 3.1719 seconds, a relaxation delay of 1 second, and a total of 16-32 scans. All samples were placed in capillary tubes inside a 5 mm NMR glass tubes and analysed using deuterated chloroform (CDCl3) or dimethyl sulfoxide (DMSO-d6) as an external reference. The spectra were acquired after setting the temperature to 25 or 60 ºC using a Bruker Variable Temperature BVT 3000.
Measurements of the density of the DES were made by difference in weight in an Ohaus Explorer Analytical 110G Balance, for which 6 measurements were carried out in which the standard deviation of the samples were calculated. The density obtained was 0.886±0.003 g/mL.
The viscosity of the DESs were determined using a viscometer PCE-RVI2. The analysis was performed at 20 ºC. Temperature control was carried out in a water bath. 4 measurements were carried out in which the standard deviation and error of the samples were calculated. The medium viscosity obtained was 608.90±0.03 cp.
3.3. Preparation of Solutions from Black Masses
Five black masses, provided by a Spanish company, have been employed to study the selective separation of Co(II). BM6, BM8 and BM9 are black masses originates from the crushing, magnetic separation and eddy current separation of NMC 662-type battery cells. Samples BM5 and BM1 come from carbo-reduction pyrometallurgical processes for pre-recovery of Li (Alcaraz et al., 2024). The samples were leached using a 10 M HCl solution (black mass/hydrochloric acid ratio S/L = 100 g/L; temperature = 70 ºC, re-action time = 2 h). The resulting suspensions were filtered and the solutions were treated with the DES (3 Aliquat 326:7 L-Menthol) utilized for this study. The preliminary extraction of Cu(II) contained in the black masses (samples BM5 and TUC2), leached using a 3 M HCl solution, was carried out with Aliquat 336 in kerosene.
Supplementary Information Tabla S1.
3.4. Extraction Experiments
The extractions of the metals (Co/Cu) were carried out with aqueous solutions of fixed concentration for each test in a shaking funnel with the corresponding amount of DES (3 Aliquat 326:7 L-Menthol) (no solvent was used in the test) or Aliquat 336 in kerosene (with 10% decanoic acid). Solvent extractions were carried out by stirring the mixture for different times (equilibrium time = 60 minutes). The temperature was maintained constant at 25°C during all experiments. The metal concentration in the aqueous solution was analyzed by aqueous adsorption spectrophotometry in an atomic absorption spectrophotometer (AAS) model contraAA 800 of Analytikjena.
The metal concentration in the organic phase was estimated according to the mass balance. In the case of the stripping stage, the aqueous phase is analyzed by AAS and in the same way by mass balance the concentration of metal remaining in the organic phase is known.
The metal (M) extraction percentage is obtained by Equation (1):
Where [M]org corresponds to the metal concentration in the organic or DES phase after the equilibration time and [M]Aq0 corresponds to the cobalt concentration in the initial aqueous phase. VOrg and VAq0 are respectively the volume in the organic phase or DES phase after the equilibration time and in the initial aqueous phase.
The cobalt distribution coefficient is defined as Equation (2):
Where [M]org corresponds to the M concentration in the organic phase or DES and [Co]aq indicates the cobalt content in the aqueous phase, both after the equilibrium time. VOrg and VAq0 are respectively the volume in the organic phase or DES phase after the equilibration time and in the aqueous phase.
The stripping percentages were calculated according to the following Equation:
, corresponds to the concentration of metal in the aqueous phase of stripping and in the organic phase, respectively. VAqrex and VOrg are respectively the volume in the stripping aqueous phase and in the organic phase.
3.5. Synthesis of Cobalt(II) Salts
The stripping solutions were evaporated in a BUCHI R-100 Rotavapor equipped with a V-100 Vacuum Pump and a B-100 Heating Bath. The solids obtained were dried in an oven (80°C, 24 h) and their mineralogical characterization was performed by X-ray diffraction using an X’Pert Pro MPD diffractometer (PANalytical) equipped with a Cu anode (Cu K radiation) with a step size of 0.03° (2Ɵ) in the range 10-80.
4. Conclusions
The DES used in this work (3 Aliquat 336:7 L-Menthol) achieved a cobalt extraction of 91% (experimental conditions: 6 g/L Co, 10 M HCl, teq = 1 h, Aqueous phase / Organic phase = 1, T: 25ºC). The extraction of cobalt contained in the black masses studied was 88% (sample BM8), after a leaching process in a hydrochloric medium and liquid-liquid extraction under the most favorable conditions tested, managing to re-extract 85% (sample BM6-1) of the cobalt contained in the organic phase or DES. Cobalt is recovered as CoSO4(H2O) using a 0.5 M H2SO4 stripping solution. DES 3 Aliquat 336:7 L-Menthol is selective in extracting Co contained in black masses of NMC batteries compared to other elements present in these samples (Li, Ni and Mn). Based on the results obtained, the process has been optimized to achieve greater selectivity in the recovery of Co(II) over Cu(II), achieving the prior separation of Cu(II) in 3 M HCl medium (94% extraction in sample TUC2) using Aliquat 336 in kerosene. Once copper has been removed from the sample, cobalt is extracted under the most favorable conditions studied, obtaining a Co(II) extraction of 93% with stripping > 82% (sample BM5). Liquid-liquid extraction using the studied DES (3 Aliquat 336:7 L-Menthol) as an extraction agent is a very promising method for the Co recovery from lithium batteries NMC 622, a metal currently in high demand for the energy transition. It offers advantages over liquid-liquid extraction with organic solvents and extraction agents due to its lower environmental impact and high percentage of metal recovery.
It is observed that some residual manganese is extracted during the extraction process in some of the samples. Optimizing this process is an ongoing research project that will be the subject of new work in the near future.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Assignment of the H of Aliquat 336 and of L-Menthol.
Author Contributions
F.A.L. and M.I.M.-H.; methodology, M.I.M.-H. and F.A.L.; validation, M.I.M.-H.; formal analysis, F.A.L. and M.I.M-H.; investigation, M.I.M-H., M.L.R., G.B.-C., O.R-L. and L.A.; data curation, M.I.M-H..; writing—original draft preparation, M.I.M.-H.; writing-review and editing, F.A.L., M.I.M-H. G.B.-C., L.A. and O.R.-L.; supervision, F.A.L.; funding acquisition, F.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by CDTI-Misiones, BATERURGIA Project, grant number MIG-2022-1014, European Union, Next Generation, RELOAD Project, grant number VEC-010000-2022-52 and European Union Horizon Europe programme, FREE4LIB project, HORIZON CL5-2021-D2-01-06, grant number 1069890.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abbott, A. P., Boothby, D., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Journal of the American Chemical Society, 126(29), 9142–9147. [CrossRef]
- Alcaraz, L., Rodríguez-Largo, O., Barquero-Carmona, G., & López, F. A. (2024). Recovery of lithium from spent NMC batteries through water leaching, carbothermic reduction, and evaporative crystallization process. Journal of Power Sources, 619, 235215. [CrossRef]
- Biniaz, P., Gol, R., Askari, S., Hora, Y., Chakraborty Banerjee, P., & Bhattacharya, S. (2025). Selective recovery of critical metals from spent lithium-ion batteries using maleic acid-based deep eutectic solvent. Resources, Conservation and Recycling, 217, 108177. [CrossRef]
- Chabhadiya, K., Srivastava, R. R., & Pathak, P. (2021). Two-step leaching process and kinetics for an eco-friendly recycling of critical metals from spent Li-ion batteries. Journal of Environmental Chemical Engineering, 9(3), 105232. [CrossRef]
- Dar, A. A., Chen, Z., Zhang, G., Hu, J., Zaghib, K., Deng, S., Wang, X., Haghighat, F., Mulligan, C. N., An, C., Ramirez, A. A., & Sun, S. (2025). Sustainable Extraction of Critical Minerals from Waste Batteries: A Green Solvent Approach in Resource Recovery. Batteries, 11(2), 51. [CrossRef]
- Dias, R. M., da Costa, M. C., & Jimenez, Y. P. (2022). Perspectives of Using DES-Based Systems for Solid–Liquid and Liquid–Liquid Extraction of Metals from E-Waste. Minerals, 12(6), 710. [CrossRef]
- Ilyas, S., Srivastava, R. R., & Kim, H. (2023). Cradle-to-cradle recycling of spent NMC batteries with emphasis on novel Co2+/Ni2+ separation from HCl leached solution and synthesis of new ternary precursor. Process Safety and Environmental Protection, 170, 584–595. [CrossRef]
- Kovačević, A., Tolazzi, M., Sanadar, M., & Melchior, A. (2024). Hydrometallurgical recovery of metals from spent lithium-ion batteries with ionic liquids and deep eutectic solvents. Journal of Environmental Chemical Engineering, 12(4), 113248. [CrossRef]
- Kozhevnikova, A. V., Zinov’eva, I. V., Zakhodyaeva, Y. A., Baranovskaya, V. B., & Voshkin, A. A. (2022). Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries. Processes, 10(12), 2671. [CrossRef]
- Martín, M. I., García-Díaz, I., & López, F. A. (2023). Properties and perspective of using deep eutectic solvents for hydrometallurgy metal recovery. Minerals Engineering, 203, 108306. [CrossRef]
- Martín, M. I., García-Díaz, I., Rodríguez, M. L., Gutiérrez, M. C., del Monte, F., & López, F. A. (2024). Synthesis and Properties of Hydrophilic and Hydrophobic Deep Eutectic Solvents via Heating-Stirring and Ultrasound. Molecules, 29(13), 3089. [CrossRef]
- Milevskii, N. A., Zinov’eva, I. V., Zakhodyaeva, Y. A., & Voshkin, A. A. (2022). Separation of Li(I), Co(II), Ni(II), Mn(II), and Fe(III) from hydrochloric acid solution using a menthol-based hydrophobic deep eutectic solvent. Hydrometallurgy, 207, 105777. [CrossRef]
- Ola, P. D., & Matsumoto, M. (2019). Use of deep eutectic solvent as extractant for separation of Fe (III) and Mn (II) from aqueous solution. Separation Science and Technology, 54(5), 759–765. [CrossRef]
- Palluzzi, M., Tsurumaki, A., Adenusi, H., Navarra, M. A., & Passerini, S. (2023). Ionic liquids and their derivatives for lithium batteries: role, design strategy, and perspectives. Energy Materials, 3(6), 300049. [CrossRef]
- Parliament, E. (2022). https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en.
- Phaechamud, T., Tuntarawongsa, S., & Charoensuksai, P. (2016). Evaporation Behavior and Characterization of Eutectic Solvent and Ibuprofen Eutectic Solution. AAPS PharmSciTech, 17(5), 1213–1220. [CrossRef]
- Prabhune, A., & Dey, R. (2023). Green and sustainable solvents of the future: Deep eutectic solvents. Journal of Molecular Liquids, 379, 121676. [CrossRef]
- Sellami, F., Kebiche-Senhadji, O., Marais, S., Lanel, C., & Fatyeyeva, K. (2021). Novel Poly(Vinylidene Fluoride)/Montmorillonite Polymer Inclusion Membrane: Application to Cr(VI) Extraction from Polluted Water. Membranes, 11(9), 682. [CrossRef]
- Smith, E. L., Abbott, A. P., & Ryder, K. S. (2014). Deep Eutectic Solvents (DESs) and Their Applications. Chemical Reviews, 114(21), 11060–11082. [CrossRef]
- Structural and Physical Properties of Biofield Treated Thymol and Menthol. (2015). Journal of Molecular Pharmaceutics & Organic Process Research, 03(02). [CrossRef]
- Tereshatov, E. E., Boltoeva, M. Y., & Folden, C. M. (2016). First evidence of metal transfer into hydrophobic deep eutectic and low-transition-temperature mixtures: indium extraction from hydrochloric and oxalic acids. Green Chemistry, 18(17), 4616–4622. [CrossRef]
- Thompson, D. L., Pateli, I. M., Lei, C., Jarvis, A., Abbott, A. P., & Hartley, J. M. (2022). Separation of nickel from cobalt and manganese in lithium ion batteries using deep eutectic solvents. Green Chemistry, 24(12), 4877–4886. [CrossRef]
- Vera, R., Anticó, E., Eguiazábal, J. I., Aranburu, N., & Fontàs, C. (2019). First Report on a Solvent-Free Preparation of Polymer Inclusion Membranes with an Ionic Liquid. Molecules, 24(10), 1845. [CrossRef]
- Vieceli, N., Casasola, R., Lombardo, G., Ebin, B., & Petranikova, M. (2021). Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Management, 125, 192–203. [CrossRef]
- Xue, K., Fan, D., Wang, X., Dong, Z., Zhu, Z., Cui, P., Meng, F., Wang, Y., & Qi, J. (2023). Lithium extraction from aqueous medium using hydrophobic deep eutectic solvents. Journal of Environmental Chemical Engineering, 11(5), 110490. [CrossRef]
- Zanoletti, A., Mannu, A., & Cornelio, A. (2025). Solvometallurgy as Alternative to Pyro- and Hydrometallurgy for Lithium, Cobalt, Nickel, and Manganese Extraction from Black Mass Processing: State of the Art. Materials, 18(12), 2761. [CrossRef]
Figure 1.
FTIR spectra of Aliquat 336, L-Menthol and DES (3 Aliquat 336:7 L-Menthol).
Figure 1.
FTIR spectra of Aliquat 336, L-Menthol and DES (3 Aliquat 336:7 L-Menthol).
Figure 2.
Differential scanning calorimetry (DSC) (a) and thermogravimetry (TGA) (b) of DES (3 Aliquat 336:7 L-Menthol).
Figure 2.
Differential scanning calorimetry (DSC) (a) and thermogravimetry (TGA) (b) of DES (3 Aliquat 336:7 L-Menthol).
Figure 5.
Effect of equilibrium time. Aqueous phase: [Co]0 = 1 g/L Co, 6 M HCl. Organic-to- aqueous ratio 1/1. Temperature 25°C.
Figure 5.
Effect of equilibrium time. Aqueous phase: [Co]0 = 1 g/L Co, 6 M HCl. Organic-to- aqueous ratio 1/1. Temperature 25°C.
Figure 6.
Effect of HCl concentration on Co(II) extraction Aqueous phase: [Co]0 = 2 g/L, Temperature: 25ºC. Equilibrium time: 60 min.
Figure 6.
Effect of HCl concentration on Co(II) extraction Aqueous phase: [Co]0 = 2 g/L, Temperature: 25ºC. Equilibrium time: 60 min.
Figure 7.
Influence of NaCl concentration on Co(II) extraction. Aqueous phase: [Co]0 = 2 or 6 g/L de Co, 6 M HCl, [NaCl] 12.5 y 25 g/L Aqueous-to-organic ratio 1/1; Temperature: 25ºC, Equilibrium time: 60 min.
Figure 7.
Influence of NaCl concentration on Co(II) extraction. Aqueous phase: [Co]0 = 2 or 6 g/L de Co, 6 M HCl, [NaCl] 12.5 y 25 g/L Aqueous-to-organic ratio 1/1; Temperature: 25ºC, Equilibrium time: 60 min.
Figure 9.
1H-NMR spectra of DES-Co after stripping process, (500 MHz, DMSO-d6) at 60ºC.
Figure 9.
1H-NMR spectra of DES-Co after stripping process, (500 MHz, DMSO-d6) at 60ºC.
Figure 10.
X-ray diffraction patterns of the salts obtained using H2SO4 concentration in the stripping stage of (a) 0.01M and (b) 0.5 M.
Figure 10.
X-ray diffraction patterns of the salts obtained using H2SO4 concentration in the stripping stage of (a) 0.01M and (b) 0.5 M.
Figure 11.
X-ray diffraction pattern of the obtained solid (Aqueous Phase: 0.5 M H2SO4) from the treatment of BM6.
Figure 11.
X-ray diffraction pattern of the obtained solid (Aqueous Phase: 0.5 M H2SO4) from the treatment of BM6.
Figure 12.
Scheme of separation of Cu(II) process.
Figure 12.
Scheme of separation of Cu(II) process.
Figure 13.
Effect of Aliquat 336 concentration on the extraction of Cu(II) from 3 M HCl solution; Aqueous phase: [Co]0 = 5 g/L, [Cu]0 = 6 g/L, Aqueous-to-organic ratio 1/1; Temperature: 25ºC. Equilibrium time: 60 min.
Figure 13.
Effect of Aliquat 336 concentration on the extraction of Cu(II) from 3 M HCl solution; Aqueous phase: [Co]0 = 5 g/L, [Cu]0 = 6 g/L, Aqueous-to-organic ratio 1/1; Temperature: 25ºC. Equilibrium time: 60 min.
Figure 14.
Effect of equilibrium time.
Figure 14.
Effect of equilibrium time.
Table 1.
Distribution coefficients as a function of reaction time.
Table 1.
Distribution coefficients as a function of reaction time.
| Reaction time (min) |
DCo |
5
10
20
30
60
120 |
0.955
1.064
1.108
1.200
1.494
1.443 |
| 180 |
1.344 |
| 240 |
1.333 |
Table 2.
Influence of initial Co concentration.
Table 2.
Influence of initial Co concentration.
| [Co]0 (g/L) |
E Co (%) |
DCo |
| 1 |
57 |
1.344 |
| 2 |
55 |
1.207 |
| 4 |
59 |
1.410 |
| 6 |
55 |
1.235 |
Table 3.
Influence of initial HCl concentration in the solution.
Table 3.
Influence of initial HCl concentration in the solution.
| [HCl]0 (M) |
[Cl-] (M) |
DCo |
| 2 |
4.472*103
|
0.000 |
| 4 |
6.324*103
|
0.130 |
| 6 |
6.324*103
|
1.207 |
8
10
12 |
8.944*103
1.095*104
1.095*104
|
4.896
9.794
8.489 |
Table 4.
Influence of NaCl concentration on Co(II) extraction.
Table 4.
Influence of NaCl concentration on Co(II) extraction.
| a) [Co]0 = 2 g/L |
|
|
|
| [NaCl]0 (g/L) |
[Cl-]total (M) |
ECo(%) |
DCo |
| 0 |
4.472*103
|
55 |
1.207 |
| 12.5 |
4.472*103
|
58 |
1.360 |
| 25 |
4.472*103
|
61 |
1.590 |
| b) [Co]0 = 6 g/L |
|
|
|
| [NaCl]0 (g/L) |
[Cl-]total (M) |
ECo(%) |
DCo |
| 0 |
7.745*103
|
55 |
1.235 |
| 12.5 |
7.745*103
|
59 |
1.447 |
| 25 |
7.745*103
|
61 |
1.579 |
Table 5.
Results obtained in the two consecutive stages of Co(II) extraction.
Table 5.
Results obtained in the two consecutive stages of Co(II) extraction.
| a) [Co]0 = 2 g/L |
|
|
| Stage |
ECo (%) |
DCo |
| 1 |
68 |
2.094 |
| 2 |
52 |
1.073 |
| Total |
84 |
5.413 |
|
b) [Co]0 = 6 g/L
|
| Stage |
ECo (%) |
DCo
|
| 1 |
63 |
1.716 |
| 2 |
53 |
1.111 |
| Total |
83 |
4.734 |
Table 6.
Influence of the aqueous-organic phase ratio on Co(II) extraction.
Table 6.
Influence of the aqueous-organic phase ratio on Co(II) extraction.
| Aqueous-to-organic ratio |
ECo (%) |
DCo |
| 10/30 |
73 |
2.770 |
| 10/20 |
59 |
1.454 |
| 20/20 |
55 |
1.207 |
| 20/10 |
52 |
1.141 |
Table 7.
Influence of the concentration of the stripping solution (HCl) on the percentage of recovered Co(II).
Table 7.
Influence of the concentration of the stripping solution (HCl) on the percentage of recovered Co(II).
| HCl [M] |
Co stripping (%) |
| 0.01 |
100 |
| 0.1 |
100 |
Table 8.
Influence of the concentration of the stripping aqueous solution (H2SO4) on the percentage of recovered Co(II).
Table 8.
Influence of the concentration of the stripping aqueous solution (H2SO4) on the percentage of recovered Co(II).
| H2SO4 [M] |
Co stripping (%) |
| 0.01 |
100 |
| 0.5 |
84 |
| 1 |
84 |
| 3 |
76 |
| 5 |
58 |
Table 9.
Extraction and stripping percentages of Co(II) and distribution coefficients after two consecutive stages, for different Co(II) concentrations.
Table 9.
Extraction and stripping percentages of Co(II) and distribution coefficients after two consecutive stages, for different Co(II) concentrations.
| a) [Co]0 = 2 g/L |
|
|
| |
E Co (%) |
DCo |
| 1st cycle |
|
|
| Extraction |
65 |
1.839 |
| Stripping |
91 |
10.560 |
| 2nd cycle |
|
|
| Extraction |
35 |
0.546 |
| Stripping |
100 |
- |
| b) [Co]0 = 6 g/L |
| |
E Co (%) |
DCo
|
| 1st cycle |
|
|
| Extraction |
60 |
1.520 |
| Stripping |
91 |
10.071 |
| 2nd cycle |
|
|
| Extraction |
37 |
0.597 |
| Stripping |
100 |
- |
Table 10.
Percentages and distribution coefficients under the best conditions of extraction and stripping stages.
Table 10.
Percentages and distribution coefficients under the best conditions of extraction and stripping stages.
| |
E Co (%) |
DCo |
| Extraction |
91 |
9.998 |
| Stripping |
84 |
5.438 |
Table 11.
Chemical composition of the solutions after leaching in HCl medium (10 M) of the studied black masses, ratio S/L = 100 g/L, reaction time = 2 h, temperature = 70ºC.
Table 11.
Chemical composition of the solutions after leaching in HCl medium (10 M) of the studied black masses, ratio S/L = 100 g/L, reaction time = 2 h, temperature = 70ºC.
| Metal |
BM6 (g/L) |
BM8 (g/L) |
BM9 (g/L) |
BM5 (g/L) |
BM1 (g/L) |
Co
Ni
Mn
Cu
Li
IR* |
10.3
33.8
10.3
1.2
6.6
31.3 |
7.3
12.8
13.8
7.3
2.8
32.6 |
12.1
50.4
13.9
8.7
1.2
18.0 |
4.4
24.4
4.5
3.8
0.7
53.9 |
12.9
27.7
10.2
1.1
1.3*10-3
41.3 |
Table 12.
Extraction percentages for leached black masses.
Table 12.
Extraction percentages for leached black masses.
| Metal |
E [BM6]
(%)
|
E [BM8]
(%)
|
E [BM9]
(%)
|
E [BM9-5]
(%)
|
E [BM6-1]
(%)
|
Co
Ni
Mn
Cu
Li |
80.2
0
2.9
87.1
0 |
87.8
0
14.9
92.5
0 |
81.2
0
0
84.4
0 |
84.7
0
8.8
83.3
0 |
83.1
0
0
85.3
0 |
Table 13.
Stripping percentages for leached black masses (with 0.5 M H2SO4).
Table 13.
Stripping percentages for leached black masses (with 0.5 M H2SO4).
| Metal |
RE [BM6]
(%)
|
RE [BM8]
(%)
|
RE [BM9]
(%)
|
RE [BM9-5]
(%)
|
RE [BM6-1]
(%)
|
Co
Ni
Mn
Cu
Li |
82.7
0
100
19.8
0 |
75.8
0
100
18.7
0 |
79.7
0
0
39.6
0 |
100
0
100
57.6
0 |
84.8
0
0
31.6
0 |
Table 14.
Stages of extraction for removal of Cu(II). Aqueous phase: [Co]0 = 5 g/L, [Cu]0 = 6 g/L, 3 M HCl. Organic phase: Aliquat 336 0.6 / 0.7 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
Table 14.
Stages of extraction for removal of Cu(II). Aqueous phase: [Co]0 = 5 g/L, [Cu]0 = 6 g/L, 3 M HCl. Organic phase: Aliquat 336 0.6 / 0.7 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
[Aliquat 336]
mg/L
|
Stage |
[Co]AP*
(g/L)
|
[Cu]AP*
(g/L)
|
E Co
(%)
|
E Cu
(%)
|
0.6
0.6
0.6
0.6
0.7
0.7
0.7
0.7 |
1
2
3
4
1
2
3
4 |
-
-
-
-
4.5
4.5
4.0
3.7 |
3.0
1.4
0.7
0.3
1.9
0.7
0.3
0.2 |
0
0
0
0
12.9
11.5
20.9
27.1 |
54.1
78.4
88.7
95.1
67.5
88.3
95.1
97.5 |
Table 15.
Chemical composition of leaching solutions in HCl medium (3 M) of the studied black masses, ratio S/L = 100 g/L, reaction time = 2 h, temperature = 70 ºC.
Table 15.
Chemical composition of leaching solutions in HCl medium (3 M) of the studied black masses, ratio S/L = 100 g/L, reaction time = 2 h, temperature = 70 ºC.
| Metal |
BM5 (g/L) |
TUC2 (g/L) |
Co
Ni
Mn
Cu
Li
IR* |
4.7
21.8
5.5
0.7
0.6
58.9 |
8.6
22.9
6.2
0.1
1.8
46.6 |
Table 16.
Extraction percentages of Cu(II) and Co(II) for leached black masses. Organic phase: Aliquat 336 0.6 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
Table 16.
Extraction percentages of Cu(II) and Co(II) for leached black masses. Organic phase: Aliquat 336 0.6 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
| Black mass |
Stage |
[Co]AP* (g/L) |
[Cu]AP* (g/L) |
E Co (%) |
E Cu (%) |
BM5 |
1
2
3
4 |
4.8
4.0
3.5
2.7 |
0.2
0.06
0.01
0.003 |
17.6
24.4
28.7
45.3 |
72.9
92.1
97.9
99.5 |
TUC2 |
1
2
3
4 |
6.9
6.2
5.2
3.9 |
0.03
0.006
0.002
0.5*10-3
|
3.6
12.9
27.9
44.8 |
79.3
95.0
98.7
99.6 |
Table 17.
Extraction percentages of Cu(II) and Co(II) for leached black masses. Organic phase: Aliquat 336 0.7 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
Table 17.
Extraction percentages of Cu(II) and Co(II) for leached black masses. Organic phase: Aliquat 336 0.7 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
| Black mass |
Stage |
[Co]AP* (g/L) |
[Cu]AP* (g/L) |
E Co (%) |
E Cu (%) |
BM5 |
1
2
3
4 |
4.0
3.2
2.3
2.1 |
0.1
0.03
0.006
0.001 |
15.4
32.2
50.5
54.6 |
77.8
95.4
99.0
99.8 |
TUC2 |
1
2
3
4 |
5.5
4.8
4.2
3.5 |
0.01
0.003
0.9*10-3
0.3*10-3
|
36.9
44.3
51.9
59.0 |
89.4
99.5
99.9
100 |
Table 18.
Extraction percentages of Cu(II) and Co(II) for leached black masses. Organic phase: Aliquat 336 0.6 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
Table 18.
Extraction percentages of Cu(II) and Co(II) for leached black masses. Organic phase: Aliquat 336 0.6 M in kerosene with 10% decanol. Aqueous-to-organic ratio 1/1. Temperature 25°C.
| Black mass |
Stage |
[Co]AP* (g/L) |
[Cu]AP* (g/L) |
E Co (%) |
E Cu (%) |
| BM5 |
1
2 |
4.0
3.2 |
0.2
0.05 |
5.1
23.0 |
73.3
91.8 |
| TUC2 |
1
2 |
6.9
5.7 |
0.03
0.007 |
12.2
27.7 |
77.1
93.8 |
Table 19.
Stripping percentages of Cu(II). Aqueous phase: H2SO4 1 M. Aqueous-to-organic ratio 1/1. Temperature 25°C.
Table 19.
Stripping percentages of Cu(II). Aqueous phase: H2SO4 1 M. Aqueous-to-organic ratio 1/1. Temperature 25°C.
| Black mass |
Stage |
[Cu]AP* (g/L) |
RE Cu (%) |
| BM5 |
2 |
0.116 |
95.82 |
| TUC2 |
2 |
0.019 |
100 |
Table 20.
Extraction percentages for studied samples.
Table 20.
Extraction percentages for studied samples.
| Metal |
BM5
(g/L)
|
TUC2
(g/L)
|
[BM5]AP*
(g/L)
|
[TUC2]AP*
(g/L)
|
E [BM5]
(%)
|
E [TUC2]
(%)
|
Co
Ni
Mn
Cu
Li |
0.55
4.87
1.04
0.009
0.11 |
1.00
4.96
1.47
0.001
0.38 |
0.038
4.96
0.68
0.003
0.14 |
0.067
6.31
0.93
0.5*10-3
0.46 |
92.99034.36
70.460 |
93.26036.83
67.150 |
Table 21.
Stripping percentages of Co for studied samples.
Table 21.
Stripping percentages of Co for studied samples.
| Metal |
[BM5]AP* (g/L) |
[TUC2]AP* (g/L) |
RE [BM5] (%) |
RE [TUC2] (%) |
| Co |
0.42 |
0.66 |
82.51 |
71.39 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).